Identification of Larvicidal Constituents of the Essential Oil of Echinops grijsii Roots against the Three Species of Mosquitoes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oil Chemical Composition
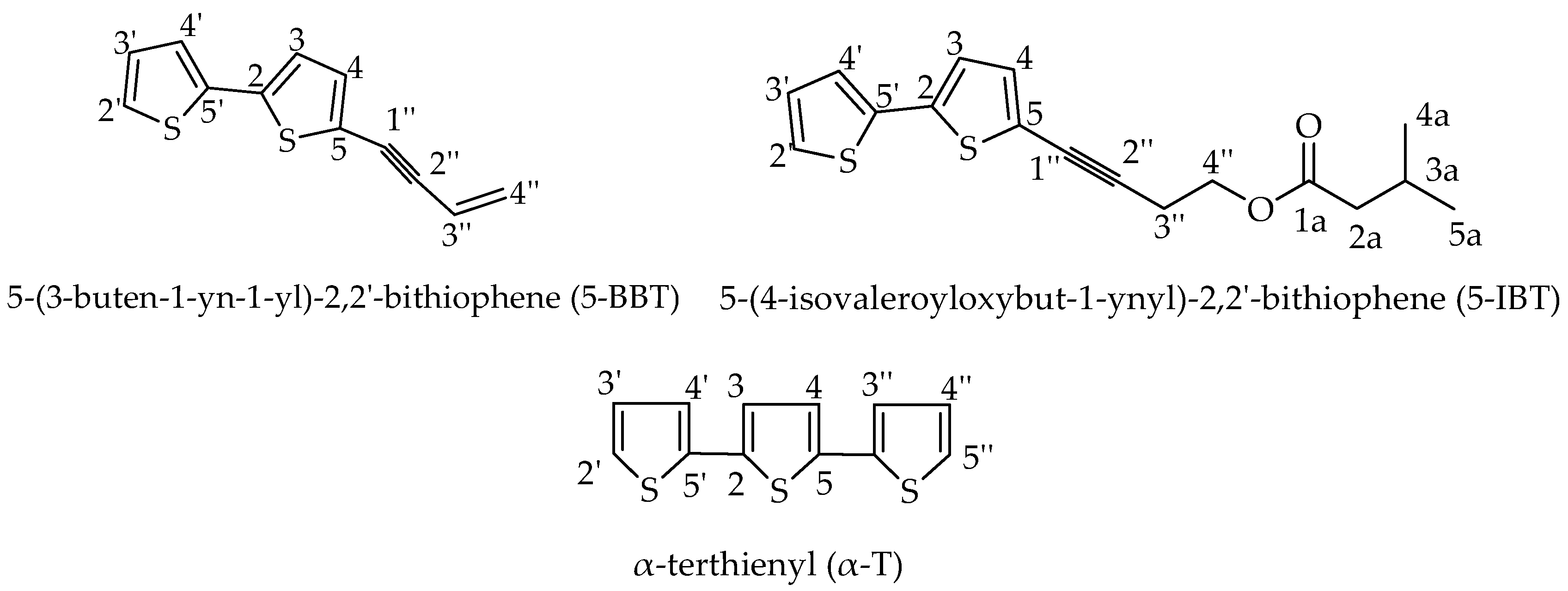
2.2. Isolated Bioactive Compounds
2.3. Larvicidial Activity
3. Experimental
3.1. General
3.2. Plant Material and Essential Oil
3.3. Gas Chromatography-Mass Spectrometry
3.4. Bioassay-Directed Fractionation
3.5. Insects
3.6. Larvicidal Activity Bioassays
3.7. Isolated Constituent Compounds
3.8. Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cui, F.; Raymond, M.; Qiao, C.L. Insecticide resistance in vector mosquitoes in China. Pest Manag. Sci. 2006, 62, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhong, D.; Fang, Q.; Hartsel, J.; Zhou, G.; Shi, L.; Fang, F.; Zhu, C.; Yan, G.; Mutuku, F. Multiple resistances and complex mechanisms of Anopheles sinensis mosquito: A major obstacle to mosquito-borne diseases control and elimination in China. PLoS Negl. Trop. Dis. 2014, 8, e2889. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Liu, Q.Z.; Du, S.S.; Deng, Z.W. Mosquito larvicidal activity of alkaloids and limonoids derived from Evodia rutaecarpa unripe fruits against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2012, 111, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; He, Q.; Chu, S.S.; Wang, C.F.; Du, S.S.; Deng, Z.W. Essential oil composition and larvicidal activity of Saussurea lappa roots against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2012, 110, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Perumalsamy, H.; Wang, M.; Shu, S.H.; Ahn, Y.J. Larvicidal activity of Magnolia denudata seed hydrodistillate constituents and related compounds and liquid formulations towards two susceptible and two wild mosquito species. Pest Manag. Sci. 2016, 72, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.S.; Shin, E.H.; Yoo, D.H.; Ahn, Y.J. Enhanced toxicity of binary mixtures of Bacillus thuringiensis subsp. israelensis and three essential oil major constituents to wild Anopheles sinensis (Diptera: Culicidae) and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 804–810. [Google Scholar] [PubMed]
- Liang, Y.P.; Li, X.W.; Gu, Z.M.; Qin, P.W.; Ji, M.S. Toxicity of amorphigenin from the seeds of Amorphafruticosa against the larvae of Culexpipienspallens (Diptera: Culicidae). Molecules 2015, 20, 3238–3254. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Zhou, L.; Liu, Q.; Liu, Z.L. Laboratory evaluation of larvicidal activity of the essential oil of Allium tuberosum roots and its selected major constituent compounds against Aedes albopictus. J. Med. Entomol. 2015, 52, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Liu, Q.; Chen, X.B.; Zhou, L.; Liu, Z.L. Mosquito larvicidal constituents from the essential oil of Tetradium glabrifolium fruits against Aedes albopictus. Pest Manag. Sci. 2015, 71, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.L.; Liu, X.C.; Liu, Z.L.; Xu, X.F. Chemical composition of Salvia plebeian R. Br. essential oil and its larvicidal activity against Aedesaegypti L. Trop. J. Pharm. Res. 2015, 14, 831–836. [Google Scholar] [CrossRef]
- Liu, X.C.; Liu, Q.; Chen, X.B.; Zhou, L.; Liu, Z.L. Larvicidal activity of the essential oil of Youngia japonica aerial parts and its constituents against Aedes albopictus. Z. Naturforsch. 2015, 70C, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Liu, Q.; Zhou, L.; Liu, Z.L. Larvicidal activity of essential oil derived from Illicium henryi Diels (Illiciaceae) leaf. Trop. J. Pharm. Res. 2015, 14, 111–116. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.C.; Liu, Q.; Niu, C.; Liu, Z.L. Evaluation of larvicidal activity of the essential oil of Illicium difengpi and its major constituents against the Aedes aegypti mosquito. Trop. J. Pharm. Res. 2015, 14, 103–109. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Peter, H.R.; Hong, D.Y. Flora of China; Science Press: Beijing, China, 2009; Volume 20–21, pp. 33–35. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200023861 (accessed on 23 October 2016).
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2005; pp. 183–184. [Google Scholar]
- Guo, D.A.; Cui, Y.J.; Lou, Z.C.; Gao, Y.; Huang, L.R. Chemical constituents of east China globe thistle (Echinops grijisii). Chin. Tradit. Herb. Drug 1992, 23, 3–5, (In Chinese with English abstract). [Google Scholar]
- Lin, Y.L.; Huang, R.L.; Kuo, Y.H.; Chen, C.F. Thiophenes from Echinops grijsii Hance. Chin. Pharm. J. 1999, 51, 201–211. [Google Scholar]
- Koike, K.; Jia, Z.H.; Guo, H.Z.; Nikaido, T.; Liu, Y.; Zhao, Y.Y.; Guo, D.A. A new neolignan glycoside from the roots of Echinops grijissii. Nat. Med. 2002, 56, 255–257. [Google Scholar]
- Liu, Y.; Ye, M.; Guo, H.Z.; Zhao, Y.Y.; Guo, D.A. New thiophenes from Echinops grijisii. J. Asian Nat. Prod. Res. 2002, 4, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jin, W.R.; Shi, Q.; He, H.; Ma, Z.J.; Qu, H.B. Two novel thiophenes from Echinops grijissi Hance. J. Asian Nat. Prod. Res. 2008, 10, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liang, D.; Jin, W.R.; Qu, H.B.; Cheng, Y.Y.; Li, X.; Ma, Z.J. Cytotoxic thiophenes from the root of Echinops grijisii Hance. Z. Naturforsch. 2009, 64C, 193–196. [Google Scholar]
- Guo, D.A.; Lou, Z.C.; Liu, Z.A. Chemical components of volatile oil from Echinops grijsii Hance. Chin. J. Chin. Mater. Med. 1994, 19, 100–101, (In Chinese with English abstract). [Google Scholar]
- Liu, X.C.; Liu, Q.; Zhou, L.; Liu, Z.L. Evaluation of larvicidal activity of the essential oil of Allium macrostemon Bunge and its selected major constituent compounds against Aedes albopictus (Diptera: Culicidae). Parasite Vector 2014, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Huang, C.G.; Chen, Y.J.; Yu, J.J.; Chen, W.J.; Chang, S.T. Chemical compositions and larvicidal activities of leaf essential oils from two eucalyptus species. Bioresour. Technol. 2009, 100, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Canale, A.; Bertoli, A.; Gozzini, F.; Pistelli, L. Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2010, 107, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Dong, H.W.; Zhou, L.; Du, S.S.; Liu, Z.L. Essential oil composition and larvicidal activity of Toddalia asiatica roots against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2012, 112, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.K.N.; Dutra, K.A.; Lira, C.S.; Lima, B.N.; Napoleão, T.H.; Paiva, P.M.G.; Maranhão, C.A.; Brandão, S.S.F.; Navarro, D.M.A.F. Effects of Croton rhamnifolioides essential oil on oviposition, larval toxicity and trypsin activity. Molecules 2014, 19, 16573–16587. [Google Scholar] [CrossRef] [PubMed]
- Arnason, J.T.; Philogene, B.J.R.; Morand, P.; Imrie, K.; Iyengar, S.; Duval, F.; Soucy-Breau, C.; Scaiano, J.C.; Werstiuk, N.H.; Hasspieler, B.; et al. Naturally occurring and synthetic thiophenes as photoactivated insecticides. ACS Symp. Ser. 1989, 387, 164–172. [Google Scholar]
- Marles, R.J.; Compadre, R.L.; Compadre, C.M.; Soucy-Breau, C.; Redmond, R.W.; Duval, F.; Mehta, B.; Morand, P.; Scaiano, J.C.; Arnason, J.T. Thiophenes as mosquito larvicides: Structure-toxicity relationship analysis. Pestic. Biochem. Physiol. 1991, 41, 89–100. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Wei, X.Y.; Xu, H.H. Photoactivated insecticidal thiophene derivatives from Xanthopappus subacaulis. J. Nat. Prod. 2006, 69, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Arnason, J.T.; Philogene, B.J.R.; Berg, C.; MacEachern, A.; Kaminski, J.; Leitch, L.C.; Morand, P.; Lam, J. Phototoxicity of naturally occurring and synthetic thiophene and acetylenean alogues to mosquito larvae. Phytochemistry 1986, 25, 1609–1611. [Google Scholar] [CrossRef]
- Arnason, J.T.; Swain, T.; Wat, C.K.; Graham, E.A.; Partington, S.; Towers, G.H.N.; Lam, J. Mosquito larvicidal activity of polyacetylenes from species in the Asteraceae. Biochem. Syst. Ecol. 1991, 9, 63–68. [Google Scholar] [CrossRef]
- Nakano, H.; Ali, A.; Rehman, J.U.; Mamonov, L.K.; Cantrell, C.L.; Khan, I.A. Toxicity of thiophenes from Echinops transiliensis (Asteraceae) against Aedes aegypti (Diptera: Culicidae) larvae. Chem. Biodivers. 2014, 11, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Philogene, B.J.; Arnason, J.T.; Berg, C.W.; Duval, F.; Champagne, D.; Taylor, R.G.; Leitch, L.C.; Morand, P. Synthesis and evaluation of the naturally occurring phototoxin, α-terthienyl, as a control agent for larvae of Aedes intrudens, Aedes atropalpus (Diptera: Culicidae) and Simulium verecundum (Diptera: Simuliidae). J. Econ. Entomol. 1985, 78, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.; Kagan, E.; Patel, S.; Perrine, D.; Bindokas, V. Light-dependent effects of α-terthienyl in eggs, larvae, and pupae of mosquito Aedes aegypti. J. Chem. Ecol. 1987, 13, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Zhang, Q.; Lv, H.F.; Zhu, P.X. Toxicity of α-terthienyl to larvae of deltamethrin- resistant strains of Aedes albopictus. J. Jinan Univ. 2005, 26, 771–775, (In Chinese with English abstract). [Google Scholar]
- Nivsarkar, M.; Cherian, B.; Padh, H. α-Terthienyl: A plant-derived new generation insecticide. Curr. Sci. 2001, 81, 667–672. [Google Scholar]
- Jin, W.R.; Shi, Q.; Hong, C.T.; Cheng, Y.Y.; Ma, Z.J.; Qu, H.B. Cytotoxic properties of thiophenes from Echinops grijissi Hance. Phytomedicine 2008, 15, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Fokialakis, N.; Cantrell, C.L.; Duke, S.O.; Skaltsounis, A.L.; Wedge, D.E. Antifungal activity of thiophenes from Echinops ritro. J. Agric. Food Chem. 2006, 54, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.M.M.G.; Cirio, A.T.; Galindo, V.M.R.; Aranda, R.S.; Torres, N.W.; Perez-Lopez, L.A. Activity against Streptococcus pneumoniae of the essential oil and 5-(3-buten-1-ynyl)-2,2′-bithienyl isolated from Chrysactinia mexicana roots. Nat. Prod. Commun. 2011, 6, 1035–1038. [Google Scholar]
- Champagne, D.E.; Arnason, J.T.; Philogene, B.J.R.; Morand, P.; Lam, J. Light-mediated allelochemical effects of naturally occurring polyacetylenes and thiophenes from Asteraceae on herbivorous insects. J. Chem. Ecol. 1986, 12, 835–858. [Google Scholar] [CrossRef] [PubMed]
- Fokialakis, N.; Osbrink, W.L.A.; Mamonov, L.K.; Gemejieva, N.G.; Mims, A.B.; Skaltsounis, A.L.; Lax, A.R.; Cantrell, C.L. Antifeedant and toxicity effects of thiophenes from four Echinops species against the formosan subterranean termite, Coptotermes formosanus. Pest Manag. Sci. 2006, 62, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Marles, R.; Durst, T.; Kobaisy, M.; Soucy-Breau, C.; Abou-Zaid, M.; Arnason, J.T.; Kacew, S.; Kanjanapothi, D.; Rujjanawate, C.; Meckes, M.; et al. Pharmacokinetics, metabolism and toxicity of the plant-derived phototoxin α-terthienyl. Pharmacol. Toxicol. 1995, 77, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- World Health Organization. Report of the WHO Informal Consultation, on the Evaluation and Testing of Insecticides; CTD/WHOPES/IC/96.1; WHO: Geneva, Switzerland, 1996; pp. 32–36. [Google Scholar]
- Wang, Y.; Li, X.; Meng, D.L.; Li, Z.L.; Zhang, P.; Xu, J. Thiophenes from Echinops latifolius. J. Asian Nat. Prod. Res. 2006, 8, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 33, 339–347. [Google Scholar]
- Sample Availability: Samples of the crude extracts and pure compounds are available from the authors.
| Peak No | Compound | RRI a | RI b | Percent Composition |
|---|---|---|---|---|
| Monoterpenoids | 22.63 | |||
| 1 | α-Pinene * | 939 | 939 | 0.89 |
| 2 | β-Pinene * | 978 | 980 | 3.92 |
| 3 | β-Myrcene * | 990 | 991 | 1.94 |
| 4 | 1,8-Cineole * | 1031 | 1033 | 5.56 |
| 5 | cis-β-Ocimene | 1038 | 1037 | 5.01 |
| 6 | Artemisia ketone | 1064 | 1062 | 0.89 |
| 7 | Linalool * | 1098 | 1097 | 1.54 |
| 8 | Carvone * | 1243 | 1242 | 2.88 |
| Sesquiterpenoids | 25.96 | |||
| 9 | β-Cubebene | 1388 | 1388 | 0.97 |
| 10 | β-Maaliene | 1411 | 1411 | 0.29 |
| 11 | Caryophyllene * | 1420 | 1418 | 3.84 |
| 12 | α-Santalene | 1424 | 1424 | 0.52 |
| 13 | cis-Thujopsene | 1427 | 1429 | 0.96 |
| 14 | α-Bergamotene | 1433 | 1436 | 0.42 |
| 15 | α-Caryophyllene | 1453 | 1455 | 0.84 |
| 16 | cis-β-Farnesene | 1458 | 1457 | 4.71 |
| 17 | cis-β-Guaiene | 1485 | 1488 | 0.50 |
| 18 | α-Selinene | 1494 | 1493 | 1.66 |
| 19 | Virdiflorene | 1497 | 1497 | 1.06 |
| 20 | α-Bulnesene | 1505 | 1505 | 0.95 |
| 21 | β-Bisabolene | 1506 | 1509 | 0.46 |
| 22 | δ-Cadinene | 1524 | 1524 | 2.32 |
| 23 | Spathulenol | 1578 | 1576 | 1.56 |
| 24 | Caryophyllene oxide * | 1583 | 1581 | 3.53 |
| 25 | τ-Cadinol | 1642 | 1640 | 0.25 |
| 26 | trans-α-Bergamotol | 1714 | - | 1.12 |
| Thiophenes | 47.62 | |||
| 27 | 5-(3-Buten-1-yn-1-yl)-2,2’-bithiophene | 1941 | 1935 | 27.63 |
| 28 | 5-(4-Isovaleroyloxybut-1-ynyl)-2,2’-bithiophene | 2062 | - | 2.34 |
| 29 | α-Terthienyl | 2243 | 2240 | 14.95 |
| Others | 2.32 | |||
| 30 | Eugenol * | 1356 | 1356 | 1.54 |
| 31 | Methyleugenol * | 1403 | 1401 | 0.78 |
| Total identified | 98.53 |
| Insects | Treatment * | LC50 (μg/mL) | LC95 (μg/mL) | Slope ± SD | χ2 | p |
|---|---|---|---|---|---|---|
| (95% CL) | (95% CL) | |||||
| Aedes albopictus | Essential oil | 2.65 | 4.65 | 5.23 ± 0.52 | 17.79 | 0.0001 |
| (2.54–2.71) | (4.17–5.42) | |||||
| 5-BBT | 0.34 | 0.72 | 4.63 ± 0.43 | 8.05 | 0.0179 | |
| (0.29–0.39) | (0.66–0.81) | |||||
| 5-IBT | 0.45 | 0.66 | 7.67 ± 0.66 | 7.84 | 0.0214 | |
| (0.38–0.49) | (0.61–0.75) | |||||
| α-T | 1.41 | 2.19 | 6.72 ± 0.61 | 7.71 | 0.0212 | |
| (1.33–1.60) | (1.89–2.41) | |||||
| Rotenone | 3.75 | 9.45 | 4.12 ± 0.65 | 9.11 | 0.0001 | |
| (3.55–3.98) | (8.65–10.32) | |||||
| Anopheles sinensis | Essential oil | 3.43 | 5.67 | 6.78 ± 0.63 | 11.09 | 0.0089 |
| (3.11–3.69) | (5.04–6.21) | |||||
| 5-BBT | 1.36 | 1.93 | 8.47 ± 0.67 | 12.03 | 0.0121 | |
| (1.27–1.45) | (1.75–2.09) | |||||
| 5-IBT | 5.36 | 11.26 | 3.97 ± 0.37 | 13.64 | 0.0219 | |
| (4.64–6.09) | (10.21–12.05) | |||||
| α-T | 1.79 | 2.54 | 8.38 ± 0.76 | 12.19 | 0.0154 | |
| (1.67–1.91) | (2.36–2.81) | |||||
| Rotenone | 1.25 | 2.24 | 2.37 ± 0.18 | 16.21 | 0.0033 | |
| (1.07–1.33) | (2.01–2.45) | |||||
| Culex pipiens pallens | Essential oil | 1.47 | 2.21 | 7.18 ± 0.67 | 5.71 | 0.0577 |
| (1.34–1.52) | (1.99–2.48) | |||||
| 5-BBT | 0.12 | 0.18 | 6.64 ± 0.59 | 8.69 | 0.0182 | |
| (0.09–0.14) | (0.16–0.21) | |||||
| 5-IBT | 0.33 | 0.54 | 5.81 ± 0.56 | 13.41 | 0.0129 | |
| (0.26–0.41) | (0.47–0.63) | |||||
| α-T | 1.38 | 2.15 | 6.65 ± 0.66 | 13.29 | 0.0193 | |
| (1.69–1.49) | (1.88–2.35) | |||||
| Rotenone | 1.88 | 3.74 | 5.33 ± 0.51 | 11.67 | 0.0044 | |
| (1.63–1.93) | (3.51–4.03) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.P.; Liu, Q.Z.; Liu, Q.; Liu, Z.L. Identification of Larvicidal Constituents of the Essential Oil of Echinops grijsii Roots against the Three Species of Mosquitoes. Molecules 2017, 22, 205. https://doi.org/10.3390/molecules22020205
Zhao MP, Liu QZ, Liu Q, Liu ZL. Identification of Larvicidal Constituents of the Essential Oil of Echinops grijsii Roots against the Three Species of Mosquitoes. Molecules. 2017; 22(2):205. https://doi.org/10.3390/molecules22020205
Chicago/Turabian StyleZhao, Mei Ping, Qi Zhi Liu, Qiyong Liu, and Zhi Long Liu. 2017. "Identification of Larvicidal Constituents of the Essential Oil of Echinops grijsii Roots against the Three Species of Mosquitoes" Molecules 22, no. 2: 205. https://doi.org/10.3390/molecules22020205
APA StyleZhao, M. P., Liu, Q. Z., Liu, Q., & Liu, Z. L. (2017). Identification of Larvicidal Constituents of the Essential Oil of Echinops grijsii Roots against the Three Species of Mosquitoes. Molecules, 22(2), 205. https://doi.org/10.3390/molecules22020205





