Abstract
Baccharis reticularia DC. is a plant species from the Asteraceae family that is endemic to Brazil. Despite the great importance of Baccharis genus, no study has been carried out regarding either the phytochemical composition of B. reticularia or the evaluation of its larvicidal potential. Considering the intrinsic immiscibility of essential oils, this study shows larvicidal nanoemulsions containing the B. reticularia phytochemically characterized essential oil and its main constituent against Aedes aegypti. The major compound found was d-limonene (25.7%). The essential oil inhibited the acetylcholinesterase, one of the main targets of insecticides. The required hydrophile-lipophile balance of both nanoemulsions was 15.0. The mean droplet sizes were around 90.0 nm, and no major alterations were observed after 24 h of preparation for both formulations. After 48 h of treatment, the estimated LC50 values were 118.94 μg mL−1 and 81.19 μg mL−1 for B. reticularia essential oil and d-limonene nanoemulsions, respectively. Morphological alterations evidenced by scanning electron micrography were observed on the larvae treated with the d-limonene nanoemulsion. This paper demonstrated a simple and ecofriendly method for obtaining B. reticularia essential oil and d-limonene aqueous nanoemulsions by a non-heating and solvent-free method, as promising alternatives for Aedes aegypti control.
1. Introduction
Aedes aegypti (Diptera: Culicidae) is the vector of neglected and emergent tropical diseases. It is the primary dengue and chikungunya vector and, more recently, it was associated to Zika virus outbreak. This is a critical public health problem of international concern due to a possible correlation between infection of pregnant women and neurological disorders, such as microcephaly, in newborns [1]. Several practices of vector control are used against A. aegypti, including the mechanical elimination of breeding sites, adulticidal and larvicidal agents [2]. In addition to the removal of breeding sites (also called environmental methods), the mechanical methods may make use of traps. The chemical methods using conventional insecticides may be used either on adults or larvae. However, the problems associated to inducement of resistance is a main issue related to this approach. On the other hand, the biological methods, including those with essential oils have been considered promising [3]. Domestic host breeding sites, such earthenware vases, barrels, cisterns, gutters, cans, tyres and plant saucers, are the main targets for the control. For example, the presence of fertilizers (e.g., NPK) in the water of plant saucers is considered a possible attractant for gravid females [4]. Therefore, the development of alternative larvicides, such those from natural origin, for domestic use should be encouraged.
Asteraceae is considered one of the most representative botanical families among the Angiosperms. In Brazil, around 280 genera and 2075 species can be found [5]. The genus Baccharis belongs to this family and has around 178 species distributed in this country [6]. Baccharis reticularia DC is endemic and native to Brazil, being found on caatinga, cerrado (Brazilian savanna) and Atlantic forests more specifically, on restinga vegetations (sandy coastal plains) [7]. It is found in open Clusia scrub vegetation and open Ericaceae scrub vegetation on the Restinga de Jurubatiba National Park (Rio de Janeiro state, Brazil), being commonly known at this location as alecrim-da-areia (sand-rosemary) due to the fact that it is a high aromatic plant [8]. The antifungal properties of B. reticularia have been investigated [9]. However, studies concerning its biological activities are scarce. Moreover, to our knowledge, the chemical constituents of this plant remains unknown, including its volatile constituents.
Essential oils are complex mixtures of volatiles mainly extracted by hydrodistillation or stem distillation, being also able to be extracted by pressing and centrifugation, specifically in the case of citric fruits. They are recognized by several biological properties, such as repellent [10], antimicrobial [11,12], antioxidant [12,13] and larvicidal actions, including against A. aegypti larvae [14,15]. Regarding Baccharis species, their essential oils were previously reported as antibacterial [16,17,18], repellent [18,19], antiparasitic [16,20], antifungal [16,18] and insecticide [18] agents. However, these complex mixtures have an intrinsic low water miscibility, configuring a technological challenge for aqueous products. Nanoemulsions are disperse systems constituted by two immiscible liquids that are oftenstabilized by one or more surfactants. They have a mean droplet size below 200 nm, kinetic stability, improved bioavailability and enhanced chemical and physical stability of the bioactive compounds [21,22]. In recent years, several studies have been carried out in order to developed new larvicidal formulations using nanotechnology. Nanostructured products prepared with natural herbal oils [23,24,25], including essential oils [26,27,28,29], are considered an excellent eco-friendly option when compared to synthetic pesticides.
However, to our knowledge, no efforts have been carried out to prepare a nanostructured product with the essential oil of B. reticularia or to evaluate its larvicidal activity against A. aegypti. Thus, the aims of the present study were to elucidate the chemical composition of the essential oil from B. reticularia and to prepare and characterize larvicidal nanoemulsions with this natural raw material and its major constituent, using a non-heating and solvent free low energy method, against A. aegypti larvae.
2. Results
2.1. Chemical Composition and Anticholinesterase Activity of the Essential Oil of B. reticularia
The extraction of B. reticularia leaves by hydrodistillation yielded 0.30% (w/w) of an essential oil with slightly green appearance. The phytochemical analysis by gas chromatography with mass spectrometric detection (GC-MS) revealed the presence of 16 identified compounds (Table 1) with a majority of mono- and sesquiterpenes. The relative quantification analysis by gas chromatography with flame ionization detection GC-FID (Table 1) indicated that the most abundant compound was d-limonene (25.7%), a precursor of monoterpene biosynthesis. An unusual component of essential oils was also found (kaurene = 0.7%).

Table 1.
Chemical constituents of the essential oil from B. reticularia and their relative abundance.
The essential oil from B. reticularia was able to inhibit the acetylcholinesterase enzyme with an IC50 value of 301.9 μg mL−1 (263.2–354.2).
2.2. Production and Characterization of B. reticularia Essential Oil and d-Limonene Nanoemulsions
On the day of preparation, most of the nanoemulsions (with hydrophile-lipophile balance–HLB, ranging between 8 and 12) presented a milky aspect which is associated to conventional macroemulsions, in addition to creaming. All the nanoemulsions presented a negative superficial charge. High mean droplet size and polydispersity index were observed mainly for low HLB formulations (Table 2 and Table 3). The best results were obtained with nanoemulsions prepared solely with polysorbate 80 as surfactant (HLB 15), which presented the best maintenance of the physicochemical characteristics after one day of preparation, including a mean droplet size below 200 nm. Considering the observations above, it can be suggested that the required HLB (rHLB) value of both B. reticularia essential oil and d-limonene is 15.0.

Table 2.
Physicochemical characterization of nanoemulsions containing B. reticularia essential oil.

Table 3.
Physicochemical characterization of nanoemulsions containing d-limonene.
2.3. Larvicidal Assay
Considering the best observed parameters, the nanoemulsions of B. reticularia essential oil and d-limonene at rHLB = 15 were chosen for further larvicidal assays at different concentrations, as shown in Figure 1 and Figure 2. No mortality level was observed in the control group after 24 h and 48 h, which did not present a statistical significant difference in all periods to the group treated at 25 μg mL−1 (p > 0.05). No statistical significant difference (p > 0.05) in all periods was observed between the group tested at higher concentration (250 μg mL−1), when compared to groups treated at 125 and 175 μg mL−1. Mortality was time-dependent (p < 0.05) in the groups treated with B. reticularia nanoemulsion (expressed as essential oil content in water) at 125 μg mL−1 (t24h = 36.67 ± 15.28%/t48h = 56.67 ± 20.82%), 175 μg mL−1 (t24h = 53.33 ± 11.55%/t48h = 73.33 ± 5.77%) and 250 μg mL−1 (t24h = 43.33 ± 5.77%/t48h = 63.33 ± 5.77%).
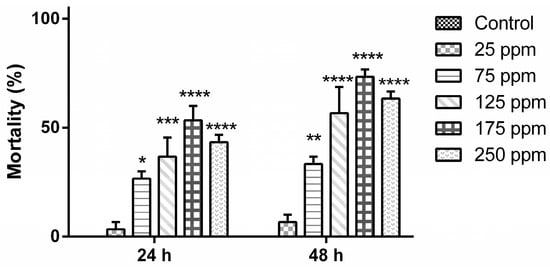
Figure 1.
Mortality levels (%) of Aedes aegypti (early fourth-instar larvae) after treatment with Baccharis reticularia essential oil-based nanoemulsion. Significance: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
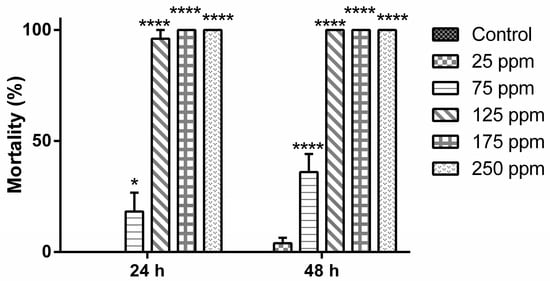
Figure 2.
Mortality levels (%) of Aedes aegypti (early fourth-instar larvae) after treatment with d-limonene -based nanoemulsion. Significance: * p < 0.05; **** p < 0.0001.
After 24 h of treatment with B. reticularia nanoemulsion, analysis of the data indicated that the percentage of deviance explained by the model was 59.8709 and adjusted percentage was 10.4797. The equation of fitted estimated regression model was y = −1.20057 + 0.00542573x, while p-value for the model and p-value for the residuals were, respectively, 0.0277 and 0.3547. These results are in agreement with the observed statistical significant differences between the variables and with the idea that the model is not significantly worse than the best possible model at the 95.0% or higher confidence level. The estimated median lethal concentration (LC50) and the 90% lethal concentration (LC90) values with the lower limit and upper limit are, respectively, 221.273 (151.563–979.895) μg mL−1 and 457.472 (299.055–3323.08) μg mL−1 (Table 4). After 48 h of treatment, analysis of the data indicated that the percentage of deviance explained by the model was 69.6843 and adjusted percentage was 38.4743. The equation of fitted estimated regression model was y = −1.04355 + 0.00721255x, while p-value for the model and p-value for the residuals were, respectively, 0.0028 and 0.2741. These results These results are in agreement with the observed statistical significant differences between the variables and with the idea that the model is not significantly worse than the best possible model at the 95.0% or higher confidence level. The estimated LC50 and LC90 values with the lower limit and upper limit are, respectively, 144.685 (84.1297–228.743) μg mL−1 and 322.368 (234.914–748.635) μg mL−1 (Table 4).

Table 4.
Larvicidal activity of nanoemulsions of B. reticularia essential oil and d-limonene.
The main constituent of the B. reticularia essential oil, the monoterpene d-limonene, was also subjected for preparation of a larvicidal nanoemulsion. According to Figure 2, no statistical significant difference was observed in the mortality induced by the group treated at 25 μg mL−1, in all periods (24 and 48 h) when compared to control group (p > 0.05). A significant time-dependent mortality (p < 0.0001) was observed only in the group treated at 75 μg mL−1 (t24h = 20.0 ± 17.32/t48h = 36.0 ± 18.17). The highest mortality levels were reached during the first 24 h in the groups treated at 125 μg mL−1 (t24h,48h = 96.0 ± 8.94%), 175 and 250 μg mL−1 (t24h,48h = 100%), presenting a statistical significant difference to the control group, 25 and 75 μg mL−1 treated groups (p < 0.0001).
After 24 h of treatment, analysis of the data indicated that the percentage of deviance explained by the model was 99.9887 and the adjusted percentage was 92.3582. The equation of the fitted estimated regression model was y = −4.74948 + 0.0520471x, while the p-value for the model and p-value for the residuals were, respectively, 0.0000 and 0.9999. These results corroborate statistical significant differences between the variables and that the model is not significantly worse than the best possible model at the 95.0% or higher confidence level. The estimated LC50 and LC90 values with the lower limit and upper limit are, respectively, 91.2534 (74.1662–111.616) μg mL−1 and 115.876 (99.85–167.279) μg mL−1 (Table 4). After 48 h of treatment, analysis of the data indicated that the percentage of deviance explained by the model was 99.2858 and adjusted percentage was 90.0882. The equation of fitted estimated regression model was −2.8997 + 0.0357126x, while the p-value for the model and p-value for the residuals were, respectively, 0.0000 and 0.9580. These results are in agreement with the observed statistical significant differences between the variables and with the idea that the model is not significantly worse than the best possible model at the 95.0% or higher confidence level. The estimated LC50 and LC90 values with the lower limit and upper limit are, respectively, 81.1953 (60.1436–102.036) μg mL−1 and 117.08 (97.5348–169.639) μg mL−1 (Table 4).
2.4. A. Aegypti Morphology by Scanning Electron Microscopy
The evaluating of A. aegypti morphology after exposure to the nanoemulsion prepared with the B. reticularia major compound was performed, since highest mortality (100%) was reached with the nanoemulsion prepared with d-limonene, together with a lower LC50 value for this nanoemulsion, when compared to the essential oil-based nanoemulsion. Photomicrographs of A. aegypti after incubation with the nanoemulsion containing d-limonene at 250 μg mL−1 can be seen in Figure 3. The larvae of the control group showed an elongated and vermiform appearance, with the body well defined. The head and the thorax presented a globular aspect, with greater amount of chitin in the cuticles. The abdomen was smooth and flexible, consisting of segments that provided larvae mobility in water. On the other hand, the larvae of the group treated with nanoemulsions containing d-limonene presented a fragile appearance, low resistance, with little mobility and all wrinkled body surface showing alterations on head, thorax, siphon and on cuticles of abdomen. An increase in the number of sows could be also seen (Figure 3D,E).
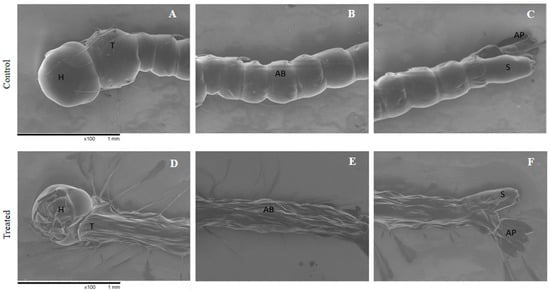
Figure 3.
A. aegypti larvae morphology by SEM. Control (A–C) showing no alteration on head (H), thorax (T), abdomen segments (AB), siphon (S) and anal papillae (AP). Larvae treated with nanoemulsion containing d-limonene at 250 ppm (D–F) showing alterations on head (H), siphon (S) and on cuticles of abdomen (AB) and thorax (T).
3. Discussion
3.1. Chemical Composition and Anticholinesterase Activity of the Essential Oil of B. reticularia
The extraction of essential oil from leaves of Baccharis reticularia by hydrodistillation yielded 0.30% (m/m) which is in accordance with the literature data for the genus, which may range from 0.01 to 1.89% [30,31]. The majority of mono and sesquiterpenes in the essential oil composition is also in accordance with the literature data for the genus Baccharis [20,32,33]. The major constituent of the essential oil, d-limonene, is a well-known precursor of monoterpene biosynthesis. d-limonene is known by its antimicrobial activities and also possesses insecticidal properties [34,35]. Although the kaurane-type diterpenes are frequently found on plants of the genus Baccharis [33], no reference was found to the presence of these compound on their essential oils. Thus, to our knowledge, this is first report of this type of natural compound as a chemical constituent of essential oils from Baccharis species.
The essential oil from B. reticularia showed moderate anticholinesterase activity when compared to other oils from the Asteraceae species [36,37]. The inhibition of the AChE is one of proposed mechanisms of insecticide action [38], causing death and paralysis on the insects by blocking neural signal transduction. Essential oils are mixtures of volatile compounds that can be produced by plants as a part of their chemical defense against phytophagous invertebrates mainly by inhibition of this enzyme [39,40]. Despite some ongoing efforts which were carried out to investigate the anticholinesterase activities of extracts and isolated compounds from Baccharis spp. [41,42], studies regarding the anticholinesterase potential of the essential oils from this genus still remain scarce. In the present study, the essential oil from B. reticularia was able to inhibit the acetylcholinesterase enzyme with an IC50 value of 301.9 μg mL−1 (263.2–354.2), demonstrating moderate anticholinesterase activity when compared to other oils from Asteraceae species [36,37]. Limonene presented known anticholinesterase activity against some insects, including from Aedes genus. Seo and coworkers [43] obtained an IC50 value around 130 μg mL−1 for the isomer l-limonene against acetylcholinesterase of Reticulitermes speratus (Japanese termite). The evaluation of the enantiomers l-limonene and d-limonene against the Culicidae Aedes albopictus demonstrated different acetylcholinesterase inhibition of 20% and 40%, respectively when assayed at 1000 μg mL−1 [44]. Anticholinesterase assay of the d-limonene against commercial enzymes from Electrophorus electricus and butyrylcholinesterase from equine serum were also performed and revealed IC50 values of 225.9 ± 1.3 μg mL−1 and 456.2 ± 5.6 μg mL−1, respectively [45]. Despite the fact that several volatile terpenoids (mono- and sesquiterpenes) show insecticidal activity by inhibitions of AChE [46], some of them may have the activity modulated by the presence of other substances, including those from complex mixtures such as essential oils [47,48]. Based on this preliminary in vitro assay and due to the fact that the essential oil presented a satisfactory IC50 value, in accordance with the literature data for its major compound, both essential oil and limonene were used for the preparation of nanoemulsions for evaluation against A. aegypti larvae.
3.2. Production and Characterization of B. reticularia Essential Oil and d-Limonene Nanoemulsions
Several nanoemulsions with B. reticularia essential oil or d-limonene were prepared by using blends of a non-ionic surfactant pair at different ratios, using a low energy and solvent-free method without heating. Despite some studies aiming to generate essential oil-based nanoemulsions focus on high energy methods to generate small size droplets, the utilization of low energy methods that makes use the physicochemical properties of the system are also a good alternative [23,24,25] and should be encouraged, due to the reduced costs of the process. The utilization of non-heating methods is desirable, due to the volatile nature of the compounds of an essential oil [27,28]. Moreover, a solvent-free preparation would lead to less impairment to the environment, being in accordance with a sustainable and eco-friendly approach. rHLB can be predicted based on a series of emulsions prepared with known ratios of a pair of two non-ionic surfactants. It is also a satisfactory strategy to achieve low mean droplet size and its determination has been used to develop larvicidal nanoemulsions [23,24,28].
3.3. Larvicidal Assay
The essential oils from some Baccharis species were previously subjected to a screening procedure in order to verify their larvicidal activity against late-third/early-fourth A. aegypti larvae. At a concentration of 100 μg mL−1, following percentages of mortality were observed: B. dracunculifolia (55–65%), B. genistelloides (20%) B. pentandlii (40%) and B. salicifolia (40%). Due to different collection places, B. latifolia induced 35% of mortality or absence of any activity. However, LC50 values of aforementioned essential oils were not estimated [49].
The decrease of 34% on the LC50 values of B. reticularia nanoemulsion as a function of time observed in this study is in accordance with literature data. Oliveira and coworkers [28] showed 42% of reduction on LC50 from 24 to 48 h (371.6 to 213.7 μg mL−1) after a larvicidal assay with Pterodon emarginatus essential oil-based nanoemulsion against A. aegypti larvae. d-limonene nanoemulsions also showed a decrease on its LC50 values (11%) from 24 h to 48 h in this study, which is in accordance with the literature. Zahran and coworkers [50] observed a reduction about 11.4% on LC50 from 24 h to 48 h from 140 μg mL−1 to 124 μg mL−1, respectively, after incubation of l-limonene against another Culicidae species (C. pipens). Kassir and coworkers [51] observed a 20% LC50 reduction from 24 to 48 h (from 53.8 to 32.52 μg mL−1) after incubation of pure limonene with Culex quinquefasciatus. The enhancement of activity may be associated to gradative release of the larvicidal compounds from nanostructure systems as nanoemulsions [52]. Further studies aiming to correlate the release of compounds with mortality as function of time should be performed to better understanding of the phenomena involved in the larvicidal action of nanoemulsions, including those based on B. reticularia.
The estimated LC50 value for d-limonene nanoemulsions obtained in this study is close to the one reported d-limonene non-nanoemulsified against fourth-instar larvae of A. aegypti (71.9 μg mL−1) [53]. The highest mortality levels were reached during the first 24 h on the groups treated at 125, 175 and 250 μg mL−1. This data is in accordance with Pavela and coworkers [54] that found 100% of mortality induced by d-limonene on Culex quinquefasciatus larvae at 250 μg mL−1.
3.4. A. Aegypti Morphology by Scanning Electron Microscopy
The observed morphological alterations in treated A. aegypti larvae are in accordance with previous works [25] and may affect larvae development and motility contributing to the observed high mortality. For example, the increase in the number of sows can hamper the exoskeleton exchange process, as seen by Borges and coworkers [55]. However, other factors can contribute for larvicidal activity on mosquito larvae, such as the damage to the digestive tube which is associated to anti-feedant behavior [56].
4. Materials and Methods
4.1. Chemicals
Polysorbate 80 and sorbitan monooleate were obtained from Praid (São Roque, SP, Brazil). n-alkanes (C7–C40), limonene, acetylthiocholine iodide (ATCI) and 5,5-dithiobis-2-nitrobenzoic acid (DTNB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Distilled water was used for general procedures. All chemicals were of analytical grade.
4.2. Plant Material
The leaves of B. reticularia (400 g) were collected at Restinga de Jurubatiba National Park, Rio de Janeiro State, Brazil (22°14.105′ S, 41°35.822′ W). The identification was performed by the Botanist Dr. Marcelo Guerra Santos, and voucher specimen of B. reticularia was deposited at the herbarium of the Faculdade de Formação de Professores (Universidade do Estado do Rio de Janeiro, São Gonçalo, RJ, Brazil) under the register number RFFP 2097. The nomenclatural update was realized in Lista de Espécies da Flora do Brazil (http://floradobrasil.jbrj.gov.br), and The Plant List: A Working List of All Plant Species (http://www.theplantlist.org/).
4.3. Gas-Chromatographic Conditions and Identification of Chemical Constituents
The essential oil was analyzed by a GC-MS-QP2010 gas chromatograph equipped with a mass spectrometer using electron ionization (Shimadzu, Barueri, SP, Brazil). The GC conditions were as follows: Injector temperature, 260 °C; detector temperature, 290 °C; carrier gas (Helium), flow rate 1 mL min−1, and split injection with split ratio 1:40. Oven temperature was initially 60 °C and then raised to 290 °C at a rate of 3 °C min−1. The sample was diluted with n-hexane (1:100, v/v) and injected on a ZB-5 column (i.d. = 0.25 mm, length 30 m, film thickness = 0.25 μm). The MS conditions were voltage, 70 eV, and scan rate; 1 scan s−1. The retention index was calculated by the interpolation of each substance retention time and the retention time of a mixture of aliphatic hydrocarbons analyzed in the same conditions [57]. The identification of substances was performed by comparison of their retention index and mass spectra with those reported in the literature [58]. MS fragmentation pattern of compounds was also checked with NIST (National Institute of Standards and Technology) mass spectra libraries. Quantitative analysis of the chemical constituents was performed by GC-FID (Shimadzu, Barueri, SP, Brazil), under the same conditions of GC-MS analysis and percentages obtained by GC-FID were performed by peak area normalization method.
4.4. Quantitative Determination of B. reticularia Essential Oil Anticholinesterase Activity
Acetylcholinesterase (AChE) activity assay was performed using a method that uses acetylthiocholine iodide as substrate [59], with some modifications. 340 μL of test solution (1.25 mg mL−1 in MeOH), 1660 μL of 0.1 mM sodium phosphate buffer (pH 7.5) and 200 μL of AChE solution (30 mU/mL, sodium phosphate buffer 0.1 M pH 7.5) were mixed and incubated for 10 min at 25 °C. The reaction started with the addition of 1000 μL of 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB, 0.68 mM) and 200 μL of acetylthiocholine iodide (17 mM). The hydrolysis of acetylthiocholine iodide was monitored by the formation of the yellow 5-thio-2-nitrobenzoate anion as a result of the reaction of DTNB with thiocholine at 412 nm. The IC50 values (the concentration of test compounds that inhibit the hydrolysis of substrates by 50%) were estimated by linear regression of the natural log of concentration of essential oil versus percentage of remaining enzyme activity in the presence of essential oil and then solving the resulting equation for a 50% remaining activity [60]. The experiments were carried out in triplicate. Physostigmine was used as positive control. One unit of enzyme activity was defined as the amount of enzyme that produced 1 μmol of 5-thio-2-nitrobenzoate anion in 1 min under the conditions defined.
4.5. Determination of Required Hydrophile-Lipophile Balance (rHLB) of B. reticularia Essential Oil and Its Major Compound
Two non-ionic surfactants with low and high hydrophile-lipophile balance value (HLB) were blended together in order to achieve a wide range of HLB values (8.0–15.0). rHLB value of each blend was calculated as follows: rHLB = [(HLBsm × mSm) + (HLBp80 × mP80)]/(mSm + mP80), where HLBsm is the HLB of sorbitan monooleate, HLBp80 is the HLB of polysorbate 80, mSm is the mass (g) of sorbitan monooleate and mP80 is the mass of polysorbate 80. rHLB value of the B. reticularia essential oil and its major compound were determined as the HLB value of single surfactant or surfactant blend that was able to induce formation of most stable nanoemulsion.
4.6. Nanoemulsification
The nanoemulsions were prepared according to a non-heating and low energy method [61]. The B. reticularia essential oil and surfactant(s) were pooled together and homogenized for 30 min. Then, distilled water was added dropwise and the system was submitted to magnetic stirring for 1 h. The final concentration of B. reticularia essential oil was 2500 μg mL−1 and surfactant to oil ratio (SOR) was 1:1. This same procedure was used for the preparation of a nanoemulsion with the main constituent of B. reticularia essential oil.
4.7. Particle Size Distribution and Zeta Potential Measurements
Photon correlation spectroscopy (PCS) analysis was carried out using a Zetasizer Nano ZS, (Malvern Instruments, Malvern, UK) equipped with a 10 mW “red” laser (X = 632.8 nm). Samples were measured at a 90° scattering detector angle immediately after preparation (Day 0) and after 24 h (Day 1). The nanoemulsions were diluted with deionized water (1:25, v/v) for analysis. The measurements of droplet size, polydispersity index and zeta potential were performed in triplicate. Data was expressed as the mean ± standard deviation.
4.8. Larvicidal Activity
Aedes aegypti larvae were obtained from the Arthropoda Laboratory (Universidade Federal do Amapá, Macapá, AP, Brazil). Biological assay was performed under controlled conditions, being early fourth-instar larvae kept at 25 ± 2 °C, relative humidity of 75 ± 5% and a 12 h light-dark cycle. The experimental evaluation was performed according to World Health Organization protocol [62] with some modifications. All the experiments were performed in triplicate with 10 early stage fourth-instar larvae in each sample. B. reticularia essential oil and d-limonene nanoemulsions were diluted separately in distilled water at 25, 75, 125, 175 and 250 μg mL−1 (concentration expressed as essential oil or major compound content on aqueous media). The control group was constituted by deionized water. Mortality levels were recorded after 24 and 48 h of exposure.
4.9. Morphological Aedes Aegypti Larvae Study
The morphology of larvae was obtained according to Oliveira and coworkers [25]. Briefly, the larvae were incubated with the nanoemulsion containing the major compound at 250 μg mL−1, since it induced the highest mortality. After, they were fixed on ethanol 70% and evaluated by scanning electron microscopy under low vacuum using a Tabletop Microscope TM3030Plus (Hitachi, Ibaraki, Japan).
4.10. Statistical Analysis
Analysis of variance (two-way ANOVA) followed by Tukey’s test or Bonferroni’s test and linear regression for IC50 determination were conducted using the Software GraphPad Prism 6.0 (San Diego, CA, USA). Differences were considered significant when p < 0.05. Probit analysis was performed with 95% confidence interval for LC50 and LC90 determination using the software Statgraphics Centurion XV version 15.2.11 (Statpoint Technologies, The Plains, VA, USA).
5. Conclusions
Few studies about preparation of nanoemulsions by low energy methods with essential oils are available when compared to high-energy methods. In addition to the successful preparation of nanoemulsions with B. reticularia essential oil and d-limonene by a titration non-heating and solvent-free method, we showed the larvicidal potential of these nanostructured systems against A. aegypti, the main vector of the dengue, zika and chikungunya viruses. The facility of nanoemulsion preparation using an ecofriendly approach and the larvicidal activity indicate great perspectives for the further utilization of these raw materials for nanophytoproducts, which are potentially useful to control the mosquito vector by dispersing low water soluble compounds in aqueous media through innovative nanoemulsions.
Acknowledgments
Authors would like to thank FAPEAP and CNPQ for the financial support.
Author Contributions
G.d.S.B., F.B.d.A., R.F. and J.L.D. performed the experiments; R.A.S.C. and L.R. analyzed the chemical data; R.S.A. performed the nanoemulsion experiments; R.N.P.S. and J.C.T.C. analyzed the biological data; M.G.S. collected and identified the plant species; C.P.F. and V.L.P.P. conceived and designed the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yakob, L.; Funk, S.; Camacho, A.; Brady, O.; Edmunds, W.J. Aedes aegypti Control Through Modernized, Integrated Vector Management. PLoS Curr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dusfour, I.; Zorrilla, P.; Guidez, A.; Issaly, J.; Girod, R.; Guillaumot, L.; Robello, C.; Strode, C. Deltamethrin Resistance Mechanisms in Aedes aegypti Populations from Three French Overseas Territories Worldwide. PLoS Negl. Trop. Dis. 2015, 9, e0004226. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, F.; Caputo, B.; Chandre, F.; Drago, A.; della Torre, A.; Montarsi, F.; Rizzoli, A. Control methods against invasive Aedes mosquitoes in Europe: A review. Pest Manag. Sci. 2015, 71, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Darriet, F. An anti-mosquito mixture for domestic use, combining a fertiliser and a chemical or biological larvicide. Pest Manag. Sci. 2016, 72, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Heiden, G.; Schneider, A. Asteraceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB55 (accessed on 18 November 2016).
- The Brazil Flora Group (BFG). Growing knowledge: An overview of Seed Plant diversity in Brazil. Rodriguésia 2015, 66, 1085–1113. [Google Scholar] [CrossRef]
- Heiden, G.; Schneider, A. Baccharis, in Flora do Brasil 2020 em Construção, Jardim Botânico do Rio de Janeiro, Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB5151 (accessed on 8 November 2016).
- Santos, M.G.; Fevereiro, P.C.A.; Reis, G.L.; Barcelos, J.I. Recursos vegetais da Restinga de Carapebus, Rio de Janeiro, Brasil. Rev. Biol. Neotrop. 2009, 6, 35–54. [Google Scholar] [CrossRef]
- Sales, M.D.C.; Costa, H.B.; Fernandes, P.M.B.; Ventura, J.A.; Meira, D.D. Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pac. J. Trop. Biomed. 2016, 6, 26–31. [Google Scholar] [CrossRef]
- Gleiser, R.M.; Bonino, M.A.; Zygadlo, J.A. Repellence of essential oils of aromatic plants growing in Argentina against Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2011, 108, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bhat, B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic, D.; Sokovic, M.; Glamoclijz, J.; Dzamic, A.; Ciric, A.; Ristic, M.; Grubišic, D. Chemical composition and antimicrobial activity of Vitex agnus-castus L. fruits and leaves essential oils. Food Chem. 2011, 128, 1017–1022. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Arisoy, K.; Tepe, B.; Cakir, A.; Abali, G.; Mete, E. Studies on the antioxidant activity of essential oil and different solvente extracts of Vitex agnus castus L. fruits from Turkey. Food Chem. Toxicol. 2009, 47, 2479–2483. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.C.; Silva, T.K.M.; Silva, F.M.; Filho, J.M.B.; Marques, M.O.M.; Santos, R.L.C.; Cavalcanti, S.C.H.; Sousa, D.P. Larvicidal activity of Mentha x villosa Hudson essential oil, rotundifolone and derivatives. Chemosphere 2014, 104, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, X.C.; Liu, Z.L.; Xu, X. Chemical Composition of Salvia plebeian R.Br. Essential Oil and its Larvicidal Activity against Aedes aegypti L. Trop. J. Pharm. Res. 2015, 14, 831–836. [Google Scholar] [CrossRef]
- Parreira, N.A.; Magalhaes, L.G.; Morais, D.R.; Caixeta, S.C.; Sousa, J.P.B.; Bastos, J.K.; Cunha, W.R.; Silva, M.L.A.; Nanayakkara, N.P.D.; Rodrigues, V.; et al. Antiprotozoal, schistosomicidal, and antimicrobial activities of the essential oil from the leaves of Baccharis dracunculifolia. Chem. Biodivers. 2010, 7, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.C.; Ponzi, M.; Ardanaz, C.; Tonn, C.E.; Donadel, O.J. Chemical Composition of Baccharis salicifolia (Ruiz & Pavon) Pers. and Antibacterial Activity. J. Chil. Chem. Soc. 2009, 54, 475–476. [Google Scholar] [CrossRef]
- Kurdelas, R.R.; López, S.; Lima, B.; Feresin, G.E.; Zygadlo, J.; Zacchino, S.; López, M.L.; Tapia, A.; Freile, M.L. Chemical composition, anti-insect and antimicrobial activity of Baccharis darwinii essential oil from Argentina, Patagonia. Ind. Crops Prod. 2012, 40, 261–267. [Google Scholar] [CrossRef]
- García, M.; Donadel, O.J.; Ardanaz, C.E.; Tonn, C.E.; Sosa, M.E. Toxic and repellent effects of Baccharis salicifolia essential oil on Tribolium castaneum. Pest Manag. Sci. 2005, 61, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.N.; Rehder, V.L.G.; Oliveira, A.S.S.; Júnior, I.M.; Carvalho, J.E.; Ruiz, A.L.T.G.; Jeraldo, V.L.S.; Linhares, A.X.; Allegretti, S.M. Schistosoma mansoni: In vitro schistosomicidal activity of essential oil of Baccharis trimera (less) DC. Exp. Parasitol. 2012, 132, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, R.; Ghaderi, L.; Rafati, H.; Aliahmadi, A.; McClements, D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016, 194, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.E.; Duarte, J.L.; Amado, J.R.; Cruz, R.A.; Rocha, C.F.; Souto, R.N.; Ferreira, R.M.; Santos, K.; da Conceição, E.C.; de Oliveira, L.A.; et al. Development of a Larvicidal Nanoemulsion with Pterodon emarginatus Vogel Oil. PLoS ONE 2016, 11, e0145835. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.C.R.; Ferreira, A.M.; Vilhena, J.C.E.; Almeida, F.B.; Cruz, R.A.S.; Florentino, A.C.; Souto, R.N.P.; Carvalho, J.C.T.; Fernandes, C.P. Development of a larvicidal nanoemulsion with Copaiba (Copaifera duckei) oleoresin. Rev. Bras. Farmacogn. 2014, 24, 699–705. [Google Scholar] [CrossRef]
- Oliveira, A.E.M.F.M.; Duarte, J.L.; Cruz, R.A.S.; Souto, R.N.P.; Ferreira, R.M.A.; Peniche, T.; Conceição, E.C.; Oliveira, L.A.R.; Faustino, S.M.M.; Florentino, A.C.; et al. Pterodon emarginatus oleoresin-based nanoemulsion as a promising tool for Culex quinquefasciatus (Diptera: Culicidae) control. J. Nanobiotechnol. 2017, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Montefuscoli, A.R.; Werdin González, J.O.; Palma, S.D.; Ferrero, A.A.; Fernández Band, B. Design and development of aqueous nanoformulations for mosquito control. Parasitol. Res. 2014, 113, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.L.; Amado, J.R.R.; Oliveira, A.E.M.F.M.; Cruz, R.A.S.; Ferreira, A.M.; Souto, R.N.P.; Falcão, D.Q.; Carvalho, J.C.T.; Fernandes, C.P. Evaluation of larvicidal activity of a nanoemulsion of Rosmarinus officinalis essential oil. Rev. Bras. Farmacogn. 2015, 25, 189–192. [Google Scholar] [CrossRef]
- Oliveira, A.E.M.F.M.; Bezerra, D.C.; Duarte, J.L.; Cruz, R.A.S.; Souto, R.N.P.; Ferreira, R.M.A.; Nogueira, J.; Conceição, E.C.; Leitão, S.; Bizzo, H.R.; et al. Essential oil from Pterodon emarginatus as a promising natural raw material for larvicidal nanoemulsions against a tropical disease vector. Sustain. Chem. Pharm. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Optimization of Process Parameters to Formulate Nanoemulsion by Spontaneous Emulsification: Evaluation of Larvicidal Activity Against Culex quinquefasciatus Larva. BioNanoScience 2014, 4, 157–165. [Google Scholar] [CrossRef]
- Trombin-Souza, M.; Amaral, W.; Pascoalino, J.A.L.; Oliveira, R.A.; Bizzo, H.R.; Deschamps, C. Chemical composition of the essential oils of Baccharis species from southern Brazil: A comparative study using multivariate statistical analysis. J. Essent. Oil Res. 2017, 29, 400–406. [Google Scholar] [CrossRef]
- Agostini, F.; Santos, A.C.A.; Rossato, M.; Pansera, M.R.; Zattera, F.; Wasum, R.; Serafini, L.A. Studies on the essential oils from several Baccharis (Asteraceae) from Southern Brazil. Rev. Bras. Farmacogn. 2005, 15, 215–219. [Google Scholar] [CrossRef]
- Valarezo, E.; Rosillo, M.; Cartuche, L.; Malagón, O.; Meneses, M.; Morocho, V. Chemical composition, antifungal and antibacterial activity of the essential oil from Baccharis latifolia (Ruiz & Pav.) Pers. (Asteraceae) from Loja, Ecuador. J. Essent. Oil Res. 2013, 25, 233–238. [Google Scholar] [CrossRef]
- Ramos Campos, F.; Bressan, J.; Godoy Jasinski, V.C.; Zuccolotto, T.; da Silva, L.E.; Bonancio Cerqueira, L. Baccharis (Asteraceae): Chemical Constituents and Biological Activities. Chem. Biodivers. 2016, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Espina, L.; Gelaw, T.K.; Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. Mechanism of Bacterial Inactivation by (+)-Limonene and Its Potential Use in Food Preservation Combined Processes. PLoS ONE 2013, 8, e56769. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Kainulainen, P.; Aflatuni, A.; Tiilikkala, K.; Holopainen, J.K. Insecticidal, repellent, antimicrobial activity and phytotoxicity of essential oils: With special reference to limonene and its suitability for control of insect pests. Agric. Food Sci. 2001, 10, 243–259. [Google Scholar]
- Dohi, S.; Terasaki, M.; Makino, M. Acetylcholinesterase Inhibitory Activity and Chemical Composition of Commercial Essential Oils. J. Agric. Food Chem. 2009, 57, 4313–4318. [Google Scholar] [CrossRef] [PubMed]
- Savelev, S.U.; Okello, E.J.; Perry, E.K. Butyryl- and Acetyl-cholinesterase Inhibitory Activities in Essential Oils of Salvia Species and Their Constituents. Phytother. Res. 2004, 18, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.F.; Byrne, O. Plant-insect coevolution and inhibition of acetyl-cholinesterase. J. Chem. Ecol. 1998, 14, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Blenau, W.; Rademacher, E.; Baumann, A. Plant essential oils and formamidines as insecticides/acaricides: what are the molecular targets? Apidologie 2012, 43, 334. [Google Scholar] [CrossRef]
- Carpinella, M.C.; Andrione, D.G.; Ruiz, G.; Palacios, S.M. Screening for acetyl-cholinesterase inhibitory activity in plant extracts from Argentina. Phytother. Res. 2010, 24, 259–263. [Google Scholar] [CrossRef] [PubMed]
- San-Martín, A.; Astudillo, L.; Gutiérrez, M.; Chamy, M.C.; Orejarena, S.; Rivera, P.; Vergara, K. 13- Epi-Neoclerodanes from Baccharis marginalis. J. Chil. Chem. Soc. 2010, 55, 118–120. [Google Scholar] [CrossRef]
- Seo, S.; Kim, J.; Kang, J.; Koh, S.; Ahn, Y.; Kang, K.; Park, I. Fumigant toxicity and acetylcholinesterase inhibitory activity of 4 Asteraceae plant essential oils and their constituents against Japanese termite (Kolbe). Pestic. Biochem. Physiol. 2014, 113, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.M.; Jung, C.S.; Kang, J.; Lee, H.R.; Kim, S.W.; Hyun, J.; Park, I.K. Larvicidal and acetylcholinesterase inhibitory activities of Apiaceae plant essential oils and their constituents against aedes albopictus and formulation development. J. Agric. Food Chem. 2015, 63, 9977–9986. [Google Scholar] [CrossRef] [PubMed]
- Menichini, F.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; Marrelli, M.; Statti, G.A.; Menichini, F.; Conforti, F. Acetylcholinesterase and butyrylcholinesterase inhibition of ethanolic extract and monoterpenes from Pimpinella anisoides V Brig. (Apiaceae). Fitoterapia 2009, 80, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Borloz, A.; Urbain, A.; Marston, A. Natural Product Inhibitors of Acetylcholinesterase. Curr. Org. Chem. 2006, 10, 825–847. [Google Scholar] [CrossRef]
- Miyazawa, M.; Tougo, H.; Ishihara, M. Inhibition of acetylcholinesterase activity by essential oil from Citrus paradisi. Nat. Prod. Lett. 2001, 15, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Savelev, S.; Okello, E.; Perry, N.S.L.; Wilkins, R.M.; Perry, E.K. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 2003, 75, 661–668. [Google Scholar] [CrossRef]
- Chantraine, J.M.; Laurent, D.; Ballivian, C.; Saavedra, G.; Ibañez, R.; Vilaseca, L.A. Insecticidal activity of essential oils on Aedes aegypti larvae. Phytother. Res. 1998, 12, 350–354. [Google Scholar] [CrossRef]
- Zahran, H.E.M.; Abdelgaleil, S.A.M. Insecticidal and developmental inhibitory properties of monoterpenes on Culex pipiens L. (Diptera: Culicidae). J. Asia-Pac. Entomol. 2011, 14, 46–51. [Google Scholar] [CrossRef]
- Kassir, J.T.; Mohsen, Z.H.; Mehdi, N.S. Toxic effects of limonene against Culex quinquefasciatus Say larvae and its interference with oviposition. Anzeiger Schädlingskunde Pflanzenschutz Umweltschutz 1989, 62, 19–21. [Google Scholar] [CrossRef]
- Jesus, F.L.M.; Almeida, F.B.; Duarte, J.L.; Oliveira, A.E.M.F.M.; Cruz, R.A.S.; Souto, R.N.P.; Ferreira, R.M.A.; Kelmann, R.G.; Carvalho, J.C.T.; Lira-Guedes, A.C.; et al. Preparation of a Nanoemulsion with Carapa guianensis Aublet (Meliaceae) Oil by a Low-Energy/Solvent-Free Method and Evaluation of Its Preliminary Residual Larvicidal Activity. Evid.-Based. Complement. Altern. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Lin, C.Y.; Chung, M.J.; Liu, Y.H.; Huang, C.G.; Chang, S.T. Larvicidal activities of wood and leaf essential oils and ethanolic extracts from Cunninghamia konishii Hayata against the dengue mosquitoes. Ind. Crops Prod. 2013, 47, 310–315. [Google Scholar] [CrossRef]
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.A.; Arruda, W.; Oliveira, E.S.F.; Cavasin, G.M.; Silva, H.H.G.; Silva, I.G. Mecanismos da ação larvicida do diflubenzuron sobre Aedes aegypti evidenciados pelas alterações ultraestruturais. Rev. Patol. Trop. 2012, 41, 222–232. [Google Scholar] [CrossRef]
- Alves, S.N.; Serrao, J.E.; Melo, A.L. Alterations in the fat body and midgut of Culex quinquefasciatus larvae following exposure to different insecticides. Micron 2010, 41, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Van Den Doll, H.; Kratz, D.J. A generalization of the retention index system including liner temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–467. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Kemp, R.J.; Wallace, B.K. Molecular Determinants of Species-Selective Inhibition of Brain Acetylcholinesterase. Toxicol. Appl. Pharmacol. 1990, 104, 246–258. [Google Scholar] [CrossRef]
- Ostertag, F.; Weiss, J.; McClements, D.J. Low-energy formation of edible nanoemulsions: Factors influencing droplet size produced by emulsion phase inversion. J. Colloid Interface Sci. 2012, 388, 95–102. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines for Laboratory and Field Testing of Mosquito Larvicides; Communicable Disease Control, Prevention and Eradication, WHO Pesticide Evaluation Scheme; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
Sample Availability: Samples of the essential oil from Baccharis reticularia are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).