Daucosterol Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Wnt/β-Catenin Signaling
Abstract
1. Introduction
2. Results
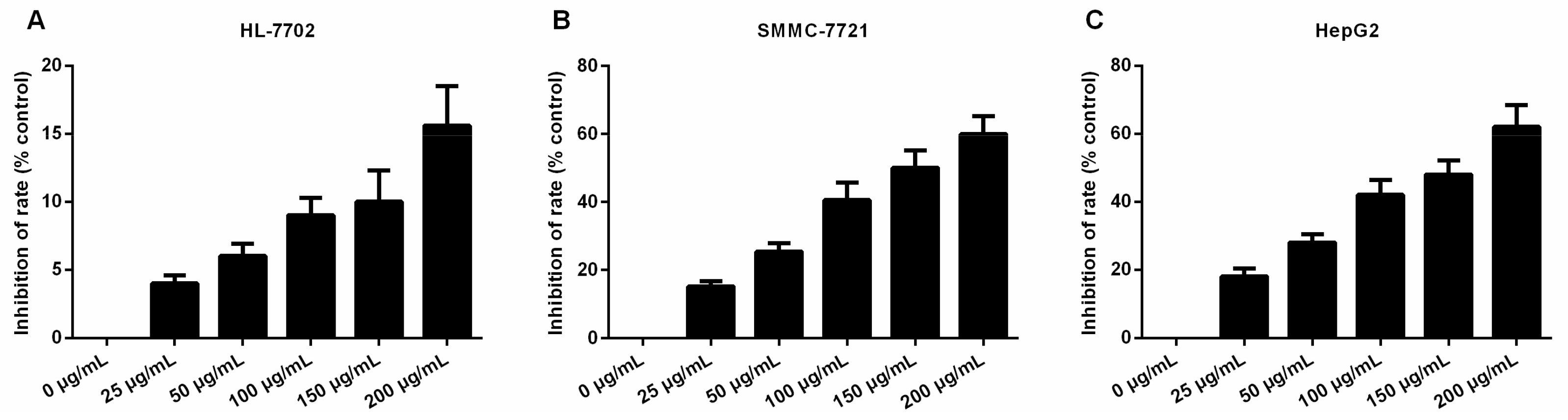
2.1. Daucosterol Inhibited the Proliferation of HepG2 and SMMC-7721 Cells
2.2. Daucosterol Decreased Cell Migration and Invasion Abilities of HepG2 and SMMC-7721 Cells
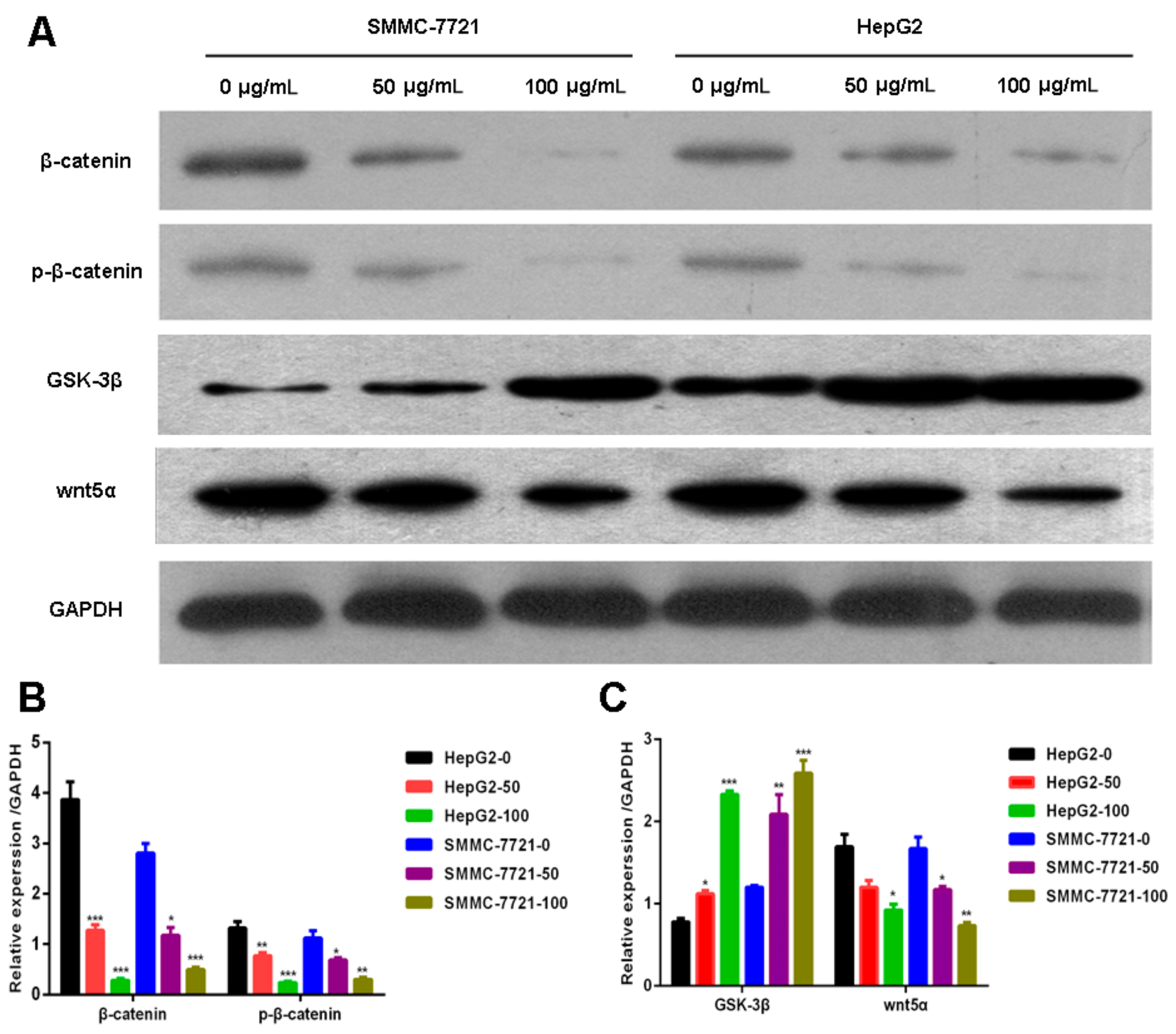
2.3. Daucosterol Suppressed the Expression of Wnt/β-Catenin Signaling Proteins in HepG2 and SMMC-7721 Cells
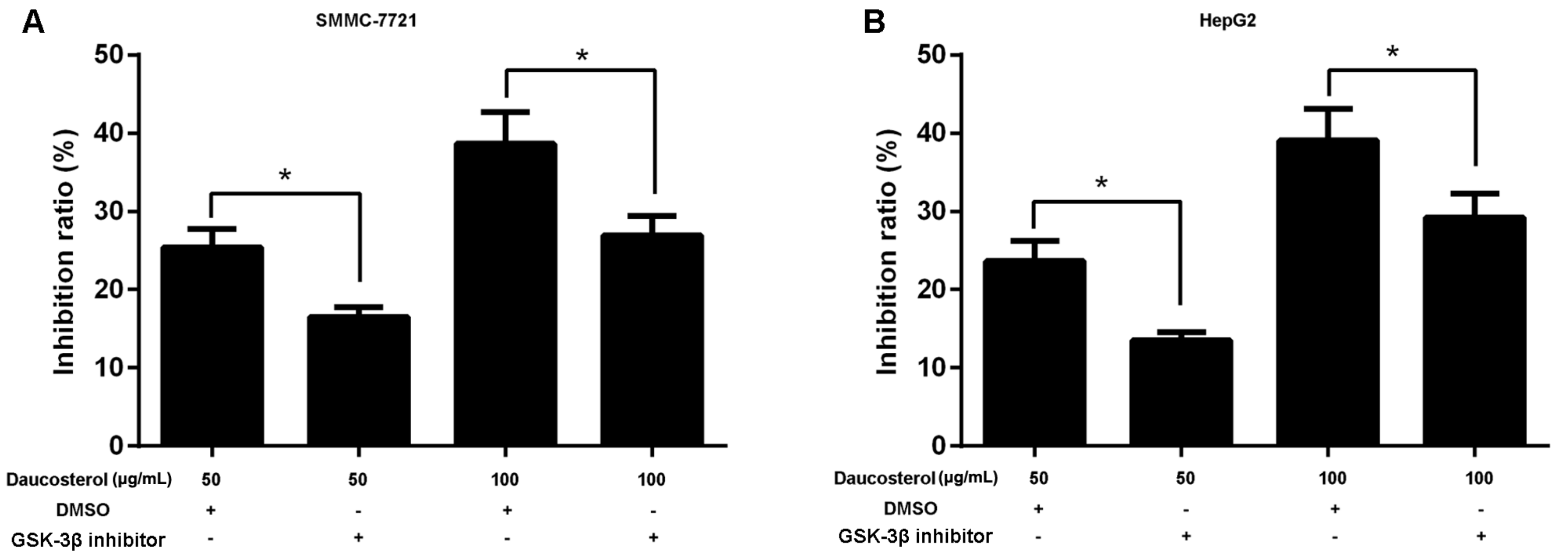
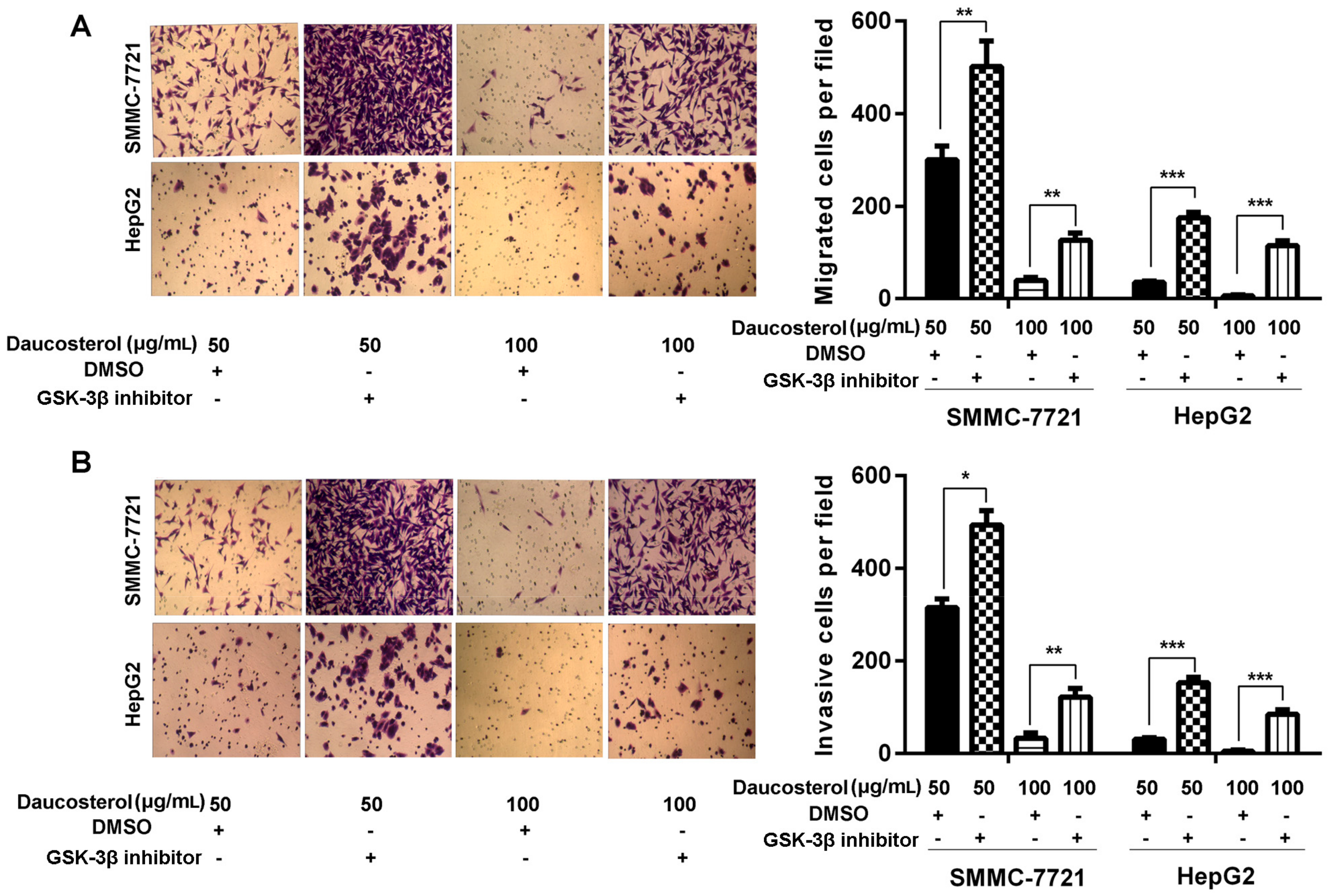
2.4. Daucosterol Inhibited the Proliferation, Migration, and Invasion of HCC Cells through Wnt/β-Catenin Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Proliferation Assay
4.3. Transwell Assays
4.4. Western Blot Analysis
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Z.; Zhang, C.; Lou, C.; Yan, F.; Mao, Y.; Hong, X.; Zhang, Y. Comparison of percutaneous cryosurgery and surgical resection for the treatment of small hepatocellular carcinoma. Oncol. Lett. 2013, 6, 239–245. [Google Scholar] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Asia-Pacific Working Party on Prevention of Hepatocellular Carcinoma. Prevention of hepatocellular carcinoma in the Asia-Pacific region: Consensus statements. J. Gastroenterol. Hepatol. 2010, 25, 657–663. [Google Scholar]
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Baffy, G. Hepatocellular carcinoma in non-alcoholic fatty liver disease: Epidemiology, pathogenesis, and prevention. J. Clin. Transl. Hepatol. 2013, 1, 131–137. [Google Scholar] [PubMed]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F.M. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.P.; Li, S.; Li, H.B. Spices for prevention and treatment of cancers. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.H.; Clausen, L.M.; Allred, K.F.; Almada, A.L.; Helferich, W.G. Beta-sitosterol, beta-sitosterol glucoside, and a mixture of beta-sitosterol and beta-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells in vitro and in ovariectomized athymic mice. J. Nutr. 2004, 134, 1145–1151. [Google Scholar] [PubMed]
- Paniagua-Perez, R.; Madrigal-Bujaidar, E.; Reyes-Cadena, S.; Alvarez-Gonzalez, I.; Sanchez-Chapul, L.; Perez-Gallaga, J.; Hernandez, N.; Flores-Mondragon, G.; Velasco, O. Cell protection induced by beta-sitosterol: Inhibition of genotoxic damage, stimulation of lymphocyte production, and determination of its antioxidant capacity. Arch. Toxicol. 2008, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Meng, X.; Li, Y.; Zhou, Y.; Xu, D.P.; Li, S.; Li, H.B. Effects of melatonin on liver injuries and diseases. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Raicht, R.F.; Cohen, B.I.; Fazzini, E.P.; Sarwal, A.N.; Takahashi, M. Protective effect of plant sterols against chemically induced colon tumors in rats. Cancer Res. 1980, 40, 403–405. [Google Scholar] [PubMed]
- Deschner, E.E.; Cohen, B.I.; Raicht, R.F. The kinetics of the protective effect of beta-sitosterol against MNU-induced colonic neoplasia. J. Cancer Res. Clin. Oncol. 1982, 103, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Janezic, S.A.; Rao, A.V. Dose-dependent effects of dietary phytosterol on epithelial cell proliferation of the murine colon. Food Chem. Toxicol. 1992, 30, 611–616. [Google Scholar] [CrossRef]
- Awad, A.B.; Hernandez, A.Y.; Fink, C.S.; Mendel, S.L. Effect of dietary phytosterols on cell proliferation and protein kinase C activity in rat colonic mucosa. Nutr. Cancer 1997, 27, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Q.; Gu, J.F.; Gao, Y.C.; Dai, Y.J. Daucosterol inhibits colon cancer growth by inducing apoptosis, inhibiting cell migration and invasion and targeting caspase signalling pathway. Bangladesh J. Pharmacol. 2016, 11, 395–401. [Google Scholar] [CrossRef]
- Zhao, C.; She, T.; Wang, L.; Su, Y.; Qu, L.; Gao, Y.; Xu, S.; Cai, S.; Shou, C. Daucosterol inhibits cancer cell proliferation by inducing autophagy through reactive oxygen species-dependent manner. Life Sci. 2015, 137, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bouic, P.J.; Etsebeth, S.; Liebenberg, R.W.; Albrecht, C.F.; Pegel, K.; van Jaarsveld, P.P. Beta-Sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 1996, 18, 693–700. [Google Scholar] [CrossRef]
- Choi, J.N.; Choi, Y.H.; Lee, J.M.; Noh, I.C.; Park, J.W.; Choi, W.S.; Choi, J.H. Anti-inflammatory effects of beta-sitosterol-beta-d-glucoside from Trachelospermum jasminoides (Apocynaceae) in lipopolysaccharide-stimulated RAW 264.7 murine macrophages. Nat. Prod. Res. 2012, 26, 2340–2343. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J.Y.; Park, J.H.; Jung, H.S.; Kim, J.S.; Kang, S.S.; Kim, Y.S.; Han, Y. Immunoregulatory activity by daucosterol, a beta-sitosterol glycoside, induces protective Th1 immune response against disseminated Candidiasis in mice. Vaccine 2007, 25, 3834–3840. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.H.; Yang, N.Y.; Yuan, X.L.; Zou, Y.J.; Zhao, F.M.; Chen, J.P.; Wang, M.Y.; Lu, D.X. Daucosterol promotes the proliferation of neural stem cells. J. Steroid Biochem. Mol. Biol. 2014, 140, 90–99. [Google Scholar] [CrossRef] [PubMed]
- King, T.D.; Suto, M.J.; Li, Y.H. The wnt/beta-catenin signaling pathway: A potential therapeutic target in the treatment of triple negative breast cancer. J. Cell. Biochem. 2012, 113, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Polakis, P. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 2007, 17, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.H.; Yuan, X.L.; Yang, N.Y.; Ren, L.; Zhao, F.M.; Luo, B.X.; Bian, Y.Y.; Xu, J.Y.; Lu, D.X.; Zheng, Y.Y. Daucosterol protects neurons against oxygen-glucose deprivation/reperfusion-mediated injury by activating IGF1 signaling pathway. J. Steroid Biochem. Mol. Biol. 2015, 152, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Yanaga, F.; Kahn, M. Targeting Wnt signaling: Can we safely eradicate cancer stem cells? Clin. Cancer Res. 2010, 16, 3153–3162. [Google Scholar] [CrossRef] [PubMed]
- Heidel, F.H.; Bullinger, L.; Feng, Z.H.; Wang, Z.; Neff, T.A.; Stein, L.; Kalaitzidis, D.; Lane, S.W.; Armstrong, S.A. Genetic and pharmacologic inhibition of beta-catenin targets imatinib-resistant leukemia stem cells in CML. Cell Stem Cell 2012, 10, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Anastas, J.N.; Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 2013, 13, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Yousuf Dar, M.; Shah, W.A.; Mubashir, S.; Rather, M.A. Chromatographic analysis, anti-proliferative and radical scavenging activity of Pinus wallichina essential oil growing in high altitude areas of Kashmir, India. Phytomedicine 2012, 19, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Strnad, M. Plant biochemistry as a source of new anticancer drugs. FEBS J. 2009, 276, 68. [Google Scholar]
- Christen, P.; Cuendet, M. Plants as a source of therapeutic and health products. Chimia 2012, 66, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Marston, A. Plants as a still unexploited source of new drugs. Nat. Prod. Commun. 2008, 3, 1307–1315. [Google Scholar]
- Weihrauch, J.L.; Gardner, J.M. Sterol content of foods of plant origin. J. Am. Diet. Assoc. 1978, 73, 39–47. [Google Scholar] [PubMed]
- Awad, A.B.; Williams, H.; Fink, C.S. Phytosterols reduce in vitro metastatic ability of MDA-MB-231 human breast cancer cells. Nutr. Cancer 2001, 40, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.A.; Farimani, M.M. Inactivation of PI3K/Akt pathway and upregulation of PTEN gene are involved in daucosterol, isolated from Salvia sahendica, induced apoptosis in human breast adenocarcinoma cells. S. Afr. J. Bot. 2014, 93, 37–47. [Google Scholar] [CrossRef]
- Yang, J.L.; Chen, J.; He, J.X.; Li, J.; Shi, J.; Cho, W.C.; Liu, X.M. Wnt signaling as potential therapeutic target in lung cancer. Expert Opin. Ther. Targets 2016, 20, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Roarty, K.; Pfefferle, A.A.; Creighton, C.J.; Perou, C.M.; Rosen, J.M. Wnt pathway dynamics in breast cancer progression. Cancer Res. 2016, 76. [Google Scholar] [CrossRef]
- Sawa, M.; Masuda, M.; Yamada, T. Targeting the Wnt signaling pathway in colorectal cancer. Expert Opin. Ther. Targets 2016, 20, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Duchartre, Y.; Kim, Y.M.; Kahn, M. The Wnt signaling pathway in cancer. Crit. Rev. Oncol. Hematol. 2016, 99, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Lu, S.Y.; Kang, S.A.; Tan, H.J.; Li, Z.L.; Wee, Z.N.A.; Guan, J.S.; Chichili, V.P.R.; Sivaraman, J.; Putti, T.; et al. Wnt signaling promotes breast cancer by blocking ITCH-mediated degradation of YAP/TAZ transcriptional coactivator WBP2. Cancer Res. 2016, 76, 6278–6289. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.M.; Lv, Q.; Zhao, Y.L.; Liu, C.F.; Sun, Y.Y.; Xi, K.M.; Xiao, J.Y.; Li, C.J. Wnt/beta-catenin pathway is required for epithelial to mesenchymal transition in CXCL12 over expressed breast cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 12357–12367. [Google Scholar] [PubMed]
- Mir, R.; Pradhan, S.J.; Patil, P.; Mulherkar, R.; Galande, S. Wnt/beta-catenin signaling regulated SATB1 promotes colorectal cancer tumorigenesis and progression. Oncogene 2016, 35, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Doble, B.W.; Woodgett, J.R. GSK-3: Tricks of the trade for a multi-tasking kinase. J. Cell. Sci. 2003, 116, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, E.M.; Sokol, S.Y. Xenopus Axin-related protein: A link between its centrosomal localization and function in the Wnt/beta-catenin pathway. Dev. Dyn. 2010, 239, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Amit, S.; Hatzubai, A.; Birman, Y.; Andersen, J.S.; Ben-Shushan, E.; Mann, M.; Ben-Neriah, Y.; Alkalay, I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: A molecular switch for the Wnt pathway. Genes Dev. 2002, 16, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Gurrieri, C.; Piazza, F.; Gnoato, M.; Montini, B.; Biasutto, L.; Gattazzo, C.; Brunetta, E.; Cabrelle, A.; Cinetto, F.; Niero, R.; et al. 3-(2,4-Dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione (SB216763), a glycogen synthase kinase-3 inhibitor, displays therapeutic properties in a mouse model of pulmonary inflammation and fibrosis. J. Pharmacol. Exp. Ther. 2010, 332, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bai, X.L.; Chen, W.; Ma, T.; Hu, Q.D.; Liang, C.; Xie, S.Z.; Chen, C.L.; Hu, L.Q.; Xu, S.G.; Liang, T.B. Wnt/beta-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1 alpha signaling. Carcinogenesis 2013, 34, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Liu, B.R.; Du, Y.J.; Chen, J.; Cheng, Y.Q.; Xu, W.; Wang, X.H. Wnt/beta-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol. Lett. 2014, 7, 1175–1178. [Google Scholar] [PubMed]
- Tang, J.; Li, L.; Huang, W.T.; Sui, C.J.; Yang, Y.C.; Lin, X.M.; Hou, G.J.; Chen, X.; Fu, J.; Yuan, S.X.; et al. MiR-429 increases the metastatic capability of HCC via regulating classic Wnt pathway rather than epithelial-mesenchymal transition. Cancer Lett. 2015, 364, 33–43. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: not available. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Liu, X.; Li, X.; Zheng, Y.; Liu, B.; Xiao, Y. Daucosterol Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Wnt/β-Catenin Signaling. Molecules 2017, 22, 862. https://doi.org/10.3390/molecules22060862
Zeng J, Liu X, Li X, Zheng Y, Liu B, Xiao Y. Daucosterol Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Wnt/β-Catenin Signaling. Molecules. 2017; 22(6):862. https://doi.org/10.3390/molecules22060862
Chicago/Turabian StyleZeng, Junquan, Xing Liu, Xiaofei Li, Yongliang Zheng, Bin Liu, and Youzhang Xiao. 2017. "Daucosterol Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Wnt/β-Catenin Signaling" Molecules 22, no. 6: 862. https://doi.org/10.3390/molecules22060862
APA StyleZeng, J., Liu, X., Li, X., Zheng, Y., Liu, B., & Xiao, Y. (2017). Daucosterol Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Wnt/β-Catenin Signaling. Molecules, 22(6), 862. https://doi.org/10.3390/molecules22060862




