Encapsulation of the Antioxidant R-(+)-α-Lipoic Acid in Permethylated α- and β-Cyclodextrins: Thermal and X-ray Structural Characterization of the 1:1 Inclusion Complexes
Abstract
:1. Introduction
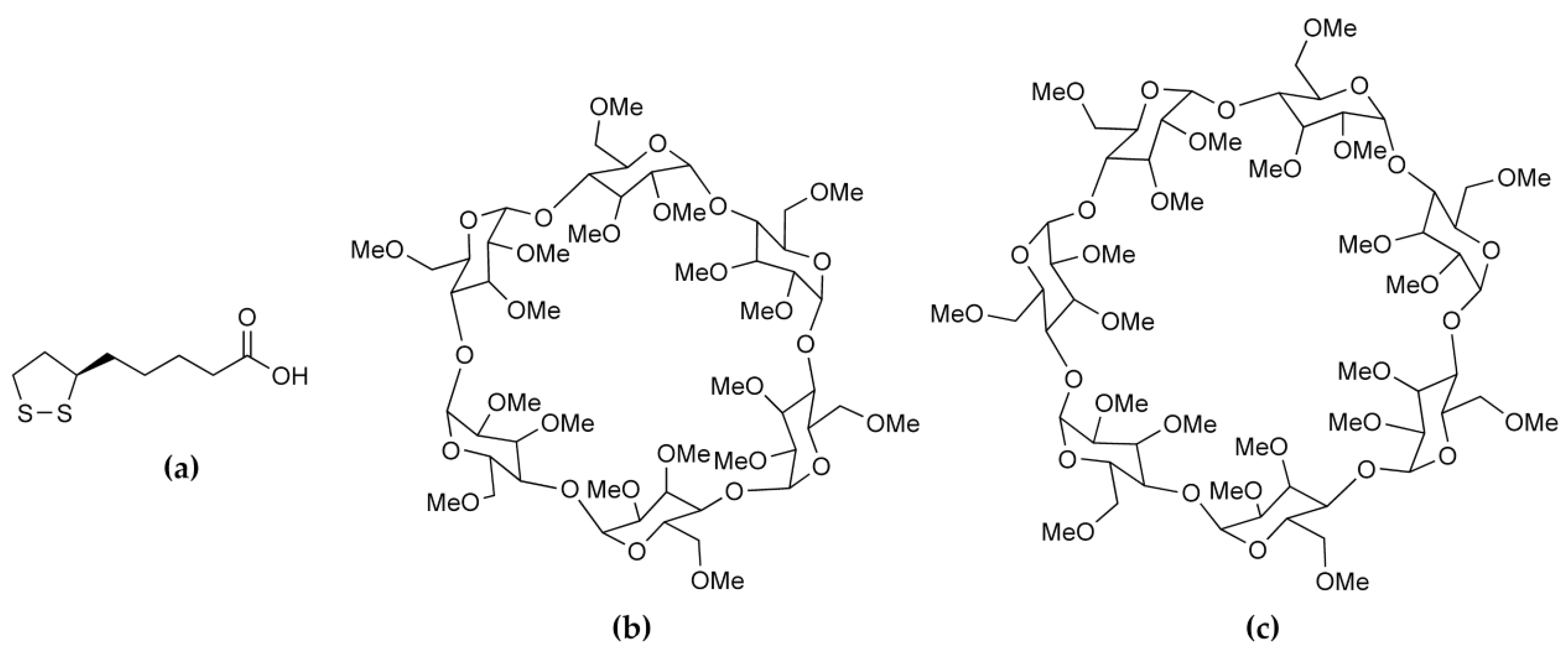
2. Results and Discussion
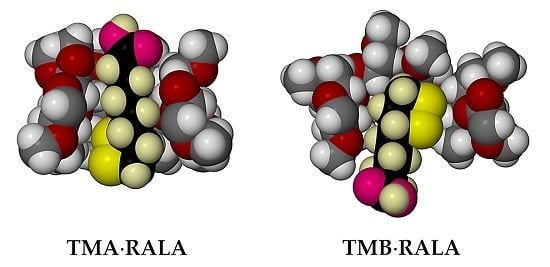
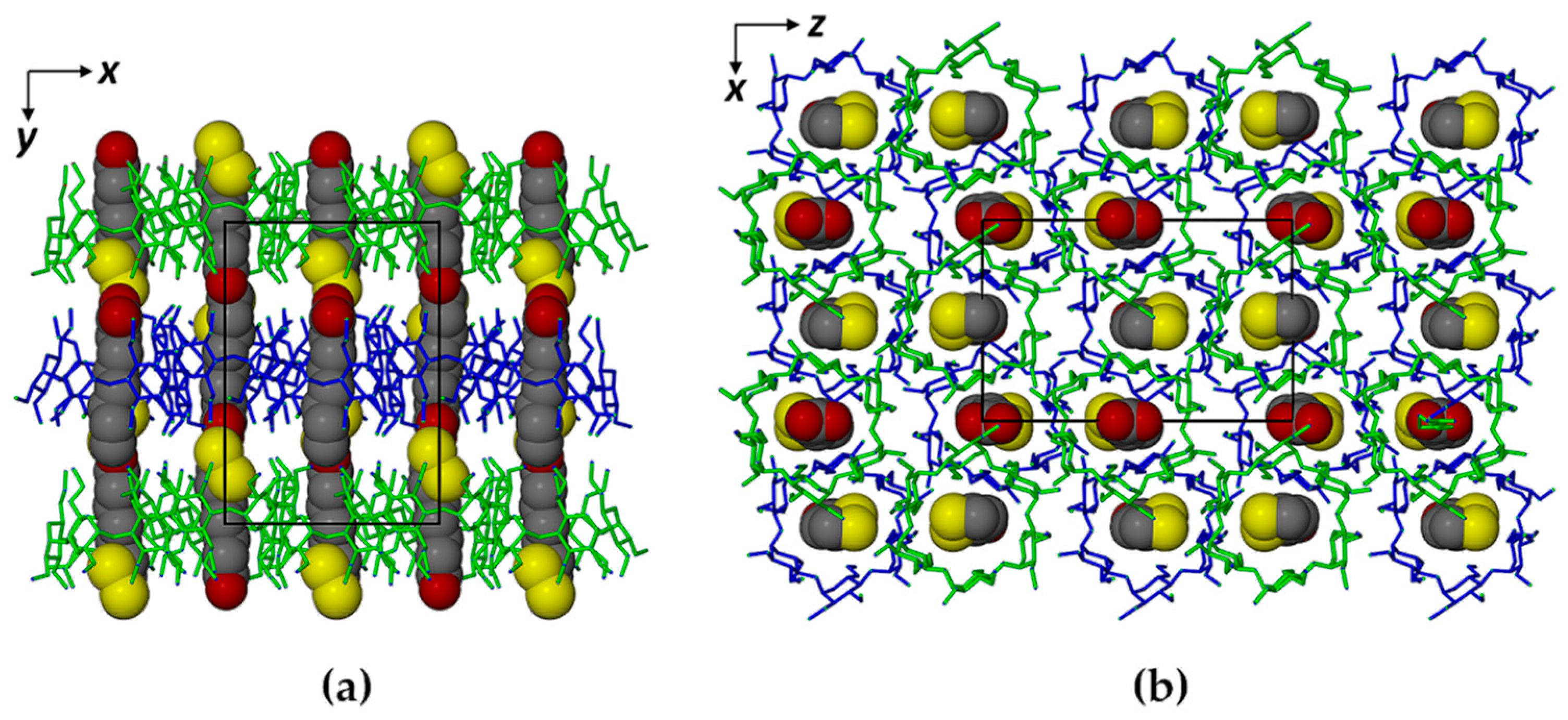
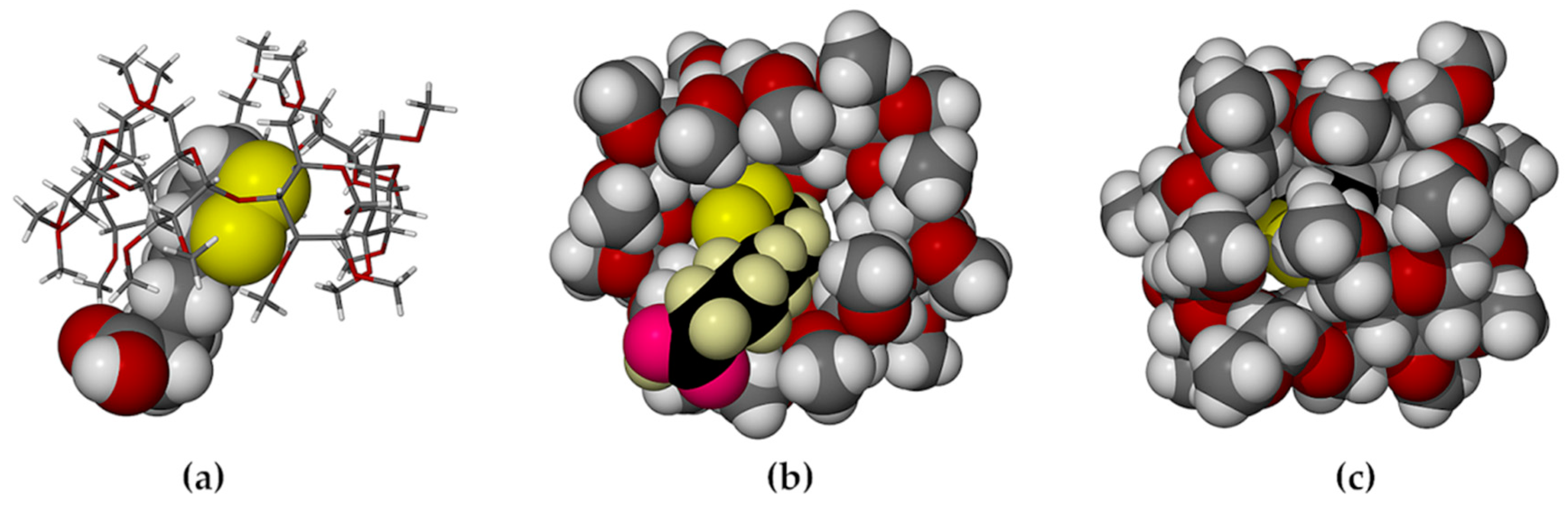
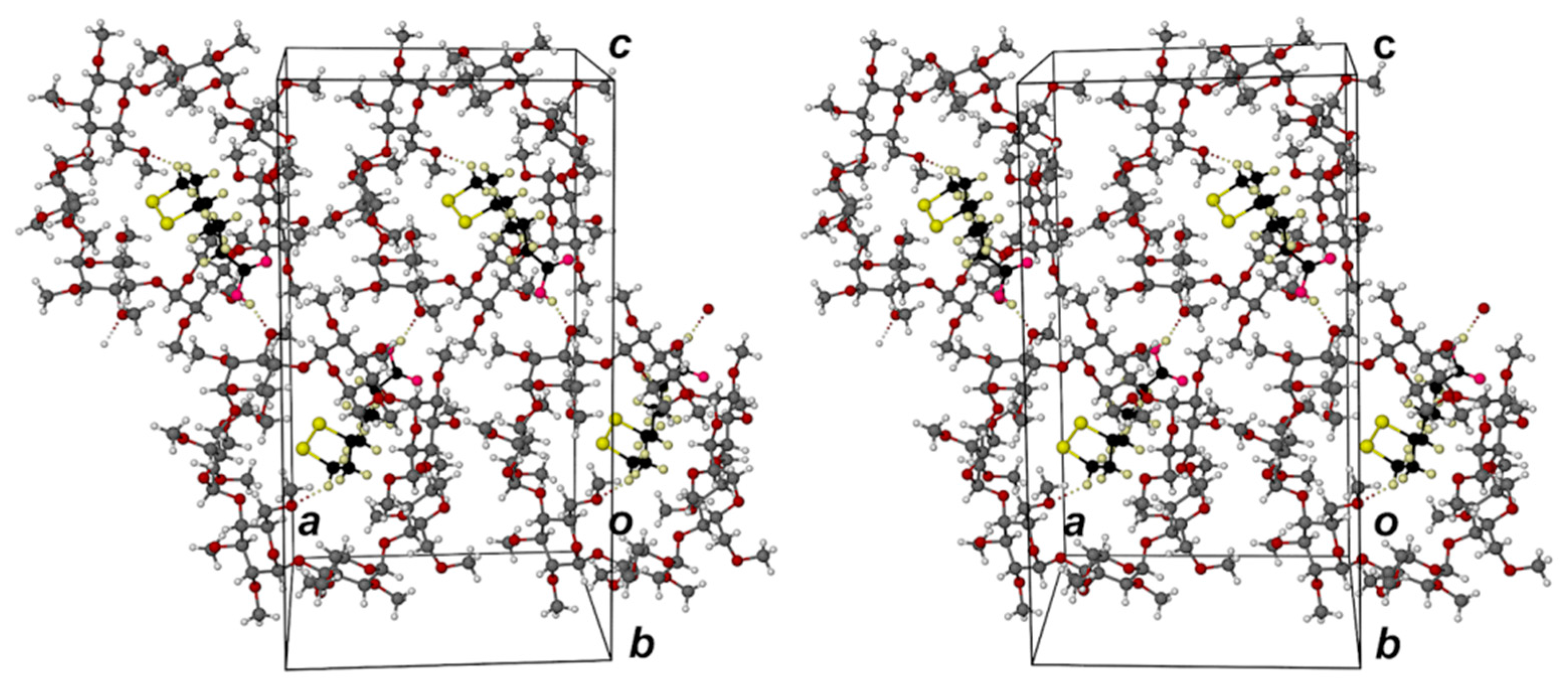
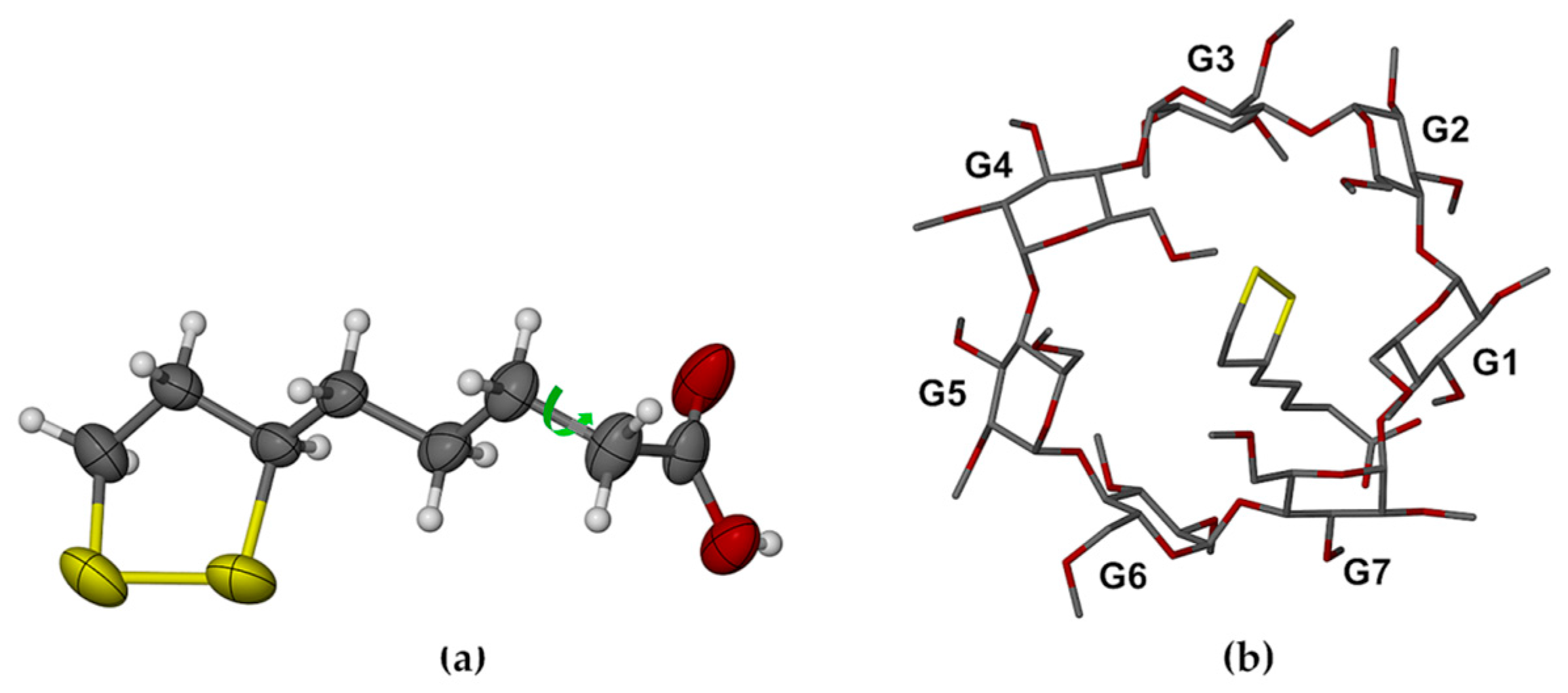
2.1. Single Crystal X-ray Diffraction
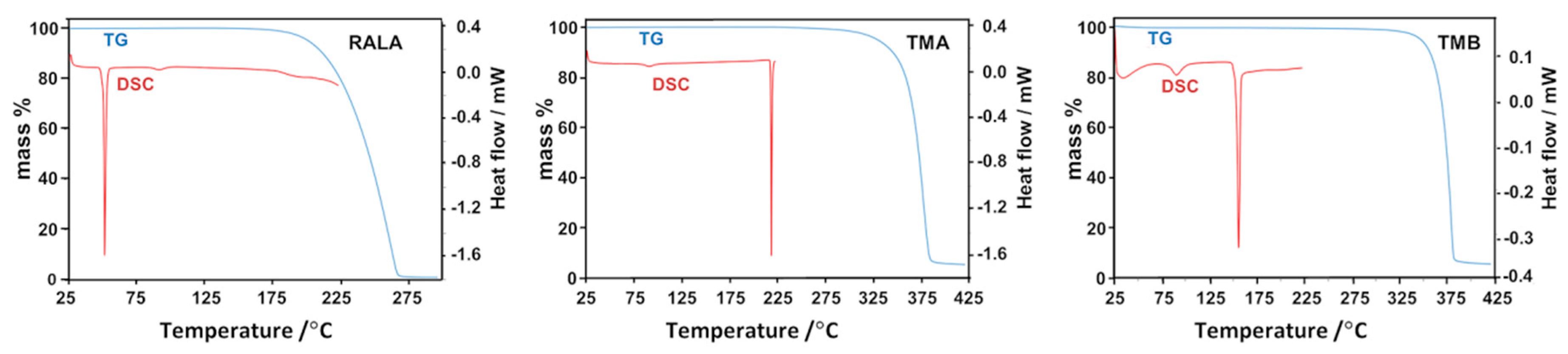
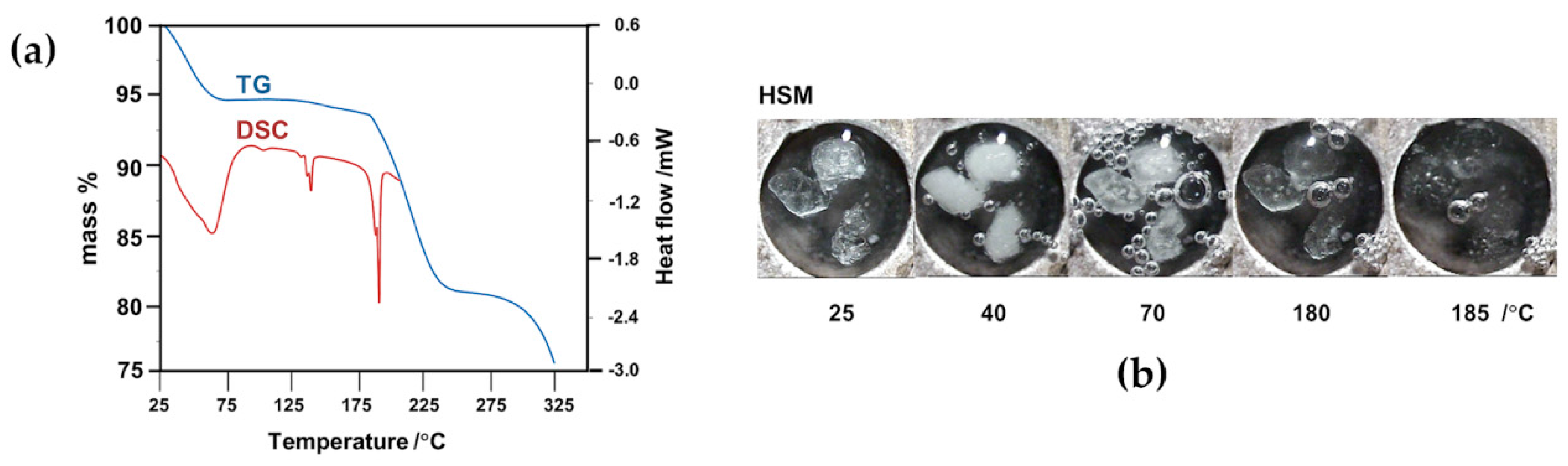
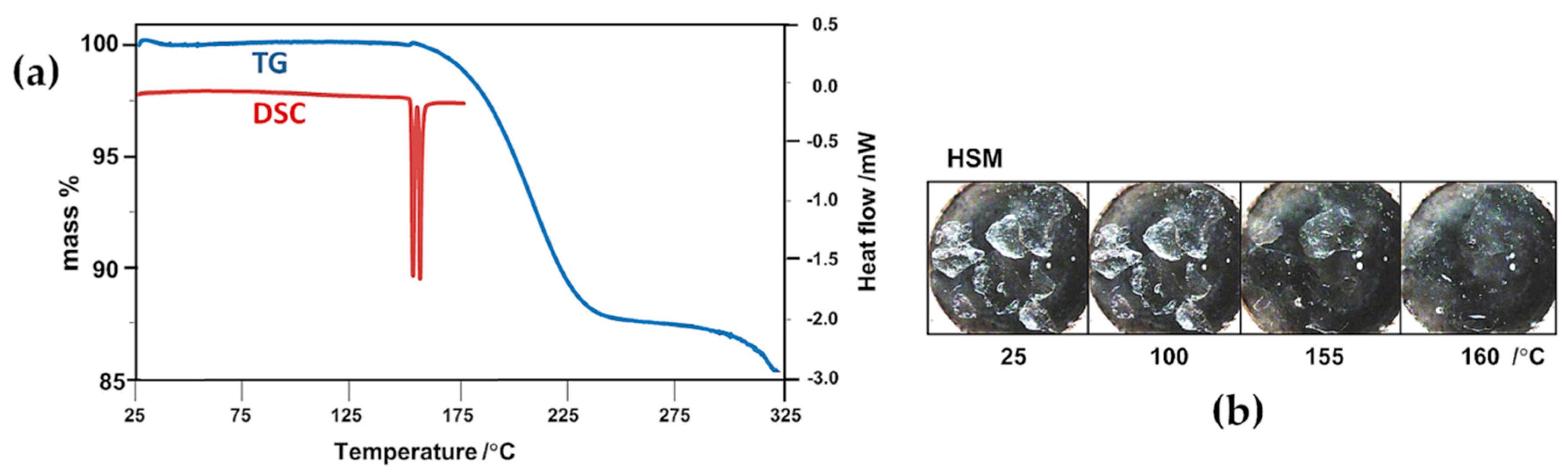
2.2. Thermal Analysis of the Starting Materials and the CD Inclusion Complexes
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hilfiker, R. Relevance of solid-state properties for pharmaceutical products. In Polymorphism in the Pharmaceutical Industry; Hilfiker, R., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 1–8. [Google Scholar]
- Schultheiss, N.; Henck, J.-O. Role of co-crystals in the pharmaceutical development continuum. In Pharmaceutical Salts and Co-Crystals; Wouters, J., Quéré, L., Eds.; RSC Drug Discovery Series No. 16; Royal Society of Chemistry: Cambridge, UK, 2012; pp. 110–127. [Google Scholar]
- Uekama, K.; Hirayama, F.; Arima, H. Pharmaceutical applications of cyclodextrins and their derivatives. In Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Dodziuk, H., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 381–422. [Google Scholar]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Alpha-lipoic acid: A potent mitochondrial nutrient for improving memory deficit, oxidative stress and mitochondrial dysfunction. In Lipoic Acid: Energy Production, Antioxidant Activity and Health Effects; Patel, M.S., Packer, L., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 475–494. [Google Scholar]
- Shay, K.P.; Moreau, R.G.; Smith, E.J.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; DeBusk, B.G.; Gunsalus, I.C.; Hornberger, C.S. Crystalline α-lipoic acid: A catalytic agent associated with pyruvate dehydrogenase. Science 1951, 114, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Monastra, G.; de Grazia, S.; Cilaker Micili, S.; Goker, A.; Unfer, V. Immunomodulatory activities of alpha lipoic acid with a special focus on its efficacy in preventing miscarriage. Expert Opin. Drug Deliv. 2016, 13, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S.; Shrivastava, R.; Mittal, M. Chemistry and pharmacological properties of some natural antioxidants for heavy metal toxicity. Curr. Med. Chem. 2013, 20, 4540–4574. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Ghibu, S.; Muresan, A.; Vergely, C. Alpha-lipoic acid: Molecular mechanisms and therapeutical potential in diabetes1. Can. J. Physiol. Pharmacol. 2015, 93, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, T.M.; Guimaraes, D.D.; Mendes, L.G., Jr.; Braga, V.A. α-Lipoic acid reduces hypertension and increases baroreflex sensitivity in renovascular hypertensive rats. Molecules 2012, 17, 13357–13367. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Smith, A.; Hagen, T.M.; Frei, B. Vascular oxidative stress and inflammation increase with age: Ameliorating effects of α-lipoic acid supplementation. Ann. N. Y. Acad. Sci. 2010, 1203, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Fava, A.; Pirritano, D.; Plastino, M.; Cristiano, D.; Puccio, G.; Colica, C.; Ermio, C.; de Bartolo, M.; Mauro, G.; Bosco, D. The effect of lipoic acid therapy on cognitive functioning in patients with Alzheimer’s disease. J. Neurodegener. Dis. 2013, 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Venigalla, M.; Gyengesi, E.; Sharman, M.J.; Munch, G. Novel promising therapeutics against neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2016, 95, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Bungo, Y.; Mikuni, K. The aqueous solubility and thermal stability of α-lipoic acid are enhanced by cyclodextrin. Biosci. Biotechnol. Biochem. 2011, 75, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Sugiyama, H.; Shimosegawa, H.; Nakane, R.; Ishida, Y.; Uekaji, Y.; Nakata, D.; Pallauf, K.; Rimbach, G.; Terao, K.; et al. Analysis of the enhanced stability of R(+)-alpha lipoic acid by the complex formation with cyclodextrins. Int. J. Mol. Sci. 2013, 14, 3639–3655. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Endo, T.; Hosomi, S.; Setou, K.; Tanaka, S.; Ogawa, N.; Yamamoto, H.; Mizukami, T.; Arai, S.; Okuno, M.; et al. Structural analysis of crystalline R(+)-α-lipoic acid-α-cyclodextrin complex based on microscopic and spectroscopic studies. Int. J. Mol. Sci. 2015, 16, 24614–24628. [Google Scholar] [CrossRef] [PubMed]
- Racz, C.-P.; Santa, S.; Tomoaia-Cotisel, M.; Borodi, G.; Kacso, I.; Pirnau, A.; Bratu, I. Inclusion of α-lipoic acid in β-cyclodextrin. Physical-chemical and structural characterization. J. Incl. Phenom. Macrocycl. Chem. 2013, 76, 193–199. [Google Scholar] [CrossRef]
- Racz, C.-P.; Borodi, G.; Pop, M.; Kacso, I.; Santa, S.; Tomoaia-Cotisel, M. Structure of the inclusion complex of β-cyclodextrin with lipoic acid from laboratory powder diffraction data. Acta Crystallogr. Sect. B Struct. Sci. 2012, 68, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Structural Database and Cambridge Structural Database System, Version 5.38 (February 2017 update); Cambridge Crystallographic Centre, University Chemical Laboratory: Cambridge, UK, 2016.
- Trollope, L.; Cruickshank, D.L.; Noonan, T.; Bourne, S.A.; Sorrenti, M.; Catenacci, L.; Caira, M.R. Inclusion of trans-resveratrol in methylated cyclodextrins: Synthesis and solid-state structures. Beilstein J. Org. Chem. 2014, 10, 3136–3151. [Google Scholar] [CrossRef] [PubMed]
- Caira, M.R.; Bourne, S.A.; Samsodien, H.; Smith, V.J. Inclusion complexes of 2-methoxyestradiol with dimethylated and permethylated β-cyclodextrins: Models for cyclodextrin–steroid interaction. Beilstein J. Org. Chem. 2015, 11, 2616–2630. [Google Scholar] [CrossRef] [PubMed]
- Biewenga, G.P.; Haenen, G.R.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997, 29, 315–331. [Google Scholar] [CrossRef]
- Gębka, A.; Serkies-Minuth, E.; Raczyńska, D. Effect of the administration of alpha-lipoic acid on contrast sensitivity in patients with type 1 and type 2 diabetes. Mediat. Inflamm. 2014, 55, 7. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Hisada, H.; Fujita, M. Mutual Induced Fit in a Synthetic Host–Guest System. J. Am. Chem. Soc. 2014, 136, 4449–4451. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Nutley, M.; MacLean, E.J.; Cameron, K.; Fielding, L.; Mestres, J.; Palin, R. Mutual induced fit in cyclodextrin–rocuronium complexes. Org. Biomol. Chem. 2005, 3, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Makedonopoulou, S.; Yannakopoulou, K.; Mentzafos, D.; Lamzin, V.; Popov, A.; Mavridis, I.M. Non-covalent interactions in the crystallization of the enantiomers of 1,7-dioxaspiro[5.5]undecane (olive fly sex pheromone) by enantiospecific cyclodextrin hosts, hexaks(2,3,6-tri-O-methyl)-α-cyclodextrin and heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin. Acta Crystallogr. 2001, B57, 399–409. [Google Scholar] [CrossRef]
- Yvon, K.; Jeitschko, W.; Parthe, E. LAZY PULVERIX, a computer program, for calculating X-ray and neutron diffraction powder patterns. J. Appl. Crystallogr. 1997, 10, 73–74. [Google Scholar] [CrossRef]
- Caira, M.R.; Bourne, S.A.; Dean, P.M.; Mhlongo, W.T. New crystalline forms of permethylated β-cyclodextrin. Chem. Commun. 2004, 2216–2217. [Google Scholar] [CrossRef] [PubMed]
- Mzondo, B. A Physicochemical Study of the Inclusion of the Antioxidant α-Lipoic Acid and Selected Derivatives in Native and Methylated Derivatives. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2013. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caira, M.R.; Bourne, S.A.; Mzondo, B. Encapsulation of the Antioxidant R-(+)-α-Lipoic Acid in Permethylated α- and β-Cyclodextrins: Thermal and X-ray Structural Characterization of the 1:1 Inclusion Complexes. Molecules 2017, 22, 866. https://doi.org/10.3390/molecules22060866
Caira MR, Bourne SA, Mzondo B. Encapsulation of the Antioxidant R-(+)-α-Lipoic Acid in Permethylated α- and β-Cyclodextrins: Thermal and X-ray Structural Characterization of the 1:1 Inclusion Complexes. Molecules. 2017; 22(6):866. https://doi.org/10.3390/molecules22060866
Chicago/Turabian StyleCaira, Mino R., Susan A. Bourne, and Buntubonke Mzondo. 2017. "Encapsulation of the Antioxidant R-(+)-α-Lipoic Acid in Permethylated α- and β-Cyclodextrins: Thermal and X-ray Structural Characterization of the 1:1 Inclusion Complexes" Molecules 22, no. 6: 866. https://doi.org/10.3390/molecules22060866
APA StyleCaira, M. R., Bourne, S. A., & Mzondo, B. (2017). Encapsulation of the Antioxidant R-(+)-α-Lipoic Acid in Permethylated α- and β-Cyclodextrins: Thermal and X-ray Structural Characterization of the 1:1 Inclusion Complexes. Molecules, 22(6), 866. https://doi.org/10.3390/molecules22060866