Laurus nobilis: Composition of Essential Oil and Its Biological Activities
Abstract
:1. Introduction
2. Results
2.1. Essential Oil Yield and Composition
2.2. Antimicrobial Activity
2.3. Antifungal Activity
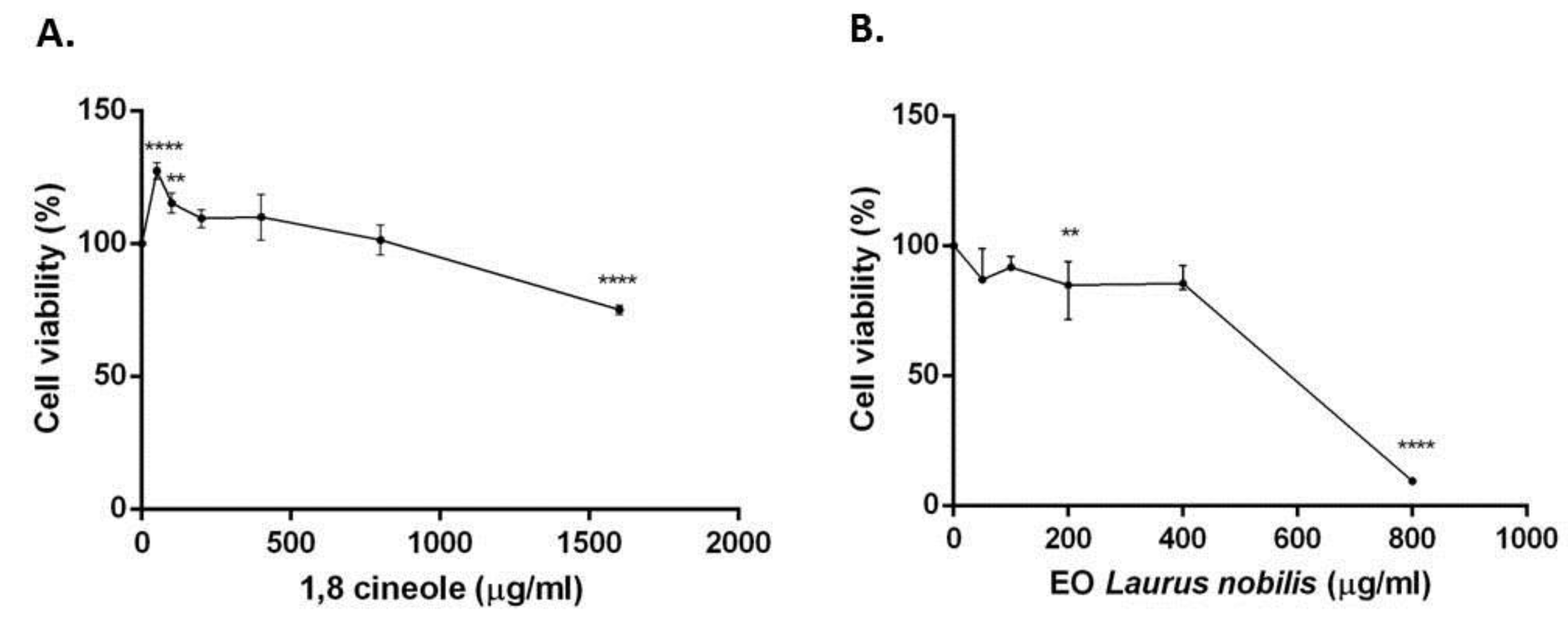
2.4. Cytotoxicity of 1,8-cineole and Laurus nobilis Essential oil
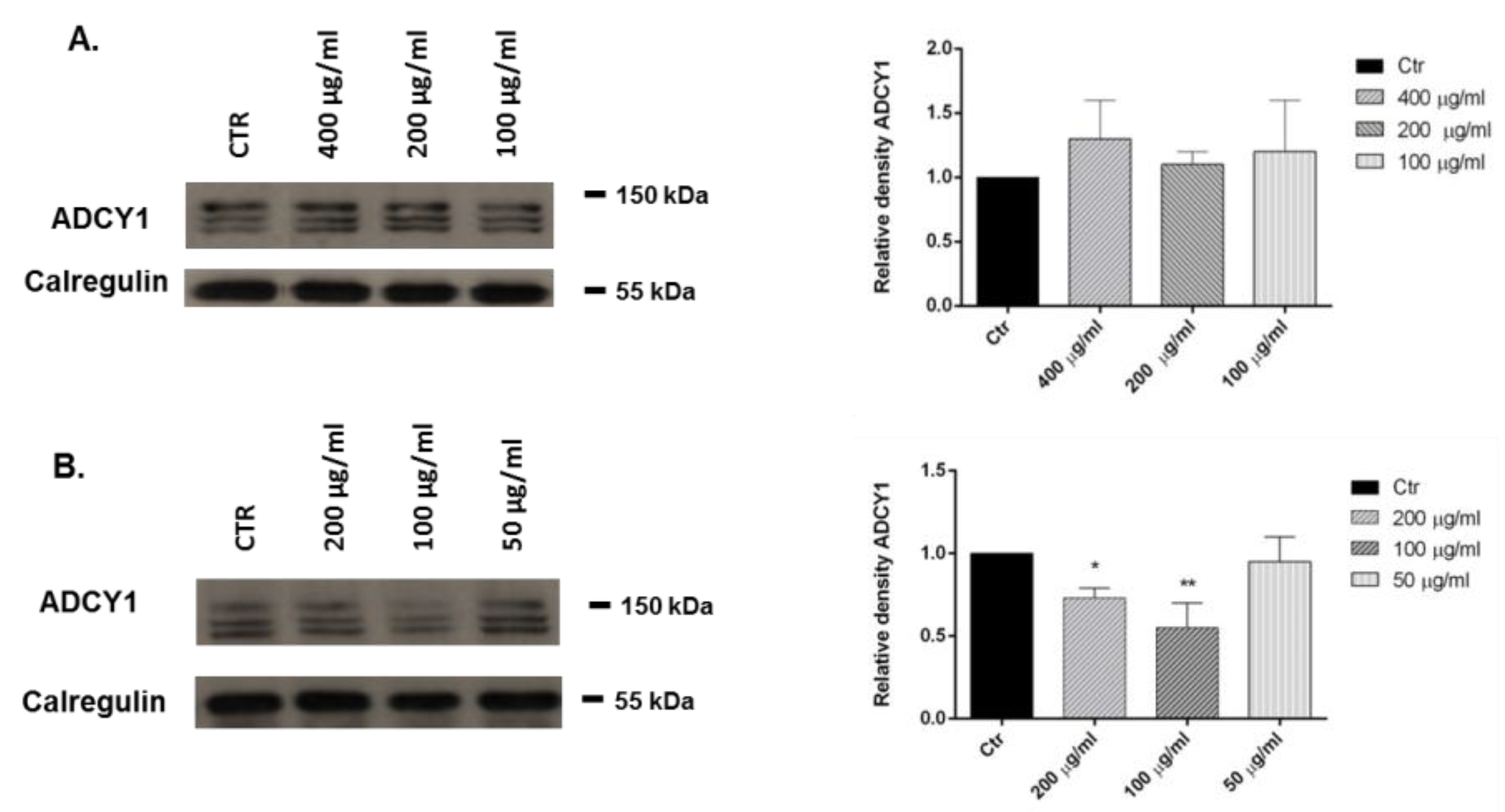
2.5. Adenylate Cyclase (ADCY1): Western Blot Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Isolation of Volatile Oil
4.3. GC-FID Analysis
4.4. GC/MS Analysis
4.5. Identification of Essential Oil Components
4.6. Antimicrobial Activity
4.7. Minimum Inhibitory Concentration (MIC)
4.8. Antifungal Activity
4.9. Cell Cultures
4.10. MTT Assay
4.11. Extraction Proteins and Western Blotting
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aqili Khorasani, M.S. Collection of Drugs (Materia Media); Enqelab-e-Eslami Publishing and Educational Organization: Teheran, Iran, 1992; pp. 624–630. [Google Scholar]
- Zargari, A. Medicinal Plants; Tehran University Press: Tehran, Iran, 1990; Volume 4, pp. 325–328. [Google Scholar]
- Abu-Dahab, R.; Kasabri, V.; Afifi, F.U. Evaluation of the volatile oil composition and antiproliferative activity of Laurus nobilis L. (Lauraceae) on breast cancer cell line models. Rec. Nat. Prod. 2014, 8, 136–147. [Google Scholar]
- Santoyo, S.; Lloria, R.; Jaime, L.; Ibañez, E. Supercritical fluid extraction of antioxidant and antimicrobial compounds from Laurus nobilis L. chemical and functional characterization. Eur. Food Res. Technol. 2006, 222, 565–571. [Google Scholar] [CrossRef]
- Derwich, E.; Benziane, Z.; Boukir, A. Chemical composition and antibacterial activity of leaves essential oil of Laurus nobilis from Morocco. Aust. J. Basic Appl. Sci. 2009, 3, 3818–3824. [Google Scholar]
- Ozcan, B.; Esen, M.; Sangun, M.K.; Coleri, A.; Caliskan, M. Effective antibacterial and antioxidant properties of methanolic extract of Laurus nobilis seed oil. J. Environ. Biol. 2010, 31, 637–641. [Google Scholar] [PubMed]
- Qnais, E.Y.; Abdulla, F.A.; Kaddumi, E.G.; Abdalla, S.S. Antidiarrheal activity of Laurus nobilis L. leaf extract in rats. J. Med. Food 2012, 15, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kosar, M.; Tunalier, Z.; Özek, T.; Kürkcüoglu, M.; Can Baser, K.H. A simple method to obtain essential oils from Salvia triloba L. and Laurus nobilis L. by using microwave-assisted hydrodistillation. Z. Naturforsch C 2005, 60, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Patrakar, R.; Mansuriya, M.; Patil, P. Phytochemical and pharmacological review on Laurus nobilis. Int. J. Pharm. Chem. Sci. 2012, 1, 595–602. [Google Scholar]
- Ekren, S.; Yerlikaya, O.; Tokul, H.E.; Akpınar, A.; Accedil, M. Chemical composition, antimicrobial activity and antioxidant capacity of some medicinal and aromatic plant extracts. Afr. J. Microbiol. Res. 2013, 7, 383–388. [Google Scholar]
- Snuossi, M.; Trabelsi, N.; Ben Taleb, S.; Dehmeni, A.; Flamini, G.; de Feo, V. Laurus nobilis, Zingiber officinale and Anethum graveolens Essential Oils: Composition, Antioxidant and Antibacterial Activities against Bacteria Isolated from Fish and Shellfish. Molecules 2016, 21, 1414. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, H.; Anik, M.; Sanda, M.A.; Cakir, A. Gas chromatography/mass spectrometry analysis of Laurus nobilis essential oil composition of northern Cyprus. J. Med. Food 2007, 10, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Jemâa, J.M.B.; Tersim, N.; Toudert, K.T.; Khouja, M.L. Insecticidal activities of essential oils from leaves of Laurus nobilis L. from Tunisia, Algeria and Morocco, and comparative chemical composition. J. Stored Prod. Res. 2012, 48, 97–104. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; de Martino, L.; Coppola, R.; de Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Sada, A.; Masucci, A.; Cipriano, L.; Nazzaro, F. Biochemical characteristics, antimicrobial and mutagenic activity in organically and conventionally produced Malus domestica, Annurca. Open Food Sci. J. 2007, 1, 10–16. [Google Scholar] [CrossRef]
- Aliberti, L.; Caputo, L.; de Feo, V.; de Martino, L.; Nazzaro, F.; Souza, L.F. Chemical Composition and in vitro Antimicrobial, Cytotoxic, and Central Nervous System Activities of the Essential Oils of Citrus medica L. cv.‘Liscia’ and C. medica cv. ‘Rugosa’ Cultivated in Southern Italy. Molecules 2016, 21, 1244. [Google Scholar] [CrossRef] [PubMed]
- Biondi, D.; Cianci, P.; Geraci, C.; Ruberto, G.; Piattelli, M. Antimicrobial activity and chemical composition of essential oils from sicilian aromatic plants. Flav. Fragr. J. 1993, 8, 331–337. [Google Scholar] [CrossRef]
- Bouzouita, N.; Kachouri, F.; Hamdi, M.; Chaabouni, M.M. Antimicrobial activity of essential oils from Tunisian aromatic plants. Flav. Fragr. J. 2003, 18, 380–383. [Google Scholar] [CrossRef]
- Goudjil, M.B.; Ladjel, S.; Bencheikh, S.E.; Zighmi, S.; Hamada, D. Study of the chemical composition, antibacterial and antioxidant activities of the essential oil extracted from the leaves of Algerian Laurus nobilis Lauraceae. J. Chem. Pharm. Res. 2015, 7, 379–385. [Google Scholar]
- Dadalioǧlu, I.; Evrendilek, GA. Chemical Compositions and Antibacterial Effects of Essential Oils of Turkish Oregano (Origanum minutiflorum), Bay Laurel (Laurus nobilis), Spanish Lavender (Lavandula. stoechas L.), and Fennel (Foeniculum vulgare) on Common Foodborne Pathogens. J. Agric. Food Chem. 2004, 52, 8255–8260. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsiha, S.; Sporera, F.; Zimmermannb, S.; Reichlinga, J.; Winka, M. Synergistic properties of the terpenoids aromadendrene and 1, 8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of eucalyptus oil and 1, 8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Simić, A.; Soković, M.D.; Ristić, M.; Grujić-Jovanović, S.; Vukojević, J.; Marin, P.D. The chemical composition of some Lauraceae essential oils and their antifungal activities. Phytother. Res. 2004, 18, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.; Arcand, Y. (Eds.) Green Technologies in Food Production and Processing; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Mejlholm, O.; Dalgaard, P. Antimicrobial effect of essential oils on the seafood spoilage microorganism Photobacterium phosphoreum in liquid media and fish products. Lett. Appl. Microbiol. 2002, 34, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Mello da Silveira, S.; Bittencourt Luciano, F.; Fronza, N.; Cunha, A., Jr.; Neudí Scheuermann, G.; Werneck Vieirad, C.R. Chemical composition and antibacterial activity of Laurus nobilis essential oil towards foodborne pathogens and its application in fresh Tuscan sausage stored at 7 °C. LWT-Food Sci. Technol. 2014, 59, 86–93. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; Macdonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumours and other biological systems. Cancer Chemother. Rep. 1972, 3, 59–61. [Google Scholar]
- Hiroyukimoteki, H.H.; Yamada, Y.; Hirotakakatsuzaki, K.I.; Komiya, T. Specific induction of apoptosis by 1, 8-cineole in two human leukemia cell lines, but not a in human stomach cancer cell line. Oncol. Rep. 2002, 9, 757–760. [Google Scholar]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res. 2007, 27, 3293–3299. [Google Scholar] [PubMed]
- Pacifico, S.; Gallicchio, M.; Lorenz, P.; Potenza, N.; Galasso, S.; Marciano, S.; Monaco, P. Apolar Laurus nobilis leaf extracts induce cytotoxicity and apoptosis towards three nervous system cell lines. Food Chem. Toxicol. 2013, 62, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.; Perry, E. Aromatherapy in the management of psychiatric disorders. CNS Drugs 2006, 20, 257–280. [Google Scholar] [CrossRef] [PubMed]
- Elisabetsky, E.; Silva Brum, L.F.; Souza, D.O. Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine 1999, 6, 107–113. [Google Scholar] [CrossRef]
- Caputo, L.; Souza, L.F.; Alloisio, S.; Cornara, L.; de Feo, V. Coriandrum Sativum and Lavandula angustifolia Essential Oils: Chemical Composition and Activity on Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1999. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982; Volume I, p. 351. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg Cedex, France, 2004; Volume I, p. 217. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Wiley Registry of Mass Spectral Data, with NIST Spectral Data CD Rom, 7th ed.; John Wiley & Sons: New York, NY, USA, 1998.
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Marrufo, T.; Nazzaro, F.; Mancini, E.; Fratianni, F.; Coppola, R.; de Martino, L.; Bela Agostinho, A.; de Feo, V. Chemical composition and biological activity of the essential oil from leaves of Moringa oleifera Lam. cultivated in Mozambique. Molecules 2013, 18, 10989–11000. [Google Scholar] [CrossRef] [PubMed]
- Picerno, P.; Autore, G.; Marzocco, S.; Meloni, M.; Sanogo, R.; Aquino, R.P. Anti-inflammatory activity of verminoside from Kigelia africana and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermis. J. Nat. Prod. 2005, 68, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Petrella, A.; Ercolino, S.F.; Festa, M.; Gentilella, A.; Tosco, A.; Conzen, S.D.; Parente, L. Dexamethasone inhibits TRAIL-induced apoptosis of thyroid cancer cells via Bcl-xL induction. Eur. J. Cancer 2006, 42, 3287–3293. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the essential oil and 1,8-cineole are available from the authors. |
| No. | Compound | % | Ri a | Ri b | Identification c |
|---|---|---|---|---|---|
| 1 | Methyl pentanoate | 0.1 | 850 | 828 | 1,2 |
| 2 | Ethyl isovalerate | 0.1 | 853 | 858 | 1,2 |
| 3 | α-Thujene | 0.7 | 916 | 930 | 1,2 |
| 4 | α-Pinene | 5.8 | 922 | 939 | 1,2,3 |
| 5 | Camphene | 0.8 | 935 | 954 | 1,2 |
| 6 | Sabinene | 12.2 | 962 | 975 | 1,2 |
| 7 | β-Pinene | 1.4 | 980 | 979 | 1,2,3 |
| 8 | α-Phellandrene | 0.5 | 991 | 1002 | 1,2,3 |
| 9 | δ-2-Carene | 0.4 | 997 | 1002 | 1,2 |
| 10 | α-Terpinene | 0.6 | 1004 | 1017 | 1,2,3 |
| 11 | o-Cymene | 0.3 | 1013 | 1026 | 1,2 |
| 12 | 1,8-Cineole | 31.9 | 1016 | 1031 | 1,2,3 |
| 13 | (Z)-β-Ocimene | 0.2 | 1028 | 1037 | 1,2 |
| 14 | (E)-β-Ocimene | 0.2 | 1038 | 1050 | 1,2 |
| 15 | γ-Terpinene | 1.0 | 1048 | 1059 | 1,2,3 |
| 16 | cis-Sabinene hydrate | 0.3 | 1057 | 1070 | 1,2 |
| 17 | ρ-Mentha-3,8-diene | 0.5 | 1077 | 1072 | 1,2 |
| 18 | trans-Sabinene hydrate | 10.2 | 1093 | 1098 | 1,2 |
| 19 | Linalool | 0.1 | 1096 | 1096 | 1,2,3 |
| 20 | exo-Fenchol | 0.1 | 1111 | 1121 | 1,2 |
| 21 | allo-Ocimene | 0.2 | 1118 | 1132 | 1,2 |
| 22 | trans-Sabinol | 0.2 | 1128 | 1142 | 1,2 |
| 23 | Camphor | 0.2 | 1133 | 1146 | 1,2,3 |
| 24 | β-Pinene oxide | 0.1 | 1147 | 1159 | 1,2 |
| 25 | Isoborneol | 0.5 | 1155 | 1160 | 1,2 |
| 26 | iso-Isopulegol | 0.6 | 1157 | 1159 | 1,2 |
| 27 | neoiso-Isopulegol | 2.5 | 1165 | 1171 | 1,2 |
| 28 | α-Terpineol | 3.3 | 1180 | 1188 | 1,2,3 |
| 29 | cis-Carveol | 0.2 | 1219 | 1229 | 1,2 |
| 30 | cis-p-Mentha-1(7),8-dien-2-ol | 0.1 | 1232 | 1230 | 1,2 |
| 31 | trans-Sabinene hydrate acetate | 0.7 | 1246 | 1256 | 1,2 |
| 32 | 2-(1E)-Propenyl-phenol | 0.1 | 1265 | 1267 | 1,2 |
| 33 | neo-3-Thujanol acetate | 0.4 | 1275 | 1276 | 1,2 |
| 34 | α-Terpinen-7-al | 0.3 | 1284 | 1285 | 1,2 |
| 35 | iso-Verbanol acetate | 0.3 | 1306 | 1309 | 1,2 |
| 36 | α-Terpinyl acetate | 5.9 | 1340 | 1349 | 1,2 |
| 37 | Eugenol | 1.6 | 1347 | 1359 | 1,2,3 |
| 38 | Cyclosativene | 0.1 | 1360 | 1371 | 1,2 |
| 39 | Longicyclene | 0.2 | 1373 | 1374 | 1,2 |
| 40 | β-Elemene | 0.4 | 1381 | 1390 | 1,2 |
| 41 | Methyl-eugenol | 3.3 | 1394 | 1403 | 1,2,3 |
| 42 | β-Funebrene | 0.5 | 1408 | 1414 | 1,2 |
| 43 | cis-Thujopsene | 0.2 | 1427 | 1431 | 1,2 |
| 44 | Spirolepechinene | 0.1 | 1445 | 1451 | 1,2 |
| 45 | allo-Aromadendrene | 0.1 | 1449 | 1460 | 1,2,3 |
| 46 | γ-Himachalene | 0.1 | 1474 | 1482 | 1,2 |
| 47 | a-Amorphene | 0.1 | 1483 | 1484 | 1,2 |
| 48 | δ-Amorphene | 0.1 | 1502 | 1512 | 1,2 |
| 49 | δ-Cadinene | 0.2 | 1512 | 1523 | 1,2 |
| 50 | Elemicin | 0.5 | 1546 | 1557 | 1,2 |
| 51 | Spathulenol | 0.4 | 1563 | 1578 | 1,2,3 |
| 52 | Caryophyllene oxide | 0.3 | 1572 | 1583 | 1,2,3 |
| 53 | Thujopsan-2-α-ol | 0.1 | 1580 | 1587 | 1,2 |
| 54 | Viridiflorol | 0.2 | 1591 | 1592 | 1,2 |
| 55 | Eremoligenol | 0.1 | 1630 | 1631 | 1,2 |
| Total | 91.6 | ||||
| Monoterpenes hydrocarbons | 34.0 | ||||
| Oxygenated monoterpenes | 48.6 | ||||
| Sesquiterpene hydrocarbons | 3.2 | ||||
| Oxygenated sesquiterpenes | 0.2 | ||||
| Phenolic compounds | 5.6 |
| Bacterial Strains | Inhibition Diameter (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Laurus nobilis Essential Oil | 1,8-cineole | Tetracycline | |||||
| 0.4 µL/mL | 1 µL/mL | 2 µL/mL | 0.4 µL/mL | 1 µL/mL | 2 µL/mL | 7 µg/mL | |
| B.cereus 4313 | 8.66 ± 1.54 b | 14.66 ± 0.57 c | 18 ± 0 e | 5.66 ± 1.54 e | 12 ± 2.64 c | 14.66 ± 0.57 d | 10.33 ± 0.57 a |
| B. cereus 4384 | 7.66 ± 1.54 b | 12 ± 2.64 d | 15.66 ± 0.57 e | 5.66 ± 1.54 c | 11.66 ± 1.54 c | 14.66 ± 0.57 e | 8.67 ± 1.67 a |
| S. aureus | 8.33 ± 0.57 c | 11.66 ± 1.54 a | 13.33 ± 1.54 b | 0 ± e | 7.66 ± 1.54 d | 12 ± 1.54 a | 11.33 ± 0.57 a |
| E. coli | 6.33 ± 0.57 e | 12 ± 0 a | 16 ± 2 e | 0 ± e | 0 ± e | 5.66 ± 1.54 e | 12.70 ± 1.67 a |
| P. aeruginosa | 8.33 ± 1.54 b | 12 ± 1.73 d | 15.33 ± 0.57 e | 0 ± e | 7.66 ± 1.54 c | 12 ± 1.73 d | 9.67 ± 0.57 a |
| Microorganism | MIC (µL/mL) | |
|---|---|---|
| Laurus nobilis | 1,8-cineole | |
| Bacillus cereus 4313 | 0.2 | 0.2 |
| Bacillus cereus 4384 | 0.2 | 0.4 |
| Staphylococcus aureus | 0.4 | 1 |
| Escherichia coli | 0.8 | 1.5 |
| Pseudomonas aeruginosa | 0.4 | 1 |
| A. niger | A. versicolor | P. citrinum | P. expansum | |
|---|---|---|---|---|
| Laurus nobilis EO | ||||
| 0.4 µL | 2 ± 0 | - | 2.33 ± 0.57 | 8 ± 1.73 |
| 1 µL | 4.33 ± 1.52 | 5.66 ± 1.54 | 4.66 ± 0.57 | 9.33 ± 2.08 |
| 2 µL | 6 ± 1 | 7.66 ± 1.54 | 5.66 ± 1.54 | 9.66 ± 0.57 |
| 1,8-cineole | ||||
| 2 µL | - | - | - | - |
| 4 µL | - | - | - | - |
| 8 µL | 5.66 ± 1.54 | 7.66 ± 1.54 | 5.66 ± 1.54 | 9.66 ± 0.57 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 2017, 22, 930. https://doi.org/10.3390/molecules22060930
Caputo L, Nazzaro F, Souza LF, Aliberti L, De Martino L, Fratianni F, Coppola R, De Feo V. Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules. 2017; 22(6):930. https://doi.org/10.3390/molecules22060930
Chicago/Turabian StyleCaputo, Lucia, Filomena Nazzaro, Lucéia Fatima Souza, Luigi Aliberti, Laura De Martino, Florinda Fratianni, Raffaele Coppola, and Vincenzo De Feo. 2017. "Laurus nobilis: Composition of Essential Oil and Its Biological Activities" Molecules 22, no. 6: 930. https://doi.org/10.3390/molecules22060930
APA StyleCaputo, L., Nazzaro, F., Souza, L. F., Aliberti, L., De Martino, L., Fratianni, F., Coppola, R., & De Feo, V. (2017). Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules, 22(6), 930. https://doi.org/10.3390/molecules22060930










