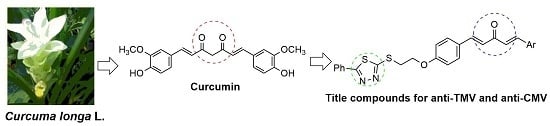
Synthesis and Antiviral Activity of Novel 1,4-Pentadien-3-one Derivatives Containing a 1,3,4-Thiadiazole Moiety
Abstract
:1. Introduction
2. Results and Discussion
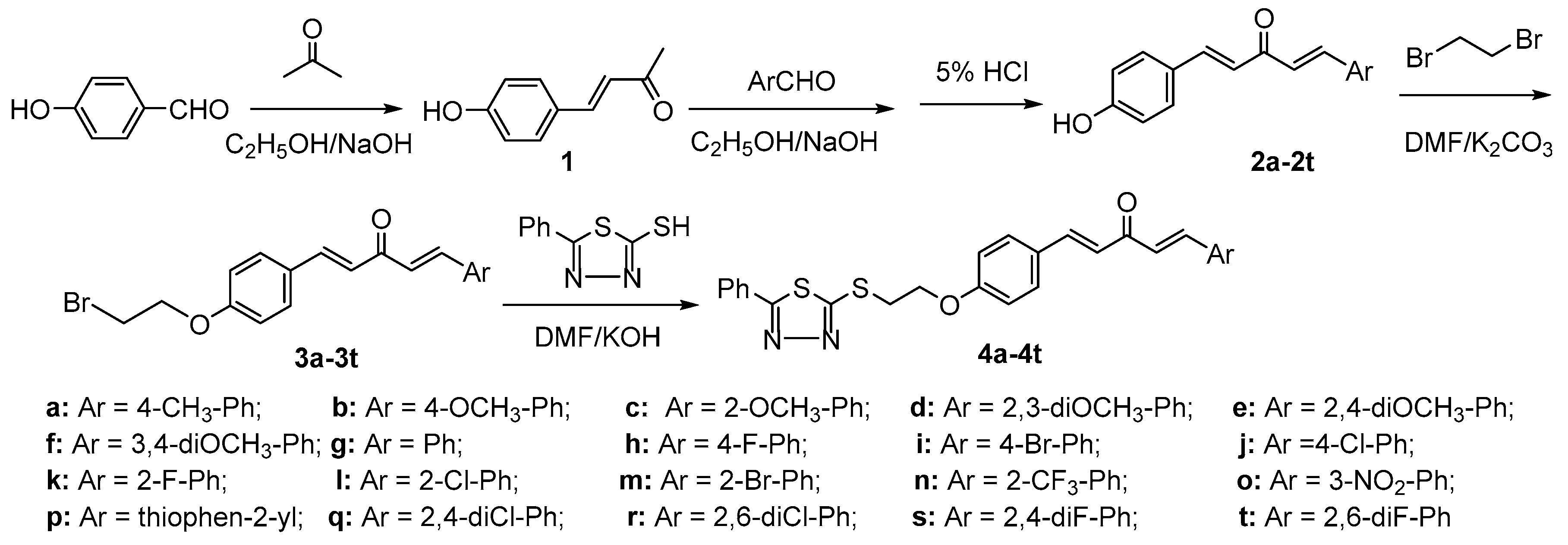
2.1. Chemistry
2.2. Antiviral Activity of Title Compounds against TMV In Vivo
2.3. Antiviral Activity of Title Compounds against CMV In Vivo
2.4. Antiviral Activity and Structure-Activity Relationships
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. General Procedure for Preparation of Intermediates 3a–3t
3.2.2. General Procedure for Preparation of Title Compounds 4a–4t
3.3. Antiviral Biological Assay
3.3.1. Purification of TMV and CMV
3.3.2. Curative Activities of Compounds against TMV and CMV In Vivo
3.3.3. Protective Activities of Compounds against TMV and CMV In Vivo
3.3.4. Inactivation Activities of Compounds aga inst TMV and CMV In Vivo
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bos, L. 100 years of virology: From vitalism via molecular biology to genetic engineering. Trends Microbiol. 2000, 8, 82–87. [Google Scholar] [CrossRef]
- Jacquemond, M. Cucumber mosaic virus. Adv. Virus Res. 2012, 84, 439–504. [Google Scholar] [PubMed]
- Wang, Z.W.; Wang, L.; Ma, S.; Liu, Y.X.; Wang, L.Z.; Wang, Q.M. Design, synthesis, antiviral activity, and SARs of 1,4-Aminophenanthroindolizidines. J. Agric. Food Chem. 2012, 60, 5825–5831. [Google Scholar] [CrossRef] [PubMed]
- Sidwell, R.W.; Huffman, J.H.; Khare, G.P.; Allen, L.B.; Witkowski, J.T.; Robins, R.K. Broad-spectrum antiviral activity of virazole: 1-β-d-Ribofuranosyl-1,2,4-triazole-3- carboxamide. Science 1972, 177, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Zhang, Z.K.; Jia, Y.T.; Shen, Y.M.; He, H.P.; Fang, R.X.; Chen, X.Y.; Hao, X.J. 3-Acetonyl-3-hydroxyoxindole: A new inducer of systemic acquired resistance in plants. Plant Biotechnol. J. 2008, 6, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Wei, P.; Wang, L.Z.; Wang, Q.M. Design, synthesis, and anti-tobacco mosaic virus (TMV) activity of phenanthroindolizidines and their analogues. J. Agric. Food Chem. 2012, 60, 10212–10219. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.H.; Liu, K.X.; Zhang, J.X.; Mu, S.Z.; Hao, X.J. The limonoids and their antitobacco mosaic virus (TMV) activities from Munronia unifoliolata Oliv. J. Agric. Food Chem. 2012, 60, 4289–4295. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, X.H.; Dong, J.H.; Sang, P.; Fang, X.; Di, Y.T.; Zhang, Z.K.; Hao, X.J. Tobacco mosaic virus (TMV) inhibitors from Picrasma quassioides Benn. J. Agric. Food Chem. 2009, 57, 6590–6595. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Zhao, W.; Xia, Z.Y.; Kong, G.H.; Lu, X.P.; Hu, Q.F.; Gao, X.M. Three novel xanthones from Garcinia paucinervis and their anti-TMV activity. Molecules 2013, 18, 9663–9669. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Yao, Y.C.; Huang, R.Q.; Fan, Z.J.; Li, G.R.; Yu, X.S. Antiviral activity of antofine from Cynanchum komarovii. Agrochemicals 2007, 46, 425–427. [Google Scholar]
- Wang, Z.W.; Wei, P.; Xu, X.Z.; Liu, Y.X.; Wang, L.Z.; Wang, Q.M. Design, synthesis, and antiviral activity evaluation of phenanthrene-based antofine derivatives. J. Agric. Food Chem. 2012, 60, 8544–8551. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Narain, U.; Mishra, R.; Misra, K. Design, development and synthesis of mixed bioconjugates of piperic acid-glycine, curcumin-glycine/alanine and curcumin-glycine-piperic acid and their antibacterial and antifungal properties. Bioorg. Med. Chem. 2005, 13, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Das, L.; Kapoor, N.; Das, A.; Dwivedi, V.; Poddar, A.; Chakraborti, G.; Janik, M.; Basu, G.; Panda, D.; et al. Curcumin recognizes a unique binding site of tubulin. J. Med. Chem. 2011, 54, 6183–6196. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, S.; Ramachandra, M.S.; Subbaraju, G.V. Synthesis and biological evaluation of polyhydroxycurcuminoids. Bioorg. Med. Chem. 2005, 13, 6374–6380. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.R., Jr.; Fesen, M.; Mazumder, A.; Yung, J.; Wang, J.; Carothers, A.M.; Grunberger, D.; Driscoll, J.; Pommier, Y.; Kohn, K. Hydroxylated aromatic inhibitors of HIV-1 integrase. J. Med. Chem. 1995, 38, 4171–4178. [Google Scholar] [CrossRef]
- Mazumder, A.; Wang, S.M.; Neamati, N.; Nicklaus, M.; Sunder, S.; Chen, J.L.; Milne, G.W.A.; Rice, W.G.; Burke, T.R., Jr.; Pommier, Y. Antiretroviral agents as inhibitors of both human immunodeficiency virus type 1 integrase and protease. J. Med. Chem. 1996, 39, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, J.J.; Jin, L.H.; Hu, D.Y.; Chen, Z.; Yang, S.; Wu, J.; Song, B.A. Synthesis and antiviral bioactivity of novel (1E,4E)-1-aryl-5-(2-(quinazolin-4-yloxy)phenyl)-1,4-pentadien-3-one derivatives. Eur. J. Med. Chem. 2013, 63, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, P.; Li, X.Y.; Shi, Q.C.; Wan, Z.H.; Hu, D.Y.; Jin, L.H.; Song, B.A. Synthesis and antiviral bioactivity of novel 3-((2-((1E,4E)-3-oxo-5-arylpenta-1,4-dien-1-yl)phenoxy)methyl)-4(3H)-quinazolinone Derivatives. J. Agric. Food Chem. 2014, 62, 8928–8934. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ding, Y.; Xie, D.D.; Hu, D.Y.; Li, P.; Li, X.Y.; Xue, W.; Jin, L.H.; Song, B.A. Design, synthesis and antiviral activity of novel rutin derivatives containing 1,4-pentadien-3-one moiety. Eur. J. Med. Chem. 2015, 92, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Long, C.W.; Li, P.; Chen, M.H.; Dong, L.R.; Hu, D.Y.; Song, B.A. Synthesis, anti-tobacco mosaic virus and cucumber mosaic virus activity, and 3D-QSAR study of novel 1,4-pentadien-3-one derivatives containing 4-thioquinazoline moiety. Eur. J. Med. Chem. 2015, 102, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Hu, D.Y.; Li, X.Y.; Yang, S.; Zhang, W.Y.; Li, P.; Song, B.A. Antiviral activity and interaction mechanisms study of novel glucopyranoside derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 3840–3844. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, P.; Hu, D.Y.; Song, B.A. Design, synthesis, and antiviral activity of novel purine derivatives containing 1,4-pentadien-3-one moiety. Res. Chem. Intermed. 2016, 42, 7153–7168. [Google Scholar] [CrossRef]
- Hu, Y.; Li, C.Y.; Wang, X.M.; Yang, Y.H.; Zhu, H.L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, T.A.; Abdallah, M.A.; Abdel Aziz, M.R. Synthesis and antimicrobial activity of aome new 1,3,4-thiadiazole derivatives. Molecules 2012, 17, 14625–14636. [Google Scholar] [CrossRef] [PubMed]
- Oruc, E.E.; Rollas, S.; Kandemirli, F.; Shvets, N.; Dimoglo, A.S. 1,3,4-thiadiazole derivatives. synthesis, structure elucidation, and structure-antituberculosis activity relationship investigation. J. Med. Chem. 2004, 47, 6760–6767. [Google Scholar] [CrossRef] [PubMed]
- Chapleo, C.B.; Myers, P.L.; Smith, A.C.; Tulloch, I.F.; Walter, D.S. Substituted 1,3,4-thiadiazolewith anticonvulsant activity. J. Med. Chem. 1987, 30, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shi, L.; Yang, X.; Yang, L.; Chen, X.W.; Wu, F.; Shi, Q.C.; Xu, W.M.; He, M.; Hu, D.Y.; et al. Design, synthesis, and antibacterial activity against rice bacterial leaf blightand leaf streak of 2,5-substituted-1,3,4-oxadiazole/thiadiazolesulfonederivative. Bioorg. Med. Chem. Lett. 2014, 24, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, C.L.; Sotelo-Hitschfeld, P.; Brauchi, S.; Olavarría, M.Z. Design and synthesis of conformationally restricted capsaicin analogues based in the 1,3,4-thiadiazole heterocycle reveal a novel family of transient receptor potential vanilloid 1 (TRPV1) antagonists. Eur. J. Med. Chem. 2013, 66, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Moise, M.; Sunel, V.; Profire, L.; Popa, M.; Desbrieres, J.; Peptu, C. Synthesis and biological activity of some new 1,3,4-thiadiazole and 1,2,4-triazole compounds containing a phenylalanine moiety. Molecules 2009, 14, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Kheder, N.A.; Abdelhamid, A.O.; Mabkhot, Y.N. One pot single step synthesis and biological evaluation of some novel bis(1,3,4-thiadiazole) derivatives as potential cytotoxic agents. Molecules 2016, 21, 1532. [Google Scholar] [CrossRef] [PubMed]
- Flefel, E.M.; El-Sayed, W.A.; Mohamed, A.M.; El-Sofany, W.I.; Awad, H.M. Synthesis and anticancer activity of new 1-thia-4-azaspiro[4.5]decane, their derived thiazolopyrimidine and 1,3,4-thiadiazole thioglycosides. Molecules 2017, 22, 170. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Can, Ö.D.; Özkay, Ü.D.; Kaplancıklı, U.A. Synthesis and evaluation of new 1,3,4-thiadiazole derivatives as antinociceptive agents. Molecules 2016, 21, 1004. [Google Scholar] [CrossRef] [PubMed]
- El-Sadek, M.M.; Hassan, S.Y.; Abdelwahab, H.E.; Yacout, G.A. Synthesis of new 1,3,4-thiadiazole and 1,2,3,4-oxathiadiazole derivatives from carbohydrate precursors and study of their effect on tyrosinase enzyme. Molecules 2012, 17, 8378–8396. [Google Scholar] [CrossRef] [PubMed]
- Clerici, F.; Pocar, D.; Maddalena, G.; Loche, A.; Perlini, V.; Brufani, M. Synthesis of 2-amino-5-sulfanyl-1,3,4-thiadiazole derivatives and evaluation of their antidepressant and anxiolytic activity. J. Med. Chem. 2001, 44, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, W.M.; Liu, K.M.; Yang, S.; Fan, H.T.; Bhadury, P.S.; Hu, D.Y.; Zhang, Y.P. Synthesis and antiviral activity of 5-(4-chlorophenyl)-1,3,4-thiadiazole sulfonamides. Molecules 2010, 15, 9046–9056. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.M.; Li, S.Z.; He, M.; Yang, S.; Li, X.Y.; Li, P. Synthesis and bioactivities of novel thioether/sulfone derivatives containing 1,2,3-thiadiazole and 1,3,4-oxadiazole/thiadiazole moiety. Bioorg. Med. Chem. Lett. 2013, 23, 5821–5824. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.H.; Hu, D.Y.; Li, P.; Wu, J.; Chen, X.W.; Xue, W.; Song, B.A. Design, synthesis, antiviral activity and three-dimensional quantitative structure-activity relationship study of novel 1,4-pentadien-3-one derivatives containing the 1,3,4-oxadiazole moiety. Pest Manag. Sci. 2016, 72, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.C.; Leal, J.; Stefanello, S.T.; Leite, M.T.B.; Souza, M.B.; Soares, F.A.A.; Rodrigues, O.E.D.; Dornelles, L. Synthesis and antioxidant properties of organosulfur and organoselenium compounds derived from 5-substituted-1,3,4-oxadiazole/thiadiazole-2-thiols. Tetrahedron Lett. 2017, 58, 87–91. [Google Scholar] [CrossRef]
- Song, B.A.; Zhang, H.P.; Wang, H.; Yang, S.; Jin, L.H.; Hu, D.Y.; Pang, L.L.; Xue, W. Synthesis and antiviral activity of novel chiral cyanoacrylate derivatives. J. Agric. Food. Chem. 2005, 53, 7886–7891. [Google Scholar] [CrossRef] [PubMed]
- Gooding, G.V., Jr.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1287. [Google Scholar]
Sample Availability: Samples of the compounds 4a–4t are available from the authors. |
| No. | Solvent | Base | Temperature (°C) | Yield (%) a |
|---|---|---|---|---|
| 1 | DMF | Et3N | 25 | 0 |
| 2 | DMF | K2CO3 | 25 | 21 |
| 3 | DMF | K2CO3 | 50 | 38 |
| 4 | acetone | KOH | 25 | 18 |
| 5 | THF | KOH | 25 | 26 |
| 6 | CH3CN | KOH | 25 | 38 |
| 7 | DMF | KOH | 25 | 56 |
| 8 | DMF | KOH | 40 | 87 |
| 9 | DMF | NaOH | 40 | 72 |
| Compound | Curative Activity (%) a | Protection Activity (%) a | Inactivation Activity (%) a | EC50 of Protection Activity (µg/mL) a |
|---|---|---|---|---|
| 4a | 55.8 ± 3.1 | 55.9 ± 3.5 | 84.1 ± 4.8 | 344.23 ± 2.35 |
| 4b | 56.4 ± 1.5 | 64.5 ± 1.1 | 84.1 ± 4.8 | 319.67 ± 1.89 |
| 4c | 36.8 ± 0.6 | 52.2 ± 1.4 | 91.8 ± 3.8 | 411.42 ± 2.68 |
| 4d | 21.2 ± 1.5 | 24.2 ± 2.1 | 64.3 ± 2.6 | 1058.25 ± 2.11 |
| 4e | 45.0 ± 1.6 | 30.4 ± 2.1 | 82.4 ± 1.8 | 1042.09 ± 1.28 |
| 4f | 40.6 ± 0.8 | 61.6 ± 3.3 | 90.1 ± 0.3 | 389.46 ± 2.32 |
| 4g | 56.3 ± 2.7 | 46.3 ± 1.4 | 78.5 ± 1.9 | 592.44 ± 1.89 |
| 4h | 56.2 ± 3.5 | 70.2 ± 1.3 | 93.8 ± 1.7 | 105.01 ± 3.15 |
| 4i | 53.7 ± 1.6 | 64.3 ± 2.7 | 85.3 ± 2.0 | 254.77 ± 1.66 |
| 4j | 44.6 ± 3.2 | 53.6 ± 3.7 | 85.2 ± 2.2 | 388.31 ± 2.05 |
| 4k | 56.5 ± 2.1 | 68.4 ± 1.8 | 87.1 ± 3.6 | 135.38 ± 3.12 |
| 4l | 45.9 ± 3.1 | 63.4 ± 4.4 | 83.0 ± 1.9 | 297.40 ± 4.10 |
| 4m | 40.5 ± 1.8 | 54.8 ± 2.5 | 81.5 ± 1.2 | 334.03 ± 1.08 |
| 4n | 47.9 ± 2.5 | 59.8 ± 4.3 | 89.3 ± 2.5 | 309.09 ± 2.56 |
| 4o | 51.7 ± 2.8 | 64.8 ± 3.1 | 77.6 ± 1.3 | 248.18 ± 4.14 |
| 4p | 24.2 ± 4.5 | 50.7 ± 1.5 | 88.6 ± 1.1 | 466.15 ± 1.98 |
| 4q | 58.7 ± 3.0 | 68.4 ± 1.6 | 84.7 ± 2.6 | 129.87 ± 3.55 |
| 4r | 42.3 ± 2.4 | 56.7 ± 2.8 | 87.4 ± 2.9 | 316.77 ± 2.54 |
| 4s | 28.2 ± 3.8 | 59.0 ± 1.7 | 82.7 ± 1.6 | 316.52 ± 4.29 |
| 4t | 54.5 ± 4.4 | 54.1 ± 1.2 | 82.7 ± 1.6 | 425.71 ± 3.17 |
| Ribavirin b | 37.9 ± 1.9 | 51.8 ± 2.3 | 72.9 ± 2.4 | 457.25± 2.68 |
| Compound | Curative Activity (%) a | Protection Activity (%) a | Inactivation Activity (%) a |
|---|---|---|---|
| 4a | 29.3 ± 1.3 | 40.1 ± 2.5 | 71.3 ± 3.1 |
| 4b | 23.6 ± 2.2 | 38.9 ± 2.9 | 65.2 ± 2.5 |
| 4c | 37.9 ± 3.7 | 43.4 ± 3.1 | 61.5 ± 2.2 |
| 4d | 18.5 ± 1.8 | 33.6 ± 2.1 | 51.2 ± 3.4 |
| 4e | 55.9 ± 1.8 | 42.3 ± 1.1 | 79.6 ± 4.2 |
| 4f | 50.2 ± 2.7 | 46.6 ± 2.3 | 62.5 ± 1.9 |
| 4g | 39.8 ± 2.5 | 48.5 ± 2.7 | 66.7 ± 2.2 |
| 4h | 39.8 ± 2.5 | 36.2 ± 2.2 | 62.1 ±1.9 |
| 4i | 37.3 ± 2.5 | 35.6 ± 2.1 | 51.8 ± 1.1 |
| 4j | 40.6 ± 3.7 | 45.9 ± 1.6 | 79.1± 2.5 |
| 4k | 26.4 ± 2.3 | 35.6 ± 1.9 | 49.9± 3.6 |
| 4l | 29.7 ± 1.1 | 43.5 ± 2.5 | 66.5 ± 2.0 |
| 4m | 28.5 ± 2.3 | 42.2 ± 1.4 | 68.9 ± 2.8 |
| 4n | 18.9 ± 2.9 | 25.4 ± 1.7 | 46.2 ± 0.9 |
| 4o | 31.8 ± 2.8 | 49.9 ± 1.9 | 58.2 ± 3.2 |
| 4p | 29.5 ± 1.4 | 38.5 ± 2.1 | 59.8 ± 1.9 |
| 4q | 44.5 ± 1.8 | 48.1 ± 2.4 | 43.9 ± 1.2 |
| 4r | 26.4 ± 2.2 | 40.5 ± 1.9 | 42.5 ± 2.8 |
| 4s | 41.0 ± 1.7 | 54.7 ± 2.4 | 78.5 ± 1.9 |
| 4t | 34.8 ± 2.3 | 48.1 ± 1.6 | 69.5 ± 2.0 |
| Ribavirin b | 36.8 ± 1.6 | 47.9 ± 2.7 | 71.2 ± 1.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Gan, X.; Zhou, D.; He, F.; Zeng, S.; Hu, D. Synthesis and Antiviral Activity of Novel 1,4-Pentadien-3-one Derivatives Containing a 1,3,4-Thiadiazole Moiety. Molecules 2017, 22, 658. https://doi.org/10.3390/molecules22040658
Yu L, Gan X, Zhou D, He F, Zeng S, Hu D. Synthesis and Antiviral Activity of Novel 1,4-Pentadien-3-one Derivatives Containing a 1,3,4-Thiadiazole Moiety. Molecules. 2017; 22(4):658. https://doi.org/10.3390/molecules22040658
Chicago/Turabian StyleYu, Lu, Xiuhai Gan, Dagui Zhou, Fangcheng He, Song Zeng, and Deyu Hu. 2017. "Synthesis and Antiviral Activity of Novel 1,4-Pentadien-3-one Derivatives Containing a 1,3,4-Thiadiazole Moiety" Molecules 22, no. 4: 658. https://doi.org/10.3390/molecules22040658
APA StyleYu, L., Gan, X., Zhou, D., He, F., Zeng, S., & Hu, D. (2017). Synthesis and Antiviral Activity of Novel 1,4-Pentadien-3-one Derivatives Containing a 1,3,4-Thiadiazole Moiety. Molecules, 22(4), 658. https://doi.org/10.3390/molecules22040658