The Oligomeric Form of the Escherichia coli Dps Protein Depends on the Availability of Iron Ions
Abstract
:1. Introduction
2. Results
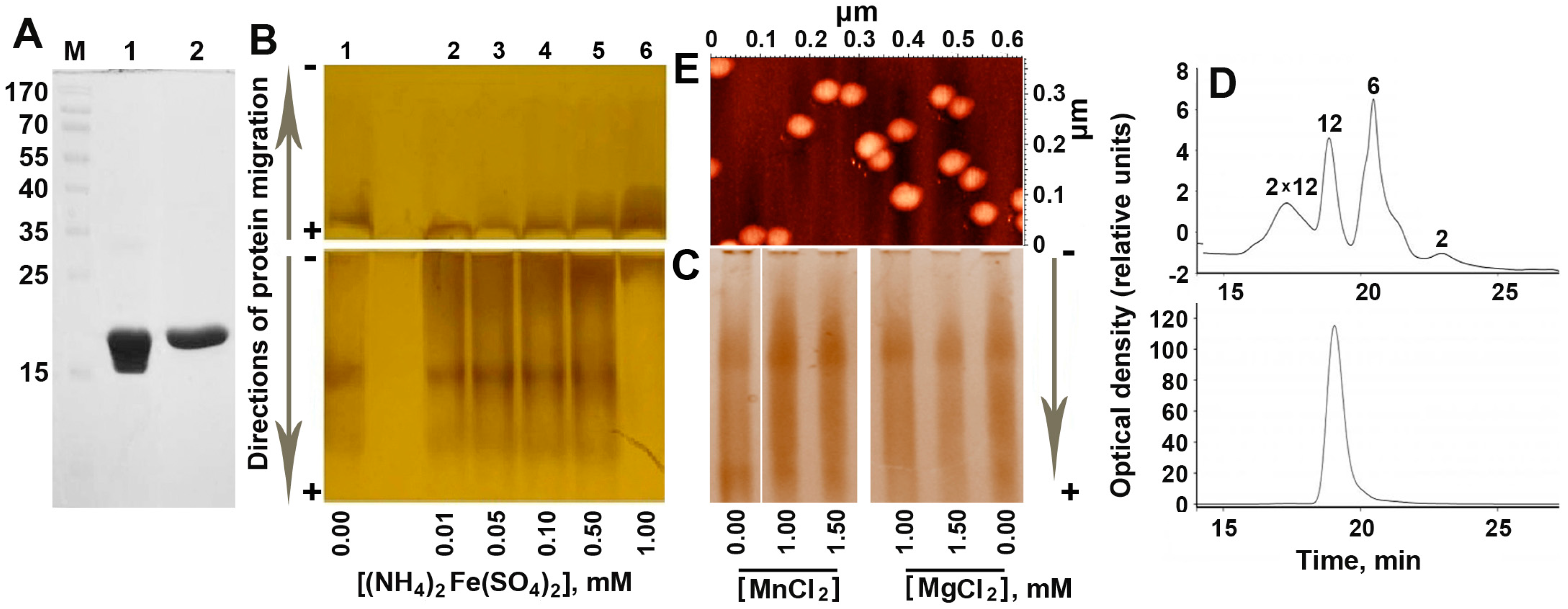
2.1. Increasing Concentration of Ferrous Ions Stabilizes Dodecameric Form of Dps
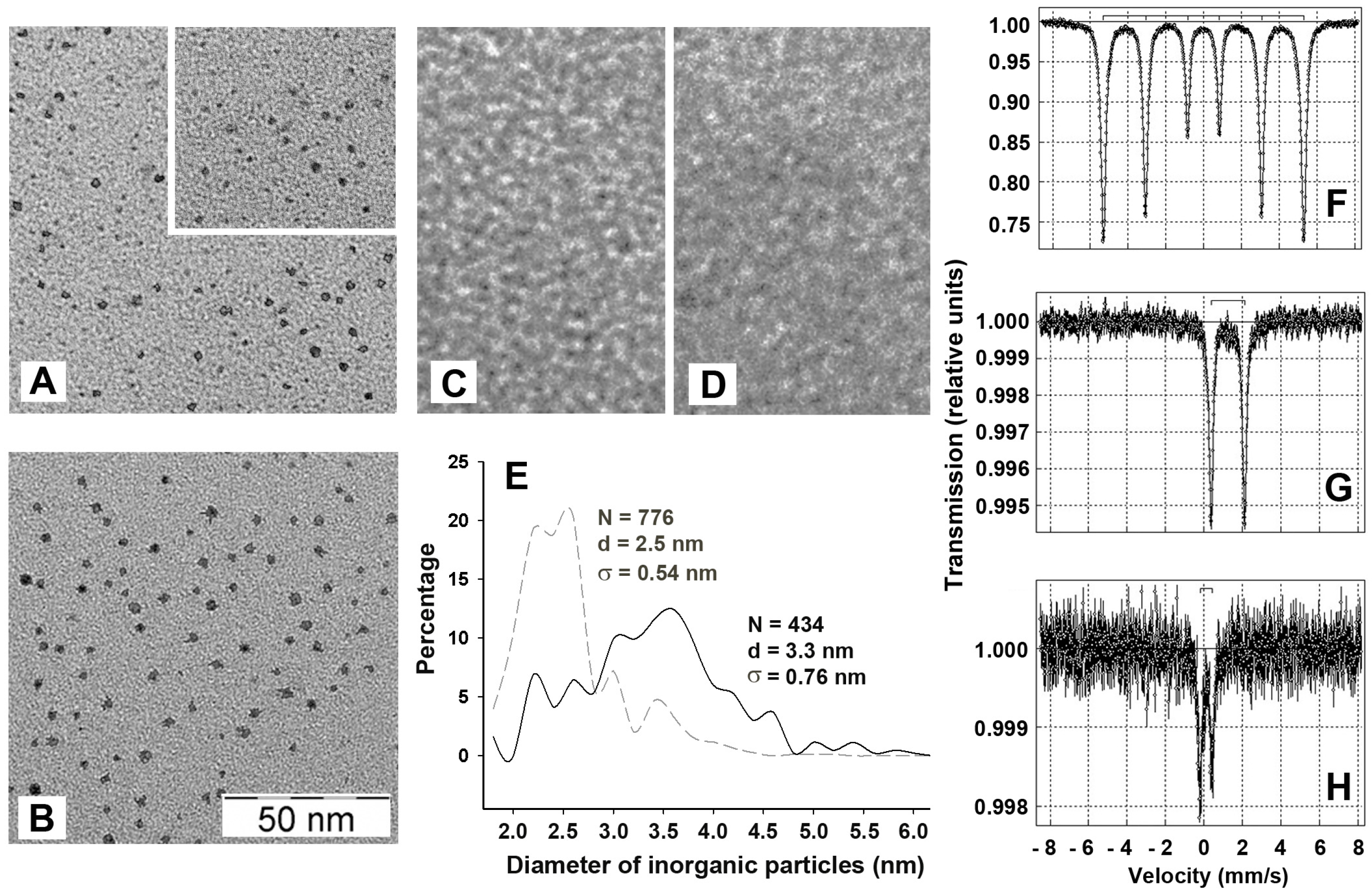
2.2. The Inorganic Cores in the Inner Cavity of the Dps Protein are Non-Uniform in Size
2.3. Mössbauer Spectroscopy Testified the Presence of Fe3O4 in the Inorganic Content of Dps
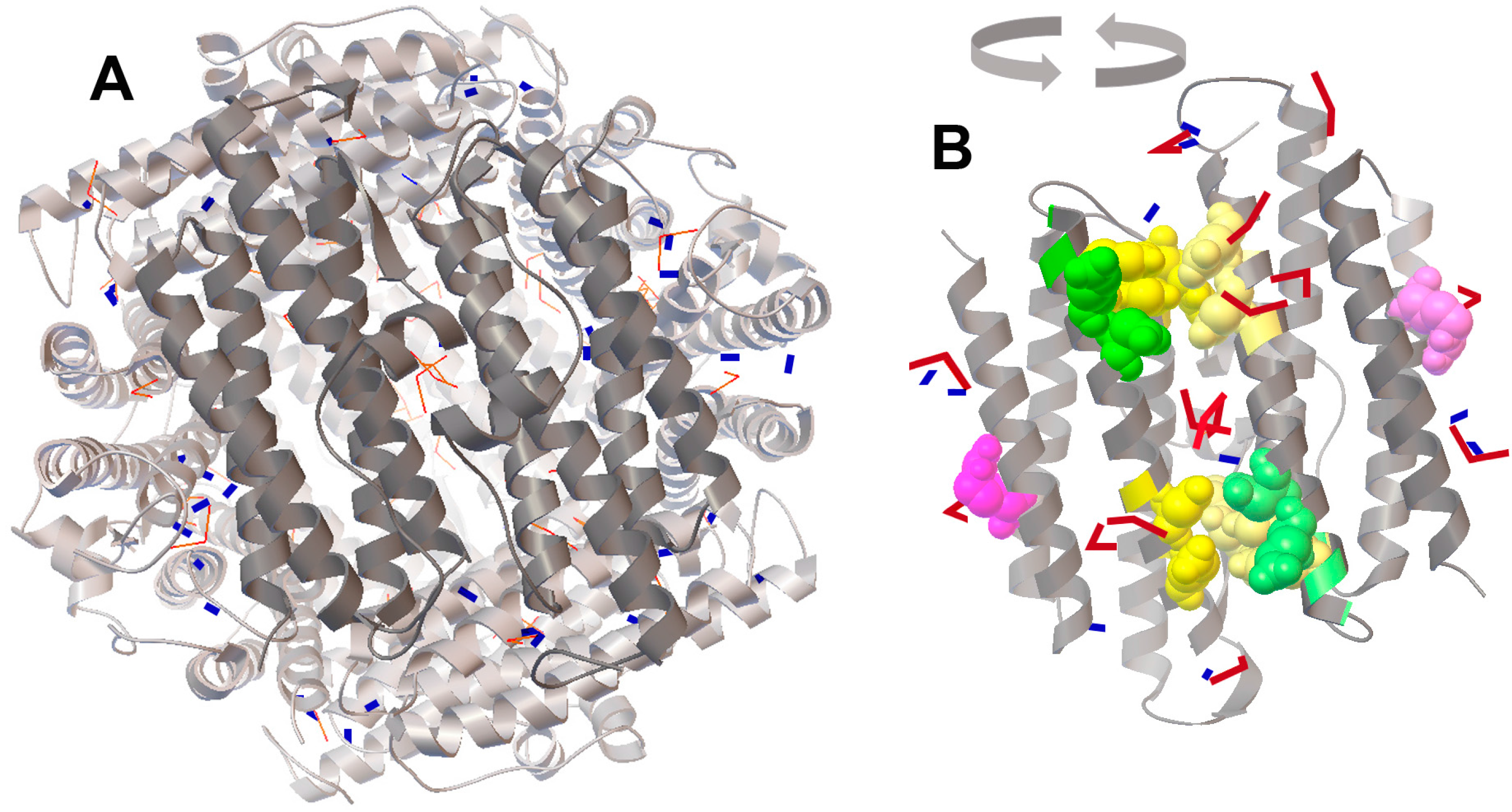
2.4. Molecular Docking of FeO and Fe2O3 on the Protein Surface of Dps Revealed a Potential Binding Site for Fe2O3
3. Discussion
4. Materials and Methods
4.1. Isolation and Purification of Dps Protein
4.2. Electrophoretic Fractionation
4.3. Size Exclusion Chromatography
4.4. Atomic Force Microscopy
4.5. Transmission Electron Microscopy
4.6. Registration of Mössbauer Spectra
4.7. Molecular Docking
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Almiron, M.A.; Link, J.; Furlong, D.; Kolter, R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992, 6, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Azam, T.A.; Ishihama, A. Twelve species of the nucleoid-associated protein from Escherichia coli. Sequence recognition specificity and DNA binding affinity. J. Biol. Chem. 1999, 274, 33105–33113. [Google Scholar] [CrossRef] [PubMed]
- Talukder, A.A.; Ishihama, A. Dps is a Stationary Phase-Specific Protein of Escherichia coli Nucleoid. Adv. Microbiol. 2014, 4, 1095–1104. [Google Scholar] [CrossRef]
- Ishihama, A.; Kori, A.; Koshio, E.; Yamada, K.; Maeda, H.; Shimada, T.; Makinoshima, H.; Iwata, A.; Fujita, N. Intracellular concentrations of 65 species of transcription factors with known regulatory functions in Escherichia coli. J. Bact. 2014, 196, 2718–2727. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.A.; Filman, D.J.; Finkel, S.E.; Kolter, R.; Hogle, J.M. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat. Struct. Biol. 1998, 5, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Ceci, P.; Cellai, S.; Falvo, E.; Rivetti, C.; Rossi, G.L.; Chiancone, E. DNA condensation and self-aggregation of Escherichia coli Dps are coupled phenomena related to the properties of the N-terminus. Nucl. Acids Res. 2004, 32, 5935–5944. [Google Scholar] [CrossRef] [PubMed]
- Ilari, A.; Ceci, P.; Ferrari, D.; Rossi, G.L.; Chiancone, E. Iron incorporation into Escherichia coli Dps gives rise to a ferritin-like microcrystalline core. J. Biol. Chem. 2002, 277, 37619–37623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ceci, P.; Ilari, A.; Giangiacomo, L.; Laue, T.M.; Chiancone, E.; Chasteen, D.N. Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells. J. Biol. Chem. 2002, 277, 27689–27696. [Google Scholar] [CrossRef] [PubMed]
- Zeth, K.; Offermann, S.; Essen, L.O.; Oesterhelt, D. Iron-oxo clusters biomineralizing on protein surfaces: Structural analysis of Halobacterium salinarum DpsA in its low- and high-iron states. Proc. Natl. Acad. Sci. USA 2004, 101, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, L.N.; Kwon, Y.M. Structure, function and regulation of the DNA-binding protein Dps and its role in acid and oxidative stress resistance in Escherichia coli: A review. J. Appl. Microbiol. 2010, 110, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Bellapadrona, G.; Stefanini, S.; Zamparelli, C.; Theil, E.C.; Chiancone, E. Iron translocation into and out of Listeria innocua Dps and size distribution of the protein-enclosed nanomineral are modulated by the electrostatic gradient at the 3-fold “ferritin-like” pores. J. Biol. Chem. 2009, 284, 19101–19109. [Google Scholar] [CrossRef] [PubMed]
- Pesek, J.; Buchler, R.; Albrecht, R.; Boland, W.; Zeth, K. Structure and mechanism of iron translocation by a Dps protein from Microbacterium arborescen. J. Biol. Chem. 2011, 286, 34872–34882. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Theil, E.C. Ferritins: Dynamic management of biological iron and oxygen chemistry. Acc. Chem. Res. 2005, 38, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Bessonova, T.; Shumeiko, S.; Purtov, Y.; Antipov, S.; Preobrazhenskaya, E.; Tutukina, M.; Ozoline, O. Hexuronates affect oligomeric form of a structural protein of bacterial nucleoid Dps and its ability to bind linear DNA fragments. Biophysics 2016, 61, 1059–1067. [Google Scholar] [CrossRef]
- Roy, S.; Saraswathi, R.; Chatterji, D.; Vijayan, M. Structural studies on the second Mycobacterium smegmatis Dps: Invariant and variable features of structure, assembly and function. J. Mol. Biol. 2008, 375, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Huergo, L.F.; Rahman, H.; Ibrahimovic, A.; Day, C.J.; Korolika, V. The Campylobacter jejuni Dps protein binds DNA in the presence of iron or hydrogen peroxide. J. Bacteriol. 2013, 195, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Purtov, Y.; Glazunova, O.; Antipov, S.; Pokusaeva, V.; Fesenko, E.; Preobrazhenskaya, E.; Shavkunov, K.; Tutukina, M.; Lukyanov, V.; Ozoline, O.N. Promoter islands as a platform for interaction with nucleoid proteins and transcription factors. J. Bioinform. Comput. Biol. 2014, 12, 1441006. [Google Scholar] [CrossRef] [PubMed]
- Melekhov, V.; Shvyreva, U.; Timchenko, A.; Tutukina, M.; Preobrazhenskaya, E.; Burkova, D.; Artiukhov, V.; Ozoline, O.; Antipov, S. Modes of Escherichia coli Dps interaction with DNA as revealed by atomic force microscopy. PLoS ONE 2015, 10, e0126504. [Google Scholar] [CrossRef] [PubMed]
- Grove, A.; Wilkinson, S.P. Differential DNA binding and protection by dimeric and dodecameric forms of the ferritin homolog Dps from Deinococcus radiodurans. J. Mol. Biol. 2005, 347, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Turishchev, S.; Antipov, S.; Novolokina, N.; Chuvenkova, O.; Melekhov, V.; Ovsyannikov, R.; Senkovskii, B.; Timchenko, A.; Ozoline, O.; Domashevskaya, E.A. Soft X-ray synchrotron study of the charge state of iron ions in the ferrihydrite core of the ferritin Dps protein in Escherichia coli. Biophysics 2016, 61, 705–710. [Google Scholar] [CrossRef]
- Ceci, P.; Ilari, A.; Falvo, E.; Giangiacomo, L.; Chiancone, E. Reassessment of protein stability, DNA binding, and protection of Mycobacterium smegmatis Dps. J. Biol. Chem. 2005, 280, 34776–34785. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.P.; Vijayabaskar, M.S.; Vishveshwara, S.; Chatterji, D. Molecular mechanism of in vitro oligomerization of Dps from Mycobacterium smegmatis: Mutations of the residues identified by “Interface cluster” analysis. Biochemistry 2008, 47, 11110–11117. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, M.; Mignogna, G.; Stefanini, S.; Barra, D.; Longhi, C.; Valenti, P.; Chiancone, E. A novel non-heme iron-binding ferritin related to the DNA binding proteins of the Dps family in Listeria innocua. J. Biol. Chem. 1997, 272, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Chiancone, E.; Ceci, P. The multifaceted capacity of Dps proteins to combat bacterial stress conditions: Detoxification of iron and hydrogen peroxide and DNA binding. Biochim. Biophys. Acta 2010, 1800, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Skoropata, E.; Desautels, R.D.; Rowe, M.; Lierop, J. Structure and composition of iron nanoparticles synthesized using a novelanionic-element complex. J. Appl. Phys. 2015, 117, 17D515. [Google Scholar] [CrossRef]
- Lyubutin, I.S.; Lin, C.-R.; Funtov, K.O.; Dmitrieva, T.V.; Starchikov, S.S.; Siao, Y.-J.; Chen, M.-L. Structural, magnetic, and electronic properties of iron selenide Fe6-7Se8 nanoparticles obtained by thermal decomposition in high-temperature organic solvents. J. Chem. Phys. 2014, 141, 044704. [Google Scholar] [CrossRef] [PubMed]
- Goya, G.F.; Berquo, T.S.; Fonseca, F.C.; Morales, M.P. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Chem. Phys. 2003, 94, 3520. [Google Scholar] [CrossRef]
- St. Pierre, T.G.; Bell, S.H.; Dickson, D.P.; Mann, S.; Webb, J.; Moore, G.R.; Williams, R.J. Mössbauer spectroscopic studies of the cores of human, limpet and bacterial ferritins. Biochem. Biophys. Acta 1986, 870, 127–134. [Google Scholar] [CrossRef]
- Walker, L.R.; Werthheim, G.K.; Jaccarino, V. Interpretation of the Fe57 isomer shift. Phys. Rev. Lett. 1961, 6, 98. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, J.; Chee, S.Y.; Ang, E.X.W.; Orner, B.P. Rational disruption of the oligomerization of the mini-ferritin E. coli DPS through protein-protein interface mutation. Protein Sci. 2011, 20, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Sayle, R.A.; Milner-White, E.J. RasMol: Biomolecular graphics for all. Trends Biochem. Sci. 1995, 20, 374–376. [Google Scholar] [CrossRef]
- Lacqua, A.; Wanner, O.; Colangelo, T.; Martinotti, M.G.; Landini, P. Emergence of biofilm-forming subpopulations upon exposure of Escherichia coli to environmental bacteriophages. Appl. Environ. Microbiol. 2006, 72, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Theoret, J.R.; Cooper, K.K.; Zekarias, B.; Roland, K.L.; Law, B.F.; Curtiss, R., III; Joens, L.A. The Campylobacter jejuni Dps homologue is important for in vitro biofilm formation and cecal colonization of poultry and may serve as a protective antigen for vaccination. Clin. Vaccine Immunol. 2012, 19, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, P.; Karmakar, K.; Kasetty, S.; Chatterji, D. Unveiling the role of Dps in the organization of mycobacterial nucleoid. PLoS ONE 2011, 6, e16019. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.G.; Frenkiel, D.; Arad, T.; Finkel, S.E.; Kolter, R.; Minsky, A. DNA protection by stress-induced biocristallization. Nature 1999, 400, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Frenkiel-Krispin, D.; Levin-Zaidman, S.; Shimoni, E.; Wolf, S.G.; Wachtel, E.J.; Arad, T. Regulated phase transitions of bacterial chromatin: A non-enzymatic pathway for generic DNA protection. EMBO J. 2001, 20, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Frenkiel-Krispin, D.; Ben-Avraham, I.; Englander, J.; Shimoni, E.; Wolf, S.G.; Minsky, A. Nucleoid restructuring in stationary-state bacteria. Mol. Microbiol. 2004, 51, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoshimura, S.H.; Hizume, K.; Ohniwa, R.L.; Ishihama, A.; Takeyasu, K. Fundamental structural units of the Escherichia coli nucleoid revealed by atomic force microscopy. Nucl. Acids Res. 2004, 32, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, M.D.; Rice, C.J.; Lucchini, S.; Pin, C.; Thompson, A.; Cameron, A.D.; Alston, M.; Stringer, M.F.; Betts, R.P.; Baranyi, J.; et al. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J. Bacteriol. 2012, 194, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Karas, V.A.; Westerlaken, I.; Meyer, A.S. Application of an in vitro DNA protection assay to visualize stress mediation properties of the Dps protein. J. Vis. Exp. 2013, 75, e50390. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Chemin. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, J.A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreadin. J. Comp. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The plasmid PGEM_dps, containing the native gene of dps under the control of IPTG inducible promoter is available from the authors. |
| Sample | Approximation | δ, mm/s | ε, mm/s | G, mm/s | S, % |
|---|---|---|---|---|---|
| α-Fe | Sexstet_1 | 0 | |||
| Mohr salt | Doublet_1 | 1.39 | |||
| Dps with Mohr salt | Doublet_1 | 0.25 ± 0.02 | 0.60 | 0.22 | 37 |
| Doublet_2 | 0.27 ± 0.02 | 0.62 | 0.22 | 63 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antipov, S.; Turishchev, S.; Purtov, Y.; Shvyreva, U.; Sinelnikov, A.; Semov, Y.; Preobrazhenskaya, E.; Berezhnoy, A.; Shusharina, N.; Novolokina, N.; et al. The Oligomeric Form of the Escherichia coli Dps Protein Depends on the Availability of Iron Ions. Molecules 2017, 22, 1904. https://doi.org/10.3390/molecules22111904
Antipov S, Turishchev S, Purtov Y, Shvyreva U, Sinelnikov A, Semov Y, Preobrazhenskaya E, Berezhnoy A, Shusharina N, Novolokina N, et al. The Oligomeric Form of the Escherichia coli Dps Protein Depends on the Availability of Iron Ions. Molecules. 2017; 22(11):1904. https://doi.org/10.3390/molecules22111904
Chicago/Turabian StyleAntipov, Sergey, Sergey Turishchev, Yuriy Purtov, Uliana Shvyreva, Alexander Sinelnikov, Yuriy Semov, Elena Preobrazhenskaya, Andrey Berezhnoy, Natalia Shusharina, Natalia Novolokina, and et al. 2017. "The Oligomeric Form of the Escherichia coli Dps Protein Depends on the Availability of Iron Ions" Molecules 22, no. 11: 1904. https://doi.org/10.3390/molecules22111904
APA StyleAntipov, S., Turishchev, S., Purtov, Y., Shvyreva, U., Sinelnikov, A., Semov, Y., Preobrazhenskaya, E., Berezhnoy, A., Shusharina, N., Novolokina, N., Vakhtel, V., Artyukhov, V., & Ozoline, O. (2017). The Oligomeric Form of the Escherichia coli Dps Protein Depends on the Availability of Iron Ions. Molecules, 22(11), 1904. https://doi.org/10.3390/molecules22111904






