Polish Yellow Sweet Clover (Melilotus officinalis L.) Honey, Chromatographic Fingerprints, and Chemical Markers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Volatile Compounds Identified by GC-MS
2.2. Phenolic Compounds
2.2.1. Total Phenolic and Flavonoids Content (TPC and TFC)
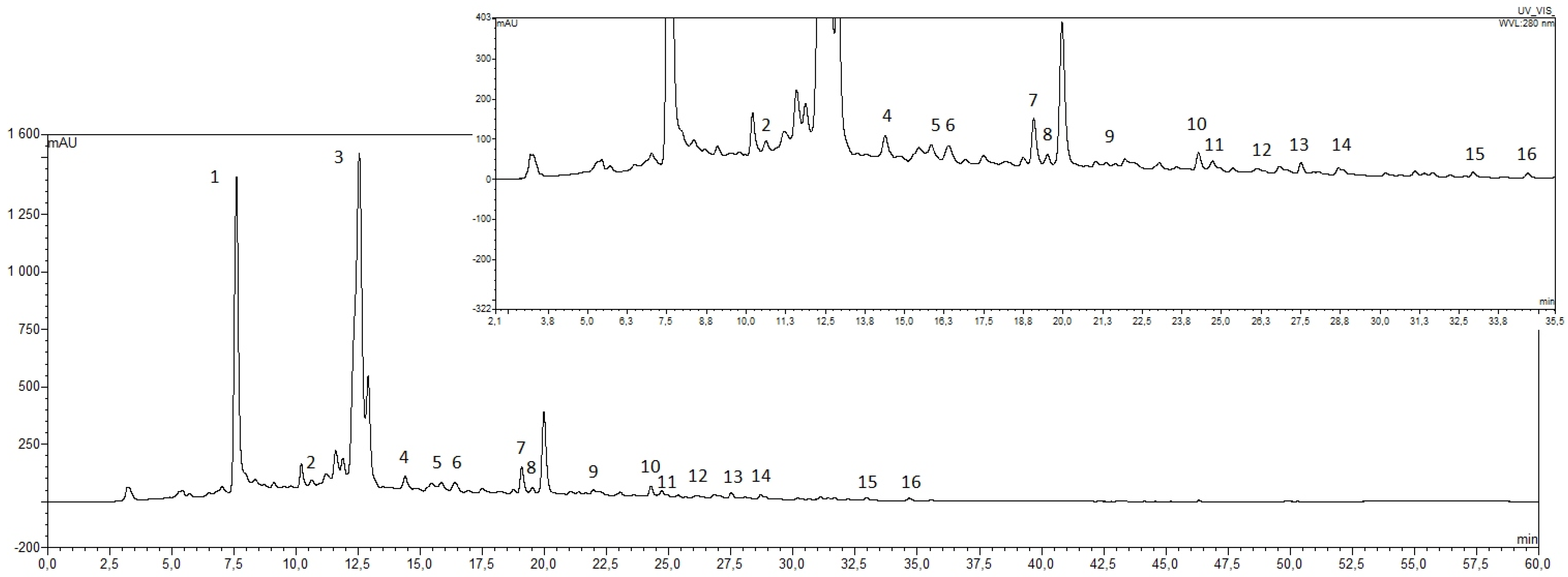
2.2.2. HPLC-PAD Analysis of Honey Samples
2.2.3. Antiradical Activity (DPPH Test)
3. Materials and Methods
3.1. Reagents and Honey Samples
3.2. Extraction and Determination of Volatile Compounds
3.2.1. Steam distillation
3.2.2. Soxhlet Extraction
3.2.3. Ultrasound Solvent Extraction (USE)
3.2.4. Solid Phase Extraction (SPE)
3.2.5. Quantification of Coumarin Content by GC-MS
3.2.6. GC-MS Analysis
3.3. Extraction and Determination of Phenolic Compounds
3.3.1. HPLC/PAD Analysis
3.3.2. Total Phenolic and Flavonoids Content
3.3.3. Antiradical Activity (DPPH Test)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bunney, S. The Illustrated Encyclopedia of Herb. Their Medicinal and Culinary Uses; Chancellor Press: London, UK, 1992; pp. 190–198. [Google Scholar]
- Chorepsima, S.; Tentolouris, K.; Dimitroulis, D.; Tentolouris, N. Melilotus: Contribution to wound healing in the diabetic foot. J. Herb. Med. 2013, 3, 81–86. [Google Scholar] [CrossRef]
- Matławska, I. Leki roślinne w terapii guzków krwawniczych odbytu. Herbal remedies in the treatment of hemorrhoids. Postępy Fitoter. 2002, 3–4, 70–74. [Google Scholar]
- Ehlers, D.; Platte, S.; Bork, W.R.; Gerard, D.; Quirin, K.W. HPLC analysis of sweet clover extracts. Deut. Lebensm. Rundsch. 1997, 93, 77–79. [Google Scholar]
- Keating, G.; O’Kennedy, R. The chemistry and occurrence of coumarins. In Coumarins—Biology, Applications and Mode of Action; O’Kennedy, R., Thornes, R.D., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1997; pp. 315–323. [Google Scholar]
- Smith, W.K.; Gorz, H.J. Sweet clover improvement. Adv. Agron. 1965, 17, 163–231. [Google Scholar]
- Locatelli, M.; Zengin, G.; Uysal, A.; Carradori, S.; de Luca, E.; Bellagamba, G.; Aktumsek, A.; Lazarova, I. Multicomponent pattern and biological activities of seven Asphodeline taxa: Potential sources of natural-functional ingredients for bioactive formulations. J. Enzyme Inhib. Med. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.; Zengin, G.; Mollica, A.; Gunes, E.; Locatelli, M.; Yilmaz, T.; Aktumsek, A. Chemical and biological insights on Cotoneaster integerrimus: A new (−)-epicatechin source for food and medicinal applications. Phytomedicine 2016, 23, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Locatelli, M.; Ceylan, R.; Aktumsek, A. Anthraquinone profile, antioxidant and enzyme inhibitory effect of root extracts of eight Asphodeline taxa from Turkey: Can Asphodeline roots be considered as a new source of natural compounds? J. Enzyme Inhib. Med. Chem. 2016, 31, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Lake, B. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Thada, R.; Chockalingam, S.; Dhandapani, R.K.; Panchamoorthy, R. Extraction and quantitation of coumarin from cinnamon and its effect on enzymatic browning in fresh apple juice: A bioinformatics approach to illuminate its antibrowning activity. J. Agric. Food Chem. 2013, 61, 5385–5390. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Marijanović, Z.; Staver, M.M. Screening of natural organic volatiles from Prunus mahaleb L. Honey: Coumarin and vomifoliol as nonspecific biomarkers. Molecules 2011, 16, 2507–2518. [Google Scholar] [PubMed]
- Guyot–Declerck, C. Floral quality and discrimination of Lavandula stoechas, Lavandula angustifolia, and Lavandula angustifolia x latifolia honeys. Food Chem. 2002, 79, 453–459. [Google Scholar] [CrossRef]
- Martino, E.; Ramaiola, I.; Urbano, M.; Bracco, F.; Collina, S. Microwave-assisted extraction of coumarin and related compounds from Melilotus officinalis (L.) Pallas as an alternative to Soxhlet and ultrasound-assisted extraction. J. Chromatogr. A 2006, 1125, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Bouseta, A.; Scheirman, V.; Collin, S. Flavor and free amino acid composition of lavender and Eucalyptus honeys. J. Food Sci. 1996, 61, 683–687. [Google Scholar] [CrossRef]
- Matiru, V.N.; Dakora, F.D. Xylem transport and shoot accumulation of lumichrome, a newly recognized rhizobial signal, alters root respiration, stomatal conductance, leaf transpiration and photosynthetic rates in legumes and cereals. New Phytol. 2005, 165, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Jerković, I.; Bifulco, E.; Marijanovic, Z.; Congiu, F.; Bubalo, D. Riboflavin and lumichrome in Dalmatian sage honey and other unifloral honeys determined by LC–DAD technique. Food Chem. 2012, 135, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Bifulco, E.; Caboni, P.; Sarais, G.; Cottiglia, F.; Floris, I. Lumichrome and phenyllactic acid as chemical markers of thistle (Galactites tomentosa Moench) honey. J. Agric. Food Chem. 2011, 59, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.M.; Jerković, I.; Tuberoso, C.I.G.; Marijanović, Z.; Congiu, F. Cornflower (Centaurea cyanus L.) honey quality parameters: Chromatographic fingerprints, chemical biomarkers, antioxidant capacity and others. Food Chem. 2014, 142, 12–18. [Google Scholar]
- Jasicka-Misiak, I.; Poliwoda, A.; Dereń, M.; Kafarski, P. Phenolic compounds and abscisic acid as potential markers for the floral origin of two Polish unifloral honeys. Food Chem. 2012, 131, 1149–1156. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Turumtay, E.A.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profile. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Piljac-Zegarac, J.; Stipcevic, T.; Belscak, A. Antioxidant properties and phenolic content of different floral origin honeys. J. Apiprod. Apimed. Sci. 2009, 1, 43–50. [Google Scholar] [CrossRef]
- Dimitrova, B.; Gevrenova, R.; Anklam, E. Analysis of phenolic acids in honeys of different floral origin by solid-phase extraction and highperformance liquid chromatography. Phytochem. Anal. 2007, 18, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic acid composition and antioxidant properties of Malaysian honeys. J. Food Sci. 2011, 76, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.Z.; Yusoff, K.M.; Makpol, S.; Yusof, Y.A.M. Antioxidant capacities and total phenolic contents increase with gamma irradiation in two types of Malaysian honey. Molecules 2011, 16, 6378–6395. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R.M. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Wilczyńska, A. Phenolic content and antioxidant activity of different types of Polish honey—A short report. Pol. J. Food Nutr. Sci. 2010, 60, 309–313. [Google Scholar]
- Rababah, T.M.; Al-Omoush, M.; Brewer, S.; Alhamad, M.; Yang, W.; Alrababah, M.; Al-Ghzawi, A.A.; Al-u’datt, M.; Ereifej, K.; Alsheyab, F.; et al. Total phenol, antioxidant activity, flavonoids, anthocyanins and color of honey as affected by floral origin found in the arid and semiarid Mediterranean areas. J. Food Process. Preserv. 2014, 38, 1119–1128. [Google Scholar] [CrossRef]
- Da Silva, I.A.; da Silva, T.M.; Camara, C.A.; Queiroz, N.; Magnani, M.; de Novais, J.S.; Soledade, L.E.; Lima Ede, O.; de Souza, A.L.; de Souza, A.G. Phenolic profile, antioxidant activity and palynological analysis of stingless bee honey from Amazonas, Northern Brazil. Food Chem. 2013, 141, 3552–3558. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.; Čanadanović-Brunet, J.; Ćetković, G.; Djilas, S.; Tumbas Šaponjac, V. Antioxidant and sensorial properties of polyfloral honey with dried apricots after one year of storage. J. Chem. 2015, 2015, 858049. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Daferera, D.; Tarantilis, P.A.; Polissiou, M.; Harizanis, P.C. Ultrasound—Assisted extraction of volatile compounds from citrus flowers and citrus honey. Food Chem. 2003, 82, 575–582. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Consuelo Díaz-Maroto, M.; Guchu, E.; Soledad Pérez-Coello, M. Analysis of volatile compounds of eucalyptus honey by solid phase extraction followed by gas chromatography coupled to mass spectrometry. Eur. Food Res. Technol. 2006, 224, 27–31. [Google Scholar] [CrossRef]
- Martos, I.; Cossentini, M.; Ferreres, F.; Tomas-Barberan, F.A. Flavonoid composition of Tunisian honeys and propolis. J. Agric. Food Chem. 1997, 45, 2824–2829. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, Ch.; Romito, M.; Millogo, J.; Nacoulma, O. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Sample Availability: The honey samples are available from the authors for limited time.
| No. | Compounds | Peak Area (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||||
| H14 | H15 | H16 | H14 | H15 | H16 | H14 | H15 | H16 | ||
| Av. (n = 3) | Av. (n = 3) | Av. (n = 3) | ||||||||
| 1 | (1S,15S)-Bicyclo[13.1.0]hexadecane | - | - | - | 0.00 ± 0.00 | 0.69 ± 0.09 | 0.00 ± 0.00 | - | - | - |
| 2 | β-Phellandrene | 1.96 ± 0.21 | 0.61 ± 0.28 | 1.36 ± 0.45 | - | - | - | - | - | - |
| 3 | δ-Selinene | 0.10 ± 0.02 | 0.12 ± 0.05 | 0.10 ± 0.05 | - | - | - | - | - | - |
| 4 | 11-Tricosene | - | - | - | 0.92 ± 0.00 | 0.92 ± 0.20 | 1.00 ± 0.31 | - | - | - |
| 5 | 17-Pentatriacontene | - | - | - | 0.70 ± 0.23 | 0.59 ± 0.03 | 0.56 ± 0.12 | |||
| 6 | Docosene | 0.51 ± 0.03 | 0.56 ± 0.04 | 0.52 ± 0.03 | 0.96 ± 0.05 | 1.56 ± 0.05 | 1.60 ± 0.12 | 0.00 ± 0.00 | 1.48 ± 0.63 | 1.12 ± 0.27 |
| 7 | 1-Heptacosanol | - | - | - | - | - | - | 1.46 ± 0.65 | 1.70 ± 0.04 | 1.51 ± 0.63 |
| 8 | 1-Heptacosane | - | - | - | - | - | - | 1.06 ± 0.26 | 0.55 ± 0.21 | 0.59 ± 0.06 |
| 9 | 1-Hexacosene | 0.71 ± 0.09 | 0.80 ± 0.05 | 0.69 ± 0.10 | - | - | - | - | - | - |
| 10 | 13-Epimanool | - | - | - | 0.00 ± 0.00 | 2.15 ± 0.98 | 1.46 ± 0.41 | - | - | - |
| 11 | 1-Nonadecene | 0.25 ± 0.02 | 0.25 ± 0.01 | 0.26 ± 0.01 | - | - | - | - | - | - |
| 12 | 1-Oxa-spiro[4.5]deca-6,9-diene-2,8-dione | 0.32 ± 0.10 | 0.28 ± 0.01 | 0.28 ± 0.03 | - | - | - | - | - | - |
| 13 | 2,6,10,14-Tetramethyl-7-(3-methylpent-4-enylidene) pentadecane | - | - | - | 0.96 ± 0.00 | 1.19 ± 0.00 | 0.00 ± 0.00 | - | - | - |
| 14 | 2-Ethyl-5-n-propylphenol | - | - | - | 0.23 ± 0.06 | 0.27 ± 0.16 | 0.00 ± 0.00 | - | - | - |
| 15 | 2-Ethylhexyl trans-4-methoxycinnamate | 0.51 ± 0.02 | 0.48 ± 0.05 | 0.00 ± 0.00 | - | - | - | - | - | - |
| 16 | 2-Phenyl-3-(2-furyl)-propenal | 0.18 ± 0.01 | 0.19 ± 0.01 | 0.19 ± 0.00 | - | - | - | - | - | - |
| 17 | 3,5-di-tert-Butyl-4-hydroxybenzaldehyde | 0.00 ± 0.00 | 0.09 ± 0.01 | 0.10 ± 0.01 | - | - | - | - | - | - |
| 18 | 3-Furanmethanol/2-Furamethanol * | 0.41 ± 0.02 | 0.35 ± 0.10 | 0.39 ± 0.20 | - | - | - | - | - | - |
| 19 | 5-Hydroxymethylfurfural | 5.94 ± 1.20 | 2.01 ± 0.01 | 1.12 ± 0.09 | 5.51 ± 1.63 | 4.32 ± 1.97 | 2.21 ± 0.69 | 15.21 ± 4.21 | 13.21 ± 3.28 | 10.26 ± 1.96 |
| 20 | (E)-9-Octadecenoic acid ** | 6.98 ± 1.02 | 7.00 ± 1.25 | 5.25 ± 0.96 | 0.96 ± 0.36 | 1.80 ± 0.64 | 1.46 ± 0.26 | 1.64 ± 0.23 | 3.60 ± 0.76 | 2.01 ± 0.36 |
| 21 | (Z)-9-Tricosene, | 1.26 ± 0.69 | 1.39 ± 0.78 | 1.33 ± 0.20 | - | - | - | 0.90 ± 0.23 | 0.89 ± 0.41 | 0.65 ± 0.09 |
| 22 | Acetophenone | 0.00 ± 0.00 | 0.13 ± 0.08 | 0.19 ± 0.10 | - | - | - | - | - | - |
| 23 | Behenic alcohol | - | - | - | 0.78 ± 0.22 | 1.03 ± 0.12 | 0.00 ± 0.00 | - | - | - |
| 24 | Benzaldehyde | 0.19 ± 0.06 | 0.25 ± 0.08 | 0.21 ± 0.42 | - | - | - | - | - | - |
| 25 | 4-Octyl-N-(4-octylphenyl)benzenamine | - | - | - | 1.23 ± 0.13 | 2.03 ± 0.29 | 1.63 ± 0.09 | - | - | - |
| 26 | Benzeneacetaldehyde | 5.69 ± 0.17 | 6.02 ± 0.10 | 5.50 ± 0.17 | - | - | - | - | - | - |
| 27 | Phenylacetic acid | - | - | - | 4.13 ± 0.06 | 6.14 ± 0.65 | 5.23 ± 0.91 | 28.12 ± 3.74 | 26.21 ± 2.78 | 24.26 ± 3.65 |
| 28 | α-(Phenylmethyl)benzeneethanol | - | - | - | - | - | - | 5.59 ± 1.61 | 8.80 ± 0.09 | 6.01 ± 1.23 |
| 29 | Benzoic acid | - | - | - | - | - | - | 1.15 ± 0.07 | 0.85 ± 0.37 | 1.06 ± 0.33 |
| 30 | 4-Hydroxy-3,5-dimethoxybenzoic acid | - | - | - | - | - | - | 1.78 ± 0.23 | 1.85 ± 0.64 | 1.79 ± 0.36 |
| 31 | Benzothiazole | 1.21 ± 0.19 | 1.32 ± 0.09 | 1.30 ± 0.09 | - | - | - | - | - | - |
| 32 | Benzyl alcohol | 0.25 ± 0.02 | 0.42 ± 0.10 | 0.40 ± 0.03 | - | - | - | - | - | - |
| 33 | 2-Methylene4,8,8-trimethyl-4-vinyl-bicyclo[5.2.0]nonane | - | - | - | 0.96 ± 0.63 | 2.93 ± 0.23 | 2.21 ± 0.09 | - | - | - |
| 34 | Bis(2-ethylhexyl) maleate | - | - | - | 1.01 ± 0.56 | 1.53 ± 0.23 | 1.26 ± 0.09 | - | - | - |
| 35 | Caryophyllene | - | - | - | 13.21 ± 3.31 | 12.26 ± 0.91 | 13.60 ± 3.69 | - | - | - |
| 36 | cis-13-Octadecenoic acid/trans-13-Octadecenoic acid ** | - | - | - | 1.71 ± 0.63 | 2.00 ± 0.06 | 1.96 ± 0.06 | - | - | - |
| 37 | Coumarin | 1.41 ± 0.15 | 0.98 ± 0.09 | 0.00 ± 0.00 | 0.22 ± 0.12 | 0.19 ± 0.09 | 0.00 ± 0.00 | - | - | - |
| 38 | Cyclohept-4-enone | 0.21 ± 0.04 | 0.19 ± 0.10 | 0.15 ± 0.10 | - | - | - | - | - | - |
| 39 | β-Menthane | 1.85 ± 0.35 | 2.61 ± 0.15 | 2.52 ± 0.31 | - | - | - | - | - | - |
| 40 | Cyclopentadecane | - | - | - | 0.65 ± 0.26 | 0.95 ± 0.06 | 0.71 ± 0.20 | - | - | - |
| 41 | Docosane | - | - | - | 1.36 ± 0.23 | 1.60 ± 0.16 | 1.09 ± 0.21 | - | - | - |
| 42 | Dodecanoic acid | 0.38 ± 0.21 | 0.28 ± 0.08 | 0.00 ± 0.00 | - | - | - | - | - | - |
| 43 | E-15-Heptadecenal | 0.41 ± 0.20 | 0.38 ± 0.09 | 0.53 ± 0.20 | - | - | - | - | - | - |
| 45 | Eicosane | 2.65 ± 0.43 | 3.45 ± 0.45 | 4.41 ± 1.01 | 0.87 ± 0.61 | 1.31 ± 0.26 | 1.16 ± 0.31 | 1.86 ± 0.71 | 1.90 ± 0.06 | 1.74 ± 0.21 |
| 46 | Eicosane/Hexadecan/Octadecane * | 1.62 ± 0.98 | 1.76 ± 0.02 | 1.82 ± 0.63 | 0.59 ± 0.27 | 0.91 ± 0.24 | 0.70 ± 0.36 | 3.28 ± 1.21 | 3.56 ± 1.96 | 0.00 ± 0.00 |
| 47 | Ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl)propan-2-yl carbonate | 2.61 ± 0.13 | 2.91 ± 0.65 | 2.79 ± 0.09 | 1.03 ± 0.52 | 1.44 ± 0.09 | 1.26 ± 0.47 | - | - | - |
| 48 | Ethyl oleate | 4.91 ± 0.96 | 6.21 ± 1.21 | 5.81 ± 1.12 | - | - | - | 2.00 ± 0.57 | 1.68 ± 0.03 | 1.80 ± 0.07 |
| 49 | Furfural | 2.09 ± 0.21 | 1.95 ± 0.09 | 1.21 ± 0.51 | - | - | - | - | - | - |
| 50 | Heneicosane | 2.09 ± 0.20 | 1.61 ± 0.51 | 1.82 ± 0.91 | - | - | - | 0.98 ± 0.14 | 1.06 ± 0.08 | 0.50 ± 0.12 |
| 51 | Hentriacontane | - | - | - | - | - | - | 0.80 ± 0.36 | 0.77 ± 0.21 | 0.80 ± 0.46 |
| 52 | Heptacosyl acetate | - | - | - | - | - | - | 1.69 ± 0.41 | 0.00 ± 0.00 | 1.56 ± 0.98 |
| 53 | Heptadecane | 3.70 ± 0.61 | 4.42 ± 0.91 | 4.02 ± 0.98 | - | - | - | - | - | - |
| 54 | Heptadecane/Hexacosane | - | - | - | - | - | - | 2.21 ± 0.97 | 1.98 ± 0.06 | 2.04 ± 0.14 |
| 55 | Methyl hexadecanoate | 0.13 ± 0.09 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | - | - | - | - | - |
| 56 | Bis(2-ethylhexyl) hexanedioate | - | - | - | 3.36 ± 0.61 | 6.05 ± 0.78 | 4.90 ± 1.76 | 1.41 ± 0.36 | 2.06 ± 0.26 | 2.01 ± 0.96 |
| 57 | Humulene | - | - | - | 0.63 ± 0.23 | 0.95 ± 0.07 | 0.77 ± 0.31 | - | - | - |
| 58 | Methyl dehydroabietate | 0.61 ± 0.36 | 0.00 ± 0.00 | 0.21 ± 0.00 | 0.96 ± 0.09 | 1.16 ± 0.45 | 1.06 ± 0.03 | 0.00 ± 0.00 | 0.35 ± 0.21 | 0.00 ± 0.00 |
| 59 | n-Hexadecanoic acid | - | - | - | 2.79 ± 0.81 | 4.00 ± 1.15 | 3.01 ± 0.05 | 2.90 ± 0.21 | 2.86 ± 0.06 | 1.16 ± 0.31 |
| 60 | Nonacosane | - | - | - | 2.39 ± 0.27 | 2.78 ± 0.97 | 2.61 ± 0.61 | 0.91 ± 0.06 | 1.00 ± 0.09 | 0.90 ± 0.36 |
| 61 | Nonadecane | 0.20 ± 0.05 | 0.15 ± 0.06 | 0.35 ± 0.12 | 0.37 ± 0.21 | 0.49 ± 0.08 | 0.41 ± 0.23 | - | - | - |
| 62 | 9-Methylnonadecane, | - | - | - | 1.00 ± 0.05 | 1.70 ± 0.07 | 1.40 ± 0.61 | - | - | - |
| 63 | Nonadecyl trifluoroacetate | - | - | - | 0.41 ± 0.26 | 0.90 ± 0.14 | 0.85 ± 0.16 | 1.44 ± 0.02 | 0.71 ± 0.21 | 1.01 ± 0.36 |
| 64 | Octacosane | 0.91 ± 0.32 | 1.26 ± 0.09 | 1.09 ± 0.26 | 0.65 ± 0.25 | 0.80 ± 0.04 | 0.23 ± 0.10 | |||
| 65 | Octadecane | 0.98 ± 0.21 | 1.79 ± 0.09 | 1.38 ± 0.31 | 1.29 ± 0.36 | 1.46 ± 0.31 | 0.00 ± 0.00 | 1.35 ± 0.41 | 2.36 ± 0.07 | 1.70 ± 0.36 |
| 66 | Octadecanoic acid | - | - | - | 2.13 ± 1.71 | 2.50 ± 0.23 | 2.41 ± 0.96 | - | - | - |
| 67 | Oleic acid | - | - | - | 0.00 ± 0.00 | 1.53 ± 0.69 | 1.49 ± 0.27 | - | - | - |
| 68 | p-Acetoxyanisole | - | - | - | 0.00 ± 0.00 | 3.21 ± 1.25 | 2.01 ± 0.09 | |||
| 69 | p-Cymen-8-ol | 0.00 ± 0.00 | 0.21 ± 0.09 | 0.35 ± 0.03 | - | - | - | - | - | - |
| 70 | p-Eugenol | - | - | - | 5.65 ± 1.21 | 7.20 ± 2.26 | 6.20 ± 0.91 | 2.26 ± 0.78 | 1.96 ± 0.21 | 2.06 ± 0.67 |
| 71 | 2-Methoxy-4-(1-propenyl)phenol | - | - | - | 0.56 ± 0.07 | 0.63 ± 0.12 | 0.59 ± 0.12 | 2.81 ± 1.21 | 3.60 ± 0.94 | 3.04 ± 0.41 |
| 72 | Phenylethyl alcohol | 1.91 ± 0.21 | 0.26 ± 0.05 | 2.01 ± 0.12 | - | - | - | - | - | - |
| 73 | Supraene/Squalene * | 0.81 ± 0.36 | 1.28 ± 0.14 | 1.13 ± 0.09 | 2.71 ± 1.21 | 2.80 ± 0.08 | 2.67 ± 0.08 | - | - | - |
| 74 | Tetracosane | - | - | - | - | - | - | |||
| 75 | Tetradecanoic acid | 0.21 ± 0.10 | 0.98 ± 0.40 | 0.41 ± 0.21 | 0.66 ± 0.12 | 1.20 ± 0.36 | 0.96 ± 0.23 | - | - | - |
| 76 | Tetradecyl trifluoroacetate | - | - | - | 0.49 ± 0.09 | 0.50 ± 0.31 | 0.49 ± 0.09 | - | - | - |
| 77 | Thymol | 2.76 ± 0.09 | 2.61 ± 0.12 | 2.85 ± 0.01 | - | - | - | - | - | - |
| 78 | Tricosane | - | - | - | 0.98 ± 0.23 | 1.65 ± 0.07 | 1.12 ± 0.36 | - | - | - |
| 79 | 2,3,4,5-Tetramethyl-tricyclo[3.2.1.02,7]oct-3-ene | 0.12 ± 0.06 | 0.25 ± 0.06 | 0.18 ± 0.09 | - | - | - | - | - | - |
| 80 | Z-10-Tetradecen-1-ol acetate | - | - | - | 0.33 ± 0.09 | 0.48 ± 0.06 | 0.40 ± 0.20 | - | - | - |
| 81 | Z-12-Pentacosene | 0.20 ± 0.09 | 0.79 ± 0.20 | 0.71 ± 0.36 | 1.12 ± 0.23 | 0.69 ± 0.02 | 0.60 ± 0.01 | - | - | - |
| 82 | α-Terpinolene | 0.00 ± 0.00 | 0.42 ± 0.00 | 0.00 ± 0.00 | - | - | - | - | - | - |
| No. | Compounds | Peak Area (%) | ||
|---|---|---|---|---|
| H14 | H15 | H16 | ||
| Av. (n = 3) | Av. (n = 3) | Av. (n = 3) | ||
| 1 | trans-Linallol oxide/cis-Linallol oxide ** | 0.33 ± 0.02 | 0.00 ± 0.00 | 0.28 ± 0.04 |
| 2 | Phynylethylalcohol | 0.10 ± 0.03 | 0.16 ± 0.06 | 0.00 ± 0.00 |
| 3 | Hotrienol | 0.20 ± 0.09 | 0.08 ± 0.02 | 0.00 ± 0.00 |
| 4 | p-cymen-8-ol | 0.16 ± 0.09 | 0.48 ± 0.2 | 0.09 ± 0.01 |
| 5 | Benzoic acid | 0.00 ± 0.00 | 0.82 ± 0.09 | 0.12 ± 0.09 |
| 6 | Phenylacetic acid | 6.21 ± 1.21 | 19.21 ± 0.21 | 17.65 ± 2.24 |
| 7 | α-Methylbenzeneethanol | 0.19 ± 0.09 | 3.2 ± 0.91 | 4.75 ± 1.21 |
| 8 | Ethyl 4-ethoxybenzoate | 0.35 ± 0.09 | 0.51 ± 0.2 | 0.63 ± 0.31 |
| 9 | N-Butylbenzenesulfonamide | 9.64 ± 0.06 | 12.06 ± 1.25 | 12.65 ± 4.52 |
| 10 | 9-Hexadecanoic acid | 0.96 ± 0.23 | 0.00 ± 0.00 | 1.17 ± 0.42 |
| 11 | Estra-1,3,5(10)-trien-17-ol | 0.96 ± 0.25 | 3.12 ± 1.09 | 2.59 ± 0.05 |
| 12 | Oleic acid | 1.83 ± 0.06 | 1.90 ± 0.36 | 2.01 ± 0.08 |
| 13 | Octadecanoic acid/cis-13-Octadecenoic acid/cis-Vaccenic acid */** | 1.45 ± 0.91 | 1.85 ± 0.03 | 1.90 ± 0.35 |
| 14 | 1-Docosene | 2.08 ± 0.31 | 2.40 ± 0.39 | 1.99 ± 0.03 |
| 15 | (Z)-9-Tricosene | 0.75 ± 0.03 | 0.89 ± 0.21 | 0.72 ± 0.01 |
| 16 | 1-Heptatriacotanol | 0.00 ± 0.00 | 0.84 ± 0.02 | 0.00 ± 0.00 |
| 17 | 2-Ehylhexyl 3-(4-methoxyphenyl)-2-propenoate | 0.71 ± 0.09 | 0.71 ± 0.21 | 0.69 ± 0.06 |
| 18 | Methyl dehydroabietate | 0.84 ± 0.09 | 0.51 ± 0.10 | 0.79 ± 0.05 |
| 19 | (Z)-9-Octadecenamide | 1.81 ± 0.36 | 3.25 ± 0.51 | 2.25 ± 0.95 |
| 20 | 1,7,11-Trimethyl-4-(1-methylethyl)-cyclotetradecane | 0.00 ± 0.00 | 0.81 ± 0.32 | 0.00 ± 0.00 |
| 21 | Pentacosane | 0.90 ± 0.21 | 1.10 ± 0.01 | 1.01 ± 0.32 |
| 22 | Nonadecane | 0.70 ± 0.01 | 1.29 ± 0.21 | 0.75 ± 0.01 |
| 23 | Lumichrome | 1.82 ± 0.30 | 1.89 ± 0.09 | 1.86 ± 0.15 |
| 24 | Squalene | 1.43 ± 0.09 | 1.62 ± 0.21 | 1.59 ± 0.21 |
| 25 | Nonadecyl trifluoroacetate | 0.39 ± 0.21 | 0.51 ± 0.09 | 0.49 ± 0.19 |
| 26 | Eicosane | 0.48 ± 0.23 | 2.18 ± 0.23 | 1.33 ± 0.91 |
| Method | R2 | Content of Coumarin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ng/g of Honey | ||||||||||
| H14 | H15 | H16 | ||||||||
| Min | Max | Avg | Min | Max | Avg | Min | Max | Avg | ||
| Steam distillation | 0.994 | 491.79 | 902.79 | 728.66 | 339.82 | 629.61 | 501.23 | Not detected | ||
| Phenolic Compounds | GA | C | 4-HBA | CA | 3-HBA | p-CA | FA | RA | EA | MC | CiA | QC | G | P | MH | Total Phenolic Content (mg GAE/100 g) n = 2 | Total Flavonoids Content (mg QE/100 g) n = 2 | Radical Scavenging Activity DPPH (%) n = 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Levels of Individual Identified Compounds of Tested Honeys (mg/100 g of Product) (RSD ≤ 5%, n = 3) | ||||||||||||||||||
| mean | 23.23 | 26.79 | 0.68 | 0.41 | 0.32 | 1.46 | 0.25 | 0.19 | 0.48 | 0.36 | 0.16 | 0.28 | 0.26 | 0.11 | 0.13 | 67.55 | 2.29 | 55.96 |
| ±SD | 4.52 | 2.99 | 0.43 | 0.11 | 0.02 | 0.12 | 0.03 | 0.04 | 0.03 | 0.05 | 0.01 | 0.03 | 0.07 | 0.01 | 0.03 | 7.51 | 0.11 | 2.35 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasicka-Misiak, I.; Makowicz, E.; Stanek, N. Polish Yellow Sweet Clover (Melilotus officinalis L.) Honey, Chromatographic Fingerprints, and Chemical Markers. Molecules 2017, 22, 138. https://doi.org/10.3390/molecules22010138
Jasicka-Misiak I, Makowicz E, Stanek N. Polish Yellow Sweet Clover (Melilotus officinalis L.) Honey, Chromatographic Fingerprints, and Chemical Markers. Molecules. 2017; 22(1):138. https://doi.org/10.3390/molecules22010138
Chicago/Turabian StyleJasicka-Misiak, Izabela, Ewa Makowicz, and Natalia Stanek. 2017. "Polish Yellow Sweet Clover (Melilotus officinalis L.) Honey, Chromatographic Fingerprints, and Chemical Markers" Molecules 22, no. 1: 138. https://doi.org/10.3390/molecules22010138
APA StyleJasicka-Misiak, I., Makowicz, E., & Stanek, N. (2017). Polish Yellow Sweet Clover (Melilotus officinalis L.) Honey, Chromatographic Fingerprints, and Chemical Markers. Molecules, 22(1), 138. https://doi.org/10.3390/molecules22010138





