Influence of Indole-3-Acetic Acid and Gibberellic Acid on Phenylpropanoid Accumulation in Common Buckwheat (Fagopyrum esculentum Moench) Sprouts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Growth Patterns of Buckwheat Sprouts after Treatment with IAA and GA
2.2. LC-MS and HPLC Analysis of Common Buckwheat Sprouts after Treatment with IAA and GA
3. Materials and Methods
3.1. Plant Materials
3.2. Chemical and Standards
3.3. Extraction and Analysis of Flavonoids
3.4. HPLC-MS Analysis of Flavonoids
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeon, Y.-J.; Kang, E.-S.; Hong, K.-W. A PCR method for rapid detection of buckwheat ingredients in food. J. Korean Soc. Appl. Biol. Chem. 2007, 50, 276–280. [Google Scholar]
- Golisz, A.; Lata, B.; Gawronski, S.W.; Fujii, Y. Specific and total activities of the allelochemicals identified in buckwheat. Weed Biol. Manag. 2007, 7, 164–171. [Google Scholar] [CrossRef]
- Kim, D.W.; Hwang, I.K.; Lim, S.S.; Yoo, K.Y.; Li, H.; Kim, Y.S.; Kwon, D.Y.; Moon, W.K.; Kim, D.W.; Won, M.H. Germinated buckwheat extract decreases blood pressure and nitrotyrosine immunoreactivity in aortic endothelial cells in spontaneously hypertensive rats. Phytother. Res. 2009, 23, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.D.; Lee, W.-K.; No, K.-O.; Park, S.-K.; Lee, M.-H.; Lim, S.R.; Roh, S.-S. Anti-allergic action of buckwheat (Fagopyrum esculentum Moench) grain extract. Int. Immunopharmacol. 2003, 3, 129–136. [Google Scholar] [CrossRef]
- Amarowicz, R.; Dykes, G.A.; Pegg, R.B. Antibacterial activity of tannin constituents from Phaseolus vulgaris, Fagoypyrum esculentum, Corylus avellana and Juglans nigra. Fitoterapia 2008, 79, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.-C.; Sun, J.-T. Changes in phenolic content, phenylalanine ammonia-lyase (PAL) activity, and antioxidant capacity of two buckwheat sprouts in relation to germination. J. Funct. Foods 2014, 7, 298–304. [Google Scholar] [CrossRef]
- Danihelová, M.; Jantová, S.; Sturdík, E. Cytotoxic and antioxidant activity of buckwheat hull extracts. J. Microbiol. Biotechnol. Food. Sci. 2013, 2, 1314–1323. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Zaidul, I.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Burczynski, F.; Campbell, C.; Pierce, G.; Austria, J.; Briggs, C. Rutin and flavonoid contents in three buckwheat species Fagopyrum esculentum, F. tataricum, and F. homotropicum and their protective effects against lipid peroxidation. Food Res. Int. 2007, 40, 356–364. [Google Scholar] [CrossRef]
- Kalinova, J.; Triska, J.; Vrchotova, N. Distribution of vitamin E, squalene, epicatechin, and rutin in common buckwheat plants (Fagopyrum esculentum Moench). J. Agric. Food Chem. 2006, 54, 5330–5335. [Google Scholar] [CrossRef] [PubMed]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Lee, L.-S.; Choi, E.-J.; Kim, C.-H.; Sung, J.-M.; Kim, Y.-B.; Seo, D.-H.; Choi, H.-W.; Choi, Y.-S.; Kum, J.-S.; Park, J.-D. Contribution of flavonoids to the antioxidant properties of common and tartary buckwheat. J. Cereal Sci. 2016, 68, 181–186. [Google Scholar] [CrossRef]
- Watanabe, M.; Ayugase, J. Effects of buckwheat sprouts on plasma and hepatic parameters in type 2 diabetic db/db mice. J. Food Sci. 2010, 75, H294–H299. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Shen, S.-R.; Lai, Y.-J.; Wu, S.-C. Rutin and quercetin, bioactive compounds from tartary buckwheat, prevent liver inflammatory injury. Food. Funct. 2013, 4, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Gulpinar, A.R.; Orhan, I.E.; Kan, A.; Senol, F.S.; Celik, S.A.; Kartal, M. Estimation of in vitro neuroprotective properties and quantification of rutin and fatty acids in buckwheat (Fagopyrum esculentum Moench) cultivated in Turkey. Food Res. Int. 2012, 46, 536–543. [Google Scholar] [CrossRef]
- Kim, S.-H.; Cui, C.-B.; Kang, I.-J.; Kim, S.Y.; Ham, S.-S. Cytotoxic effect of buckwheat (Fagopyrum esculentum Moench) hull against cancer cells. J. Med. Food 2007, 10, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-Y.; Chu, J.-X.; Li, G.-M.; Zhu, L.-S.; Shi, R.-F. Effects of rutin from leaves and flowers of buckwheat (Fagopyrum esculentum Moench.) on angiotensin II-induced hypertrophy of cardiac myocytes and proliferation of fibroblasts. Lat. Am. J. Pharm. 2010, 29, 137–140. [Google Scholar]
- Davies, P.J. The plant hormones: Their nature, occurrence, and functions. In Plant Hormones: Biosynthesis, Signal Transduction, Action, 3rd ed.; Davies, P.J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 1–15. [Google Scholar]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell. Dev. Biol. Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Crozier, A.; Kamiya, Y.; Bishop, G.; Yokota, T. Biosynthesis of Hormones and Elicitors Molecules. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; Volume 40. [Google Scholar]
- Vanisree, M.; Lee, C.-Y.; Lo, S.-F.; Nalawade, S.M.; Lin, C.Y.; Tsay, H.-S. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot. Bull. Acad. Sin. 2004, 45, 1–22. [Google Scholar]
- Cho, G.; Kim, D.; Pedersen, H.; Chin, C.K. Ethephon enhancement of secondary metabolite synthesis in plant cell cultures. Biotechnol. Prog. 1988, 4, 184–188. [Google Scholar] [CrossRef]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar]
- Rao, S.R.; Ravishankar, G. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [PubMed]
- Yeoman, M.; Yeoman, C. Manipulating secondary metabolism in cultured plant cells. New Phytol. 1996, 134, 553–569. [Google Scholar] [CrossRef]
- Hou, S.; Sun, Z.; Linghu, B.; Wang, Y.; Huang, K.; Xu, D.; Han, Y. Regeneration of buckwheat plantlets from hypocotyl and the influence of exogenous hormones on rutin content and rutin biosynthetic gene expression in vitro. Plant Cell Tissue Organ Cult. 2015, 120, 1159–1167. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Kim, S.-H.; Chung, I.-M. Exogenous phytohormones increase the accumulation of health-promoting metabolites, and influence the expression patterns of biosynthesis related genes and biological activity in Chinese cabbage (Brassica rapa spp. pekinensis). Sci. Hortic. 2015, 193, 136–146. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Gray, D.J.; Lu, J.; Gu, L. Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chem. 2011, 126, 982–988. [Google Scholar] [CrossRef]
- Yahia, A.; Kevers, C.; Gaspar, T.; Chénieux, J.-C.; Rideau, M.; Crèche, J. Cytokinins and ethylene stimulate indole alkaloid accumulation in cell suspension cultures of Catharanthus roseus by two distinct mechanisms. Plant Sci. 1998, 133, 9–15. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, W.-Y.; Wang, J.; Li, X.-L. Effects of sucrose concentration and exogenous hormones on growth and periplocin accumulation in adventitious roots of Periploca sepium Bunge. Acta Physiol. Plant. 2012, 34, 1345–1351. [Google Scholar] [CrossRef]
- Park, C.H.; AyeThwe, A.; Kim, S.J.; Park, J.S.; Arasu, M.; Al-Dhabi, N.A.; Il Park, N.; Park, S.U. Effect of Auxins on Anthocyanin Accumulation in Hairy Root Cultures of Tartary Buckwheat Cultivar Hokkai T10. Nat. Prod. Commun. 2016, 11, 1283–1286. [Google Scholar]
- Komaikul, J.; Kitisripanya, T.; Tanaka, H.; Sritularak, B.; Putalun, W. Enhanced Mulberroside A Production from Cell Suspension and Root Cultures of Morus alba Using Elicitation. Nat. Prod. Commun. 2015, 10, 1253–1256. [Google Scholar] [PubMed]
- Pitta-Alvarez, S.I.; Spollansky, T.C.; Giulietti, A.M. The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzym. Microb. Technol. 2000, 26, 252–258. [Google Scholar] [CrossRef]
- Uddin, M.R.; Thwe, A.A.; Kim, Y.B.; Park, W.T.; Chae, S.C.; Park, S.U. Effects of jasmonates on sorgoleone accumulation and expression of genes for sorgoleone biosynthesis in sorghum roots. J. Chem. Ecol. 2013, 39, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Seo, J.M.; Lee, M.-K.; Chun, J.-H.; Antonisamy, P.; Arasu, M.V.; Suzuki, T.; Al-Dhabi, N.A.; Kim, S.-J. Influence of different LED lamps on the production of phenolic compounds in common and Tartary buckwheat sprouts. Ind. Crops Prod. 2014, 54, 320–326. [Google Scholar] [CrossRef]
- Seo, J.-M.; Arasu, M.V.; Kim, Y.-B.; Park, S.U.; Kim, S.-J. Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chem. 2015, 177, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.-G.; Lee, S.M.; Park, J.-H.; Kim, D.-O.; Baek, N.-I.; Eom, S.H. Flavonoid analysis of buckwheat sprouts. Food Chem. 2015, 170, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [PubMed]
- Sun, T.P.; Gubler, F. Molecular mechanism of gibberellin signaling in plants. Annu. Rev. Plant Biol. 2004, 55, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Phillips, A.L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Huang, J.; Ding, J. Interaction between exogenous brassinolide, IAA and BAP in secondary metabolism of cultured Onosma paniculatum cells. Plant Growth Regul. 2003, 39, 253–261. [Google Scholar] [CrossRef]
- Cui, X.-H.; Chakrabarty, D.; Lee, E.-J.; Paek, K.-Y. Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresour. Technol. 2010, 101, 4708–4716. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-T.; Woo, J.-C.; Park, D.-H. Effect of plant growth regulators on growth and biosynthesis of phenolic compounds in genetically transformed hairy roots of Panax ginseng CA Meyer. Biotechnol. Bioprocess. Eng. 2007, 12, 86–91. [Google Scholar] [CrossRef]
- Farkya, S.; Bisaria, V.S. Exogenous hormones affecting morphology and biosynthetic potential of hairy root line (LYR2i) of Linum album. J. Biosci. Bioeng. 2008, 105, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.; Sudha, G.; George, J.; Ravishankar, G. Influence of exogenous hormones on growth and secondary metabolite production in hairy root cultures of Cichorium intybus L. cv. Lucknow local. In Vitro Cell. Dev. Biol. Plant 2001, 37, 293–299. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Stiles, A.R.; Saxena, P.K.; Liu, C.-Z. Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl. Biochem. Biotechnol. 2012, 168, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Weathers, P.; Bunk, G.; McCoy, M. The effect of phytohormones on growth and artemisinin production in Artemisia annua hairy roots. In Vitro Cell. Dev. Biol. Plant 2005, 41, 47–53. [Google Scholar] [CrossRef]
- Li, X.; Kim, J.K.; Park, S.-Y.; Zhao, S.; Kim, Y.B.; Lee, S.; Park, S.U. Comparative analysis of flavonoids and polar metabolite profiling of tanno-original and tanno-high rutin buckwheat. J. Agric. Food Chem. 2014, 62, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
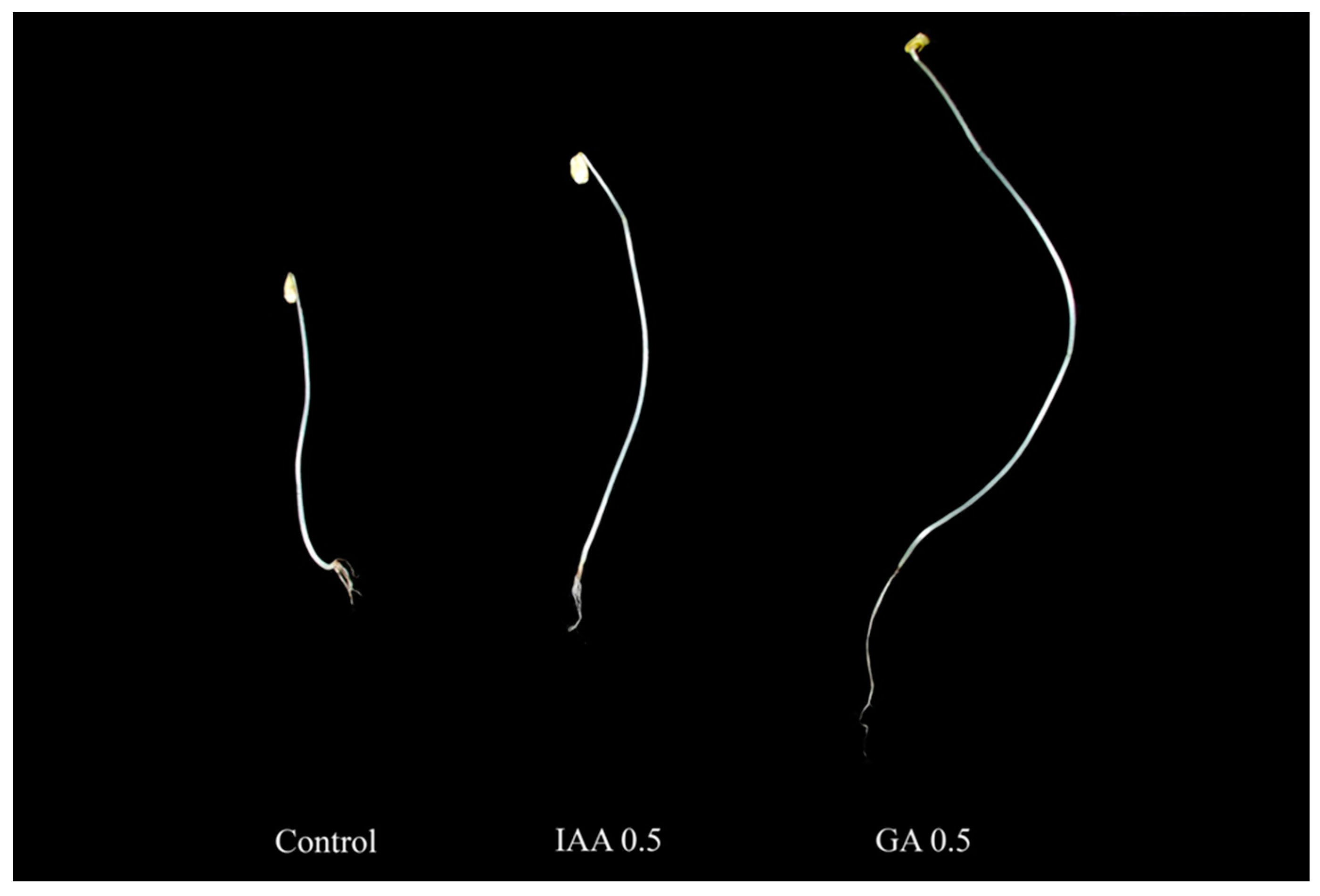
| Hormones | Concentration (mg/L) | Shoot Length (cm) | Root Length (cm) | Fresh Weight (g) |
|---|---|---|---|---|
| Control | 0 | 6.77 ± 0.29c 1 | 1.67 ± 0.09d | 0.09 ± 0.01de |
| IAA | 0.10 | 11.63 ± 0.50a | 2.50 ± 0.17c | 0.17 ± 0.01ab |
| 0.50 | 12.10 ± 0.71a | 5.63 ± 0.21a | 0.21 ± 0.04a | |
| 1.00 | 10.07 ± 0.35b | 3.67 ± 0.14b | 0.14 ± 0.04bc | |
| 3.00 | 5.83 ± 0.10d | 1.90 ± 0.11cd | 0.11 ± 0.01cd | |
| 5.00 | 2.03 ± 0.26e | 1.20 ± 0.06d | 0.06 ± 0.01e | |
| Control | 0 | 6.77 ± 0.29c | 1.67 ± 0.09bc | 0.09 ± 0.01b |
| GA | 0.10 | 8.30 ± 0.40b | 2.93 ± 0.13a | 0.13 ± 0.02b |
| 0.50 | 12.90 ± 0.36a | 3.30 ± 0.20a | 0.20 ± 0.05a | |
| 1.00 | 8.07 ± 0.64b | 2.23 ± 0.13b | 0.13 ± 0.00b | |
| 3.00 | 5.27 ± 0.21d | 1.07 ± 0.09cd | 0.09 ± 0.01b | |
| 5.00 | 1.57 ± 0.15e | 0.63 ± 0.04d | 0.04 ± 0.01c |
| No | Name | Molecular Formula | Molecular Weight | tR (min) | [M − H]− | [M − Na]− |
|---|---|---|---|---|---|---|
| 1 | Gallic acid | C7H6O5 | 170.12 | 41.80 | 169.5 | |
| 2 | 4-hydroxybenzoic acid | C7H6O3 | 138.12 | 20.10 | 137.3 | |
| 3 | Catechin | C15H14O6 | 290.27 | 20.30 | 289.2 | |
| 4 | Chlorogenic acid | C16H18O9 | 354.31 | 32.60 | 353.0 | |
| 5 | 4-hydroxy-3-methoxybenzoic acid | C8H8O4 | 168.15 | 49.60 | 167.9 | |
| 6 | Caffeic acid | C9H8O4 | 180.16 | 36.50 | 179.7 | |
| 7 | Epi-catechin | C15H14O5 | 290.27 | 42.20 | 289.5 | |
| 8 | P-coumaric acid | C9H8O3 | 164.16 | 84.90 | 163.8 | |
| 9 | Ferulic acid | C10H10O4 | 194.18 | 28.50 | 193.5 | |
| 10 | Rutin | C27H30O16 | 610.52 | 66.23 | 609.8 | |
| 11 | Quercetin | C15H10O7 | 302.24 | 79.71 | 301.9 | |
| 12 | Kaempferol | C15H10O6 | 286.23 | 81.55 | 285.3 | |
| 13 | Quercetin-3-O-neohesperidoside | C27H30O16 | 610.52 | 67.11 | 609.9 | |
| 14 | Apigenin glucoside | C21H20O10 | 432.38 | 63.68 | 431.6 | |
| 15 | Syringic acid | C9H10O5 | 198.17 | 59.52 | 175.6 | |
| 16 | Caffeic acid hexose | C15H17O9 | 341.08 | 63.50 | 341.8 | |
| 17 | Procyanidin B2 | C30H25O12 | 578.13 | 59.70 | 577.7 |
| Natural Plant Hormones | Concentrations (mg/L) | Phenolic Compounds (μg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 4-hydroxybenzoic acid | Catechin | Chlorogenic Acid | Caffeic Acid | Epicatechin | Rutin | Quercetin | Total | ||
| Control | 0 | 7.02 ± 0.72a 1 | 91.77 ± 7.45b | 123.28 ± 9.98a | 16.19 ± 3.47b | 455.38 ± 50.05a | 704.22 ± 21.61c | 52.11 ± 4.74a | 1449.97 ± 11.19b |
| IAA | 0.1 | 1.79 ± 0.11d | 90.90 ± 11.46b | 112.86 ± 15.44ab | 18.06 ± 1.95b | 356.76 ± 0.27b | 977.1 ± 31.68a | 23.02 ± 1.23c | 1580.49 ± 14.96a |
| 0.5 | 0.44 ± 0.27e | 113.35 ± 5.26a | 100.13 ± 3.78b | 26.34 ± 0.21a | 327.90 ± 28.95bc | 852.46 ± 11.88b | 20.23 ± 3.67c | 1440.85 ± 10.93b | |
| 1.0 | 3.98 ± 0.02c | 113.01 ± 9.51a | 101.79 ± 3.93b | 24.03 ± 4.87a | 307.31 ± 7.52bcd | 982.15 ± 62.65a | 45.85 ± 1.70b | 1578.12 ± 10.93a | |
| 3.0 | 1.31 ± 0.22d | 84.88 ± 13.16b | 97.59± 9.78b | 14.48 ± 3.53bc | 290.73 ± 34.52cd | 495.58 ± 12.06d | 18.12 ± 3.02c | 1002.69 ± 55.49c | |
| 5.0 | 5.78 ± 0.74b | 89.08 ± 3.75b | 74.33 ± 4.63c | 9.04 ± 3.84c | 261.52 ± 35.80d | 414.54 ± 9.40e | 7.46 ± 2.10d | 861.74 ± 47.43d | |
| Control | 0 | 7.02 ± 0.72a | 91.77 ± 7.45c | 123.28 ± 9.98a | 16.19 ± 3.47b | 455.38 ± 50.05a | 704.22 ± 21.61c | 52.11 ± 4.74b | 1449.97 ± 11.19a |
| GA | 0.1 | 1.28 ± 0.38b | 115.93 ± 5.69a | 111.85 ± 6.75a | 27.77 ± 1.07a | 248.27 ± 34.82b | 866.13 ± 37.41a | 62.27 ± 10.49a | 1433.51 ±19.21a |
| 0.5 | 0.00 ± 0.00c | 103.96 ± 4.71b | 114.07 ± 8.30a | 27.80 ± 3.02a | 251.46 ± 0.75b | 756.98 ± 40.04b | 70.70 ± 5.12a | 1324.97 ± 10.93b | |
| 1.0 | 1.11 ± 0.33b | 103.43 ± 1.94b | 97.39 ± 8.88b | 24.11 ± 3.53a | 266.19 ± 8.06b | 644.23 ± 12.04d | 31.07 ± 2.19c | 1167.53 ± 10.93c | |
| 3.0 | 0.46 ± 0.80bc | 76.96 ± 6.72d | 62.60 ± 5.58c | 15.06 ± 5.02b | 234.31 ± 21.80b | 311.49 ± 5.88e | 15.26 ± 1.32d | 716.14 ± 25.48d | |
| 5.0 | 0.13 ± 0.23c | 96.63 ± 3.27bc | 54.13 ± 2.60c | 12.43 ± 2.59b | 268.42 ± 12.23b | 289.61 ± 6.36e | 10.59 ± 0.60d | 731.94 ± 12.68d | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.H.; Yeo, H.J.; Park, Y.J.; Morgan, A.M.A.; Valan Arasu, M.; Al-Dhabi, N.A.; Park, S.U. Influence of Indole-3-Acetic Acid and Gibberellic Acid on Phenylpropanoid Accumulation in Common Buckwheat (Fagopyrum esculentum Moench) Sprouts. Molecules 2017, 22, 374. https://doi.org/10.3390/molecules22030374
Park CH, Yeo HJ, Park YJ, Morgan AMA, Valan Arasu M, Al-Dhabi NA, Park SU. Influence of Indole-3-Acetic Acid and Gibberellic Acid on Phenylpropanoid Accumulation in Common Buckwheat (Fagopyrum esculentum Moench) Sprouts. Molecules. 2017; 22(3):374. https://doi.org/10.3390/molecules22030374
Chicago/Turabian StylePark, Chang Ha, Hyeon Ji Yeo, Yun Ji Park, Abubaker M. A. Morgan, Mariadhas Valan Arasu, Naif Abdullah Al-Dhabi, and Sang Un Park. 2017. "Influence of Indole-3-Acetic Acid and Gibberellic Acid on Phenylpropanoid Accumulation in Common Buckwheat (Fagopyrum esculentum Moench) Sprouts" Molecules 22, no. 3: 374. https://doi.org/10.3390/molecules22030374
APA StylePark, C. H., Yeo, H. J., Park, Y. J., Morgan, A. M. A., Valan Arasu, M., Al-Dhabi, N. A., & Park, S. U. (2017). Influence of Indole-3-Acetic Acid and Gibberellic Acid on Phenylpropanoid Accumulation in Common Buckwheat (Fagopyrum esculentum Moench) Sprouts. Molecules, 22(3), 374. https://doi.org/10.3390/molecules22030374






