Hydrophilic Dogwood Extracts as Materials for Reducing the Skin Irritation Potential of Body Wash Cosmetics
Abstract
:1. Introduction
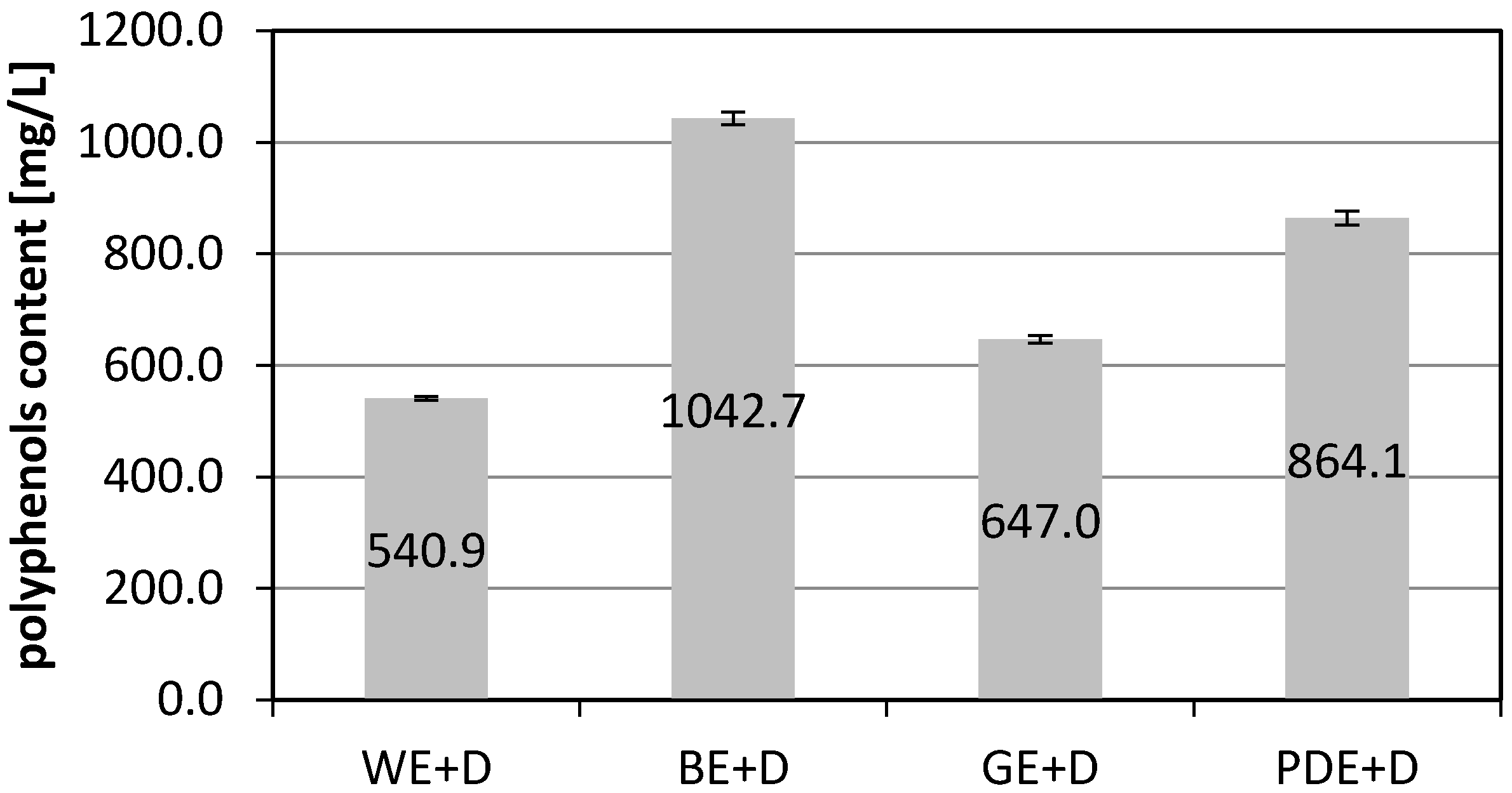
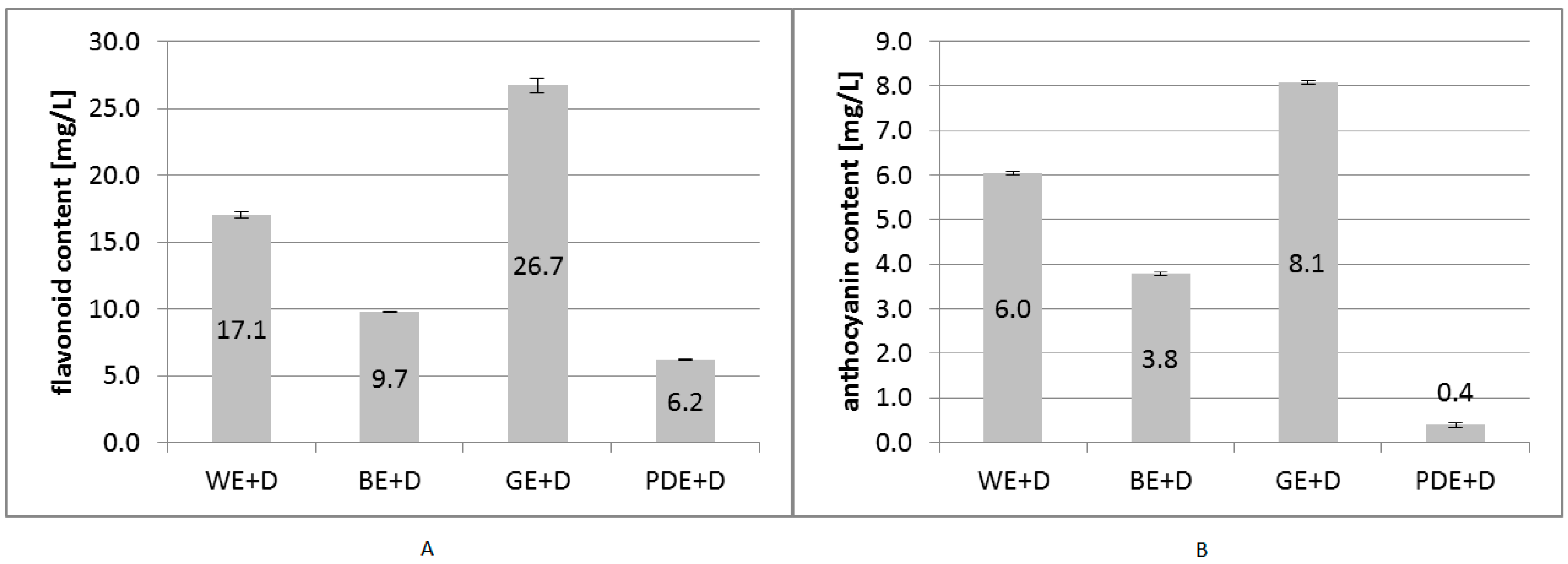
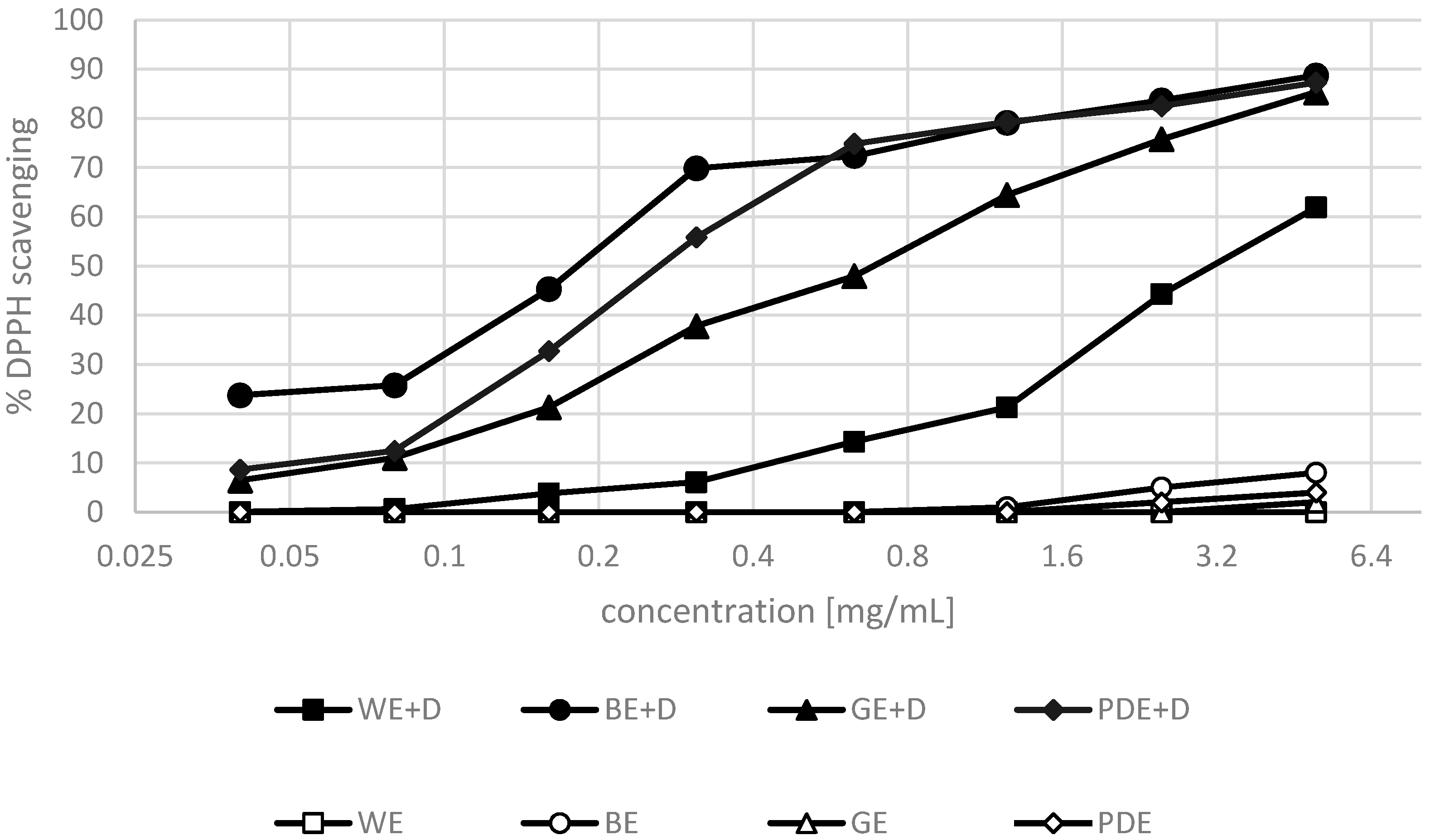
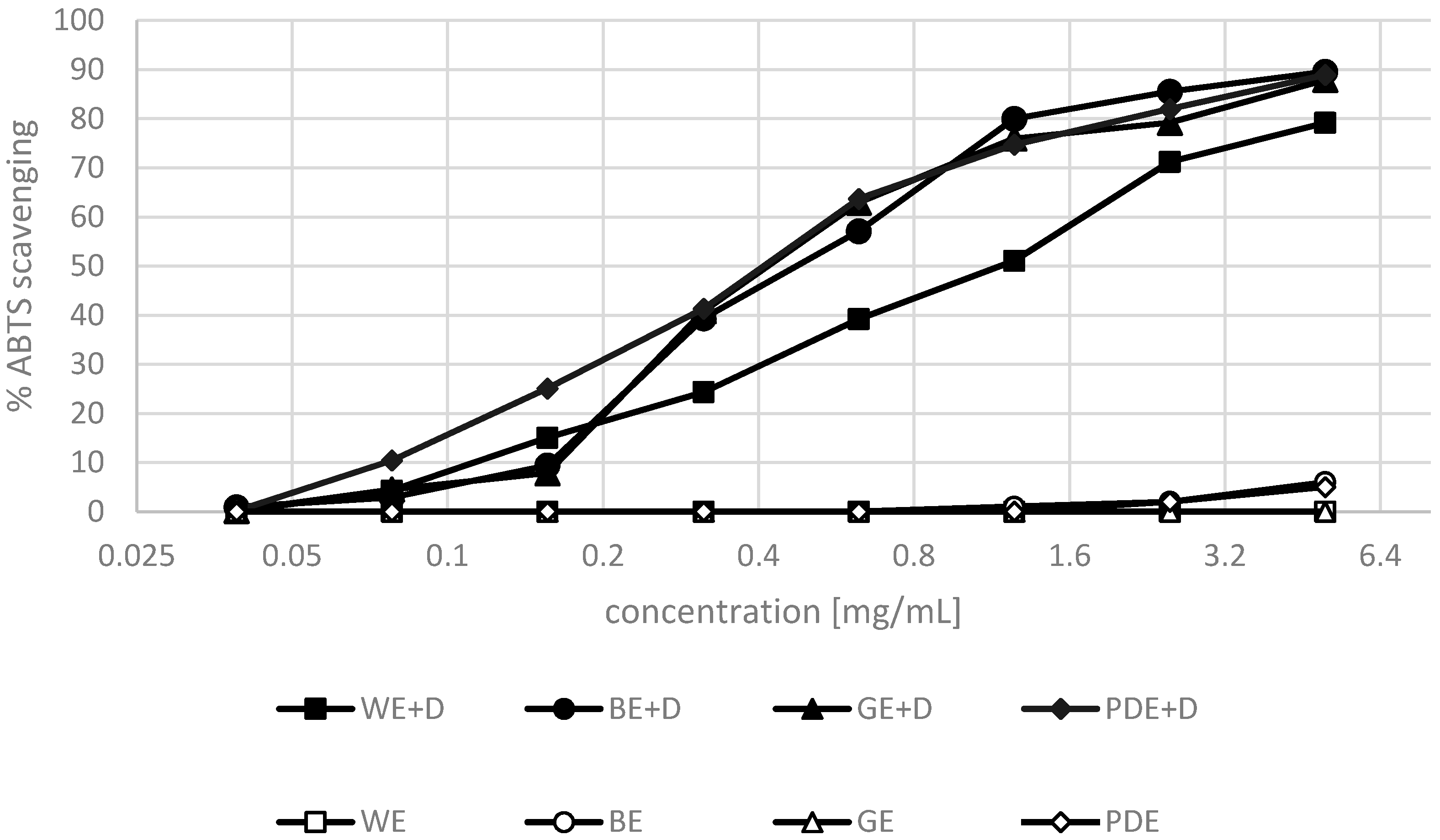
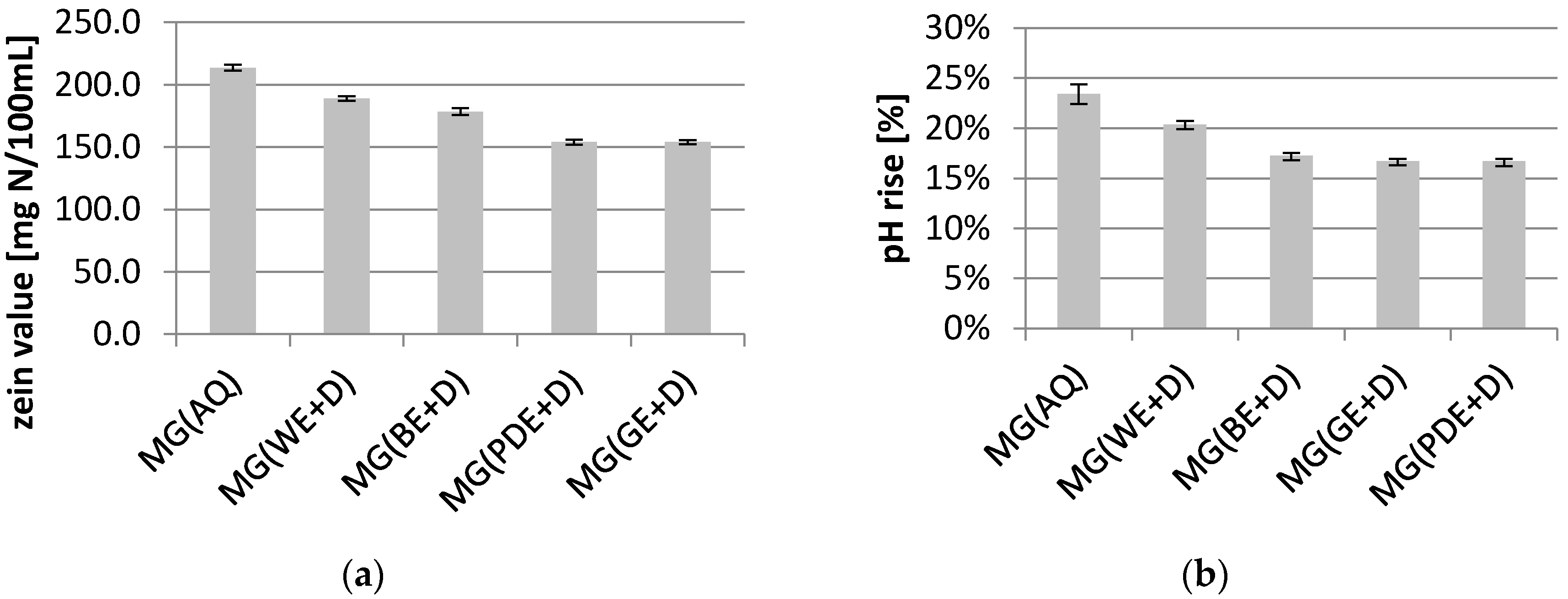
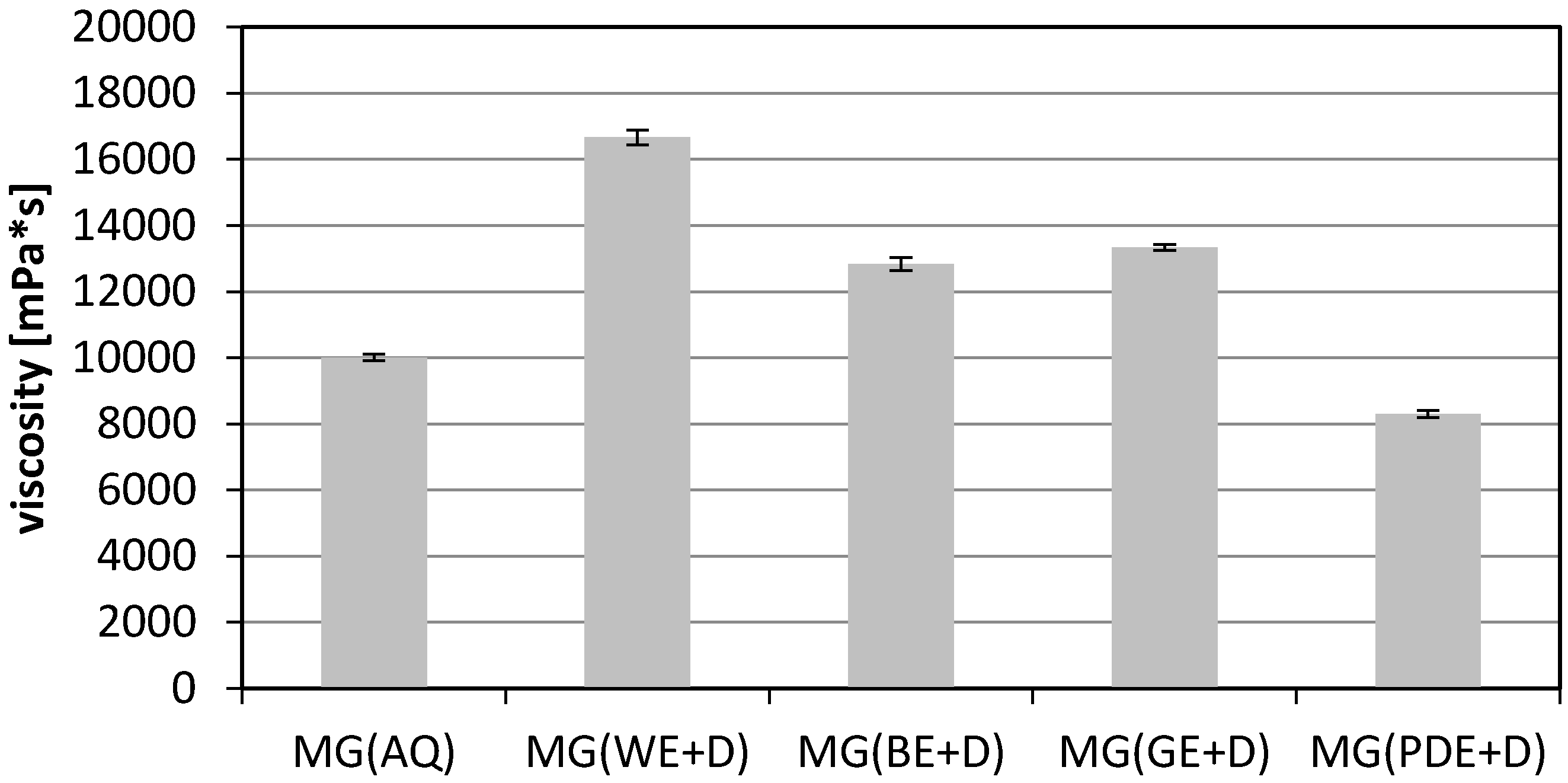
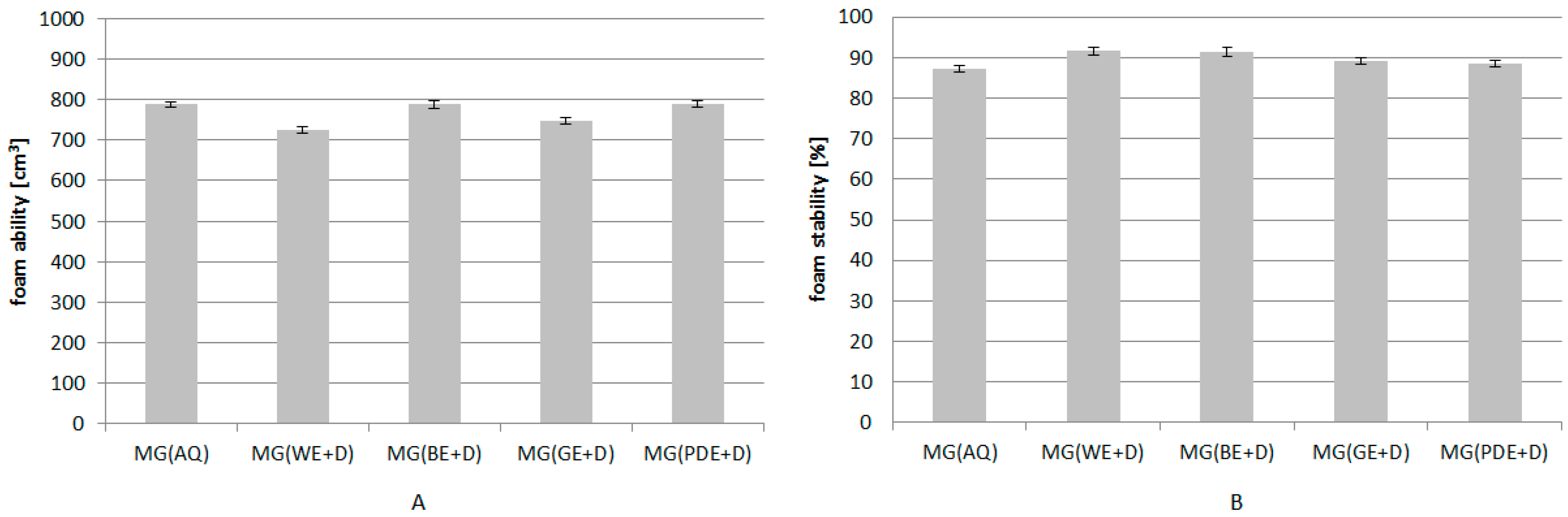
2. Results and Discussion
3. Material and Methods
3.1. Extract Derivation Method
3.2. Determination of Total Phenolic Content
3.3. Determination of Flavonoid Content
3.4. Determination of Total Monomeric Anthocyanin Pigment Content
3.5. DPPH• Radical Scavenging Activity
3.6. ABTS•+ Radical Scavenging Activity
3.7. Technology for Obtaining Prototypical Body Wash Gels
3.8. Zein Test
3.9. Determination of Irritant Potential—pH Rise Test with Bovine Albumin Serum (BSA)
3.10. Viscosity Measurements
3.11. Determination of the Foaming Properties
3.12. Error Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Barel, M.; Paye, M. Handbook of Cosmetic Science and Technology, 4th ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 353–365. [Google Scholar]
- Rhein, L.; Schlossman, M. Surfactants in Personal Care Products and Decorative Cosmetics, 3rd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- Broze, G. Handbook of Detergents. Part A: Properties; Marcel Dekker: New York, NY, USA, 1999. [Google Scholar]
- Farn, R.J. Chemistry and Technology of Surfactants; Blackwell Publishing: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2006. [Google Scholar]
- Agner, T.; Serup, J. Sodium lauryl sulphate for irritant patch testing—A dose-response study using bioengineering methods for determination of skin irritation. J. Investig. Dermatol. 1990, 95, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Charmonnier, V.; Morrison, B.M.; Paye, M.; Maibach, H.I. Subclinical, non-erythematous irritation with an open assay model (washing): Sodium lauryl sulfate (SLS) versus sodium laureth sulfate (SLES). Food Chem. Toxicol. 2001, 39, 279–286. [Google Scholar] [CrossRef]
- Dasilva, S.C.; Sahu, R.P.; Konger, R.L.; Perkins, S.M.; Kaplan, M.H.; Travers, J.B. Increased skin barrier disruption by sodium lauryl sulfate in mice expressing a constitutively active STAT6 in T cells. Arch. Dermatol. Res. 2012, 304, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.G.; Balaguer, F.; Parra, J.L.; Pelejero, C.M. The inhibitory effect of some amphoteric surfactants on the irritation potential of alkylsulphates. Int. J. Cosmet. Sci. 1981, 3, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Faucher, J.A.; Goddard, E.D. Interaction of keratinous substrates with sodium lauryl sulfate. I. Sorption. J. Soc. Cosmet. Chem. 1978, 29, 323–337. [Google Scholar]
- Hall-Manning, T.J.; Holland, G.H.; Rennie, G.; Revell, P.; Hines, J.; Barratt, M.D.; Basketter, D.A. Skin irritation potential of mixed surfactant systems. Food Chem. Toxicol. 1998, 36, 233–238. [Google Scholar] [CrossRef]
- Loffer, H.; Happle, R. Profile of irritant patch testing with detergents: Sodium lauryl sulfate, sodium laureth sulfate and alkyl polyglucoside. Contact Dermat. 2003, 48, 26–32. [Google Scholar] [CrossRef]
- McFadden, J.P.; Holloway, D.B.; Whittle, E.G.; Basketter, D.A. Benzalkonium chloride neutralizes the irritant effect of sodium dodecyl sulfate. Contact Dermat. 2000, 43, 264–266. [Google Scholar] [CrossRef]
- Moore, P.N.; Puvvada, S.; Blankschtein, D. Challenging the surfactant monomer skin penetration model: Penetration of sodium dodecyl sulfate micelles into the epidermis. J. Cosmet. Sci. 2003, 54, 29–46. [Google Scholar] [PubMed]
- Nielsen, G.D.; Nielsen, J.B.; Andersen, K.E.; Grandjean, P. Effects of industrial detergents on the barrier function of human skin. Int. J. Occup. Environ. Health 2000, 6, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Paye, M.; Block, C.; Hamaide, N.; Hüttman, G.E.; Kirkwood, S.; Lally, C.; Lloyd, P.H.; Makela, P.; Razenberg, H.; Young, R. Antagonisms between Surfactants: The Case of Laundry Detergents. Tenside. Surfactants Deterg. 2006, 4, 290. [Google Scholar] [CrossRef]
- Teglia, A.; Secchi, G. Minimizing the cutaneous effect of anionic detergents. Cosmet. Toilet. 1996, 111, 61. [Google Scholar]
- Tadros, T.F. Applied Surfactants: Principles and Applications; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Bujak, T.; Wasilewski, T.; Nizioł-Łukaszewska, Z. Role of macromolecules in the safety of use of body wash cosmetics. Colloids Surf. B Biointerfaces 2015, 135, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Seweryn, A.; Bujak, T. Supercritical carbon dioxide blackcurrant seed extract as an anti-irritant additive for hand dishwashing liquids. Green Chem. Lett. Rev. 2016, 9, 114–121. [Google Scholar] [CrossRef]
- Katsarou, A.; Davoy, E.; Xenos, K.; Armenaka, M.; Theoharides, T.C. Effect of an antioxidant (quercetin) on sodium-lauryl-sulfate-induced skin irritation. Contact Dermat. 2000, 42, 85–89. [Google Scholar] [CrossRef]
- Wang, W.; Sun, F.; An, Y.; Ai, H.; Zhang, L.; Huang, W.; Li, L. Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur. J. Pharmacol. 2009, 613, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Nair, M.G. Inhibition of lipid peroxidation and structure-activity-related studies of the dietary constituents anthocyanins, anthocyanidins, and catechins. J. Argic. Food Chem. 2002, 50, 5308–5312. [Google Scholar] [CrossRef]
- Kyriakopoulos, A.M.; Dinda, B. Cornus mas (Linnaeus) Novel Devised Medicinal Preparations: Bactericidal Effect against Staphylococcus aureus and Pseudomonas aeruginosa. Molecules 2015, 20, 11202–11218. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A. Związki Aktywne Owoców Derenia; Uniwersytet Przyrodniczy we Wrocławiu: Wrocław, Poland, 2012. [Google Scholar]
- Gülçin, I.; Beydemir, S.; Küfrevioğlu, Ö.İ. Evaluation of antioxidant activity of cornelian cherry (Cornus mas L.). Acta Aliment. 2005, 34, 193–202. [Google Scholar] [CrossRef]
- Sineiro, J.; Franco, D.; Rubilar, M.; Sanchez, M.; Jerz, M.; Pinelo, M.; Costoya, N.; Jose Nunez, M. Polyphenols from Plant Materials: Extraction and Antioxidant Power. EJEAFChe 2008, 7, 3210–3216. [Google Scholar]
- Singleton, V.L.; Rossi, J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 21, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Victório, C.P.; Celso Luiz, S.; Lage, R.; Kuster, M. Flavonoid extraction from Alpinia zerumbet (Pers.) Burtt et Smith leaves using different techniques and solvents. Eclética Química 2004, 34, 19–24. [Google Scholar]
- Tural, S.; Koca, I. Physicochemical and antioxidant properties of Cornelian Cherry fruits (Cornus mas L.) grown in Turkey. Sci. Hortic. 2008, 116, 362–366. [Google Scholar] [CrossRef]
- Klimenko, S.W. The Cornelian Cherry (Cornus mas L.): Collection, Preservation, and Utilization of Genetic Resources. J. Fruit Ornam. Plant Res. 2004, 12, 93–98. [Google Scholar]
- Pawlowska, A.M.; Camangi, F.; Braca, A. Quali-quantitative analysis of flavonoids of Cornus mas L. (Cornaceae) fruits. Food Chem. 2010, 119, 1257–1261. [Google Scholar] [CrossRef]
- Vareed, S.K.; Reddy, M.K.; Schutzki, R.E.; Nair, M.G. Anthocyanins in Cornus alternifolia, Cornus controversa, Cornus kousa and Cornus florida fruits with health benefits. Life Sci. 2006, 78, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Medvidovic-Kosanovic, M.; Seruga, M.; Jakobek, L.; Novak, I. Eletrochemical and antioxidant properties of (+)-catechin, quercetin and rutin. Croat. Chem. Acta 2010, 83, 197–207. [Google Scholar]
- Lim, S.H.; Choi, S.H.; Oh, Y.I.; Kim, S.J. Anti-oxidative effects of flavonoids enriched Corni fructus extract and the mechanism. African Journal of Pharmacy and Pharmacology 2011, 5, 506–514. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrostland, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, rubus, and ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Wermerris, W.; Nicholson, R. Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Amerah, A.M. The differences between natural betaine and betaine hydrochloride. Int. Poult. Prod. 2014, 22, 11–13. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. A review on structure-activity relationship of dietary polyphenols inhibiting α-amylase. Crit. Rev. Food. Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Richelle, M.; Sabatier, M.; Steiling, H.; Williamson, G. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br. J. Nutr. 2006, 96, 227–238. [Google Scholar] [PubMed]
- Schiffer, R.; Neis, M.; Höller, D.; Rodríguez, F.; Geier, A.; Gartung, C.; Lammert, F.; Dreuw, A.; Zwadlo-Klarwasser, G.; Merk, H.; et al. Active influx transport is mediated by members of the organic anion transporting polypeptide family in human epidermal keratinocytes. J. Investig. Dermatol. 2003, 120, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Mezrag, A.; Malafronte, N.; Bouheroum, M.; Travaglino, C.; Russo, D.; Milella, L.; Severino, L.; de Tommasi, N.; Braca, A.; Piaz, F.D. Phytochemical and antioxidant activity studies on Ononis angustissima L. aerial parts: isolation of two new flavonoids. Nat. Prod. Res. 2017, 31, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Tomaino, A.; Trombetta, D.; Giacchi, M.; Pasquale, A.; De Bonina, F. Influence of different penetration enhancers on in vitro skin permeation and in vivo photoprotective effect of flavonoids. Int. J. Pharm. 1998, 175, 85–94. [Google Scholar] [CrossRef]
- Norlia, M.; Siti, A.M.; Mashitah, M.Y.; Jolius, G. Influence of Solvent Polarity and Conditions on Extraction of Antioxidant, Flavonoids and Phenolic Content from Averrhoa bilimbi. J. Food Sci. Eng. 2014, 4, 255. [Google Scholar]
- Mehling, A.; Kleber, M.; Hensen, H. Comparative studies on the ocular and dermal irritationpotential of surfactants. Food Chem. Toxicol. 2007, 45, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Sumura, K.; Katsumi, M. Study on skin roughness caused by surfactants: II. Correlation between protein denaturation and skin roughness. J. Am. Oil Chem. Soc. 1975, 52, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Tavss, E.A.; Eigen, E.; Kligman, A.M. Anionic detergent-induced skin irritation and anionic detergent-induced pH rise of bovine serum albumin. J. Soc. Cosmet. Chem. 1998, 39, 267–272. [Google Scholar]
- Huang, J.B.; Mao, M.; Zhu, B.Y. The surface physico-chemical properties of surfactants in ethanol–water mixtures. Colloids Surf. A Physicochem. Eng. Asp. 1999, 155, 339–348. [Google Scholar] [CrossRef]
- Sansanwal, P.K. Effect of co-solutes on the physico-chemical properties of surfactant solutions. J. Sci. Ind. Res. 2006, 65, 57–64. [Google Scholar]
- Nagarajan, R.; Chien-Chung, W. Theory of Surfactant Aggregation in Water/Ethylene Glycol Mixed Solvents. Langmuir 2000, 16, 5242–5251. [Google Scholar] [CrossRef]
- Sidim, T.; Acar, G. Alcohols Effect on Critic Micelle Concentration of Polysorbate 20 and Cetyl Trimethyl Ammonium Bromine Mixed Solutions. J. Surfact. Deterg. 2013, 16, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Zana, R.; Yiv, S.; Strazielle, C.; Lianos, P. Effect of alcohol on the properties of micellar systems: I. Critical micellization concentration, micelle molecular weight and ionization degree, and solubility of alcohols in micellar solutions. J. Colloid Interface Sci. 1981, 80, 208–223. [Google Scholar] [CrossRef]
- Woisky, R.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Matejic, J.S.; Dzamic, A.; Randjelovic, V.; Marin, P. Total phenolic content, flavonoid concentration, antioxidant and antimicrobial activity of methanol extracts from three Seseli L. taxa. Cent. Eur. J. Biol. 2012, 7, 1116–1122. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC INTERNATIONAL, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Protegente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bartosz, G. Reactive oxygen species: Destroyers or messengers? Biochem. Pharmacol. 2009, 77, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
| Ingredient | Ingredient Content (wt %) | ||||
|---|---|---|---|---|---|
| MG(AQ) | MG(WE+D) | MG(BE+D) | MG(GE+D) | MG(PDE+D) | |
| Aqua | To 100 | ||||
| Sodium Lauryl Sulfate | 7.0 | ||||
| Cocamidopropyl Betaine | 2.4 | ||||
| Lauryl Glucoside | 2.0 | ||||
| Citric Acid | 0.2 | ||||
| Sodium Benzoate and Potassium Sorbate | 0.9 | ||||
| Sodium Chloride | 1.0 | ||||
| Cornus Extract (water) WE+D | - | 7.0 | - | - | - |
| Cornus Extract (water-betaine) BE+D | - | - | 7.0 | - | - |
| Cornus Extract (water-glycerine) GE+D | - | - | - | 7.0 | - |
| Cornus Extract (water-propanediol) PPD+D | - | - | - | - | 7.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nizioł-Łukaszewska, Z.; Osika, P.; Wasilewski, T.; Bujak, T. Hydrophilic Dogwood Extracts as Materials for Reducing the Skin Irritation Potential of Body Wash Cosmetics. Molecules 2017, 22, 320. https://doi.org/10.3390/molecules22020320
Nizioł-Łukaszewska Z, Osika P, Wasilewski T, Bujak T. Hydrophilic Dogwood Extracts as Materials for Reducing the Skin Irritation Potential of Body Wash Cosmetics. Molecules. 2017; 22(2):320. https://doi.org/10.3390/molecules22020320
Chicago/Turabian StyleNizioł-Łukaszewska, Zofia, Paweł Osika, Tomasz Wasilewski, and Tomasz Bujak. 2017. "Hydrophilic Dogwood Extracts as Materials for Reducing the Skin Irritation Potential of Body Wash Cosmetics" Molecules 22, no. 2: 320. https://doi.org/10.3390/molecules22020320
APA StyleNizioł-Łukaszewska, Z., Osika, P., Wasilewski, T., & Bujak, T. (2017). Hydrophilic Dogwood Extracts as Materials for Reducing the Skin Irritation Potential of Body Wash Cosmetics. Molecules, 22(2), 320. https://doi.org/10.3390/molecules22020320