Health-Promoting Phytochemicals from 11 Mustard Cultivars at Baby Leaf and Mature Stages
Abstract
1. Introduction
2. Results and Discussion
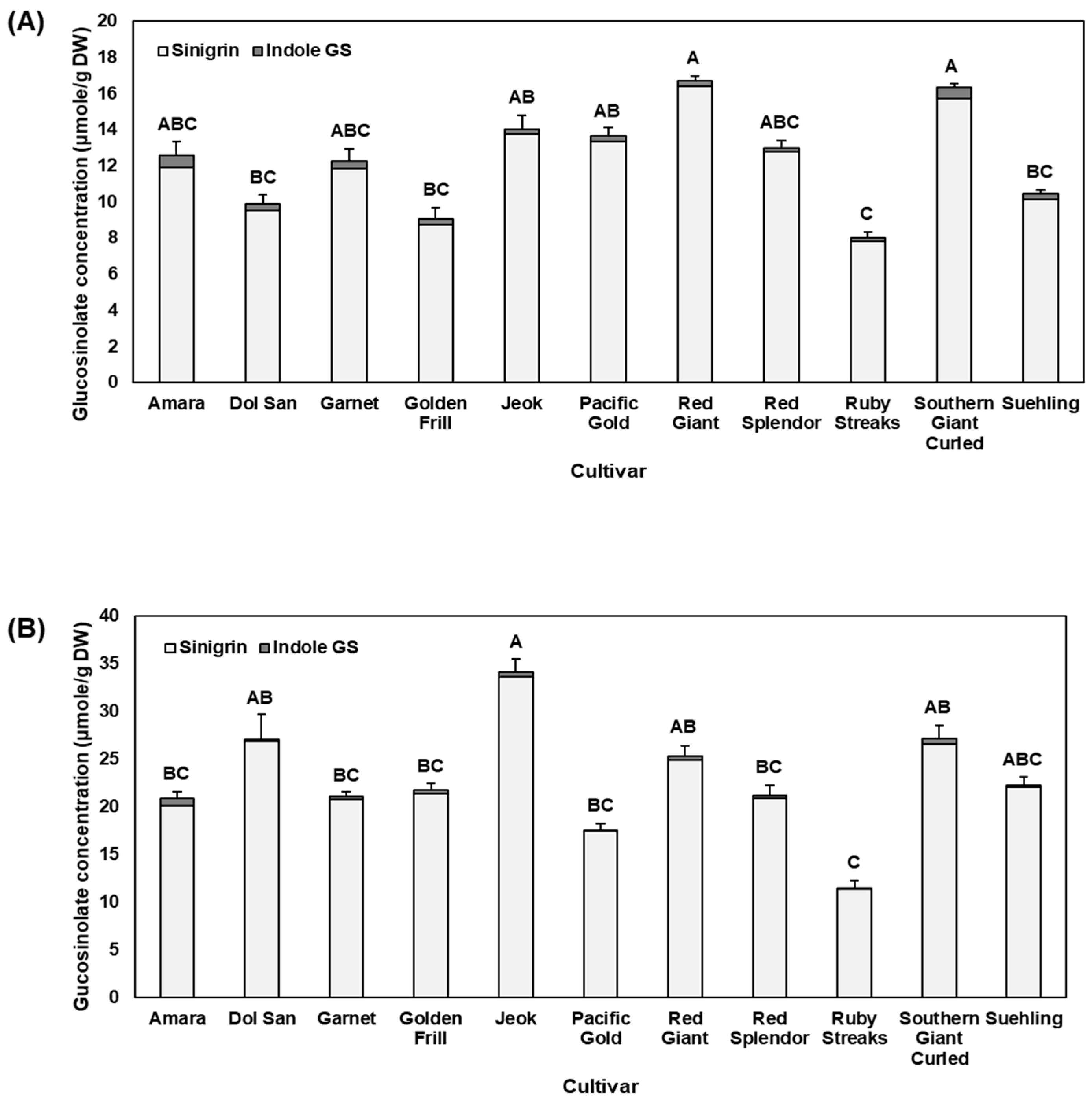
2.1. Glucosinolates
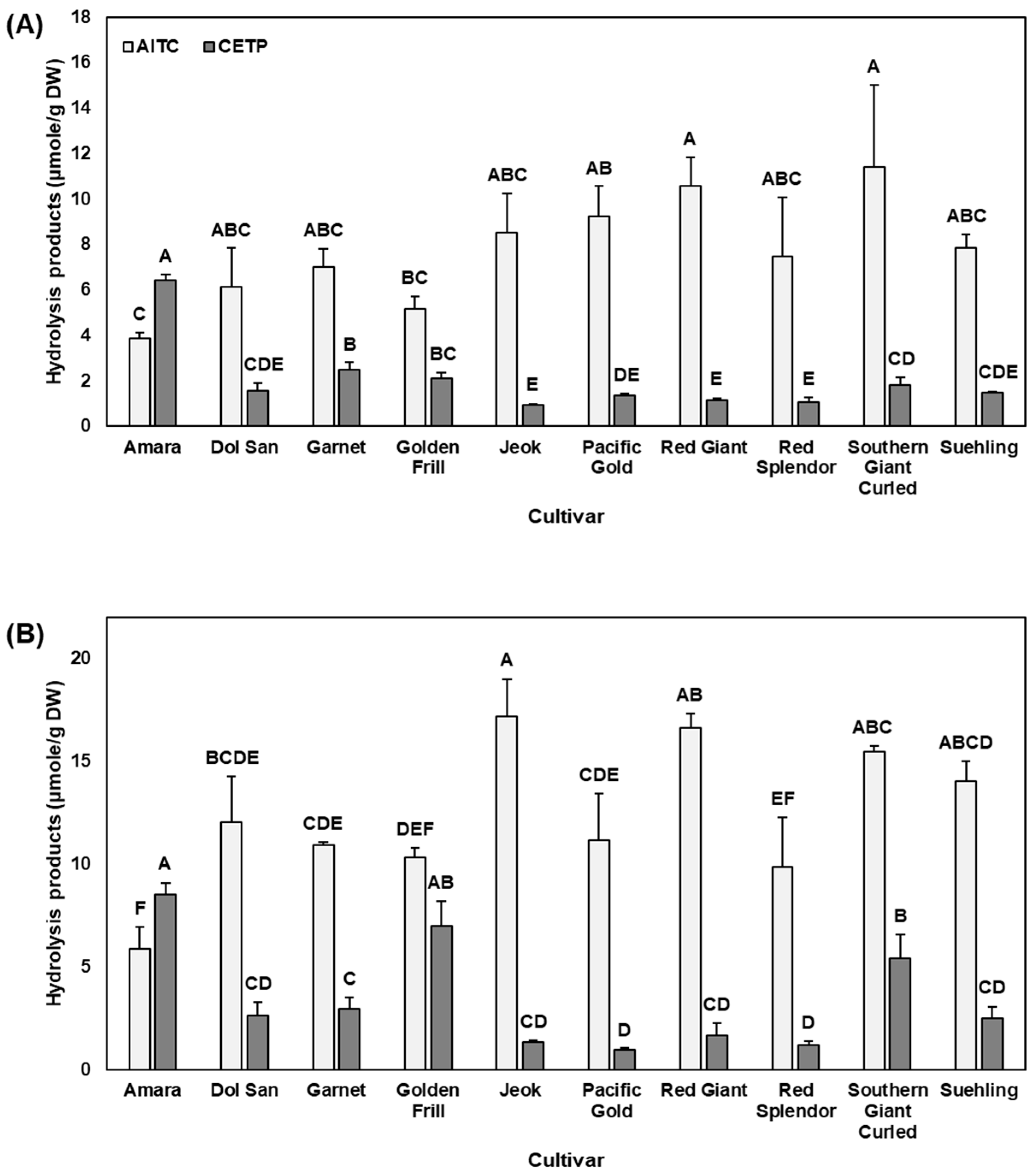
2.2. Hydrolysis Products and Nitrile Formation (%)
2.3. Carotenoids and Recommended Dietary Allowances (RDA)
2.4. Total Anthocyanin Concentrations (TAC)
2.5. Total Phenolics and Antioxidant Capacity
3. Materials and Methods
3.1. Mustard Cultivation
3.2. Quantitation of Glucosinolate
3.3. Hydrolysis Products and Nitrile FORMATION (%)
3.4. Carotenoid Analysis
3.5. Total Anthocyanin Analysis
3.6. Total Phenolic Content and Antioxidant Capacity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, X.L.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.F.; Sun, J.; Wu, X.Z.; Liu, R.H. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, Z.; Zhang, L. Anthocyanin accumulation, antioxidant ability and stability, and a transcriptional analysis of anthocyanin biosynthesis in purple heading Chinese cabbage. J. Agric. Food Chem. 2016, 64, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Mushtaq, M.A.; Pan, Q.; Chen, D.; Zhang, Q.; Ge, X.; Li, Z. Comparative leaves transcriptome analysis emphasizing on accumulation of anthocyanins in Brassica: Molecular regulation and potential interaction with photosynthesis. Front. Plant Sci. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Peto, R.; Doll, J.D.; Buckley, M.B. Can dietary beta-carotene materially reduce human cancer rates? Nature 1981, 290, 201–208. [Google Scholar] [CrossRef] [PubMed]
- West, K.; Darnton-Hill, I. Vitamin A deficiency. In Nutrition and Health in Developing Countries; Semba, R.D., Bloem, M.W., Eds.; Humana Press: New York, NY, USA, 2008; pp. 377–433. [Google Scholar]
- West, K. Extent of vitamin A deficiency among preschool children and women of reproductive age. J. Nutr. 2002, 132, 2857S–2866S. [Google Scholar] [PubMed]
- Rice, A.; West, K.J.; Black, R.E. Vitamin A deficiency. In Comparative Quantification of Health Risks; Ezzati, M., Lopez, A.D., Rodgers, A., Murray, C.J.L., Eds.; World Health Organization: Geneva, Switzerland, 2004; Volume 1, pp. 211–256. [Google Scholar]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Ko, H.C.; Baek, H.J.; Cho, S.M.; Jang, H.H.; Lee, Y.M.; Kim, J.B. Identification and quantification of glucosinolates in Korean leaf mustard germplasm (Brassica juncea var. integrifolia) by liquid chromatography-electrospray ionization/tandem mass spectrometry. Eur. Food Res. Technol. 2016, 242, 1479–1484. [Google Scholar]
- Halkier, B.A.; Gershenzon, J. Bioloy and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.; Yim, B.; Winkelmann, T.; Smalla, K.; Schreiner, M. Degredation of biofumigant isothiocyanates and allyl glucosinolate in soil and their effects on the microbial community composition. PLoS ONE 2015, 10, e0132931. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.G.; Hannahan, H.N.; Sams, C.E. Indian mustard and allyl isothiocyanate inhibit Sclerotium rolfsii. J. Am. Soc. Hortic. Sci. 2002, 127, 27–31. [Google Scholar]
- Ku, K.-M.; Jeffery, E.H.; Juvik, J.A.; Kushad, M.M. Correlation of quinone reductase qctivity and allyl isothiocyanate formation among different genotypes and grades of horseradish roots. J. Agric. Food Chem. 2015, 63, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Juvik, J.A.; Jeffery, E.H. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry 2004, 65, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Waterland, N.L.; Moon, Y.; Tou, J.C.; Kim, M.J.; Pena-Yewtukhiw, E.M.; Park, S. Mineral content differs among microgreen, baby leaf, and adult stages in three cultivars of kale. HortScience 2017, 52, 566–571. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.P.; Minchinton, I.R.; Johnstone, P.K.; Truscott, R.J.W. Glucosinolate profiles in the seed, root and leaf tissue of cabbage, mustard, rapeseed, radish and swede. Can. J. Plant Sci. 1984, 64, 77–93. [Google Scholar] [CrossRef]
- Gupta, S.; Sangha, M.K.; Kaur, G.; Atwal, A.K.; Banga, S.; Banga, S.S. Variability for leaf and seed glucosinolate contents and profiles in a germplasm collection of the Brassica juncea. Biochem. Anal. Biochem. 2012, 1, 120. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Jo, J.S.; Lee, J.G. Comparison of glucosinolate profiles in different tissues of nine Brassica crops. Molecules 2015, 20, 15827–15841. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Jeffery, E.H. Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. J. Agric. Food Chem. 2001, 49, 5743–5749. [Google Scholar] [CrossRef] [PubMed]
- Matusheski, N.V.; Swarup, R.; Juvik, J.A.; Mithen, R.; Bennett, M.; Jeffery, E.H. Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. italica) inhibits formation of the anticancer agent sulforaphane. J. Agric. Food Chem. 2006, 54, 2069–2076. [Google Scholar] [PubMed]
- Zhang, Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 2010, 54, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, M.O.; McMahon, M.; Eggleston, I.M.; Dixon, M.J.; Taguchi, K.; Yamamoto, M.; Hayes, J.D. 1-Cyano-2,3-epithiopropane is a novel plant-derived chemopreventive agent which induces cytoprotective genes that afford resistance against the genotoxic α,β-unsaturated aldehyde acrolein. Carcinogenesis 2009, 30, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Chin, H.-W.; Zeng, Q.; Lindsay, R.C. Occurrence and flavor properties of sinigrin hydrolysis products in fresh cabbage. J. Food Sci. 1996, 61, 101–104. [Google Scholar] [CrossRef]
- Earlywine, D.T.; Smeda, R.J.; Teuton, T.C.; Sams, C.E.; Xiong, X. Evaluation of oriental mustard (Brassica juncea) seed meal for weed suppression in turf. Weed Technol. 2010, 24, 440–445. [Google Scholar] [CrossRef]
- Björkman, T.; Lowry, C.; Shail, J.W., Jr.; Brainard, D.C.; Anderson, D.S.; Masiunas, J.B. Mustard cover crops for biomass production and weed suppression in the Great Lakes region. Agron. J. 2015, 107, 1235–1249. [Google Scholar] [CrossRef]
- De Azevedo, C.H.; Rodriguez-Amaya, D.B. Carotenoid composition of kale as influencedby maturity, season and minimal processing. J. Sci. Food Agric. 2005, 85, 591–597. [Google Scholar] [CrossRef]
- Lefsrud, M.; Kopsell, D.; Wenzel, A.; Sheehan, J. Changes in kale (Brassica oleracea L. var. acephala) carotenoid and chlorophyll pigment concentrations during leaf ontogeny. Sci. Hortic. 2007, 112, 136–141. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; Institute of Medicine: Washington, DC, USA, 2000. [Google Scholar]
- USDA. National Nutrient Database for Standard Reference Release 28. Available online: http://ndb.nal.usda.gov/ndb/ (accessed on 20 March 2017).
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; Institute of Medicine: Washington, DC, USA, 2001. [Google Scholar]
- Haskell, M.J. The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion-evidence in humans. Am. J. Clin. Nutr. 2012, 96, 1193S–1203S. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Hu, Z.; Zhang, Y.; Tian, S.; Wang, Z.; Zhao, Z.; Yang, Y.; Chen, G. Accumulation and molecular regulation of anthocyanin in purple tumorous stem mustard (Brassica juncea var. tumida Tsen et Lee). J. Agric. Food Chem. 2014, 62, 7813–7821. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Z.; Sun, J.; Chen, P.; Harnly, J. UHPLC-PDA-ESI/HRMS/MSn analysis of anthocyanins, flavonol glycosides, and hydroxycinnamic acid derivatives in red mustard greens (Brassica juncea Coss variety). J. Agric. Food Chem. 2011, 59, 12059–12072. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.M.; Pogrebnyak, N.; Kuhn, P.; Krueger, C.G.; Johnson, W.D.; Raskin, I. Development and phytochemical characterization of high polyphenol red lettuce with anti-diabetic properties. PLoS ONE 2014, 9, e91571. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, C.; Cardinault, N.; Gueux, E.; Jaffrelo, L.; Rock, E.; Mazur, A.; Amouroux, P.; Rémésy, C. Health effect of vegetable-based diet: Lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin. Nutr. 2004, 23, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Hu, Y.; Liu, D.; Chen, J.; Ye, X. Changes of phenolic acids and antioxidant activities during potherb mustard (Brassica juncea, Coss.) pickling. Food Chem. 2008, 108, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Harbaum, B.; Hubbermann, E.M.; Zhu, Z.; Schwarz, K. Impact of fermentation on phenolic compounds in leaves of pak choi (Brassica campestris L. ssp. chinensis var. communis) and Chinese leaf mustard (Brassica juncea Coss). J. Agric. Food Chem. 2008, 56, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Kroymann, J.; Brown, P.; Figuth, A.; Pedersen, D.; Gershenzon, J.; Mitchell-Olds, T. Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 2001, 126, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A. Optimization of methyl jasmonate application to broccoli florets to enhance health-promoting phytochemical content. J. Sci. Food Agric. 2014, 94, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.-M.; Kim, M.J.; Jeffery, E.H.; Kang, Y.-H.; Juvik, J.A. Profiles of glucosinolates, their hydrolysis products, and quinone reductase inducing activity from 39 arugula (Eruca Sativa Mill.) accessions. J. Agric. Food Chem. 2016, 64, 6524–6532. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Chiu, Y.-C.; Kim, N.K.; Park, H.M.; Lee, C.H.; Juvik, J.A.; Ku, K.-M. Cultivar-specific changes in primary and secondary metabolites in pak choi (Brassica rapa, Chinensis group) by methyl jasmonate. Int. J. Mol. Sci. 2017, 18, 1004. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.M.; Mein, J.R.; Chaudhuri, S.K.; Constant, H.L. An improved UHPLC-UV method for separation and quantification of carotenoids in vegetable crops. Food Chem. 2014, 165, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Moon, Y.; Kopsell, D.A.; Park, S.; Tou, J.C.; Waterland, N.L. Nutritional value of crisphead ‘Iceberg’ and romaine lettuces (Lactuca sativa L.). J. Agric. Sci. 2016, 8. [Google Scholar] [CrossRef]
- Ku, K.M.; Choi, J.N.; Kim, J.; Kim, J.K.; Yoo, L.G.; Lee, S.J.; Hong, Y.-S.; Lee, C.H. Metabolomics analysis reveals the compositional differences of shade grown tea (Camellia sinensis L.). J. Agric. Food Chem. 2010, 58, 418–426. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Freeze dried samples are available from the authors. |
| Variable | by Variable | Correlation Coefficient |
|---|---|---|
| Glucobrassicin (mature) | Glucobrassicin (baby leaf) | 0.8072 **,a |
| Neoglucobrassicin (mature) | Neoglucobrassicin (baby leaf) | 0.7275 ** |
| Total indole glulcosinolates (mature) | Total indole glulcosinolates (baby leaf) | 0.7783 ** |
| Nitrile formation from sinigrin (mature) | Nitrile formation from sinigrin (baby leaf) | 0.8785 ** |
| Nitrile formation from glucobrassicin (mature) | Nitrile formation from glucobrassicin (mature) | 0.9164 *** |
| Total phenolics (mature) | Total phenolics (baby leaf) | 0.7495 ** |
| Anthocyanins (mature) | Anthocyanins (baby leaf) | 0.8647 *** |
| Total phenolics (mature) | Anthocyanins (mature) | 0.8262 ** |
| Total phenolics (mature) | Antioxidant capacity—DPPH (mature) | 0.7609 ** |
| Antioxidant capacity—DPPH (mature) | Anthocyanins (mature) | 0.6608 * |
| Cultivar Name | β-Carotene | Violaxanthin | Neoxanthin | Lutein |
|---|---|---|---|---|
| μmole/g DW | ||||
| Amara | 476 ± 26 c a | 830 ± 32 bcd | 213 ± 12 ab | 1445 ± 88 a |
| 580 ± 108 c | 1153 ± 185 abc | 294 ± 51 a | 1485 ± 289 ab | |
| Dol San | 931 ± 258 abc | 838 ± 52 bcd | 158 ± 8 bc | 1261 ± 71 a |
| 1113 ± 163 ab | 1093 ± 104 abc | 299 ± 17 a | 2039 ± 11 a | |
| Garnet | 912 ± 67 bc | 654 ± 12 d | 142 ± 3 c | 1335 ± 55 a |
| 804 ± 50 bc | 1056 ± 14 abc | 273 ± 13 a | 1637 ± 31 ab | |
| Golden Frill | 710 ± 58 bc | 771 ± 23 cd | 154 ± 3 bc | 1388 ± 61 a |
| 664 ± 207 bc | 957 ± 120 bc | 268 ± 20 a | 1582 ± 96 ab | |
| Jeok | 1438 ± 21 a | 647 ± 30 d | 192 ± 5 abc | 1260 ± 40 a |
| 1386 ± 98 a | 767 ± 80 c | 272 ± 31 a | 1901 ± 218 ab | |
| Pacific Gold | 696 ± 84 bc | 1027 ± 10 ab | 227 ± 8 a | 1533 ± 23 a |
| 704 ± 20 bc | 1461 ± 31 a | 293 ± 9 a | 1649 ± 48 ab | |
| Red Giant | 717 ± 94 bc | 1114 ± 88 a | 228 ± 30 a | 1595 ± 118 a |
| 644 ± 21 bc | 1205 ± 53 abc | 272 ± 16 a | 1458 ± 52 ab | |
| Red Splendor | 666 ± 24 bc | 866 ± 24 bc | 189 ± 6 abc | 1375 ± 57 a |
| 637 ± 23 bc | 1278 ± 115 ab | 303 ± 28 a | 1627 ± 193 ab | |
| Ruby Streaks | 675 ± 147 bc | 879 ± 42 bc | 182 ± 12 a | 1239 ± 116 a |
| 863 ± 93 bc | 992 ± 34 bc | 267 ± 7 a | 1655 ± 36 ab | |
| Southern Giant Curled | 1114 ± 44 ab | 815 ± 39 cd | 178 ± 11 abc | 1557 ± 79 a |
| 573 ± 37 c | 988 ± 38 bc | 256 ± 9 a | 1482 ± 62 ab | |
| Suehling | 782 ± 60 bc | 869 ± 15 bc | 185 ± 4 abc | 1407 ± 73 a |
| 539 ± 12 c | 1071 ± 68 abc | 261 ± 26 a | 1339 ± 84 b | |
| Cultivar Name | TAC | Total Phenolics | Antioxidant Capacity |
|---|---|---|---|
| μg/g DW | mg/g DW | % | |
| Amara | 1.9 ± 1.3 c a | 4.15 ± 0.11 c | 40.6 ± 1.9 a |
| 7.8 ± 2.2 d | 2.83 ± 0.12 cde | 29.2 ± 1.3 bc | |
| Dol San | 9.3 ± 2.9 c | 4.63 ± 0.19 bc | 18.8 ± 0.8 d |
| 4.8 ± 2.0 d | 2.73 ± 0.06 e | 23.9 ± 0.2 c | |
| Garnet | 1477.8 ± 98.7 a | 5.71 ± 0.26 b | 30.2 ± 2.1 bc |
| 1985.4 ± 127.5 a | 4.14 ± 0.13 a | 37.9 ± 1.2 a | |
| Golden Frill | 5.6 ± 1.9 c | 4.18 ± 0.13 c | 22.0 ± 1.2 cd |
| 16.0 ± 4.3 d | 3.11 ± 0.08 cde | 26.6 ± 1.7 bc | |
| Jeok | 40.4 ± 4.5 c | 4.68 ± 0.19 bc | 21.7 ± 2.0 cd |
| 39.3 ± 9.2 d | 2.60 ± 0.15 e | 20.8 ± 1.3 c | |
| Pacific Gold | 13.0 ± 2.0 c | 4.14 ± 0.10 c | 31.0 ± 1.6 bc |
| 4.18 ± 0.7 d | 2.74 ± 0.11 de | 28.2 ± 1.9 bc | |
| Red Giant | 300.1 ± 8.7 c | 5.36 ± 0.19 bc | 33.9 ± 2.4 ab |
| 335.5 ± 90.0 c | 3.00 ± 0.09 cde | 24.9 ± 1.5 c | |
| Red Splendor | 733.8 ± 51.5 b | 7.48 ± 0.71 a | 37.9 ± 1.0 ab |
| 673.9 ± 35.3 b | 3.85 ± 0.19 ab | 33.7 ± 3.7 ab | |
| Ruby Streaks | 1261.2 ± 214.6 a | 5.36 ± 0.19 bc | 40.7 ± 1.4 a |
| 449.3 ± 81.1 bc | 3.40 ± 0.25 bcd | 41.4 ± 1.8 a | |
| Southern Giant Curled | 10.4 ± 3.2 c | 5.36 ± 0.10 bc | 35.2 ± 2.7 ab |
| 21.2 ± 13.7 d | 3.43 ± 0.03 bc | 26.4 ± 0.3 bc | |
| Suehling | 12.4 ± 7.2 c | 4.65 ± 0.03 bc | 32.7 ± 2.3 ab |
| 10.8 ± 2.6 d | 2.68 ± 0.05 e | 21.7 ± 0.9 c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frazie, M.D.; Kim, M.J.; Ku, K.-M. Health-Promoting Phytochemicals from 11 Mustard Cultivars at Baby Leaf and Mature Stages. Molecules 2017, 22, 1749. https://doi.org/10.3390/molecules22101749
Frazie MD, Kim MJ, Ku K-M. Health-Promoting Phytochemicals from 11 Mustard Cultivars at Baby Leaf and Mature Stages. Molecules. 2017; 22(10):1749. https://doi.org/10.3390/molecules22101749
Chicago/Turabian StyleFrazie, Marissa D., Moo Jung Kim, and Kang-Mo Ku. 2017. "Health-Promoting Phytochemicals from 11 Mustard Cultivars at Baby Leaf and Mature Stages" Molecules 22, no. 10: 1749. https://doi.org/10.3390/molecules22101749
APA StyleFrazie, M. D., Kim, M. J., & Ku, K.-M. (2017). Health-Promoting Phytochemicals from 11 Mustard Cultivars at Baby Leaf and Mature Stages. Molecules, 22(10), 1749. https://doi.org/10.3390/molecules22101749







