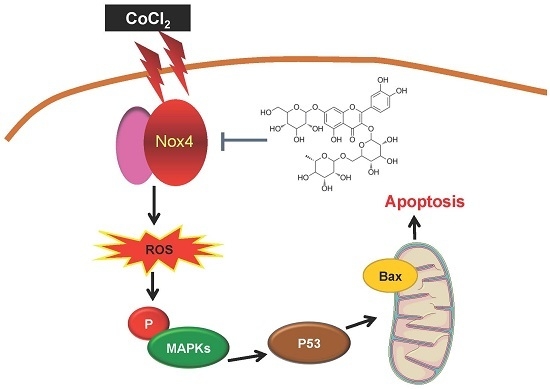
ZYZ-772 Prevents Cardiomyocyte Injury by Suppressing Nox4-Derived ROS Production and Apoptosis
Abstract
:1. Introduction
2. Results
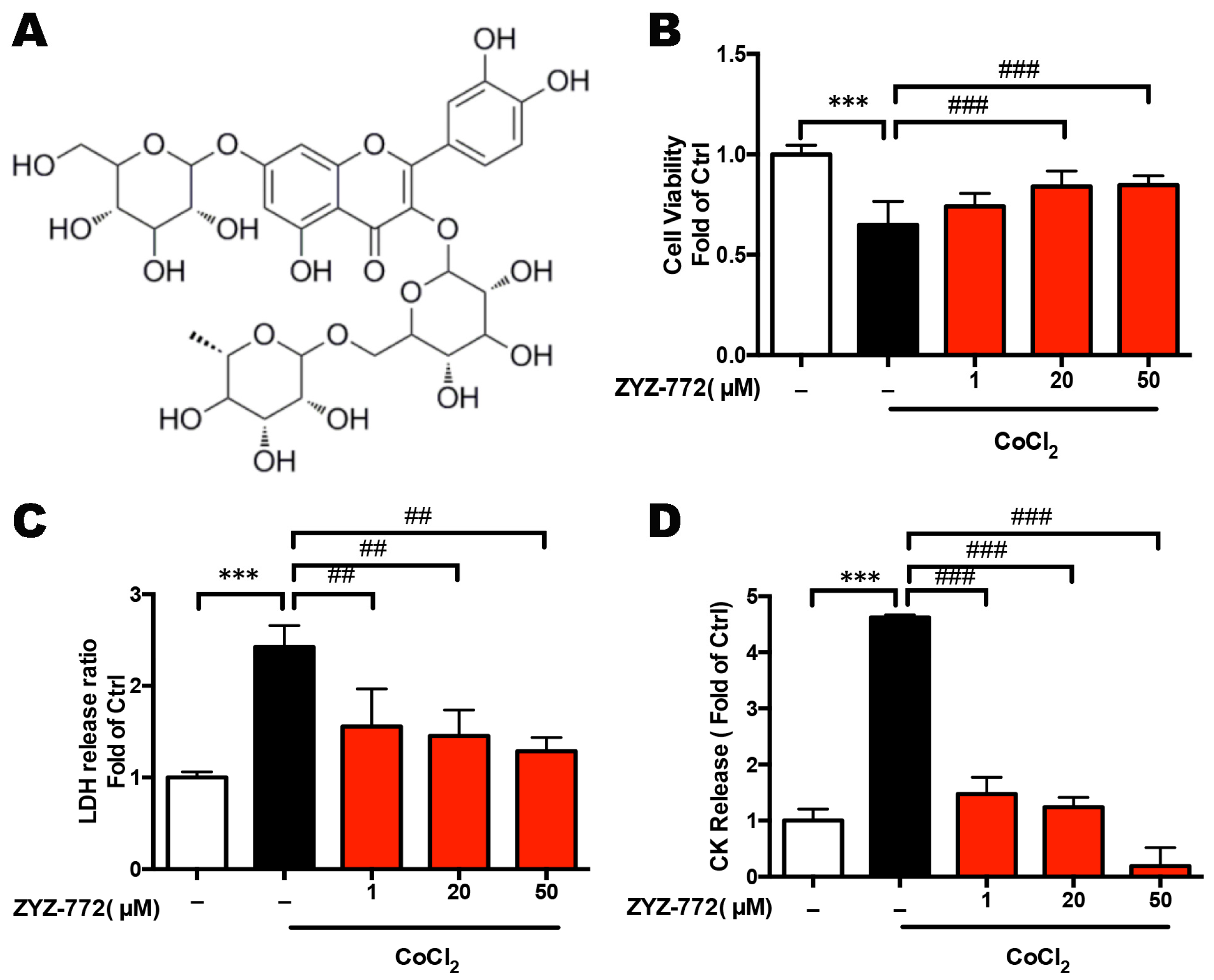
2.1. Quercetin-3-O-(6''-O-α-l-rhamnopyransoyl)-β-d-glucopyranoside-7-O-β-d-glucopyranoside (ZYZ-772) Protects against CoCl2 Induced Hypoxia Injury in H9c2 Rat Ventricular Cells
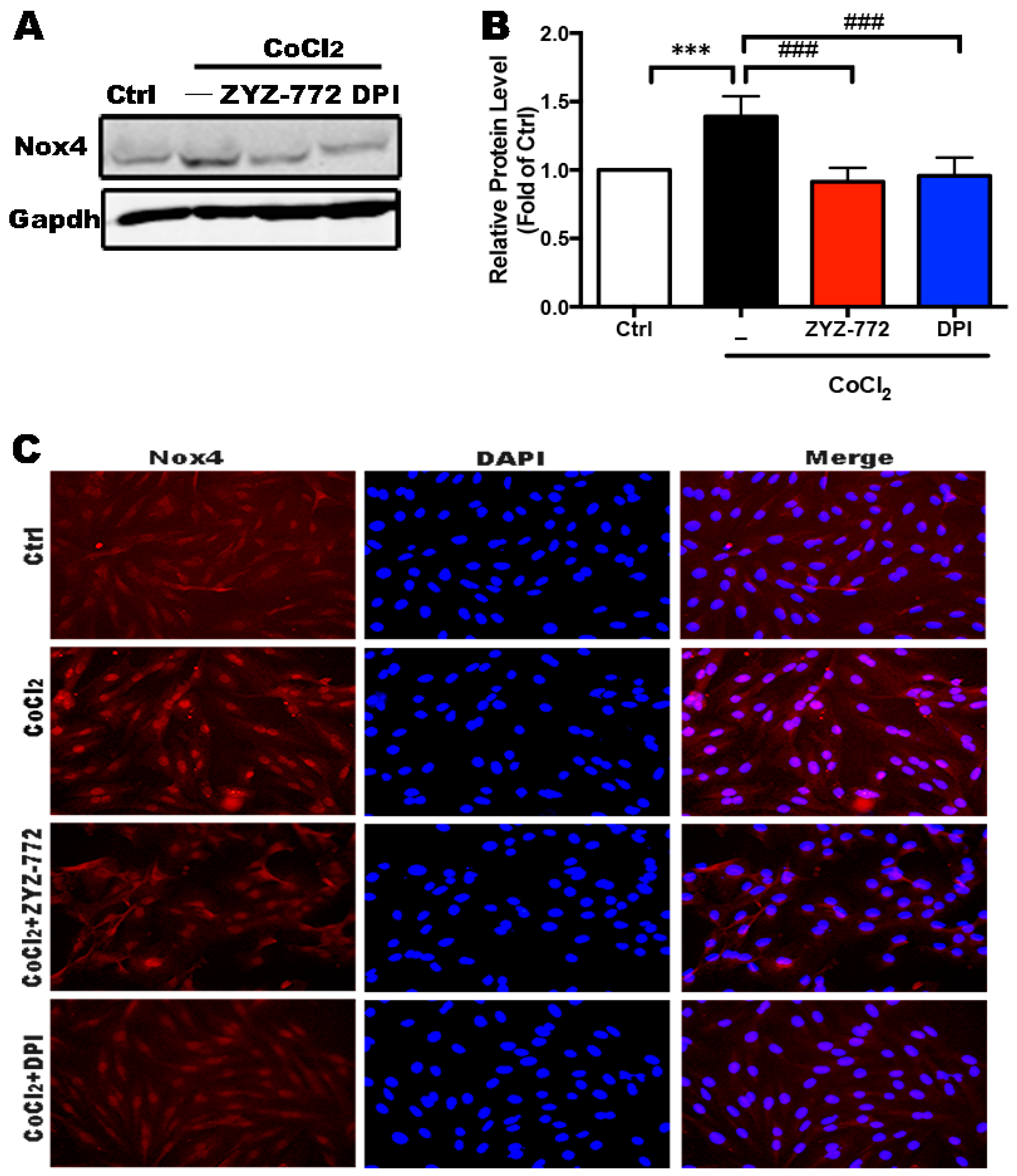
2.2. Activation of Nox4 Was Inhibited by ZYZ-772 in H9c2 Cells
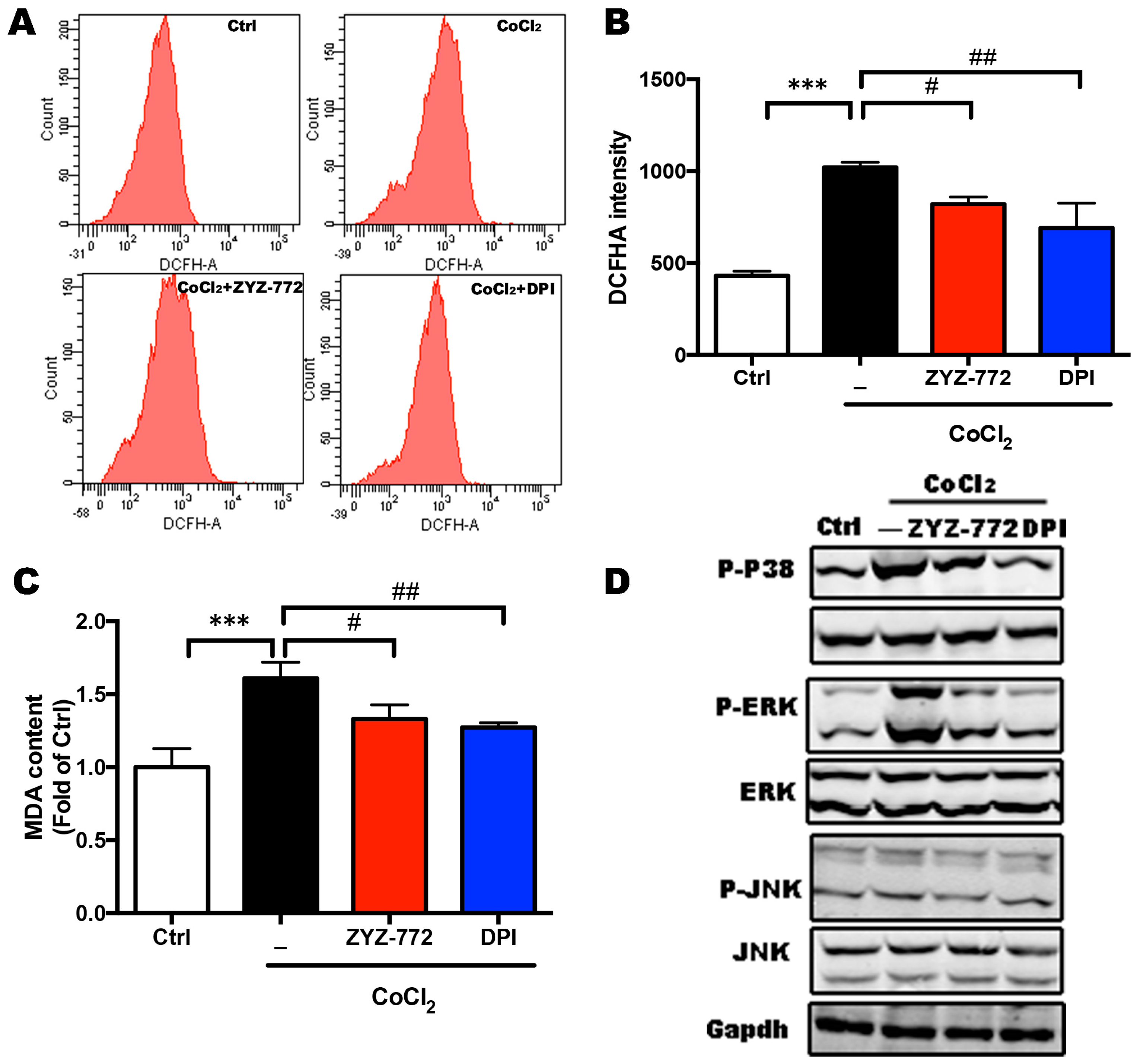
2.3. CoCl2-Induced ROS Generation and MAPKs Signaling Phosphorylation Were Alleviated by ZYZ-772
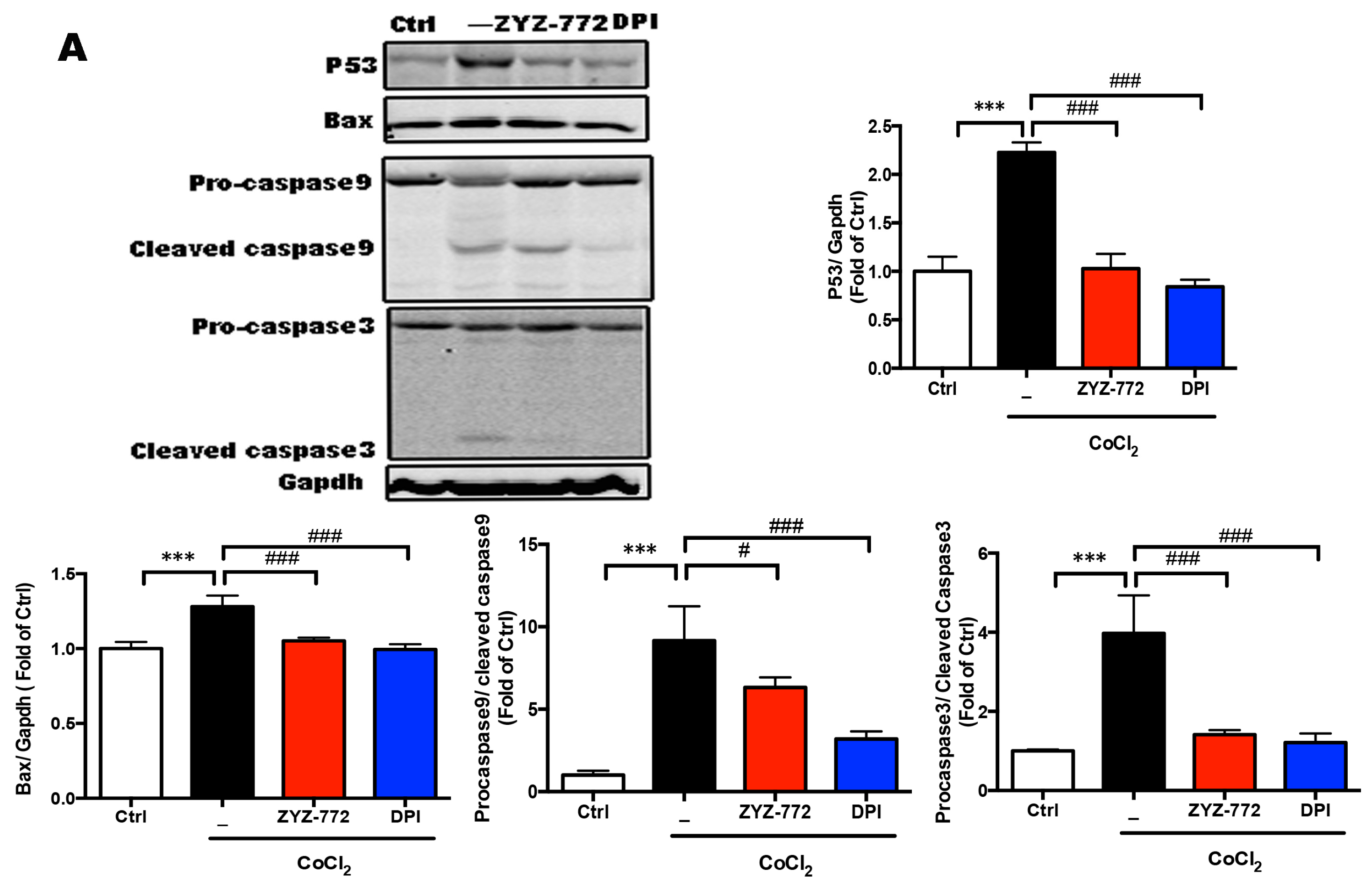
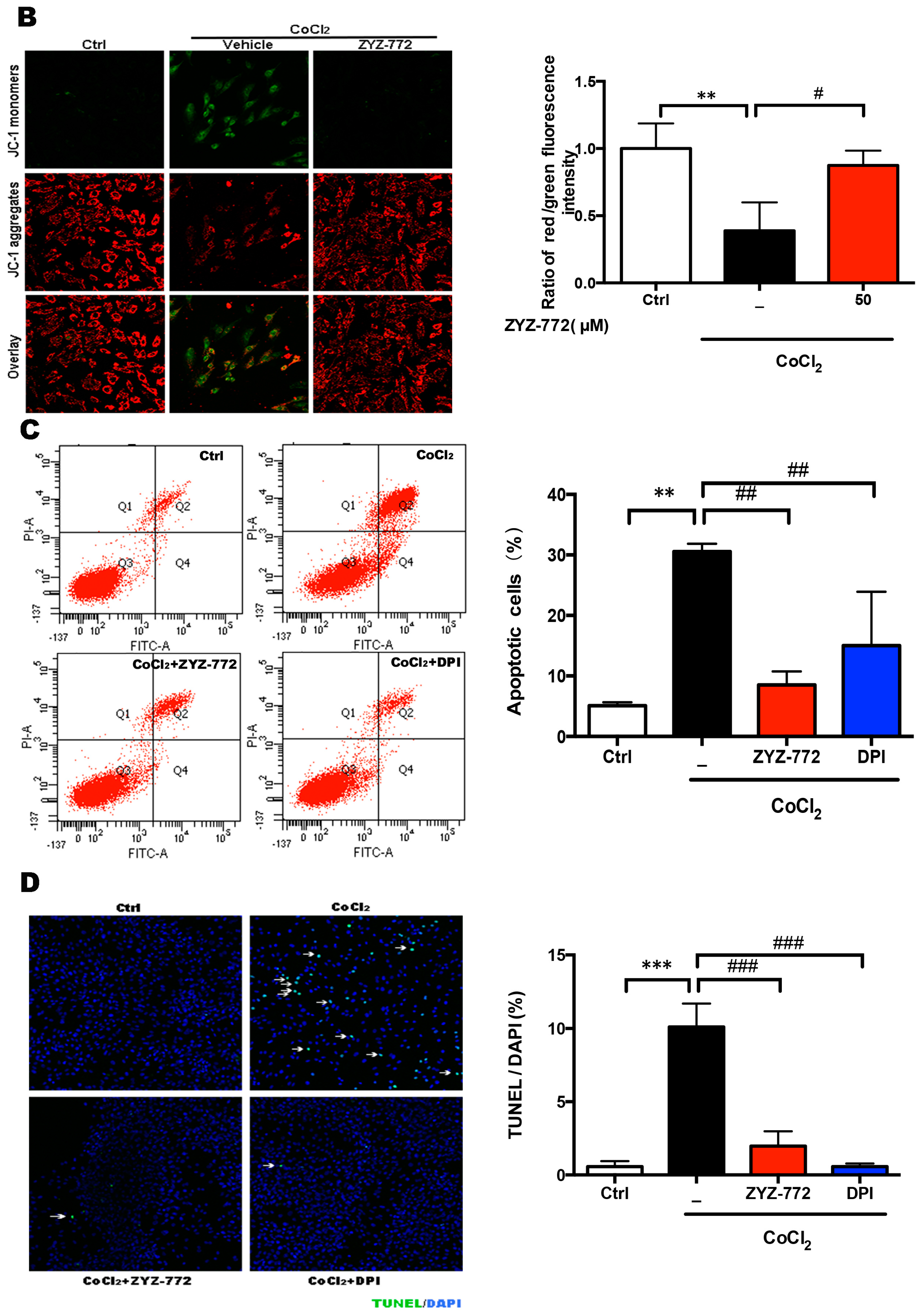
2.4. Inhibitory Effects of ZYZ-772 on CoCl2-Induced H9c2 Cells Apoptosis
3. Discussion
4. Materials and Methods
4.1. Extraction and Isolation of Quercetin-3-O-(6''-O-α-l-rhamnopyransoyl)-β-d-glucopyranoside-7-O-β-d-glucopyranoside (ZYZ-772)
4.2. Drugs and Reagents
4.3. Cell Culture and Treatments
4.4. Cell Survival Assay
4.5. LDH and CK Leakage Measurement
4.6. Western Blot Analysis
4.7. Assay of Mitochondrial Membrane Potential (ΔΨm)
4.8. TUNEL Staining
4.9. Assessment of Apoptosis by Flow Cytometry
4.10. ROS Assay
4.11. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cesselli, D.; Jakoniuk, I.; Barlucchi, L.; Beltrami, A.P.; Hintze, T.H.; Nadal-Ginard, B.; Kajstura, J.; Leri, A.; Anversa, P. Oxidative stress–Mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ. Res. 2001, 89, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.A.; Langille, B.L.; Srivastava, D. Apoptosis during cardiovascular development. Circ. Res. 2000, 87, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Biondi-Zoccai, G.G.; Baldi, A. Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J. Cell. Physiol. 2002, 193, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, K.A.; Bonda, T.A.; Korecki, J.; Musial, W.J. Oxidative stress and neutrophil activation—The two keystones of ischemia/reperfusion injury. Int. J. Cardiol. 2002, 86, 41–59. [Google Scholar] [CrossRef]
- Aikawa, R.; Komuro, I.; Yamazaki, T.; Zou, Y.; Kudoh, S.; Tanaka, M.; Shiojima, I.; Hiroi, Y.; Yazaki, Y. Oxidative stress activates extracellular signal-Regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J. Clin. Investig. 1997, 100, 1813. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Zou, Y.; Hasegawa, H.; Akazawa, H.; Nagai, T.; Komuro, I. Oxidative stress-induced signal transduction pathways in cardiac myocytes: Involvement of ROS in heart diseases. Antioxid. Redox Signal. 2003, 5, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Sadoshima, J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. PNAS 2010, 107, 15565–15570. [Google Scholar] [CrossRef] [PubMed]
- Ago, T.; Kuroda, J.; Pain, J.; Fu, C.; Li, H.; Sadoshima, J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circul. Res. 2010, 106, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Sadoshima, J. NADPH oxidase and cardiac failure. J. Cardiovasc. Transl. Res. 2010, 3, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Yang, L.; Zhou, D.; Zhang, J. Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity. PLoS ONE 2014, 9, e105725. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, J.; Liu, L.; Jin, X. GC-MS analysis of supercritical carbon dioxide extraction products from pericarp of Zanthoxylum bungeanum. J. Chin. Med. Mater. 2001, 24, 572–573. [Google Scholar]
- Tirillini, B.; Stoppini, A.M. Volatile constituents of the fruit secretory glands of Zanthoxylum bungeanum Maxim. J. Essent. Oil Res. 1994, 6, 249–252. [Google Scholar] [CrossRef]
- Wu, T.; Zhong, L.; Hong, Z.; Li, Y.; Liu, X.; Pan, L.; Xin, H.; Zhu, Y. The effects of Zanthoxylum bungeanum extract on lipid metabolism induced by sterols. J. Pharm. Sci. 2015, 127, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Qiao, Z.; Kuai, Q.; Wang, Y.; Wang, X.; He, M.; Li, W.; He, Y.; Ren, S. Valproic acid–Mediated myocardial protection of acute hemorrhagic rat via the BCL-2 pathway. J. Trauma Acute Care Surg. 2016, 80, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Perino, A.; Ghigo, A.; Hirsch, E.; Shah, A.M. NADPH oxidases in heart failure: Poachers or gamekeepers? Antioxid. Redox Signal. 2013, 18, 1024–1041. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Kuroda, J.; Ago, T.; Zhai, P.; Park, J.Y.; Xie, L.; Tian, B.; Sadoshima, J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circul. Res. 2013, 112, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Infanger, D.W.; Cao, X.; Butler, S.D.; Burmeister, M.A.; Zhou, Y.; Stupinski, J.A.; Sharma, R.V.; Davisson, R.L. Silencing Nox4 in the Paraventricular Nucleus Improves Myocardial Infarction–Induced Cardiac Dysfunction by Attenuating Sympathoexcitation and Periinfarct Apoptosis. Circul. Res. 2010, 106, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Galán, A.; García-Bermejo, L.; Troyano, A.; Vilaboa, N.E.; Fernández, C.; de Blas, E.; Aller, P. The role of intracellular oxidation in death induction (apoptosis and necrosis) in human promonocytic cells treated with stress inducers (cadmium, heat, X-rays). Eur. J. Cell Biol. 2001, 80, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, Y.; Zhu, Q.; Gu, X.; Zhu, Y.Z. The discovery of a novel inhibitor of apoptotic protease activating factor-1 (Apaf-1) for ischemic heart: Synthesis, activity and target identification. Sci. Rep. 2016, 6, 29820. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; Ciriolo, M.R. Redox control of apoptosis: An update. Antioxid. Redox Signal. 2006, 8, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.L.; Ramana, C.V.; Hamilton, M.; Taylor, W.R.; DePrimo, S.E.; Bean, L.; Agarwal, A.; Agarwal, M.K.; Wolfman, A.; Stark, G.R. Regulation of p53 expression by the RAS-MAP kinase pathway. Oncogene 2001, 20, 2527. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S. The functional interactions between the MAPK and p53 signaling pathways. Cancer Biol. Ther. 2004, 3, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Dogliotti, G.; Galliera, E.; Dozio, E.; Vianello, E.; Villa, R.E.; Licastro, F.; Barajon, I.; Corsi, M.M. Okadaic acid induces apoptosis in Down syndrome fibroblasts. Toxicol. In Vitro 2010, 24, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.; Lawen, A. The mitochondrial membrane potential (Δψm) in apoptosis; An update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, W.; Lin, S.Z.; Wang, Z.J.; Kan, J.T.; Chen, S.Y.; Zhu, Y.Z. Gp130-mediated STAT3 activation by S-propargyl-cysteine, an endogenous hydrogen sulfide initiator, prevents doxorubicin-induced cardiotoxicity. Cell Death Dis. 2016, 7, e2339. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Cao, W.; Zhou, X.; Cao, W.; Xie, Y.; Wang, S. Anti-thrombotic effects of α-linolenic acid isolated from Zanthoxylum bungeanum Maxim seeds. BMC Complement. Alternat. Med. 2014, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Su, Y.; Li, Y.; Wu, C.; Meng, Y.; Peng, X.; Cui, Y. Tetramethylpyrazine inhibits CoCl2-induced neurotoxicity through enhancement of Nrf2/GCLc/GSH and suppression of HIF1alpha/NOX2/ROS pathways. J. Neurochem. 2015, 134, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Katare, P.B.; Banerjee, S.K. Repositioning of Drugs in Cardiometabolic Disorders: Importance and Current Scenario. Curr. Top. Med. Chem. 2016, 16, 2189–2200. [Google Scholar] [CrossRef]
- Segers, V.F.; Lee, R.T. Stem-cell therapy for cardiac disease. Nature 2008, 451, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, Z.; Miao, L.; Xin, X.; Lin, S.; Zhu, Y.; Guo, W.; Zhu, Y.Z. miRNA-30 family inhibition protects against cardiac ischemic injury by regulating cystathionine-γ-lyase expression. Antioxid. Redox Signal. 2015, 22, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, C.B.; Gersh, B.J.; Stone, G.W.; Granger, C.B. Novel therapeutics in myocardial infarction: Targeting microvascular dysfunction and reperfusion injury. Trends Pharm. Sci. 2015, 36, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Vuorela, P.; Leinonen, M.; Saikku, P.; Tammela, P.; Rauha, J.P.; Wennberg, T.; Vuorela, H. Natural products in the process of finding new drug candidates. Curr. Med. Chem. 2004, 11, 1375–1389. [Google Scholar] [CrossRef]
- Yang, Q.; Cao, W.; Zhou, X.; Cao, W.; Xie, Y.; Wang, Si. Anti-thrombotic effects of α-linolenic acid isolated from Zanthoxylum bungeanum Maxim seeds. BMC Complement. Altern. Med. 2014, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, R.C.; Emani, V.R.; Hess, M.L. Activated oxygen species in heart failure. Heart Fail. Rev. 1999, 4, 1–12. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compound is available from the authors.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhong, L.; Liu, X.; Zhu, Y.Z. ZYZ-772 Prevents Cardiomyocyte Injury by Suppressing Nox4-Derived ROS Production and Apoptosis. Molecules 2017, 22, 331. https://doi.org/10.3390/molecules22020331
Wang Y, Zhong L, Liu X, Zhu YZ. ZYZ-772 Prevents Cardiomyocyte Injury by Suppressing Nox4-Derived ROS Production and Apoptosis. Molecules. 2017; 22(2):331. https://doi.org/10.3390/molecules22020331
Chicago/Turabian StyleWang, Ying, Liangjie Zhong, Xinhua Liu, and Yi Zhun Zhu. 2017. "ZYZ-772 Prevents Cardiomyocyte Injury by Suppressing Nox4-Derived ROS Production and Apoptosis" Molecules 22, no. 2: 331. https://doi.org/10.3390/molecules22020331
APA StyleWang, Y., Zhong, L., Liu, X., & Zhu, Y. Z. (2017). ZYZ-772 Prevents Cardiomyocyte Injury by Suppressing Nox4-Derived ROS Production and Apoptosis. Molecules, 22(2), 331. https://doi.org/10.3390/molecules22020331