1. Introduction
The purpose of an implant is to not only replace the damaged tissue but also to support the body in the process of regenerating the damaged area. In the case of implantable bone devices, there are two groups: active and inert products. Inert materials are those that, after implantation, do not integrate into the adjacent tissue. Consequently, over time, fibrosis of surrounding tissues occurs, as does degeneration and a loosening of the prosthesis. The term ‘active bone implant’ refers to products that fully integrate with the surrounding tissue. One method of activation of the implanted devices is to develop a porous structure, which allows the penetration of adjacent tissue cells into the implant and enables its osteointegration. There are a number of published studies indicating that a porous structure supports the processes of both the regeneration of the damaged area and the integration of the implant [
1,
2,
3,
4]. In particular, a porous structure allows osteoconduction and vascularisation, which is always important in cases of implants. Research carried out mainly on soft tissues indicates that, if the implanted material has a structure that reproduces the natural tissue, the process of healing and integration takes place more quickly [
5,
6,
7]. Recently, a number of studies have been conducted on polymer-biological composite bone implant materials with engineered structures [
8,
9,
10], which may be an alternative to xenogenic implants. In the manufacture of such implants, a number of textile techniques are used, including electrospinning and rapid prototyping and also techniques intended to produce porous materials with nonfibrous structures such as phase separation, the formation of foams, and other techniques [
11,
12,
13,
14,
15]. In the majority of publications, emphasis is placed on the significance of the influence of the porous structure of materials for bone implantation and scaffolds in the process of tissue regeneration [
16,
17]. The materials for bone implantation, depending upon the manufacturing technique, are characterized by a porosity of up to 90%, with an average pore diameter of 1 μm to 800 μm [
1]. At the same time, a porous structure with a pore size preferred for the proliferation of cells often results in the ‘fall out’ of cells from the scaffold. For this reason, a number of multilayer structures and composite models have been developed and are characterized by a different pore structure in each layer [
3]. Another issue is the immobilization of bioactive substances in the structure of the material [
12]. There is still a great need for drug delivery systems that allow for improved release kinetics of multiple growth factors or other compounds in order to enhance their therapeutic efficacy [
18]. One of these is the local microsphere growth factor release system. The research has shown that a number of growth factors, promote tissue repair and regeneration, including bone formation; moreover, the efficacy of growth factor therapies in periodontology and implantology has been well characterized in a variety of in vitro and in vivo studies [
19]. One of the recommended growth factors is bone morphogenetic protein (BMP), with known and accepted efficacy, but recent studies have shown that the interaction of BMP and insulin-like growth factor- 1 (IGF-1) was more beneficial in bone regeneration than the use of a single BMP [
18]. The mechanisms of interaction and the role of IGF1 in cell and tissue metabolism are still being explored. Numerous IGF1 functions have demonstrated their effects on cell proliferation and differentiation, and on angiogenesis, which facilitates the overgrowth of the implant [
20]. The clinical observations and animal data suggest that the essential pool of IGF1 in the bone matrix may not be sufficiently available for new bone formation during the aging process and in disease states. Therefore, the modulation of IGF1 deposition in the bone matrix could potentially be a therapeutic approach to delay or prevent bone loss [
21]. Similarly, hydroxyapatite supplementation may be used to promote local regeneration.
Another important factor influencing the quality of the implant material for tissue regeneration is its resorbability. Among resorbable polyesters, polylactide (PLA) and its copolymers with glycolide (PLGA), as well as polyhydroxybutyrates (PHB), play a leading role in biomedical applications. An accelerated process of degradation occurs when the PLA used is atactic, built from heterochiral chains l,d-PLA. Additionally, another possibility of modification is copolymerisation with a glycolide repeat unit, which allows the production of the poly(lactide-co-glycolide) (PLGA) copolymer. All the mentioned polymers undergo degradation by hydrolysis over time, and the extent of degradation depends on the chemical composition, the presence of a biocatalyst, the pH level, and the temperature. Bioresorbability is a very desirable property of implanted materials destined for temporal contact with tissues because it can help prevent subsequent revisions, potential pain, and infections.
Reports on the biofunctionality of implantable devices based on PLA, PLGA, and PHB indicate that the impact is not always beneficial. After the clinical use of surgical nails using PLA in the knee, swelling was observed, and the patient required a surgical revision [
22]. The same authors showed a severe reaction of the giant cells around the PLA materials. Adverse reactions were associated with changes in the crystallinity of the polymers in the biodegradation process. The positive impact and high biocompatibility of PLGA microparticles as carriers were also demonstrated [
23]. Research is being conducted on new biodegradable polyesters that are synthesized using less toxic compounds. As an example, Dobrzynski et al. synthesised poly(lactide-
co-glycolide) PLGA using Zr(AcAc)
4 as an initiator [
24,
25,
26,
27].
Implantable medical devices can cause an immunologically mediated cutaneous reaction to a substance. The programmed bioactivity of PLGA and PLGA + PHB implants requires efficacy and safety testing, not only in vitro but also in vivo.
The aim of the present study is to assess the allergenicity and the local response of bone tissue after the implantation of two newly developed prototypes of fibrous implants for osseous tissue regeneration, which are characterized by high biocompatibility in vitro and by resorbability, based on PLGA polymers manufactured with a zirconium initiator. Alloplastic PLGA and PLGA/PHB implants with potential bioactivity by IGF1 and HAp supplementation were prepared.
2. Materials
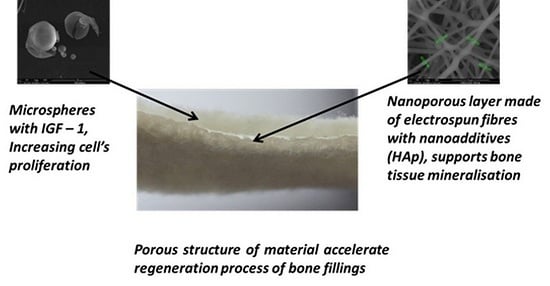
Two prototypes of bone implants were produced. The raw material that was used to produce the first test material was a copolymer of lactide and glycolide, designated as PLGA [
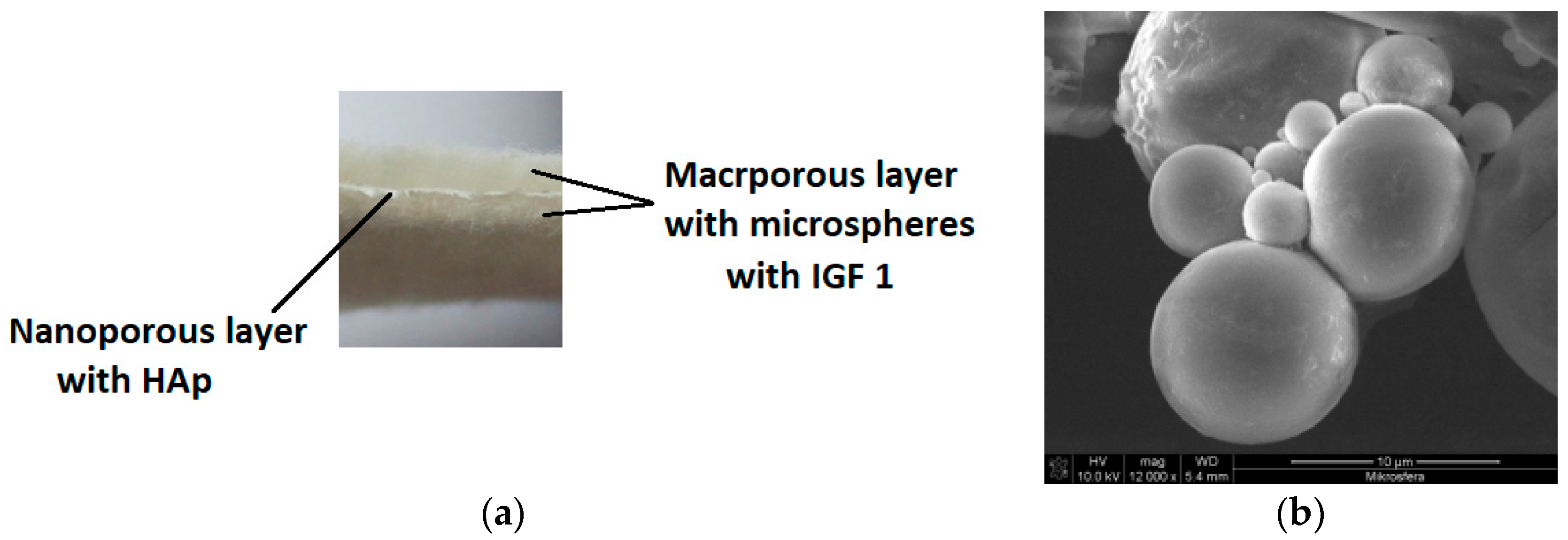
26]. The second prototype was produced from a mixture of PLGA and poly(hydroxybutyrate), designated as PLGA/PHB. The amount of PHB in the mixture was 10% wt. Both designed implants had a multilayer structure with a dominant role for a nonwoven material with large pores, which had a mean diameter of 150 μm, forming a space for the the cell culture (
Figure 1a).
Macroporous layers were produced by a card system, with the average surface mass at the level of 325 g/m
2 and average thickness equal to 2.4 mm. Two layers with a pore size of several hundred micrometres were separated by another layer with nanometer pores of an average pore diameter of 10 to 17 nm; the role of those layers was to overgrow and maintain the cells in the material structure. The nanoporous layer was produced from the same polymer as the layers with large pores, with the addition of a 1% wt. solution of hydroxyapatite. The hydroxylapatite was in the form of nanoparticles delivered by Sigma Aldrich (St. Louis, MO, USA), with an average grain size of 200 nm. Moreover, the bioactivity of the proposed product was induced by introducing a biologically active agent: insulin-like growth factor IGF1 (Sigma Aldrich, USA). This active agent was encapsulated in calcium alginate microspheres (
Figure 1b). The microspheres were formed from a 5% wt. solution of sodium alginate in biologically pure water, to which was added 25 μg IGF1 per 100 mL polymer solution. Microspheres were formed on a laboratory stand for that purpose and solidified in a 10 wt. % solution of calcium carbonate Ca
2Cl·6H
2O in biologically pure water. During the solidification, the replacement of the Na
+ by Ca
2+ ions in the alginate salt was observed. The decanted microspheres were filtered and then suspended in the biologically pure water. The suspension of the microspheres was filtered through the three-layer material. The activity of the IGF1 was confirmed during biological tests. Final products in the form of prototypes of the PLGA + IGF1 and PLGA/PHB + IGF1 implantable products were assessed from the point of view of their structure. Implants made from PLGA were characterised by a surface mass of 665.95 g/m
2 and a thickness of 2.8 mm. This multilayer structure was characterised by an average pore diameter of 172,877.1 nm and a total pore area of 0.139 m
2/g. The implantable material produced from PLGA/PHB + IGF1 had a surface mass equal to 692.34 g/m
2 and a thickness of 2.7 mm. The porous structure of this material was characterised by an average pore diameter of 181,042.3 nm and a total pore area of 0.133 m
2/g. Prototypes of the implantable products containing active insulin-like growth factor (IGF1) were tested on animals. As the control material, the same product was applied without IGF1. The Local Ethics Committee for Animal Experimentation in Wroclaw accepted the proposed and currently shown animal studies as admissible (consent No. 11/2008 and 88/2012, 89/2012, and 90/2012). For the implantation research, samples of the layered implant materials with dimensions of 3 × 3 × 6 mm were used. All materials were sterilized using a radiation dose of 28 kGy (before the IGF1 introduction) and a second dose of 15 kGy (after the IGF1 introduction into the fibrous implant). In the preliminary biological studies that were conducted to assess the potential risk of toxicity of the test materials, no cytotoxic or mutagenic activity was found in vitro. In animal model studies using both genders, there was no evidence of systemic toxicity after chronic implantation, the blood parameters were within the reference values, and the internal organs showed normal structures and functions. The IGF1 levels in the rabbits’ serum following the implantation of samples of PHB/PLGA + IGF1 and PLGA + IGF1 were normal.
3. Methodology
3.1. Sensitisation Test
A maximisation test using extracts (Guinea-Pig Maximisation Test, GPMT) was performed to assess the potential causes of delayed-type hypersensitivity for PLGA + IGF1 and PLGA/PHB + IGF1 implants [
28,
29,
30,
31].
The study was carried out on albino guinea pigs of both sexes with an average initial body weight of 355.68 g (300 to 430 g). The animals for testing came from a certified supplier of laboratory animals. During the experiment, the guinea pigs were kept in cages under controlled humidity (55% to 65%) and temperature (20 °C to 25 °C) and were given wholesome granulated food and water. with the addition of ascorbic acid in the amount of 200 mg/kg of their body mass, ad libitum.
For the preparation of the extracts, a saline solution was used. The extracts for testing were prepared with a proportion of a 1 g sample of PLGA + IGF1/10 mL + 10.1 mL of 0.9% NaCl and a 1 g sample of PLGA/PHB + IGF1/10 mL + 10.89 mL of 0.9% NaCl, taking into account their absorptivity. The extracts were incubated at 37 °C for 72 h.
After the acclimatisation period, the animals were randomly divided into three groups:
- -
The experimental group with PLGA + IGF1 has 10 animals,
- -
The experimental group with PLGA/PHB + IGF1 has 10 animals,
- -
The control group with 0.9% NaCl had five animals.
The day before the examination, during the intradermal induction phase, the guinea pigs’ fur was cut on the back in an area of 5 × 10 cm. Within each group of animals, each animal received three pairs of injections of 0.1 mL of the following solutions: the first pair of injections was comprised of a stable emulsion of complete Freund’s adjuvant, mixed 50:50 with physiological saline; the second pair of injections was comprised of an extract from the samples to be tested; and the third pair of injections was comprised of the extract of the tested samples emulsified in a volume ratio of 50:50 stable emulsion of complete Freund’s adjuvant and physiological saline.
The animals classified as the control group were also given three pairs of injections: the first pair was comprised of a stable emulsion of complete Freund’s adjuvant, mixed 50:50 with saline; the second pair was comprised of physiological saline; and the third pair was comprised of saline emulsified in a volume ratio of 50:50 by a stable emulsion of complete Freund’s adjuvant and solvent.
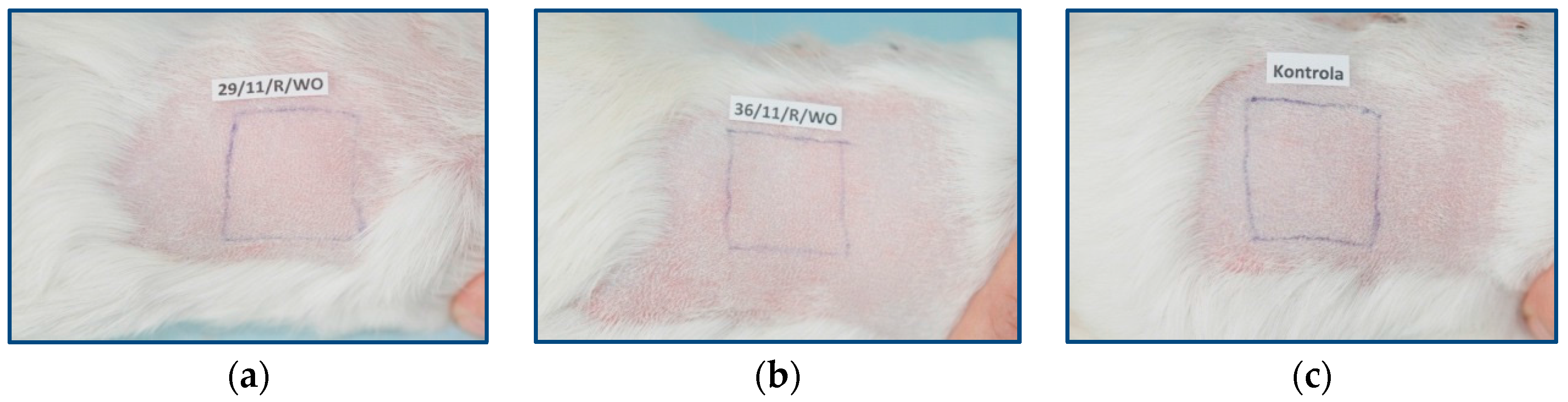
In the local induction phase, six days after the end of the intradermal induction phase, the fur was cut on the back of the animal again, and the injection site was checked. In the injection sites of extracts from the samples of PLGA + IGF1 and PLGA/PHB + IGF1, there was no skin irritation. In the nonirritated areas of skin, 10% sodium dodecyl sulphate was massaged.
After 24 h, the injection site was covered with filter paper and soaked with the tested extracts (experimental groups) or physiological saline (control group). Flakes of paper were secured with occlusive dressings (Blenderm 3M, 3M Poland Sp. z o.o., Kajetany, Poland). The dressings and paper flakes were removed after 48 h.
In the challenge phase, 13 days after the end of the local induction phase, all of the animals’ fur was cut on the right side. On day 14, in the cut-off space, the filter paper was applied and soaked with the tested extracts for the experimental group and with physiological saline solution for the control group. The tested areas were secured with Blenderm 3M dressings. The dressings and flakes were removed after 24 h.
3.2. Evaluation of Local Reactions after Implantation
The implantation research was done in four groups. These were two experimental groups of PLGA + IGF1 and PLGA/PHB + IGF1, control PLGA, and PLGA/PHB without microspheres and with IGF1.
3.3. Surgical Procedures
The tests were carried out on 24 New Zealand white rabbits of both sexes that had an average weight of 2.7 kg (± 200 g). The rabbits were kept singly in cages under controlled humidity (28% to 37%) and temperature (16°C to 20 °C). The animals had free access to water and were fed with a standard pelleted feed for rabbits (LSK), with an average daily consumption of between 50 to 70 g per rabbit. For each planned date of autopsy and all types of material tested, a minimum of three were rabbits were sacrificed, (five rabbits at later time points).
Beginning 24 h before the scheduled surgical procedures, the rabbits were subjected to fasting with access to water. An area of approximately 5 cm × 5 cm of the fur around the hip was removed mechanically. The rabbits were anesthetized with an intramuscular injection of anaesthetic mixture: Xylazine at a dose of 5 mg/kg and Ketamine at a dose of 35 mg/kg. Full analgesia was obtained 10 to 15 min after the injection and lasted for 60 to 80 min. The full effect lasted for 120 to 140 min. After complete analgesia, at the height of the hips, the skin was disinfected with SkinSept Color (Ecolab, St. Paul, MN, USA) and incisions of 4 to 5 cm long were made, running along the base of the proximal femur. Then, the lesser and the greater trochanter were exposed, in which two holes having a diameter of 3 mm and a length of 6 mm were drilled. In these cavities, the two test samples were placed on the right, and the control samples were placed on the opposite side. The muscles and soft tissues were closed with a single surgeon’s knot of MonoPlus 3-0 absorbable sutures (B Braun Medical Coe., Rubi, Spain). The skin was closed with a single stitch of Novosyn 2/0 (B Braun Medical Coe., Rubi, Spain).
In the postoperative period, the animals were housed in cages with free access to water and food under constant medical-veterinary care. The overall health of the rabbit was evaluated, with special emphasis on the healing of the surgical wounds, the active and passive mobility of the hip, and food intake [
28,
29,
32].
3.4. Post Mortem Examinations
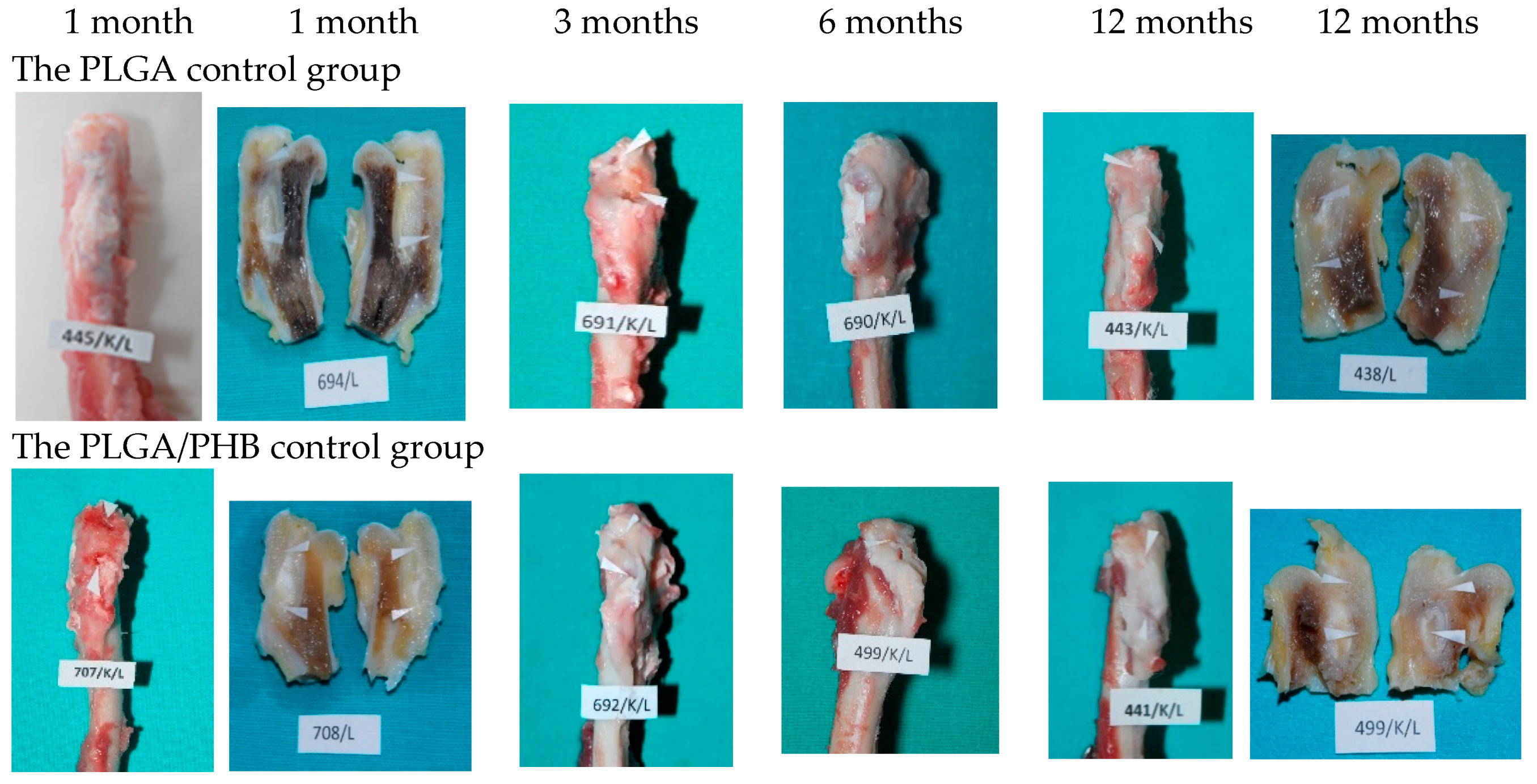
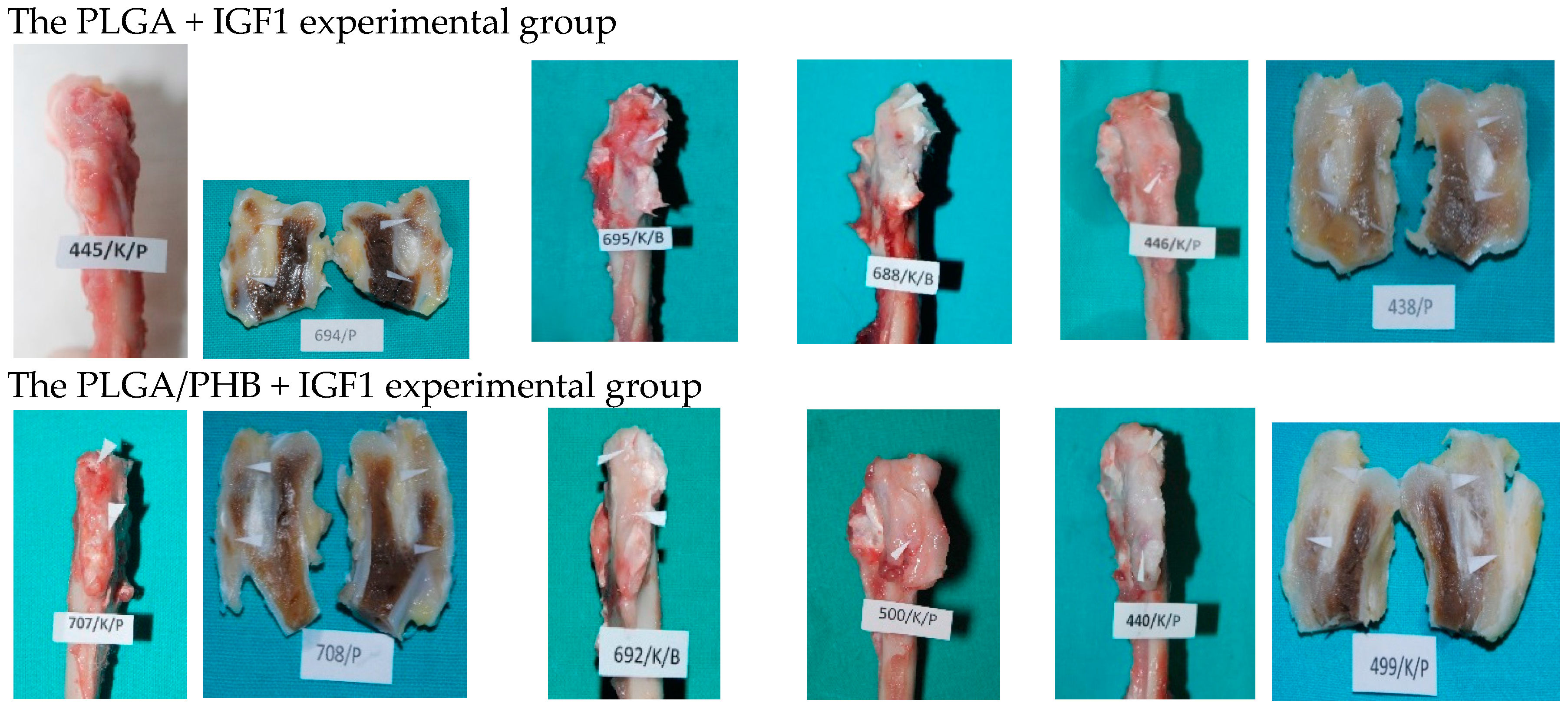
At the planned study time points of one, two, three, six, nine, and 12 months after implantation, euthanasia was performed on the rabbits by an intravenous injection of pentobarbital (trade name: Morbital, producer: Biowet, Pulawy, Poland) at doses of up to 80 mg/kg, administered in fractionated doses to achieve respiratory arrest and the cessation of heart function. Prior to pentobarbital administration, the general health status of the animals was assessed. During the autopsies, first, the postoperative wound was evaluated macroscopically, along with the appearance of the tissue at the implantation site. Afterward, the appearance of the selected internal organs was assessed. A macroscopic evaluation of tissue after the implantation of the PLGA + IGF1 and PLGA/PHB + IGF1 materials was carried out in relation to the tissues of the animals in the control group, which were implanted with PLGA and PLGA/PHB. For further radiological and histological studies, the femur bones were collected along with the implants.
3.5. X-ray Examinations
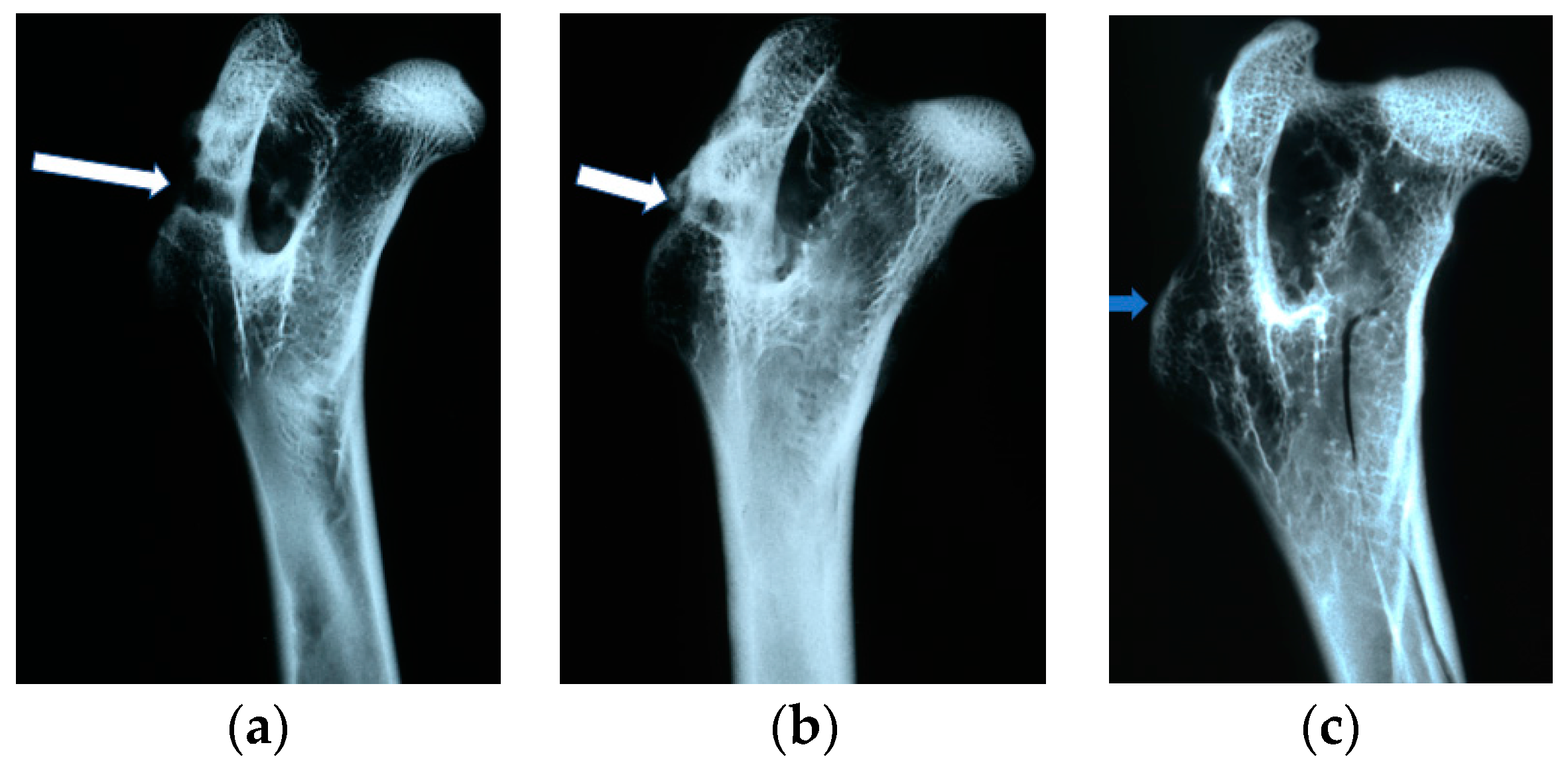
The X-ray examinations were performed in analog version on a Siemens Mammomat Nova 3000 X-ray unit with the following exposure parameters: tube voltage of 35 kV, tube current of 10 mA, exposure time of 100 ms, 0.3 mm lamp focus, and Mo/Mo filtration. Each of the implants was shown in two projections: antero-posterior and lateral.
On the obtained images the visibility and the number and location of the implants in the femoral bones of the rabbits were assessed. For implantation evaluation, the following parameters were identified and analyzed: the presence of osteolysis and osteosclerosis around the implants; closure of the borehole in the femoral bone through so-called ‘bone cap’ creation; bone canal callus filling; the quality of bone trabeculae; and the presence of periosteal reactions.
3.6. Histological Studies
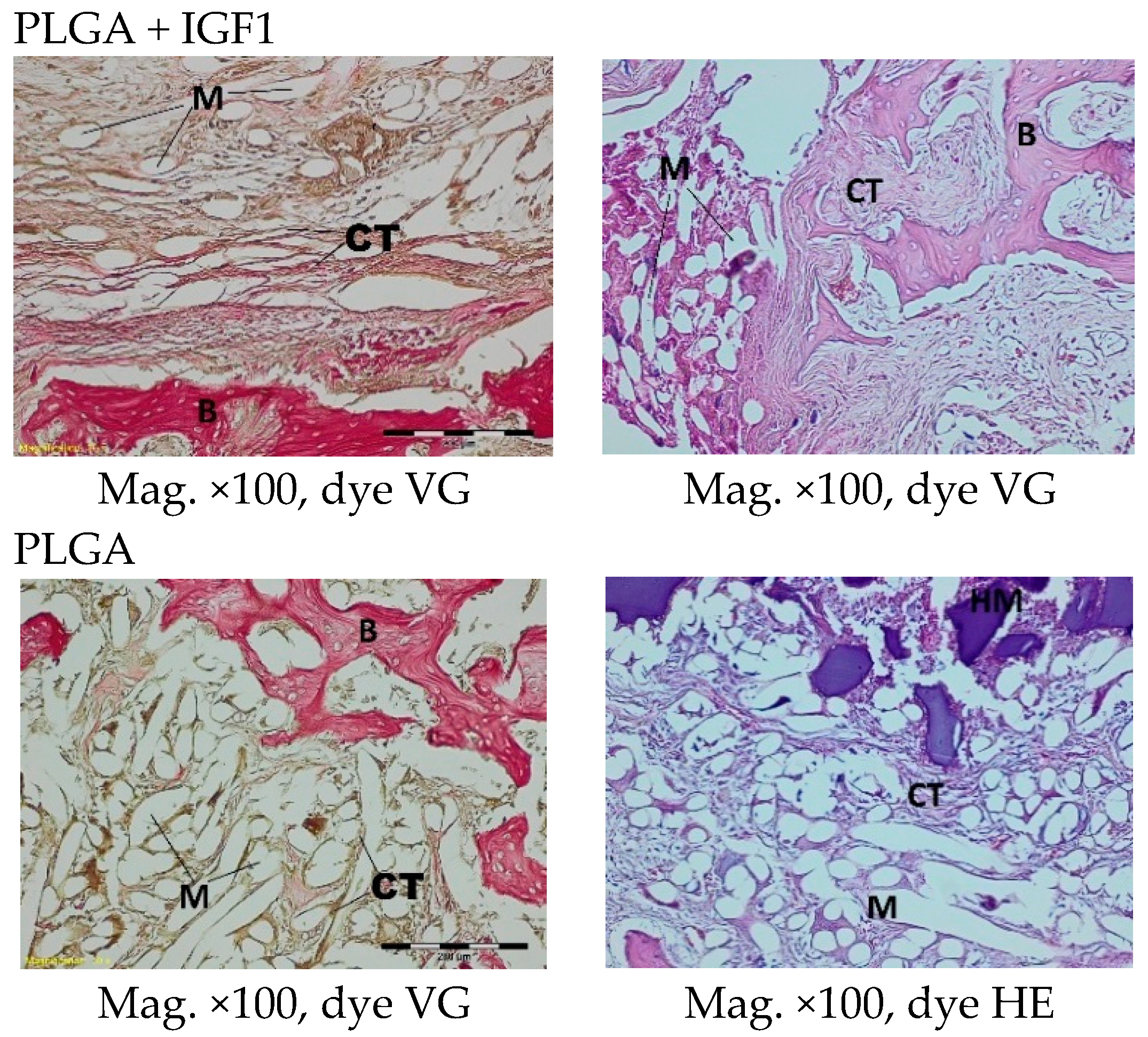
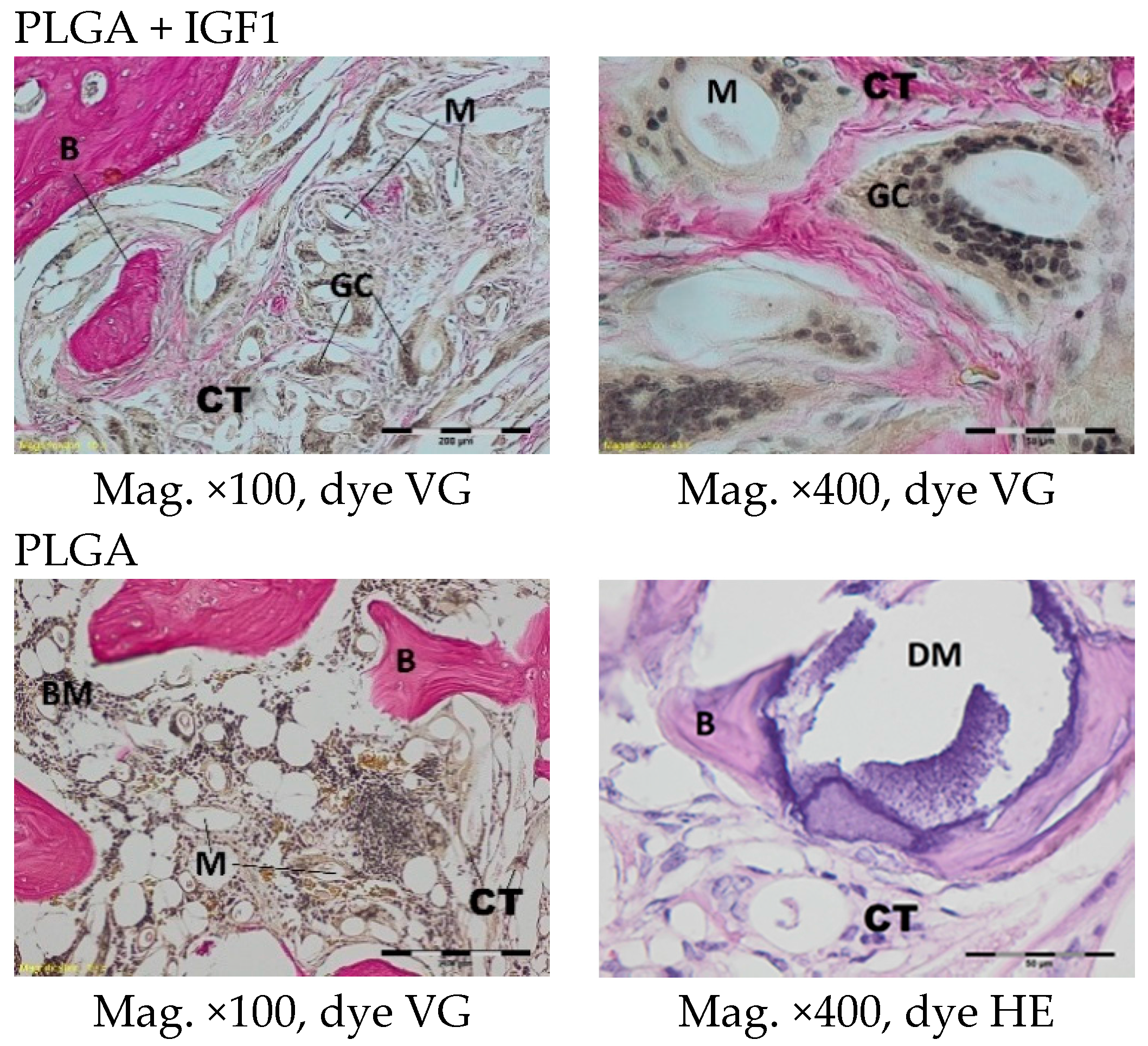
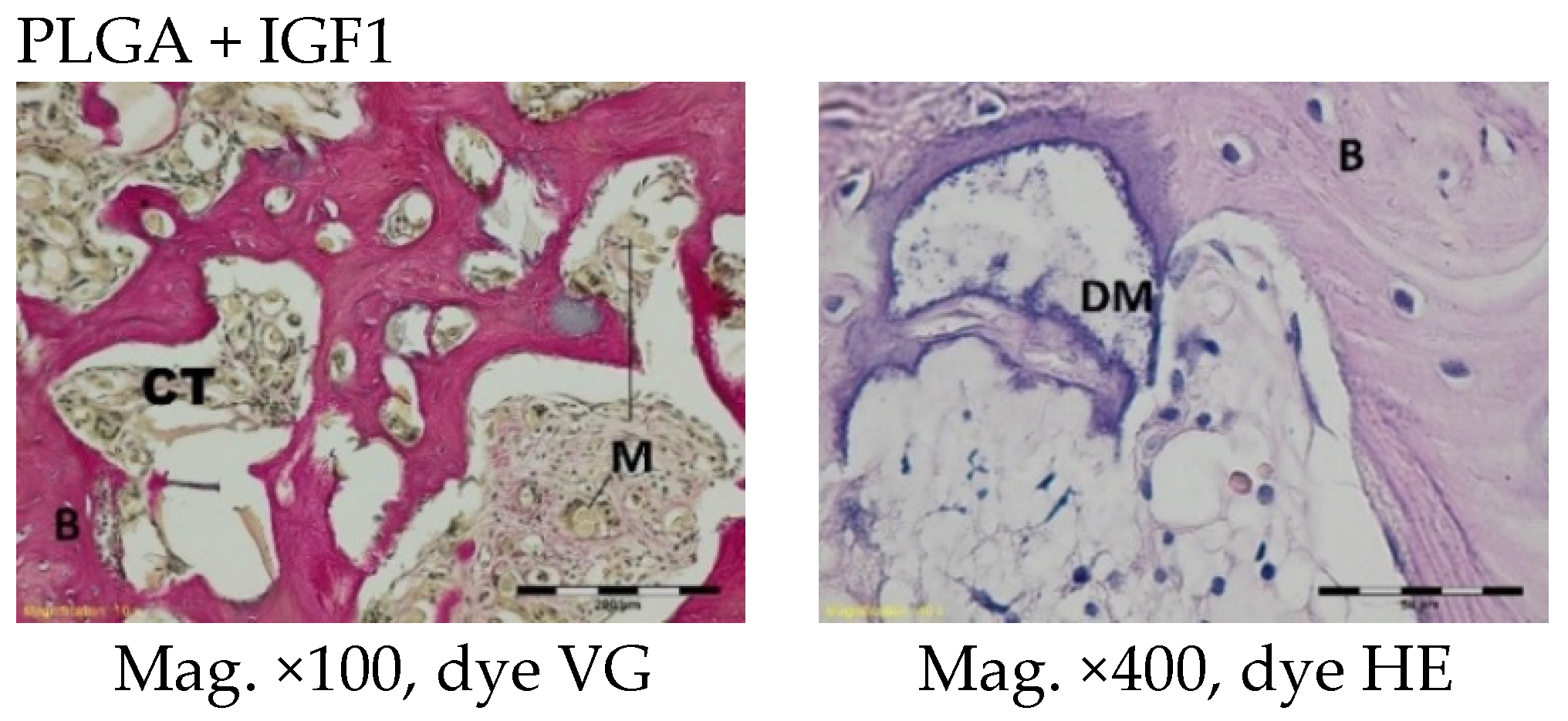
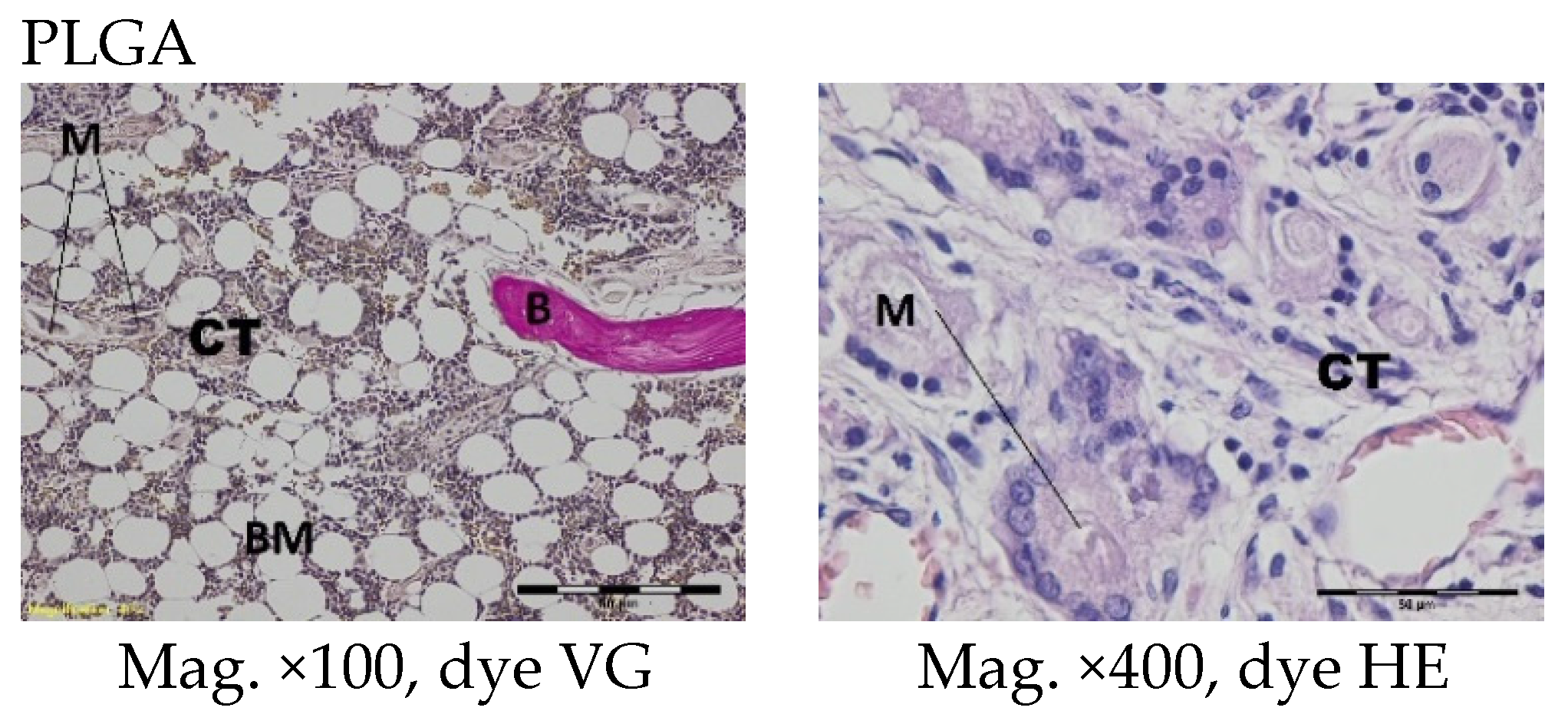
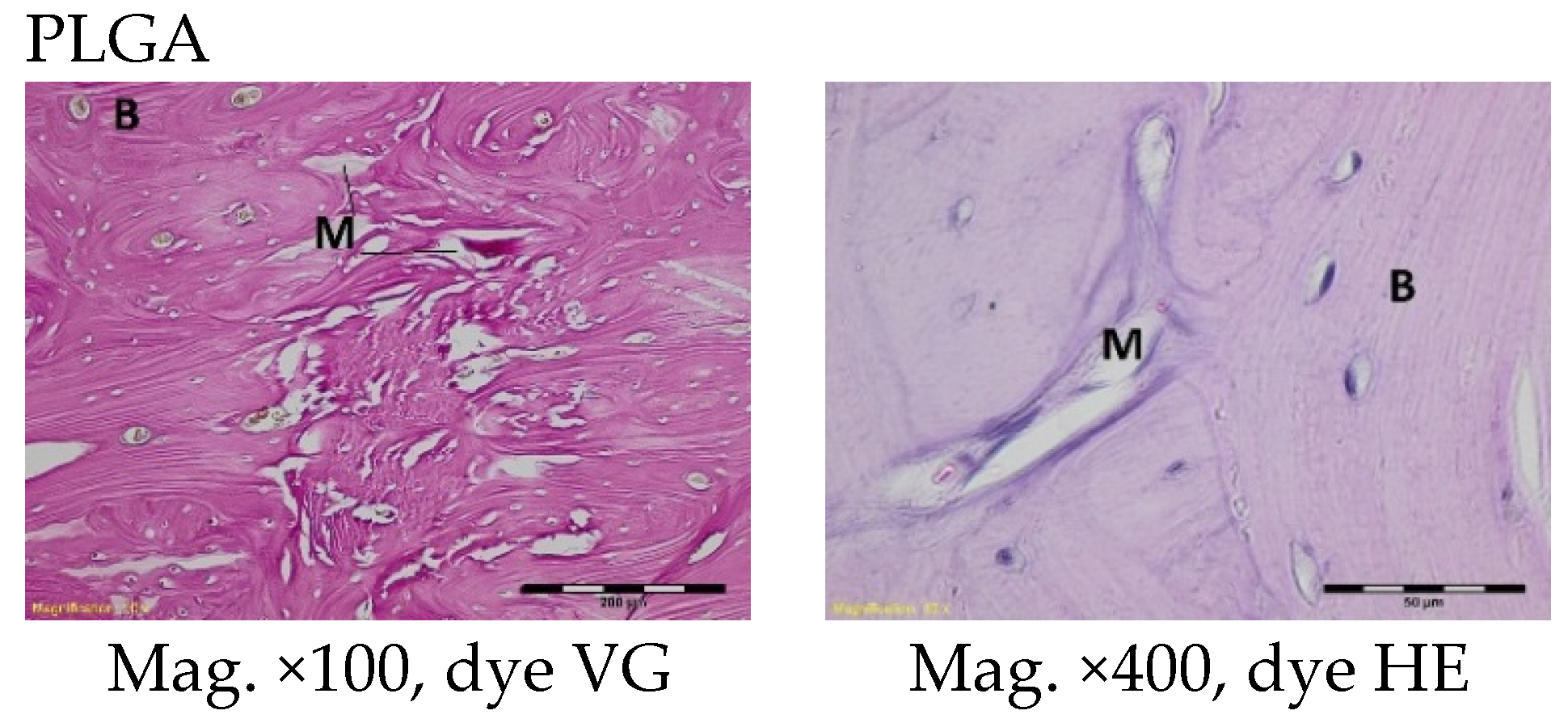
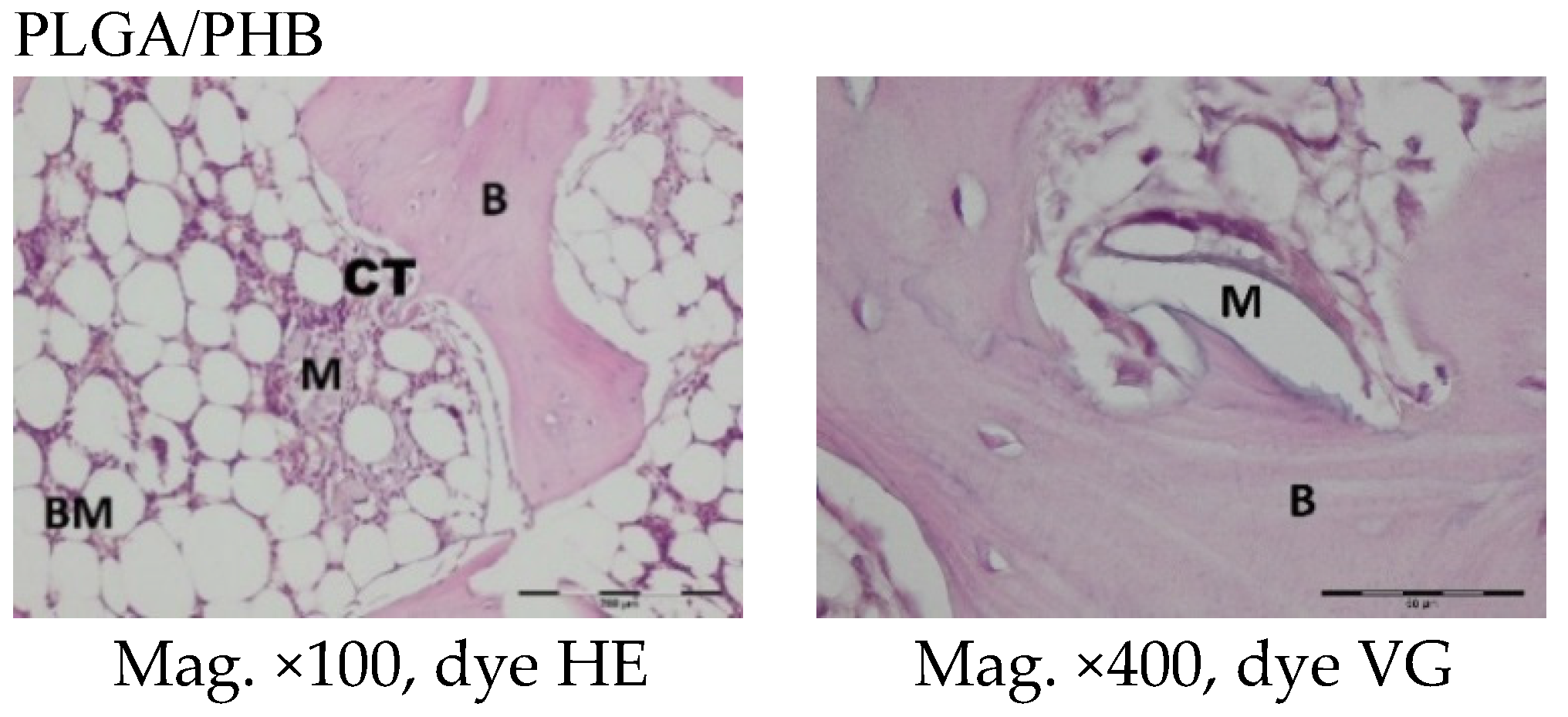
The femoral fragments with the implants were fixed for 72 h in 10% aqueous formic formaldehyde in phosphate buffer. Then, the samples were decalcified in a solution of formic acid and hydrochloric acid, dehydrated in acetone (at a temperature of 56 °C), X-rayed in xylene at room temperature, and embedded in paraffin blocks. With the use of a microtome (Lecica Microsystems Inc., Bannockburn, IL, USA) thick sections of approximately 4 μm were cut. The prepared samples were dyed with haematoxylin and eosin (HE) by the Van Gieson method (VG) to differentiate stromal connective tissues. Histological specimens were evaluated under a light microscope (Olympus BX43, Olympus, Tokyo, Japan) using a computer program for analysis and image acquisition (cellSens Standard, Olympus).
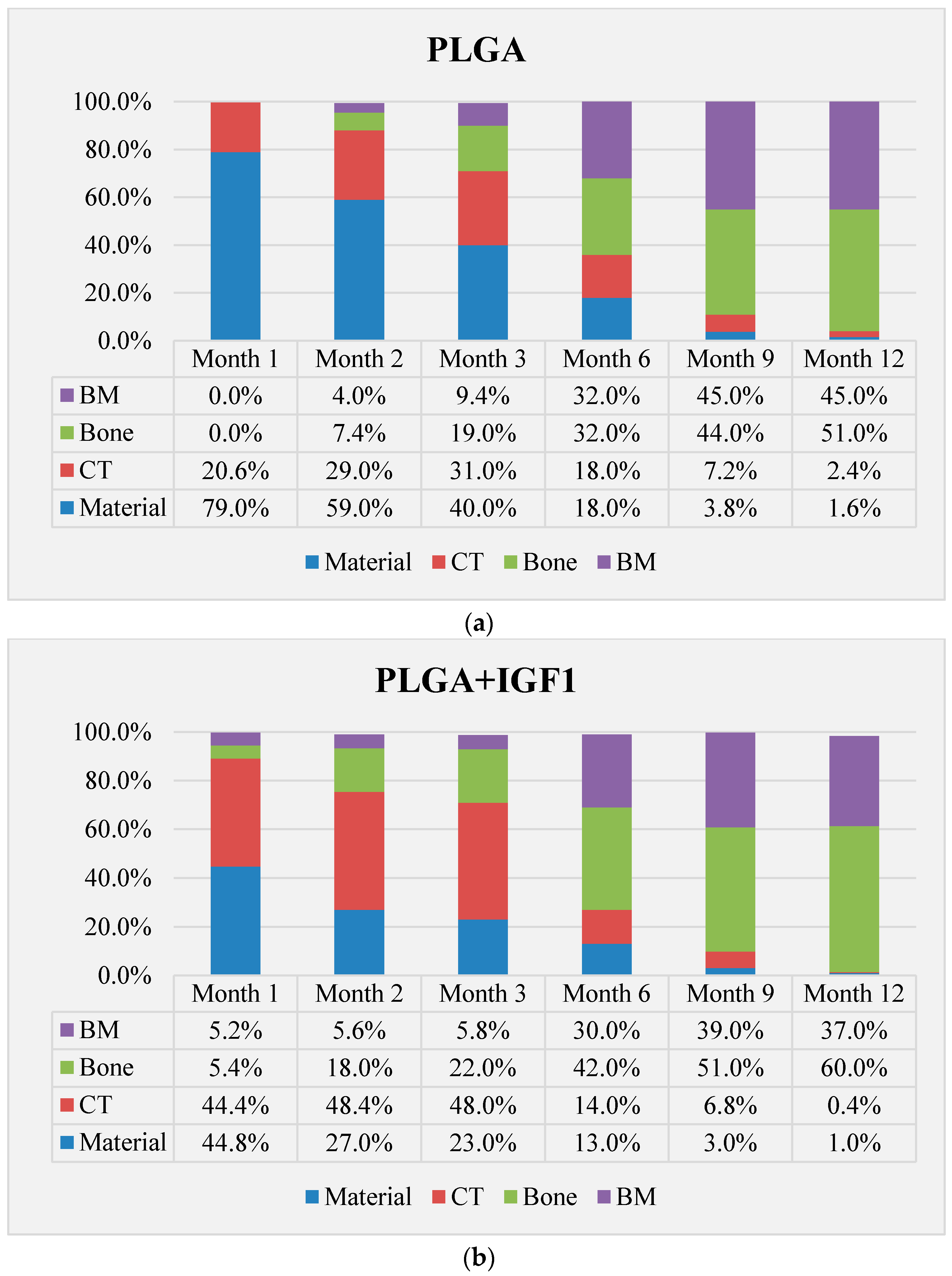
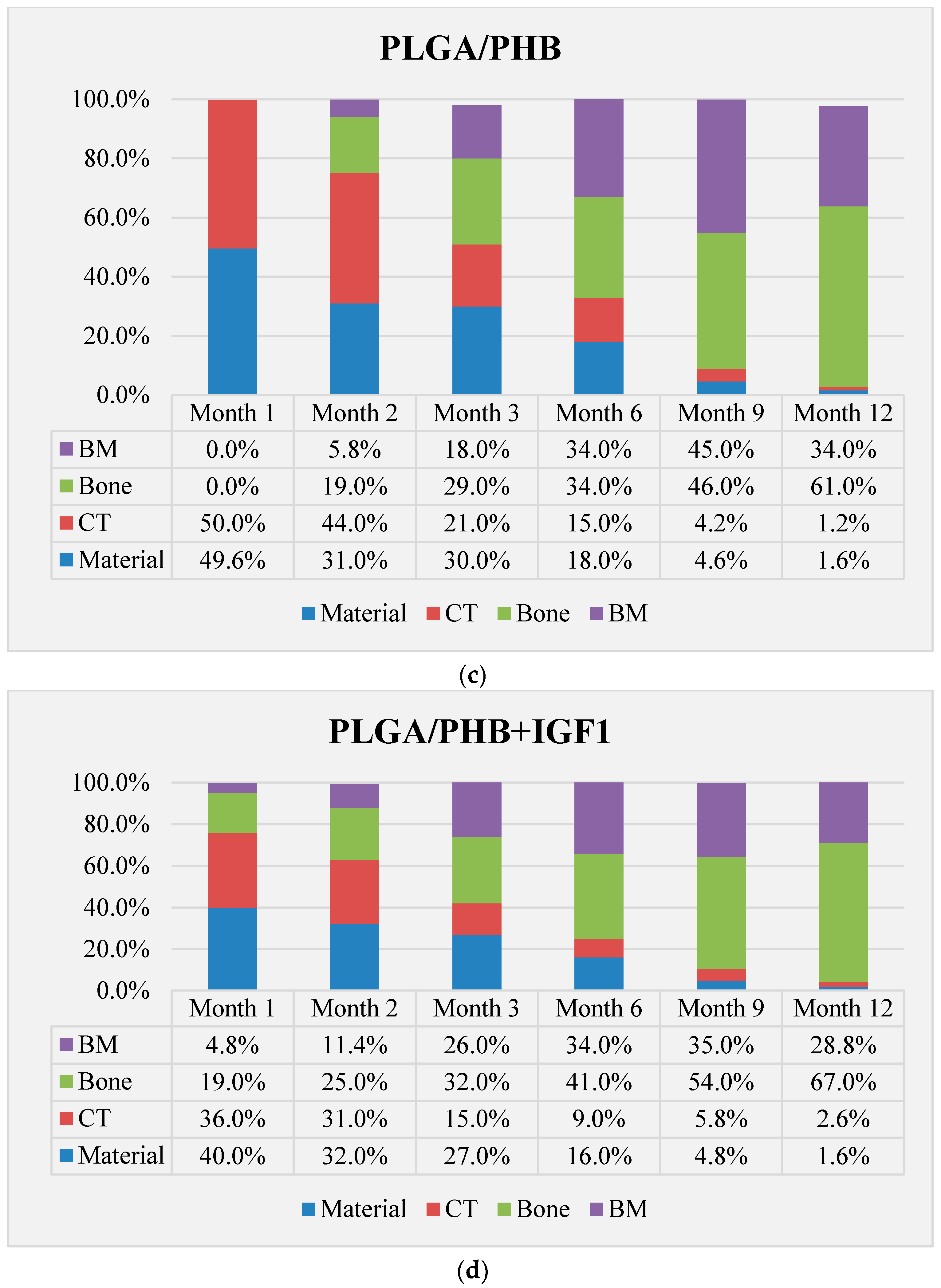
The qualitative assessment included: (1) the type of tissue filling the area of the implant in the intraosseous defect; (2) the amount of resorption of the biomaterial; (3) the method of integrating the alloplastic material with the surrounding tissues filling the intraosseous defect; and (4) the presence of cells indicating an inflammatory process. The quantitative assessment included, first, the relative area of newly formed bone and, second, the surface of the relatively non-resorbed implanted material. The relative surfaces examined were presented as a percentage of the total surface area of the test for each group.
The degree of degradation of the fibrous implants examined was assessed on the basis of the measurements of the diameter of individual circular filaments. The t-test was used for the statistical evaluation.
5. Discussion
Biodegradable fibrous products based on a newly developed polymer with reduced toxicity, PLGA with poly(hydroxybutyrate) (PHB), were designed and prepared. The newly developed implants had a porous structure and contained microspheres with growth factor IGF1, as well as Hap nanoparticles, to support regenerative processes and increase bioactivity. The use of multilayer systems allowed the creation of a material with varied porosity. Combining macroporous and mesoporous layers increased the total area of the pores. The increase in average pore size resulted from the process of connecting the two layers during needle-punching. This significant increase in apparent density suggests increased packing of the fibres in the process of combining layers, as well as an association with the introduction of microspheres with growth factors, which partially fill the pores of the material.
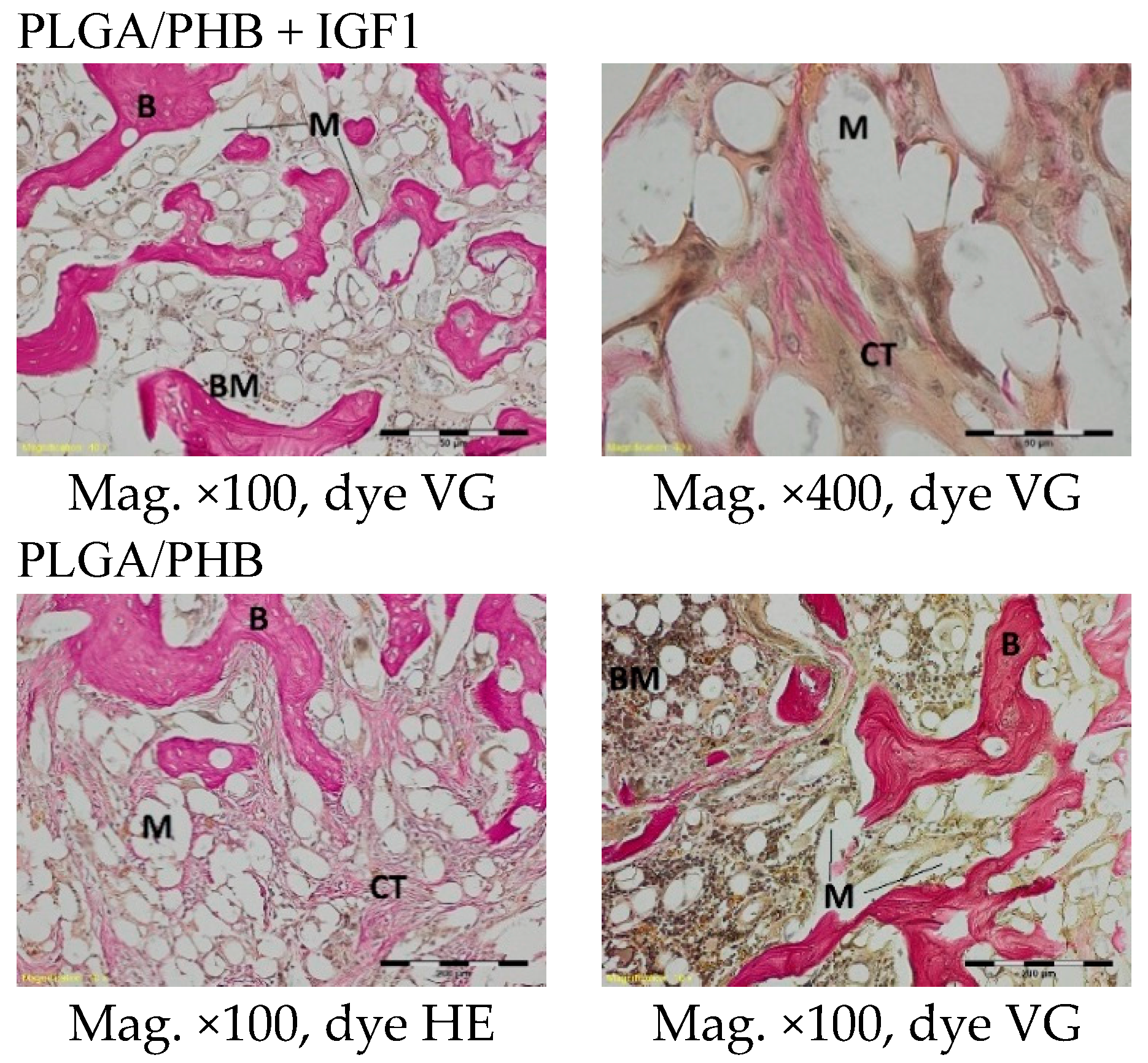
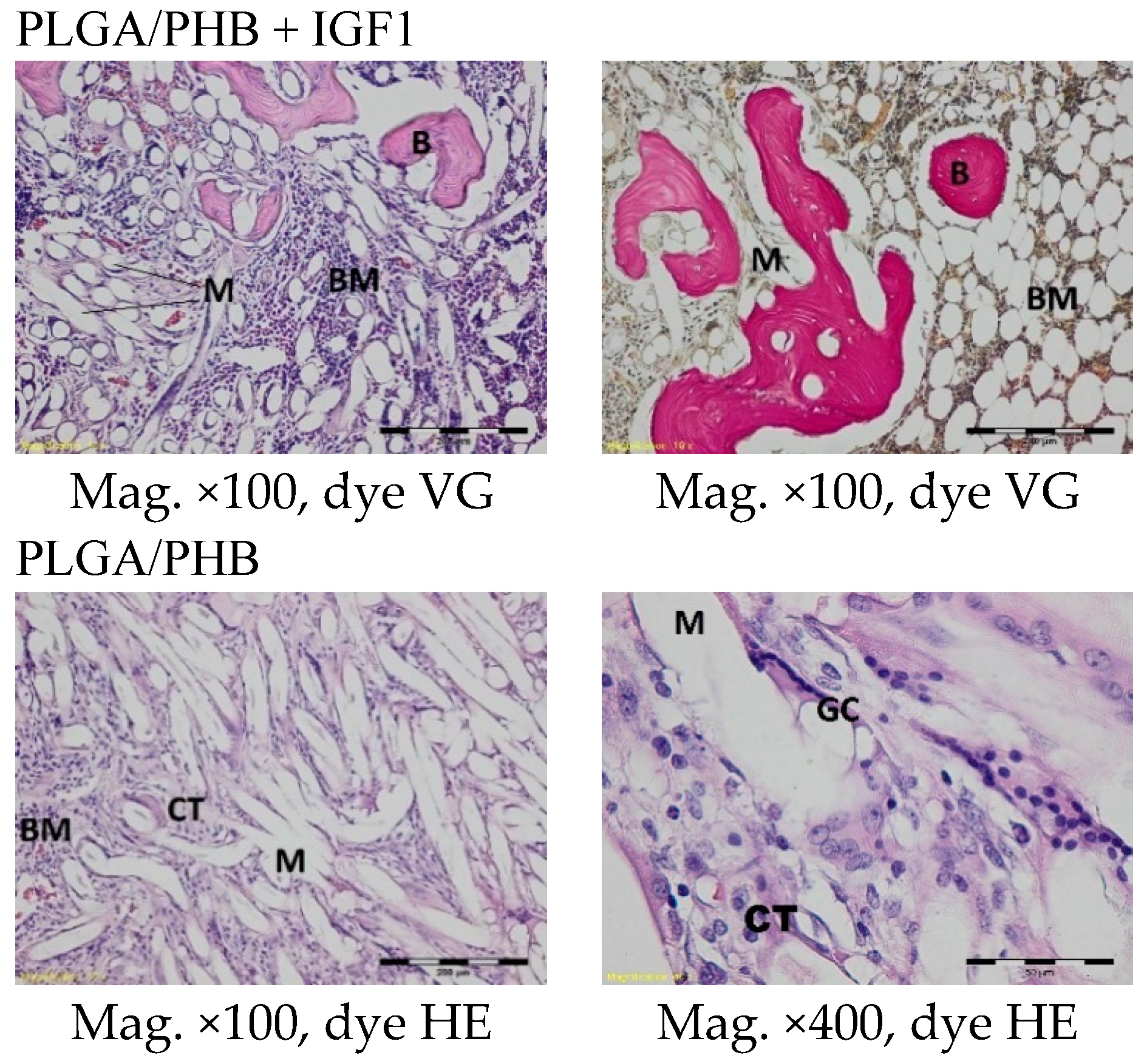
In the evaluation of the biocompatibility and bioactivity of the porous, fibrous bone implants of experimental PLGA + IGF1 and PLGA/PHB + IGF1 and control PLGA and PLGA + PHB, tests for allergenic reaction and tests for local bone tissue response after implantation for a period of one, two, three, six, nine, and 12 months were carried out. In the scheduled postmortem periods, macroscopic and radiological evaluations were performed, followed by microscopic histological evaluations of the healing process and the degradation time of the implanted materials. Our research revealed similar and correct clinical pictures for all types of implants. Every animal in both the experimental and control groups survived. The surgical wounds were healed by first intention. The animals retained active and passive mobility of the hip. Individual animals in the early period, one to two months after surgery, demonstrated slightly larger surroundings of the hip joint. During later periods, no change was noticed. Macroscopically, the soft tissues around the hip, following the implantation of all investigated implants, were correct and similar. During autopsy, moderate amounts of colourless exudate around the implant were observed in both the control group and the experimental group for individually tested animals one month after implantation. The macroscopic images of the experimental group and the control group in the early period showed the locations of the implantation on the trochanters’ surface. In subsequent periods, the implants were covered by periosteum and were barely visible. For individual animals from the experimental group (with a supplement of IGF1), a slight increase of femur trochanters was found in the macroscopic images; for the other animals, the shape and size of the trochanters were comparable to those in the control group. In X-ray imaging, all the implants remained translucent and invisible. Upon examination one month after implantation, the canals with PLGA + IGF1 and PLGA/PHB + IGF1 were partially filled with calluses, while, in those with control materials, only traces of callus were visible. After two months the implant holes remained open in the PLGA + IGF1 samples, while, in the PLGA/PHB + IGF1 samples, most of the implant holes were closed with osseous lamina. At later observations in all the groups the implant canals were filled with spongy calluses and closed by osseous lamina, partially after three months and entirely after six, nine, and 12 months. Compared to the control samples, those implanted with the tested materials were filled with thicker bone trabeculae.
In the histological studies, in the spongy bone tissue at the implantation site for all implants (both experimental and control), within one month after implantation, a narrow band of loose connective tissue, characteristic of inflammatory granulation tissue, surrounded the implant, and the individual filaments of the implant were revealed. In the centre of the implantation site in individual cases, within one to two months after implantation, small amounts of homogeneous masses (corresponding to exudate) were visible. In addition, in the experimental groups (with IGF1), newly developed trabeculae could be observed around the implants within one month after implantation. In the surrounding tissue, increased osteoblast activity was observed. The formation of trabecular bone tissue after only one month has also been described in studies following the augmentation of animal mandible defects with xenogenic implants based on bovine bone with collagen-containing polypeptide growth factors [
7,
33,
34].
More intensive expansion of the trabeculae was observed within one to two months after implantation for experimental group PLGA/PHB + IGF1 than for experimental group PLGA + IGF1. Furthermore, the implanted materials gradually degraded while the loose and fibrous connective tissue was undergoing replacement by the spongy bone tissue. In the immediate vicinity of the residues of the implants, the presence of cell-rich connective tissue, including very few inflammatory and mesenchymal cells, was observed. Excessive PLGA process biodegradation of the material from both the experimental and control groups was observed at three months after implantation, with observed activity of multinucleated macrophages (giant cells). Some filament residues of PLGA were visible up to six months, while, after 12 months, they were hardly noticeable. Experimental and control implants of PLGA + PHB later showed insignificant increases in biodegradation, and filament residues were also visible up to nine to 12 months after implantation. In all the groups, there were statistically significant reductions in the diameters of individual filaments between three and six months.
Focally, in the newly produced bone, the decomposition of implant material incorporated into bone lamellae was observed for the control group, PLGA, after three months and in the experimental group, PLGA + IGF1, after six months. The PLGA/PHB + IGF1 implants showed a higher osteostimulation property in the early period and degraded later compared to PLGA + IGF1.
Similar observations of the beneficial effects of using a PHB membrane and the increased filling of defects with bone tissue compared to the control group were also found in other studies [
35,
36]. The transient presence of the giant cells’ reaction related to the biodegradation of the polymer was observed in histopathological studies by other researchers; e.g., in the experiment in which patches of PHB were applied, and in which, after 12 months, fragments of material and associated giant cells were found [
37]. On the basis of quantitative histological tests performed at all the observational time points, the experimental group with IGF1 demonstrated increases in the relative surface area of bone trabecular filling the intraosseous defects compared with the control group.
The results obtained are consistent with the data, which suggests that IGF1 released from the bone matrix during bone resorption produces an osteogenic micro-environment and induces the differentiation of recruited mesenchymal stem cells (MSCs) to obtain new bone. Next, the authors postulate that the primary function of IGF1 in bone matrix is to maintain bone mass and skeletal homeostasis during bone remodelling [
21]. The benefits of using IGF1 are also reported by other authors [
38]. A similar effect was reported in an animal study focused on the histological characteristics of the early osseointegration of implants with or without the addition of platelet-rich plasma (PRP) or combined platelet-derived growth factor (PDGF)/IGF1. The results showed greater new bone deposition in animals in which PDGF/IGF1 was added to the implants, compared to animals treated with PRP or to the controls [
19]. Other studies have indicated that applying polypeptide growth factors in the early period of bone healing has a favourable effect [
7].
Moreover, in our research, in the period up to three months after implementation, histological observation of the implantation sites of the experimental and control materials revealed the very good condition of the bone marrow.
In a study of the sensitisation effect, the animals’ skin, at the application site of the experimental extracts of PLGA + IGF1 and PLGA/PHB + IGF1, had a regular appearance compared to the control group. In both the experimental and control groups, the animals’ health did not deviate from the norm. The animals showed normal increases in body weight, and the gender of the animals had no effect on the results.
6. Conclusions
Studied bone implants produced from the newly developed zirconium-based copolymer of lactide and glycolide, with and without insulin-like growth factor (PLGA + IGF1 or PLGA, respectively), as well as the implant produced from the blend of this copolymer with poly([R,S]-3-hydroxybutyrate), reinforced or not with IGF1 (PLGA/PHB + IGF1 or PLGA/PHB, respectively), did not cause negative changes in the health status of animals in clinical trials up to 12 months after implantation in the experimental or control groups.
Macroscopic evaluation of the hip joint surroundings in the early stage, i.e., one to two months after implantation, showed a small amount of colourless and serous fluid at the implantation site in individual animals. At later time points, there was no exudation, and the observed image was normal. In the macroscopic evaluation, the femurs with implants were normal, and only in individual animals from the experimental group (with the addition of IGF1) were the trochanters slightly enlarged.
All materials only weakly induced an inflammation reaction, leading to the formation of increased quantities of cancellous and lamellar bone tissue and good bone marrow condition at the site of implantation within nine to 12 month. The tested materials with IGF1 induced a greater percentage of bone mass than the control implants. In the early period of bone healing, the experimental implant PLGA/PHB + IGF1 revealed higher osteostimulation properties and degraded later in comparison to the PLGA + IGF1 samples. All the materials gradually degraded. The most rapid statistically significant degradation occurred between three and six months. It should be noted that the designed porous structure of the material is beneficial for healing tissue and allows for free material overgrowth of cells. The structure of the implant, bioactive substances, and especially IGF1 allow for the acceleration of tissue regeneration. The obtained results of the maximisation test for a sensitisation effect allow us to conclude that samples of the materials, PLGA + IGF1 and PLGA/PHB + IGF1, did not cause allergic reactions on the skin of guinea pigs.