Polyphenolics from Albizia harveyi Exhibit Antioxidant Activities and Counteract Oxidative Damage and Ultra-Structural Changes of Cryopreserved Bull Semen
Abstract
1. Introduction
2. Results
2.1. Phytochemical Profiling of A. harveyi Leaf Extract
2.2. Biological Activities
2.2.1. Total Phenolic Content and Antioxidant Activities In Vitro
2.2.2. Antioxidant Activities in Bull Semen Cryopreservation
Quantification of Oxidative Stress Markers in Seminal Plasma of Post-Thawed Bull Semen
Effect of the Extract on Post-Thawing Sperm Characteristics
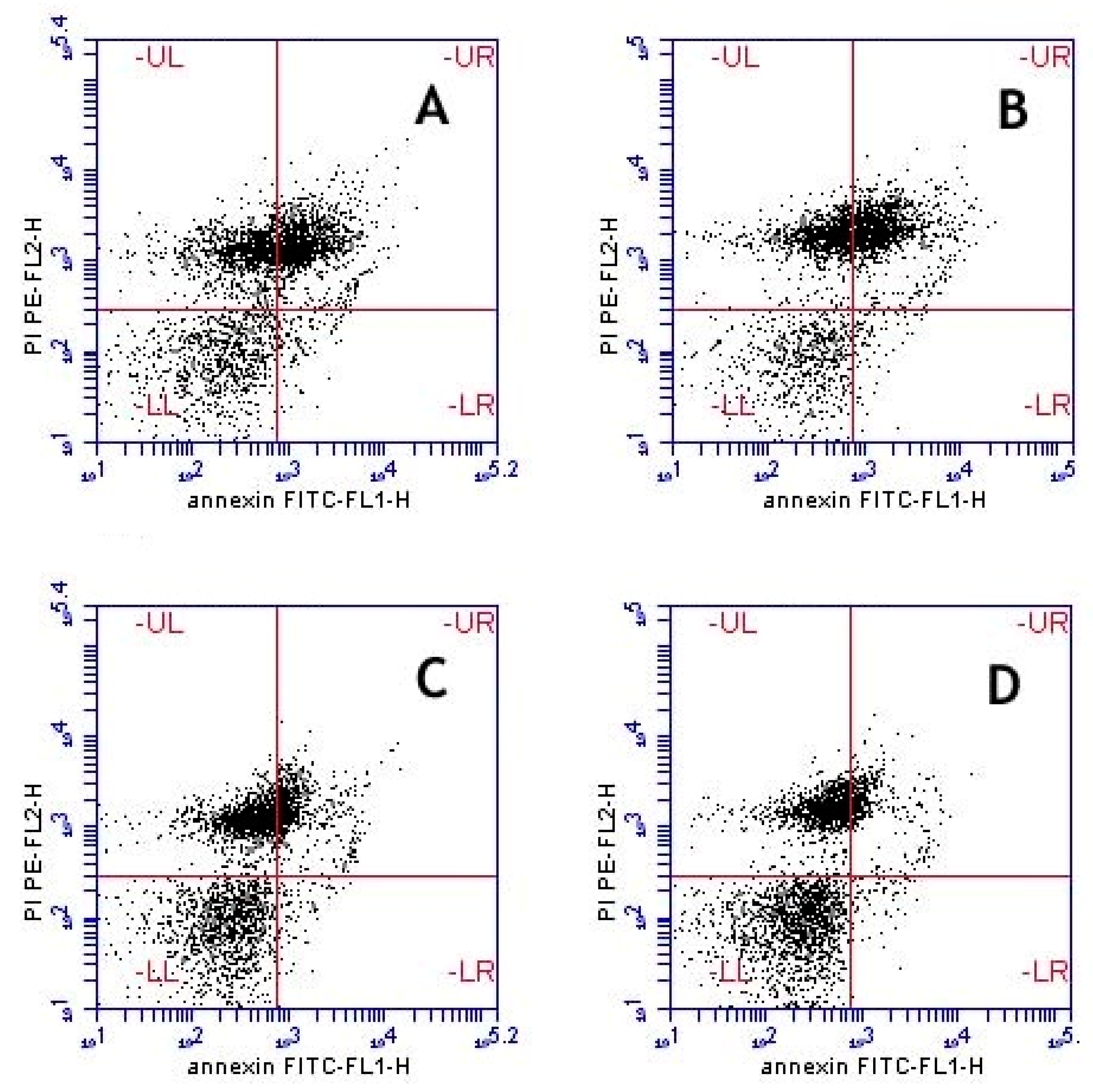
Anti-Apoptosis Effects
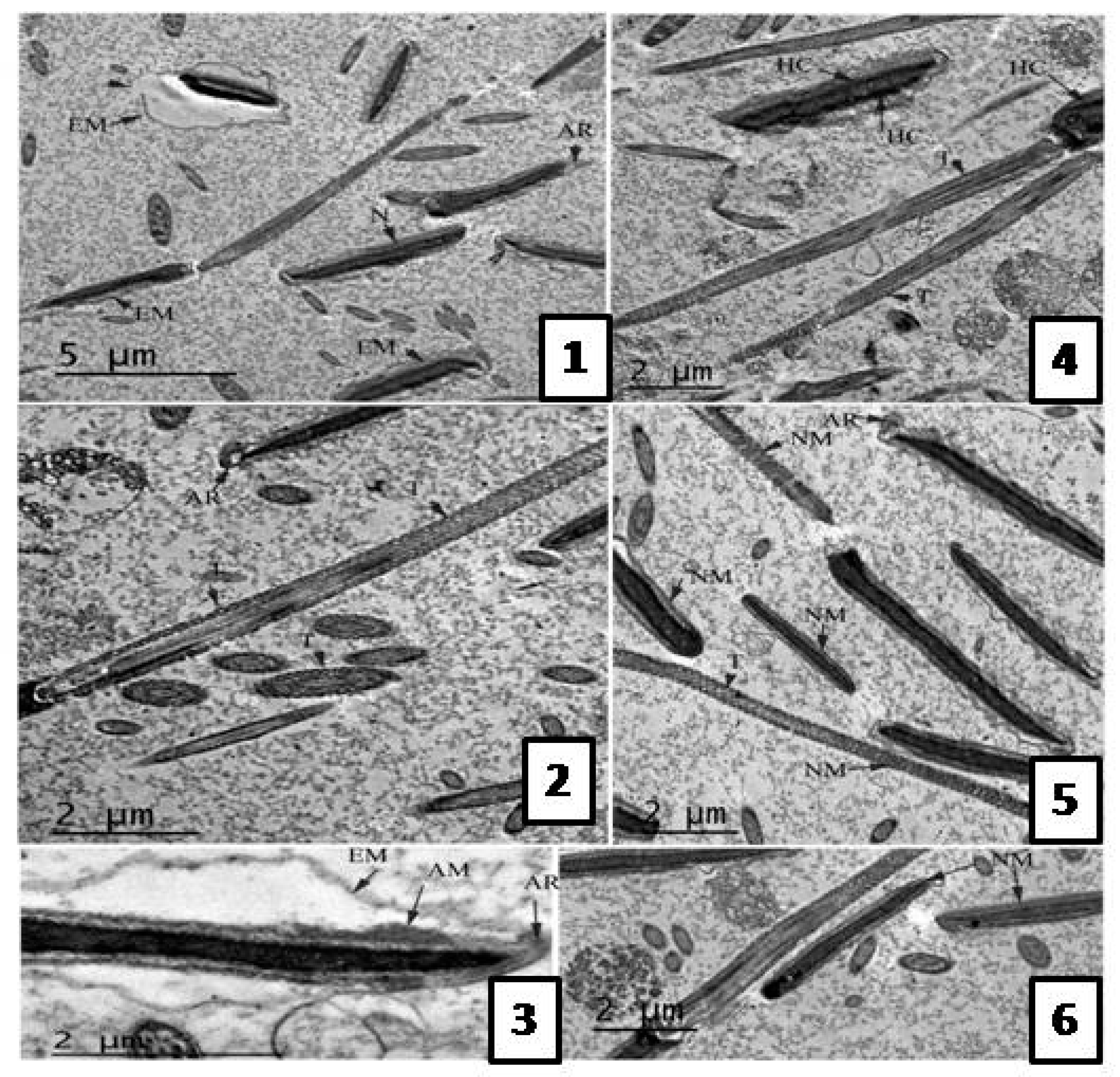
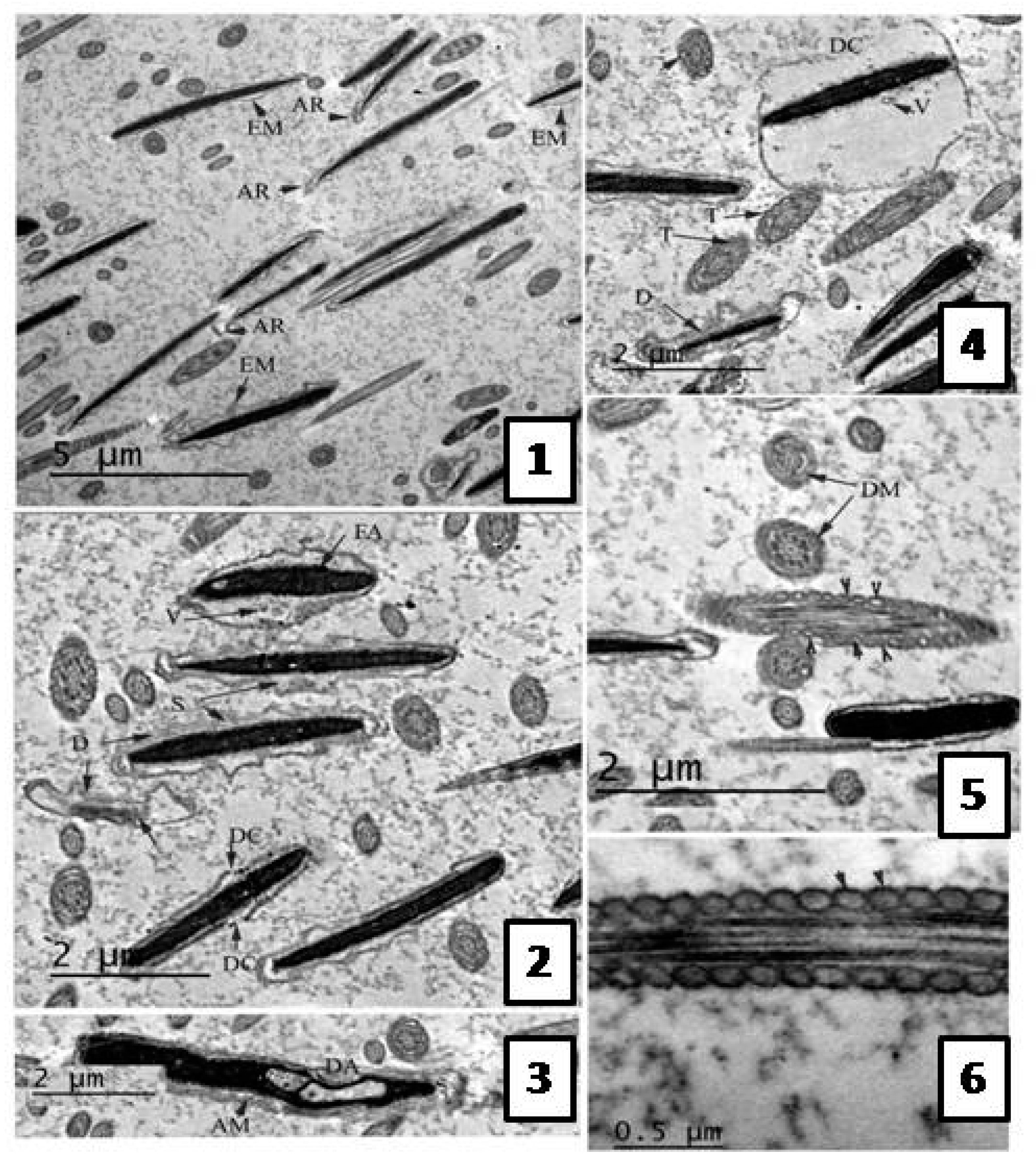
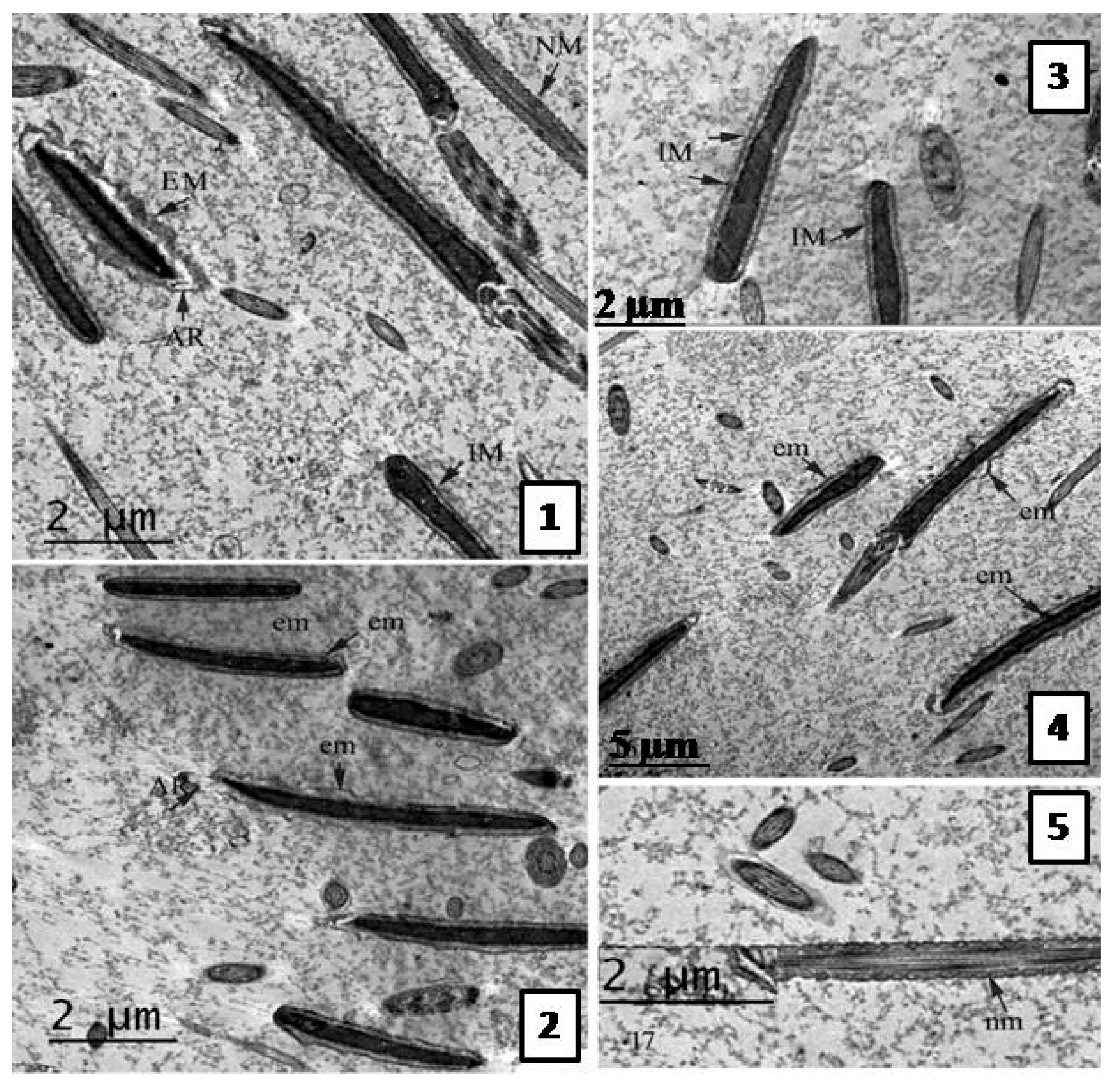
Effect of the Extract on Sperm Ultra-structure Post Thawing
3. Discussion
4. Materials and Methods
4.1. Extraction
4.2. HPLC-PDA-MS/MS
4.3. Antioxidant Activity in Vitro
4.4. Bull Semen Cryopreservation
4.4.1. Collection and Selection of Semen Samples
4.4.2. Cryopreservation Procedures
4.4.3. Experimental Design
Assessment of Sperm Progressive Motility
Assessment of Sperm Viability and Abnormalities
Determination of Membrane Integrity with Hypo-Osmotic Swelling Test
Determination of Chromatin Integrity with Toluidine Blue Staining
Fluorescent Staining of Sperm and Flow Cytometric Analysis
Biochemical Assays in Seminal Plasma
Transmission Electron Microscope (TEM) Evaluation of Semen Samples
4.5 Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ashour, M.L.; Sobeh, M.; El-Beshbishy, H.A.; Singab, A.N.; Wink, M. Eremophila maculata-Isolation of a rare naturally-occurring lignan glycoside and the hepatoprotective activity of the leaf extract. Phytomedicine 2016, 23, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Sghaier, M.B.; Ismail, M.B.; Bouhlel, I.; Ghedira, K.; Chekir-Ghedira, L. Leaf extracts from Teucrium ramosissimum protect against DNA damage in human lymphoblast cell K562 and enhance antioxidant, antigenotoxic and antiproliferative activity. Environ. Toxicol. Pharmacol. 2016, 44, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.; El-Beshbishy, H.A.; El-Shazly, A.M.; Wink, M. Albizia harveyi: Phytochemical profiling, antioxidant, antidiabetic and hepatoprotective activities of the bark extract. Med. Chem. Res. 2017, 26, 3091–3105. [Google Scholar] [CrossRef]
- Kang, T.H.; Jeong, S.J.; Kim, N.Y.; Higuchi, R.; Kim, Y.C. Sedative activity of two flavonol glycosides isolated from the flowers of Albizzia julibrissin Durazz. J. Ethnopharmacol. 2000, 71, 321–323. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, J.; Zhao, Y.; Wang, B.; Wu, L.; Liang, H. Three anti-tumor saponins from Albizia julibrissin. Bioorg. Med. Chem. Lett. 2006, 16, 2765–2768. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.O.; El-Beshbishy, H.A.; El-Shazly, A.M.; Wink, M. Hepatoprotective and hypoglycemic effects of a tannin rich extract from Ximenia americana var. caffra root. Phytomedicine 2017, 33, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.K.; Nassar, M.I.; Gaara, A.H.; El-Kashak, W.A.; Brouard, I.; El-Toumy, S.A. Secondary metabolites and bioactivities of Albizia anthelmintica. Pharmacogn. Res. 2013, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Plazonić, A.; Bucar, F.; Maleš, Ž.; Mornar, A.; Nigović, B.; Kujundžić, N. Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules 2009, 14, 2466–2490. [Google Scholar] [CrossRef]
- Celli, G.B.; Pereira-Netto, A.B.; Beta, T. Comparative analysis of total phenolic content, antioxidant activity, and flavonoids profile of fruits from two varieties of Brazilian cherry (Eugenia uniflora L.) throughout the fruit developmental stages. Food Res. Int. 2011, 44, 2442–2451. [Google Scholar] [CrossRef]
- Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of flavonoids and phenolic acids in Myrcia bella cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.; Inbaraj, B.S.; Chen, B.-H. Determination of phenolic acids and flavonoids in Taraxacum formosanum Kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vázquez, F.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, J.; Yahia, E.; Rivera-Pastrana, D.; Rojas-Molina, A.; Zavala-Sánchez, Á.M. Nutraceutical value of black cherry Prunus serotina fruits: Antioxidant and antihypertensive properties. Molecules 2013, 18, 14597–14612. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Kaur, P.; Kumar, N.; Singh, B.; Awasthi, S.; Lal, B. Chemical fingerprint analysis of phenolics of Albizia chinensis based on ultra-performance LC-electrospray ionization-quadrupole time-of-flight mass spectrometry and antioxidant activity. Nat. Prod. Commun. 2011, 6, 1617–1620. [Google Scholar] [PubMed]
- Barros, L.; Duenas, M.; Carvalho, A.M.; Ferreira, I.C.; Santos-Buelga, C. Characterization of phenolic compounds in flowers of wild medicinal plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC–MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, T.; Daikonya, A.; Kitanaka, S. Flavonol acylglycosides from flower of Albizia julibrissin and their inhibitory effects on lipid accumulation in 3T3-L1 cells. Chem. Pharm. Bull. 2012, 60, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol. Reprod. Dev. 2001, 59, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, Z.; Zhandi, M.; Zare-Shahneh, A.; Najafi, A.; Nabi, M.M.; Mohammadi-Sangcheshmeh, A. Does rosemary aqueous extract improve buck semen cryopreservation? Small Rumin. Res. 2013, 114, 120–125. [Google Scholar] [CrossRef]
- Martin, S.; Reutelingsperger, C.; McGahon, A.J.; Rader, J.A.; Van Schie, R.; LaFace, D.M.; Green, D.R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995, 182, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Falleiros, M.; Bicudo, S.D.; Júnior, J.A.; Golim, M.; Silva Filho, F.; Padovani, C.; Modolo, J.R. Tritrichomonas fetus extracellular products decrease progressive motility of bull sperm. Theriogenology 2010, 73, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Banothu, V.; Neelagiri, C.; Adepally, U.; Lingam, J.; Bommareddy, K. Phytochemical screening and evaluation of in vitro antioxidant and antimicrobial activities of the indigenous medicinal plant Albizia odoratissima. Pharm. Biol. 2017, 55, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; Johannisson, A.; Wallgren, M. Assessment of fresh and frozen–thawed boar semen using an Annexin-V assay: A new method of evaluating sperm membrane integrity. Theriogenology 2003, 60, 677–689. [Google Scholar]
- Zhong, R.-Z.; Zhou, D.-W. Oxidative stress and role of natural plant derived antioxidants in animal reproduction. J. Integr. Agric. 2013, 12, 1826–1838. [Google Scholar] [CrossRef]
- Sokunbi, O.; Ajani, O.; Lawanson, A.; Amao, E. Antibiotic potential of Moringa leaf (Moringa oleifera Lam.) crude extract in bull semen extender. Eur. J. Med. Plants 2015, 9, 1–8. [Google Scholar]
- El-Harairy, M.; Abdel-Khalek, A.; Khalil, W.; Khalifa, E.; El-Khateeb, A.; Abdulrhmn, A. Effect of aqueous extracts of Moringa oleifera leaves or Arctium lappa roots on lipid peroxidation and membrane integrity of ram sperm preserved at cool temperature. J. Anim. Poult. Prod. Mansoura Univ. 2016, 7, 467–473. [Google Scholar]
- Montón, A.; Gil, L.; Malo, C.; Olaciregui, M.; González, N.; de Blas, I. Sage (Salvia officinalis) and fennel (Foeniculum vulgare) improve cryopreserved boar epididymal semen quality study. Cryoletters 2015, 36, 83–90. [Google Scholar] [PubMed]
- Malo, C.; Gil, L.; Cano, R.; González, N.; Luño, V. Fennel (Foeniculum vulgare) provides antioxidant protection for boar semen cryopreservation. Andrologia 2012, 44, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; Johannisson, A.; Wallgren, M.; Rodriguez Martinez, H. Antioxidant supplementation in vitro improves boar sperm motility and mitochondrial membrane potential after cryopreservation of different fractions of the ejaculate. Anim. Reprod. Sci. 2003, 78, 85–98. [Google Scholar] [CrossRef]
- Zribi, N.; Chakroun, N.F.; Abdallah, F.B.; Elleuch, H.; Sellami, A.; Gargouri, J.; Rebai, T.; Fakhfakh, F.; Keskes, L.A. Effect of freezing–thawing process and quercetin on human sperm survival and DNA integrity. Cryobiology 2012, 65, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.O.; Cheng, H.; El-Shazly, A.M.; Wink, M. A proanthocyanidin-rich extract from Cassia abbreviata exhibits antioxidant and hepatoprotective activities in vivo. J. Ethnopharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.; Hasan, R.; Cheng, H.; El-Shazly, A.; Wink, M. Senna singueana: Antioxidant, hepatoprotective, antiapoptotic properties and phytochemical profiling of a methanol bark extract. Molecules 2017, 22, 1502. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.A.; Mohamed, T.; Saad, A.M.; Refahy, L.A.-G.; Sobeh, M.; Wink, M. HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. J. Pharm. Pharmacol 2017. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Foote, R.H.; Tobback, C.; Zhang, L.; Hough, S. Survival of bull spermatozoa seeded and frozen at different rates in egg yolk-tris and whole milk extenders. J. Dairy Sci. 1993, 76, 1028–1034. [Google Scholar] [CrossRef]
- Salisbury, G.W.; Van Demark, N.L.; Lodge, J.R. (Eds.) Physiology of Reproduction and Artificial Insemination of Cattle, 2nd ed.; Extenders and Extension of Unfrozen Semen; W.H. Freeman and Co.: San Francisco, CA, USA, 1978; pp. 442–493. [Google Scholar]
- Moskovtsev, S.I.; Librach, C.L. Methods of sperm vitality assessment. Methods Mol. Biol. 2013, 927, 13–19. [Google Scholar] [PubMed]
- Menon, A.G.; Thundathil, J.C.; Wilde, R.; Kastelic, J.P.; Barkema, H.W. Validating the assessment of bull sperm morphology by veterinary practitioners. Can. Vet. J. 2011, 52, 407–408. [Google Scholar] [PubMed]
- Caycho, K.; Santolaria, P.; Soler, C.; Yániz, J. Effect of hypoosmotic swelling test and water test on the distribution of sperm subpopulations in bull. Anim. Reprod. Sci. 2016, 169, 101. [Google Scholar] [CrossRef]
- Erenpreiss, J.; Jepson, K.; Giwercman, A.; Tsarev, I.; Erenpreisa, J.; Spano, M. Toluidine blue cytometry test for sperm DNA conformation: Comparison with the flow cytometric sperm chromatin structure and TUNEL assays. Hum. Reprod. 2004, 19, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Chaveiro, A.; Santos, P.; Da Silva, F. Assessment of sperm apoptosis in cryopreserved bull semen after swim-up treatment: A flow cytometric study. Reprod. Domest. Anim. 2007, 42, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Masters, A.; Harrison, P. Platelet counting with the BD AccuriTM C6 flow cytometer. Platelets 2014, 25, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Oliveira, L.Z.; Hossepian de Lima, V.F.; Levenhagen, M.A.; Santos, R.M.; Assumpcao, T.I.; Jacomini, J.O.; Andrade, A.F.; Arruda, R.P.; Beletti, M.E. Transmission electron microscopy for characterization of acrosomal damage after Percoll gradient centrifugation of cryopreserved bovine spermatozoa. J. Vet. Sci. 2011, 12, 267–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
Sample Availability: Sample of the plant material is available from the authors. |
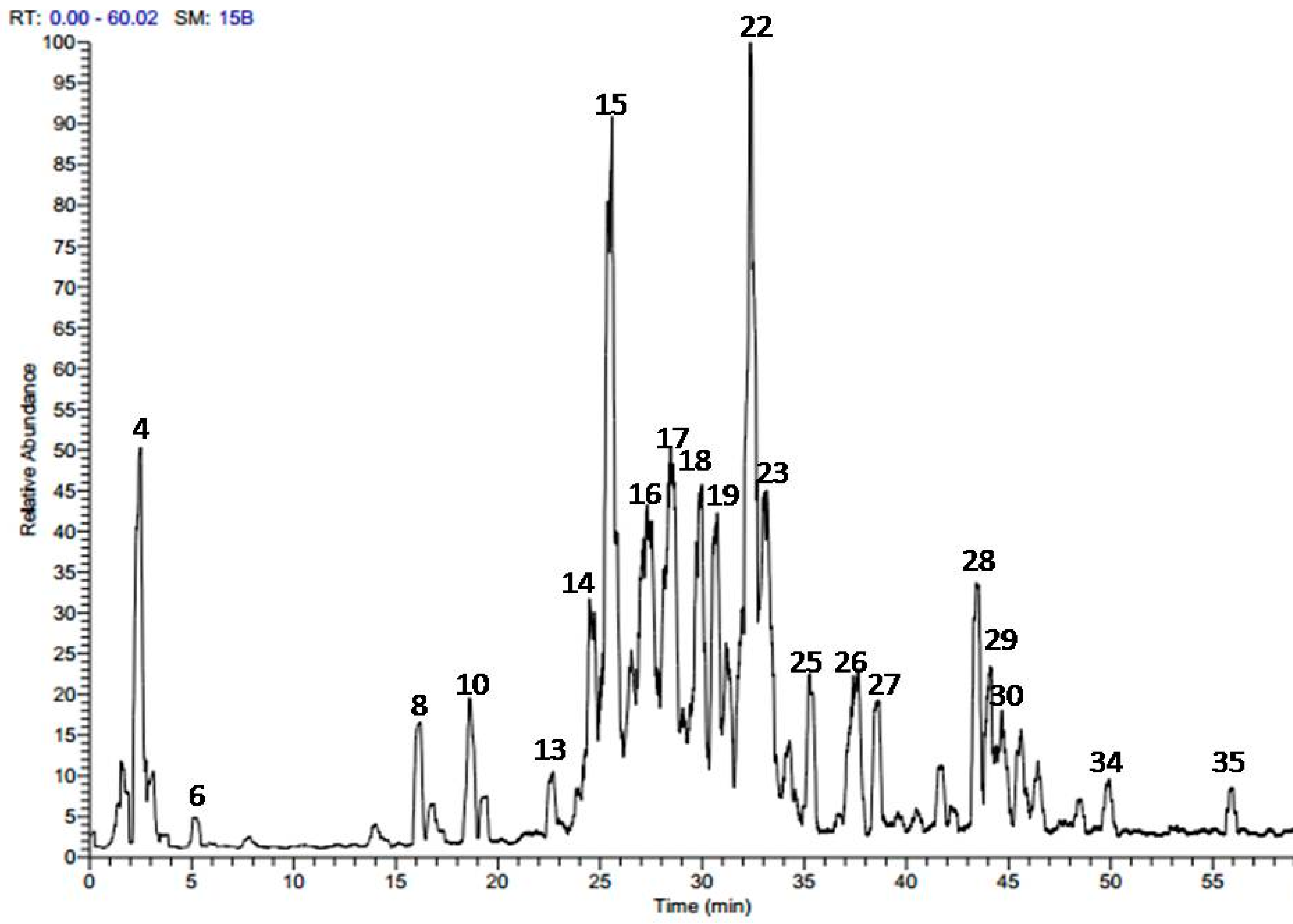
| No. | Tentatively Identified Compounds | tR (min.) | UV λmax | [M − H]− (m/z) | MS/MS | Reference |
|---|---|---|---|---|---|---|
| 1 | Malic acid | 1.37 | - | [4] | ||
| 2 | Gallic acid | 1.51 | 272 | 169 | 125 | [7,8] |
| 3 | Ferulic acid | 1.73 | 266, 307 | 193 | 147 | [9] |
| 4 | Gentisic acid-O-rhamnoside | 2.42 | 310 | 299 | 153 | |
| 5 | 3-O-p-Coumaroylquinic acid | 3.15 | 276, 315 | 337 | 163, 191 | [4] |
| 6 | 4-O-p-Coumaroylquinic acid | 5.20 | 281, 310 | 337 | 163, 191 | [4] |
| 7 | Caffeic acid derivative | 14.04 | - | 541 | 179, 389 | |
| 8 | (epi)-Catechin-(epi)-catechin | 16.75 | 277 | 577 | 289, 425 | [4] |
| 9 | Sinapic acid-O-hexoside | 17.16 | 265, 310 | 385 | 153, 223 | |
| 10 | (epi)Catechin | 18.70 | 280 | 289 | 179, 245 | [4] |
| 11 | Lyoniresinol-O-hexoside | 18.96 | 278 | 581 | 419 | |
| 12 | Rosmarinic acid-O-hexoside | 19.31 | - | 521 | 359 | |
| 13 | Myricetin-O-hexoside | 22.72 | 262, 357 | 479 | 317 | [10] |
| 14 | Quercetin-O-galloyl-hexoside | 24.61 | 265, 355 | 615 | 463, 301 | [8,11] |
| 15 | Quercetin-O-galloyl-hexoside | 25.75 | 267, 352 | 615 | 463, 301 | [8,11] |
| 16 | Myricetin-O-pentoside | 26.55 | 266, 360 | 449 | 317 | [10] |
| 17 | Quercetin-O-galactoside | 27.55 | 265, 357 | 463 | 301 | [8,12] |
| 18 | Quercetin-O-glucoside | 28.55 | 265, 355 | 463 | 301 | [8,12] |
| 19 | Kaempferol-O-galloyl-hexoside | 29.15 | 266, 352 | 599 | 447, 285 | [8] |
| 20 | Quercetin-O-pentoside | 29.98 | 265, 357 | 433 | 301 | [12] |
| 21 | Kaempferol-O-hexoside | 31.32 | 266, 352 | 447 | 151, 285 | [8,13] |
| 22 | Quercetin-O-pentoside | 32.45 | 265, 356 | 433 | 301 | [12] |
| 23 | Quercetin-O-rhamnoside | 33.33 | 265, 354 | 447 | 301 | [14] |
| 24 | Kaempferol-O-pentoside | 34.17 | 265, 350 | 417 | 285 | [13] |
| 25 | Kaempferol-O-pentoside | 35.26 | 265, 349 | 417 | 285 | [13] |
| 26 | Quercetin-O-caffeoyl-hexoside | 37.54 | 265, 335, 354 | 625 | 463, 301 | [15] |
| 27 | Quercetin-O-caffeoyl-hexoside | 38.67 | 269 | 625 | 463, 301 | [15] |
| 28 | Quercetin-O-coumaroyl-hexoside | 43.47 | 269, 280 | 609 | 463, 301 | |
| 29 | Quercetin-O-coumaroyl-hexoside | 44.12 | 268, 312 | 609 | 463, 301 | |
| 30 | Quercetin-O-feruloyl-hexoside | 44.80 | 270, 308 | 639 | 463, 301 | [16] |
| 31 | Quercetin | 45.56 | 265, 369 | 301 | 179, 151 | [11,14] |
| 32 | Kaempferol-O-coumaroyl-hexoside | 46.57 | 269, 315 | 593 | 447, 285 | |
| 33 | Ferulic acid derivative | 48.64 | - | 293 | 193, 236 | |
| 34 | Quercetin methyl galloyl-hexoside | 49.91 | 267, 303, 354 | 629 | 463, 301 | |
| 35 | Kaempferol | 55.84 | 268, 350 | 285 | 151, 285 | [8,17] |
| Sample | Extract | EGCG (Epigallocatechin gallate) |
|---|---|---|
| DPPH [EC50, µg/mL] | 16.3 | 3.5 |
| FRAP [mM FeSO4/mg extract] | 17.00 | 25 |
| Sample | TAC (mM/L) | MDA (nmol/mL) |
|---|---|---|
| Untreated control | 0.18 ± 0.00 | 67.26 ± 1.86 |
| Extract 0.5 µg/mL | 0.36 ± 0.10 | 56.76 ± 3.08 ** |
| Extract 1.0 µg/mL | 0.87 ± 0.04 *** | 43.53 ± 0.84 *** |
| Extract 1.5 µg/mL | 1.26 ± 0.16 *** | 32.43 ± 0.99 *** |
| Sample | Motility (%) | Viability (%) | Membrane Integrity (%) | Abnormality (%) | Chromatin Damage (%) |
|---|---|---|---|---|---|
| Untreated control | 46.66 ± 1.76 | 46.66 ± 2.96 | 41.33 ± 1.76 | 27.33 ± 2.84 | 8.67 ± 1.76 |
| Extract 0.5 µg/mL | 51.55 ± 1.67 | 50.33 ± 2.90 | 46.66 ± 1.85 | 23.33 ± 3.92 | 7.33 ± 0.88 |
| Extract 1.0 µg/mL | 60.00 ± 2.88 ¥ | 58.00 ± 1.53 @ | 52.33 ± 2.96 ¥ | 20.33 ± 2.60 | 5.67 ± 0.88 |
| Extract 1.5 µg/mL | 66.66 ± 1.67 ¥ | 67.00 ± 4.16 ¥ | 60.66 ± 1.76 ¥ | 18.66 ± 1.76 @ | 3.33 ± 0.33 @ |
| Sample | Viable (%) | Early Apoptosis (%) | Apoptosis (%) | Necrosis (%) |
|---|---|---|---|---|
| (A−/PI−) | (A+/PI−) | (A+/PI+) | (A−/PI+) | |
| Untreated control | 42.60 ± 2.19 | 2.65 ± 0.03 | 30.40 ± 1.56 | 24.50 ± 0.75 |
| Extract 0.5 µg/mL | 47.35 ± 1.01 | 2.20 ± 0.00 ** | 27.00 ± 0.92 * | 23.45 ± 0.09 |
| Extract 1.0 µg/mL | 58.10 ± 0.23 *** | 2.00 ± 0.00 *** | 14.25 ± 0.14 *** | 25.45 ± 0.49 |
| Extract 1.5 µg/mL | 62.65 ± 1.76 *** | 1.80 ± 0.17 *** | 10.50 ± 0.98 *** | 25.05 ± 0.61 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobeh, M.; Hassan, S.A.; El Raey, M.A.; Khalil, W.A.; Hassan, M.A.E.; Wink, M. Polyphenolics from Albizia harveyi Exhibit Antioxidant Activities and Counteract Oxidative Damage and Ultra-Structural Changes of Cryopreserved Bull Semen. Molecules 2017, 22, 1993. https://doi.org/10.3390/molecules22111993
Sobeh M, Hassan SA, El Raey MA, Khalil WA, Hassan MAE, Wink M. Polyphenolics from Albizia harveyi Exhibit Antioxidant Activities and Counteract Oxidative Damage and Ultra-Structural Changes of Cryopreserved Bull Semen. Molecules. 2017; 22(11):1993. https://doi.org/10.3390/molecules22111993
Chicago/Turabian StyleSobeh, Mansour, Soha A. Hassan, Mohamed A. El Raey, Wael A. Khalil, Mahmoud A. E. Hassan, and Michael Wink. 2017. "Polyphenolics from Albizia harveyi Exhibit Antioxidant Activities and Counteract Oxidative Damage and Ultra-Structural Changes of Cryopreserved Bull Semen" Molecules 22, no. 11: 1993. https://doi.org/10.3390/molecules22111993
APA StyleSobeh, M., Hassan, S. A., El Raey, M. A., Khalil, W. A., Hassan, M. A. E., & Wink, M. (2017). Polyphenolics from Albizia harveyi Exhibit Antioxidant Activities and Counteract Oxidative Damage and Ultra-Structural Changes of Cryopreserved Bull Semen. Molecules, 22(11), 1993. https://doi.org/10.3390/molecules22111993







