Abstract
Tyrosinase inhibitors are of far-ranging importance in cosmetics, medicinal products, and food industries. Peru is a diverse country with a wide variety of plants that may contain excellent anti-tyrosinase inhibitors. In the present study, the tyrosinase inhibitory properties of 50 medicinal plant extracts from Peru were investigated using tyrosinase assay. Among plant extracts, those that showed an inhibition rate >50% were Hypericum laricifolium Juss., Taraxacum officinale F.H.Wigg., and Muehlenbeckia vulcanica Meisn., with H. laricifolium Juss. showing the greatest anti-tyrosinase activity. Although H. laricifolium Juss. has been widely used as a medicinal plant by Peruvians, little is known regarding its bioactive components and effects on tyrosinase activity. For this reason, we attempted to discover tyrosinase inhibitors in H. laricifolium Juss. for the first time. The bioactive components were separated by Sephadex LH-20 chromatography and eluted with 100% methanol. Eight compounds were discovered and characterized by high-performance liquid chromatography coupled with diode array detection (HPLC-DAD): protocatechuic acid, p-hydroxybenzoic acid, chlorogenic acid, vanilic acid, caffeic acid, kaempferol 3-O-glucuronide, quercetin, and kaempferol. In addition, the concentration of these compounds required for 50% inhibition (IC50) of tyrosinase activity were evaluated. Quercetin exhibited the strongest tyrosinase inhibition (IC50 14.29 ± 0.3 μM). Therefore, the Peruvian plant H. laricifolium Juss. could be a novel source for anti-tyrosinase activity.
1. Introduction
Tyrosinase (E.C.1.14.18.1), also known as polyphenol oxidase, is a multifunctional glycosylated copper-containing enzyme which is widespread in many organisms including animals, plants, and microorganisms [1]. Tyrosinase is mainly involved in the initial steps of the pathway which catalyzes the orthohydroxylation of l-tyrosine to l-3,4-dihydroxyphenylalanine (l-DOPA) (monophenolase activity) and the oxidation of l-DOPA to dopaquinone (diphenolase activity), which is then converted to the end-product melanin [2,3]. Melanin is the main component responsible for the darkening of the skin and hair, and plays an important role against ultraviolet (UV) ray damage. However, the accumulation of an excessive level of melanin can cause skin damage, such as age spots or malignant melanoma. It has also been associated with Parkinson’s disease [4]. Additionally, the browning of fruits and vegetables is related to the oxidation of phenolic compounds catalyzed by tyrosinase, which results in a loss of market value for the food [2,3,4,5]. Therefore, tyrosinase inhibitors are important in cosmetics (hyperpigmentation), medicinal products, and food industries. To date, despite the existence of a large number of tyrosinase inhibitors, only a few are marketed as safe [3,6]. Thus, the search for new tyrosinase inhibitors is important for the treatment of hyperpigmentation, development of skin-whitening agents, and use as preservatives in the food industry.
Peru is a developing country characterized by a rich biodiversity, where medicinal plants still represent the main therapeutic tool in traditional medicine, especially in many ethnic groups. It is estimated that 17,144 flowering plant species exist in Peru, of which 5354 (31.3%) are endemic, while the rest are native or introduced [7,8]. Some of these may be good candidates for the development of new anti-tyrosinase agents and/or standardized phytomedicines, and may be effective as future therapies for various diseases. However, to date, no report has been published regarding the tyrosinase inhibitory activity of Peruvian plants.
For that reason, as part of our ongoing efforts to find new tyrosinase agents, we measured the anti-tyrosinase activity of 50 Peruvian plants traditionally used as medicinal infusions by Peruvian people, based on the ethnobotanical information provided by literature sources [9,10,11,12,13,14,15,16,17]. Among the extracts that were screened, we selected the most active plants against tyrosinase based on spectrophotometric analyses of their bioactive compounds. Subsequently, we found that Hypericum laricifolium Juss. (A10) extract showed significant tyrosinase inhibition. H. laricifolium Juss., is a species of Hypericum (Clusiaceae) which can be found in tropical regions of the world at high altitudes, particularly in South America and Africa. In South America, H. laricifolium Juss., is distributed from western Venezuela, along the Andes of Colombia and Ecuador through central Peru and into Bolivia. In Peru it is called “Hierba de la fortuna” and used for good health, fortune, or luck in love, while in Ecuador it is called “Romerillo” or “Hierba de San juan” and used as a diuretic and for inducing menstruation [18,19].
In one study, from the aerial parts of H. laricifolium Juss., twelve xanthones were isolated and identified [20]. Biological investigations also revealed the presence of phenolic acids, flavonoids, triterponoids [21], and dimeric and monomeric acylphloroglucinol derivatives [22,23,24]. Additionally, studies in Venezuela reported the chemical composition of H. laricifolium Juss. essential oils [25]. Scientists in Peru also found that the leaves of H. laricifolium Juss. had an anti-depressive effect in rats [22]. To the best of our knowledge, this plant has not previously been studied relative to its effect on tyrosinase. In this study, for the first time, we investigated inhibitory effects and characterization of compounds with an effect on tyrosinase extracted from H. laricifolium Juss.
2. Results and Discussion
2.1. Ethnopharmacological Data
Plants represent a rich source of bioactive chemicals, many of which are largely free from harmful adverse effects [26,27], but their individual activity is not sufficiently potent to be of practical use. Recently, safe and effective tyrosinase inhibitors have become important for their potential applications in improving the quality of food, preventing pigmentation disorders, and preventing other melanin-related health problems in human beings, in addition to cosmetic applications [28]. We selected 50 species to be studied which were bought in popular markets in Lima, Peru. These plants were chosen due to the fact that there have been no previous studies on their effect on tyrosinase activity. Table 1 provides the scientific name, the common name, the family name, as well as the traditional use of each plant. Many of the plants had two or more names which could be found in Spanish or in the native language Quechua. All the plants chosen are used by Peruvians, and have different traditional uses, such as for treatment of liver diseases, use as an anti-inflammatory, or for curing cancer, diabetes, and other diseases. These 50 species belong to 29 botanical different families. Asteraceae was the family with the largest number medicinal species (18%), followed by the Fabaceae, Lamiaceae, and Euphorbiacea families (10%, 8%, 8%).

Table 1.
Traditional uses and ethnobotanical data of the Peruvian plants used in the current study.
2.2. Screening of Tyrosinase Inhibitory Activities of Methanol Extracts
Crude extracts of 50 plants were prepared using 70% methanol and their yields were from 1.1% to 57.1% as listed in Table 2.

Table 2.
Tyrosinase inhibitory activity of 70% methanol extracts of Peruvian medicinal plants.
For the extraction of the 50 plants, an assortment of plant parts were used in our study, including leaves, flowers, aerial parts, seeds and roots, according to the traditional use by Peruvians. The effect on tyrosinase inhibition of the 50 crude methanol extracts were investigated at a concentration of 500 μg/mL and the results are reported in Table 2. Arbutin was used as the positive control. Of the 50 extracts assayed, 36 extracts demonstrated an effect on tyrosinase activity, among which three showed an inhibition rate >50%, 5 showed inhibition rates between 40% and 50%, and the rest showed in inhibition rate <40%. The best inhibitory activities were observed in extracts of leaves of H. laricifolium Juss. which showed 74% ± 2.1% inhibition, followed by Taraxacum officinale F.H. Wigg (P49, inhibition percent is 60.8% ± 4.1%), Muehlenbeckia vulcanica Meisn (P82, inhibition percent is 57.1% ± 3.0%), Salvia hispanica L. (P4, inhibition percent is 44.3% ± 3.4%), Senna sp. (A7, inhibition percent is 44.1% ± 2.3%), Anacardium occidentale L. (A41, inhibition percent is 42.5% ± 7.2%), Matricaria recutita L. (A12, inhibition percent is 40.49% ± 2.1%) and Ch. pilosa Goldm (P17, inhibition percent is 40.35% ± 2.0%).
In Korea, one study reported on the antioxidant capacity and tyrosinase inhibition activity of water extracts of various parts of T. Officinale. The leaf extract (1 mg/mL) showed the highest tyrosinase inhibition (34.2%), while the roots and whole plant showed tyrosinase inhibition of more than 20% [32]. M. volcanica contains anthraquinone-O-glycosides, and according to some investigations, some anthraquinones have an anti-tyrosinase effect [33,34]. S. hispanica L. contains compounds such as quercetin, kaemperol and caffeic acids, and these are known to have anti-tyrosinase activity [35]. A. occidentale L. was found to exhibit anti-tyrosinase activity [36], while M. recutita L. showed an ability to reduce ultraviolet B (UVB)–induced pigmentation in vivo [37]. There are no reports on anti-tyrosinase activity in the other samples.
As Figure 1 shows, the extracts which showed an inhibition rate >50%, were selected and their IC50 (concentration of each extract required for 50% inhibition of the enzyme activity) was determined with H. laricifolium Juss., T. officinale F.H. Wigg, and M. vulcanica Meisn, with an IC50 of 120.9 μg/mL, 280.1 μg/mL, and 290.4 μg/mL, respectively. Because of its low IC50 and due to its high availability, H. laricifolium Juss. was selected as a potential source of a new tyrosinase inhibitor.
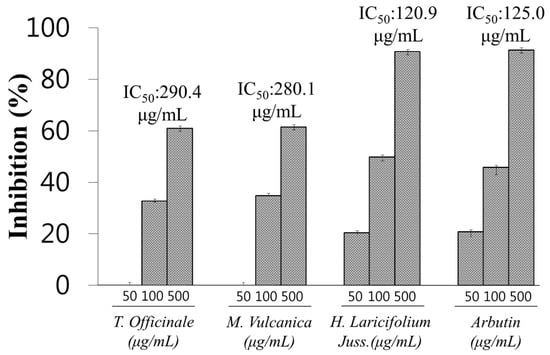
Figure 1.
Tyrosinase inhibitory effect of the most effective extracts of Peruvian medicinal plants, T. Officinale F.H. Wigg, M.Vulcanica Meisn, H. Laricifolium Juss. Arbutin was used as positive control. IC50, indicates the concentration of each extract required for 50% inhibition of the enzyme activity.
2.3. Effect of H. laricifolium Juss. on Tyrosinase Inhibition
H. laricifolium Juss. has many traditional uses. In Peru, it is distributed in Amazonas, Ancash, Cajamarca, Huanuco, La Libertad, Pasco, Piura, and San Martin, between 2000 and 4500 m above sea level [17]. Previous studies have reported that H. laricifolium Juss. has various phytochemical constituents, including two caffeic acid esters of long-chain aliphatic alcohols, sterols, triterpenoids, benzoic and cinnamic acid derivatives [21,22,23,24,25], flavonols, and flavonol glycosides. These components were tested in vitro for anti-inflammatory activity, especially quercetin, which inhibited cyclooxygenase-1 (COX-1) by 52% ± 2% and caffeic acid esters which inhibited COX-2 by 44% ± 2% [21].
In other studies, the ethanol extracts of leaves of H. laricifolium Juss. showed a weak minimum inhibitory concentration (MIC) of 250 μg/mL against Candida albicans, but the bioactive compounds were not identified [38]. In the literature, no information has been found regarding tyrosinase activity in H. laricifolium Juss., but there are reports on one species of Hypericum of which St. John’s Wort (Hypericum perforatum L.) is the most well-known. It is a medicinal herb with antidepressant activity and is possibly associated with anti-cholinesterase, anti-tyrosinase, and antioxidant properties [20,39].
Its known that genus Hypericum has a variety of molecules with different biological activities, among them the content of hypericin and various phenolics is considerable, exhibiting wide pharmacological activities such as anti-inflammatory, anti-cholinesterase, antimicrobial, antiviral and antioxidant properties [40]. The species Hypericum humifusum and Hypericum perfoliatum have great content of hypericin and phloglucinols. Hypericin is used against viruses and retroviruses such as the human immunodeficiency virus (HIV) 35, and influenza A, while phloroglucinols (hyperforin and adhyperforin) show potential antibacterial, antidiabetic, and cytotoxic activities [40,41].
According to our results mentioned above, we decided to compare the differences in anti-tyrosinase activities between 70% and 50% methanol extracts, and a methylene chloride extract of H. laricifolium Juss. at concentrations of 100, 500, and 1000 μg/mL, and arbutin. As shown in Table 3, we found that the methylene chloride extract did not show any activity and the 50% methanol extract showed low inhibition at 1000 μg/mL (22.3%). The 70% methanol extract demonstrated a good inhibition of tyrosinase activity; the inhibition rate was >50% at 500, and 1000 μg/mL (IC50 is 122.1 μg/mL). The IC50 value for arbutin was 42.0 μg/mL. According these results, a 70% methanol extract should be studied to determine the constituents responsible for tyrosinase inhibition.

Table 3.
IC50 values of tyrosinase inhibitory from various solvent extracts of H. laricifolium Juss.
2.4. Identification of Major Bioactive Components of H. laricifolium Juss.
Figure 2 shows the HPLC chromatograms of crude H. laricifolium Juss. extracts. All the sample components were separated in less than 40 minutes, with retention factors depending mainly on structural hydrophobicity, as previously demonstrated by a number of studies employing the HPLC for highly efficient separations of complex samples [42]. After the isolation, we identified and isolated eight compounds as candidates for tyrosinase inhibition, eluting in different retention times. From compound (1) to compound (5), peaks appeared between 12 and 19 min, and from compound (6) to compound (8), peaks appeared between 20 and 30 min. Figure 3 shows the chemical structures of these isolated compounds. From the 70% methanol extract of the plant, protocatechuic acid was obtained and identified as compound (1), p-hydroxybenzoic acid as compound (2), chlorogenic acid as compound (3), vanillic acid as compound (4), caffeic acid as compound (5), kaempferol 3-O-glucuronide as compound (6), quercetin as compound (7) and kaempferol compound as (8). These compounds were positively identified both on the basis of information provided by the literature, by direct comparisons with authentic materials available commercially, and by comparison with data from similar species in the same family [21,43,44].
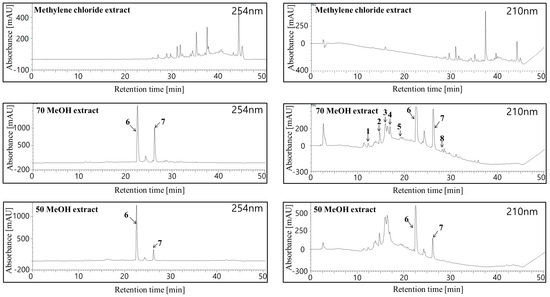
Figure 2.
HPLC chromatograms of crude H. laricifolium Juss. methylene chloride extract, 70% methanol extract, and 50% methanol extract at 254 nm and 210 nm.
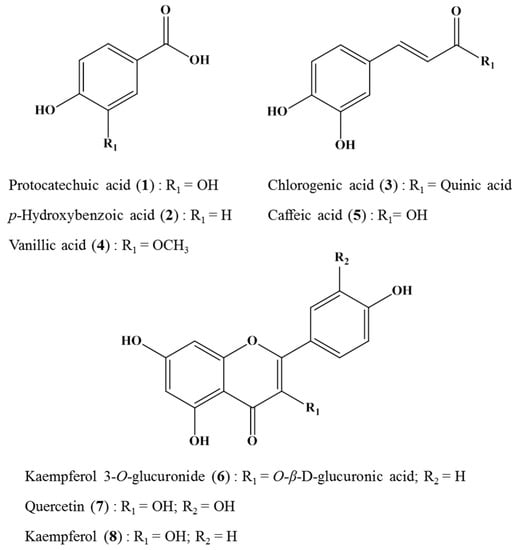
Figure 3.
Compounds isolated from crude H. laricifolium Juss. extract by Sephadex LH-20 chromatography.
2.5. Effect of Bioactive Compounds on Tyrosinase Inhibition
The tyrosinase inhibitory activities of the major bioactive components of H. laricifolium Juss. were confirmed using a tyrosinase assay. As shown in Table 4, all compounds except quercetin (compound 7), did not show any anti-tyrosinase activity. Quercetin showed strong tyrosinase inhibition with an IC50 of 14.29 ± 0.3 μM, which is seven times lower than that of arbutin (IC50 = 110.4 ± 1.9 μM) but a little higher than that of kojic acid (IC50 8.0 ± 0.5 μM), which is also a positive control produced by many species of fungi such as Aspergillus and Penicillium sp. [27]. Although some other compounds showed some tyrosinase inhibitory activities at 500 μg/mL or 1000 μg/mL (data not shown), there were no other strong inhibitors.

Table 4.
Percentage of tyrosinase inhibition and amounts of compounds from H. laricifolium Juss. and positive control: arbutin and kojic acid. The concentration of the compounds and positive control was 100 μg/mL.
The inhibition percent of original methanol extract of H. laricifolium Juss. (data not shown) and quercetin at 100 µg/mL of concentration were compared, the methanol extract show around 50% inhibition, while quercetin show high inhibition over 95%. Therefore, it is revealed that quercetin has a significant inhibition on tyrosinase activity compared to the other compounds and is the main determinant of the observed activity for H. laricifolium Juss. The x-axis and y-axis is fuzzy.
In previous studies on flavonoids, compounds such as quercetin and kaempferol were shown to interfere with the activity of tyrosinase through chelation of copper in its active site [28]. It has been demonstrated that quercetin and kaempferol can suppress melanogenesis, directly inhibiting tyrosinase activity or reducing tyrosinase expression [45]. In our study, quercetin proved to be a good tyrosinase inhibitor, while kaempferol was a weaker inhibitor than arbutin.
Figure 4 shows % inhibition of quercetin using L-tyrosine as a substrate, we decided to study quercetin’s ability to inhibit the activity of tyrosinase at different times and in different concentrations (2 min, 5 min, 10 min, and 3 μg/mL, 6.25 μg/mL, and 12.5 μg/mL). At a concentration of 12.5 μg/mL, quercetin inhibited >50% tyrosinase at 2 min, 5 min, and 10 min, with the greatest inhibition being 88.1%.
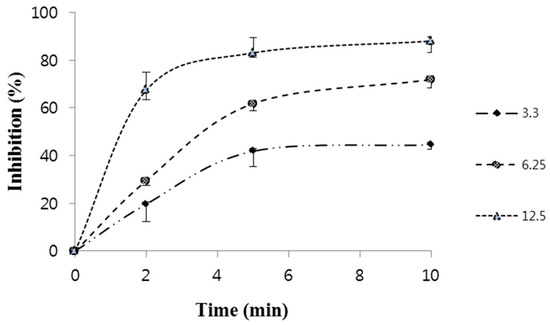
Figure 4.
Course of oxidation of L-tyrosine by tyrosinase in the presence of different concentrations of quercetin (compound 7). Concentrations were 3.3, 6.25, and 12.5 μg/mL.
As quercetin is the only active compound in H. laricifolium Juss. responsible for tyrosinase inhibition, the 70% methanol extract, with the highest concentration of quercetin, showed the strongest activity against tyrosinase, more than the 50% methanol extract and methylene chloride extract. This study is a starting point for further investigations on tyrosinase inhibitors with respect to Peruvian plant extracts. Simultaneously, we suggest that more Peruvian plants should be explored in order to identify more useful chemical compounds.
3. Materials and Methods
3.1. Ethnobotanical Search
The plants were selected according to the ethnobotanical bibliography provided by different literature sources [9,10,11,12,13,14,15,16,17,29,30,31], particularly those with a lack of scientific information about their activity. Scientific names, common names which can be found in Spanish or Quechua (native language in Peru), and traditional uses for Peruvian population are summarized in Table 1.
3.2. Chemicals
Mushroom tyrosinase (120 kDa), l-tyrosine, propylene glycol, dimethyl sulfoxide, arbutin, and kojic acid were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Potassium phosphate dibasic (K2HPO4), and potassium phosphate monobasic (KH2PO4), were obtained from Junsei Chemical Co. (Tokyo, Japan). Deionized distilled water used for all solutions, dilutions, and HPLC analysis was obtained from a Milli-Q system (Millipore, Bedford, MA, USA) with a resistance of over 18.2 MΩ cm. The Sephadex LH-20 column was purchased from GE Healthcare (Uppasala, Sweden). All organic solvents were HPLC grade and were obtained from J.T. Baker (Phillipsburg, NJ, USA). All other chemicals and solvents, unless otherwise specified, were guaranteed reagent grade and purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
3.3. Plant Materials
The 50 dried plant samples were purchased from different local markets in Lima, Peru from May to October 2015. The vouchers of all samples were deposited at the Center for Efficacy Assessment and Development of Functional Foods and Drugs, Hallym University (Chuncheon, South Korea). The samples were identified according to macroscopic characters using available literature in English and Spanish and verified by Paul H. Gonzales Arce from the Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru.
3.4. Preparation of Extracts and Isolation of Plant Samples
The dried Peruvian plants (20–50 g) were pulverized at room temperature and then were extracted by maceration at room temperature with 70% methanol for 72 h. The supernatants were filtered through filter paper (Hyundai Micro, Seoul, Korea) and evaporated under vacuum at 37 °C by means of a rotary evaporator (Eyela, Tokyo Rikakikai CO, Tokyo, Japan). The resultant extract was stored at −4 °C until use. Fifty extracts were prepared, and the extractions were performed in duplicate. A yield for each extract was obtained after the solvent was removed and expressed as the calculated weight of air-dried crushed plant material with respect to the starting material.
The major components of the H. laricifolium Juss. 70% methanol extract were isolated by column chromatography. Briefly, the 70% methanol extract of H. laricifolium Juss. (1 g), was dissolved in methanol and loaded onto a Sephadex LH-20 column (2 cm × 90 cm, Uppsala, Sweden). The column was eluted with 100% methanol at a flow rate of 2.0 mL/min. The effluents were collected (fraction size 40 mL) into test tubes as 10 separate fractions. Among them, from fractions 7 and 10 the compounds 6 and 7 were directly obtained, which showed high purities: kaempferol 3-O-glucuronide (40.3 mg, 98.73% purity) and quercetin (16.8 mg, 98.36% purity), respectively. Six other major compounds were identified by direct comparison with the authentic materials available commercially or in our laboratory, and also by comparison with references of similar species of same family [21,41,42]. Among them were protocatechuic acid (1), p-hydroxybenzoic acid (2), chlorogenic acid (3), vanillic acid (4), caffeic acid (5) and kaempferol (8).
3.5. HPLC Analysis
HPLC analysis was carried out on a Dionex system (Dionex, Sunnyvale, CA, USA) consisting of a P850 pump, an ASI-100 automated sample injector, a Synergi Hydro-RP 80A column (150 mm × 4.6 mm, 4 μm; Phenomenex, Torrance, CA, USA) maintained at 30 °C, and an UVD170S detector. Briefly, the mobile phase was composed of 0.1% trifluoroacetic acid (A-line) and methanol (B-line). The gradient elution system was modified as follows: 0–35 min, starting with 5% B-line, programmed to reach 40% B-line at 15 min using a linear gradient, followed by 100% B-line from 15–35 min. The flow rate was 0.7 mL/min and 10 μL was the sample injection volume. The detector monitored the eluent at wavelength 254 nm.
3.6. NMR Analysis
Two compounds were separated from the 70% MeOH extract of H. laricifolium Juss. by Sephadex LH-20 column chromatography. Kaempferol 3-O-glucuronide (compound 6) and quercetin (compound 7) were identified by comparing 1H-NMR and 13C-NMR spectra with previously reported data. 1H-NMR spectra of these two isolated, pure compounds were recorded with a Bruker AV600 instrument (Mundelein, IL. USA), using DMSO-d6 as a solvent. The detailed structural information was listed as following:
Compound 6. 1H-NMR (DMSO-d6, 600 MHz) δ 6.20 (1H, d, J = 2.1 Hz, H-6), δ 6.42 (1H, d, J = 2.0 Hz, H-8), δ 6.86 (2H, d, J = 8.9 Hz, H-3’, 5’), δ 8.00 (2H, d, J = 8.9 Hz, H-2’, 6’), δ 5.47 (1H, d, J = 7.5 Hz, H-1”), δ 3.23–3.18 (1H, m, H-2”), δ 3.53 (1H, d, J = 9.6 Hz, H-5”), δ 3.26–3.22 (1H, m, H-3”), δ 3.35–3.30 (1H, m, H-4”); p13 C NMR (DMSO-d6, 150 MHz) δ 157.4 (C-2), 134.3 (C-3), 177.8 (C-4), 161.0 (C-5), 99.8 (C-6), 165.5 (C-7), 94.5 (C-8), 156.8 (C-9), 103.9 (C-10), 120.6 (C-1’),160.1 (C-4’), 102.8 (C-1”), 72.0 (C-2”), 73.6 (C-3”), 76.3 (C-4”), 76.7 (C-5”), 173.8 (C-6”). The 1H-NMR, 13C-NMR data for compound 6 are identical to those reported previously [46]. Compound 6 was identified as kaempferol 3-O-glucuronide.
Compound 7. 1H-NMR (DMSO-d6, 600 MHz) δ 6.19 (1H, d, J = 2.0 Hz, H-6), δ 6.40 (1H, d, J = 2 Hz, H-8), δ 6.88 (1H, d, J = 8.4 Hz, H-5’), δ 7.54 (1H, dd, J = 2.2, 8.4 Hz, H-6’), δ 7.68 (1H, d, J = 2.2 Hz, H-2’), δ 12.50 (1H, s, 5-OH). p13C NMR (DMSO-d6, 150 MHz) δ 177.3 (C-4), 165.7 (C-7), 162.5 (C-3), 158.3 (C-9), 148.1 (C-4’), 147.9 (C-2), 146.3 (C-3’), 137.3 (C-3), 124.2 (C-1’), 121.7 (C-6’), 116.3 (C-5’), 116.0 (C-2’), 104.6 (C-10), 99.3 (C-6), 94.4 (C-8). The 1H-NMR, 13C-NMAR data for compound 7 are identical to those reported previously [47,48]. Compound 7 was identified as quercetin.
3.7. Tyrosinase Assay
Tyrosinase activity was determined by spectrophotometry, with minor modifications [26]. First, 80 μL of 0.1 M potassium phosphate buffer (pH 6.6), 20 μL of sample dissolved in dimethyl sulfoxide at the concentrations needed (final concentrations 100–500 μg/mL), and 50 μL of l-tyrosine buffer (1.5 mM) solutions were mixed. Then, 50 μL of mushroom tyrosinase solution (200 unit/mL in phosphate buffer) was added to each mixture, which was then incubated at 25 °C for 15 min. The absorbance of the mixture was observed at 450 nm using a 96-well reader and an EL-800 Universal microplate reader (Bio-Tek Instrument Inc. Winooski, VT, USA). Arbutin and kojic acid were used as positive controls. All the samples were first tested at 500 μg/mL and those showing 50% inhibition or higher in three different repetitions were further evaluated for the concentration necessary for 50% inhibition (IC50). The percent inhibition of tyrosinase was calculated as follows:
where A is the absorbance of the reaction mixture of the enzyme but without test samples, B is the absorbance of the buffer solution without test samples and enzyme, C is the absorbance of the test samples and enzyme, and D is the absorbance of the test sample but without enzyme.
3.8. Statistical Analysis
The results were expressed as mean ± standard deviation (SD), and were repeated three to five times. The inhibitory concentration (IC50) was calculated by log-Probit analysis.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A1A01059199) and by Hallym University Research Fund (HRF-201701-010).
Author Contributions
Y.N.G.Q. designed the experiment and prepared the extraction. S.H.H. performed the isolation of the compounds and conducted its elucidation. Y.N.G.Q. carried out experiments of the extracts and compounds, analyzed data and wrote the first draft. Z.W. and S.S.L. revised the manuscript. All the authors read and approved the final manuscript and all authors’ names added in manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garcia, P.; Furlan, R. Multiresponse Optimisation applied to the developement of a TLC Autography for the detection of tyrosinase inhibitors. Phytochem. Anal. 2015, 26, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, W.; Zhao, H.; Chen, Z. Tyrosinase inhibitor screening in traditional Chinese medicines by electrophoretically, mediated microanalysis. J. Sep. Sci. 2015, 38, 2887–9892. [Google Scholar] [CrossRef] [PubMed]
- Chiari, M.E.; Joray, M.B.; Ruiz, G.; Palacios, S.M.; Capinella, M.C. Tyrosinase inhibitory activity of native plants from central Argentina: Isolation of an active principle from Lithrea molleoides. Food Chem. 2010, 120, 10–14. [Google Scholar] [CrossRef]
- Hasegawa, T. Tyrosinase-Expressing Neuronal Cell Line as in Vitro Model of Parkinson’s Disease. Int. J. Mol. Sci. 2010, 11, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent Inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Devkota, H.P.; Takano, A.; Masuda, K.; Nakane, T.; Basnet, P.; Skaiko-Basnet, N. Screening of Nepalese crude drugs traditionally used to treat hyperpigmentation: in vitro tyrosinase inhibition. Int. J. Cosmet. Sci. 2008, 30, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Brack, A. Enciclopédico de Plantas Utiles del Perú; CBC: Cuzco, Perú, 1999; p. 550. [Google Scholar]
- Carraz, M.; Lavergne, C.; Jullian, V.; Wright, M.; Gairin, JE.; Gonzales de la Cruz, M.; Bourdy, G. Antiproliferative activity and phenotypic modification induced by selected Peruvian medicinal plants on human hepatocellular carcinoma Hep3B cells. J. Ethnopharmacol. 2015, 166, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Monigatti, M.; Bussmann, R.W.; Weckerle, C. Medicinal plant use in two Andean communities located at different altitudes in the Bolivar Province, Peru. J. Ethnopharmacol. 2013, 145, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Bizet, J.; Campos de la Cruz, J.; Epiquien-Rivera, M.A.; Canigueral, S. A first survey on the medicinal plants of the Chazuta valley (Peruvian Amazon). J. Etnopharmacol. 2009, 122, 333–362. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.E. OMD. Review of Antiviral and Immunomodulating Properties of Plants of the Peruvian Rainforest with a Particular Emphasis on Una de Gato and Sangre de Grado. Altern. Med. Rev. 2001, 6, 6. [Google Scholar]
- Neto, C.C.; Owens, C.W.; Langfield, R.D.; Comeau, A.B.; Onge, J.St.; Vaisberg, A.J.; Hammond, G.B. Antibacterial activity of some Peruvian medicinal plants from the Callejon de Huaylas. J. Ethnopharmacol. 2002, 79, 133–138. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Malca, G.; Glenn, A.; Sharon, D.; Nilsen, B.; Parris, B.; Dubose, D.; Ruiz, D.; Saleda, J.; Martinez, M.; et al. Toxicity of medicinal plants used in traditional medicine in Northern Peru. J. Ethnopharmacol. 2011, 137, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, R.; Pedreschi, R.; Rogez, H.; Larondelle, Y.; Campos, D. Phenolic compound contents and antioxidant activity in plants with nutritional and/or medicinal properties from the Peruvian Andean region. Ind. Crop Prod. 2013, 47, 145–152. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Glenn, A.; Sharon, D. Antibacterial activity plants of Northern Peru – can traditional applications provide leads for modern science? Ind. J. Tradit. Knowl. 2010, 9, 742–753. [Google Scholar]
- Berlowski, A.; Zawada, K.; Wawer, I.; Paradowska, K. Antioxidants Properties of Medicinal Plants from Peru. Food Nutr. Sci. 2013, 4, 71–77. [Google Scholar] [CrossRef]
- Huamani, M.E.; Ruiz, J. Determinacion de la Actividad Antifungica Contra Candida Albicans y Aspergillus Niger de 10 Plantas Medicinales de 3 Departamentos del Peru; Universidad Nacional Mayor de San Marcos: Lima, Peru, 2005. [Google Scholar]
- Duke, J.A.; Vasquez, R. Amazonian Ethnobotanical Dictionary; CRC Press: Boca Raton, FL, USA, 1994; p. 215. [Google Scholar]
- Solis, M.A. Vademecum de plantas medicinales del Ecuador. Available online: http://bases.bireme.br/cgi-bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&src=google&base=LILACS&lang=p&nextAction=lnk&exprSearch=389748&indexSearch=ID (Accessed on 6 July 2016).
- Ramírez-Gonzáilez, I.; Amaro-Luis, J.M.; Bahsas, A. Xanthones from aerial parts of Hypericum laricifolium Juss. Nat. Prod. Commun. 2013, 8, 1731–1732. [Google Scholar] [PubMed]
- El-Seedi, H.R.; Ringbom, T.; Torssell, K.; Bohlin, L. Constituents of Hypericum laricifolium and their cyclooxygenase (COX) enzyme activities. Chem. Pharm. Bull. 2003, 51, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Ccana-Ccapatinta, G.V.; Barros, F.M.C.; Bridi, H.; Von Poser, G.L. Dimeric acylphloroglucinols in Hypericum species from Brathys and Trigynobrathys. Phytochem. Rev. 2015, 14, 25–50. [Google Scholar] [CrossRef]
- Ccana-Ccapatinta, G.V.; Serrano, C.F.; Urrunaga, E.J.S.; Choquenaira, J.P.; Galiano, W.S.; Crockett, S.L.; Von Poser, G.L.; del Carpio, C.J. Assessing the phytochemical profiles and antidepressant-like activity of four Peruvian Hypericum species using the murine forced swimming test. Phytochem. Lett. 2014, 10, 107–112. [Google Scholar] [CrossRef]
- Ccana-Ccapatinta, G.V.; Von Poser, G.L. Acylphoroglucinol derivates from Hypericum laricifolium Juss. Phytochem. Rev. 2015, 12, 63–66. [Google Scholar]
- Rojas, J.; Buitrago, A.; Rojas, L.B.; Morales, A. Chemical composition of Hypericum laricifolium Juss. essential oil collected from Merida-Venezuela. Med. Aromat. Plants 2013, 2, 132–134. [Google Scholar]
- Chen, Y.S.; Lee, S.M.; Lin, C.C.; Liu, C.Y.; Wu, M.C.; Shi, W.L. Kinetic study on the tyrosinase and melanin formation inhibitory activities of carthamus yellow isolated from Carthamus tinctorius L. J. Biosci. Bioeng. 2013, 115, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.F.; Chen, Y.W.; Chang, C.T.; Chou, S.T. Studies on the inhitory effect of Graptopetalum paraguayense E. Walter extracts on mushroom tyrosinase. Food Chem. 2005, 89, 583–587. [Google Scholar] [CrossRef]
- Chen, Q.X.; Kubo, I. Kinetics of mushroom tyrosinase inhibition by quercetin. J. Agric. Food Chem. 2002, 50, 4108–4112. [Google Scholar] [CrossRef] [PubMed]
- Repo-Carrasco, R.; Acevedo de la Cruz, A.; Icochea, J. Chemical and Functional Characterization of Kañiwa (Chenopodium pallidicaule) Grain, Extrudate and Bran. Plant Foods Hum. Nutr. 2009, 64, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kaul Chahal, K.; Bhardwaj, U.; Kaushal, S.; Kaur Sandhu, A. Chemical composition and biological properties of Chrysopogon zizanioides (L.) Roberty syn. Vetiveria zizanioides (L.) Nash. Ind. J. Nat. Prod. Resour. 2015, 6, 251–260. [Google Scholar]
- Bussmann, R.W.; Glenn, A.; Sharon, D.; Meyer, K.; kuhlman, A.; Townesmith, A.; Pourmand, K.; Jonat, B.; Guardado, C.G.; Aguirre, R.; et al. Proving that traditional knowledge works: The antibacterial acitivity of Northern Peruvian medicinal plants. Ethnobot. J. 2011, 9, 67–96. [Google Scholar] [CrossRef]
- Han, E.; Lee, J.; Jung, E.; Jin, Y.; Chung, Ch. Antioxidative Activities of Water Extracts from Different Parts of Taraxacum officinale. J. Korean Soc. Food Sci. Nutr. 2010, 39, 1580–1586. [Google Scholar] [CrossRef]
- Mellado, M.; Madrid, A.; Pena-Cortes, H.; Lopez, R.; Jara, C.; Espinoza, L. Antioxidant Activity of Anthraquinones isolated from leaves of Muehlenbeckia Hastulata (J.E. SM) Johnst. (Polygonaceae). J. Chil. Chem. Soc. 2013, 58, 2. [Google Scholar] [CrossRef]
- Lu, T.; Ko, H. A new anthraquinone glycoside from Rhamnus nakaharai and anti-tyrosinase effect of 6-methoxysorigenin. Nat. Prod. Res. 2016, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Norlaily, A.S.; Keong, Y.; Wan, Y.H. The promising future of chia, salvia hispanica L. J. Biomed. Biotechnol. 2012, 17, 1956. [Google Scholar]
- Kubo, I.; Kinst-Hori, I.; Yokokawa, Y. Tyrosinase inhibitors from Anacardium occidentale fruits. J. Nat. Prod. 1994, 57, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.; Sivamani, J.J.R.; Howard, P.; Maibach, I. Cosmeceuticals and Active Cosmetics, 3rd ed.; Taylor & Francis Group: London, UK, 2015; p. 458. [Google Scholar]
- Crockett, S.; Eberhardt, M.; Kunert, O.; Schuhly, W. Hypericum species in the Paramos of Central and South America: a special focus upon H. irazuense Kuntze ex N. Robson. Phytochem. Rev. 2010, 9, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Levent, M.A.; Sever, Y.B.; Erdogan, O.I.; Saltan, C.G. Assessment of cholinesterase and tyrosinase inhibitory and antioxidant effects of Hypericum perforatum L. (Jhon’s wort). Ind. Crops Prod. 2013, 43, 87–92. [Google Scholar]
- Bejaoui, A.; Ben Salem, I.; Rokbeni, N.; M’rabet, Y.; Boussaid, M.; Boulila, A. Bioactive compounds from Hypericum humifusum and Hypericum perfoliatum: Inhibition potential of polyphenols with acetylcholinesterase and key enzymes linked to type- 2 diabetes. Pharm. Biol. 2017, 1, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Hosni, K.; Msaada, K.; Taarit, M.B.; Hammami, M.; Marzouk, B. Bioactive components of three Hypericum species from Tunisia: A comparative study. Ind. Crops Prod. 2010, 31, 158–163. [Google Scholar] [CrossRef]
- Germanò, M.P.; Cacciola, F.; Donato, P.; Dugo, P.; Certo, G.; D’Angelo, V.; Mondello, L.; Rapisarda, A. Betula pendula leaves: Polyphenolic characterization and potential innovative use in skin whitening products. Fitoterapia 2012, 83, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, I.; Szydlowska, A.; Kawka, B.; Beerhues, L.; Ekiert, H. Accumulation of biologically active phenolic acids in agitated shoot cultures of three Hy Hypericum perforatum cultivars: Elixir, Helos, and Topas. Plant Cell Tiss. Organ Cult. 2015, 123, 273–281. [Google Scholar] [CrossRef]
- Filipiak-Szok, A.; Kurzawa, M.; Szlyk, E. Optimazation of extraction procedure and determination by high performance liquid chromstography of flavonols and phenolic acids from Hypericum Perforatum L. Copern. Lett. 2010, 1, 62–73. [Google Scholar] [CrossRef]
- Taherkhani, N.; Nematollah, G. Inhibitory Effects of Quercetin and Kaempferol as two Propolis Derived Flavonoids on Tyrosinase Enzyme. Biotech. Health Sci. 2014, 1, 22242. [Google Scholar] [CrossRef]
- Badria, F.; Ameen, M.R.; Akl, M. Evaluation of Cytotoxic Compounds from Calligonum comosum L. Growing in Egypt. Z. Naturforsch. 2007, 62, 656–660. [Google Scholar] [CrossRef]
- Huang, W.; Wan, C.; Shouran, Z. Quercetin—A Flavonoid Compound from Sarcopyramis bodinieri var delicate with Potential Apoptotic Activity in HepG2.Liver Cancer Cells. Trop. J. Pharm. Res. 2013, 12, 529–533. [Google Scholar] [CrossRef]
- Selvara, K.; Chowdhury, R.; Bhattacharjee, C. Isolation and structural elucidation of flavonoids from aquatic fern Azolla Microphyla and evaluation of free radical scavenging activity. Int. J. Pharm. Pharm. Sci. 2013, 5, 743–749. [Google Scholar]
- Sample Availability: Samples of the compounds from Hypericum laricifolium Juss. are available from the authors.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).