Characterization of Metabolite Profile in Phyllanthus niruri and Correlation with Bioactivity Elucidated by Nuclear Magnetic Resonance Based Metabolomics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Amino Acid Profile of the Dried Phyllanthus niruri
2.2. 1H Nuclear Magnetic Resonance Spectra of the Samples and Metabolites Assignment
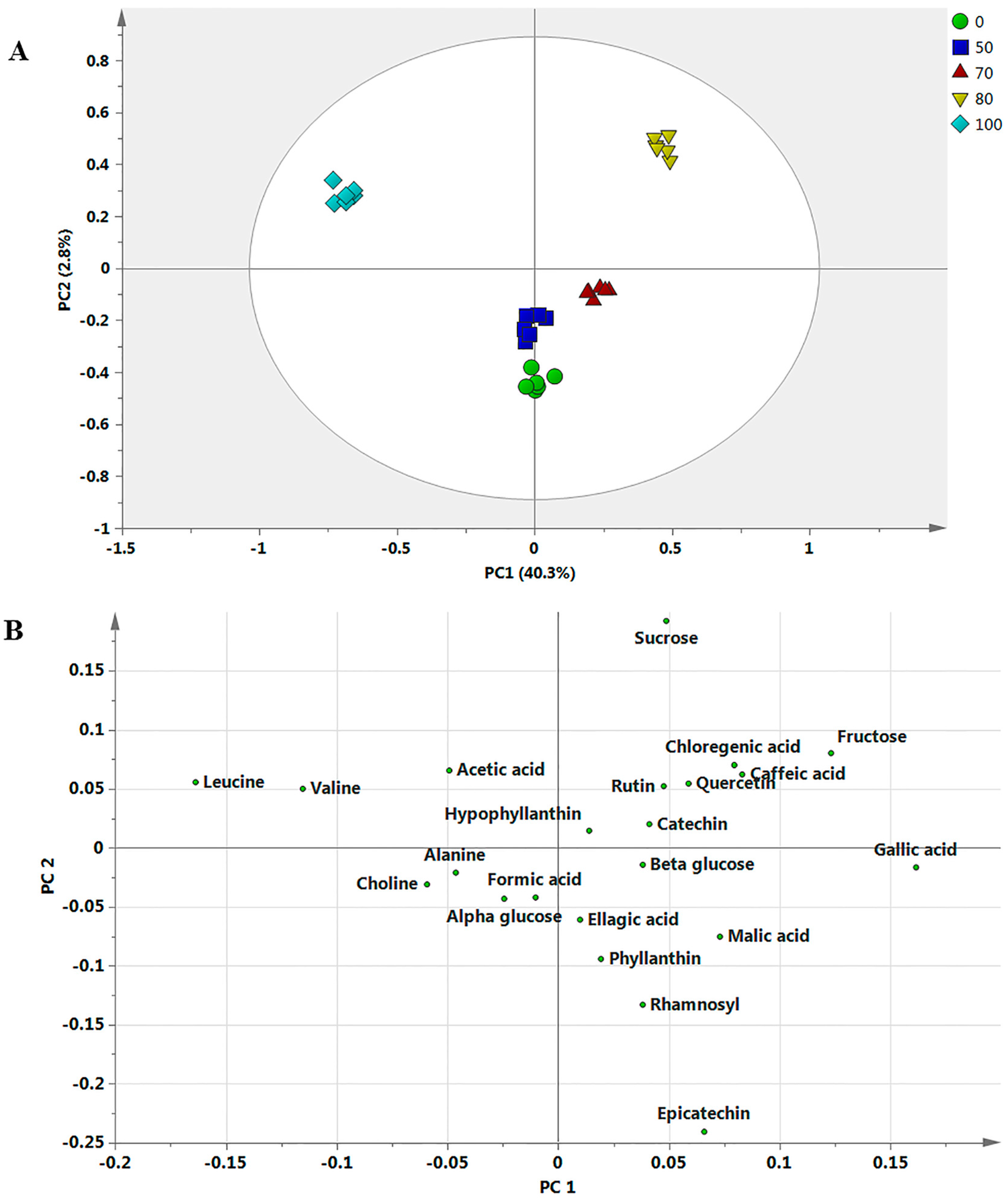
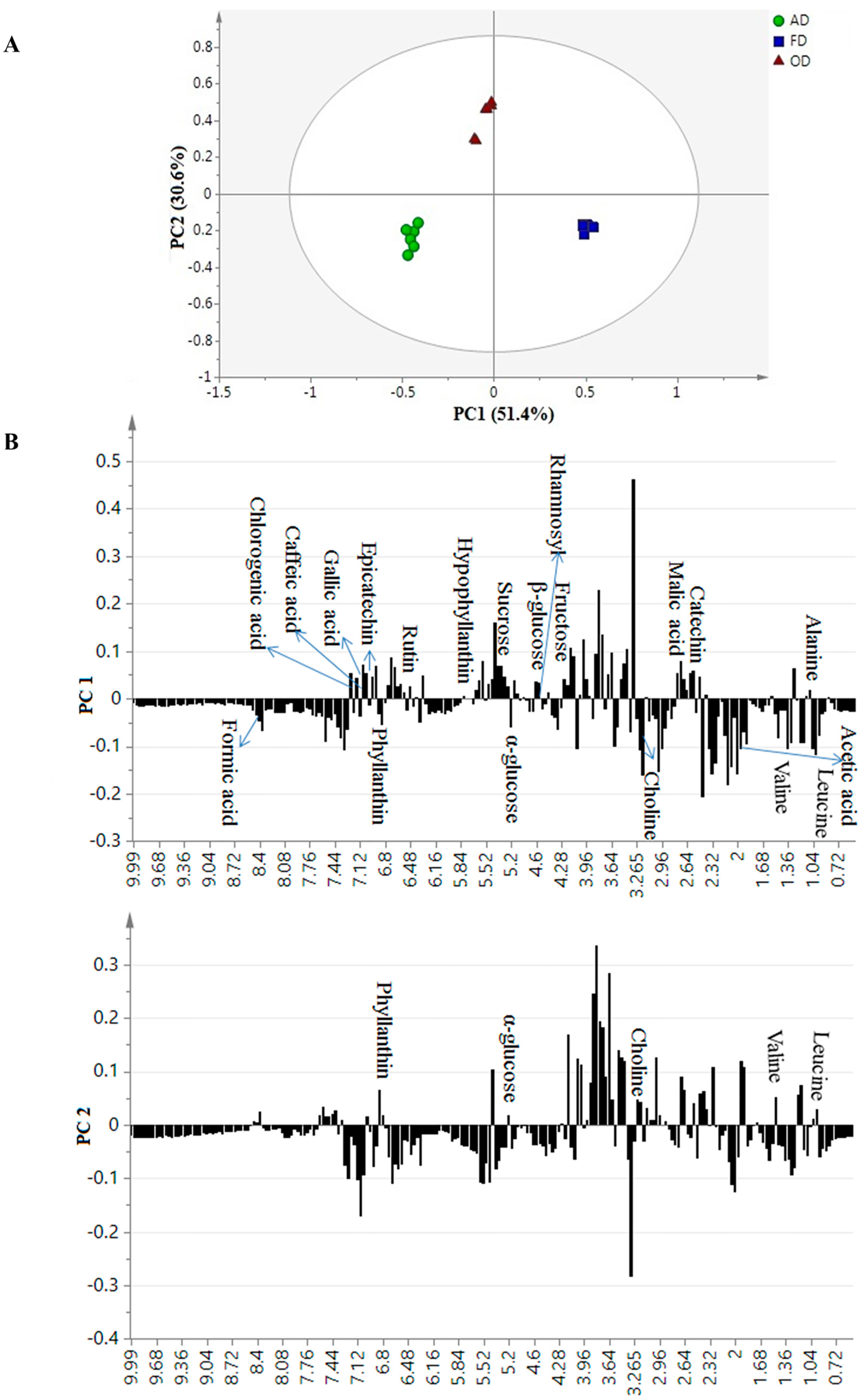
2.3. Discrimination of the Dried Samples Extracted with Different Ethanol Ratios by Principal Component Analysis
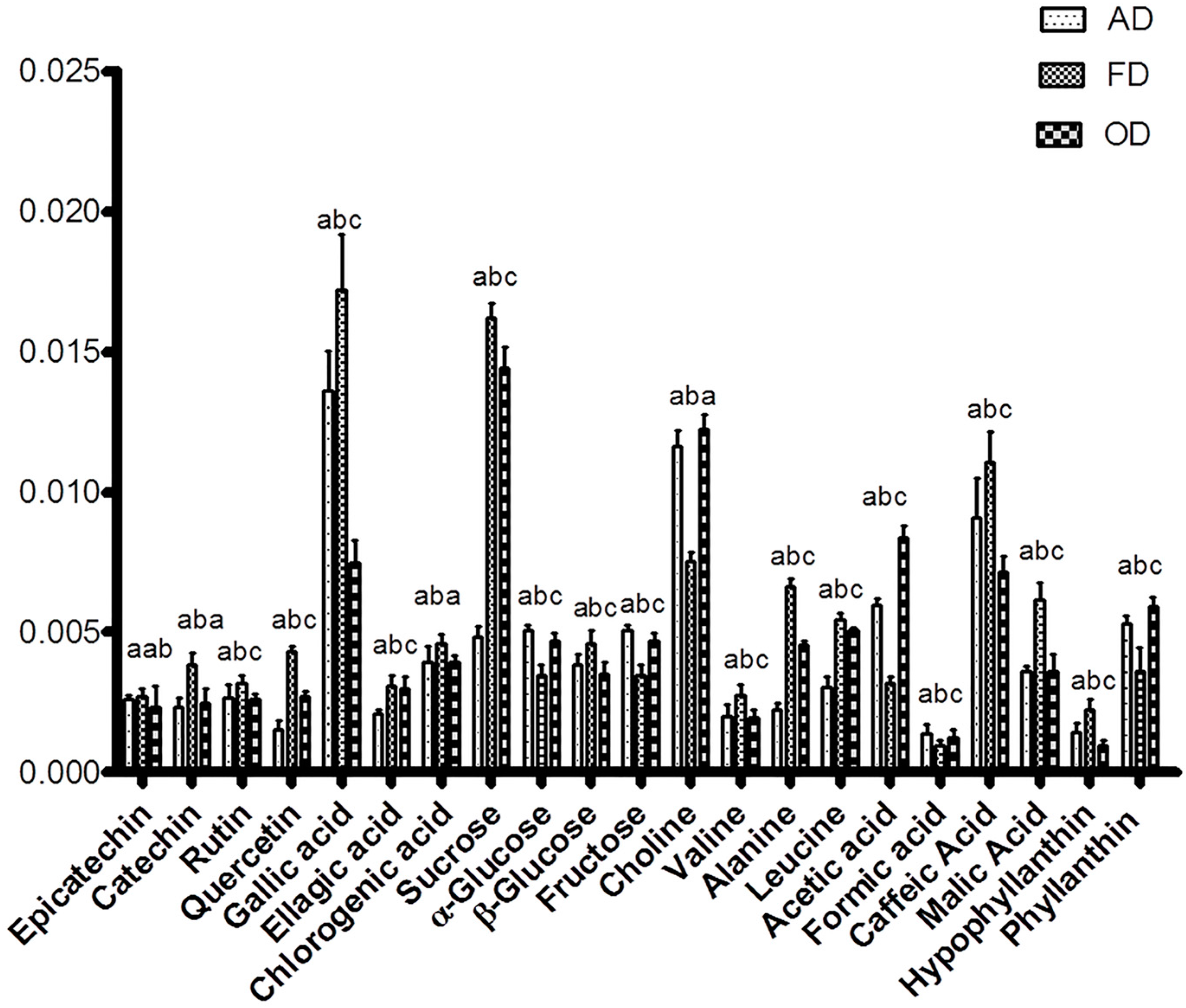
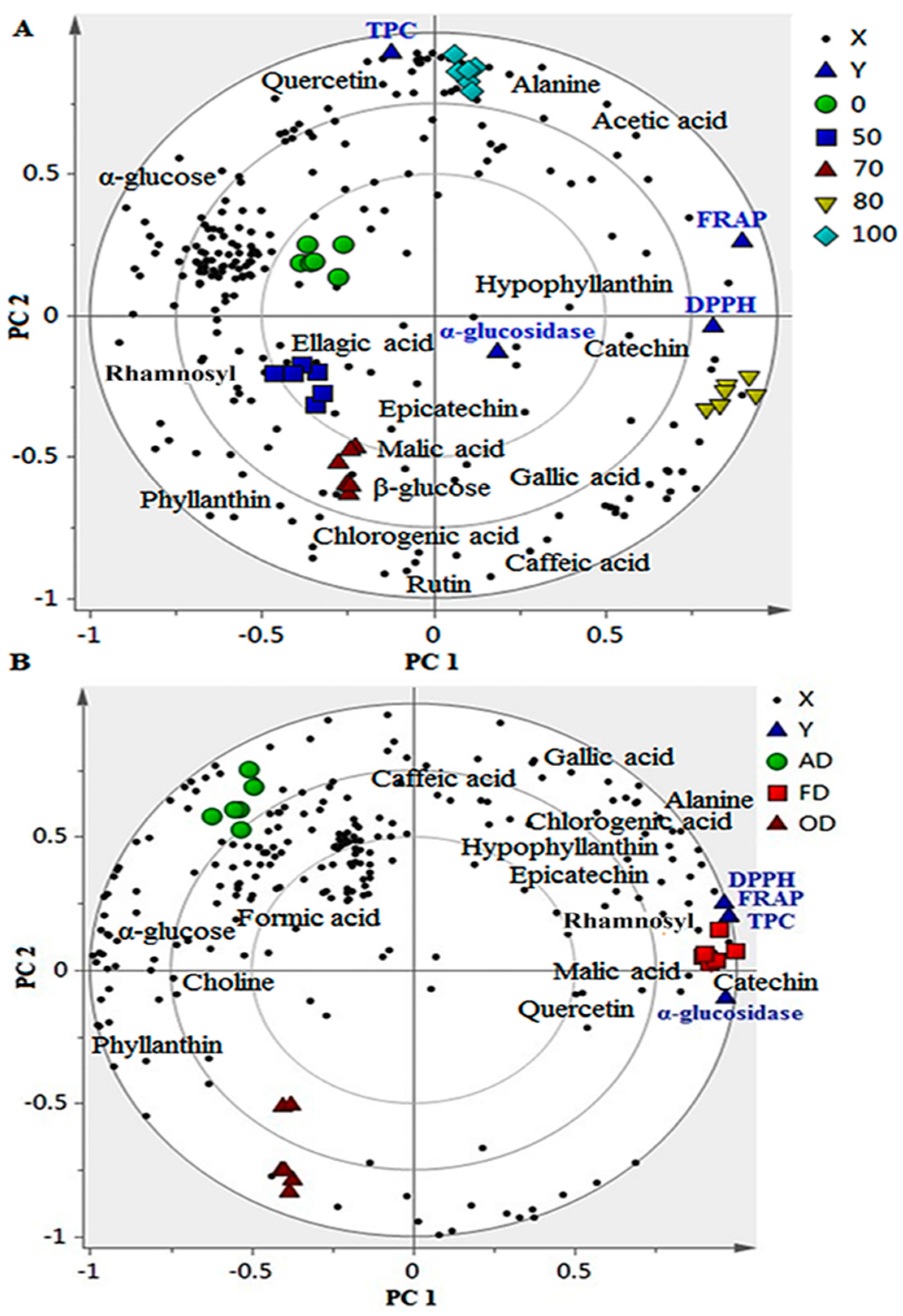
2.4. Correlation between Bioactivities and Metabolite Changes among the Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Drying Treatments
3.3. Extraction of Samples
3.4. Amino Acid Content Determination
3.5. Nuclear Magnetic Resonance Measurement
3.6. Bucketing of 1H Nuclear Magnetic Resonance Spectra and Multivariate Data Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Leyva-López, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2016, 187, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Bang, W.Y.; Nair, V.; Angulo-Escalante, M.A.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of flavonoids and lipophilic compounds from Jatropha platyphylla on the suppression of lipopolysaccharide (LPS)-induced inflammation in macrophage cells. J. Agric. Food Chem. 2016, 64, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective role of ternatinanthocyanins and quercetin glycosides from butterfly pea (Clitoriaterna tea Leguminosae) blue flower petals against lipopolysaccharide (LPS)-induced inflammation in macrophage cells. J. Agric. Food Chem. 2015, 63, 6355–6365. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.K.; Kim, Y.S.; Natarajan, S.K.; Kim, W.S.; Hwang, J.W.; Jeon, N.J.; Jeong, J.H.; Moon, S.H.; Jeon, B.T.; Park, P.J. Antioxidant and anti-inflammatory effects of Chaenomeles sinensis leaf extracts on LPS- stimulated RAW 264.7 cells. Molecules 2016, 21, 422. [Google Scholar] [CrossRef] [PubMed]
- Abas, F.; Lajis, N.H.; Israf, D.A.; Khozirah, S.; Umi Kalsom, Y. Antioxidant and nitric oxide inhibition activities of selected Malay traditional vegetables. Food Chem. 2006, 95, 566–573. [Google Scholar] [CrossRef]
- Markom, M.; Hasan, M.; Daud, W.R.W.; Singh, H.; Jahim, J.M. Extraction of hydrolysable tannins from Phyllanthus niruri Linn: Effects of solvents and extraction methods. Sep. Purif. Technol. 2007, 52, 487–496. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Murtijaya, J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT Food Sci. Technol. 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Moreira, J.; Klein-Júnior, L.C.; Filho, V.C.; Buzzi, F.C. Anti-hyperalgesic activity of corilagin, a tannin isolated from Phyllanthus niruri L. (Euphorbiaceae). J. Ethnopharmacol. 2013, 146, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.R.; Tripathi, P.; Sharma, V.; Chauhan, N.S.; Dixit, V.K. Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: A review. J. Ethnopharmacol. 2011, 138, 286–313. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chandra, P.; Bajpai, V.; Singh, A.; Srivastava, M.; Mishra, D.; Kumar, B. Rapid qualitative and quantitative analysis of bioactive compounds from Phyllanthus amarus using LC/MS/MS techniques. Ind. Crop Prod. 2015, 69, 143–152. [Google Scholar] [CrossRef]
- Maulidiani, H.; Khatib, A.; Shaari, K.; Abas, F.; Shitan, M.; Kneer, R.; Neto, V.; Lajis, N.H. Discrimination of three pegaga (Centella.) varieties and determination of growth-lighting effects on metabolites content based on the chemometry of 1H nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2012, 60, 410–417. [Google Scholar]
- Verpoorte, R.; Choi, Y.; Kim, H. NMR-based metabolomics at work in phytochemistry. Phytochem. Rev. 2007, 6, 3–14. [Google Scholar] [CrossRef]
- Mediani, A.; Abas, F.; Khatib, A.; Maulidiani, H.; Shaari, K.; Choi, Y.H.; Lajis, N.H. 1H-NMR-based metabolomics approach to understanding the drying effects on the phytochemicals in Cosmos caudatus. Food Res. Int. 2012, 49, 763–770. [Google Scholar] [CrossRef]
- Shuib, N.H.; Shaari, K.; Khatib, A.; Maulidiani; Kneer, R.; Zareen, S.; Raof, S.M.; Lajis, N.H.; Neto, V. Discrimination of young and mature leaves of Melicope ptelefolia using 1H-NMR and multivariate data analysis. Food Chem. 2011, 126, 640–645. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Supriatno; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Mediani, A.; Abas, F.; Khatib, A.; Tan, C.P.; Ismail, I.S.; Shaari, K.; Ismail, A.; Lajis, N.H. Relationship between metabolites composition and biological activities of Phyllanthus niruri extracts prepared by different drying methods and solvents extraction. Plant Food Hum. Nutr. 2015, 70, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hamid, N.A.; Mediani, A.; Maulidiani, M.; Abas, F.; Ismail, I.S.; Shaari, K.; Lajis, N.H. Discrimination and nitric oxide inhibitory activity correlation of ajwa dates from different grades and origin. Molecules 2016, 21, 1423. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, Y.H.; Verberne, M.; Lefeber, A.W.M.; Erkelens, C.; Verpoorte, R. Metabolic fingerprinting of wild type and transgenic tobacco plants by 1H-NMR and multivariate analysis technique. Phytochemistry 2004, 65, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.; Mastrorilli, P.; Cafagna, I.; Nitti, G.I.; Latronico, M.; Longobardi, F.; Minoja, A.P.; Napoli, C.; Romito, V.A.; Schäfer, H.; et al. Effects of agronomical practices on chemical composition of table grapes evaluated by NMR spectroscopy. J. Food Compos. Anal. 2014, 35, 44–52. [Google Scholar] [CrossRef]
- Kirk, H.; Cheng, D.; Choi, Y.H.; Vrieling, K.; Klinkhamer, P.G. Transgressive segregation of primary and secondary metabolites in F2 hybrids between Jacobaea aquatica and J. vulgaris. Metabolomics 2012, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Igwe, C.U.; Nwaogu, L.A.; Ujuwondu, C.O. Assessment of the hepatic effects, phytochemical and proximate compositions of Phyllanthus amarus. Afr. J. Biotechnol. 2007, 6, 728–731. [Google Scholar]
- Chan, E.W.C.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009, 113, 166–172. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S.; Singh, B. Antioxidant activity of barley as affected by extrusion cooking. Food Chem. 2012, 131, 1406–1413. [Google Scholar] [CrossRef]
- Coman, C.; Ruginǎ, O.D.; Socaciu, C. Plants and natural compounds with antidiabetic action. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 314–325. [Google Scholar]
- Wansi, J.D.; Lallemand, M.; Chiozem, D.D.; Toze, F.A.A.; Mbaze, L.M.; Naharkhan, S.; Iqbal, M.C.; Tillequin, F.; Wandji, J.; Fomum, Z.T. α-Glucosidase inhibitory constituents from stem bark of Terminalia superba (Combretaceae). Phytochemistry 2007, 68, 2096–2100. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Paul, P.; Mudgal, J.; Nayak, P.; Thomas Pannakal, S.; Priyadarsini, K.; Unnikrishnan, M. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp. Toxicol. Pathol. 2011, 64, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.; Rashmi, R.; Rajan, M.; Sambaiah, K.; Salimath, P. Antidiabetic influence of quercetin in streptozotocin-induced diabetic rats. Nutr. Res. 2004, 24, 373–381. [Google Scholar] [CrossRef]
- Shobana, S.; Sreerama, Y.; Malleshi, N. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Javadi, N.; Abas, F.; Hamid, A.A.; Simoh, S.; Shaari, K.; Ismail, I.S.; Mediani, A.; Khatib, A. GC-MS-based metabolite profiling of Cosmos caudatus leaves possessing alpha-glucosidase inhibitory activity. J. Food Sci. 2014, 79, C1130–C1136. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the extracts are available from the authors. |
| Amino Acids | Freeze Drying (FD) | Oven Drying (OD) | Air Drying (AD) |
|---|---|---|---|
| Aspartic acid | 3.18 a ± 0.13 | 1.85 b ± 0.19 | 2.10 c ± 0.11 |
| Glutamic acid | 9.33 a ± 0.18 | 4.32 b ± 0.03 | 4.59 b ± 0.32 |
| Serine | 2.33 a ± 0.14 | 0.98 b ± 0.09 | 0.95 b ± 0.14 |
| Glycine | 2.10 a ± 0.16 | 0.91 b ± 0.06 | 0.97 b ± 0.09 |
| Histidine | 1.24 a ± 0.10 | 0.56 b ± 0.18 | 0.42 c ± 0.02 |
| Arginine | 2.48 a ± 0.03 | 1.48 b ± 0.33 | 0.69 c ± 0.08 |
| Threonine | 1.55 a ± 0.03 | 0.61 b ± 0.24 | 0.71 b ± 0.02 |
| Alanine | 2.48 a ± 0.11 | 1.50 b ± 0.19 | 1.46 b ± 0.30 |
| Proline | 2.05 a ± 0.06 | 1.20 b ± 0.04 | 2.88 c ± 0.05 |
| Tyrosine | 1.00 a ± 0.10 | 0.24 b ± 0.03 | 0.34 b ± 0.05 |
| Valine | 2.60 a ± 0.10 | 1.15 b ± 0.03 | 1.08 b ± 0.09 |
| Methionine | 0.34 a ± 0.06 | 0.19 b ± 0.04 | 0.11 b ± 0.01 |
| Cystine | 9.50 a ± 0.54 | 4.17 b ± 0.22 | 4.79 b ± 0.52 |
| Isoleucine | 2.08 a ± 0.04 | 0.87 b ± 0.02 | 0.82 b ± 0.08 |
| Leucine | 3.49 a ± 0.08 | 1.43 b ± 0.02 | 1.34 b ± 0.08 |
| Phenylalanine | 4.01 a ± 0.42 | 2.89 b ± 0.06 | 2.84 b ± 0.16 |
| Lysine | 14.10 a ± 0.32 | 5.76 b ± 0.06 | 6.42 b ± 0.58 |
| Metabolites | 1H-NMR Characteristic Signals |
|---|---|
| Primary metabolites: | |
| β glucose | δ 4.59 (d, J = 8.0 Hz) |
| α glucose | δ 5.19 (d, J = 4.0 Hz) |
| Sucrose | δ 5.41 (d, J = 3.5 Hz) |
| Fructose | δ 4.18 (d, J = 8.5 Hz) |
| Fatty acid | δ 1.33–1.25 (m) |
| Formic acid | δ 8.47 (s) |
| Acetic acid | δ 1.93 (s) |
| Choline | δ 3.21 (s) |
| Alanine | δ 1.49 (d, J = 7.5 Hz), δ 3.72 (q) |
| d-l-Valine | δ 1.07 (d, J = 7.0 Hz), δ 1.02 (d, J = 7.0 Hz) |
| Leucine or isoleucine | δ 0.98 (d, J = 7.5 Hz) |
| Secondary metabolites: | |
| Quercetin 3-O-glucoside | δ 6.45 (d, J = 2.0 Hz), δ 7.37 (d, J = 2.0 Hz), δ 7.01 (d, J = 8.0 Hz), δ 7.33 (dd, J = 8.5, 2.0 Hz), δ 5.16 (d, J = 8.0 Hz) |
| Catechin | δ 6.45 (d, J = 2.0 Hz), δ 7.37 (d, J = 2.0 Hz), δ 7.01 (d, J = 8.0 Hz), δ 7.33 (dd, J = 8.5, 2.0 Hz), δ 5.40 (d, J = 7.6 Hz), δ 2.56 (dd, J = 7.5, 16.0 Hz), δ 2.84 (m) |
| Quercetin 3-O-α-rhamnoside | δ 6.45 (d, J = 2.0 Hz), δ 7.37 (d, J = 2.0 Hz), δ 7.01 (d, J = 8.0 Hz), δ 7.33 (dd, J = 8.5, 2.0 Hz), δ 5.48 (d, J = 1.0 Hz). Methyl signal: δ 0.91 (d, J = 1.1 Hz) |
| Epicatechin | δ 6.45 (d, J = 2.0 Hz), δ 7.37 (d, J = 2.0 Hz), δ 7.01 (d, J = 8.0 Hz), δ 7.33 (dd, J = 8.5, 2.0 Hz), δ 4.97 (d, J = 8.0 Hz), δ 7.05 (s), δ 6.95 (s) |
| Rutin | δ 6.51 (d, J = 2.0 Hz), δ 7.59 (dd, J = 8.5, 2.0 Hz), δ 7.67 (d, J = 2.0 Hz). Anomeric protons (glucosyl δ 4.97 (d, J = 8.0 Hz), rhamnosyl δ 4.54 (d, J = 1.0 Hz) |
| Chlorogenic acid (5-O-caffeoyl quinic acid) | δ 7.20 (s), signal for caffeoyl δ 7.62 (d, J = 16.0 Hz), δ 6.37 (d, J = 16.0 Hz) and quinic δ 4.09 (m), δ 2.63 (dd, J = 1.3, 5.0 Hz), δ 1.9 (d, J = 10.0 Hz), δ 7.20 (s) |
| Gallic acid | δ 7.07 (s), 7.15 (s) |
| Ellagic acid | δ 7.46 (s), 7.69 (s) |
| Malic acid | δ 2.72 (d, J = 16.0 Hz), δ 6.37 (d, J = 16.0 Hz) |
| Quinic acid | δ 4.09 (m), δ 2.63 (dd, J = 1.3, 5.0 Hz), δ 4.23 (d, J = 7.0 Hz) |
| Caffeic acid | δ 7.13 (d, J = 2.0 Hz), δ 6.87 (d, J = 9.0 Hz) |
| Phyllanthin | δ 2.05 (m), δ 2.70 (d, J = 8.0 Hz), δ 3.29 (d, J = 9.0 Hz), δ 3.33 (s), δ 3.81 (s), δ 6.61 (dd, J = 8.5, 2.0 Hz), δ 6.82 (d, J = 8.0 Hz) |
| Hypophyllanthin | δ 1.93 (m), δ 2.79 (d, J = 8.0 Hz), δ 3.29 (s), δ 3.31 (s), δ 3.85 (s), δ 4.08 (d, J = 7.2 Hz), δ 5.61 (d, J = 2.0 Hz), δ 5.72 (d, J = 2.0 Hz), δ 6.29 (s), δ 6.65 (d, J = 2.0 Hz), δ 6.71 (d, J = 5.0 Hz) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mediani, A.; Abas, F.; Maulidiani, M.; Khatib, A.; Tan, C.P.; Ismail, I.S.; Shaari, K.; Ismail, A. Characterization of Metabolite Profile in Phyllanthus niruri and Correlation with Bioactivity Elucidated by Nuclear Magnetic Resonance Based Metabolomics. Molecules 2017, 22, 902. https://doi.org/10.3390/molecules22060902
Mediani A, Abas F, Maulidiani M, Khatib A, Tan CP, Ismail IS, Shaari K, Ismail A. Characterization of Metabolite Profile in Phyllanthus niruri and Correlation with Bioactivity Elucidated by Nuclear Magnetic Resonance Based Metabolomics. Molecules. 2017; 22(6):902. https://doi.org/10.3390/molecules22060902
Chicago/Turabian StyleMediani, Ahmed, Faridah Abas, M. Maulidiani, Alfi Khatib, Chin Ping Tan, Intan Safinar Ismail, Khozirah Shaari, and Amin Ismail. 2017. "Characterization of Metabolite Profile in Phyllanthus niruri and Correlation with Bioactivity Elucidated by Nuclear Magnetic Resonance Based Metabolomics" Molecules 22, no. 6: 902. https://doi.org/10.3390/molecules22060902
APA StyleMediani, A., Abas, F., Maulidiani, M., Khatib, A., Tan, C. P., Ismail, I. S., Shaari, K., & Ismail, A. (2017). Characterization of Metabolite Profile in Phyllanthus niruri and Correlation with Bioactivity Elucidated by Nuclear Magnetic Resonance Based Metabolomics. Molecules, 22(6), 902. https://doi.org/10.3390/molecules22060902







