Plant Growth Biostimulants, Dietary Feed Supplements and Cosmetics Formulated with Supercritical CO2 Algal Extracts
Abstract
:1. Introduction
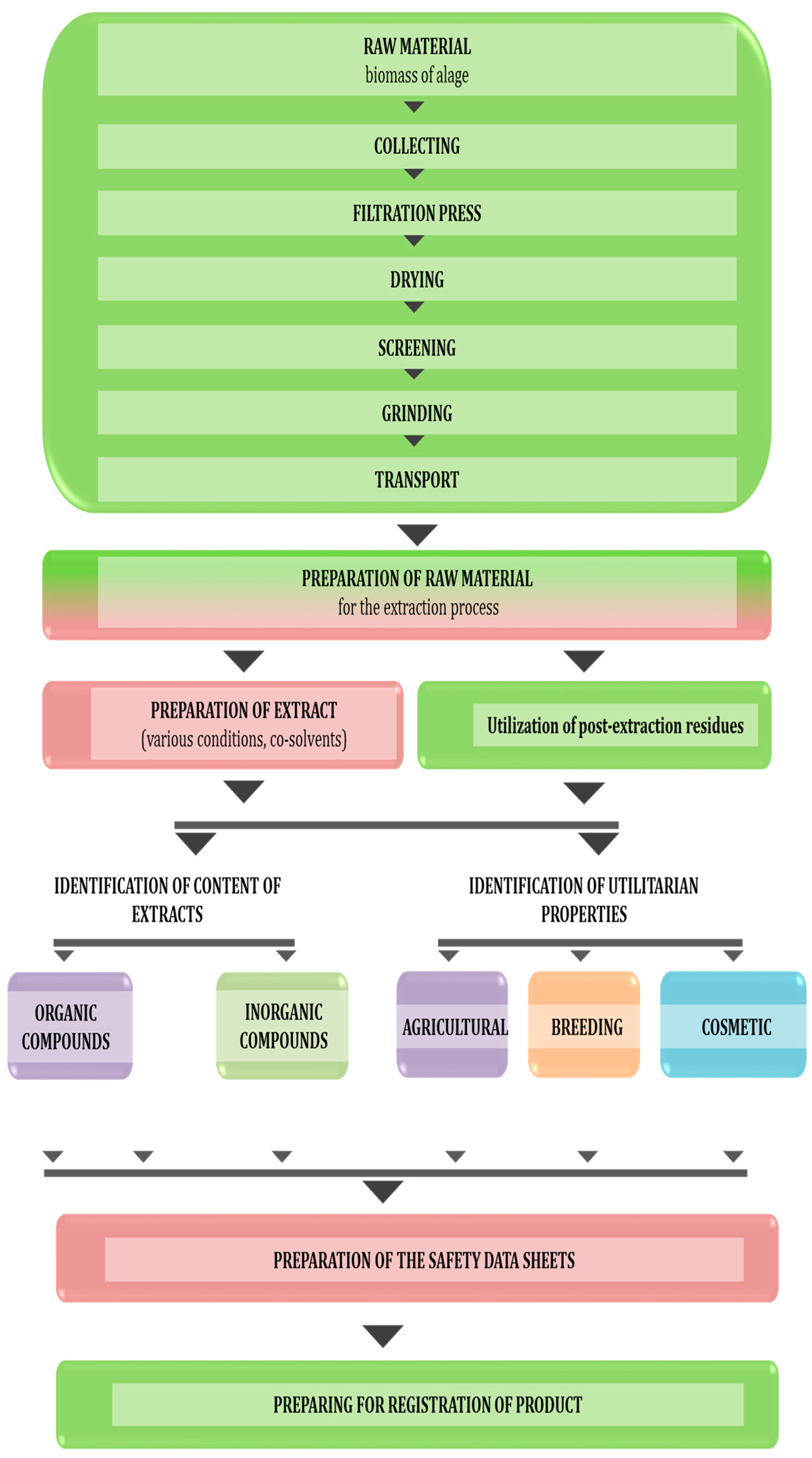
Multiple Use of Algal Extracts—Concept of the Work
2. Sources of Algae for the Extraction
3. Extraction Techniques
Supercritical Fluid Extraction of Baltic and Freshwater Seaweeds and Microalgae
4. Analytical Methods Used for the Identification of Compounds Extracted from Algae
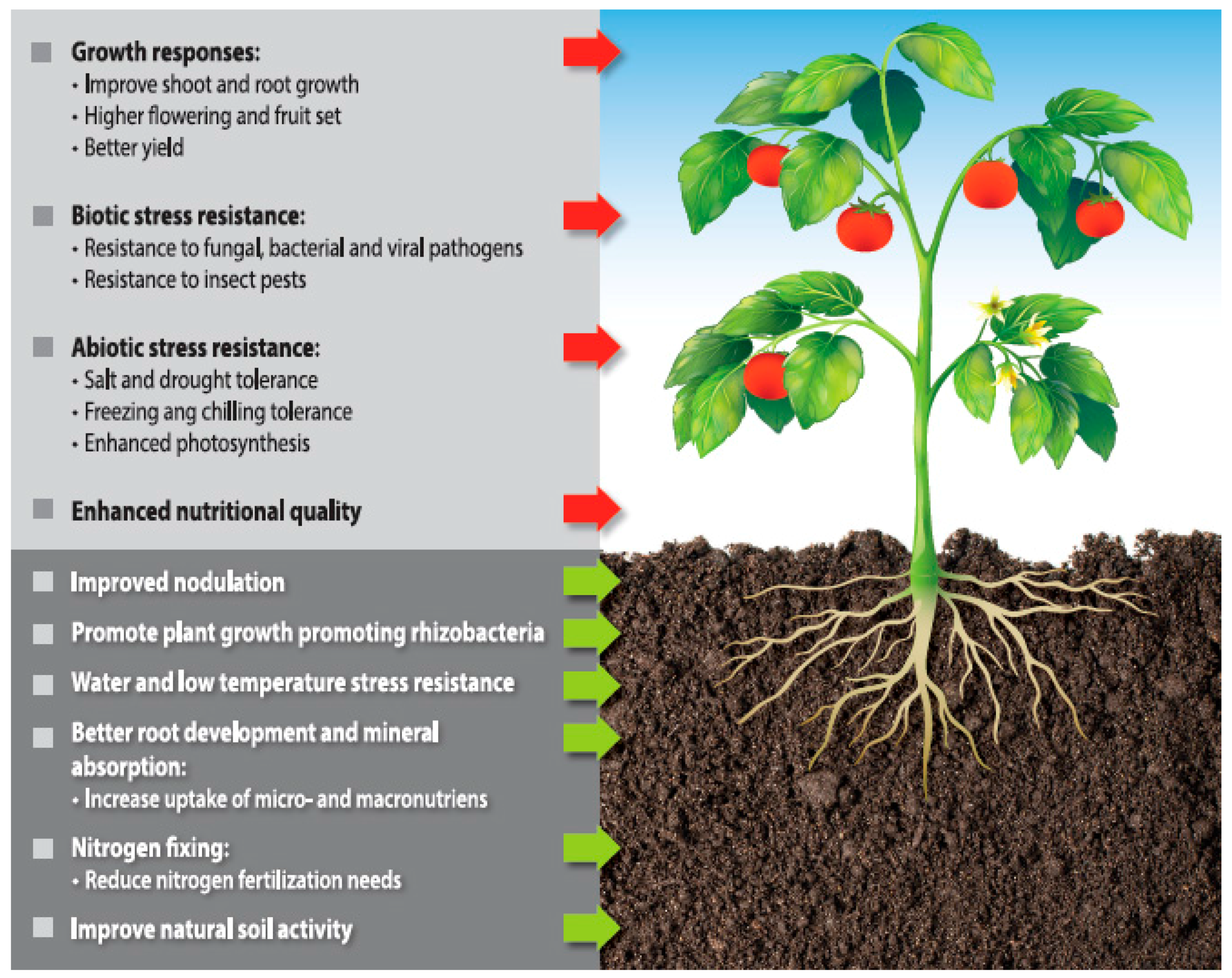
5. Algal Extracts as Plant Growth Biostimulants
6. Algal Extracts as Components of Cosmetics
7. Algal Extracts as Components of Feed Additives
8. Possible Commercialization of Supercritical Algal Extracts
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, S.K. Marine Nutraceuticals: Prospects and Perspectives; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Cannell, R.J.P. Algae as a source of biologically active products. Pestic. Sci. 1993, 39, 147–153. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compound. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Shalaby, E.A. Algae as promising organisms for environment and health. Plant Signal. Behav. 2011, 6, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K. Algal polysaccharides: Properties and applications. Biochem. Anal. Biochem. 2015, 4. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chou, H.N. Screening of red algae filaments as a potential alternative source of eicosapentaenoic acid. Mar. Biotechnol. 2002, 4, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.; Muhammad, A.; Hameed, U. Comparative assessment of Cladophora, Spirogyra and Oedogonium biomass for the production of fatty acid methyl esters. Appl. Biochem. Microbiol. 2014, 50, 69–72. [Google Scholar] [CrossRef]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.; Kim, M.; Son, K.T.; Jeong, Y.; Jeon, Y.J. Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.P.; Montero, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Application of Hansen solubility approach for the subcritical and supercritical selective extraction of phlorotannins from Cystoseira abies-marina. RSC Adv. 2016, 6, 94884–94895. [Google Scholar] [CrossRef]
- Saravana, P.S.; Getachew, A.T.; Cho, Y.J.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Influence of co-solvents on fucoxanthin and phlorotannin recovery from brown seaweed using supercritical CO2. J. Supercrit. Fluids 2017, 120, 295–303. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 86–399. [Google Scholar] [CrossRef]
- Michalak, I.; Górka, B.; Wieczorek, P.P.; Rój, E.; Lipok, J.; Łęska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzyńska-Inger, A.; et al. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur. J. Phycol. 2015, 51, 243–252. [Google Scholar] [CrossRef]
- Wieczorek, P.P.; Lipok, J.; Górka, B. Biologically active compounds isolated from algae and their application as a plant growth regulators. In Proceedings of the IX International Scientific Conference daRostim, Lviv, Ukraine, 7–10 October 2013.
- Wieczorek, P.P.; Lipok, J.; Górka, B. Separation and identification of biologically active compounds from algae and their use in nutrition of plants. Przem. Chem. 2013, 92, 1061–1066. (In Polish) [Google Scholar]
- Górka, B.; Lipok, J.; Wieczorek, P.P. Biologically active organic compounds, especially plant promoters, in algae extracts and their potential application in plant cultivation. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 659–680. [Google Scholar]
- Burketová, L.; Trdá, L.; Ott, P.G.; Valentová, O. Bio-based resistance inducers for sustainable plant protection against pathogens. Biotechnol. Adv. 2015, 33, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Saravana, S.P.; Yin, S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar. Drugs 2015, 13, 3422–3442. [Google Scholar]
- Saravana, P.S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Evaluation of the chemical composition of brown seaweed (Saccharina japonica) hydrolysate by pressurized hot water extraction. Algal Res. 2016, 13, 246–254. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.J.; Park, Y.B.; Woo, H.C.; Chun, B.S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr Polym. 2016, 20, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, G.; Łęska, B.; Messyasz, B.; Pikosz, M.; Fabrowska, J. Extraction of macroalgae biomass for cosmetics industry. Przem. Chem. 2015, 94, 405–407. (In Polish) [Google Scholar]
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibanez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Anaëlle, T.; Serrano, L.E.; Laurent, V.; Elena, I.; Mendiola, J.A.; Stéphane, C.; Nelly, K.; Stéphane, L.B.; Luc, M.; Valérie, S.P. Green improved processes to extract bioactive phenolic compounds from brown macroalgae using Sargassum muticum as model. Talanta 2013, 104, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Production of seaweed extracts by biological and chemical methods. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 121–143. [Google Scholar]
- Chojnacka, K.; Michalak, I.; Dmytryk, A.; Wilk, R.; Górecki, H. Innovative natural plant growth biostimulants. In Advances in Fertilizer Technology; Shishir, S., Pant, K.K., Eds.; Studium Press LLC: Houston, TX, USA, 2014; Volume 21, pp. 452–489. [Google Scholar]
- European Food Safety Authority (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance sea-algae extract. EFSA J. 2012, 10. [Google Scholar] [CrossRef]
- Korczyński, M.; Witkowska, Z.; Opaliński, S.; Świniarska, M.; Dobrzański, Z. Algae extract as a potential feed additive. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 603–626. [Google Scholar]
- Fabrowska, J.; Łęska, B.; Schroeder, G.; Messyasz, B.; Pikosz, M. Biomass and extracts of algae as material for cosmetic. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 681–706. [Google Scholar]
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Hong, J.W.; Kim, O.H.; Kim, H.; Jo, S.W.; Cho, H.W.; Yoon, H.S. Mass cultivation from a Korean raceway pond system of indigenous microalgae as potential biofuel feedstock. Oil Gas Res. 2016, 2. [Google Scholar] [CrossRef]
- Dayananda, C.; Kumudha, A.; Sarada, R.; Ravishankar, G.A. Isolation, characterization and outdoor cultivation of green microalgae Botryococcus sp. Sci. Res. Essays 2010, 5, 2497–2505. [Google Scholar]
- Schroeder, G.; Łęska, B.; Fabrowska, J.; Messyasz, B.; Pikosz, M. Analysis of green algae extracts. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 81–99. [Google Scholar]
- Messyasz, B.; Pikosz, M.; Schroeder, G.; Łęska, B.; Fabrowska, J. Identyfication and ecology of macroalgae species existing in Poland. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 17–39. [Google Scholar]
- Messyasz, B.; Łęska, B.; Fabrowska, J.; Pikosz, M.; Rój, E.; Cieślak, A.; Schroeder, G. Biomass of freshwater Cladophora as a raw material for agriculture and the cosmetic industry. Open Chem. 2015, 13, 1108–1118. [Google Scholar] [CrossRef]
- Dederen, L.H.T. Marine eutrophication in Europe: Similarities and regional differences in appearance. In Proceedings of Marine Coastal Eutrophication, Proceedings of an International Conference, Bologna, Italy, 21–24 March 1990; Vollenweider, R.A., Marchetti, R., Viviani, R., Eds.; Elsevier Science: New York, NY, USA, 1992; pp. 663–672. [Google Scholar]
- Messyasz, B.; Pikosz, M.; Łęska, B.; Pankiewicz, R. The freshwater species of Cladophora (Chlorophyta) from Poland (Central Europe). Eur. J. Phycol. 2015, 50, 132. [Google Scholar]
- Messyasz, B.; Łęska, B.; Pikosz, M.; Fabrowska, J.; Schroeder, G. Characteristic of bioactive compounds from biomass of freshwater Cladophora glomerata. Eur. J. Phycol. 2015, 50, 140–141. [Google Scholar]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bhattacharjee, P. Supercritical carbon dioxide extraction of antioxidant rich fraction from Phormidium valderianum: Optimization of experimental process parameters. Algal Res. 2014, 3, 49–54. [Google Scholar] [CrossRef]
- Li, Y.; Naghdi, F.G.; Garg, S.; Adarme-Vega, T.C.; Thurecht, K.J.; Ghafor, W.A.; Tannock, S.; Schenk, P.M. A comparative study: The impact of different lipid extraction methods on current microalgal lipid research microbial cell factories. Microb. Cell Fact. 2014, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tuhy, Ł.; Witkowska, Z.; Saeid, A.; Chojnacka, K. Use of seaweed extracts for production of fertilizers, feed, food and cosmetics. Przem. Chem. 2012, 91, 1031–1034. (In Polish) [Google Scholar]
- Gil-Chávez, J.G.; Villa, J.A.; Ayala-Zavala, F.J.; Heredia, B.J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Starmans, D.; Nijhuis, H. Extraction of secondary metabolites from plant material: A review. Trends Food Sci. Technol. 1996, 7, 191–197. [Google Scholar] [CrossRef]
- Rój, E.; Dobrzyńska-Inger, A.; Dębczak, A.; Kostrzewa, D.; Stępnik, K. Algae extract production methods and process optimization. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 101–120. [Google Scholar]
- Abbas, K.A.; Mohamed, A.; Abdulamir, A.S.; Abas, H.A. A review on Supercritical Fluid extraction as new analytical method. Am. J. Biochem. Biotechnol. 2008, 4, 345–353. [Google Scholar]
- Chojnacka, K.; Kim, S.K. Introduction of marine algae extracts. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; Volume 1, pp. 1–13. [Google Scholar]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibanez, E.; del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef]
- Mendes, R.L.; Nobre, B.P.; Cardoso, M.T.; Pereira, A.P.; Palavra, A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg. Chim. Acta 2003, 356, 328–334. [Google Scholar] [CrossRef]
- Fujii, K. Process integration of supercritical carbon dioxide extraction and acid treatment for astaxanthin extraction from a vegetative microalga. Food Bioprod. Process. 2012, 90, 762–766. [Google Scholar] [CrossRef]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Supercritical Carbon Dioxide extraction of fucoxanthin from Undaria pinnatifida. J. Agric. Food Chem. 2013, 61, 5792–5797. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.L.; Fernandes, H.L.; Coelho, J.P.; Reis, E.C.; Cabral, J.M.S.; Novais, J.M.; Palavra, A.F. Supercritical CO2 extraction of carotenoids and other lipids from Chlorella vulgaris. Food Chem. 1995, 53, 99–103. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Mantell, C.; Rodríguez, M.; Martínez de la Ossa, E.; Lubián, L.M.; Montero, O. Comparison of supercritical fluid and ultrasound-assisted extraction of carotenoids and chlorophyll a from Dunaliella salina. Talanta 2009, 77, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Roh, M.K.; Uddin, M.S.; Chun, B.S. Extraction of fucoxanthin and polyphenol from Undaria pinnatifida using Supercritical Carbon Dioxide with co-solvent. Biotechnol. Bioprocess Eng. 2008, 13, 724–729. [Google Scholar] [CrossRef]
- Cheung, P.C.K.; Leung, A.Y.H.; Ang, P.O., Jr. Comparison of supercritical carbon dioxide and Soxhlet extraction of lipids from a brown seaweed, Sargassum hemiphyllum (Turn.) C. Ag. J. Agric. Food Chem. 1998, 46, 4228–4232. [Google Scholar] [CrossRef]
- Cheung, P.C.K. Temperature and pressure effects on supercritical carbon dioxide extraction of n-3 fatty acids from red seaweed. Food Chem. 1999, 65, 399–403. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Carone, M.; Colonna, T.; Fragale, C. Production of biodiesel from macroalgae by supercritical CO2 extraction and thermochemical liquefaction. Environ. Chem. Lett. 2005, 3, 136–139. [Google Scholar] [CrossRef]
- Dejoye, C.; Abert Vian, M.; Lumia, G.; Bouscarle, C.; Charton, F.; Chemat, F. Combined extraction processes of lipid from Chlorella vulgaris microalgae: Microwave prior to supercritical carbon dioxide extraction. Int. J. Mol. Sci. 2011, 12, 9332–9341. [Google Scholar] [CrossRef] [PubMed]
- Wilk, R.; Górecki, H.; Chojnacka, K.; Rój, E. Supercritical CO2 extraction of rockweed. A new concept for production of algae extracts. Short communication. Przem. Chem. 2013, 92, 750–752. (In Polish) [Google Scholar]
- Dmytryk, A.; Rój, E.; Wilk, R.; Chojnacka, K. Innovative bioformulations for seed treatment. Preliminary assessment of functional properties in the initial plant growth phase. Przem. Chem. 2014, 93, 959–963. (In Polish) [Google Scholar]
- Dmytryk, A.; Rój, E.; Wilk, R.; Chojnacka, K.; Górecki, H. Effect of new biostimulators on the initial phase of plant growth. Przem. Chem. 2014, 93, 1020–1025. (In Polish) [Google Scholar]
- Wilk, R.; Chojnacka, K. Upstream processing in technology of algal extracts—Biomass harvesting and preparation for extraction process. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 145–159. [Google Scholar]
- Wilk, R.; Chojnacka, K. Downstream processing in technology of algal extracts—From the component to the final formulations. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 161–178. [Google Scholar]
- Saeid, A.; Chojnacka, K. Algae biomass as a raw material for production of algal extracts. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 179–188. [Google Scholar]
- Chojnacka, K.; Wilk, R.; Michalak, I.; Rój, E.; Górecka, H.; Górecki, H. New algal biostimulant: From the extract to formulation. In Proceedings of the 5th International Conference on Engineering for Waste and Biomass Valorisation, Rio de Janeiro, Brazil, 25–28 August 2014; Nzihou, A., Ed.; pp. 189–202.
- Chen, K.T.; Cheng, C.H.; Wu, Y.H.; Lu, W.C.; Lin, Y.H.; Lee, H.T. Continuous lipid extraction of microalgae using high-pressure carbon dioxide. Bioresour. Technol. 2013, 146, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Dmytryk, A.; Wieczorek, P.P.; Rój, E.; Łęska, B.; Górka, B.; Messyasz, B.; Lipok, J.; Mikulewicz, M.; Wilk, R.; et al. Supercritical algal extracts: A source of biologically active compounds from nature. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Górka, B.; Kucab, K.; Lipok, J.; Wieczorek, P.P. Application of HPLC-PDA method for determination of phytohormones in extracts from biomass. In Proceedings of the 2nd World Biostimulants Congress Florence, Italy, 16–19 November 2015.
- Chojnacka, K.; Michalak, I.; Dmytryk, A; Gramza, M.; Słowiński, A.; Górecki, H. Algal extracts as plant growth biostimulators. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 189–211. [Google Scholar]
- Michalak, I.; Chojnacka, K. The potential usefulness of a new generation of agro-products based on raw materials of biological origin. Acta Sci. Pol. Hortic. 2016, 15, 97–120. [Google Scholar]
- Shin, T.; Ahn, M.; Hyun, J.W.; Kim, S.H.; Moon, C. Antioxidant marine algae phlorotannins and radioprotection: A review of experimental evidence. Acta Histochem. 2014, 116, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Tuhy, Ł.; Chojnacka, K.; Michalak, I.; Witek-Krowiak, A. Algal extracts as a carrier of micronutrients—Utilitarian properties of new formulations. In Marine Algae Extracts: Processes, Products, Applications; Kim, S.K., Chojnacka, K., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 467–488. [Google Scholar]
- Sivasankari, S.; Venkatesalu, V.; Anantharaj, M.; Chandrasekaran, M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour. Technol. 2006, 97, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Briceño-Domínguez, D.; Hernández-Carmona, G.; Moyo, M.; Stirk, W.; van Staden, J. Plant growth promoting activity of seaweed liquid extracts produced from Macrocystis pyrifera under different pH and temperature conditions. J. Appl. Phycol. 2014, 26, 2203–2210. [Google Scholar] [CrossRef]
- Chojnacka, K.; Dmytryk, A.; Wilk, R.; Michalak, I.; Rój, E.; Wieczorek, P.P.; Schroeder, G.; Górecki, H.; Górka, B. Bioregulator of Plant Growth and Method of Production. Polish Patent P.412073, 20 April 2015. [Google Scholar]
- Michalak, I.; Chojnacka, K.; Dmytryk, A.; Wilk, R.; Gramza, M.; Rój, E. Evaluation of supercritical extracts of algae as biostimulants of plant growth in field trials. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Dmytryk, A.; Michalak, I.; Wilk, R.; Chojnacka, K.; Górecka, H.; Górecki, H. Innovative seed treatment with algae homogenate. Waste Biomass Valoriz. 2015, 6, 441–448. [Google Scholar] [CrossRef]
- Świniarska, M.; Opaliński, S.; Korczyński, M.; Wilk, R.; Rój, E.; Kalus, K.; Chojnacka, K. Toxicity of the Spirulina sp. extract-containing plant biostimulant. Przem. Chem. 2015, 94, 1793–1797. (In Polish) [Google Scholar]
- Kraan, S. Algal polysaccharides, novel applications and outlook. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; Chang, C.F., Ed.; InTech: New York, NY, USA, 2012; pp. 489–532. [Google Scholar]
- Agatonovic-Kustrin, S.; Morton, D.W. Cosmeceuticals derived from bioactive substances found in marine algae. Oceanography 2013, 1. [Google Scholar] [CrossRef]
- Schroeder, G.; Messyasz, B.; Łęska, B.; Fabrowska, J.; Pikosz, M.; Rybak, A. Biomass of freshwater algae as raw material for the industry and agriculture. Przem. Chem. 2013, 92, 1380–1384. (In Polish) [Google Scholar]
- Shields, R.J.; Lupatsch, I. Algae for aquaculture and animal feeds. Theor. Prax. 2012, 1, 23–37. [Google Scholar]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Molina Grima, E.; Belarbi, E.H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]

| Extraction | Algae | Temperature (°C) | Pressure (bar) | Extraction Time (min) | Extracted Compound | Reference |
|---|---|---|---|---|---|---|
| PIGMENTS | ||||||
| SFE with CO2 | Undaria pinnatifida (MA) | 25–60 (40 *) | 200–400 * | 180 | fucoxanthin | [58] |
| SFE with CO2 and with EtOH | Saccharina japonica, Sargassum horneri (MA) | 45 | 250 | 120 | fucoxanthin | [22] |
| SFE with CO2 and with sunflower oil/soybean oil/canola oil/ethanol/water | Saccharina japonica (MA) | 45–55 | 200–300 | 120 | fucoxanthin | [14] |
| SFE with CO2 | Dunaliella salina (MI) | 40, 60 | 200 | n.a. | β-carotene | [56] |
| SFE with CO2 | Chlorella vulgaris (MI) | 40 *, 55 | 150, 200, 275, 350 * | n.a. | total carotenoids, astaxanthin, canthaxanthin | [59] |
| SFE with CO2 | Chlorella vulgaris (MI) | 40, 55 | 100–350 (275 and 350 *) | n.a. | carotenoids (astaxanthin, canthaxanthin) | [56] |
| SFE with CO2 | Dunaliella salina (MI) | 40, 50, 60 * | 100, 200, 300 *, 400, 500 | 180 | carotenoids, chlorophylls | [60] |
| SFE with CO2 SFE with CO2 and with EtOH | Haematococcus pluvialis (MI) | 30 *, 45, 60 | 200 | 15 *, 30, 60 | chlorophyll, astaxanthin | [57] |
| SFE with CO2 and with EtOH | Undaria pinnatifida (MA) | 30, 40, 50 *, 60 | 80, 100, 150, 200 *, 250, 300 | 50 | fucoxanthin | [61] |
| POLYPHENOLS | ||||||
| SFE with CO2 | Polysiphonia, Ulva, Cladophora (MA) | 40 | 500 | 320 *, 360, 810 | polyphenols | [17] |
| SFE with CO2 (modified with 12% EtOH) | Sargassum muticum (MA) | 60 | 152 | 90 | polyphenols | [28] |
| SFE with CO2 and with EtOH | Undaria pinnatifida (MA) | 30, 40, 50, 60 * | 80, 100, 150, 200, 250 *, 300 | 50 | polyphenols | [61] |
| LIPIDS | ||||||
| SFE with CO2 | Sargassum hemiphyllum (MA) | 40 *, 50 * | 241–379 * | 60 | fatty acid profile of lipids (n-3: C18:3, C18:4, C20:5, C22:5, C22:6) | [62] |
| SFE with CO2 | Hypnea charoides (MA) | 40–50 * | 241–379 * | 120 | fatty acid profile of lipids (n-3: C18:3, C18:4, C20:4, C20:5, C22:5, C22:6) | [63] |
| SFE with CO2 | Chaetomorpha linum (MA) | 50 | 260 | 420 | oil | [64] |
| SFE with CO2 | Bangia atropurpurea, Porphyra angusta, P. dentate (Pyropia dentate), Helminthocladia australis, Liagora orientalis (Izziella orientalis), Liagora boergesenii, Scinaia monoliformis, Tricleocarpa cylindrica (Galaxaura cylindrical), Grateloupia filicina, Halymenia microcarpa (H. ceylanica) (MA) | 55 | 34.5 | 180 | fatty acid (e.g., eicosapentaenoic acid, EPA, C20:5, n-3) | [9] |
| SFE with CO2 | Chlorella vulgaris (MI) | 40, 55 | 150, 200, 275, 350 | n.a. | lipids | [59] |
| SFE with CO2 | Cladophora glomerata (MA) | 45 | 300, 500, 700 | n.a. | polyunsaturated, saturated fatty acid | [41] |
| SFE with CO2 | Chlorella vulgaris (MI) | 40 *–70 | 200–280 * | 540 | lipids (saturated, mono- and polyunsaturated fatty acids) | [65] |
| SFE with CO2 | Tetraselmis sp. (MI) | 40 | 150 | 720 | lipids (saturated, mono- and polyunsaturated fatty acids) | [47] |
| SFE with CO2 SFE with CO2 and with EtOH (10%) | Arthrospira maxima (Spirulina maxima) (MI) | (1) 50 (2) 50 *, 60 | (1) 250 (2) 250 * | n.a. | fatty acids: γ-linolenic acid (GLA, C18:3, n-6) | [56] |
| PLANT GROWTH PROMOTING SUBSTANCES | ||||||
| SFE with CO2 | Polysiphonia, Ulva, Cladophora (MA) | 40 | 500 | 320 *, 360, 810 | auxins, cytokinins | [17] |
| MICRO- AND MACROELEMENTS | ||||||
| SFE with CO2 | Polysiphonia, Ulva, Cladophora (MA) | 40 | 500 | 320 *, 360, 810 | micro- and macroelements | [17] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, I.; Chojnacka, K.; Saeid, A. Plant Growth Biostimulants, Dietary Feed Supplements and Cosmetics Formulated with Supercritical CO2 Algal Extracts. Molecules 2017, 22, 66. https://doi.org/10.3390/molecules22010066
Michalak I, Chojnacka K, Saeid A. Plant Growth Biostimulants, Dietary Feed Supplements and Cosmetics Formulated with Supercritical CO2 Algal Extracts. Molecules. 2017; 22(1):66. https://doi.org/10.3390/molecules22010066
Chicago/Turabian StyleMichalak, Izabela, Katarzyna Chojnacka, and Agnieszka Saeid. 2017. "Plant Growth Biostimulants, Dietary Feed Supplements and Cosmetics Formulated with Supercritical CO2 Algal Extracts" Molecules 22, no. 1: 66. https://doi.org/10.3390/molecules22010066
APA StyleMichalak, I., Chojnacka, K., & Saeid, A. (2017). Plant Growth Biostimulants, Dietary Feed Supplements and Cosmetics Formulated with Supercritical CO2 Algal Extracts. Molecules, 22(1), 66. https://doi.org/10.3390/molecules22010066








