Abstract
Sulfonyl-containing compounds, which exhibit a broad spectrum of biological activities, comprise a substantial proportion of and play a vital role, not only in medicines but also in agrochemicals. As a result increasing attention has been paid to the research and development of sulfonyl derivatives. A series of thirty-eight 2-substituted phenyl-2-oxo- III, 2-hydroxy- IV and 2-acyloxyethylsulfonamides V were obtained and their structures confirmed by IR, 1H-NMR, and elemental analysis. The in vitro and in vivo bioactivities against two Botrytis cinerea strains, DL-11 and HLD-15, which differ in their sensitivity to procymidone, were evaluated. The in vitro activity results showed that the EC50 values of compounds V-1 and V-9 were 0.10, 0.01 mg L−1 against the sensitive strain DL-11 and 3.32, 7.72 mg L−1 against the resistant strain HLD-15, respectively. For in vivo activity against B. cinerea, compound V-13 and V-14 showed better control effect than the commercial fungicides procymidone and pyrimethanil. The further in vitro bioassay showed that compounds III, IV and V had broad fungicidal spectra against different phytopathogenic fungi. Most of the title compounds showed high fungicidal activities, which could be used as lead compounds for further developing novel fungicidal compounds against Botrytis cinerea.
1. Introduction
Sulfonyl-containing compounds, which exhibit a broad spectrum of biological activities, comprise a substantial proportion of and play an important role not only in medicines but also in agrochemicals [1,2,3,4,5]. The sulfonyl group is widely applied in the field of functional organic molecule design due to its stable structure and high polarity with strong electron withdrawing ability. Sulfonyl groups can supply two hydrogen-bond acceptors, and a monosubstituted sulfonamide can supply an additional hydrogen-bond donor [6]. Structurally, the sulfonyl group possesses similar molecular size and charge distribution properties as carbonyl, carboxyl and phosphate groups, which can be replaced by a sulfonyl group as a bioisostere to retain or improve bioactivity [7,8,9]. In addition, the introduction of a sulfonyl group can also modulate the solubility and acid-base property of the functional molecules [10,11]. Since the sulfonyl group contains two hydrogen-bond acceptor centers, reasonable introduction of sulfonyl group can enhance the binding affinity of functional molecules with their target proteins to improve activity through hydrogen bond interactions [12,13]. Moreover, the introduction of a sulfonyl group can increase the metabolic stability of functional molecules to prolong the duration of action by blocking metabolically labile sites and increase the bioavailability [14,15,16]. Therefore, the development of sulfonyl-containing compounds has attracted considerable attention.
Sulfonamides were the first drugs with a selective effect on bacteria that could be used systemically against bacterial infections [17]. Hence, great attention has increasingly been paid to developing compounds containing sulfonyl groups as drugs and agrochemicals [18,19,20]. Sulfonamide fungicides, such as tolnifanide, cyazofamid and amisulbrom, have been commercialized, however, there are less sulfonamide species fungicides in field applications, as the advantages and action characteristics of these fungicides are sometimes not obvious in the field. Therefore, there is an urgent need to develop novel sulfonamide fungicides with improved properties [21]. Recently, a series of novel sulfonyl-containing compounds with different scaffolds, such as cycloalkylsulfonamide, benzenesulfonamide and their derivatives containing 1,3,4-thiadiazoles, coumarins and pyrans were reported for obvious and diverse fungicidal activity against Phomopsis asparagi, Cladosporium fulvum and Fusarium oxysporum, etc. [22,23,24,25,26]. The study of cycloalkylsulfonamides started from 2-oxocyclo-dodecylsulfonamide which showed good inhibitory activity against Venturia nashicola and Fusarium graminearum [27]. Using it as a lead compound, 2-oxocycloalkylsulfonamides were further investigated, and the novel candidate fungicide chesulfamide (codename CAUWL-2004-L-13) [28] has been developed to control phytopathogenic fungi including Botrytis cinerea and Corynespora cassiicola.
In our previous work, different scaffolds were introduced onto lead 2-oxocycloalkyl-sulfonamides, and the structure-activity relationships (SAR) were studied [29,30,31,32]. According to the results and following a program of extension and change of these compounds’ structures, we changed the naphthenic group linked with the sulfur bond in the sulfonamide group into an alkyl chain and found that the ethylsulfonamide group was the essential bioactive moiety and therefore, the ethylsulfonamide group was identified as a key building block for compound activity. Meanwhile, some benzoylmethanesulfonamide which showed better fungicidal activity than the cycloalkylsulfonamides were also synthesized [33]. Based on these results, 105 kinds of N-substituted-2-oxo-2-phenylethylsulfonamide derivatives were synthesized using combinatorial chemistry, and the fungicidal activity against B. cinerea were evaluated. Therefore, the SAR of substituent groups on the N atom such as monosubstituted anilines, multi-substituted anilines, substituted benzylamines and alkylamines were systematically studied [34].
On the basis of the above, in order to explore novel structure compounds with high fungicidal activity, and according to our SAR study, we designed and developed a series of new 2-substituted phenyl-2-oxo- III, 2-hydroxy- IV and 2-acyloxyethylsulfonamides V (Figure 1 and Scheme 1). The in vitro and in vivo activities against different strains of Botrytis cinerea were tested, and the fungicidal spectra of the title compounds were also determined.
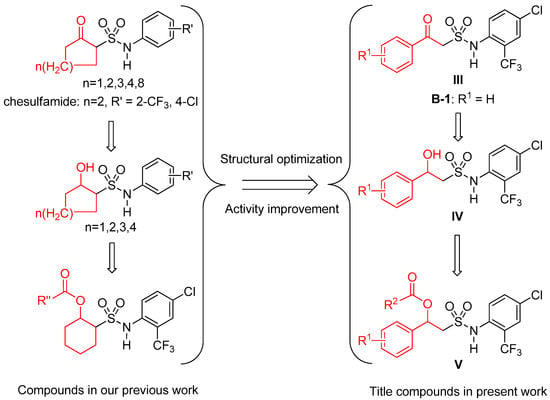
Figure 1.
Design strategy of the title compounds.
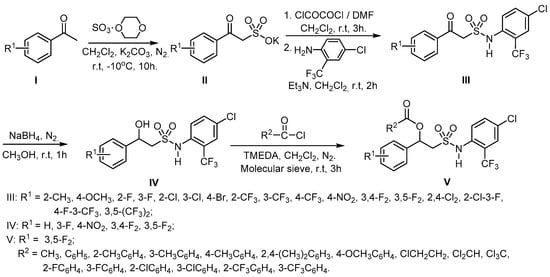
Scheme 1.
Synthesis of compounds III, IV and V.
2. Results
2.1. Chemistry
Potassium 2-substituted-phenyl-2-oxoethylsulfonates II, prepared from commercially available substituted acetophenones I by sulfonation with a sulfur trioxide-dioxane adduct and neutralized with potassium bicarbonate, were reacted with oxalyl chloride to give the corresponding 2-substituted-phenyl-2-oxoethylsulfonyl chlorides, which were converted into the title compounds III by amination with 2-trifluoromethyl-4-chlorophenylamine using triethylamine (Et3N) as catalyst.
According to the structure-activity relationships between substituents on the phenyl ring and fungicidal activity, some of these N-(2-trifluoromethyl-4-chlorophenyl)-2-substituted-phenyl-2-oxoethylsulfonamides III with high fungicidal activity were selected for further reaction with sodium borohydride, giving N-(2-trifluoromethyl-4-chlorophenyl)-2-hydroxy-2-substituted-phenylethyl-sulfonamides IV. Among these compounds N-(2-trifluoromethyl-4-chlorophenyl)-2-hydroxy-2-(3,5-difluorophenyl)ethylsulfonamide (IV-5) showed excellent fungicidal activity. Therefore, we further synthesized a series of N-(2-trifluoromethyl-4-chlorophenyl)-2-acyloxy-2-(3,5-difluorophenyl)ethyl-sulfonamides V by the reaction of IV-5 with acyl chlorides in the presence of TMEDA and molecular sieves as catalysts. Finally, a total of 38 2-substituted-phenyl-2-oxo-, 2-hydroxy- and 2-acyloxy- ethylsulfonamides were synthesized. Flash chromatography was used for separation and purification and the structures of all the title compounds were confirmed by IR, 1H-NMR, and elemental analysis. The 1H-NMR spectra is available in Supplementary materials.
2.2. Biological Assay
The fungicidal activities of all the target compounds against two different strains of Botrytis cinerea with different sensitivity to procymidone collected from different areas in Liaoning, China were tested by in vitro mycelium growth inhibition assay and in vivo greenhouse pot experiments. Moreover, the fungicidal spectra against seven phytopathogenic fungi (Pyricularia grisea, Exserohilum turcicum (Pass.) Leonard et Suggs, Pythium aphanidermatum, Phytophthora capsici Leonian, Fusarium graminearum Schw., Corynespora cassiicola and Thanatephorus cucumeris) were also evaluated.
2.2.1. In Vitro Fungicidal Activity against Botrytis cinerea
The fungicidal activities of N-(2-trifluoromethyl-4-chlorophenyl)-2-substituted-phenyl-2-oxoethylsulfonamides III against two different B. cinerea strains DL-11 (a sensitive strain) and HLD-15 (a resistant strain) were evaluated by an in vitro mycelium growth inhibition assay, and the commercial fungicides procymidone, chlorothalonil and pyrimethanil were used as the positive controls. The synthetic compound (N-(2-trifluoromethyl-4-chlorophenyl)-2-phenyl-2-oxoethyl-sulfonamide (B-1) was also used as the positive control to compare the difference between the pre- and post-structure optimization. The inhibition of mycelial growth was evaluated by measuring colony diameters in the presence and absence of the tested compounds. The results of preliminary bioactivity screening, expressed as the percentage of inhibition, showed that most of the compounds III showed high inhibition rate (more than 70% at 50 mg L−1) and better activity than that of B-1. Based on the experimental results, the EC50 values of the compounds III were next evaluated to confirm their fungicidal effects on the two different kinds of B. cinerea strains DL-11 and HLD-15. The values are listed in Table 1.

Table 1.
The EC50 values of all the title compounds against 2 kinds of B. cinerea in vitro.
As shown in Table 1, the EC50 values of compounds III against B. cinerea strains DL-11 and HLD-15 were within 1.58–10.81 mg L−1 and 3.48–17.70 mg L−1, respectively. Among them, compounds III-13, with EC50 values of 1.58 mg L−1 and 3.48 mg L−1 against DL-11 and HLD-15, respectively, exhibited the best fungicidal activity against B. cinerea. The commercial fungicide procymidone showed EC50 values of 2.59 mg L−1 and 15.95 mg L−1, chlorothalonil gave EC50 values of 1.66 mg L−1 and 17.52 mg L−1 and pyrimethanil provided EC50 values of 32.73 mg L−1 and 71.25 mg L−1 against the two strains DL-11 and HLD-15, against which B-1, another positive control, provided EC50 values of 14.25 mg L−1 and 22.79 mg L−1. The antifungal activity of compounds III-8, III-14 and III-15 was close to that of the control compound procymidone. With procymidone as a standard fungicide against B. cinerea strain DL-11, the relative activities of compounds III-13 and III-15 were 1.64- and 1.57-fold higher, respectively; with B-1 as a standard fungicide, the relative activities of compounds III-13 and III-15 were 9.02- and 8.69 fold better, respectively. As regards to the control efficiency of B. cinerea strain HLD-15, compounds III-13 and III-15, with 14.42 and 13.89 times higher activity than that of B-1, were 10.09 and 9.72 times better than procymidone, respectively.
The fungicidal activity of N-(2-trifluoromethyl-4-chlorophenyl)-2-hydroxy-2-substituted-phenyl ethylsulfonamides IV and N-(2-trifluoromethyl-4-chlorophenyl)-2-acyloxy-2-(3,5-difluoro-phenyl)ethylsulfonamides V against B. cinerea was also evaluated and the EC50 values are summarized in Table 1. The EC50 values of compounds IV against B. cinerea strains DL-11 and HLD-15 were within 0.70–13.33 mg L−1 and 0.61–33.16 mg L−1, respectively, and with compounds V, within 0.01–1279.16 mg L−1 and 3.32–3648.50 mg L−1, respectively. Some of compounds IV and V which were derived from compounds III showed better activity against B. cinerea. Compound V-9 showed the best in vitro fungicidal activity against DL-11, with an EC50 value of 0.01 mg L−1, and IV-5, with an EC50 value of 0.61 mg L−1, exhibited the best activity against HLD-15 in vitro. With procymidone as a standard fungicide against the B. cinerea strain DL-11, the relative activities of compounds V-1 and V-9 were 25.9- and 259-fold higher, respectively. As regards to the control effect of the strain HLD-15, compounds IV-5 and V-1 were 26.15- and 4.8-times more active than procymidone, respectively. Additionally, there is significantly different in the activity level among compounds V. For instance, compound V-1 showed an EC50 values of 0.1 mg L−1 against the B. cinerea strain DL-11, but compound V-6 showed an EC50 value of 131.12 mg L−1.
Finally, it was important to stress that most compounds III showed better fungicidal activity against B. cinerea strain DL-11 (sensitive strain) than that against HLD-15 (resistant strain) because of the resistance, but some individual compounds, on the contrary, exhibited better activity against HLD-15. In general, compounds should exhibit better activity against sensitive strains than resistant strains. Moreover, the same compound gave different control effects on the different B. cinerea strains owing to the difference of biological characteristics, pathogenicity and resistant to fungicides, but on the whole, there were consistent trends in the control effects against both DL-11 and HLD-15.
2.2.2. In Vivo Fungicidal Activity against Botrytis cinerea
The in vivo fungicidal activity of compounds III, IV and V against B. cinerea was evaluated and the results listed in Table 2. The results showed that compounds III, IV and V showed good inhibition, and some of them exhibited better control than the commercial fungicides procymidone and pyrimethanil. Among them, compounds V-13 demonstrated the best activity, with a control efficiency of 74.6%. Besides, the control efficiencies of V-1, V-6, V-8, V-10, V-13 and V-14 were 70.8%, 71.3%, 70.8%, 71.3%, 74.6% and 73.3%, respectively, which were similar to that of the commercial fungicide procymidone (with a control efficiency of 72.5%).

Table 2.
Control efficiency of all the title compounds against B. cinerea in vivo (500 mg L−1).
The tested results showed that the whole level of the control effects of compounds IV and V was somewhat increased in comparison to compounds III, especially compounds V, all of which showed control efficiencies of more than 60%. In addition, the in vitro fungicidal activity of the tested compounds showed no consistent correlation with that in vivo. Compound V-13, with the high EC50 values of 35.18 mg L−1 in vitro, had the highest control efficiency of 74.6% against the B. cinerea strain DL-11. The reason may be that, there were interactions between reagents and plants in vivo experiments, not just between the reagents and the fungi. Therefore, characteristics of the tested compounds such as polarity, conductibility and degradation in vivo also had influence on the fungicidal activity, which is worthy of further study.
2.2.3. Fungicidal Activity against Different Phytopathogenic Fungi
Different kinds of phytopathogenic fungi were chosen to evaluate the fungicidal spectrum of the title compounds III, IV and V. We summarize the bioassay results of inhibitory activities of these compounds against seven phytopathogenic fungi at 50 mg L−1. Overall, the title compounds showed different levels of fungicidal activity. Compounds III-2, III-10, III-16, IV-5 and V-10 exhibited wide spectrum antifungal activity and compound V-10 displayed excellent activities against all the tested phytopathogentic fungi. Moreover, some compounds showed highly specific pathogen activity, whereby compounds III-1 and III-4 exhibited activities against Phytophthora capsici Leonian with inhibition rates 90.4% and 91.0%, respectively. It is noteworthy that nearly all compounds III showed excellent fungicidal activity against Phytophthora capsici Leonian and Fusarium graminearum Schw., and most of the inhibition rate was more than 70%. In general, compounds V possessed an improvement of fungicidal activity in comparison to compounds III. However, compounds V were only slightly active against Exserohilum turcicum (Pass.) Leonard et Suggs and Pythium aphanidermatum, being less active than compounds III. Besides, some individual compound showed unexpected antifungal activity against a certain pathogen. For example, Compound V-16 exhibited good activity against P. aphanidermatum with inhibition rate of 72.9%, although the inhibition rates of most compounds V were less than 25%.
Based on the inhibition rate of compounds III, IV and V against different phytopathogentic fungi, EC50 values of some highly bioactive compounds were evaluated against four different phytopathogentic fungi (Fusarium graminearum Schw., Thanatephorus cucumeris, Pythium aphanidermatum and Phytophthora capsici Leonian). Compound B-1 and chlorothalonil were used as the positive controls. As shown in Table 3, compounds III-6, III-10, III-13 and V-10 exhibited the best fungicidal activity against the four fungi, with EC50 values of 1.41, 0.99, 7.25, 2.25 mg L−1; 1.05, 0.60, 8.65, 4.47 mg L−1; 0.79, 1.86, 8.62, 2.74 mg L−1 and 2.41, 1.07, 6.96, 3.22 mg L−1, respectively.

Table 3.
The EC50 values of selected compounds III, IV and V against different phytopathogenic fungi in vitro.
3. Discussion
3.1. Synthesis and Structure Elucidation
High yield syntheses of compounds III, IV and V were accomplished in short reaction times and using mild reaction conditions. Oleum was often used as an efficient sulfonating agent, but lots of byproducts are generated during the sulphonation process with that reagent. In our reactions, sulfur trioxide-dioxane adduct was used as the sulfonating agent, prepared by a standard procedure (mixing a solution of sulfur trioxide in 1,2-dichloroethane with a solution of dioxane in the same solvent in a certain order to give 1:1 sulfur trioxide-dioxane adducts), and high yields of products were achieved. Besides, we also tried to react sulfur trioxide-pyridine adduct with the acetophenones, but the yields of this reaction were very low, and in some cases no product was obtained. A tentative inference from this result was that the relative stability and reactivity of sulfur trioxide-pyridine adduct and sulfur trioxide-dioxane adduct has an influence on the sulfonation of acetophenones. Moreover, oxalyl chloride was used as the chlorinating agent in these experiments to give the corresponding sulfonyl chlorides and acyl chlorides, which were reacted with DMF catalysis. The reaction proceeded smoothly in high yields and the product separation and purification were simple. In most cases, the title compounds III, IV and V were obtained in acceptable yield.
The chemical structures of all the synthesized compounds in this work were mainly characterized by nuclear magnetic resonance (NMR) and infrared (IR) absorption spectroscopy. The 1H-NMR spectra of N-(2-trifluoromethyl-4-chlorophenyl)-2-substituted-phenyl-2-oxoethyl-sulfonamides III showed some characteristic peaks, i.e., the 1H chemical shifts of the phenyl rings in the sulfonamides were seen at around 7.50 ppm. The 1H-NMR spectrum of compounds III showed a singlet signal around 4.70 ppm, which was assigned to the methylene protons between the sulfonyl and carbonyl groups. Additionally, a signal appearing at approximately 7.25 ppm was assigned to the NH group in the sulfonamide functionality (Figure 2a).
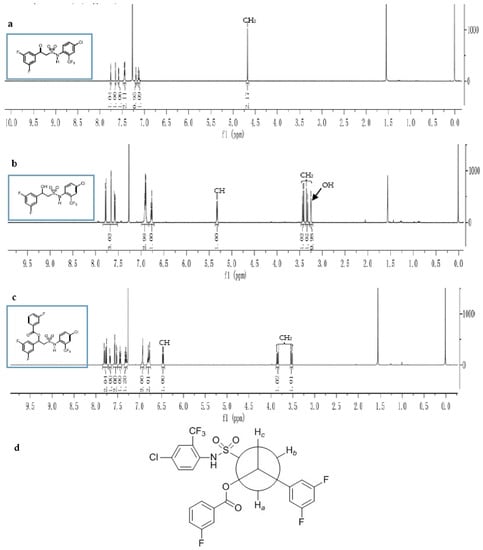
Figure 2.
Structure elucidation of compounds III, IV and V: (a) 1H-NMR spectrum of compound III-13; (b) 1H-NMR spectrum of the compound IV-5; (c) 1H-NMR spectrum of compound V-12; (d) The proposed stable conformation of compound V-12.
In the IR spectra, a carbonyl stretching vibration appeared, as expected, around 1680 cm−1 and 1700 cm−1. The NH stretching vibration was around 3250 cm−1 to 3350 cm−1. The spectra of compounds IV, which were obtained in high yield (more than 85%) by reduction of compounds III, showed significant changes compared with those of compounds III. The signal of the methylene group in the 1H-NMR spectra appeared as a multiplet due to the effect of the chiral carbon in the side chain. Therefore, the 1H-NMR chemical shifts of the CH2 combined with the sulfonyl and CH linked with the hydroxyl group were at about 3.40 ppm and 5.30 ppm, respectively. A signal appearing at approximately 3.20 ppm was assigned to the OH group (Figure 2b). In the IR spectrum, it was found that carbonyl absorption peak at 1680 cm−1 disappeared and that of a hydroxyl with an obvious stretching vibration at 3400 cm−1 appeared. For compounds V, the ester carbonyl stretching vibration appeared around 1700 cm−1. In addition, the most stable conformation of compound V-12 (Figure 2c,d) could be proposed. As shown in Figure 2d, the two hydrogens (Ha, Hb) of methylene were of chemical unequivalent. The 1H-NMR analysis showed that the coupling constant of Ha and Hb was 1J = 7.35 Hz, and that of Ha and Hc, Hb and Hc were 2J = 1.56 Hz, 3J = 4.83 Hz, respectively (Figure 2c).
3.2. Screening of Fungicidal Activity and Structure-Activity Relationships
In this paper, the fungicidal activities of compounds III, IV and V against two Botrytis cinerea strains collected from different areas in Liaoning, China were tested by an in vitro mycelium growth inhibition assay and in vivo greenhouse pot experiments. By adopting different populations of the same species, the analytical activity screening results were more accurate and reliable, and then this made the activity evaluation of synthetic compounds against fungi more comprehensive and persuasive. Comprehensively, the fungicidal activities of the same compound against different Botrytis cinerea strains showed less differences owing to the difference of biological characteristics, pathogenicity and resistance to fungicides between isolates. Hence, certain compounds showed excellent antifungal activity against a certain strain, whereas for another isolate, the activity may be unsatisfactory. For the purpose of screening new compounds worthy of further research, we selected compounds with excellent fungicidal activities against all the tested Botrytis cinerea strains, especially for the resistant strain. Thus, compounds such as III-12, III-13 were selected for further activity screening and structure optimization.
From the results of the fungicidal activity screening against Botrytis cinerea and the structure-activity relationships (SAR), it could be seen that the substituents on the benzene ring might exert a greater influence on the activity. As for N-(2-trifluoromethyl-4-chlorophenyl)-2-substituted-phenyl-2-oxoethylsulfonamides III, the biological experiments showed that the introduction of any substituent in the phenyl ring increased the inhibition activity to Botrytis cinerea. The activity was significantly increased when the benzene ring contained fluorine-containing groups (F and CF3) which show significant functions in organisms due to their osmotic and electronic effects, etc. The number of fluorine-containing groups also influenced the antifungal activity. Thus, when the substituent was fluorine, the fungicidal activity increased with the increase of the number of substituents. If the substituent was a trifluoromethyl group, contrarily the fungicidal activity decreased. Moreover, there was no significant difference between different positions of halogen atoms on the benzene ring and their fungicidal activities. However, for trifluoromethyl, it seemed that the compound with a trifluoromethyl group on the para position of the benzene ring showed higher activities than when it was at the meta position, and the compound with a trifluoromethyl group on the para position exhibited the best activity. We also revealed that the strong electron-donating capacity of the substituent on the phenyl ring reduced the fungicidal activity of compounds III. For example, compounds III-1 and III-3 (with the EC50 values of 8.67, and 7.51 mg L−1 against the Botrytis cinerea strain DL-11, respectively) had lower fungicidal activity than the compound III-13 (with an EC50 value of 1.58 mg L−1 against the Botrytis cinerea strain DL-11). The structure-activity relationship of N-(2-trifluoromethyl-4-chlorophenyl)-2-hydroxy-2-substituted-phenyl ethyl-sulfonamides IV was similar to that of compounds III, while for N-(2-trifluoromethyl-4-chlorophenyl)-2-acyloxy-2-(3,5-difluorophenyl)ethylsulfonamides V, it could be seen that the acyloxy substituent showed higher fungicidal activity when it was a small group. The compounds with an alkylacyloxy groups showed higher activities than that with an arylacyloxy group, and generally substituted benzoyloxy compounds showed lower activity than benzoyloxy compounds. For instance, compounds V-1, V-10 and V-15 provided EC50 values of 0.10, 1.18 and 20.33 mg L−1 against the Botrytis cinerea strain DL-11, respectively. The fungicidal activities showed no significant difference between compounds V with the change of substituent position of the substituted benzoyloxy group.
It is worth mentioning that the fungicidal activities showed no significant difference between compounds III and IV, most of which had inhibition rates of more than 75.0% against Botrytis cinerea, although for compounds V, there were obvious differences between their individual fungicidal activities. This suggests that these compounds have different modes of action, which need to be further studied. Additionally, the results of in vitro and in vivo fungicidal activities were inconsistent. For example, with procymidone as a standard fungicide against Botrytis cinerea strains DL-11, the relative toxicity of compound V-9 was 259, which represented the best activity in vitro. However, the in vivo activity of V-10 only showed the control efficiency of 65.4%, and the positive control procymidone had a control efficiency of 58.8%. Further investigation are needed to explain these findings.
4. Materials and Methods
4.1. General Information
The melting points were measured using an X-5 melting-point apparatus (Beijing Second Optical Instrument Factory, Beijing, China). Infrared (IR) spectra were recorded in potassium bromide disks on a Spectrum 65 spectrophotometer (Perkin Elmer, Waltham, MA, USA). Nuclear magnetic resonance (NMR) spectra were recorded in CDCl3 on Bruker 400 or 600 MHz spectrometers (Bruker, Shanghai, China), using tetramethylsilane (TMS) as an internal standard. Elemental analyses were determined on a Vario EL III elemental analyser (Elementar Analysensysteme GmbH, Frankfurt, Germany).
4.2. Synthetic Procedures
The synthetic route of the title compounds is shown in Scheme 1. Potassium 2-substituted phenyl-2-oxoethylsulfonates II, were prepared from readily commercially available substituted acetophenones I by sulfonation with a sulfur trioxide-dioxane adduct and neutralization with potassium carbonate according to the method given in references [27,29,30]. The title compounds were synthesized by a method developed by our group described as follows:
4.2.1. General Synthetic Procedure for the Target Compounds III
To a slurry of potassium 2-substituted0phenyl-2-oxoethylsulfonate II (0.03 mol) and N,N-dimethylformamide (DMF, 0.15 mL) in dichloromethane (CH2Cl2, 30 mL), oxalyl chloride (0.033 mol) was added dropwise at −5 °C. The mixture was stirred at room temperature for 3 h. After cooling in an ice-water bath, it was filtered under reduced pressure. The filtrate was added dropwise to a solution of 2-amino-5-chlorobenzotrifluoride (0.015 mol) and triethylamine (Et3N, 0.03 mol) in CH2Cl2 (20 mL) at −5 °C. After stirring at room temperature for 3 h, the reaction mixture was washed successively with 3 mol L−1 hydrochloric acid (HCl, 15 mL), saturated sodium bicarbonate solution (15 mL) and saturated salt water (15 mL), and then dried over sodium sulfate. After evaporating the solvent under vacuum, the crude product was further purified by silica column chromatography to give pure compounds III-1 to III-17 using the mixture of ethyl acetate and petroleum ether (10:1) as the eluent.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(2-methylphenyl)-2-oxoethylsulfonamide (III-1): white solid, 88.1% yield: m.p. 59–61 °C; IR (KBr) νmax 3255, 3045, 2917, 1689, 1338, 1130 cm−1; 1H-NMR (400 MHz), δ (ppm) 2.25 (s, 3H, CH3), 4.71 (s, 2H, CH2), 7.37 (S, 1H, NH), 7.29–7.75 (m, 7H, C6H4 + C6H3); Elemental anal. calc. for C16H13ClF3NO3S (%): C, 49.05; H, 3.34; N, 3.58; found: C, 49.33; H, 3.20; N, 3.78.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(4-methoxylphenyl)-2-oxoethylsulfonamide (III-2): light brown solid, 83.6% yield: m.p. 83–85 °C; IR (KBr) νmax 3350, 3080, 2968, 1670, 1344, 1161 cm−1; 1H-NMR (400 MHz), δ (ppm) 3.90 (s, 3H, CH3), 4.71 (s, 2H, CH2), 7.37 (S, 1H, NH), 6.97–7.92 (m, 7H, C6H3 + C6H4); Elemental anal. calc. for C16H13ClF3NO4S (%): C, 47.13; H, 3.21; N, 3.43; found: C, 47.36; H, 3.48; N, 3.66.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(2-fluorophenyl)-2-oxoethylsulfonamide (III-3): white solid, 78.9% yield: m.p. 122–124 °C; IR (KBr) νmax 3296, 3033, 2941, 1689, 1311, 1116 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.78 (d, 2H, CH2), 7.17–7.20 (q, 1H, NH), 7.30–7.97 (m, 7H, C6H4 + C6H3); Elemental anal. calc. for C15H10ClF4NO3S (%): C, 45.52; H, 2.55; N, 3.54; found: C, 45.68; H, 2.34; N, 3.74.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3-fluorophenyl)-2-oxoethylsulfonamide (III-4): light yellow solid, 81.5% yield: m.p. 109–111 °C; IR (KBr) νmax 3342, 3078, 2974, 1687, 1311, 1122 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.72 (s, 2H, CH2), 7.25 (s, 1H, NH), 7.35–7.77 (m, 7H, C6H4 + C6H3); Elemental anal. calc. for C15H10ClF4NO3S (%): C, 45.52; H, 2.55; N, 3.54; found: C, 45.36; H, 2.37; N, 3.68.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(2-chlorophenyl)-2-oxoethylsulfonamide (III-5): light yellow solid, 76.6% yield: m.p. 85–87 °C; IR (KBr) νmax 3253, 3064, 2927, 1689, 1307, 1168 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.84 (s, 2H, CH2), 7.25 (s, 1H, NH), 7.29–7.75 (m, 7H, C6H4 + C6H3); Elemental anal. calc. for C15H10Cl2F3NO3S (%): C, 43.71; H, 2.45; N, 3.40; found: C, 43.90; H, 2.59; N, 3.28.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3-chlorophenyl)-2-oxoethylsulfonamide (III-6): light yellow solid, 68.4% yield: m.p. 109–111 °C; IR (KBr) νmax 3340, 3056, 2974, 1687, 1338, 1116 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.72 (s, 2H, CH2), 7.25 (s, 1H, NH), 7.45–7.91 (m, 7H, C6H4 + C6H3); Elemental anal. calc. for C15H10Cl2F3NO3S (%): C, 43.71; H, 2.45; N, 3.40; found: C, 44.01; H, 2.19; N, 3.25.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(4-bromophenyl)-2-oxoethylsulfonamide (III-7): light yellow solid, 78.9% yield: m.p. 89–91 °C; IR (KBr) νmax 3292, 3033, 2926, 1687, 1396, 1120 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.71 (s, 2H, CH2), 7.25 (s, 1H, NH), 7.55–7.81 (m, 7H, C6H4 + C6H3); Elemental anal. calc. for C15H10BrClF3NO3S (%): C, 39.45; H, 2.21; N, 3.07; found: C, 39.25; H, 2.03; N, 3.24.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(2-trifluoromethylphenyl)-2-oxoethylsulfonamide (III-8): light yellow solid, 74.3% yield: m.p. 79–81 °C; IR (KBr) νmax 3298, 3032, 2943, 1705, 1315, 1114 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.62 (s, 2H, CH2), 7.19 (s, 1H, NH), 7.55–7.78 (m, 7H, C6H4 + C6H3); Elemental anal. calc. for C16H10ClF6NO3S (%): C, 43.11; H, 2.26; N, 3.14; found: C, 43.35; H, 2.05; N, 3.36.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3-trifluoromethylphenyl)-2-oxoethylsulfonamide (III-9): white solid, 77.6% yield: m.p. 85–87 °C; IR (KBr) νmax 3348, 3027, 2972, 1689, 1338, 1184 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.77 (s, 2H, CH2), 7.24 (s, 1H, NH), 7.55–8.18 (m, 7H, C6H4 + C6H3); Elemental anal. calc. for C16H10ClF6NO3S (%): C, 43.11; H, 2.26; N, 3.14; found: C, 43.31; H, 2.04; N, 2.99.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(4-trifluoromethylphenyl)-2-oxoethylsulfonamide (III-10): light yellow solid, 79.8% yield: m.p. 116–118 °C; IR (KBr) νmax 3311, 3021, 2960, 1680, 1323, 1134 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.75 (s, 2H, CH2), 7.22 (s, 1H, NH), 7.57–8.05 (m, 7H, C6H4 + C6H3); Elemental anal. calc. for C16H10ClF6NO3S (%): C, 43.11; H, 2.26; N, 3.14; found: C, 42.92; H, 2.50; N, 3.32.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(4-nitrophenyl)-2-oxoethylsulfonamide (III-11): light yellow solid, 70.5% yield: m.p. 119–121 °C; IR (KBr) νmax 3273, 3010, 2954, 1703, 1396, 1122 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.77 (s, 2H, CH2), 7.17 (s, 1H, NH), 7.58–8.39 (m, 7H, C6H4 + C6H3); Elemental anal. calc. for C15H10ClF3N2O5S (%): C, 42.62; H, 2.38; N, 6.63; found: C, 42.84; H, 2.18; N, 6.47.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,4-difluorophenyl)-2-oxoethylsulfonamide (III-12): white solid, 71.5% yield: m.p. 89–91 °C; IR (KBr) νmax 3356, 3078, 2972, 1687, 1313, 1176 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.69 (s, 2H, CH2), 7.21 (s, 1H, NH), 7.30–7.82 (m, 6H, C6H3 + C6H3); Elemental anal. calc. for C15H9ClF5NO3S (%): C, 43.55; H, 2.19; N, 3.39; found: C, 43.82; H, 1.98; N, 3.54.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-oxoethylsulfonamide (III-13): light yellow solid, 75.5% yield: m.p. 93–95 °C; IR (KBr) νmax 3334, 3011, 2926, 1680, 1338, 1122 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.67 (s, 2H, CH2), 7.19 (s, 1H, NH), 7.17–7.77 (m, 6H, C6H3 + C6H3); Elemental anal. calc. for C15H9ClF5NO3S (%): C, 43.55; H, 2.19; N, 3.39; found: C, 43.72; H, 2.35; N, 3.18.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(2,4-dichlorophenyl)-2-oxoethylsulfonamide (III-14): light yellow solid, 80.6% yield: m.p. 106–108 °C; IR (KBr) νmax 3290, 3017, 2946, 1687, 1340, 1120 cm−1; 1H-NMR (CDCl3, 400 MHz), δ (ppm) 4.80 (s, 2H, CH2), 7.20 (s, 1H, NH), 7.38–7.75 (m, 6H, C6H3 + C6H3); Elemental anal. calc. for C15H9Cl2F3NO3S (%): C, 40.34; H, 2.03; N, 3.14; found: C, 40.58; H, 2.31; N, 3.32.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(2-chloro-3-fluorophenyl)-2-oxoethylsulfonamide (III-15): white solid, 72.8% yield: m.p. 115–117 °C; IR (KBr) νmax 3309, 3014, 2916, 1730, 1338, 1122 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.53 (s, 2H, CH2), 7.15(s, 1H, NH), 7.22–7.78 (m, 6H, C6H3 + C6H3); Elemental anal. calc. for C15H9Cl2F4NO3S (%): C, 41.88; H, 2.11; N, 3.26; found: C, 42.01; H, 1.97; N, 3.42.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(4-fluoro-3-trifluoromethylphenyl)-2-oxoethylsulfonamide (III-16): light yellow solid, 84.6% yield: m.p. 99–101 °C; IR (KBr) νmax 3354, 3072, 2964, 1685, 1350, 1174 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.73 (s, 2H, CH2), 7.20 (s, 1H, NH), 7.37–8.23 (m, 6H, C6H3 + C6H3); Elemental anal. calc. for C16H9ClF7NO3S (%): C, 41.44; H, 1.96; N, 3.02; found: C, 41.66; H, 2.10; N, 3.29.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-ditrifluoromethylphenyl)-2-oxoethylsulfonamide (III-17): light yellow solid, 69.7% yield: m.p. 119–121 °C; IR (KBr) νmax 3354, 3072, 2964, 1685, 1350, 1174 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.77 (s, 2H, CH2), 7.15 (s, 1H, NH), 7.57–8.35 (m, 6H, C6H3 + C6H3); Elemental anal. calc. for C17H9ClF9NO3S (%): C, 39.74; H, 1.77; N, 2.73; found: C, 39.55; H, 1.52; N, 2.99.
4.2.2. General Synthetic Procedure for the Target Compounds IV
To a solution of N-(2-trifluoromethyl-4-chlorophenyl)-2-phenyl-2-oxoethylsulfonamide (0.01 mol) in methanol (30 mL) at 0–5 °C, sodium borohydride solution (0.016 mol NaBH4 + 8 mL of 1% NaOH + 8 mL of CH3OH) was added dropwise. The mixture was stirred at 5–25 °C for 1–3 h. After methanol was evaporated in vacuum, the residue was dissolved in ethyl acetate (80 mL), washed with 5% HCl (30 mL) and water (20 mL), and dried over sodium sulfate. After the solvent was evaporated under vacuum, the crude product was recrystallized from acetone/petroleum ether to give pure compounds IV-1 to IV-5.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-phenyl-2-hydroxyethylsulfonamide (IV-1): white solid, 87.2% yield: m.p. 95–96 °C; IR (KBr) νmax 3242, 3061, 1369, 1315, 1128, 975; 1H-NMR (400 MHz), δ (ppm) 3.03 (s, 1H, OH), 3.37–3.67 (m, 2H, CH2), 5.35–5.36 (d, 1H, CH), 7.06–7.79 (m, 9H, C6H5 + C6H3 + NH); Elemental anal. calc. for C15H13ClF3NO3S (%): C, 47.44; H, 3.45; N, 3.69; found: C, 47.69; H, 3.56; N, 3.49.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3-fluorophenyl)-2-hydroxyethylsulfonamide (IV-2): white solid, 88.6% yield: m.p. 78–80 °C; IR (KBr) νmax 3241, 3024, 1368, 1316, 1134, 984; 1H-NMR (400 MHz), δ (ppm) 3.12 (s, 1H, OH), 3.36–3.54 (m, 2H, CH2), 5.35 (d, 1H, CH), 6.96–7.79 (m, 8H, C6H4 + C6H3 + NH); Elemental anal. calc. for C15H12ClF4NO3S (%): C, 45.29; H, 3.04; N, 3.52; found: C, 45.06; H, 3.21; N, 3.67.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(4-nitrophenyl)-2-hydroxyethylsulfonamide (IV-3): white solid, 94.1% yield: m.p. 110–111 °C; IR (KBr) νmax 3256, 3054, 1385, 1320, 1122, 956; 1H-NMR (400 MHz), δ (ppm) 3.35–3.47 (m, 3H, CH2+OH), 5.45–5.47 (q, 1H, CH), 6.91–8.25 (m, 8H, C6H4 + C6H3 + NH); Elemental anal. calc. for C15H12ClF3N2O5S (%): C, 42.41; H, 2.85; N, 6.60; found: C, 42.58; H, 3.01; N, 6.48.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,4-difluorophenyl)-2-hydroxyethylsulfonamide (IV-4): white solid, 88.7% yield: m.p. 104–105 °C; IR (KBr) νmax 3215, 3042, 1349, 1320, 1156, 964; 1H NMR (400 MHz), δ (ppm) 3.19 (s, 1H, OH), 3.32–3.50 (m, 2H, CH2), 5.30–5.32 (q, 1H, CH), 6.92–7.79 (m, 7H, C6H3 + C6H3 + NH); Elemental anal. calc. for C15H11ClF5NO3S (%): C, 43.33; H, 2.67; N, 3.37; found: C, 43.54; H, 2.39; N, 3.47.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-hydroxyethylsulfonamide (IV-5): white solid, 98.8% yield: m.p. 98–100 °C; IR (KBr) νmax 3273, 3025, 1396, 1311, 1122, 989; 1H-NMR (400 MHz), δ (ppm) 3.25 (s, 1H, OH), 3.34–3.47(m, 2H, CH2), 5.31–5.33 (d, 1H, CH), 6.75–7.78 (m, 7H, C6H3 + C6H3 + NH); Elemental anal. calc. for C15H11ClF5NO3S (%): C, 43.33; H, 2.67; N, 3.37; found: C, 43.16; H, 2.88; N, 3.24.
4.2.3. General Synthetic Procedure for the Target Compounds V
To the solution of N-(2-trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-hydroxyethyl-sulfonamide (0.01 mol), N,N,N′,N′-tetramethylenediamine (TMEDA, 0.006 mol) and 3Å molecular sieves (2 g) in dry CH2Cl2 (30 mL), acyl chloride (0.011 mol) was added dropwise at room temperature. The mixture was stirred at room temperature for 2 h. The reaction was quenched with ice water (20 mL × 2), filtered and dried over anhydrous magnesium sulfate. After filtering and evaporating the solvent under vacuum, the crude product was purified by silica gel chromatography using petroleum ether/ethyl acetate (10/1, v/v) as eluent to obtain pure compounds V-1 to V-16.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-acetoxyethylsulfonamide (V-1): white solid, 65.5% yield: m.p. 96–98 °C; IR (KBr) νmax 3350, 3097, 2954, 1761, 1307, 1220 cm−1; 1H-NMR (400 MHz), δ (ppm) 2.11 (s, 3H, CH3), 3.38–3.68 (m, 2H, CH2), 6.27–6.29 (q, 1H, CH), 7.55–7.58 (d, 1H, NH), 6.78–7.75 (m, 6H, C6H3 + C6H3); Elemental anal. calc. for C17H13ClF5NO4S (%): C, 44.60; H, 2.86; N, 3.06; found: C, 44.42; H, 3.01; N, 3.25.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-benzoyloxyethylsulfonamide (V-2): white solid, 51.8% yield: m.p. 93–95 °C; IR (KBr) νmax 3307, 3032, 2952, 1724, 1338, 1269 cm−1; 1H-NMR (400 MHz), δ (ppm) 3.53–3.88 (m, 2H, CH2), 6.77–6.95 (q, 1H, CH), 6.77–6.95 (q, 3H, CH3), 7.55–7.76 (d, 1H, NH), 7.44–7.4 (t, 2H, CH2), 7.50–8.01 (m, 6H, C6H3 + C6H3); Elemental anal. calc. for C22H15ClF5NO4S (%): C, 50.83; H, 2.91; N, 2.69; found: C, 50.67; H, 3.12; N, 2.48.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-(2-methylbenzoyloxy)ethylsulfonamide (V-3): white solid, 56.3% yield: m.p. 69–71 °C; IR (KBr) νmax 3325, 3028, 2931, 1747, 1311, 736 cm−1; 1H-NMR (400 MHz), δ (ppm) 2.54 (s, 3H, CH3), 3.51–3.82 (m, 2H, CH2), 6.44–6.45 (q, 1H, CH), 6.78–8.05 (m, 11H, C6H3 + C6H3 + NH + C6H4); Elemental anal. calc. for C23H17ClF5NO4S (%): C, 51.74; H, 3.21; N, 2.62; found: C, 51.92; H, 3.10; N, 2.81.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-(3-methylbenzoyloxy)ethylsulfonamide (V-4): white solid, 81.1% yield: m.p. 114–116 °C; IR (KBr) νmax 3253, 3033, 2945, 1747, 1338, 734 cm−1; 1H-NMR (400 MHz), δ (ppm) 2.41 (s, 3H, CH3), 3.53–3.89 (m, 2H, CH2), 6.45–6.48 (q, 1H, CH), 6.77–6.79 (m, 11H, C6H3 + C6H3 + NH + C6H4); Elemental anal. calc. for C23H17ClF5NO4S (%): C, 51.74; H, 3.21; N, 2.62; found: C, 51.59; H, 3.43; N, 2.75.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-(4-methylbenzoyloxy)ethylsulfonamide (V-5): white solid, 81.8% yield: m.p. 137–139 °C; IR (KBr) νmax 3307, 3032, 2928, 1716, 1338, 827 cm−1; 1H-NMR (400 MHz), δ (ppm) 2.43 (s, 3H, CH3), 3.52–3.87 (q, 2H, CH2), 6.45–6.47 (q, 1H, CH), 6.77–7.88 (m, 11H, C6H3 + C6H3 + NH + C6H4); Elemental anal. calc. for C23H17ClF5NO4S (%): C, 51.74; H, 3.21; N, 2.62; found: C, 51.88; H, 3.10; N, 2.57.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-(4-methoxybenzoyloxy)ethylsulfonamide (V-6): white solid, 60.5% yield: m.p. 118–120 °C; IR (KBr) νmax 3307, 3057, 2943, 1716, 1305, 829 cm−1; 1H-NMR (400 MHz), δ (ppm) 3.52–3.55 (q, 1H, CH), 3.83–3.87 (q, 1H, CH), 3.88 (s, 3H, CH3), 6.43–6.45 (q, 1H, CH), 6.75–7.95 (m, 11H, C6H3 + C6H3 + NH + C6H4); Elemental anal. calc. for C23H17ClF5NO5S (%): C, 50.24; H, 3.12; N, 2.55; found: C, 50.49; H, 2.94; N, 2.72.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-(2,4-dimethylbenzoyloxy)ethylsulfonamide (V-7): white solid, 71.8% yield: m.p. 120–122 °C; IR (KBr) νmax 3307, 3054, 2927, 1745, 1392, 821 cm−1; 1H-NMR (400 MHz), δ (ppm) 2.37 (s, 3H, CH3), 2.51 (s, 3H, CH3), 3.51–3.85 (m, 2H, CH2), 6.42–6.44 (q, 1H, CH), 6.77–7.83 (m, 10H, C6H3+C6H3+NH+C6H3); Elemental anal. calc. for C24H19ClF5NO4S (%): C, 52.61; H, 3.50; N, 2.56; found: C, 52.74; H, 3.41; N, 2.68.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-(2-chloropropionyloxy)ethylsulfonamide (V-8): light yellow solid, 62.7% yield: m.p. 124–126 °C; IR (KBr) νmax 3502, 3115, 2997, 1691, 1303, 1155 cm−1; 1H-NMR (400 MHz), δ (ppm) 3.30–3.40 (d, 2H, CH2), 3.77–5.82 (m, 6H, CH2 + CH2 + CH2), 6.58–7.85 (m, 7H, C6H3 + C6H3 + NH); Elemental anal. calc. for C18H14Cl2F5NO4S (%): C, 42.70; H, 2.79; N, 2.77; found: C, 42.56; H, 2.91; N, 2.58.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-dichloroacetoxyethylsulfonamide (V-9): white solid, 51.4% yield: m.p. 150–152 °C; IR (KBr) νmax 3102, 3010, 2956, 1714, 1384, 1170 cm−1; 1H-NMR (400 MHz), δ (ppm) 3.93–4.62 (m, 2H, CH2), 6.53 (d, 1H, CH), 5.99–6.02 (d, 1H, CH), 6.35–7.88 (m, 7H, C6H3 + C6H3 + NH); Elemental anal. calc. for C17H11Cl3F5NO4S (%): C, 38.77; H, 2.11; N, 2.66; found: C, 38.86; H, 2.33; N, 2.48.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-trichloroacetoxyethylsulfonamide (V-10): white solid, 80.9% yield: m.p. 168–170 °C; IR (KBr) νmax 3082, 3010, 2954, 1705, 1379, 1224 cm−1; 1H-NMR (400 MHz), δ (ppm) 4.05–4.71 (m, 2H, CH2), 6.32–6.34 (q, 1H, CH), 6.88–7.79 (m, 7H, C6H3 + C6H3 + NH); Elemental anal. calc. for C17H10Cl4F5NO4S (%): C, 36.39; H, 1.80; N, 2.50; found: C, 36.51; H, 2.02; N, 2.31.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-(2-fluorobenzoyloxy)ethylsulfonamide (V-11): white solid, 75.6% yield: m.p. 118–120 °C; IR (KBr) νmax 3307, 3095, 2956, 1743, 756, 686 cm−1; 1H-NMR (400 MHz), δ (ppm) 3.52–3.88 (m, 2H, CH2), 6.49–6.51 (q, 1H, CH), 6.78–7.93 (m, 11H, C6H3 + C6H3 + NH + C6H4); Elemental anal. calc. for C22H14ClF6NO4S (%): C, 49.13; H, 2.62; N, 2.60; found: C, 49.32; H, 2.51; N, 2.84.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-(3-fluorobenzoyloxy)ethylsulfonamide (V-12): white solid, 32.9% yield: m.p. 97–99 °C; IR (KBr) νmax 3290, 3099, 2953, 1741, 752, 684 cm−1; 1H-NMR (400 MHz), δ (ppm) 3.51–3.97 (m, 2H, CH2), 6.45–6.47 (q, 1H, CH), 6.77–7.81 (m, 11H, C6H3 + C6H3 + NH + C6H4); Elemental anal. calc. for C22H14ClF6NO4S (%): C, 49.13; H, 2.62; N, 2.60; found: C, 48.97; H, 2.54; N, 2.77.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-(2-chlorobenzoyloxy)ethylsulfonamide (V-13): white solid, 49.6% yield: m.p. 99–101 °C; IR (KBr) νmax 3327, 3080, 2931, 1741, 754, 692 cm−1; 1H-NMR (400 MHz), δ (ppm) 3.52–3.87 (m, 2H, CH2), 6.45–6.48 (q, 1H, CH), 6.87–7.90 (m, 11H, C6H3 + C6H3 + NH + C6H4); Elemental anal. calc. for C22H14Cl2F5NO4S (%): C, 47.67; H, 2.55; N, 2.53; found: C, 47.43; H, 2.38; N, 2.69.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-(3-chlorobenzoyloxy)ethylsulfonamide (V-14): white solid, 69.5% yield: m.p. 114–116 °C; IR (KBr) νmax 3273, 3048, 2935, 1747, 1338, 742 cm−1; 1H-NMR (400 MHz), δ (ppm) 3.52–3.88 (m, 2H, CH2), 6.45–6.48 (q, 1H, CH), 6.78–7.95 (m, 11H, C6H3 + C6H3 + NH + C6H4); Elemental anal. calc. for C22H14Cl2F5NO4S (%): C, 47.67; H, 2.55; N, 2.53; found: C, 47.45; H, 2.78; N, 2.39.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-(2-trifluoromethylbenzoyloxy) ethylsulfonamide (V-15): white solid, 79.8% yield: m.p. 81–83 °C; IR (KBr) νmax 3308, 3095, 2948, 1747, 1319, 779 cm−1; 1H-NMR (400 MHz), δ (ppm) 3.51–3.84 (m, 2H, CH2), 6.45–6.48 (q, 1H, CH), 6.75–7.90 (m, 11H, C6H3 + C6H3 + NH + C6H4); Elemental anal. calc. for C23H14ClF8NO4S (%): C, 46.99; H, 2.40; N, 2.38; found: C, 46.78; H, 2.64; N, 2.14.
N-(2-Trifluoromethyl-4-chlorophenyl)-2-(3,5-difluorophenyl)-2-(3-trifluoromethylbenzoyloxy) ethylsulfonamide (V-16): white solid, 43.9% yield: m.p. 119–121 °C; IR (KBr) νmax 3273, 3054, 2931, 1747, 1338, 694 cm−1; 1H-NMR (400 MHz), δ (ppm) 3.52–4.15 (m, 2H, CH2), 6.49–6.51 (q, 1H, CH), 6.79–8.27 (m, 11H, C6H3 + C6H3 + NH + C6H4); Elemental anal. calc. for C23H14ClF8NO4S (%): C, 46.99; H, 2.40; N, 2.38; found: C, 47.11; H, 2.26; N, 2.51.
4.3. Fungicidal Activity Bioassays
The in vitro and in vivo fungicidal activities of all the title compounds against B. cinerea were tested by a mycelium growth inhibition assay and greenhouse pot experiments, respectively. The Botrytis cinerea strains (DL-11, HLD-15) were isolated from damaged parts of tomato in a greenhouse in different areas of Liaoning, China, and cultured on potato dextrose agar (PDA) for many generations. The commercial fungicides procymidone (with a purity of 96%), pyrimethanil (with a purity of 95%) and chlorothalonil (with a purity of 96%) provided by the Shenyang Research Institute of the Chemical Industry, National Pesticides Engineering Research Centre, were used as positive controls. Excel 2007 (Microsoft, Redmond, WA, USA) was used to analyze the bioassay data. Analysis of difference significances was performed using SPSS v.18.0 (SPSS Inc., Chicago, IL, USA).
4.3.1. Evaluation of Compounds III, IV, V on the Mycelia Growth of Botrytis cinerea in Solid Media
The in vitro fungicidal activity of compounds III, IV and V was assessed using a radial growth test on potato dextrose agar (PDA). The compounds were dissolved in acetone and mixed with sterile molten PDA to obtain five concentrations of 100, 25, 6.25, 1.56 and 0.39 mg L−1. PDA with different concentrations of the test compounds was poured into 90 mm Petri dishes (15 mL per dish), which were then inoculated with 5 mm plugs of B. cinerea. The plugs were obtained from a PDA culture plate by punching at the edge of the mycelia colony. Three replicates were used per treatment. The commercial fungicides were used as positive controls. After an incubation period of 72 h at 23 °C under a regular 12:12 h light:dark regimen, mycelia growth diameters were measured and the inhibition percentages relative to the control with 1% acetone were calculated. The inhibition rate was determined following a standard method [35]. The EC50 values were calculated using log-probit analysis.
4.3.2. In Vivo Fungicidal Activity against Botrytis cinerea by Greenhouse Pot Experiments
The in vivo fungicidal activity of the title compounds against B. cinerea was evaluated in the greenhouse. B. cinerea was maintained on PDA medium at 4 °C. The culture plates were cultivated at 24 ± 1 °C. Germination was conducted by soaking cucumber seeds in water for 2 h at 50 °C and then keeping the seeds moist for 24 h at 28 °C in an incubator. When the radicles were 0.5 cm, the seeds were grown in plastic pots containing a 1:1 (v/v) mixture of vermiculite and peat. Cucumber plants used for inoculations were at the stage of two seed leaves. The tested compounds were confected to 2.5% EC formulations, which were diluted to concentration of 500 μg/mL with water to obtain the solutions. Tested compounds and commercial fungicides were sprayed with a hand spray on the surface of the seed leaves. Water sprayed seed leaves were set as the CK. After drying, the upper sides of the leaves were inoculated with 5 mm plugs of B. cinerea, which was maintained on PDA. The plants were maintained at 24 ± 1 °C and above 80% relative humidity in the greenhouse. The fungicidal activity was then evaluated.
4.3.3. In vitro Fungicidal Activity of Compounds III, IV, V against Different Phytopathogenic Fungi
The fungicidal activity of compounds III, IV and V against phytopathogentic fungi (Pyricularia grisea, Exserohilum turcicum (Pass.) Leonard et Suggs, Pythium aphanidermatum, Phytophthora capsici Leonian, Fusarium graminearum Schw., Corynespora cassiicola and Thanatephorus cucumeris) was assessed using the mycelium growth test on PDA. The compounds were dissolved in acetone and mixed PDA to obtain final concentration of 50 mg L−1. Chlorothalonil was used as the positive control. Other test conditions were the same with the method given in the mycelial growth.
5. Conclusions
In summary, 38 2-substituted-phenyl-2-oxo-, 2-hydroxy- and 2-acyloxyethylsulfonamides were designed and synthesized. Their structures were characterized by 1H-NMR, IR and elemental analysis. In vitro and in vivo fungicidal activities against 2 different B. cinerea strains were tested. The results showed that some title compounds exhibited better fungicidal activities than that of the lead compound B-1. Among them, compound V-13 showed better in vivo fungicidal activity than the commercial fungicides procymidone and pyrimethanil. In addition, these new compounds had a broad spectrum of fungicidal activity. Bioassays showed that some target molecules might be used as new lead compounds for further development of novel fungicides against B. cinerea. Finally, as we all know, sulfonamides are more used as herbicides, while its application as fungicides is less common in agrochemicals. In our preliminary research, we found that Botrytis cinerea control using sulfonamides may involve a new mode of action mode [36,37], which makes our research more significant. Hence, the enhancement of the activity of these sulfonamide fungicides will be our focus in further research.
Supplementary Materials
Supplementary materials are available online. The 1H-NMR spectrogram of compounds III, IV and V.
Acknowledgments
The research was supported in part by the National Natural Science Foundation of China (31101466 and 31570122), the National Key Project for Basic Research (973 Project, 2015CB150600), the Key Laboratory of Pesticide Chemistry and Application, Ministry of Agriculture (MOAPCA201009), the Natural Science Foundation of Liaoning Province (2015020766), the Scientific Research Project of Department of Education of Liaoning Province (LSNYB201611), the Opening Foundation of the Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University (2015GDGP0101), the Pearl River S & T Nova Program of Guangzhou (201506010029), the Special Fund for the Research and Construction of Public Service Ability in Guangdong (2014A020210019). The authors declare no conflict of interest.
Author Contributions
Xinghai Li, Zining Cui, Minlong Wang and Peng Rui conceived and designed the experiments; Minlong Wang and Peng Rui performed the experiments; Minlong Wang, Ying Du and Caixiu Liu analyzed the data; Peiwen Qin, Zhiqiu Qi, Ying Du and Mingshan Ji contributed reagents, materials and strains; Minlong Wang, Xinghai Li and Zining Cui collaborated in the discussion and interpretation of results; Minlong Wang wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alsughayer, A.; Elassar, A.-Z.A.; Mustafa, S.; Sagheer, F.A. Synthesis, structure analysis and antibacterial activity of new potent sulfonamide derivatives. J. Biomater. Nanobiotechnol. 2011, 2, 144–149. [Google Scholar]
- Wang, X.L.; Wang, X.L.; Geng, R.X.; Zhou, C.H. Advance in research of antimicrobial drugs with sulfamide group. Chin. J. New Drug 2010, 19, 2050–2059. [Google Scholar]
- Wilkinson, B.L.; Bornaghi, L.F.; Wright, A.D.; Houston, T.A.; Poulsen, S.-A. Anti-mycobacterial activity of a bis-sulfonamide. Bioorg. Med. Chem. Lett. 2007, 17, 1355–1357. [Google Scholar] [CrossRef] [PubMed]
- Hen, N.; Bialer, M.; Wlodarczyk, B.; Finnell, R.H.; Yagen, B. Syntheses and evaluation of anticonvulsant profile and teratogenicity of novel amide derivatives of branched aliphatic carboxylic acids with 4-aminobenzensulfonamide. J. Med. Chem. 2010, 53, 4177–4186. [Google Scholar] [PubMed]
- Namba, K.; Zheng, X.X.; Motoshima, K.; Kobayashi, H.; Tai, A.; Takahashi, E. Design and synthesis of benzenesulfonanilides active against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Bioorg. Med. Chem. 2008, 16, 6131–6144. [Google Scholar] [PubMed]
- Zhao, F.; Wang, J.; Ding, X.; Shu, S.J.; Liu, H. Application of sulfonyl in drug design. Chin. J. Org. Chem. 2016, 36, 490–501. [Google Scholar] [CrossRef]
- Vandyck, K.; Cummings, M.D.; Nyanguile, O.; Boutton, C.W.; Vendeville, S.; McGowan, D.; Devogelaere, B.; Amssoms, K.; Last, S.; Rombauts, K.; et al. Structure-based design of a benzodiazepine scaffold yields a potent allosteric inhibitor of hepatitis C NS5B RNA polymerase. J. Med. Chem. 2009, 52, 4099–4102. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, P.; Demopoulos, V.J. A diverse series of substituted benzenesulfonamides as aldose reductase inhibitors with antioxidant activity: Design, synthesis, and in vitro activity. J. Med. Chem. 2010, 53, 7756–7766. [Google Scholar] [CrossRef] [PubMed]
- Ala, P.J.; Gonneville, L.; Hillman, M.; Becker-Pasha, M.; Yue, E.W.; Douty, B.; Wayland, B.; Polam, P.; Crawley, M.L.; McLaughlin, E.; et al. Structural insights into the design of nonpeptidic isothiazolidinone-containing inhibitors of protein-tyrosine phosphatase 1B. J. Biol. Chem. 2006, 281, 38013–38021. [Google Scholar] [CrossRef] [PubMed]
- Terrett, N.K.; Bell, A.S.; Brown, D.; Ellis, P. Sildenafil (Viagra TM), a potent and selective inhibitor of type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction. Bioorg. Med. Chem. Lett. 1996, 6, 1819–1824. [Google Scholar]
- Roda, A.; Cerrè, C.; Manetta, A.C.; Cainelli, G.; Umani-Ronchi, A.; Panunzio, M. Synthesis and physicochemical, biological, and pharmacological properties of new bile acids amidated with cyclic amino acids. J. Med. Chem. 1996, 39, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- Breslin, H.J.; Lane, B.M.; Ott, G.R.; Ghose, A.K.; Angeles, T.S.; Albom, M.S.; Cheng, M.; Wan, W.; Haltiwanger, R.C.; Wells-Knecht, K.J.; et al. Design, synthesis, and Anaplastic Lymphoma Kinase (ALK) inhibitory activity for a Novel Series of 2,4,8,22-tetraazatetracyclo [14.3.1.13,7.19,13]docosa-1(20),3(22),4,6,9(21),10,12,16,18-nonaene macrocycles. J. Med. Chem. 2012, 55, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Kamisuki, S.; Shirakawa, T.; Kugimiya, A.; Abu-Elheiga, L.; Choo, H.-Y.P.; Yamada, K.; Shimogawa, H.; Wakil, S.J.; Uesugi, M. Synthesis and evaluation of diarylthiazole derivatives that inhibit activation of sterol regulatory element-binding proteins. J. Med. Chem. 2011, 54, 4923–4927. [Google Scholar] [CrossRef] [PubMed]
- Pinard, E.; Alberati, D.; Borroni, E.; Fischer, H.; Hainzl, D.; Jolidon, S.; Moreau, J.L.; Narquizian, R.; Nettekoven, M.; Norcross, R.D. Discovery of benzoylpiperazines as a novel class of potent and selective GlyT1 inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 5134–5139. [Google Scholar] [CrossRef] [PubMed]
- Sturino, C.F.; O’Neill, G.; Lachance, N.; Boyd, M.; Berthelette, C.; Labelle, M.; Li, L.; Roy, B.; Scheigetz, J.; Tsou, N.; et al. Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]-acetic acid (MK-0524). J. Med. Chem. 2007, 50, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, P.K.; Naylor, E.M.; Chen, A.; Chang, R.S.L.; Chen, T.-B.; Faust, K.A.; Lotti, V.J.; Kivlighn, S.D.; Gable, R.A. A highly potent, orally active imidazo[4,5-b]pyridine biphenyl acylsulfonamide (MK-996; L-159,282): A new AT1-selective angiotensin II receptor antagonist. J. Med. Chem. 1994, 37, 4068–4072. [Google Scholar] [CrossRef] [PubMed]
- Sköld, O. Sulfonamide resistance: Mechanisms and trends. Drug Resist. Update 2000, 3, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Page, D.; Wei, Z.Y.; Liu, Z.P.; Tremblay, M.; Desfosses, H.; Milburn, C.; Srivastava, S.; Yang, H.; Brown, W.; Walpole, C.; et al. 5-Sulfonamide benzimidazoles: A class of cannabinoid receptors agonists with potent in vivo antinociception activity. Lett. Drug Des. Discov. 2010, 7, 208–213. [Google Scholar] [CrossRef]
- Keche, A.P.; Hatnapure, G.D.; Tale, R.H.; Rodge, A.H.; Birajdar, S.S.; Kamble, V.M. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: Synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 3445–3448. [Google Scholar] [CrossRef] [PubMed]
- Mitani, S.; Araki, S.; Yamaguchi, T.; Takii, Y.; Ohshima, T.; Matsuo, N. Antifungal activity of the novel fungicide cyazofamid against Phytophthora infestans and other plant pathogenic fungi in vitro. Pest. Biochem. Physiol. 2001, 70, 92–99. [Google Scholar] [CrossRef]
- Tomlin, C.D.S. The Pesticide Manual: A World Compendium, 15th ed.; British Crop. Production Council: Alton, UK, 2009. [Google Scholar]
- Li, X.H.; Pan, Q.; Cui, Z.N.; Ji, M.S.; Qi, Z.Q. Synthesis and fungicidal activity of N-(2,4,5-trichlorophenyl)-2-oxo- and 2-hydroxycycloalkylsulfonamides. Lett. Drug Des. Discov. 2013, 10, 353–359. [Google Scholar] [CrossRef]
- Wang, M.Y.; Li, Z.M. Synthesis and bioactivities of novel 2-methoxycarbonyl-5-arylazomethine benzenesulfonamide. Chin. J. Synth. Chem. 2012, 20, 65–68. [Google Scholar]
- Chen, Z.; Xu, W.M.; Liu, K.M.; Yang, S.; Fan, H.T.; Bhadury, P.S.; Huang, D.Y.; Zhang, Y. Synthesis and antiviral activity of 5-(4-chlorophenyl)-1,3,4-thiadiazole sulfonamides. Molecules 2010, 15, 9046–9056. [Google Scholar] [CrossRef] [PubMed]
- Basanagouda, M.; Shivashankar, K.; Kulkarni, M.V.; Rasal, V.P.; Patel, H.; Mutha, S.S.; Mohite, A.A. Synthesis and antimicrobial studies on novel sulfonamides containing 4-azidomethyl coumarin. Eur. J. Med. Chem. 2010, 45, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.M.; Chen, R.; Xing, R.G.; Chen, X.; Liu, S.; Guo, Z.Y.; Ji, X.; Wang, L.; Li, P. Synthesis and antifungal properties of sulfanilamide derivatives of chitosan. Carbohydr. Res. 2007, 342, 2390–2395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Yang, X.L.; Liang, X.M.; Kai, Z.P.; Yuan, H.Z.; Yuan, D.K.; Zhang, J.J.; Wang, R.Q.; Ran, F.X.; Qi, S.H.; et al. Synthesis and biological activities of 2-oxocycloalkylsulfonamides. Bioorg. Med. Chem. 2008, 16, 4538–4544. [Google Scholar]
- Liang, X.M.; Wang, D.Q.; Yang, H.Y.; Wu, J.P.; Zhang, J.J.; Yan, X.J. Preparation Method of 1-Oxotetralyl-2-sulfonamide and Its Usage as a Fungicide. Patent CN 101,503,381, 12 August 2009. [Google Scholar]
- Li, X.H.; Yang, X.L.; Ling, Y.; Fan, Z.J.; Liang, X.M.; Wang, D.Q.; Chen, F.H.; Li, Z.M. Synthesis and fungicidal activity of novel 2-oxocycloalkylsulfonylureas. J. Agric. Food Chem. 2005, 53, 2202–2206. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Wu, D.C.; Qi, Z.Q.; Li, X.W.; Gu, Z.M.; Wei, S.S.; Zhang, Y.; Wang, Y.Z.; Ji, M.S. Synthesis, fungicidal activity, and structure–activity relationship of 2-oxo-and 2-hydroxycycloalkylsulfonamides. J. Agric. Food Chem. 2010, 58, 11384–11389. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Ji, M.S.; Qi, Z.Q.; Li, X.W.; Shen, Y.X.; Gu, Z.M.; Zhang, Y.; Wei, S.S.; Wang, Y.Z.; Wang, D.Q. Synthesis of 2-amino-6-oxocyclohexenyl-sulfonamides and their activity against Botrytis cinerea. Pest Manag. Sci. 2011, 67, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Cui, Z.N.; Chen, X.Y.; Wu, D.C.; Qi, Z.Q.; Ji, M.S. Synthesis of 2-acyloxycyclohexylsulfonamides and evaluation on their fungicidal activity. Int. J. Mol. Sci. 2013, 14, 22544–22557. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Qi, Z.Q.; Zhong, C.J.; Zhang, Y.; Ji, M.S.; Wang, Y.Z.; Wang, D.Q. The synthesis and fungicidal activity of benzoyl methanesulfonamides. Chin. J. Pestic. Sci. 2008, 10, 136–140. [Google Scholar]
- Li, X.H.; Rui, P.; Pan, Q.; Chen, X.Y.; Qi, Z.Q.; Ji, M.S. Combined synthesis and fungicidal activity evolution of the 2-oxo-2-phenyl ethyl sulfonamide derivatives. Chin. J. Pestic. Sci. 2016, 18, 28–36. [Google Scholar]
- Hou, Z.; Yang, R.; Zhang, C.; Zhu, L.F.; Miao, F.; Yang, X.J.; Zhou, L. 2-(Substituted phenyl)-3,4-dihydroisoquinolin-2-iums as novel antifungal lead compounds: biological evaluation and structure-activity relationships. Molecules 2013, 18, 10413–10424. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.S.; He, R.H.; Li, X.H.; Xiao, S.Q.; Niu, M.Y.; Feng, H. Inhibition effect of cycloakylsulfonamide on Phytophthora capsici. Plant Prot. 2016, 42, 214–218. [Google Scholar]
- Qi, Z.Q.; Sun, Q.B.; Li, X.H.; Gu, Z.M.; Li, X.W.; Ji, M.S. Inhibitory effect of N-(2,4,5-trichlorophenyl)-2-oxocyclohexylsulfonamide against Botrytis cinerea. Chin. J. Pestic. Sci. 2014, 16, 523–528. [Google Scholar]
Sample Availability: Samples of the compounds III, IV and V are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).