Early Response Monitoring Following Radiation Therapy by Using [18F]FDG and [11C]Acetate PET in Prostate Cancer Xenograft Model with Metabolomics Corroboration
Abstract
:1. Introduction
2. Results
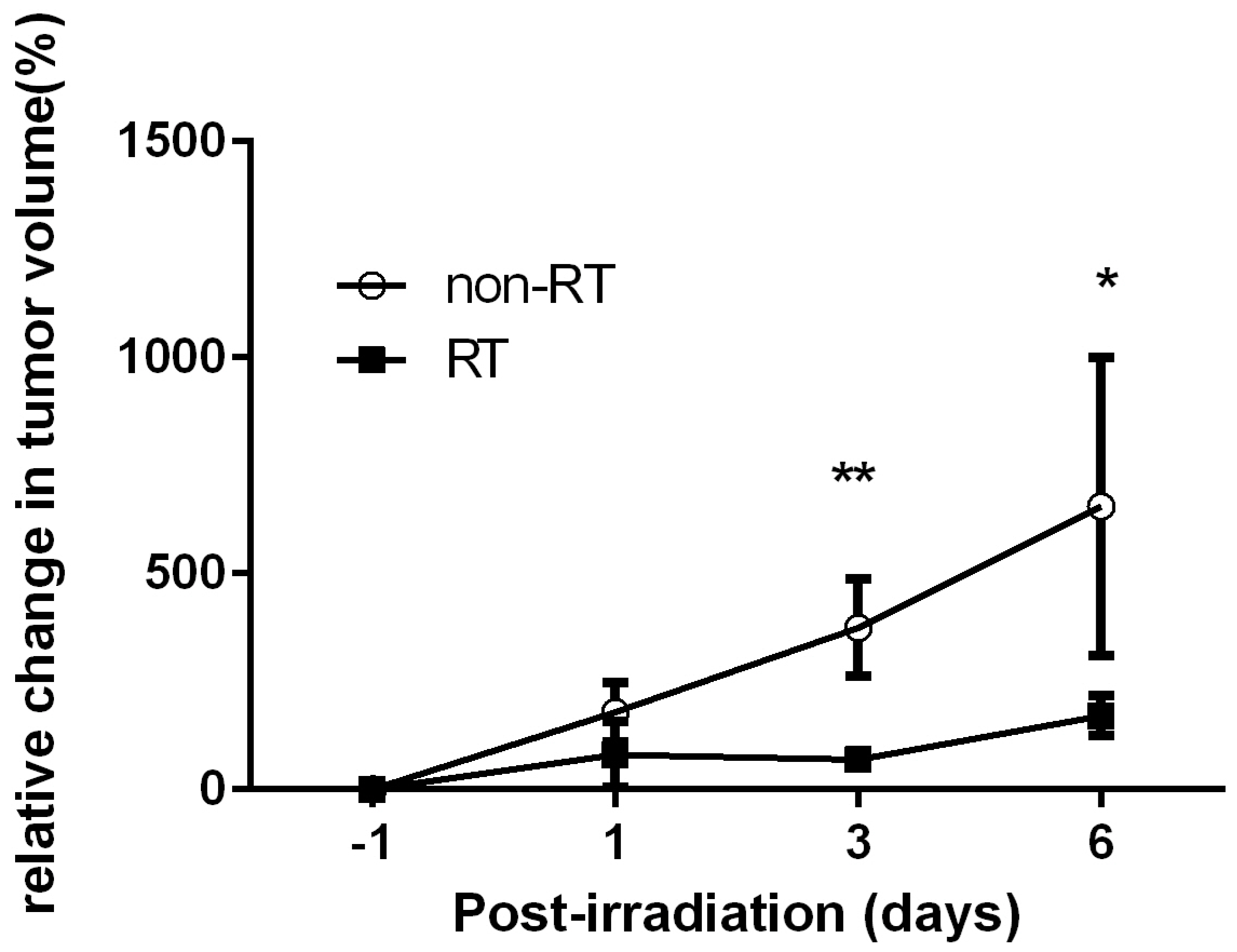
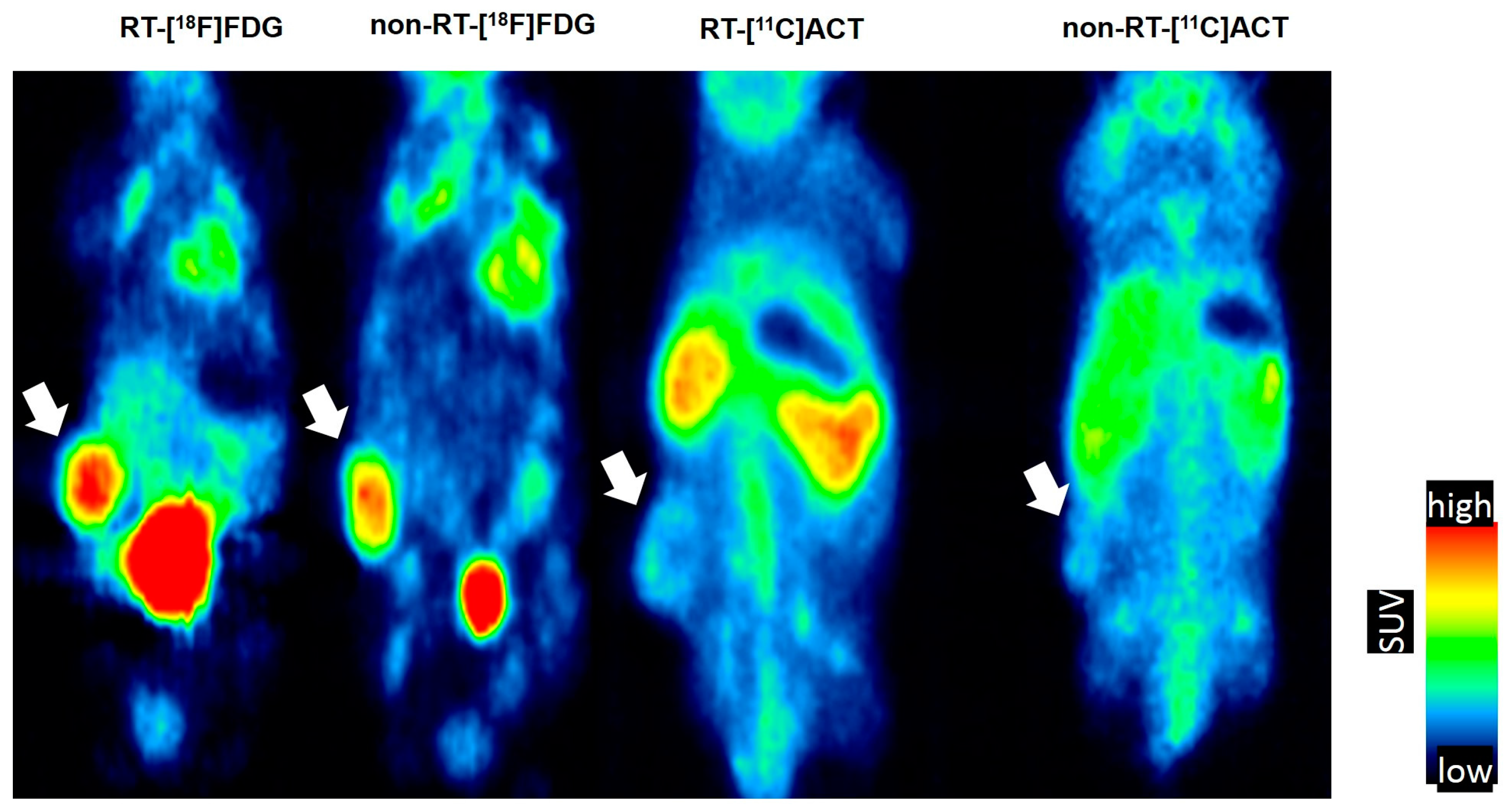
2.1. Early Changes on [18F]FDG PET of Tramp-C Prostate Tumors Following Irradiation
2.2. No Remarkable Changes on [11C]ACT PET of Tramp-C Prostate Tumors Following Irradiation
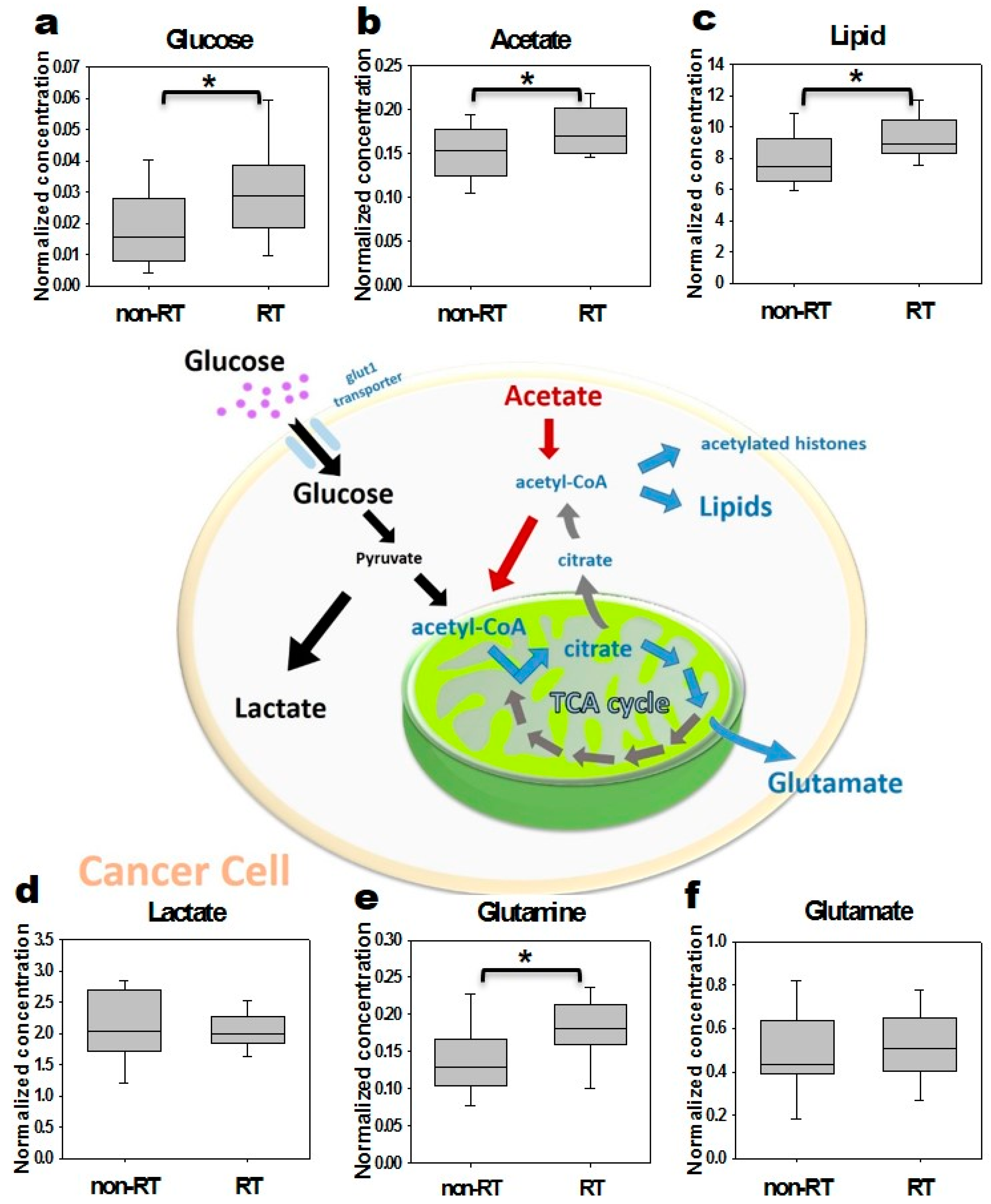
2.3. In Vitro Cells NMR Corroboration
2.4. Ex Vivo Tissue NMR Corroboration
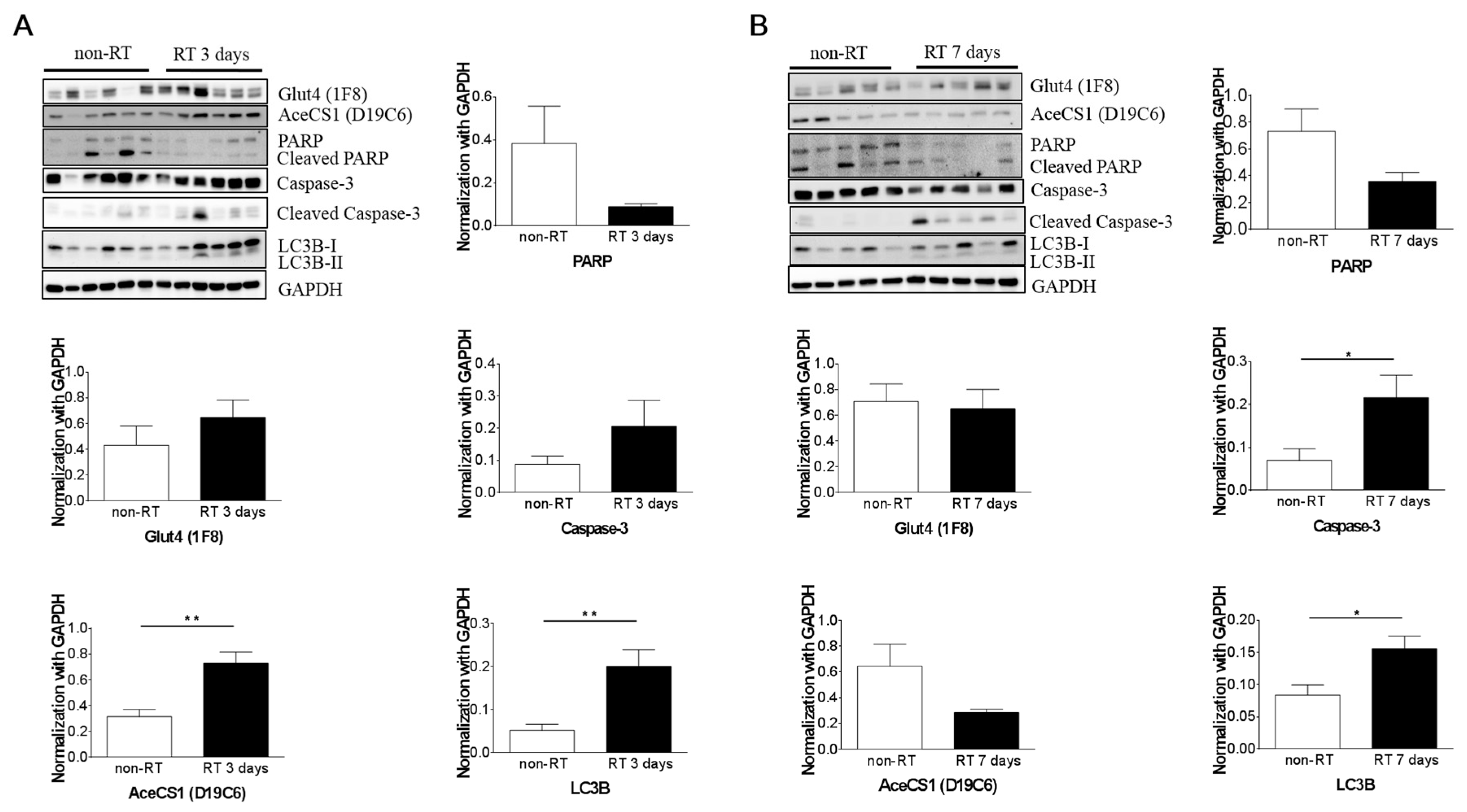
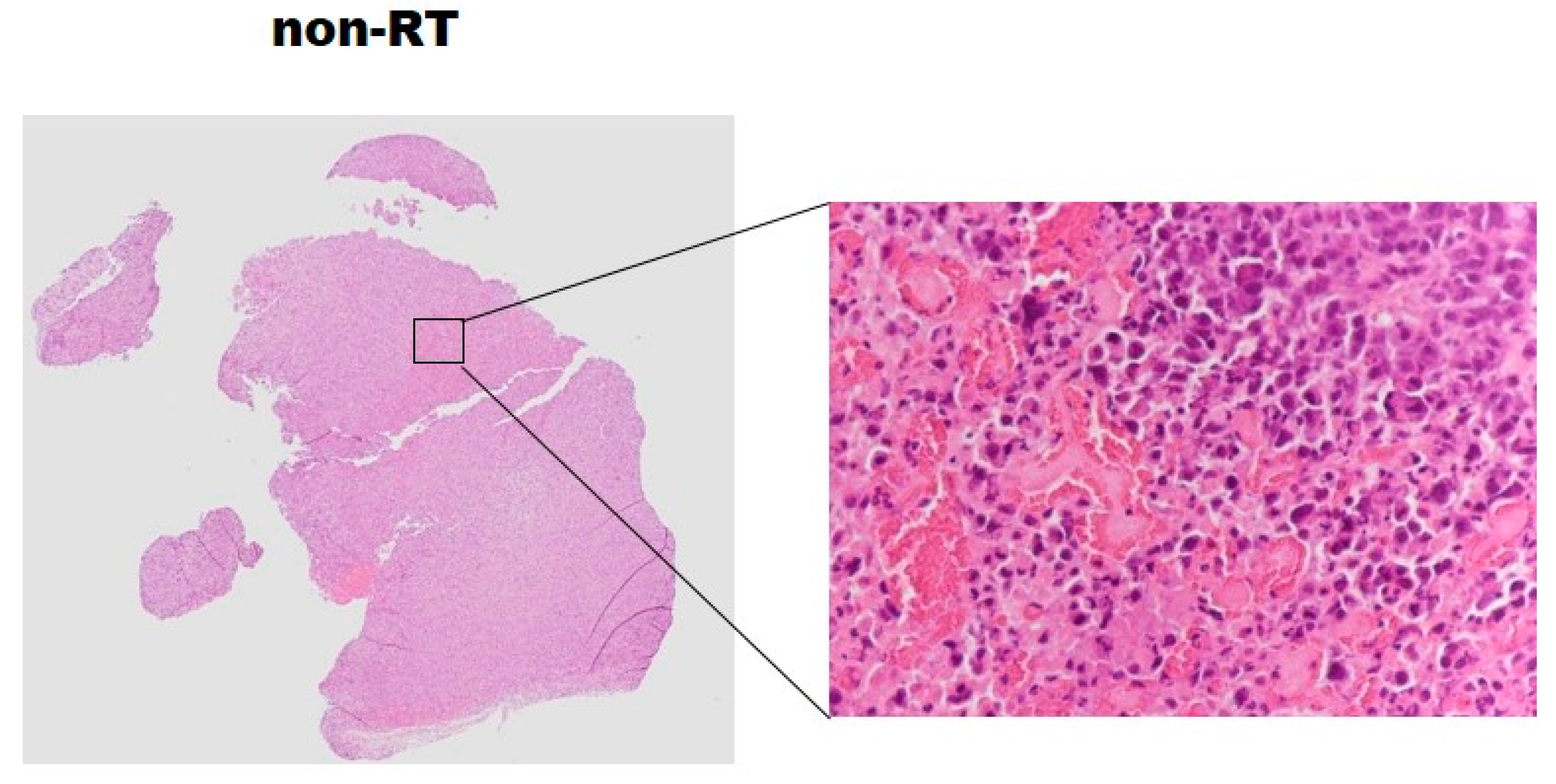
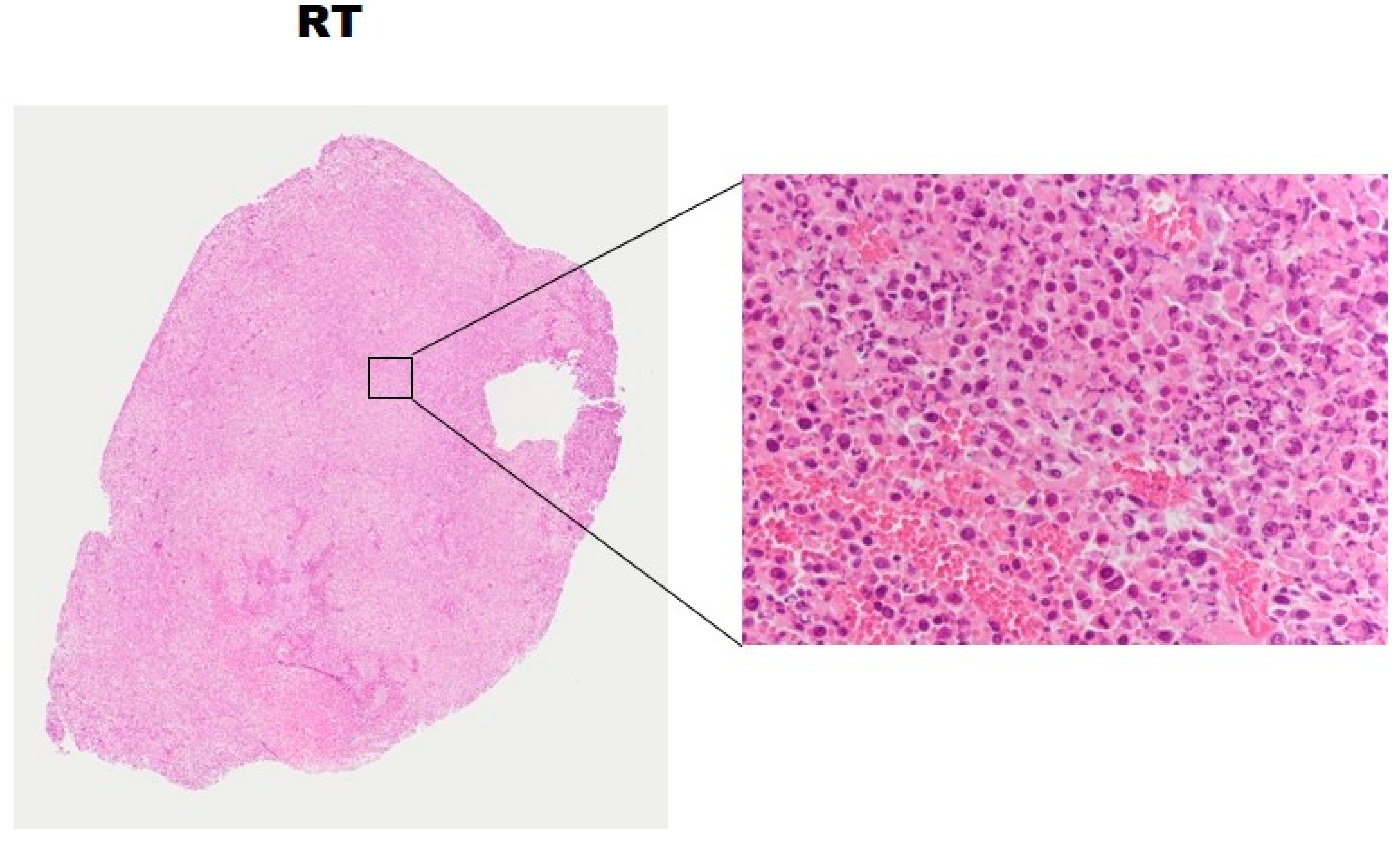
2.5. Histopathology and Western Blotting Verifications
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Animal Experiment
4.3. Irradiation Setup
4.4. [18F]FDG and [11C]ACT PET and Imaging Analysis
4.5. NMR Metabolomic Analysis
4.6. Western Blotting and Histologic Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.B.; Coia, L.R.; Hanks, G.E. Recent patterns of growth in radiation therapy facilities in the United States: A pattern of care study report. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 983–986. [Google Scholar] [CrossRef]
- Buzdar, S.A.; Afzal, M.; Nazir, A.; Gadhi, M.A. Accuracy requirements in radiotherapy treatment planning. J. Coll. Physicians Surg. Pak. 2013, 23, 418–423. [Google Scholar] [PubMed]
- Jiang, M.; Huang, Q.; Chen, P.; Ruan, X.; Luo, Z.; Zhao, L.; Fan, W.; Peng, T.; Sun, L.; Wu, H. Monitoring the early biologic response of esophageal carcinoma after irradiation with 18F-FLT: An in vitro and in vivo study. Nucl. Med. Commun. 2014, 35, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Mardor, Y.; Pfeffer, R.; Spiegelmann, R.; Roth, Y.; Maier, S.E.; Nissim, O.; Berger, R.; Glicksman, A.; Baram, J.; Orenstein, A.; et al. Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J. Clin. Oncol. 2003, 21, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, B.; Tian, J.H.; Xu, B.X.; Guan, Z.W.; Qu, B.L.; Liu, C.B.; Wang, R.M.; Chen, Y.M.; Zhang, J.M. Monitoring early responses to irradiation with dual-tracer micro-PET in dual-tumor bearing mice. World J. Gastroenterol. 2010, 16, 5416–5423. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, S.Y.; Kil, I.S.; Park, J.W. Regulation of ionizing radiation-induced apoptosis by mitochondrial NADP+-dependent isocitrate dehydrogenase. J. Biol. Chem. 2007, 282, 13385–13394. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kobayashi, T.; Nishioka, A.; Kariya, S.; Hamasato, S.; Seguchi, H.; Yoshida, S. Radiation-induced reactive oxygen species formation prior to oxidative DNA damage in human peripheral T cells. Int. J. Mol. Med. 2003, 11, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, K.B.; Devipriya, N.; Srinivasan, M.; Menon, V.P. Investigation of the radioprotective efficacy of hesperidin against gamma-radiation induced cellular damage in cultured human peripheral blood lymphocytes. Mutat. Res. 2009, 676, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Radons, J. Radiation, inflammation, and immune responses in cancer. Front. Oncol. 2012, 2, 58. [Google Scholar] [CrossRef] [PubMed]
- Dewey, W.C.; Ling, C.C.; Meyn, R.E. Radiation-induced apoptosis: Relevance to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 781–796. [Google Scholar] [CrossRef]
- Shah, R.; Vattoth, S.; Jacob, R.; Manzil, F.F.; O’Malley, J.P.; Borghei, P.; Patel, B.N.; Cure, J.K. Radiation necrosis in the brain: Imaging features and differentiation from tumor recurrence. Radiographics 2012, 32, 1343–1359. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Stoffels, G.; Filss, C.P.; Piroth, M.D.; Sabel, M.; Ruge, M.I.; Herzog, H.; Shah, N.J.; Fink, G.R.; Coenen, H.H.; et al. Role of O-(2-(18)F-fluoroethyl)-l-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J. Nucl. Med. 2012, 53, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.E.; Lim, R.; Huang, C.; Chebib, I.A.; El Fakhri, G. Synergistic role of simultaneous PET/MRI-MRS in soft tissue sarcoma metabolism imaging. Magn. Reson. Imaging 2016, 34, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Kortylewicz, Z.P.; Enke, T.; Baranowska-Kortylewicz, J. Co-targeting androgen receptor and DNA for imaging and molecular radiotherapy of prostate cancer: In vitro studies. Prostate 2014, 74, 1634–1646. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.P.; Chu, W.; Gu, Y.P.; Cunningham, C.H. Probing early tumor response to radiation therapy using hyperpolarized [1-(1)(3)C]pyruvate in MDA-MB-231 xenografts. PLoS ONE 2013, 8, e56551. [Google Scholar]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Zhang, X.; Wang, X.; Kang, Y.; Riley, B.; Bilton, S.; Mohan, R.; Komaki, R.; Cox, J.D. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Nanni, C.; Pettinato, C.; Ambrosini, V.; Spinelli, A.; Trespidi, S.; Rubello, D.; Al-Nahhas, A.; Franchi, R.; Alavi, A.; Fanti, S. Retro-orbital injection is an effective route for radiopharmaceutical administration in mice during small-animal PET studies. Nucl. Med. Commun. 2007, 28, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Monakhov, N.K.; Neistadt, E.L.; Shavlovskil, M.M.; Shvartsman, A.L.; Neifakh, S.A. Physicochemical properties and isoenzyme composition of hexokinase from normal and malignant human tissues. J. Natl. Cancer Inst. 1978, 61, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Early Breast Cancer Trialists’ Collaborative Group, Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 366, 2087–2106. [Google Scholar] [PubMed]
- Cascini, G.L.; Avallone, A.; Delrio, P.; Guida, C.; Tatangelo, F.; Marone, P.; Aloj, L.; De Martinis, F.; Comella, P.; Parisi, V.; et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J. Nucl. Med. 2006, 47, 1241–1248. [Google Scholar] [PubMed]
- Castaldi, P.; Leccisotti, L.; Bussu, F.; Micciche, F.; Rufini, V. Role of (18)F-FDG PET-CT in head and neck squamous cell carcinoma. Acta Otorhinolaryngol. Ital. 2013, 33, 1–8. [Google Scholar] [PubMed]
- Castaldi, P.; Rufini, V.; Bussu, F.; Micciche, F.; Dinapoli, N.; Autorino, R.; Lago, M.; De Corso, E.; Almadori, G.; Galli, J.; et al. Can “early” and “late” 18F-FDG PET-CT be used as prognostic factors for the clinical outcome of patients with locally advanced head and neck cancer treated with radio-chemotherapy? Radiother. Oncol. 2012, 103, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, M.; Appold, S.; Schreiber, A.; Abolmaali, N.; Abramyuk, A.; Dorr, W.; Kotzerke, J.; Baumann, M.; Zophel, K. Early FDG PET at 10 or 20 Gy under chemoradiotherapy is prognostic for locoregional control and overall survival in patients with head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, P.E.; Fletcher, J.W. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin. Ultrasound CT MR 2010, 31, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Doot, R.; Allberg, K.; Kinahan, P. Errors in serial PET SUV measurements. J. Nucl. Med. 2010, 51, 126. [Google Scholar]
- Larson, S.M.; Erdi, Y.; Akhurst, T.; Mazumdar, M.; Macapinlac, H.A.; Finn, R.D.; Casilla, C.; Fazzari, M.; Srivastava, N.; Yeung, H.W.; et al. Tumor Treatment Response Based on Visual and Quantitative Changes in Global Tumor Glycolysis Using PET-FDG Imaging. The Visual Response Score and the Change in Total Lesion Glycolysis. Clin. Positron Imaging 1999, 2, 159–171. [Google Scholar] [CrossRef]
- Choi, E.S.; Ha, S.G.; Kim, H.S.; Ha, J.H.; Paeng, J.C.; Han, I. Total lesion glycolysis by 18F-FDG PET/CT is a reliable predictor of prognosis in soft-tissue sarcoma. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Lin, P.; Lee, M.T.; Shon, I.H.; Lin, M.; Forstner, D.; Bray, V.; Chicco, A.; Tieu, M.T.; Fowler, A. Prognostic role of metabolic parameters of (18)F-FDG PET-CT scan performed during radiation therapy in locally advanced head and neck squamous cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Brogsitter, C.; Zophel, K.; Kotzerke, J. 18F-Choline, 11C-choline and 11C-acetate PET/CT: Comparative analysis for imaging prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2013, 40 (Suppl. S1), S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Oyama, N.; Kim, J.; Jones, L.A.; Mercer, N.M.; Engelbach, J.A.; Sharp, T.L.; Welch, M.J. MicroPET assessment of androgenic control of glucose and acetate uptake in the rat prostate and a prostate cancer tumor model. Nucl. Med. Biol. 2002, 29, 783–790. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Molthoff, C.F.; Klabbers, B.M.; Berkhof, J.; Felten, J.T.; van Gelder, M.; Windhorst, A.D.; Slotman, B.J.; Lammertsma, A.A. Monitoring response to radiotherapy in human squamous cell cancer bearing nude mice: Comparison of 2′-deoxy-2′-[18F]fluoro-D-glucose (FDG) and 3′-[18F]fluoro-3′-deoxythymidine (FLT). Mol. Imaging Biol. 2007, 9, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, L.K.; Boerman, O.C.; Franssen, G.M.; Kaanders, J.H.; Bussink, J. 111In-cetuximab-F(ab’)2 SPECT and 18F-FDG PET for prediction and response monitoring of combined-modality treatment of human head and neck carcinomas in a mouse model. J. Nucl. Med. 2015, 56, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, L.K.; Boerman, O.C.; Franssen, G.M.; Lok, J.; Kaanders, J.H.; Bussink, J. Early response monitoring with 18F-FDG PET and cetuximab-F(ab’)2-SPECT after radiotherapy of human head and neck squamous cell carcinomas in a mouse model. J. Nucl. Med. 2014, 55, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Jeraj, R.; Bradshaw, T.; Simoncic, U. Molecular Imaging to Plan Radiotherapy and Evaluate Its Efficacy. J. Nucl. Med. 2015, 56, 1752–1765. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, B.; Giorgio, T.; Rasoul, Z.S.; Werner, L.; Ali, G.R.; Reza, D.K.; Ramin, S. Application of C-11-acetate positron-emission tomography (PET) imaging in prostate cancer: Systematic review and meta-analysis of the literature. BJU Int. 2013, 112, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Dendooven, P.; Buitenhuis, H.J.; Diblen, F.; Heeres, P.N.; Biegun, A.K.; Fiedler, F.; van Goethem, M.J.; van der Graaf, E.R.; Brandenburg, S. Short-lived positron emitters in beam-on PET imaging during proton therapy. Phys. Med. Biol. 2015, 60, 8923–8947. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Wang, J.J.; Hong, J.H.; Lin, Y.P.; Lee, C.C.; Wai, Y.Y.; Ng, S.H.; Wu, Y.M.; Wang, C.C. Noninvasive monitoring of microvascular changes with partial irradiation using dynamic contrast-enhanced and blood oxygen level-dependent magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Kuan, F.C.; Yen, T.C.; Lu, M.S.; Lin, P.Y.; Chung, Y.H.; Chen, W.C.; Lee, K.D. IL-6-stimulated CD11b+ CD14+ HLA-DR- myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget 2014, 5, 8716–8728. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Hsu, P.H.; Huang, C.W.; Hsieh, W.C.; Huang, F.T.; Chang, W.C.; Chiu, H.; Hsu, S.T.; Yen, T.C. Evaluation of prognostic integrin alpha2beta1 PET tracer and concurrent targeting delivery using focused ultrasound for brain glioma detection. Mol. Pharm. 2014, 11, 3904–3914. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Hill, D.K.; Andrejeva, G.; Boult, J.K.; Troy, H.; Fong, A.C.; Orton, M.R.; Panek, R.; Parkes, H.G.; Jafar, M.; et al. Dichloroacetate induces autophagy in colorectal cancer cells and tumours. Br. J. Cancer 2014, 111, 375–385. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the all compounds are not available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, Y.-H.; Tsai, C.-K.; Wang, C.-C.; Chen, H.-M.; Lu, K.-Y.; Chiu, H.; Lin, Y.-C.; Yen, T.-C.; Lin, G. Early Response Monitoring Following Radiation Therapy by Using [18F]FDG and [11C]Acetate PET in Prostate Cancer Xenograft Model with Metabolomics Corroboration. Molecules 2017, 22, 1946. https://doi.org/10.3390/molecules22111946
Chung Y-H, Tsai C-K, Wang C-C, Chen H-M, Lu K-Y, Chiu H, Lin Y-C, Yen T-C, Lin G. Early Response Monitoring Following Radiation Therapy by Using [18F]FDG and [11C]Acetate PET in Prostate Cancer Xenograft Model with Metabolomics Corroboration. Molecules. 2017; 22(11):1946. https://doi.org/10.3390/molecules22111946
Chicago/Turabian StyleChung, Yi-Hsiu, Cheng-Kun Tsai, Chiun-Chieh Wang, Hsi-Mu Chen, Kuan-Ying Lu, Han Chiu, Yu-Chun Lin, Tzu-Chen Yen, and Gigin Lin. 2017. "Early Response Monitoring Following Radiation Therapy by Using [18F]FDG and [11C]Acetate PET in Prostate Cancer Xenograft Model with Metabolomics Corroboration" Molecules 22, no. 11: 1946. https://doi.org/10.3390/molecules22111946
APA StyleChung, Y.-H., Tsai, C.-K., Wang, C.-C., Chen, H.-M., Lu, K.-Y., Chiu, H., Lin, Y.-C., Yen, T.-C., & Lin, G. (2017). Early Response Monitoring Following Radiation Therapy by Using [18F]FDG and [11C]Acetate PET in Prostate Cancer Xenograft Model with Metabolomics Corroboration. Molecules, 22(11), 1946. https://doi.org/10.3390/molecules22111946






