Chemical Constituents from the Flower of Hosta plantaginea with Cyclooxygenases Inhibition and Antioxidant Activities and Their Chemotaxonomic Significance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Compounds 1–8
2.2. Biological Activities
2.3. Chemotaxonomic Significance
3. Experimental Section
3.1. General Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Acid Hydrolysis and HPLC Analysis
3.5. In Vitro COX-1 and COX-2 Inhibitory Assay
3.6. Antioxidant Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, J.X.; Zhao, C.H.; Liu, X.R.; Xi, Y.Z.; Zhang, Y.L. Pollen morphology of Hosta Tratt. in China and its taxonomic significance. Plant Syst. Evol. 2011, 294, 99–107. [Google Scholar] [CrossRef]
- State Administration of Traditional Chinese Medicine. Chinese Materia Medica; Shanghai Science and Technology Press: Shanghai, China, 1999; Volume VIII, pp. 107–108. [Google Scholar]
- He, J.W.; Yang, L.; Zhong, G.Y. Research progress in chemical constituents, pharmacological activities, clinical practices and quality control of folk medicine Hosta plantaginea. Chin. Tradit. Herb. Drugs 2016, 47, 4295–4300. [Google Scholar]
- Wang, Q.H.; Han, J.J.; Bao, B.Y. Antibacterial effects of two monoterpene glycosides from Hosta plantaginea (lam.) Aschers. J. Food Biochem. 2017, 41, e12320. [Google Scholar] [CrossRef]
- He, J.W.; Yang, L.; Zhu, J.X.; Wang, X.M.; Zhou, Z.R.; He, W.W.; Zhong, G.Y. Comparison of anti-inflammatory effects and HPLC detection on different extracts from the flower of Hosta plantaginea in mice. J. Jiangxi Norm. Univ. (Nat. Sci.) 2016, 40, 183–185. [Google Scholar]
- Wang, Y.H.; Zhang, Z.K.; Yang, F.M.; Sun, Q.Y.; He, H.P.; Di, Y.T.; Mu, S.Z.; Lu, Y.; Chang, Y.; Zheng, Q.T.; et al. Benzylphenethylamine Alkaloids from Hosta plantaginea with inhibitory activity against tobacco mosaic virus and acetylcholinesterase. J. Nat. Prod. 2007, 70, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.X.; Zhang, J.H.; Zhang, H.G.; Xue, P.F. Studies on chemical constituents from Hosta plantaginea (Lam.) Asehers, a Mongolia medieine. Chin. Pharm. J. 2009, 44, 733–735. [Google Scholar]
- Mimaki, Y.; Kameyama, A.; Kuroda, M.; Sashida, Y.; Hirano, T.; Oka, K.; Koike, K.; Nikadio, T. Steroidal glycosides from the underground parts of Hosta plantainea var. Japonica and their cytostatic activity on leukamia HL-60 cells. Phytochemistry 1997, 44, 305–310. [Google Scholar] [CrossRef]
- Li, B.; Ni, Y.; Zhu, L.J.; Wu, F.B.; Yan, F.; Zhang, X.; Yao, X.S. Flavonoids from matteuccia struthiopteris and their anti-influenza virus (H1N1) activity. J. Nat. Prod. 2015, 78, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, R.P.; Jangwan, J.S.; Kaiya, T.; Sakakibara, J. Puddumin A, a new flavanone glucoside from Prunus cerasoides. J. Nat. Prod. 1987, 50, 232–234. [Google Scholar] [CrossRef]
- Hanáková, Z.; Hošek, J.; Kutil, Z.; Temml, V.; Landa, P.; Vanĕk, T.; Schuster, D.; Dall’Acqua, S.; Cvačka, J.; Polansky, O.; Šmejkal, K. Anti-inflammatory activity of natural geranylated flavonoids: Cyclooxygenase and lipoxygenase inhibitory properties and proteomic analysis. J. Nat. Prod. 2017, 80, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Andreu, C.; Olmo, M. Potential of some yeast strains in the stereoselective synthesis of (R)-(−)-phenylacetylcarbinol and (S)-(+)-phenylacetylcarbinol and their reduced 1,2-dialcohol derivatives. Appl. Microbiol. Biotechnol. 2014, 98, 5901–5913. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Gunaratna, M.J.; Zhang, M.; Weerasekara, S.; Seiwald, S.N.; Nguyen, V.T.; Meier, A.; Hua, D.H. Chiral-substituted poly-N-vinylpyrrolidinones and bimetallic nanoclusters in catalytic asymmetric oxidation reactions. J. Am. Chem. Soc. 2016, 138, 16839–16848. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Chen, Y.Z.; Xu, Y.; Li, A.T.; Xu, Q.S.; Glieder, A.; Li, Z. Enantioselective trans-dihydroxylation of aryl olefins by cascade biocatalysis with recombinant Escherichia coli coexpressing monooxygenase and epoxide hydrolase. ACS Catal. 2014, 4, 409–420. [Google Scholar] [CrossRef]
- Kingsbury, C.A.; Cowles, C.R. Conformations of vicinal diesters. J. Org. Chem. 1975, 40, 1302–1308. [Google Scholar] [CrossRef]
- Kihumbu, D.; Stillger, T.; Hummel, W.; Liese, A. Enzymatic synthesis of all stereoisomers of 1-phenylpropane-1,2-diol. Tetrahedron Asymmetry 2002, 13, 1069–1072. [Google Scholar] [CrossRef]
- Brambilia, U.; Nasini, G.; Pava, O.V. Secondary mold metabolites, part 49. isolation, structural elucidation, and biomimetic synthesis of trametol, a new 1-arylpropane-1,2-diol produced by the fungus Trametes sp. J. Nat. Prod. 1995, 58, 1251–1253. [Google Scholar] [CrossRef]
- Mayorga, H.; Knapp, H.; Winterhalter, P.; Duque, C. Glycosidically bound flavor compounds of cape gooseberry (Physalis Peruviana L.). J. Agric. Food Chem. 2001, 49, 1904–1908. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.J.; Yang, J.Z.; Ma, J.; Zhang, D.M. Chemical constituents from stems of Clausena lansium. Chin. Tradit. Herb. Drugs 2016, 47, 32–37. [Google Scholar]
- Ma, S.J.; Mizutani, M.; Hiratake, J.; Hayashi, K.; Yagi, K.; Watanabe, N.; Sakata, K. Substrate specificity of β-primeverosidase, a key enzyme in aroma formation during oolong tea and black tea manufacturing. Biosci. Biotechnol. Biochem. 2001, 65, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Hamerski, L.; Bomm, M.D.; Silva, D.H.; Young, M.C.M.; Furlan, M.; EBerlin, M.N.; Castro-Gamboa, I.; Cavalherio, A.J.; Bolzani, V.S. Phenylpropanoid glucosides from leaves of Coussarea hydrangeifolia (Rubiaceae). Phytochemistry 2005, 66, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Deng, Z.W.; Li, J.; Fu, H.Z.; Lin, W.H. Chemical constituents from starfish Asterias rollestoni. J. Chin. Pharm. Sci. 2004, 13, 81–86. [Google Scholar]
- Yang, S.X.; Zhao, F.W.; Wang, H.; Huang, Q.Q.; Xu, J.J.; Wang, Y.H.; Long, C.L. Chemical constituents of Hosta ventricosa, an ornamental medicinal plant. J. Yunnan Agric. Univ. 2011, 26, 662–667. [Google Scholar]
- Kim, C.S.; Kim, K.Y.; Lee, K.R. Phytochemical constituents of the leaves of Hosta longipes. Nat. Prod. Sci. 2014, 20, 86–90. [Google Scholar]
- Liu, H.X.; Sun, Q.Y.; Yang, F.M.; Zhao, F.W.; Wang, Y.H.; Long, C.L. A new sesquiterpene lactone from H. ensata. Chem. Nat. Compd. 2012, 48, 580–582. [Google Scholar] [CrossRef]
- Ochieng, G.O.; Opiyo, S.A.; Mureka, E.W.; Ishola, I.O. Cyclooxygenase inhibitory compounds from Gymnosporia heterophylla aerial parts. Fitoterapia 2017, 119, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.T.; Li, C.Y.; Wang, C.H.; Wang, Y.F.; Wang, X.D.; Wang, H.T.; Zhu, Y.; Jiang, M.M.; Gao, X.M. Phenolic compounds from the roots of Rhodiola crenulata and their antioxidant and inducing IFN-γ production activities. Molecules 2015, 20, 13725–13739. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
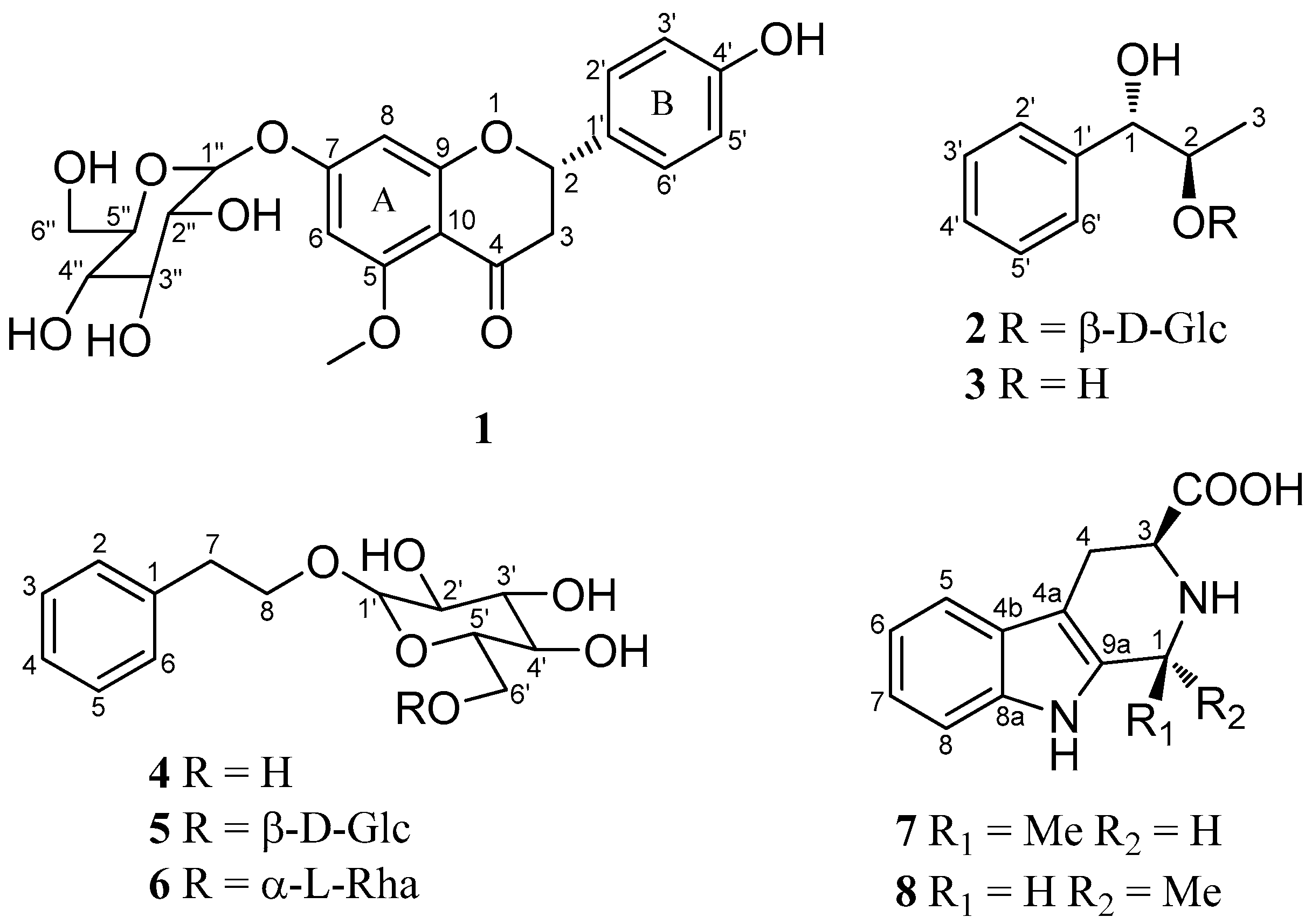
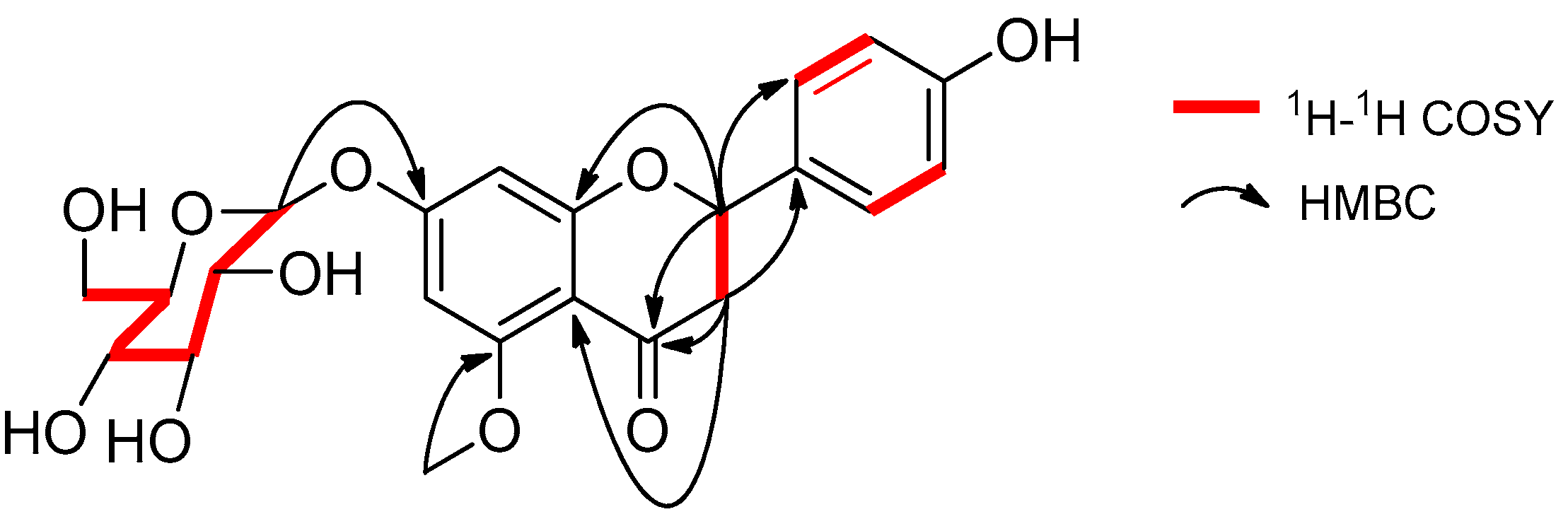
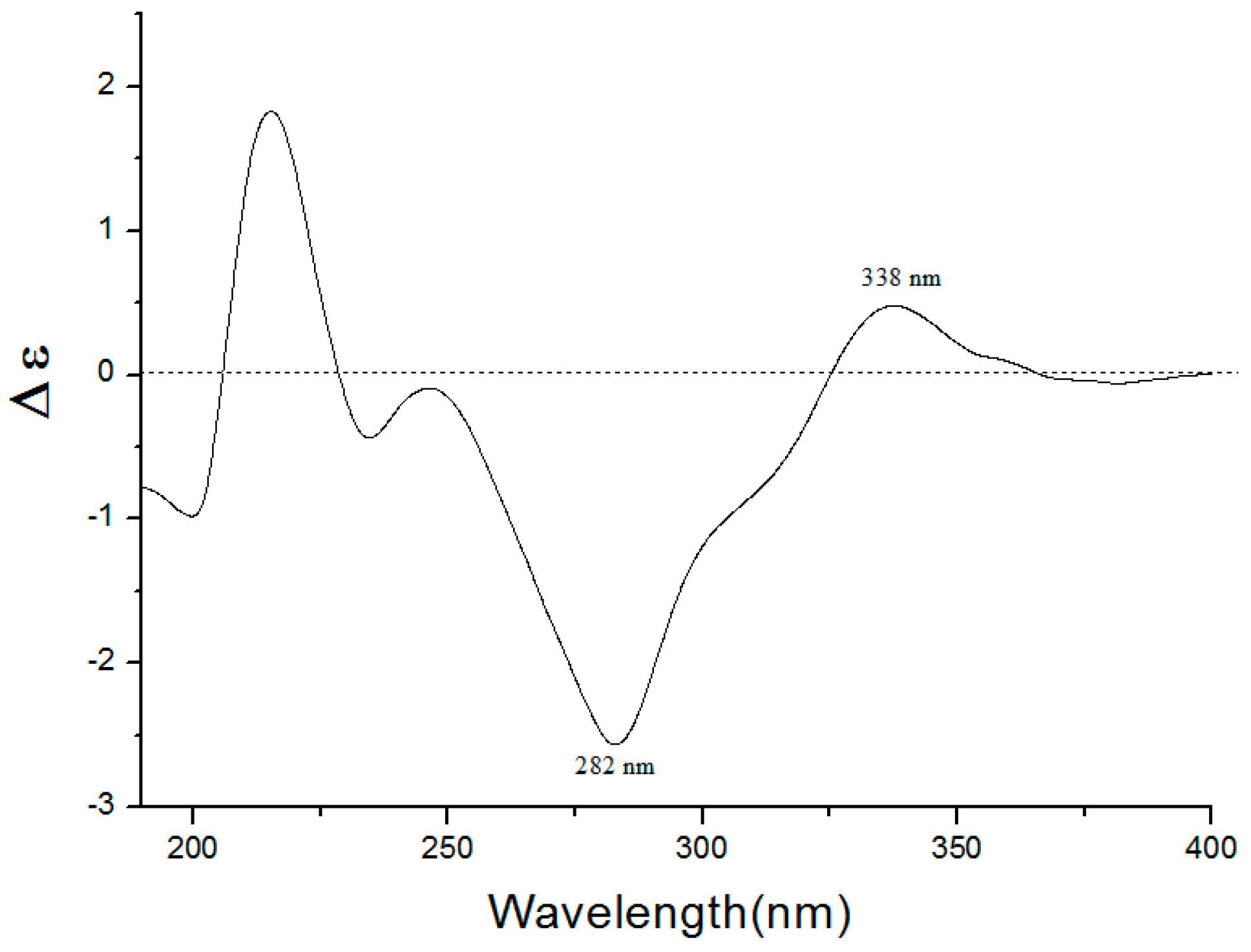
| Position | 1 | Puddumin A | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 2 | 78.3 | 5.40 (1H, m) | 78.3 | 6.15 (1H, s) |
| 3 | 44.7 | 3.07 (1H, dd, J = 16.3/12.8) | 44.5 | 3.30 (1H, d, J = 2) |
| 2.57 (1H, dd, J = 16.3/2.5) | 2.70 (1H, s) | |||
| 4 | 188.3 | - a | 192.4 | - a |
| 5 | 161.7 | - a | 159.9 | - a |
| 6 | 94.0 | 6.28 (1H, d, J = 2.5) | 93.7 | 6.40 (1H, d, J = 2) |
| 7 | 163.3 | - a | 165.6 | - a |
| 8 | 96.1 | 6.24 (1H, d, J = 2.5) | 95.1 | 6.78 (1H, d, J = 2) |
| 9 | 164.1 | - a | 165.1 | - a |
| 10 | 106.1 | - a | 106.5 | - a |
| 1′ | 129.1 | - a | 126.0 | - a |
| 2′, 6′ | 128.3 | 7.30 (2H, d, J = 8.4) | 130.8 | 7.65 (2H, d, J = 9) |
| 3′, 5′ | 115.2 | 6.79 (2H, d, J = 8.4) | 115.9 | 6.83 (2H, d, J = 9) |
| 4′ | 157.7 | - a | 159.6 | - a |
| 1′′ | 99.7 | 4.99 (1H, d, J = 7.8) | 100.3 | - b |
| 2′′ | 73.1 | 3.23 (1H, m) | 73.3 | - b |
| 3′′ | 76.5 | 3.28 (1H, m) | 76.8 | - b |
| 4′′ | 69.7 | 3.14 (1H, m) | 69.6 | - b |
| 5˝ | 77.2 | 3.39 (1H, m) | 77.5 | - b |
| 6′′ | 60.7 | 3.68 (1H, m) | 60.6 | |
| 3.43 (1H, m) | b | |||
| 5-OCH3 | 55.9 | 3.78 (3H, s) | 55.5 | 3.80 (3H, s) |
| Position | 3 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 76.1 | 4.48 (1H, d, J = 5.1) | 78.6 | 4.43 (1H, d, J = 5.1) |
| 2 | 69.4 | 4.41 (1H, m) | 73.7 | 4.40 (1H, m) |
| 3 | 19.1 | 1.08 (3H, d, J = 6.0) | 16.5 | 1.16 (3H, d, J = 6.3) |
| 1′ | 140.3 | 140.1 | ||
| 2′, 6′ | 127.8 | 7.15–7.26 (5H, m) | 127.9 | 7.16-7.26 (5H, m) |
| 3′, 5′ | 129.3 | 7.15–7.26 (5H, m) | 129.3 | 7.16-7.26 (5H, m) |
| 4′ | 125.5 | 7.15–7.26 (5H, m) | 125.6 | 7.16-7.26 (5H, m) |
| 1′′ | - b | - b | 102.9 | 4.23 (1H, d, J = 7.8) |
| 2′′ | - b | - b | 73.5 | - a |
| 3′′ | - b | - b | 76.4 | - a |
| 4′′ | - b | - b | 70.1 | - a |
| 5′′ | - b | - b | 76.8 | - a |
| 6′′ | - b | - b | 61.0 | - a |
| Compounds | IC50 (μM) | SI d | ||
|---|---|---|---|---|
| COX-1 a | COX-2 a | Antioxidant b | ||
| 1 | 21.6 ± 1.2 | 45.4 ± 3.3 | 83.2 ± 3.0 | 0.48 |
| 4 | 15.5 ± 0.6 | 38.2 ± 3.9 | 282 ± 5.2 | 0.41 |
| 5 | 38.4 ± 1.3 | 31.7 ± 2.9 | 257 ± 13.8 | 1.16 |
| 6 | 41.2 ± 1.5 | 35.4 ± 1.6 | 275 ± 14.8 | 1.21 |
| celecoxib | 9.0 ± 0.6 | 1.0 ± 0.1 | - c | 9.00 |
| l-ascorbic acid | - c | - c | 33.9 ± 1.1 | - c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Jiang, S.-T.; Zhou, Q.-G.; Zhong, G.-Y.; He, J.-W. Chemical Constituents from the Flower of Hosta plantaginea with Cyclooxygenases Inhibition and Antioxidant Activities and Their Chemotaxonomic Significance. Molecules 2017, 22, 1825. https://doi.org/10.3390/molecules22111825
Yang L, Jiang S-T, Zhou Q-G, Zhong G-Y, He J-W. Chemical Constituents from the Flower of Hosta plantaginea with Cyclooxygenases Inhibition and Antioxidant Activities and Their Chemotaxonomic Significance. Molecules. 2017; 22(11):1825. https://doi.org/10.3390/molecules22111825
Chicago/Turabian StyleYang, Li, Shu-Tai Jiang, Qin-Guang Zhou, Guo-Yue Zhong, and Jun-Wei He. 2017. "Chemical Constituents from the Flower of Hosta plantaginea with Cyclooxygenases Inhibition and Antioxidant Activities and Their Chemotaxonomic Significance" Molecules 22, no. 11: 1825. https://doi.org/10.3390/molecules22111825
APA StyleYang, L., Jiang, S.-T., Zhou, Q.-G., Zhong, G.-Y., & He, J.-W. (2017). Chemical Constituents from the Flower of Hosta plantaginea with Cyclooxygenases Inhibition and Antioxidant Activities and Their Chemotaxonomic Significance. Molecules, 22(11), 1825. https://doi.org/10.3390/molecules22111825




