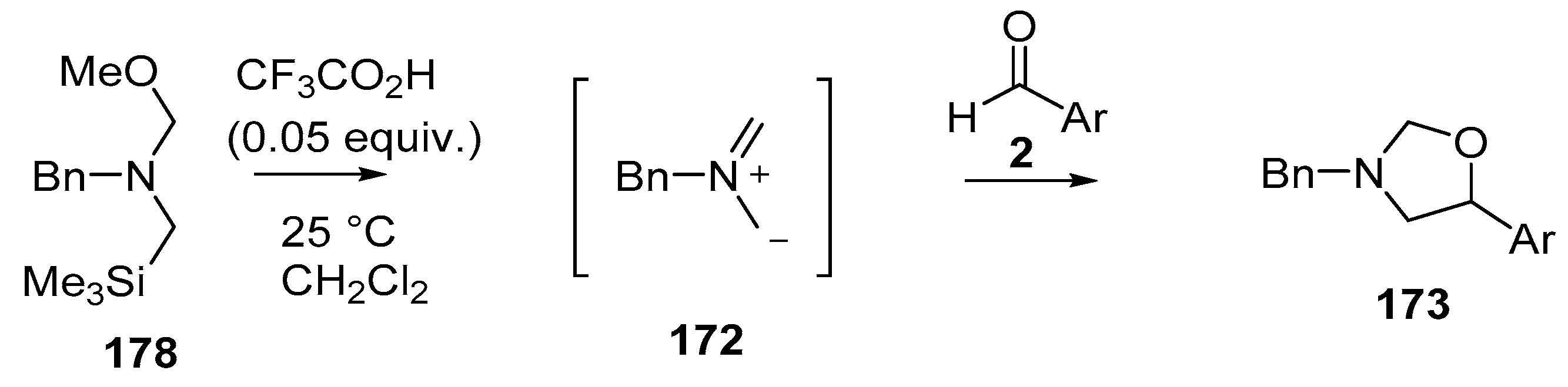Abstract
We provide a comprehensive account of the 1,3-dipolar cycloaddition reactions of azomethine ylides with carbonyl dipolarophiles. Many different azomethine ylides have been studied, including stabilized and non-stabilized ylides. Of the carbonyl dipolarophiles, aldehydes including formaldehyde are the most studied, although there are now examples of cycloadditions with ketones, ketenes and carboxyl systems, in particular isatoic anhydrides and phthalic anhydrides. Intramolecular cycloadditions with esters can also occur under certain circumstances. The oxazolidine cycloadducts undergo a range of reactions triggered by the ring-opening of the oxazolidine ring system.
Keywords:
dipolar; cycloaddition; azomethine; ylide; carbonyl; aldehyde; ketone; anhydride; oxazolidine; spiro 1. Introduction
The versatile and convergent nature of the 1,3-dipolar cycloaddition reaction has led to its development as a powerful method for the synthesis of five-membered heterocycles [1,2,3,4,5,6,7]. The reaction involves the addition of 1,3-dipoles, such as azides, nitrones, carbonyl ylides, nitrile oxides, nitrile imines and azomethine ylides to unsaturated double or triple bonds (1,2-dipolarophiles) [8,9,10,11,12,13,14,15]. Azomethine ylides 1, which contain four electrons distributed over the π orbitals of a C-N-C group, are examples of the bent allyl anion-type of 1,3-dipole [16,17,18]. Azomethine ylides can be classified as non-stabilized (where R1–R5 = H or alkyl) or stabilized either by electron-withdrawing/electron-donating groups at the appropriate termini of the ylide or by N-metalation [18]. Azomethine ylides are mostly generated in situ due to their high reactivity and/or transient existence; however, in some cases, stabilized ylides have been isolated [19,20,21]. The most frequent type of 1,3-dipolar cycloaddition reaction of azomethine ylides is that with alkenyl or alkynyl dipolarophiles substituted with electron-withdrawing groups, providing access to pyrrolidine-containing molecules of biological [18,22,23,24,25,26] or materials science interest [27,28,29,30,31]. The reactions with multiple bonded heteroatom systems such as carbonyl, thiocarbonyl, isothiocyanato, imino, isocyanato, nitrile, nitroso, and azo derivatives are also known, but less well studied [7,16,32,33,34,35,36,37,38]. Less recognized is the ability of azomethine ylides to react with aromatic dipolarophiles when the aromatic system is embedded within a polycyclic aromatic hydrocarbon, tethered with the azomethine ylide (an intramolecular process) or substituted with highly electron withdrawing nitro groups [39].
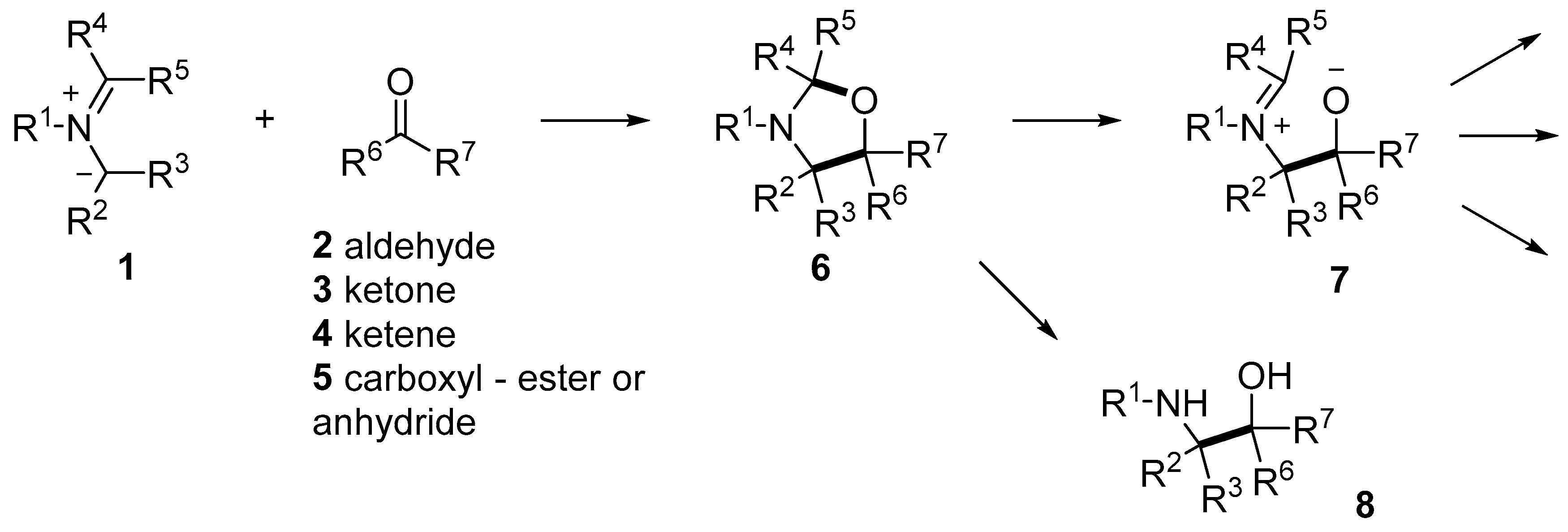
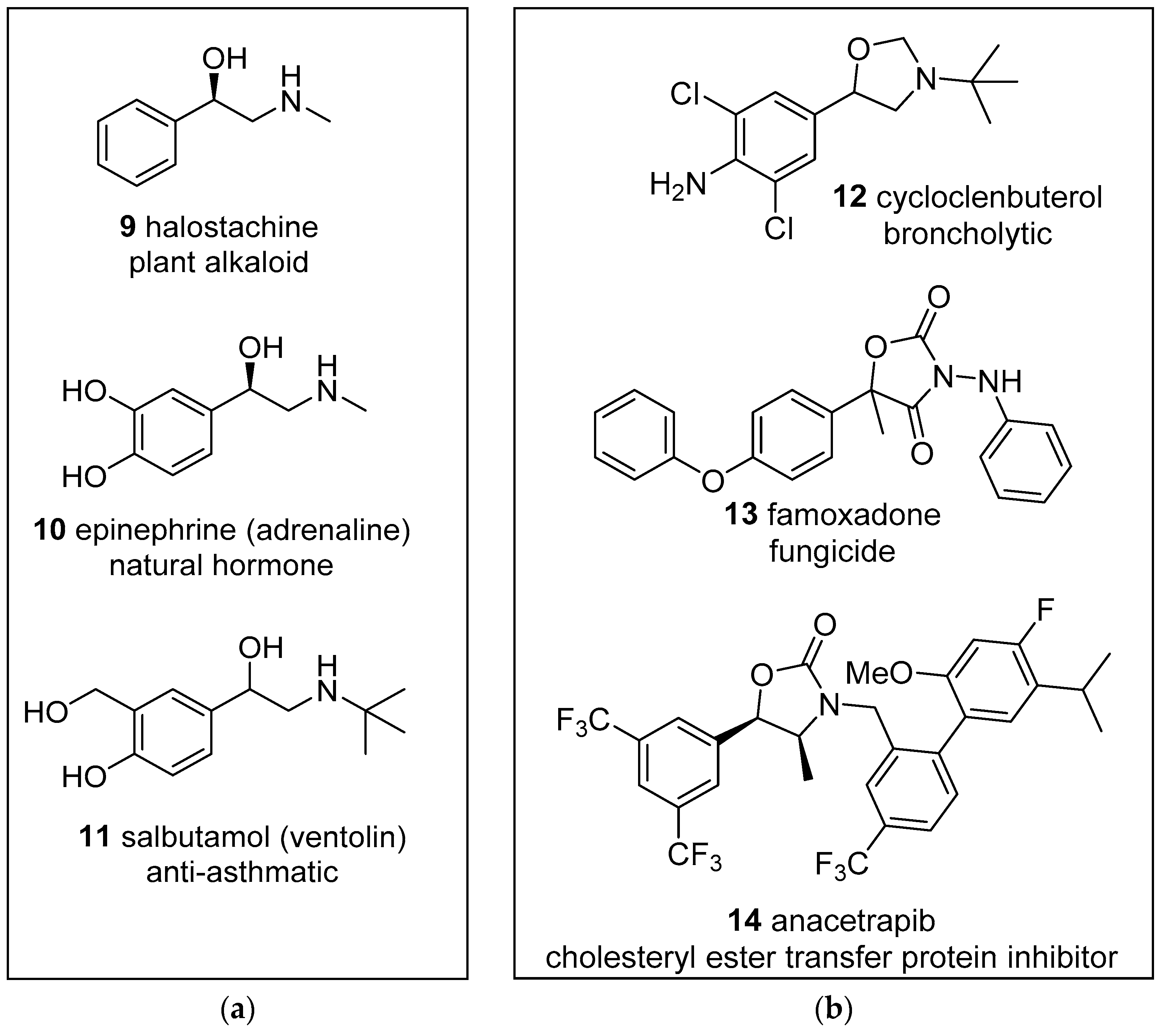
There has been a resurgence of interest in reactions with carbonyl compounds, including aldehydes 2, ketones 3, ketenes 4 or carboxylates 5 as the heterodipolarophile, which yield oxazolidine derivatives 6 (Scheme 1). This recent interest has been due to the recognition that anhydrides such as isatoic anhydride and phthalic anhydride can act as carbonyl dipolarophiles in cycloadditions with azomethine ylides [40,41]. Additionally, the oxazolidine products 6 have a propensity to ring-open to reveal alkoxide-iminium reactive intermediates 7 which can undergo a diversity of interesting and potentially useful transformations [40,42]. Furthermore, hydrolysis of the oxazolidine products 6 leads to 1,2-ethanolamines 8, the substructure of which can be found in a wide array of compounds of natural and synthetic origins, e.g., halostochine (9), epinephrine (10) and salbutamol (11) (Figure 1a) [43,44,45]. Finally, the oxazolidine ring is a core substructure of bioactive compounds of pharmaceutical and agrichemical interest, such as cycloclenbuterol (12), famoxadone (13) and anacetrapib (14) (Figure 1b) [46,47,48]. Herein, we provide a comprehensive review of the 1,3-dipolar cycloaddition reactions of azomethine ylides and carbonyl dipolarophiles and note interesting reactions of the oxazolidine products.
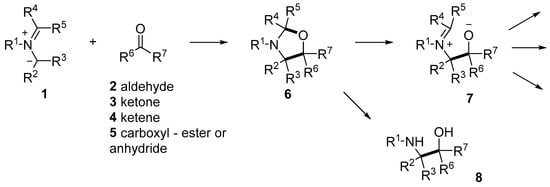
Scheme 1.
1,3-Dipolar cycloaddition reactions of azomethine ylides and carbonyl compounds to give oxazolidines and chemistry of the oxazolidines.
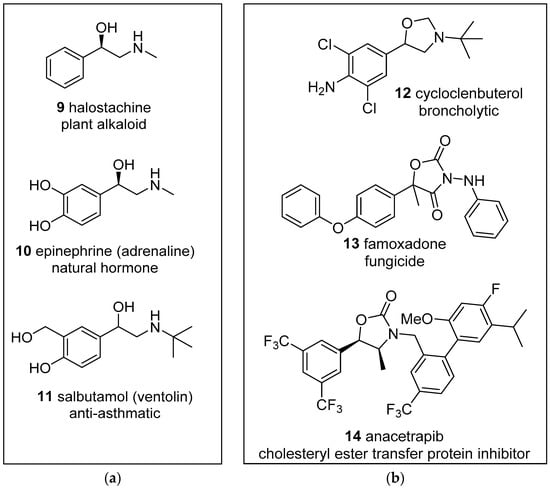
Figure 1.
Examples of biologically active: (a) 1-phenyl-1-ethanol-2-amine derivatives; (b) oxazolidine derivatives.
2. Review of Reactions of Azomethine Ylides with Carbonyl Compounds
Each section of this review refers to different types of azomethine ylide and/or different synthetic methods used to form the azomethine ylide. Within each section, examples of carbonyl dipolarophiles such as formaldehyde, aldehydes, ketones and anhydrides are provided, in that order.
2.1. Reactions with Ylides Formed from Ring-Opening of Aziridines
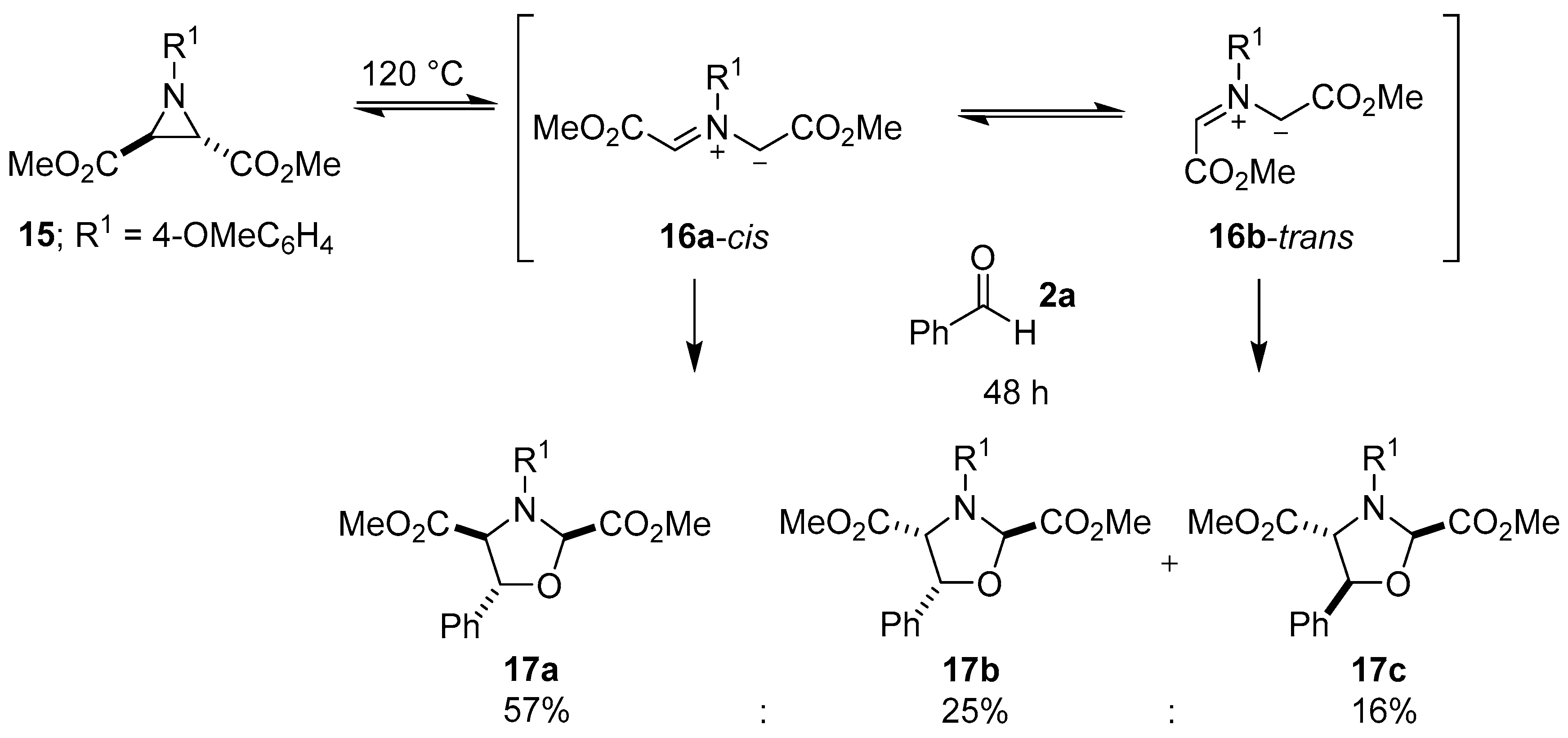
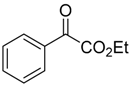
It is fitting that Huisgen in 1967 disclosed the first 1,3-dipolar cycloaddition reaction between an azomethine ylide generated by thermal carbon-carbon bond cleavage of an aziridine ring and a carbonyl group. In this brief account, a trans-2,3-dimethoxycarbonyl-1-arylaziridine was heated in the presence of benzaldehyde to give a high yield of the corresponding oxazolidine as three stereoisomers [49]. A more detailed report of this work, published in 1971, described the reaction between trans-2,3-dimethoxycarbonyl-1-(4-methoxyphenyl)aziridine (15) and an excess of benzaldehyde (2a) to form three diasteromeric oxazolidines 17a–c. When heated, trans-aziridine 15 underwent a conrotatory ring-opening (according to Woodward-Hoffmann theory) to generate cis-azomethine ylide 16a, which primarily underwent a cycloaddition reaction with the carbonyl group of 2a to form cis-2,4-oxazolidine 17a, but also interconverted to the more stable trans-isomer 16b, which then reacted with 2a to form lesser amounts of the trans-2,4-oxazolidines 17b and 17c (Scheme 2). Aziridine 15 also underwent ring-opening and diastereoselective cycloaddition with the ketone group within diethyl ketomalonate, to predominantly form the corresponding cis-2,4-oxazolidine (not shown) [50].
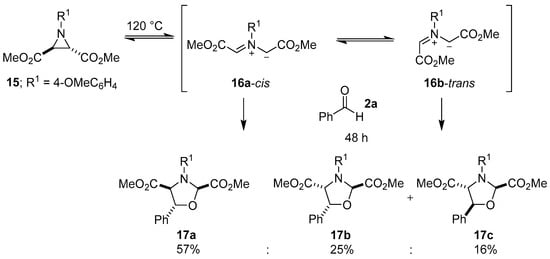
Scheme 2.
Cycloaddition of azomethine ylides 16a-cis and 16b-trans to benzaldehyde (2a).
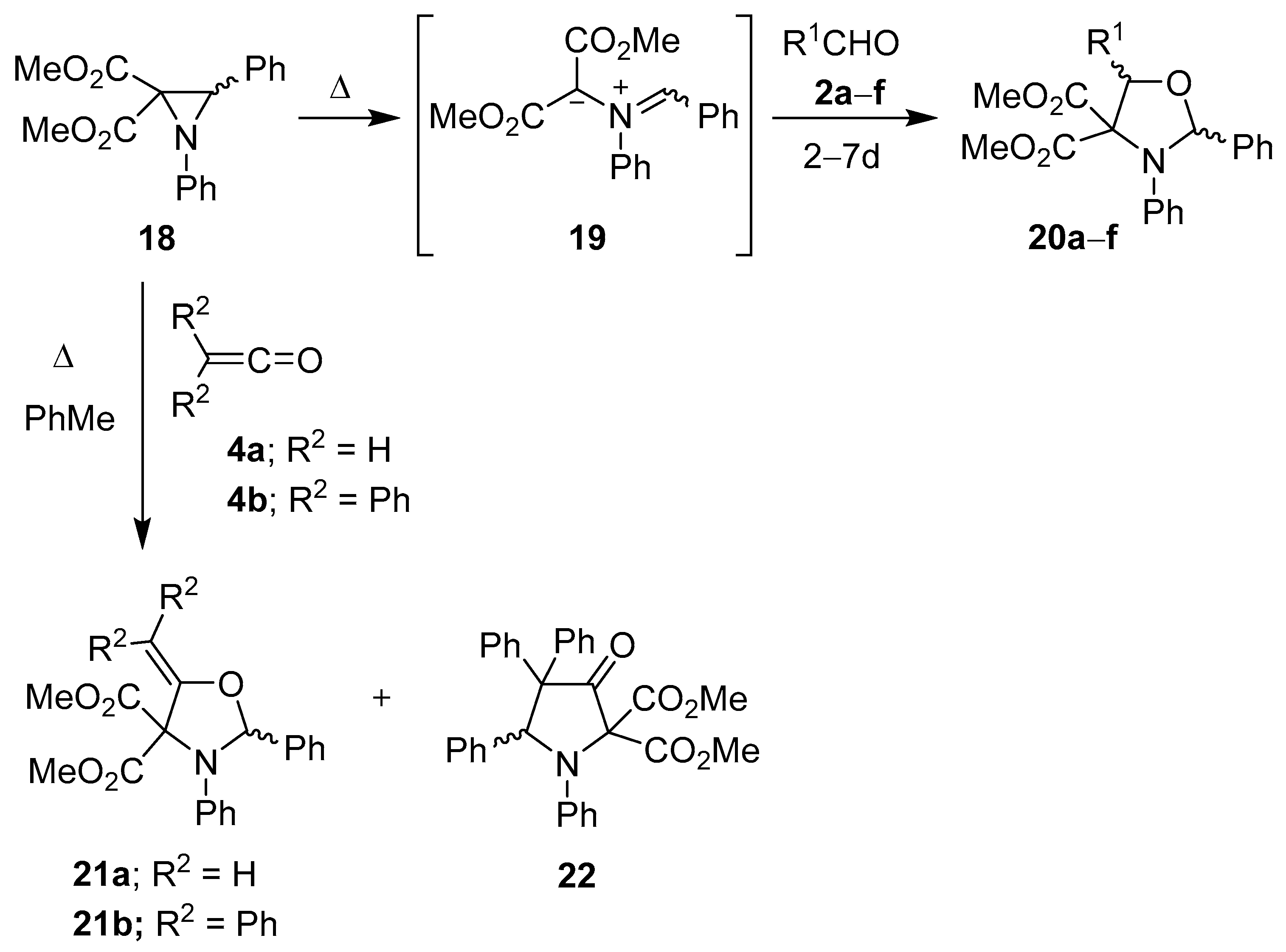
Two years after Huisgen’s initial report, Texier and Carrié reported a number of cycloaddition reactions involving the azomethine ylide generated from the thermal ring-opening of 1,3-diphenyl-2,2-methoxycarbonylaziridine (18). Cycloaddition reactions of the derived ylide 19 with benzaldehyde (2a), acetaldehyde (2b), anisaldehyde (2c) and cinnamaldehyde (2d), gave the corresponding oxazolidines 20a–d (as mostly one diastereoisomer), and with isobutyraldehyde (2e) and 2,2-diphenylacetaldehyde (2f) the oxazolidines 20e and f were formed as single diastereoisomers (Scheme 3, Table 1) [51,52]. The same workers found that the ylide 19 added preferentially to the carbonyl bond of ketene 4a to form oxazolidine 21a, and to diphenylketene 4b to form an 80:20 mixture of oxazolidine 21b and pyrrolidone 22 (Scheme 3) [53].
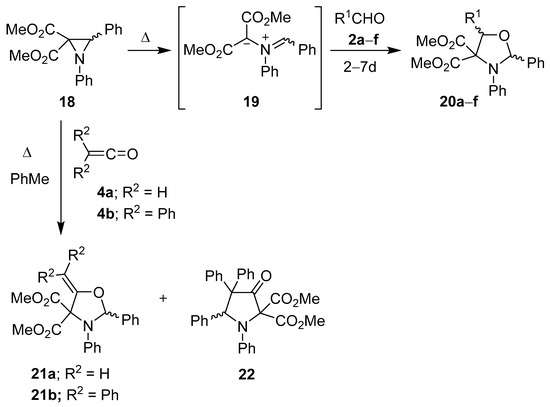
Scheme 3.
Cycloaddition of azomethine ylide 19 to aldehydes 2a–f and to ketenes 4a and 4b.

Table 1.
Cycloaddition of azomethine ylide 19 to aldehydes 2a–f (Scheme 3).
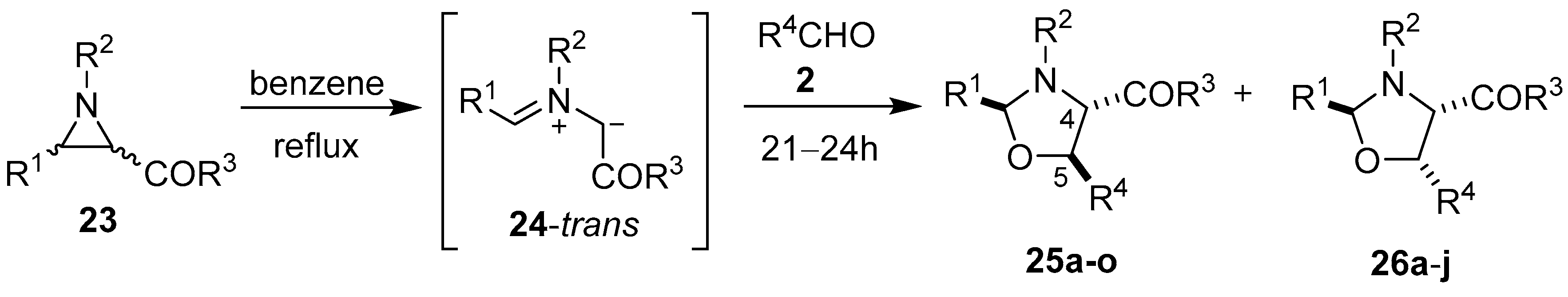
In 1970 Lown and co-workers disclosed that a variety of 2-benzoylaziridines 23 underwent thermal [3 + 2] cycloaddition reactions with a range of aromatic aldehydes 2 to give mixtures of the diastereomeric oxazolidines 25 and 26 (Scheme 4, Table 2).
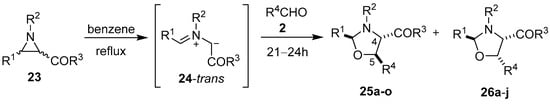
Scheme 4.
Cycloaddition of trans-azomethine ylide 24, generated from 2-benzoylaziridines 23, to aldehydes 2.

Table 2.
Cycloaddition of trans-azomethine ylide 24 to aldehydes 2 (Scheme 4).
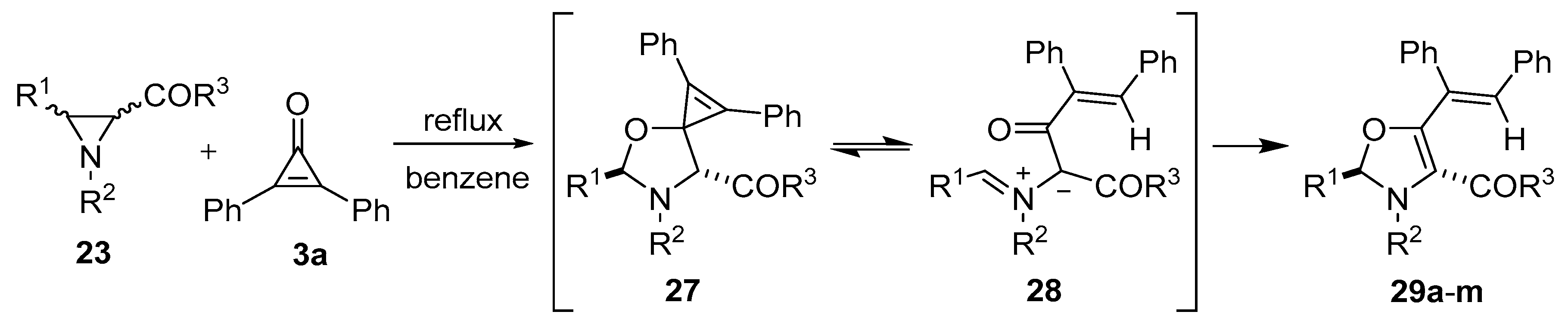
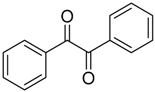
When trichloroacetaldehyde (2g) was used as the dipolarophile, the corresponding trans-4,5-oxazolidines 25k–o were obtained exclusively (Table 2, Entries 11–15). The orientation of the addition was confirmed by specific deuterium-labelling experiments, and the preference for the trans-4,5-isomer was rationalized by considering that the intermediate cis- and trans-azomethine ylides had time to equilibrate to mostly the more stable trans-azomethine ylide 24 prior to addition (owing to the sluggish dipolarophilic activity of the carbonyl bond). The addition of the azomethine ylide 24 to the dipolarophiles 2 then occurred in such a manner so as to predominantly form the trans-4,5-oxazolidines [54,55]. Two years prior, Lown’s research group also reported that azomethine ylides, derived from 3-aroyl- and 3-acylaziridines 23, added to the carbonyl group of diphenylcyclopropenone (3a). This afforded intermediate oxazolidines 27 that were postulated to open to give azomethine ylides 28 and subsequently close onto the adjacent carbonyl group to afford 4-aroyl- and 4-acyl-4-oxazolines 29 (Scheme 5, Table 3) [56,57].
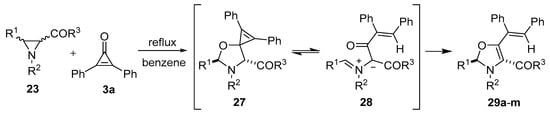
Scheme 5.
Reaction of aziridines 23 with diphenylcyclopropenone 3a to afford 4-aroyl- and 4-acyl-4-oxazolines 29.

Table 3.
Cycloaddition of the azomethine ylides derived from aziridines 23 to diphenylcyclopropenone (3a) (Scheme 5).
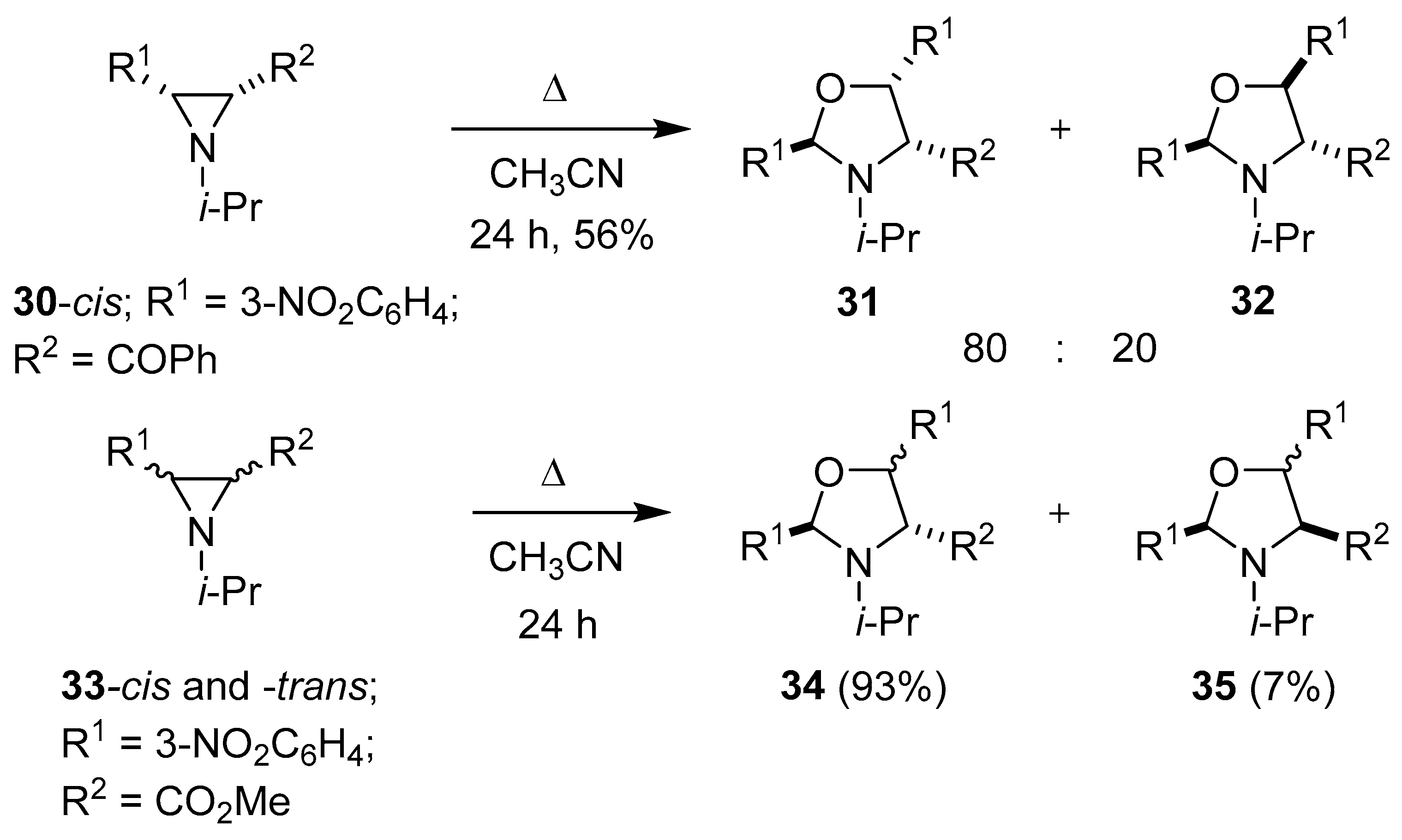
Lown and Akhtar went on to examine the thermolysis of cis-3-benzoylaziridine 30 in dry acetonitrile, which predominantly formed an 80:20 mixture of the corresponding stereoisomeric oxazolidines 31 and 32, respectively, by an autocatalytic partial hydrolytic fragmentation of one molecule of aziridine 30 to give 3-nitrobenzaldehyde (2h) and subsequent 1,3-dipolar cycloaddition of 2h to the azomethine ylide formed from ring opening of another molecule of aziridine 30. A marked difference in reactivity was observed for the corresponding trans-aziridine isomer, which underwent mainly hydrolytic ring-cleavage processes, and only small amounts of each oxazolidine were isolated. In contrast, both cis- and trans-3-carbomethoxyaziridines 33 formed the corresponding stereoisomeric oxazolidines 34 and 35 in identical ratios and yields by an analogous partial hydrolytic fragmentation/cycloaddition process (Scheme 6) [58]. The thermolysis of an N-unsubstituted aziridine, dimethyl 3-[(4-nitro)phenyl]aziridine-2,2-dicarboxylate, in toluene also lead to an oxazolidine, albeit in low yield, from a partial fragmentation/cycloaddition process [59].
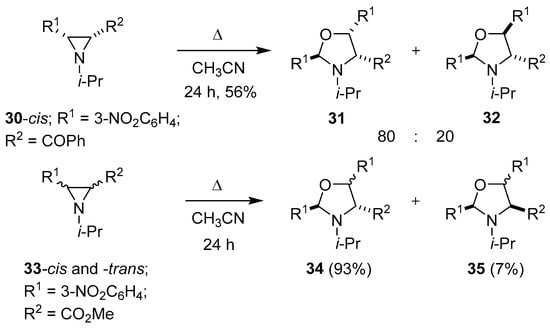
Scheme 6.
The partial fragmentation/cycloaddition reactions of aziridines 30 and 33.
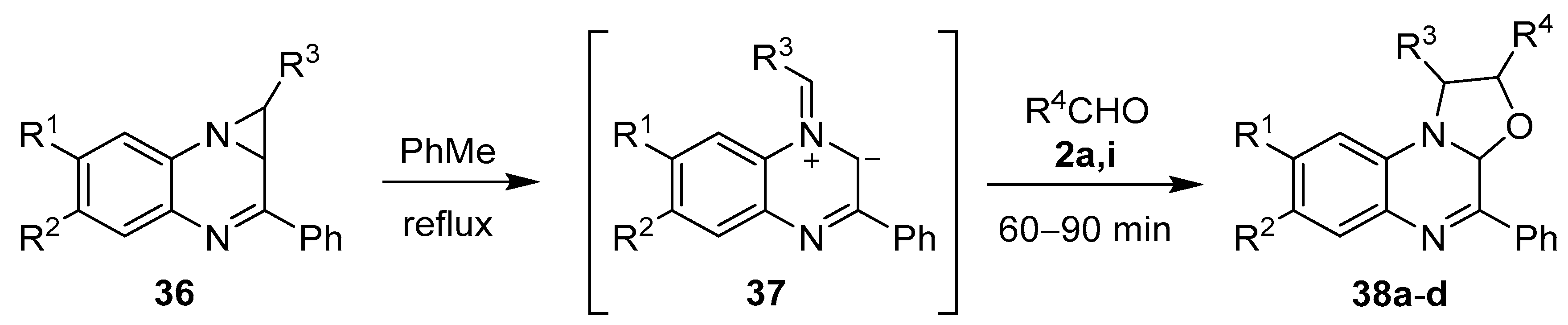
Azomethine ylides have also been generated from aziridines fused to a quinoxoline ring system. The thermolytic ring-opening of 1,1a-dihydro-1,2-diarylazirino[1,2-a]quinoxalines 36 gave the intermediate azomethine ylides 37, which underwent cycloadditions with benzaldehyde (2a) and 4-nitrobenzaldehyde (2i) to give oxazolo[3,2-a]quinoxaline derivatives 38 (Scheme 7, Table 4) [38,60].
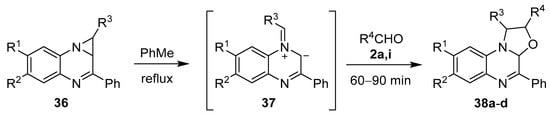
Scheme 7.
Cycloaddition of azomethine ylide 37, generated from 1,1a-dihydro-1,2-diarylazirino[1,2-a]quinoxalines 36, to aromatic aldehydes 2a and 2i.

Table 4.
Cycloaddition of azomethine ylide 37 to aromatic aldehydes 2a and 2i (Scheme 7).
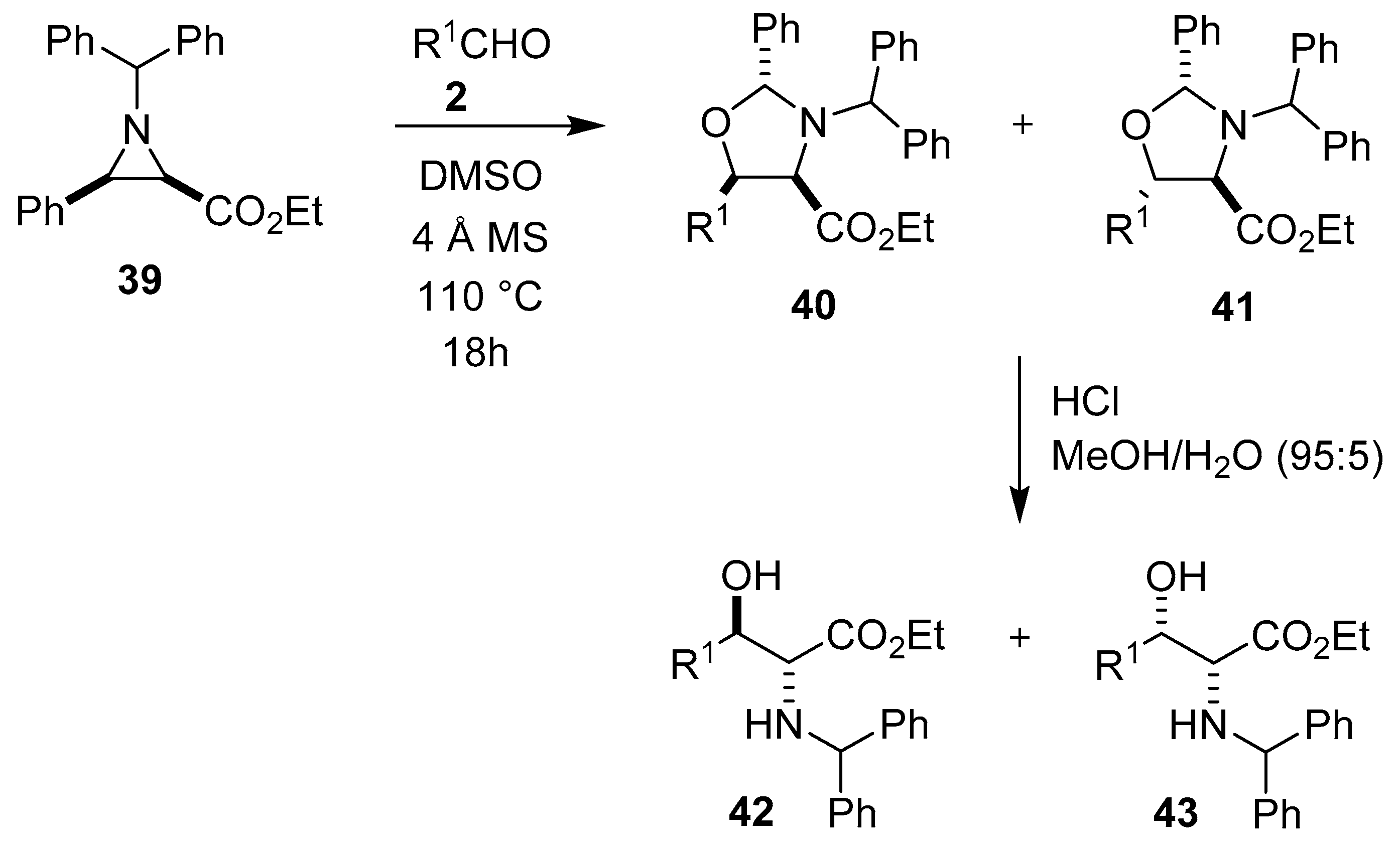
The thermal electrocyclic ring-opening of benzhydryl-protected aziridine 39 gives a cis-azomethine ylide in equilibrium with a trans-azomethine ylide. The trans-ylide underwent a series of [3 + 2] cycloaddition reactions with mostly aromatic aldehydes 2 to form oxazolidines 40 and 41, with the cis-4,5-oxazolidines 40 being the major stereoisomers isolated. In most cases, reaction mixtures comprising both oxazolidines 40 and 41 were directly hydrolyzed to the corresponding α-amino-β-hydroxy esters 42 and 43, with the anti-diastereomer 42 formed as the major product (Scheme 8) [61].
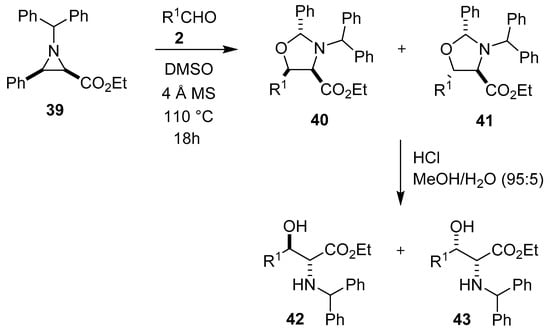
Scheme 8.
Reaction of aziridine 39 with aldehydes 2 to afford oxazolidines 40 and 41.
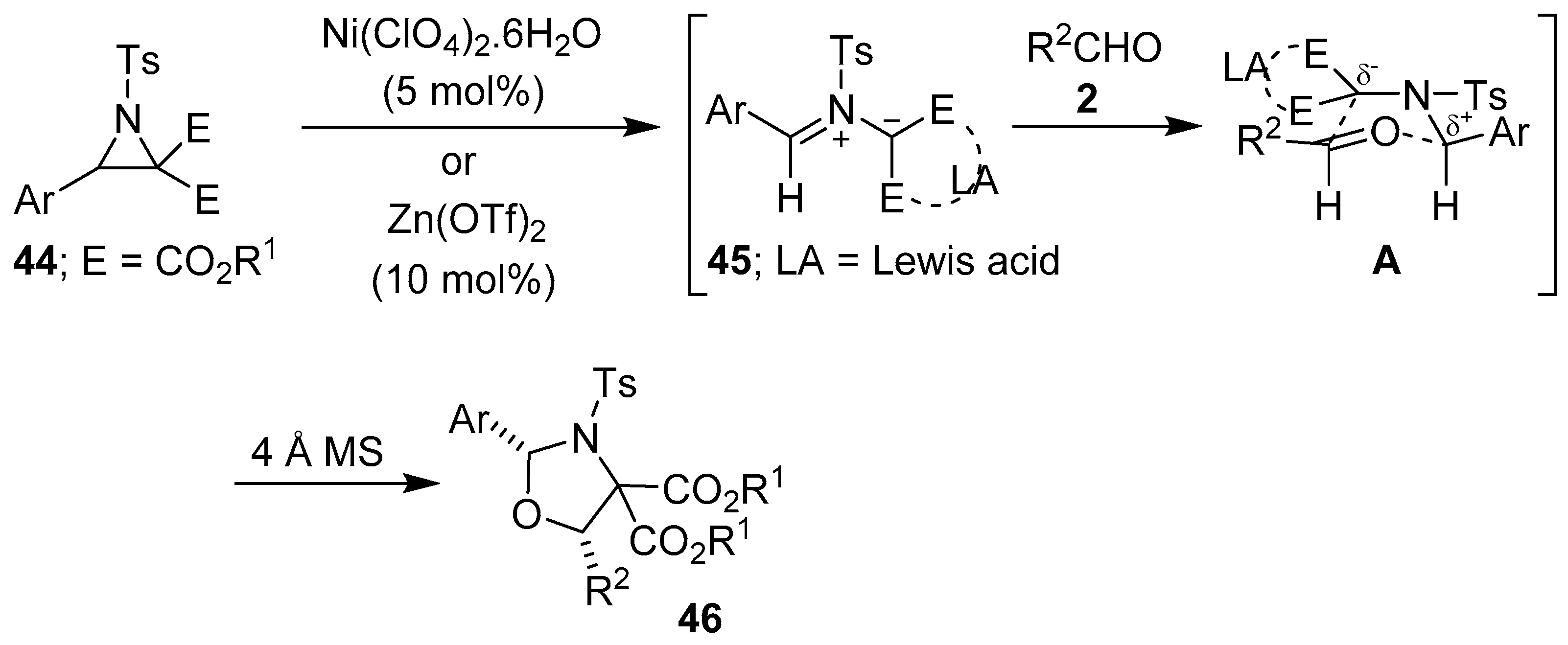
More recently, Zhang and co-workers have disclosed an efficient, mild and general nickel(II)-catalyzed diastereoselective [3 + 2] cycloaddition reaction of N-tosylaziridine 2,2-dicarboxylates 44 with aromatic aldehydes 2 to afford highly substituted 1,3-oxazolidines 46 (Scheme 9, Table 5). Presumably this process proceeds via the Lewis acid-promoted carbon-carbon bond cleavage of aziridines 44, generating Lewis acid-coordinated azomethine ylides 45, followed by [3 + 2]-cycloaddition reactions with the carbonyl group of aldehydes 2 via transition state A. The nickel-catalyzed process proved to be scalable, in contrast to the corresponding thermally-promoted process [62]. When catalyzed by Zn(OTf)2, this [3 + 2] cycloaddition process afforded exclusively the cis-2,5-disubstituted oxazolidines 46 (Scheme 9, Table 6) [63].
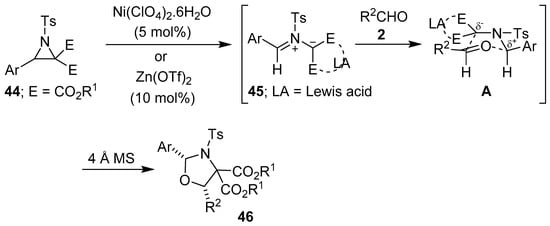
Scheme 9.
Lewis acid-promoted cycloaddition of azomethine ylides 45, generated from aziridines 44, to aldehydes 2.

Table 5.
Cycloaddition of azomethine ylides 45 to aldehydes 2 catalyzed by nickel(II) perchlorate (Scheme 9).

Table 6.
Zinc triflate-catalyzed cycloaddition of azomethine ylide 45 to aldehydes 2 (Scheme 9).
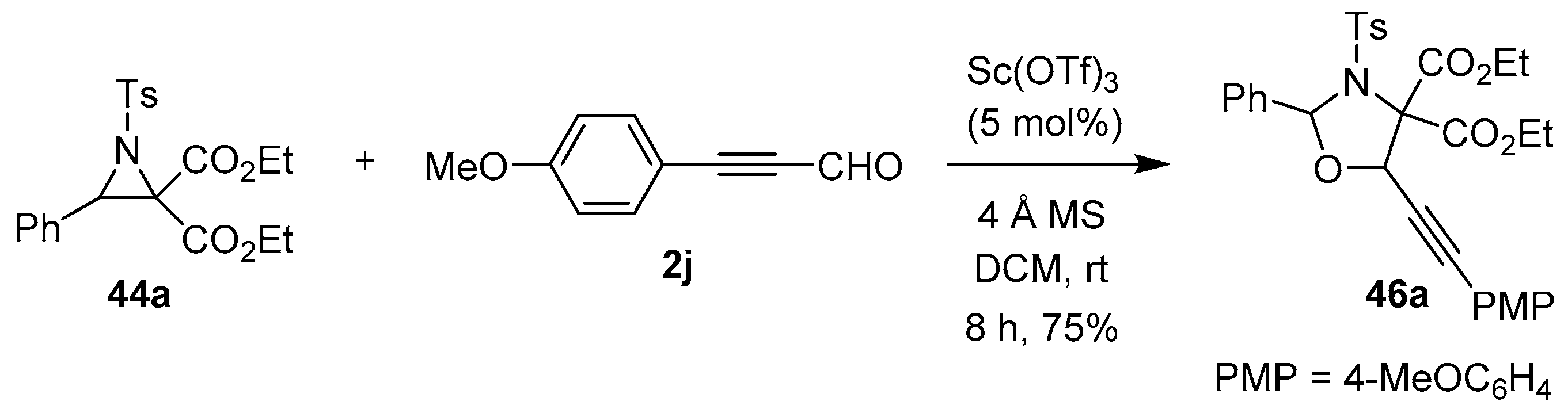
Zhang’s research group also reported a single example of a highly chemoselective Sc(III)-catalyzed [3 + 2] cycloaddition of the azomethine ylide derived from N-tosylaziridine 44a, which reacted somewhat surprisingly with the carbonyl group rather than the alkyne group of (4-methoxyphenyl)propiolaldehyde (2j) to form 1,3-oxazolidine 46a (Scheme 10) [64].
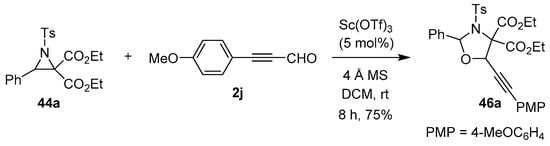
Scheme 10.
Reaction of N-tosylaziridine 44a with (4-methoxyphenyl)propiolaldehyde (2j) to form 1,3-oxazolidine 46a.
2.2. Reactions with Ylides Formed from Decarboxylative Condensation of Secondary α-Amino Acids and Carbonyl Compounds
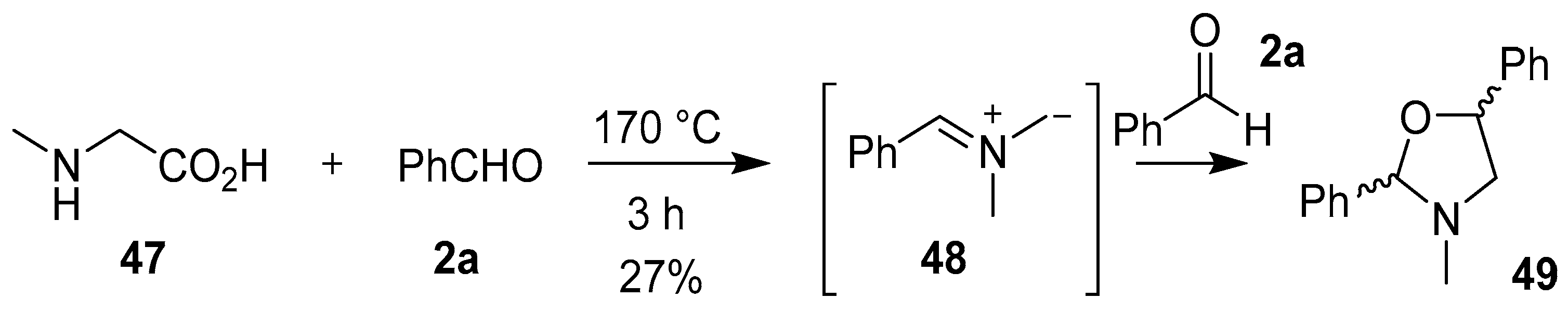
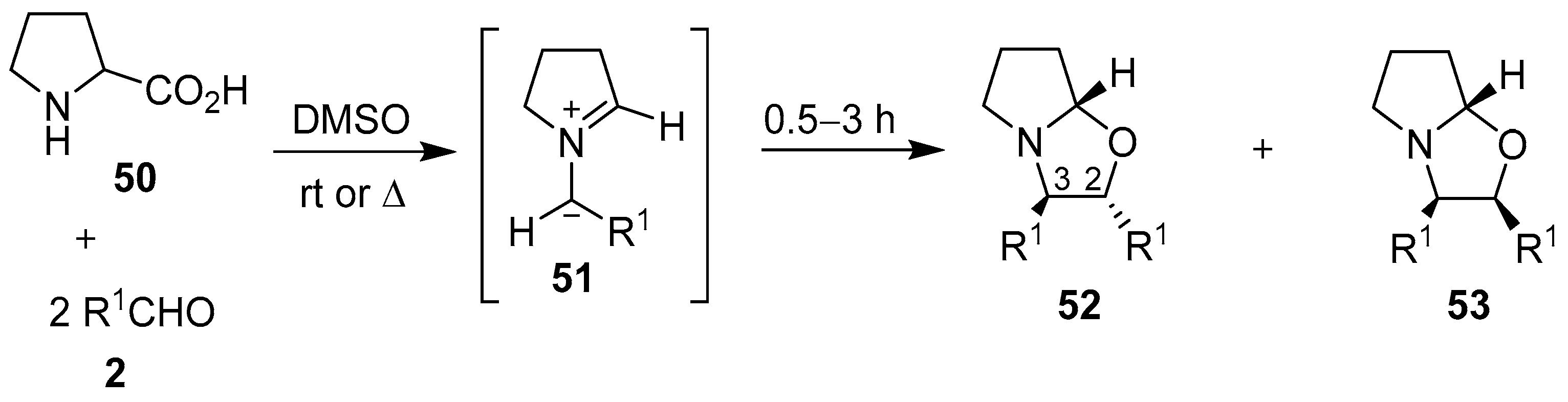
The decarboxylative condensation reaction of secondary α-amino acids and carbonyl compounds is a convenient method to generate a wide variety of non-stabilized azomethine ylides. The first evidence for the generation of a non-stabilized azomethine ylide in this manner was reported by Rizzi in 1970. This work described the decarboxylation of sarcosine (47) in an excess of benzaldehyde (2a) to form the corresponding azomethine ylide 48, which underwent a 1,3-dipolar cycloaddition reaction with a second molecule of benzaldehyde (2a) to form the oxazolidine 49, albeit in low yield (Scheme 11). A similar reaction between sarcosine and excess benzophenone gave an array of products derived from both resonance forms of the corresponding azomethine ylide, which included a small isolable amount of an oxazolidine product (not shown) [65].
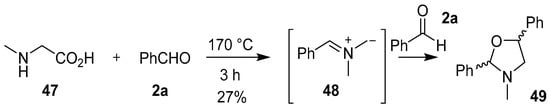
Scheme 11.
Cycloaddition of azomethine ylide 48 to benzaldehyde (2a).
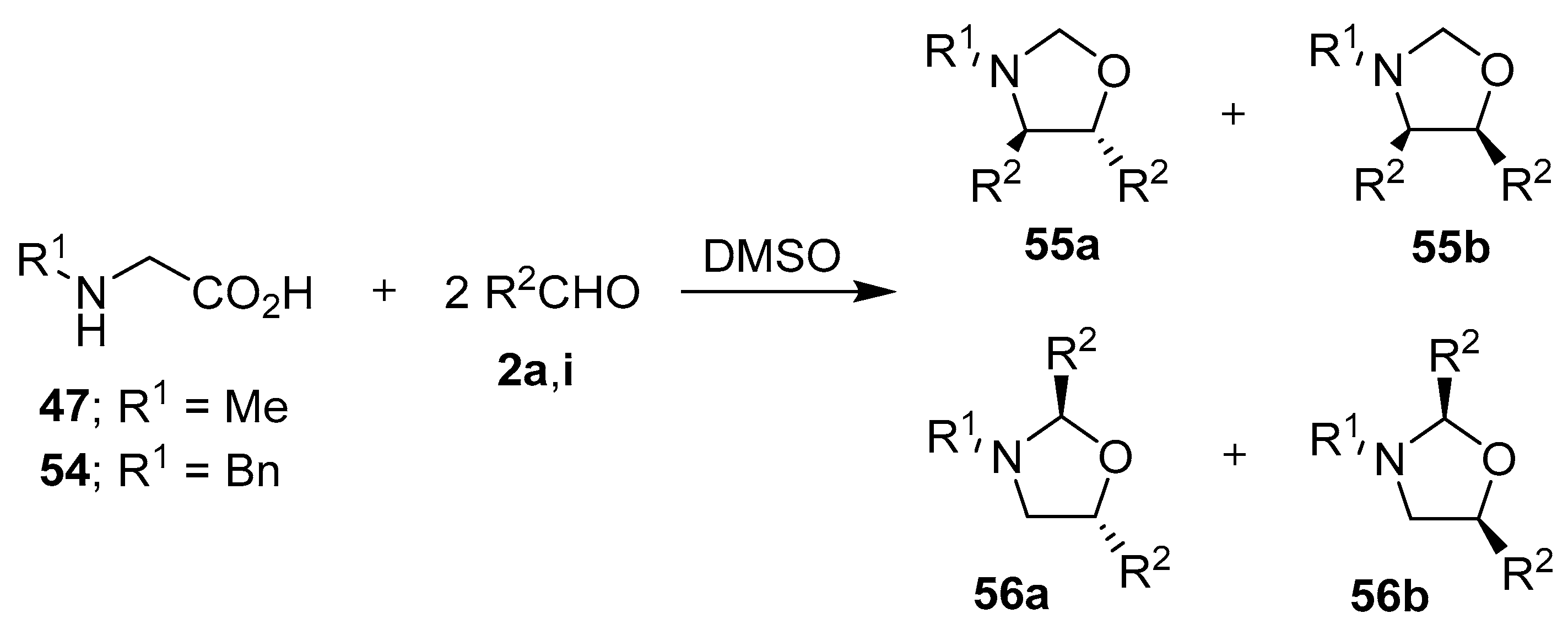
Fifteen years later, Orsini and co-workers reported a study on the generation of azomethine ylides from an aldehyde-induced decarboxylation of secondary α-amino acids, and their subsequent 1,3-dipolar cycloadditions to a second mole of aldehyde to form oxazolidines [66]. A more detailed account of their work appeared in 1988, where proline (50) was reacted with various aromatic aldehydes 2 to afford, with complete regioselectivity, the hexahydropyrrolo[2,1-b]oxazoles 52 and 53, with the trans-2,3-oxazolidine 52 being either the exclusive or major stereoisomer formed [67,68,69]. The stereoselectivity of the cycloaddition was governed by both the ylide stereochemistry, with the anti-azomethine ylide 51 being favored, and the mutual orientation of the dipole and dipolarophile (Scheme 12, Table 7). The reaction of N-substituted glycine derivatives 47 or 54 with aldehydes 2a and 2i was also investigated, with complex mixtures of the respective 4,5- and 2,5-disubstituted-1,3-oxazolidines 55 and 56 being isolated (Scheme 13, Table 8). When this method was applied to N-benzylalanine and aldehydes 2a or 2i, complex mixtures of regio- and stereoisomeric oxazolidines were also obtained.
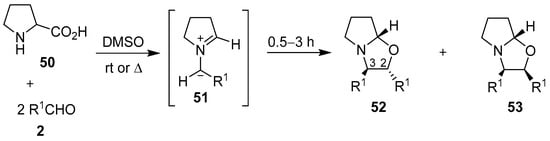
Scheme 12.
Cycloaddition of azomethine ylides 51, generated from proline (50) and aromatic aldehydes 2, to a second mole of aldehyde.

Table 7.
Cycloaddition of azomethine ylide 51 to aromatic aldehydes 2 (Scheme 12).
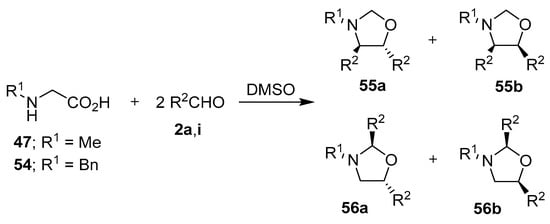
Scheme 13.
Reactions of N-substituted glycine derivatives 47 or 54 with aldehydes 2a and 2i to afford the respective 4,5- and 2,5-disubstituted-1,3-oxazolidines 55 and 56.

Table 8.
Reaction between glycine derivatives 47 or 54 and aromatic aldehydes 2a or 2i (Scheme 13).
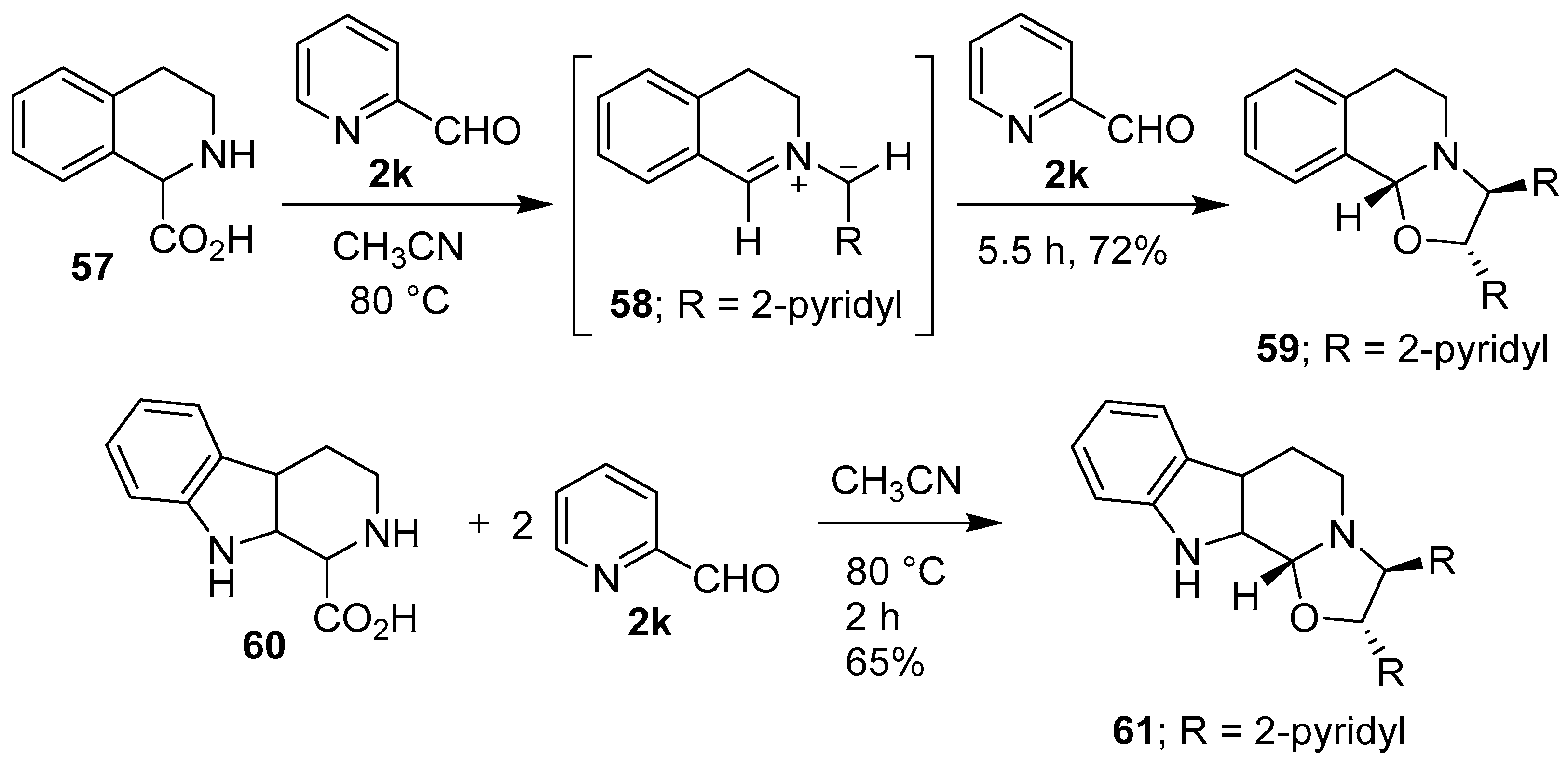
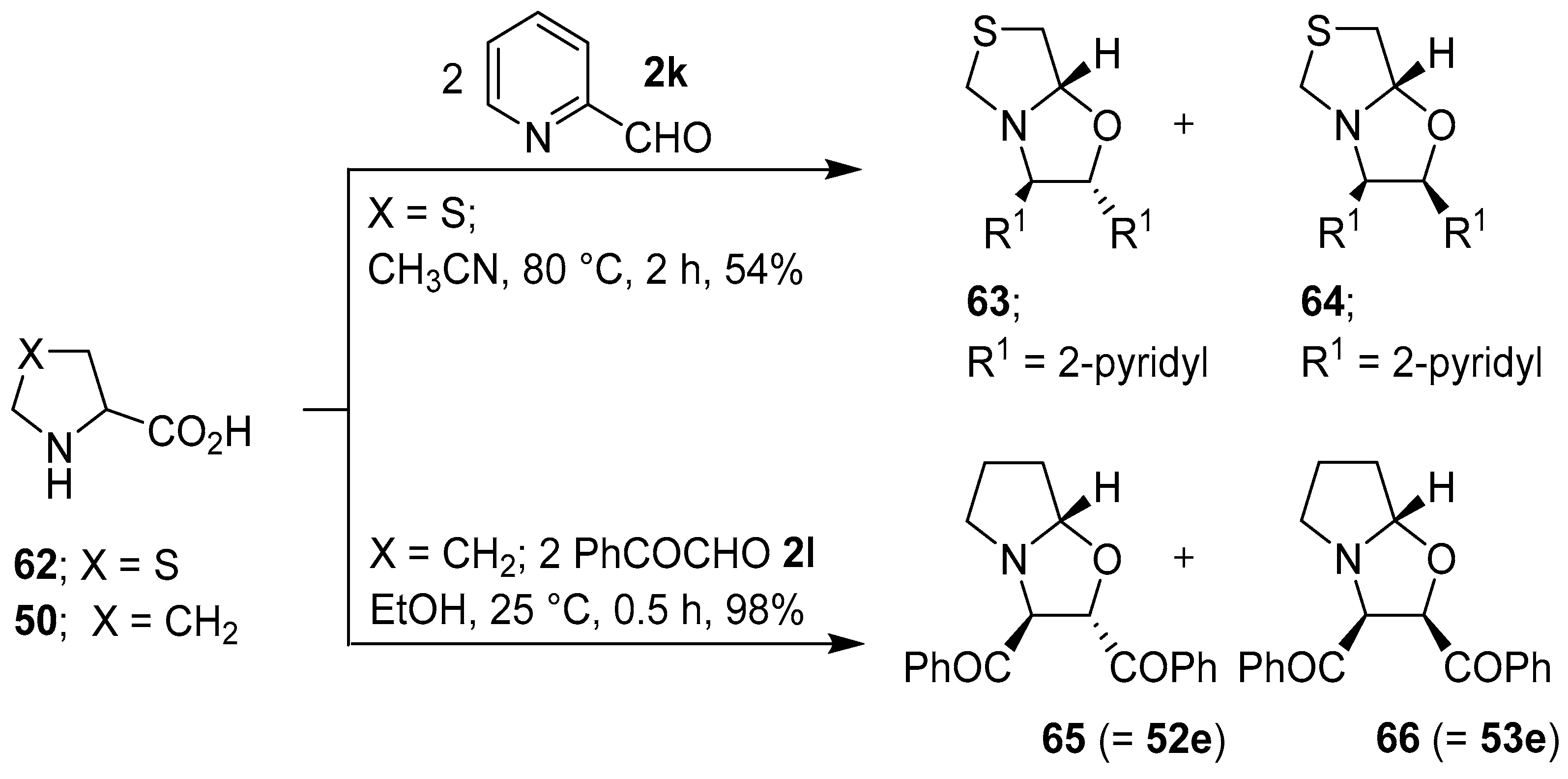
Grigg and co-workers found that cyclic secondary amino acid 57 reacted regio- and stereo-specifically with 2-pyridylcarboxaldehyde (2k), via the anti-azomethine ylide 58, to give the corresponding oxazolidine 59 in good yield. In a similar manner, β-carboline 60 reacted with aldehyde 2k to give oxazolidine 61 (Scheme 14). In further examples, thiazolidine-4-carboxylic acid (62) reacted regiospecifically with 2k to give a 2:1 mixture of 63 and 64, and proline (50) reacted with phenylglyoxal (2l) to give a 5:1 mixture of 65 and 66 (Scheme 15) [70,71]. Proline (50) also reacted with 2-(allyloxy)benzaldehyde to afford an azomethine ylide that underwent intermolecular cycloaddition with the aldehyde moiety of another equivalent of 2-(allyloxy)benzaldehyde to give the corresponding hexahydropyrrolo[2,1-b]oxazole in 51% yield rather than intramolecular cycloaddition with the unactivated internal olefin [72]. Furthermore, proline underwent a step-wise decarboxylative condensation with 2-phenylbenzaldehyde followed by cycloaddition with the same aldehyde to give the corresponding oxazolidine as a single isomer in low yield [73]. l-Proline reacted with (a) ethyl pyruvate to give the corresponding hexahydropyrrolo[2,1-b]oxazole derivative as a mixture of diastereomers in high yield [74]; and (b) with 4-nitrobenzaldehyde in a Ce(IV) oxide catalyzed cycloaddition reaction [75]. The reaction of sarcosine or L-proline with heteroaryl-2-carboxaldehydes afforded inseparable diastereomeric mixtures of the corresponding decarboxylative condensation-cycloaddition products [76].
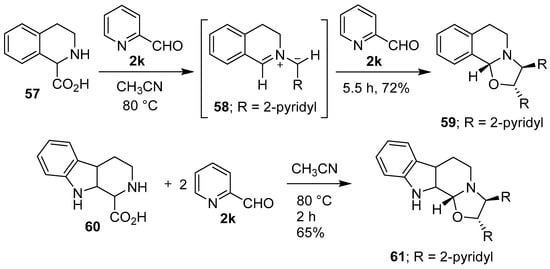
Scheme 14.
Reactions of cyclic secondary amino acids 57 and 60 with 2-pyridylcarboxaldehyde (2k).
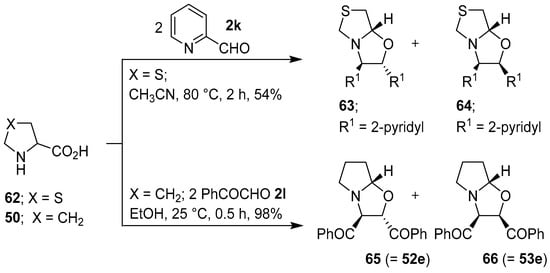
Scheme 15.
Reaction of thiazolidine-4-carboxylic acid (62) with 2-pyridylcarboxaldehyde (2k), and of proline (50) with phenylglyoxal (2l).
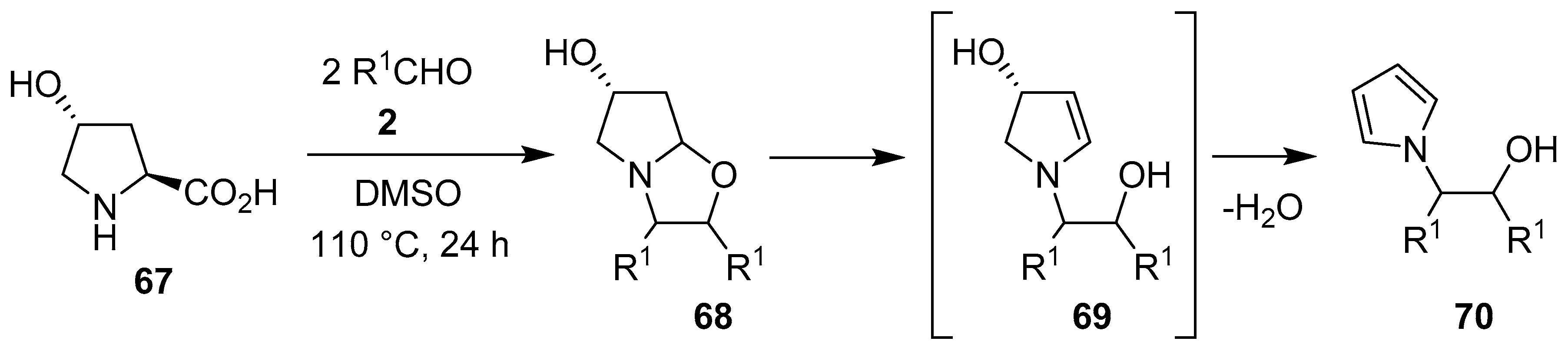
In an interesting one-pot domino process, trans-4-hydroxyproline (67) reacted with electron-deficient aromatic aldehydes 2 to give bicyclic 1,3-oxazolidines 68, which presumably underwent ring-opening to form intermediate 69, with subsequent dehydrative aromatization to furnish N-β-hydroxyethyl pyrroles 70 (Scheme 16, Table 9) [77]. In a similar fashion, indoline-2-carboxylic acid afforded the corresponding N-β-hydroxyethyl indoles in 33%–82% yield.
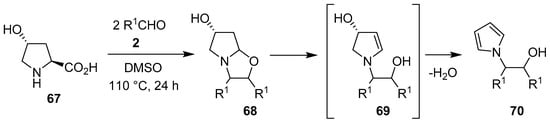
Scheme 16.
Reaction of trans-4-hydroxyproline (67) with aromatic aldehydes 2 to afford N-β-hydroxyethyl pyrroles 70.

Table 9.
Domino decarboxylative condensation, [3 + 2] cycloaddition and ring-opening aromatization process to give N-β-hydroxyethyl pyrroles 70 (Scheme 16).
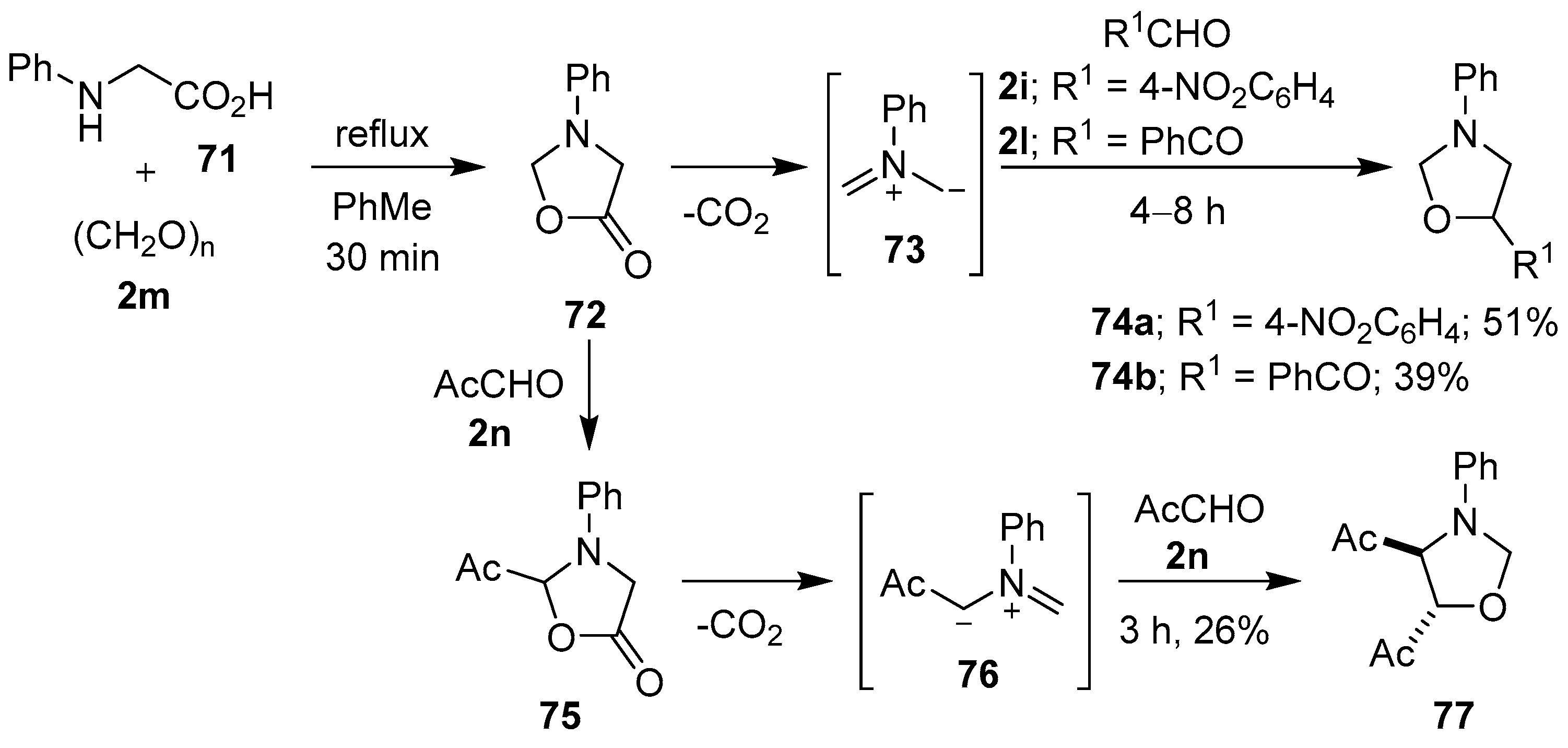
A number of research groups have provided direct evidence to support the intermediacy of azomethine ylides from the decarboxylative condensation of α-amino acids with carbonyl compounds [78,79,80,81]. Tsuge and co-workers briefly heated N-phenylglycine (71) at reflux with paraformaldehyde (2m) to produce isolable 3-phenyl-5-oxazolidinone (72). When oxazolidinone 72 was further heated, decarboxylation occurred to give non-stabilized azomethine ylide 73, which underwent cycloaddition with 4-nitrobenzaldehyde (2i) or phenylglyoxal (2l) to afford the respective oxazolidines 74 in modest yields. Oxazolidinone 72 also underwent an acetal-exchange reaction with methyl glyoxal (2n) to afford acetyl-substituted 5-oxazolidinone 75. This compound then underwent decarboxylation to generate stabilized azomethine ylide 76, which was captured by another equivalent of aldehyde 2n to give cycloadduct 77 (Scheme 17) [80].
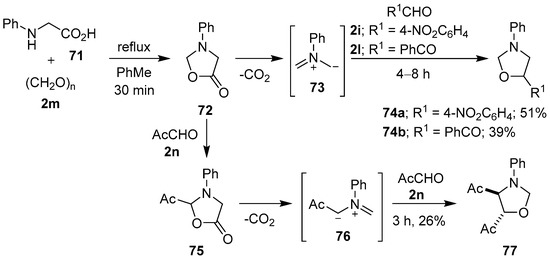
Scheme 17.
Cycloadditions of non-stabilized azomethine ylide 73 to aldehydes 2i and 2l, and of stabilized azomethine ylide 76 to methyl glyoxal (2n).
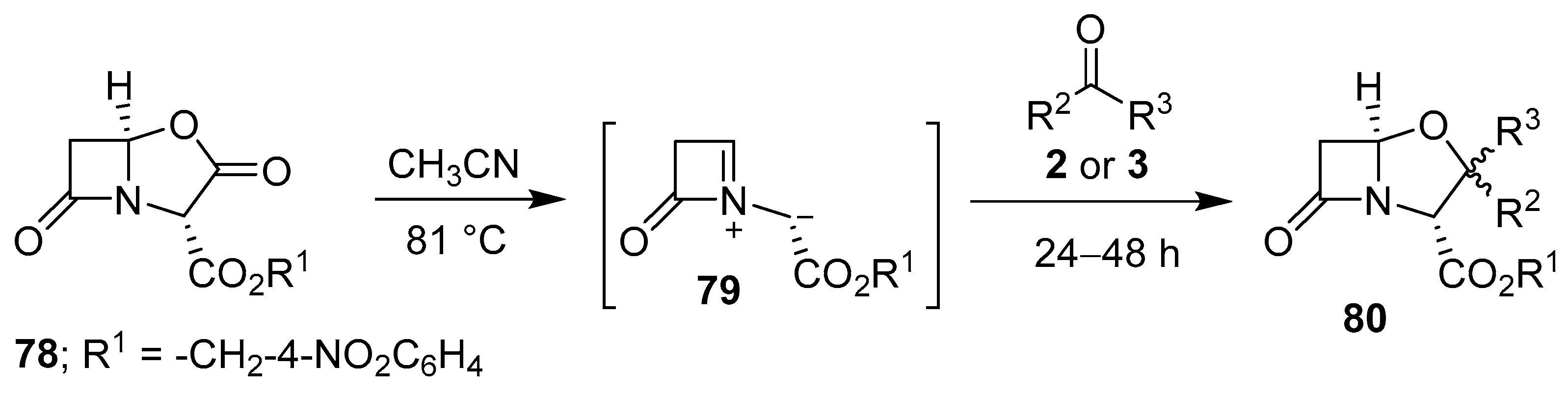
In a related decarboxylative process, thermolysis of lactam-based oxazolidinone 78 generated the corresponding four-membered lactam incorporating an azomethine ylide 79, which underwent cyloadditions with aldehydes 2 and ketones 3 to furnish mostly 1:1 diastereomeric mixtures of oxapenam derivatives 80 in low to moderate yields (Scheme 18, Table 10) [82].
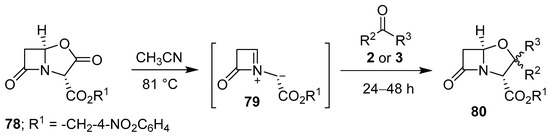
Scheme 18.
Cycloaddition of azomethine ylide 79 to aldehydes 2 or ketones 3.

Table 10.
Cycloaddition of azomethine ylide 79 to aldehydes 2 or ketones 3 (Scheme 18).
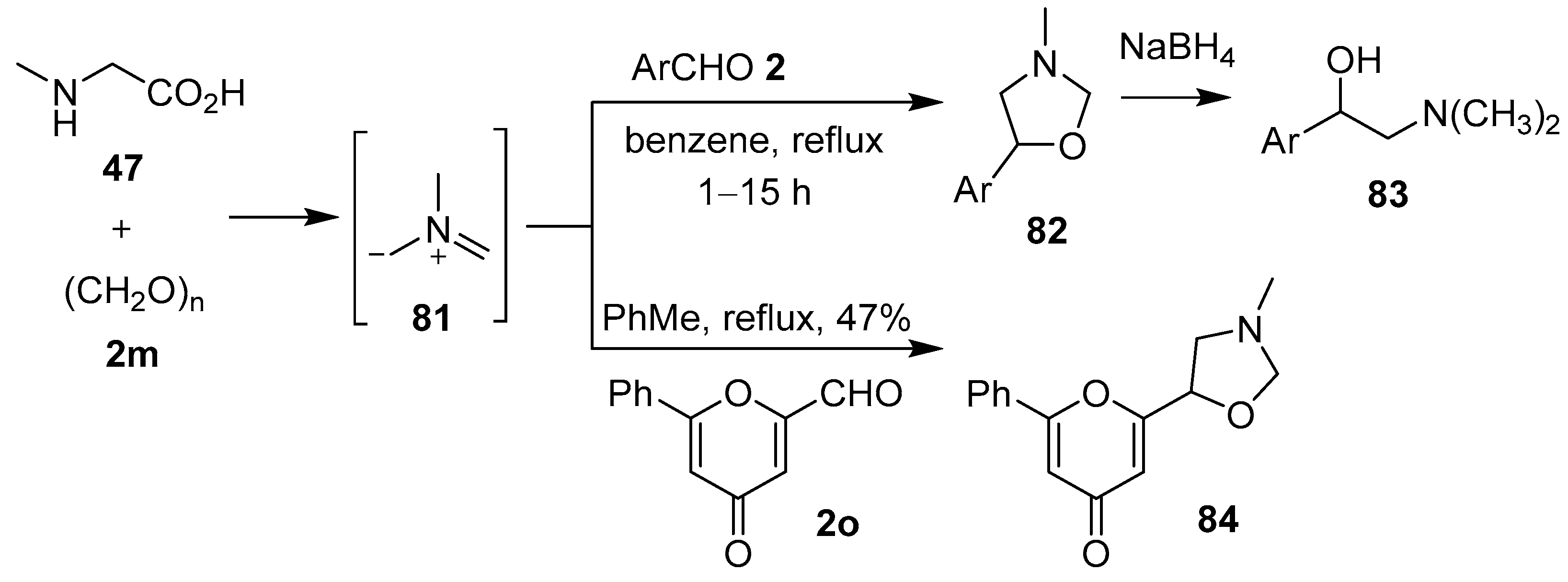
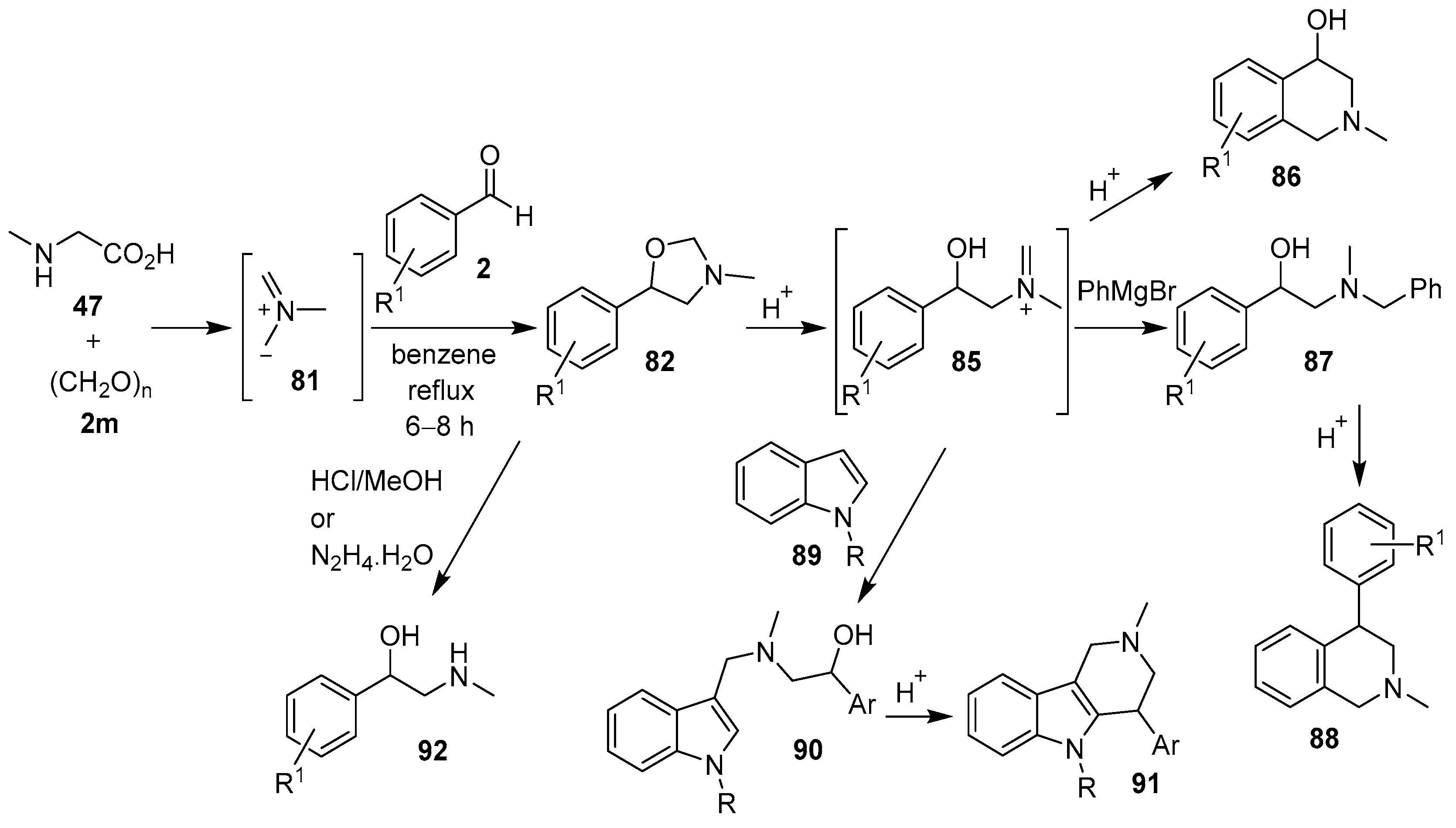
The non-stabilized azomethine ylide 81, derived in situ from sarcosine (47) and paraformaldehyde (2m), underwent 1,3-dipolar cycloaddition reactions with various aromatic aldehydes 2 to form the corresponding 5-aryl-3-methyloxazolidines 82 (Scheme 19, Table 11). Subsequent reductive ring-opening of the cycloadducts afforded 1-aryl-2-dimethylaminoethanols 83 [83]. This work was predicated on the observation that sarcosine (47), paraformaldehyde (2m) and pyrone aldehyde 2o reacted to form N-methyloxazolidine 84 [84]. Azomethine ylide 81, formed in this way, also underwent cycloaddition reactions with 2-chloro-3-quinolinecarboxaldehydes to afford the corresponding 3-quinolyl-1,3-oxazolones [85], and in an internal competition reaction with 3-formyl-2H-chromene to furnish a 2.8:1 mixture of the corresponding benzo[3,4-c]pyrrolidine and oxazolidine cycloadducts, respectively [86].
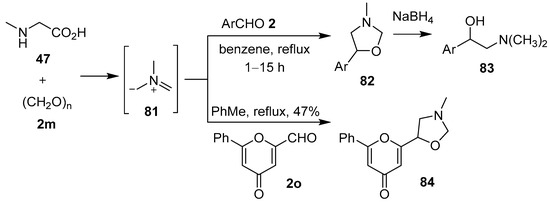
Scheme 19.
Cycloaddition of azomethine ylide 81 to aromatic aldehydes 2 or pyrone aldehyde 2o.

Table 11.
Cycloaddition of azomethine ylide 81 to aldehydes 2 (Scheme 19).
In a series of recent publications, Moshkin, Sosnovskikh and co-workers have also utilized the azomethine ylide 81 in 1,3-dipolar cycloaddition reactions with the carbonyl bond of various aromatic aldehydes 2. The synthetic utility of the corresponding 5-aryloxazolidine products was explored, and in a series of acid-catalyzed ring-opening reactions via iminium ion 85, a number of heterocycles and 2-amino-1-arylethanols were formed (Scheme 20). Thus, the 5-aryloxazolidines 82 underwent (a) acid-catalyzed rearrangement into 2-methyl-1,2,3,4-tetrahydroisoquinolin-4-ols 86 [42]; (b) reaction with arylmagnesium bromides to produce N-benzyl-β-hydroxyphenethylamines 87, which underwent subsequent acid-catalyzed cyclization to give 4-aryl-1,2,3,4-tetrahydro-isoquinolines 88 [87]; and (c) reaction with indoles 89 to afford 2-(indol-3-ylmethylamino)-1-aryl ethanols 90, which underwent acid-catalyzed cyclization to yield 4-aryl-2-methyl-1,2,3,4-tetrahydro-β-carbolines 91 [88]. Alternatively, oxazolidines 82 could be readily hydrolyzed to form 2-(alkylamino)-1-arylethanols 92 by treatment with acid or hydrazine hydrate [89].
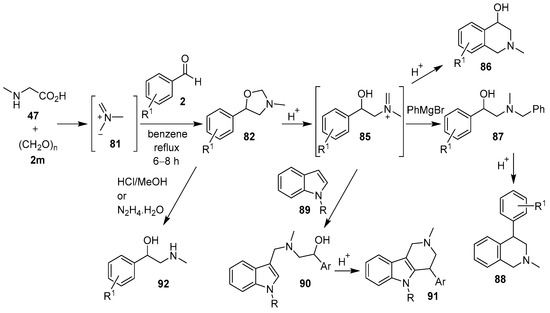
Scheme 20.
Cycloaddition of azomethine ylide 81 to aromatic aldehydes 2, and the subsequent products derived from ring-opening reactions of 5-aryloxazolidine 82.
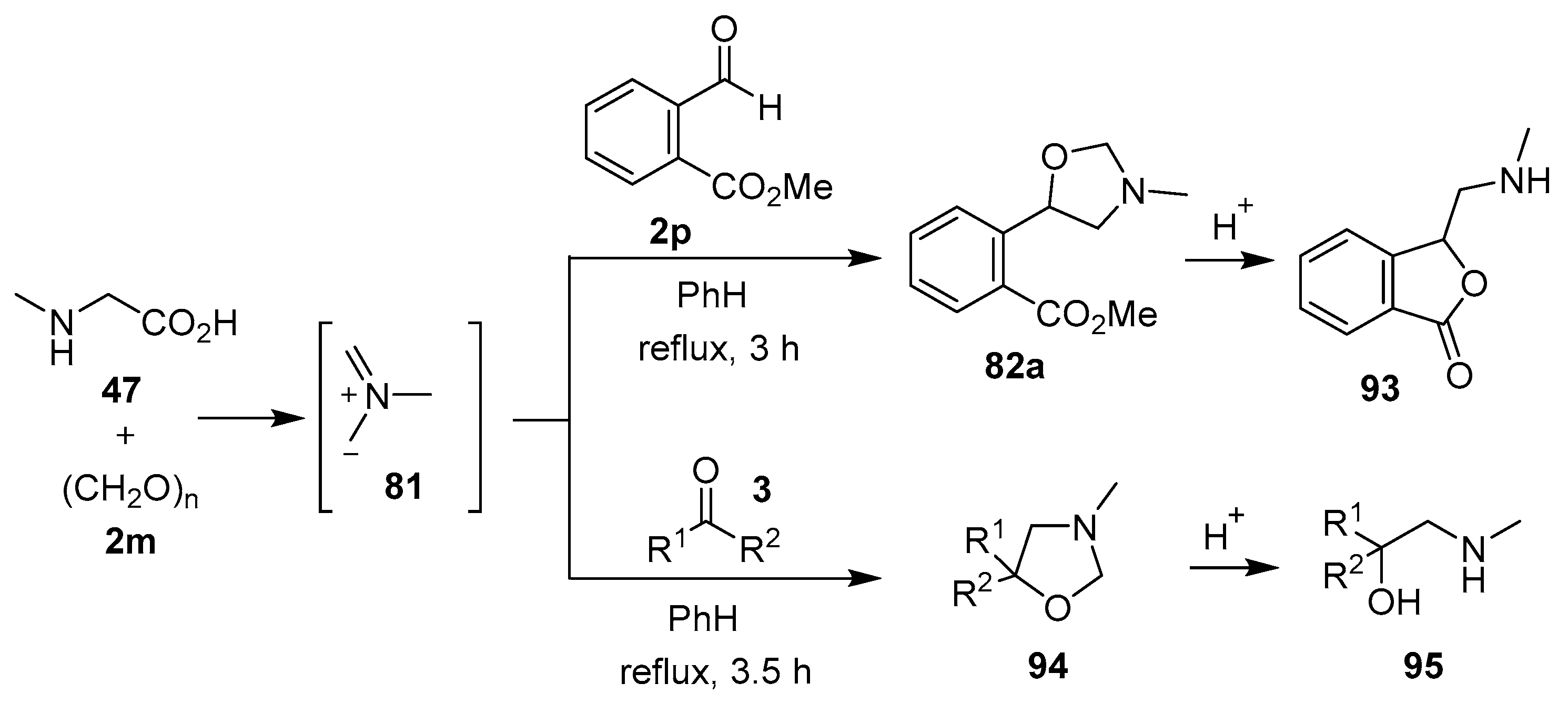
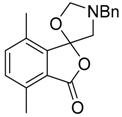
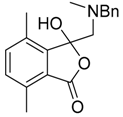
The sarcosine/formaldehyde derived azomethine ylide 81 also reacted with (a) aromatic aldehydes bearing an ester group such as 2p, which formed the corresponding oxazolidine 82a, and underwent a subsequent demethylenation/rearrangement process to form (aminomethyl)lactone 93 [90]; and (b) a variety of aromatic ketones 3 to give the 5,5-diaryloxazolidines 94 (Scheme 21, Table 12), which underwent hydrolysis to afford 2-alkylaminoethanols 95 (Scheme 21) [91].
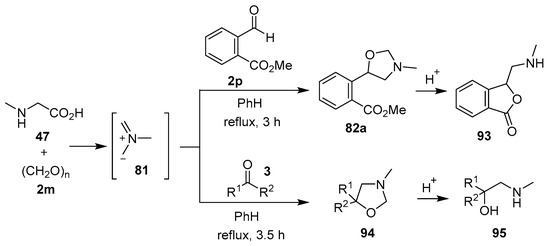
Scheme 21.
Cycloaddition of azomethine ylide 81 to aldehyde 2p and ketones 3, and the subsequent oxazolidine ring-opened products.

Table 12.
Cycloaddition of azomethine ylide 81 to ketones 3 (Scheme 21).
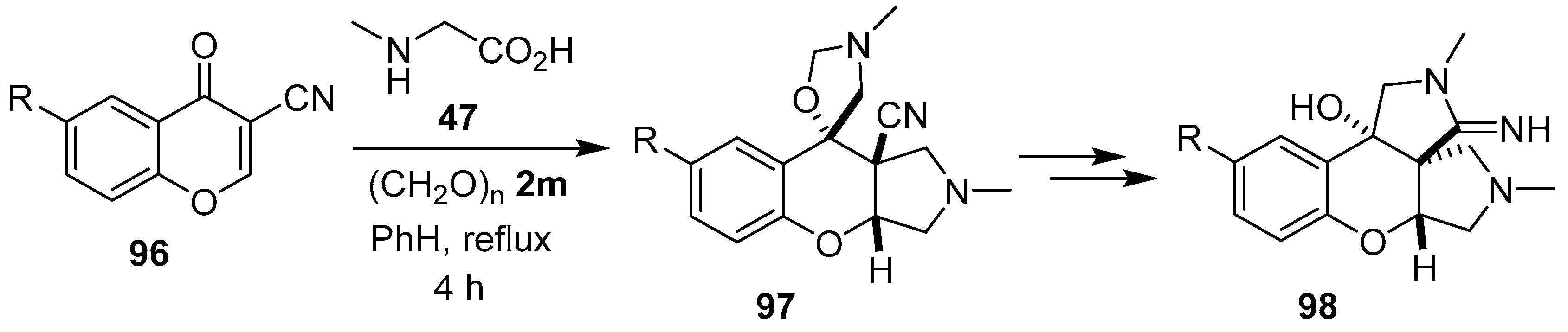
The same researchers found that an excess of the sarcosine/paraformaldehyde derived azomethine ylide 81 participated in a dual 1,3-dipolar cycloaddition reaction with 3-cyanochromones 96 at both the double bond and the carbonyl group to give diastereoselectively tetrahydro-1H-spiro[chromeno[2,3-c]pyrrol-9,5′-oxazolidine]-9a-carbonitriles 97, which subsequently underwent hydrolysis and cyclization of the amino group onto the nitrile to give hexahydrochromeno[2,3-c:3,4-c′]dipyrroles 98 (Scheme 22, Table 13) [92,93].
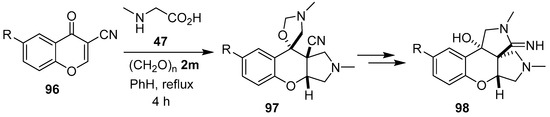
Scheme 22.
Dual cycloaddition of azomethine ylide 81 to 3-cyanochromones 96.

Table 13.
Cycloaddition of excess azomethine ylide 81 to 3-cyanochromones 96 (Scheme 22).
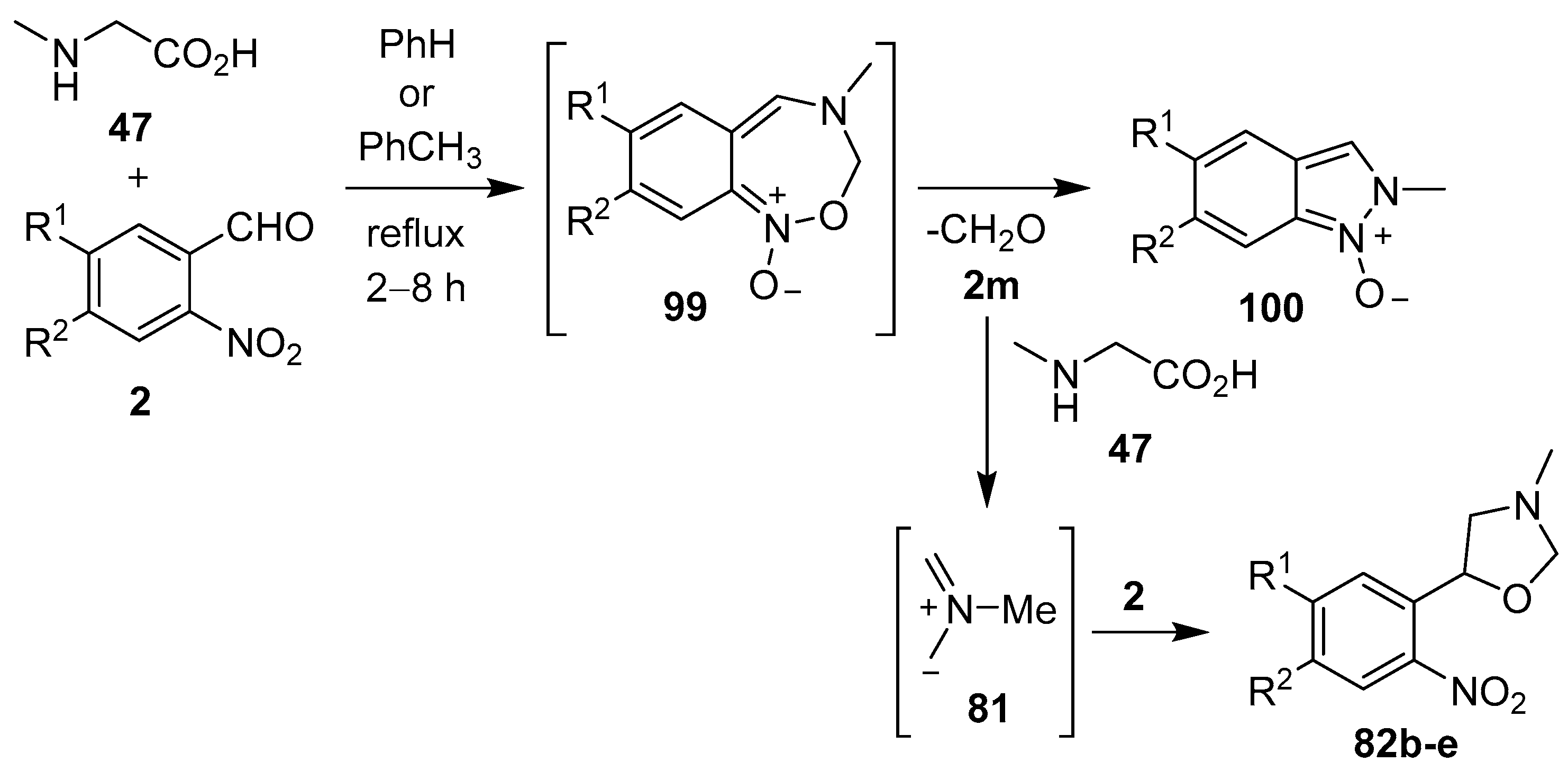
Azomethine ylides, generated from sarcosine (47) and substituted 2-nitrobenzaldehydes 2, underwent 1,7-electrocylization onto the adjacent nitro group to give unstable benz-1,2,6-oxadiazepines 99. Subsequent ring-contraction afforded mixtures of indazole-N-oxides 100 and 3-methyl-5-aryloxazolidines 82b–e. It was postulated that the oxazolidine products were derived from the paraformaldehyde (2m) eliminated during this process, that reacted with excess sarcosine (47) to generate azomethine ylide 81, which underwent cycloaddition to another molecule of a 2-nitrobenzaldehyde derivative 2 (Scheme 23, Table 14) [94,95].
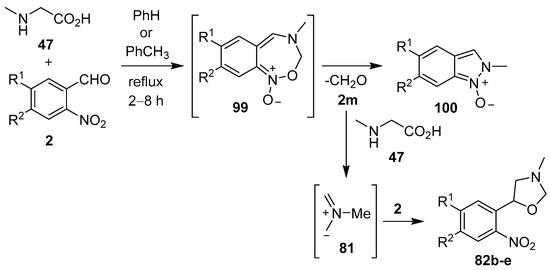
Scheme 23.
Reaction of sarcosine (47) with 2-nitrobenzaldehydes 2.

Table 14.
Ring-contraction of benz-1,2,6-oxadiazepines 99 to indazole-N-oxides 100 and formation of 3-methyl-5-aryloxazolidines 82b–e (Scheme 23).
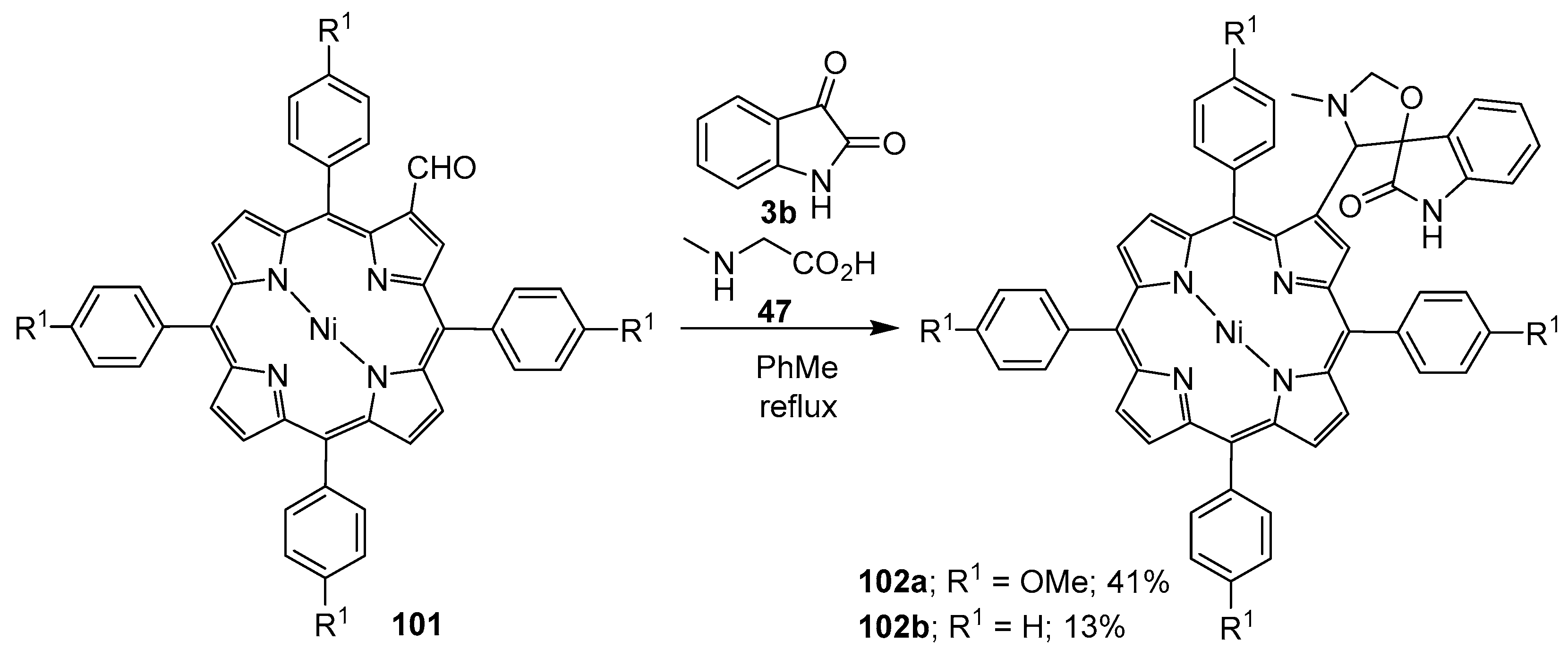
Azomethine ylides generated from sarcosine (47) and Ni(II) β-formyl-meso-tetraphenyl-porphyrins 101 underwent regioselective cycloaddition reactions with isatin (3b) to furnish the corresponding spiroporphyrin derivatives 102 in modest yields (Scheme 24) [96].
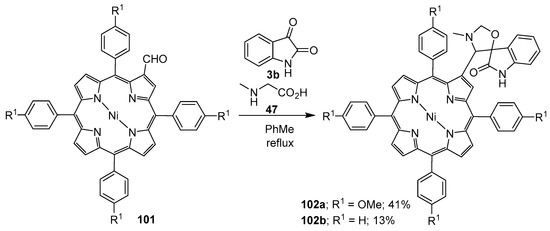
Scheme 24.
Cycloaddition of azomethine ylides generated from sarcosine (47) and porphyrins 101 to isatin (3b).
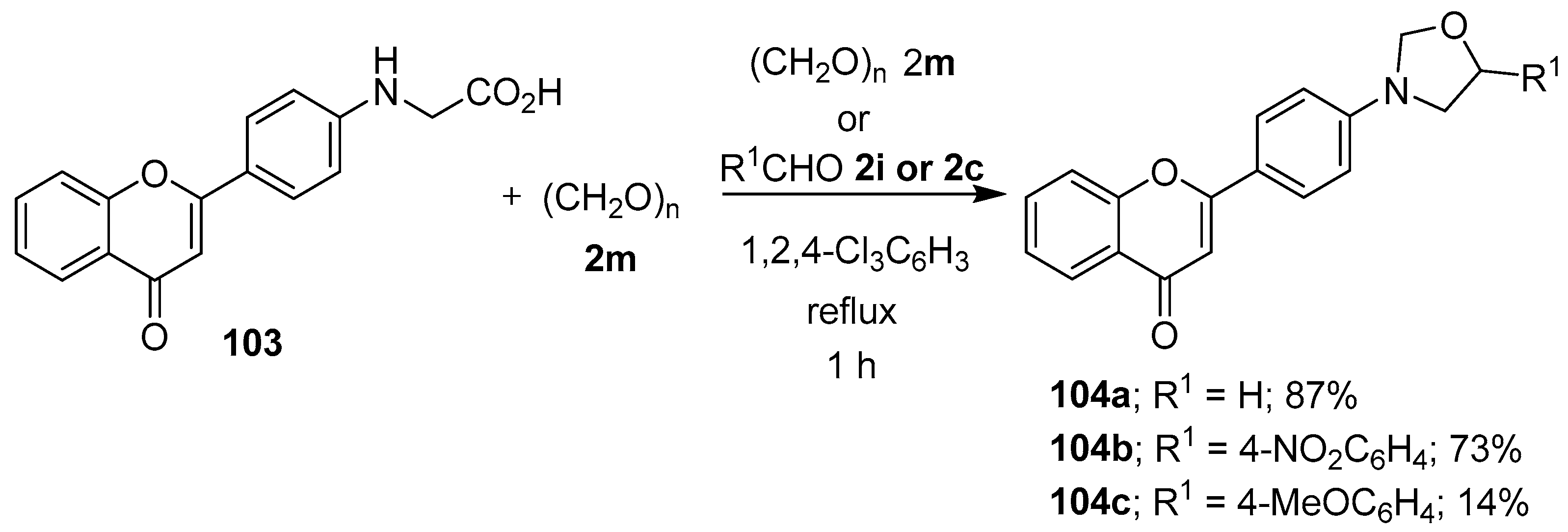
The azomethine ylide generated in situ from the reaction of N-[4-(chromon-2-yl)phenyl]glycine (103) and paraformaldehyde (2m) was trapped with either a molecule of paraformaldehyde (2m) or the aromatic aldehydes 2i or 2c to afford the corresponding flavone-oxazolidine dyads 104 (Scheme 25) [97]. Minor amounts of the corresponding oxazolidine cycloadducts have also been isolated as side products from reactions involving (a) sarcosine, phenylglyoxal and N-ethylmaleimide [98]; and (b) (S)-(−)-2-azetidinecarboxylic acid, phenylglyoxal and dimethyl fumarate [99].
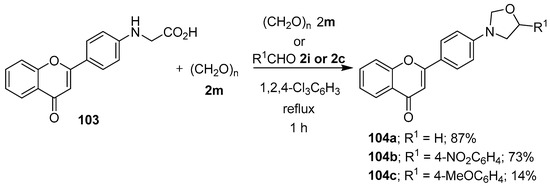
Scheme 25.
Cycloaddition of the azomethine ylide generated from N-[4-(chromon-2-yl)phenyl]glycine (103) and paraformaldehyde (2m) to a second molecule of paraformaldehyde (2m) or to aldehydes 2i or 2c.
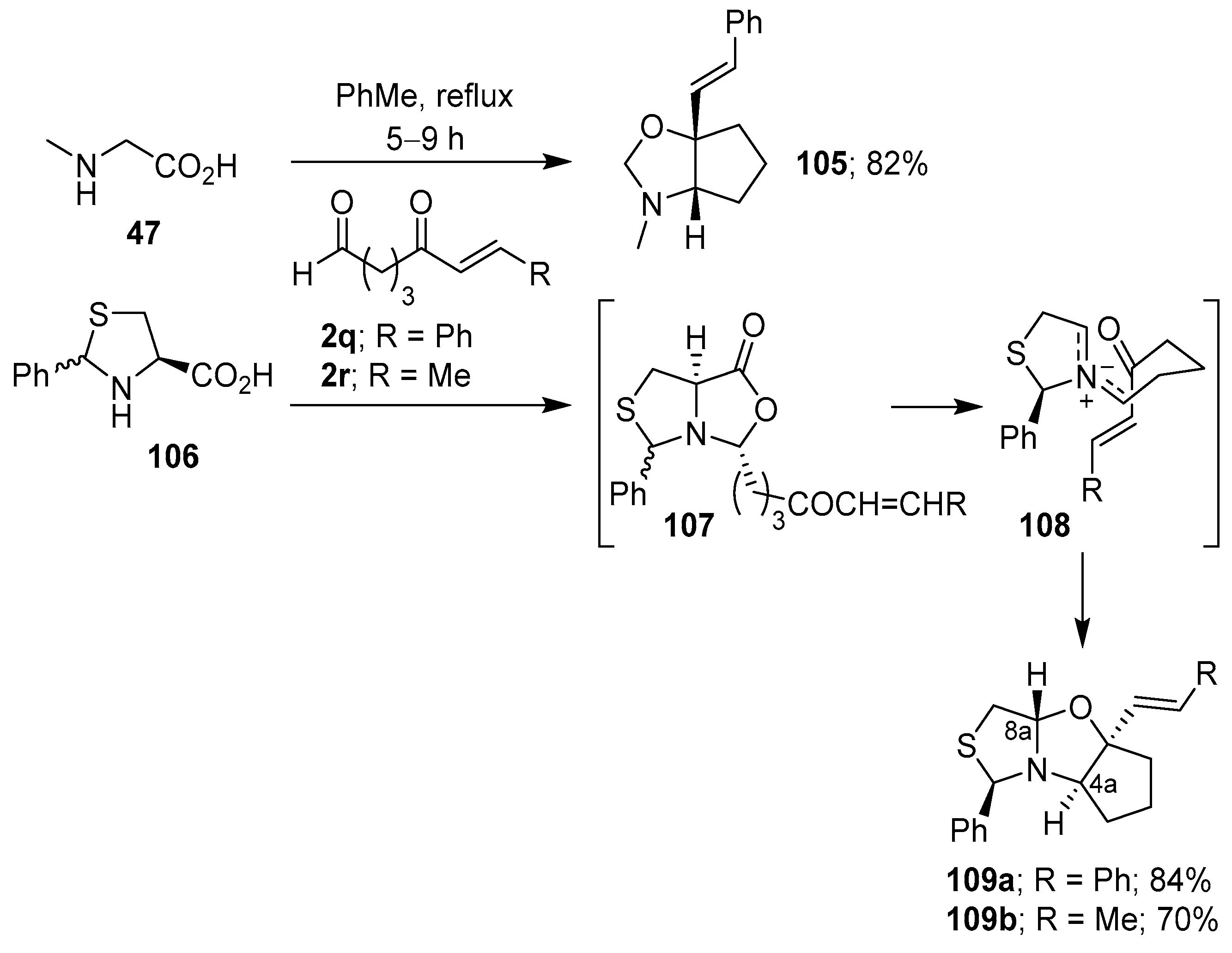
An intramolecular variant that involves the generation of non-stabilized azomethine ylides from the condensation of α-amino acids with aldehydes bearing a tethered carbonyl group has also been reported. Thus, the azomethine ylide generated from sarcosine (47) and 5-oxo-7-phenyl-6-heptenal (2q), reacted exclusively at the internal carbonyl moiety to produce regio- and stereoselectively cycloadduct 105 in 82% yield. Similarly, 2-phenyl-4-thiazolidinecarboxylic acid (106) reacted with 2q and 2r to produce 109a and 109b, respectively, as single stereoisomers in good yields. The trans-configuration between 4a-H and 8a-H was due to the selective participation of the Z,E-ylidic forms 108, which was presumably derived from the stereospecific decarboxylation of the more thermodynamically stable bicyclic lactones 107 (Scheme 26) [100].
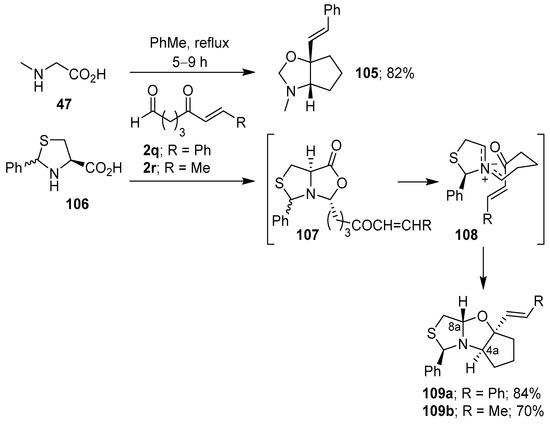
Scheme 26.
Intramolecular cycloaddition of the non-stabilized azomethine ylide generated from the condensation of α-amino acids with aldehydes bearing a tethered aldehyde.
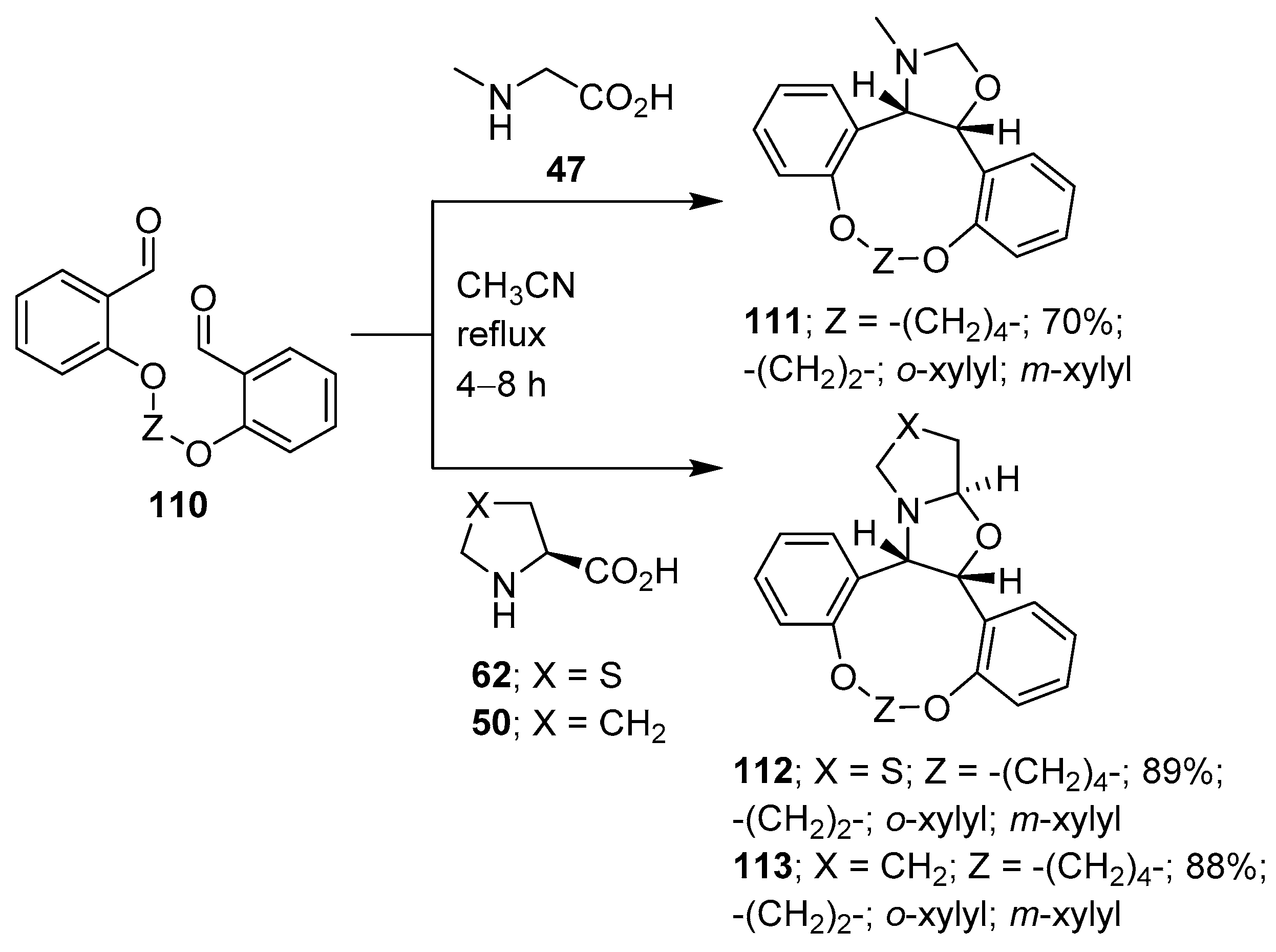
Further examples of this type of intramolecular 1,3-dipolar cycloaddition process include the reaction of intermediate azomethine ylides derived from dialdehyde 110 and (a) sarcosine (47), which resulted in the stereoselective formation of cis-fused oxazolidine-grafted macrocycles 111; (b) thiazolidine-4-carboxylic acid (62) to form tetrahydrooxazolothiazoles 112; and (c) L-proline (50) to give hexahydropyrrolooxazole grafted macrocycles 113 (Scheme 27) [101].
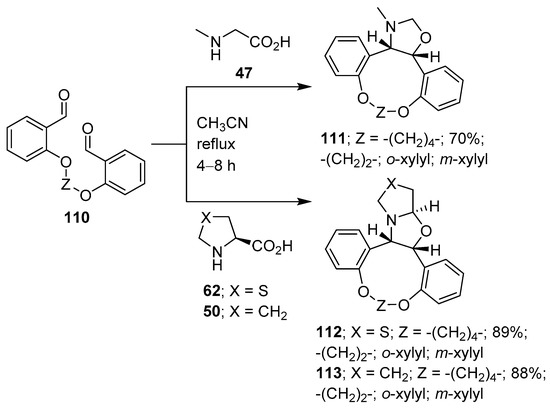
Scheme 27.
Intramolecular cycloaddition of azomethine ylides generated from dialdehyde 110 and sarcosine (47), thiazolidine-4-carboxylic acid (62) or L-proline (50).
2.3. Reactions of Ylides Formed by Condensation of Amines with Carbonyl or 1,2-Dicarbonyl Compounds
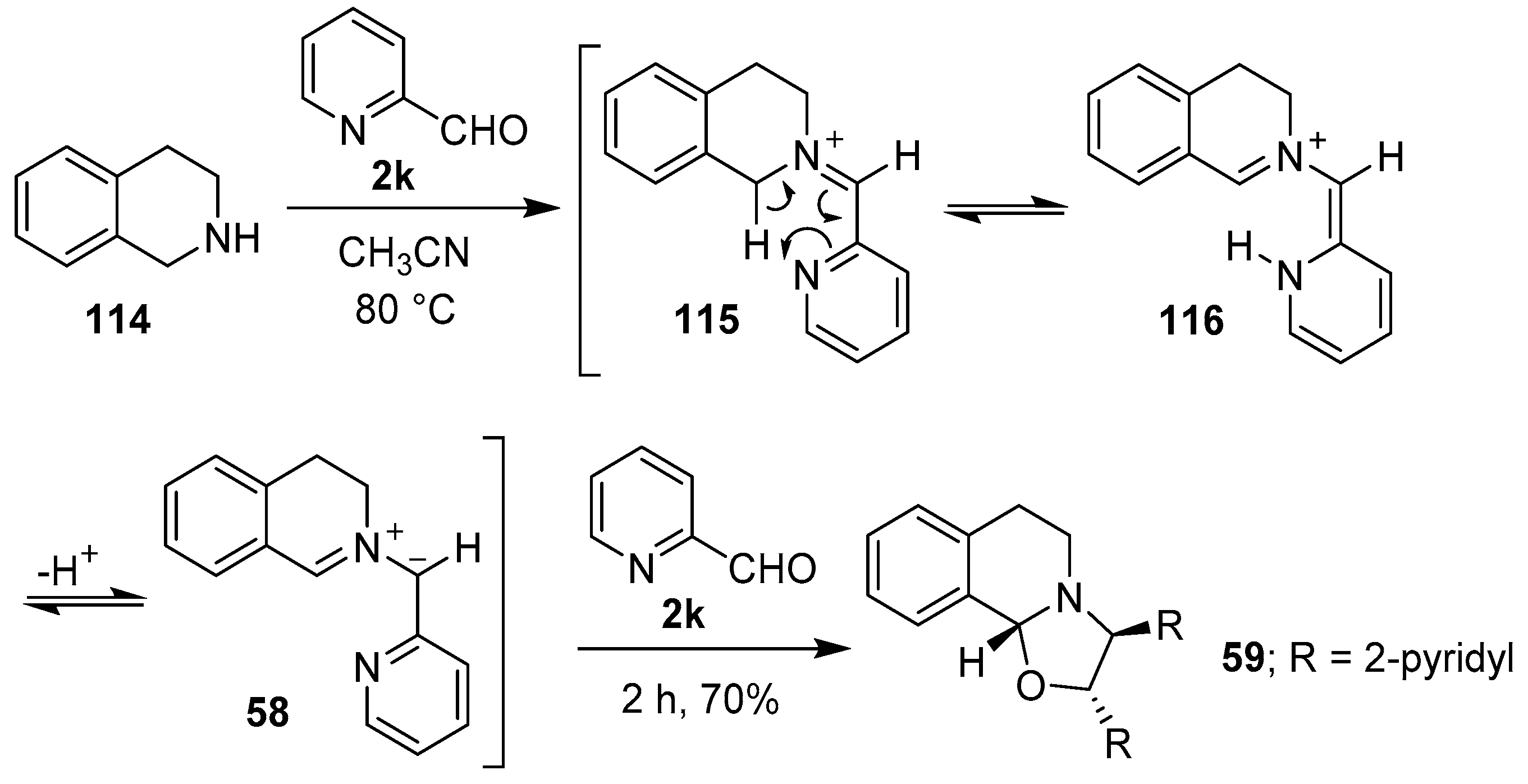
In 1986, Grigg and co-workers reported that azomethine ylides could be derived from iminium ions formed between primary or secondary amines and bifunctional carbonyl compounds. Thus, tetrahydroisoquinoline (114) and 2-pyridaldehyde (2k) reacted to form iminium ion 115, which was postulated to undergo a formal sigmatropic 1,5-H shift to generate intermediate 116, and subsequently regio- and stereospecifically, the anti-azomethine ylide 58. Cycloaddition of the derived 1,3-dipole with another molecule of the aldehyde afforded oxazolidine 59 (Scheme 28). In a similar manner, β-carboline reacted with the aldehyde 2k to give oxazolidine 61 in 64% yield [102,103].
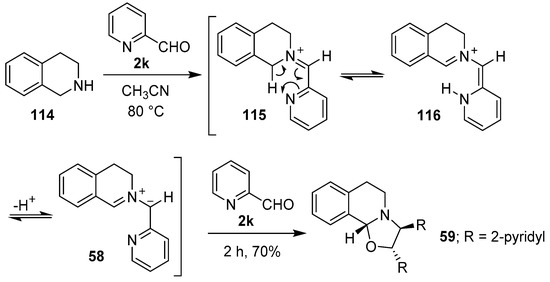
Scheme 28.
Cycloaddition of azomethine ylide 58 to 2-pyridaldehyde (2k).
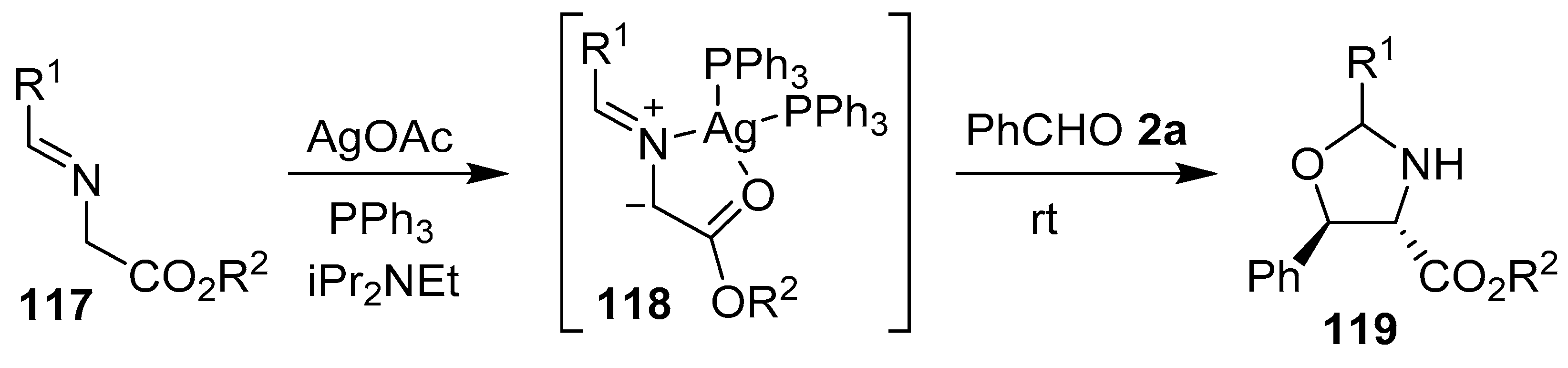
To prepare N-unsubstituted oxazolidines, a hydrogen or a metal atom must be attached to the nitrogen atom of the azomethine ylide. N-Metalated ylides of this type have most often been generated by treatment of an imine bearing an electron withdrawing group with a metal salt, which coordinates with the imine nitrogen to activate the substrate towards deprotonation by an added base. Thus, activation of aromatic aldehyde-derived α-iminoesters 117 by a Ag(I)/PPh3 catalyst generated the N-metalated azomethine ylides 118, which underwent cycloadditions with benzaldehyde 2a to yield predominantly 4,5-trans oxazolidines 119 (Scheme 29, Table 15). Subsequent hydrolysis of the oxazolidine cycloadducts furnished the corresponding syn-β-aryl-β-hydroxy-α-amino esters [104].
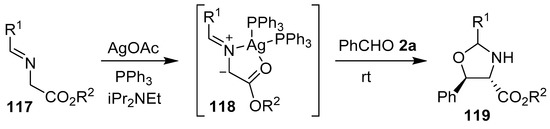
Scheme 29.
Cycloaddition of N-metalated azomethine ylides 118, generated from α-iminoesters 117, to benzaldehyde (2a).

Table 15.
Cycloaddition of N-metalated azomethine ylide 118 to benzaldehyde (2a) (Scheme 29) 1.
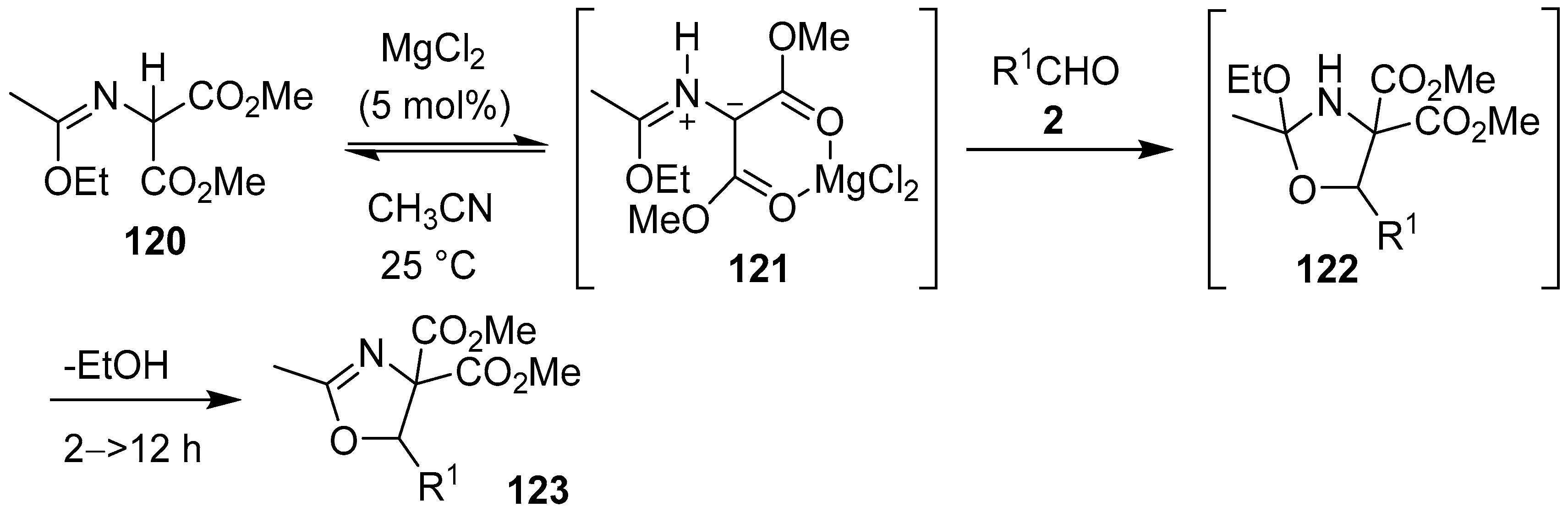
In a Lewis acid-catalyzed process, malonic imidate 120 was postulated to chelate to MgCl2 to form intermediate azomethine ylide 121 via 1,2-prototropy. The metal-coordinated ylide was subsequently trapped by various aldehydes 2 to form the corresponding oxazolidines 122, which underwent spontaneous loss of ethanol to afford oxazolines 123 (Scheme 30, Table 16) [105].
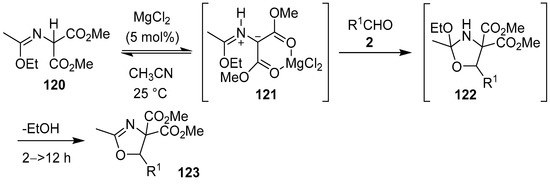
Scheme 30.
Cycloaddition of azomethine ylide 121, generated from malonic imidate 120 via 1,2-prototropy, to aldehydes 2.

Table 16.
Cycloaddition of azomethine ylide 121 to aldehydes 2 (Scheme 30).
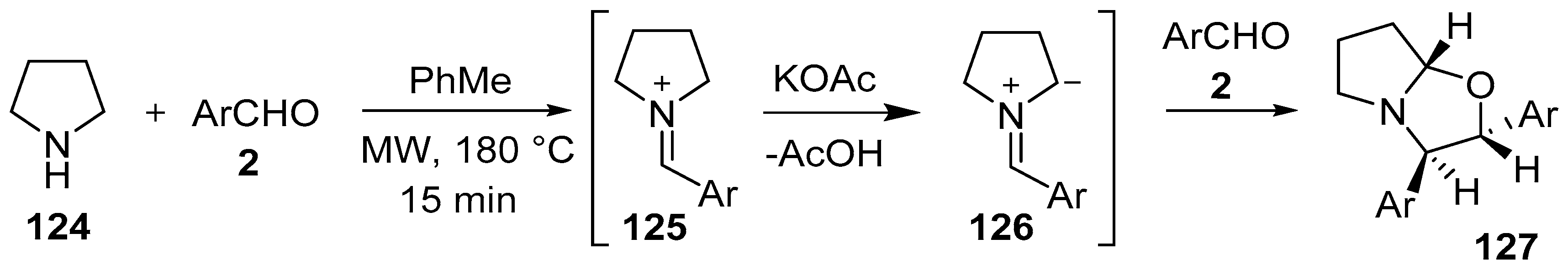
In a metal-free, base promoted process under microwave irradiation, pyrrolidine (124) reacted with aromatic aldehydes 2 to form the corresponding iminium ions 125, which were presumably transformed into azomethine ylides 126 via α-deprotonation by acetate anion. The [3 + 2] cycloaddition reaction between the derived azomethine ylide and another molecule of aldehyde afforded the corresponding hexahydropyrrolo[2,1-b]oxazoles 127, in a diastereoselective fashion (Scheme 31, Table 17). Piperidine reacted in an analogous fashion. This process can be considered as a direct functionalization of an sp3 C-H bond of an unfunctionalized cyclic amine [106]. In a similar manner, but under milder conditions, 1,2,3,4-tetrahydroisoquinoline and tetrahydro-β-carboline reacted with aromatic aldehydes to furnish the corresponding oxazolo[2,3-a]isoquinolines as single diastereomers [107].
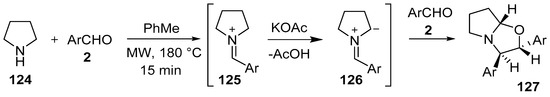
Scheme 31.
Cycloaddition of azomethine ylide 126, generated from pyrrolidine (124) and aromatic aldehydes 2, to a second molecule of aldehyde 2.

Table 17.
Cycloaddition of azomethine ylide 126 to aromatic aldehydes 2 (Scheme 31).
2.4. Reactions of Ylides from the Condensation of Secondary Alpha Amino Acid Esters with Carbonyl Compounds
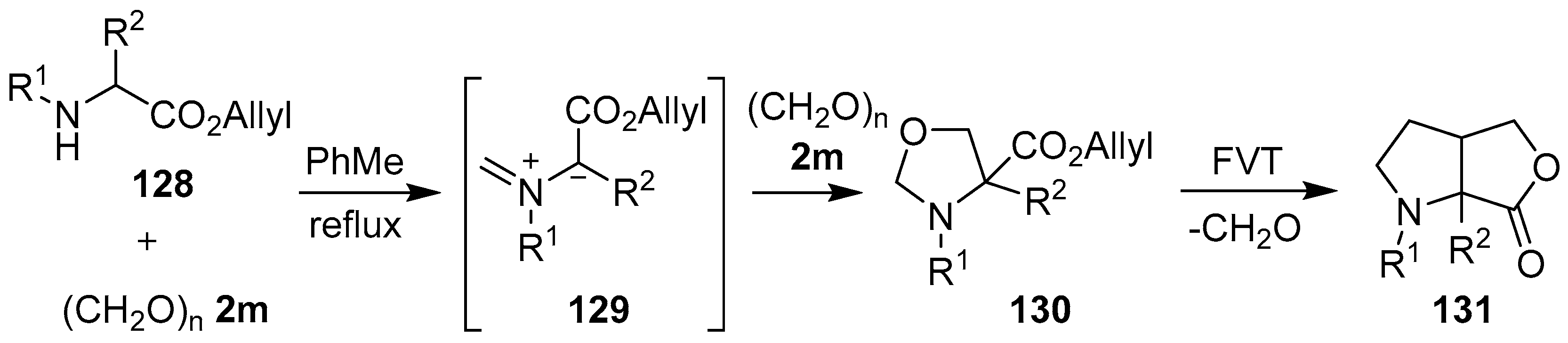
A simple approach to generate stabilized azomethine ylides is via the reaction of a secondary amine, bearing an α-carboxylic ester functionality, with an aldehyde, and the subsequent deprotonation of the resultant iminium ion. The first example of an azomethine ylide being generated in this manner and participating in a 1,3-dipolar cycloaddition with a carbonyl bond to form an oxazolidine ring, was published by Joucla and co-workers in 1987 [108]. In this report, the condensation of allylic α-amino esters 128 with paraformaldehyde (2m) presumably gave rise to the corresponding azomethine ylides 129, which underwent intermolecular cycloadditions with a second equivalent of paraformaldehyde (2m) to afford oxazolidines 130 in good yields. When these oxazolidines were subjected to flash vacuum thermolysis (FVT), a cycloreversion process occurred to regenerate azomethine ylide 129, which underwent an intramolecular cycloaddition reaction with the adjacent alkene to furnish pyrrolidines 131 (Scheme 32, Table 18).
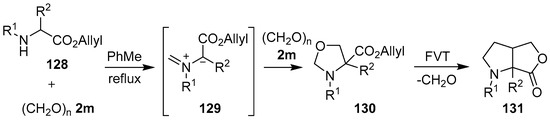
Scheme 32.
Cycloaddition of azomethine ylide 129, generated from allylic α-amino esters 128 and paraformaldehyde (2m), to a second molecule of paraformaldehyde (2m), and subsequent FVT-induced cycloreversion-intramolecular cycloaddition.

Table 18.
Cycloaddition of azomethine ylide 129 to formaldehyde (2m), and subsequent FVT-induced cycloreversion-intramolecular cycloaddition (Scheme 32).
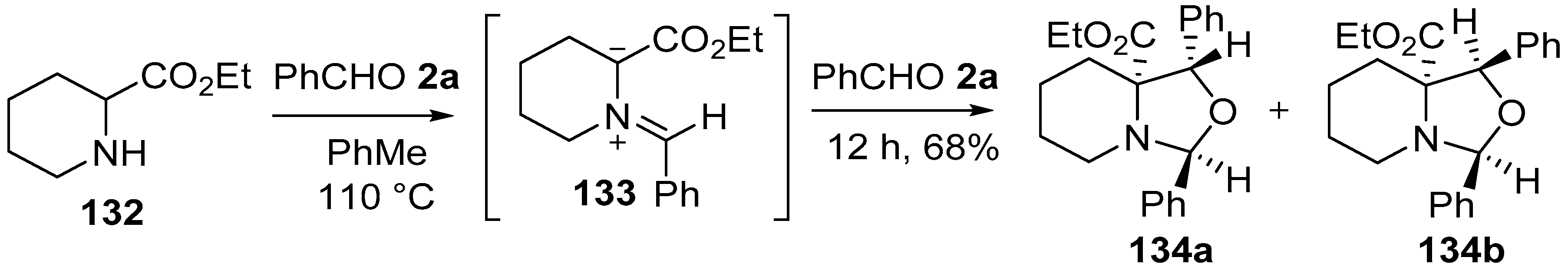
Around the same time, Joucla and co-workers reported that the azomethine ylide 133, prepared in situ from the condensation of pipecolic acid ethyl ester (132) and benzaldehyde (2a) underwent a 1,3-dipolar cycloaddition with a second molecule of benzaldehyde (2a) to form a 70:30 mixture of the oxaindolizidines 134a and 134b, respectively (Scheme 33) [109]. The stereoselectivity of the cycloaddition reaction was governed by both the conformation of the ylide, and the orientation at which the dipole and dipolarophile approached. Furthermore, the same workers disclosed the synthesis of a number of oxazolidines derived from reactions between proline, sarcosine and N-benzylglycinate methyl esters and two molar equivalents of formaldehyde or benzaldehyde [110].
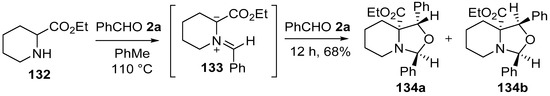
Scheme 33.
Cycloaddition of azomethine ylide 133, generated from pipecolic acid ethyl ester (132) and benzaldehyde (2a), to a second molecule of benzaldehyde (2a).
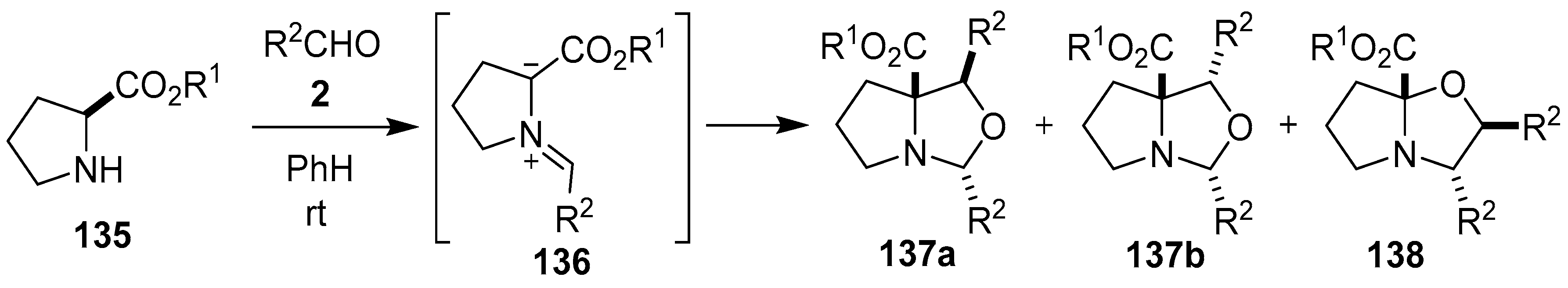
The reaction between l-proline alkyl esters 135 and aromatic aldehydes 2 generated the azomethine ylides 136, which were trapped by a second aldehyde molecule to give mostly mixtures of the oxapyrrolizidines 137a, 137b and 138 (Scheme 34, Table 19). The oxapyrrolizidines were prone to cycloreversion to regenerate azomethine ylides 136, and in the presence of a range of conjugated nitroolefins underwent cycloaddition to afford the corresponding pyrrolizidines [111]. A number of reactions of N-alkylated glycine ethyl esters and paraformaldehyde have also been reported, which were conducted neat and under microwave irradiation, to afford the corresponding oxazolidines in moderate yield [112]. During a reaction involving sarcosine methyl ester, formaldehyde and N-ethyl maleimide, the azomethine ylide generated from sarcosine methyl ester and formaldehyde was trapped by a second molecule of formaldehyde to give very small amounts of the corresponding oxazolidine cycloadduct [98].
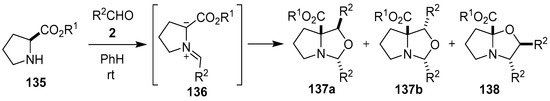
Scheme 34.
Cycloaddition of azomethine ylide 136, generated from L-proline alkyl esters 135 and aromatic aldehydes 2, to a second molecule of aldehyde 2.

Table 19.
Cycloaddition of azomethine ylide 136 to aldehydes 2 (Scheme 34).
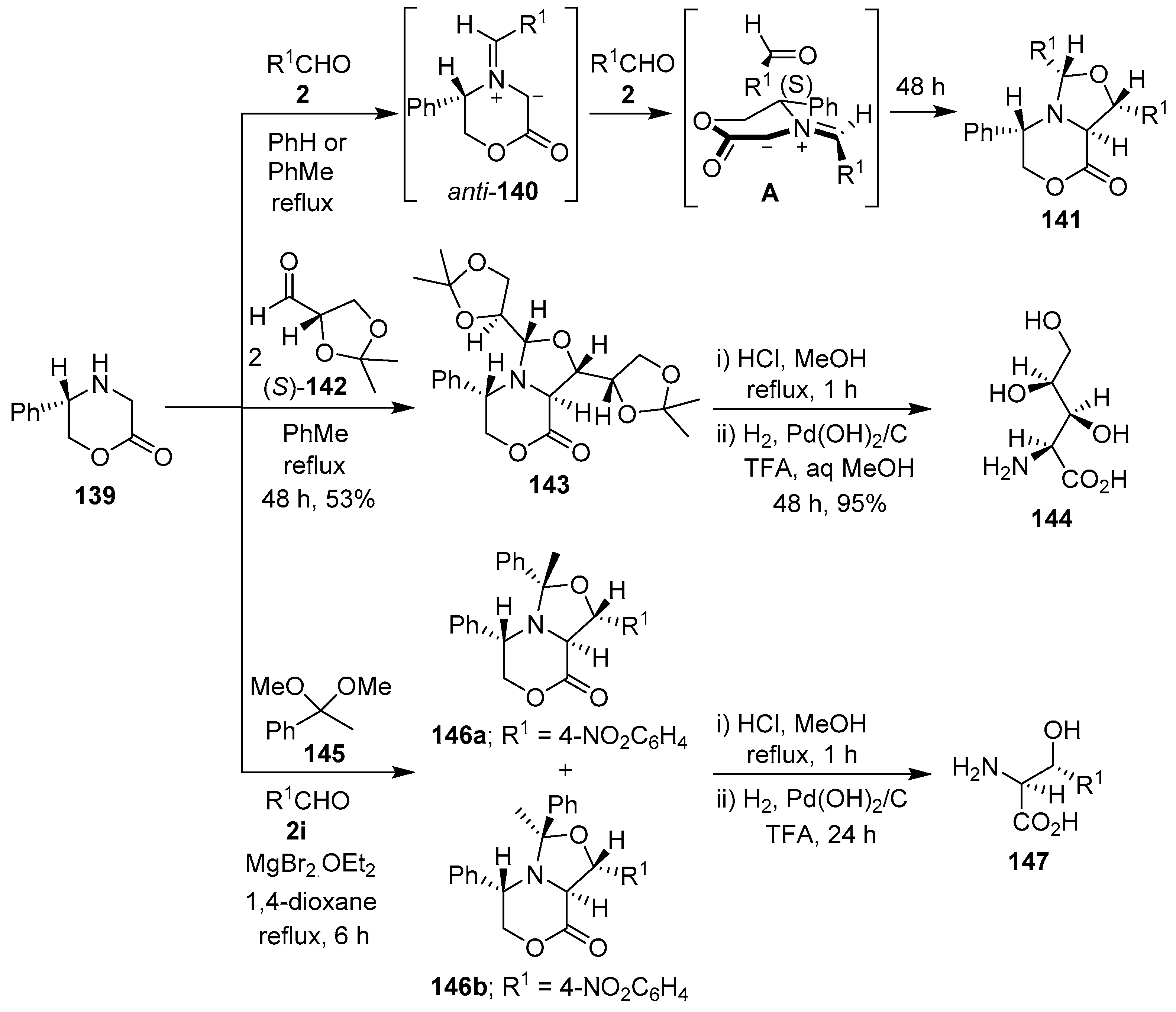
Harwood and co-workers have reported the diastereoselective synthesis of bicyclic oxazolidines via 1,3-dipolar cycloaddition reactions of azomethine ylides derived from the condensation of 5-(S)-phenylmorpholine-2-one (139) with aldehydes 2, and their subsequent trapping by the carbonyl bond of a second equivalent of the aldehyde 2. Thus, chiral morpholinone 139 reacted with aldehydes 2 to generate stabilized anti-azomethine ylides 140, which underwent highly diastereocontrolled cycloadditions with a second aldehyde 2 to afford cycloadducts 141. The high stereocontrol was rationalized by cycloaddition mode A involving the aldehyde dipolarophile approaching the least hindered side of the anti-azomethine ylide, and from the face opposite the 5-phenyl substituent (Scheme 35, Table 20). Subsequent hydrogenolysis of cycloadducts 141 formed the corresponding homochiral β-hydroxy-α-amino acids [113,114,115]. When morpholinone 139 was condensed with two equivalents of (S)-glyceraldehyde acetonide 142, a cycloaddition occurred that was diastereochemically ‘matched’ in both the ylide generation and trapping steps such that cycloadduct 143 was formed exclusively. Sequential hydrolysis and hydrogenolysis of cycloadduct 143 afforded polyoxamic acid 144 in an enantiomerically pure fashion (Scheme 35). In the same manner, the opposite enantiomer of polyoxamic acid was obtained by reacting (R)-139 with (R)-142, followed by sequential degradation of the cycloadduct [116]. Furthermore, a chiral azomethine ylide, derived from morpholinone 139 and acetophenone dimethyl acetal (145), underwent Lewis acid-promoted cycloaddition with 4-nitrobenzaldehyde (2i) to afford anti-exo 146a and syn-exo 146b cycloadducts in 42% and 18%, respectively. Hydrogenolysis of either cycloadduct afforded the same product, (2S,3R)-2-amino-3-hydroxy-3-(4-aminophenyl)propanoic acid (147) (Scheme 35), which confirmed that 146a and 146b were epimeric at C-9, the stereochemistry was retained at C-7, and that both syn- and anti-ylides were involved in the cycloaddition [115,117]. Having developed this methodology, Harwood’s research group also prepared enantiopure long chain threo-2-amino-3-hydroxyesters via chiral azomethine ylides derived from the reaction of 5-(R)-phenylmorpholine-2-one with long chain aldehydes, and their subsequent trapping with a second equivalent of aldehyde [118].
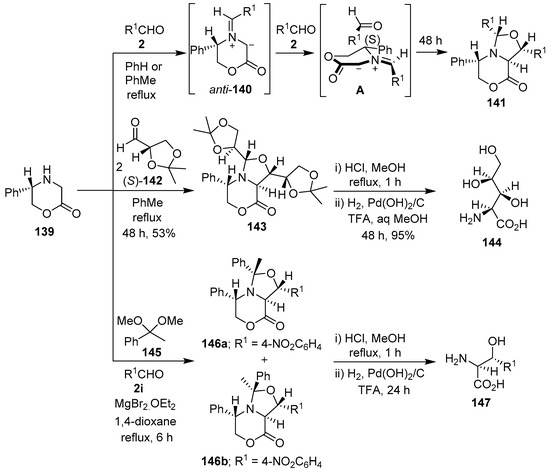
Scheme 35.
Synthesis of bicyclic oxazolidines via cycloaddition reactions of azomethine ylides, derived from 5-(S)-phenylmorpholine-2-one (139) and aldehydes 2, 142 or acetophenone dimethyl acetal (145), to a second equivalent of the same or a different aldehyde.

Table 20.
Cycloaddition of azomethine ylide 140 to aldehydes 2 (Scheme 35).
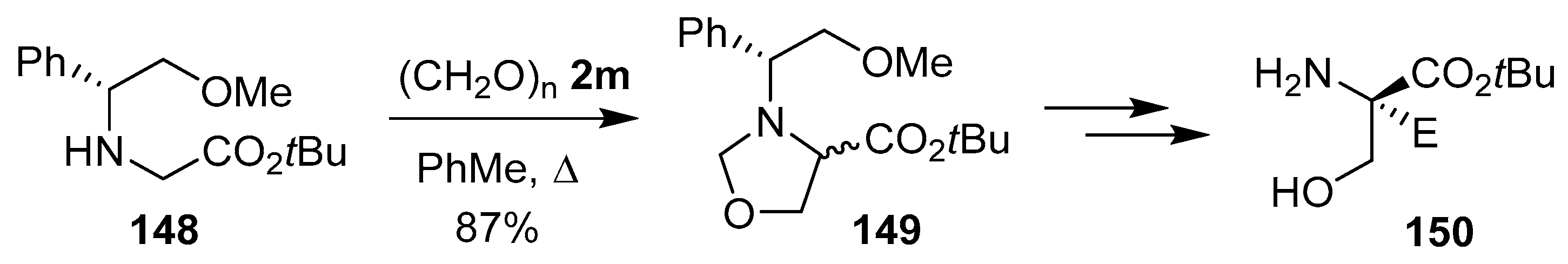
Oxazolidine 149, containing a chiral N-benzyl protecting group, was prepared as a 1:1 diastereomeric mixture from the reaction of glycine derivative 148 and paraformaldehyde (2m). Sequential diastereoselective functionalization of the corresponding oxazolidine ester enolate, then hydrolysis and hydrogenolysis afforded enantiomerically pure quaternary serine esters 150 (Scheme 36) [119].
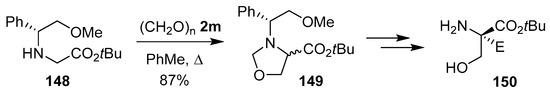
Scheme 36.
Reaction of glycine derivative 148 and paraformaldehyde (2m) to afford oxazolidine 149.
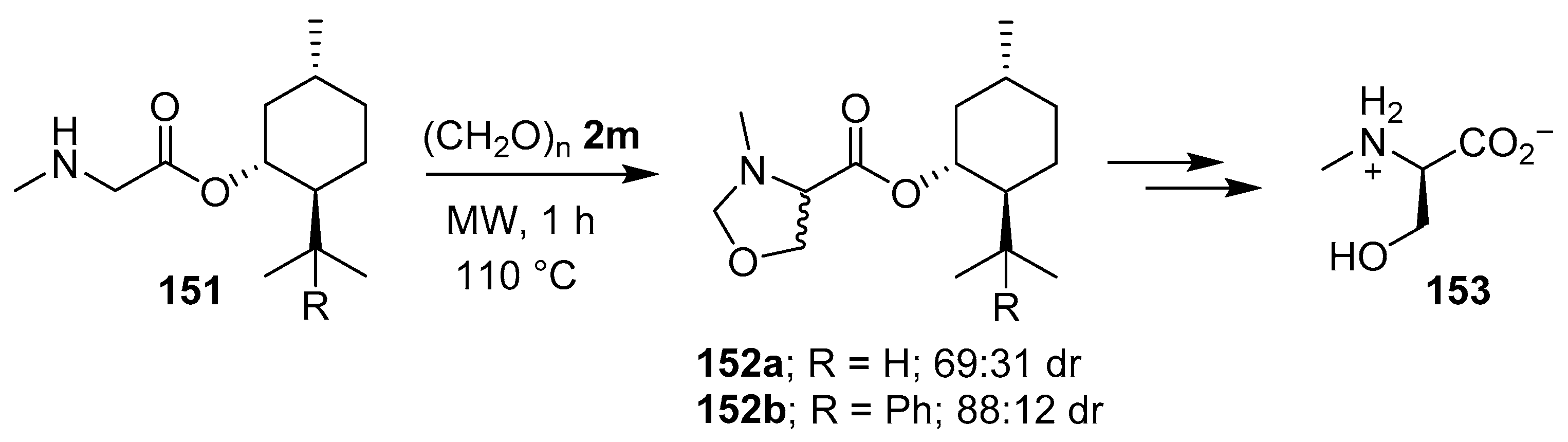
When sarcosine (−)-menthyl or (−)-8-phenylmenthyl esters, 151a and 151b respectively, were reacted with paraformaldehyde (2m) cycloaddition occurred in a diastereoselective fashion to give the respective oxazolidines 152a and 152b, which were exhaustively hydrolyzed to form predominantly N-methyl-d-serine (153) (Scheme 37) [120].
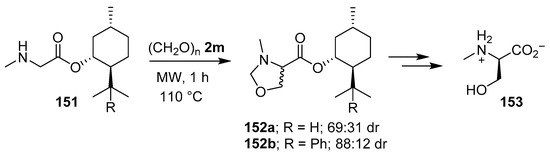
Scheme 37.
Reaction of sarcosine esters 151a and 151b with paraformaldehyde (2m) to afford oxazolidines 152a and 152b, respectively.
2.5. Reactions with Ylides Formed from Silylmethylamines and Related Reagents
A wide range of azomethine ylides generated from silylmethylamine reagents have been explored in reactions with carbonyl dipolarophiles.
2.5.1. Desilylation of (Trimethylsilylmethyl)iminium Ions
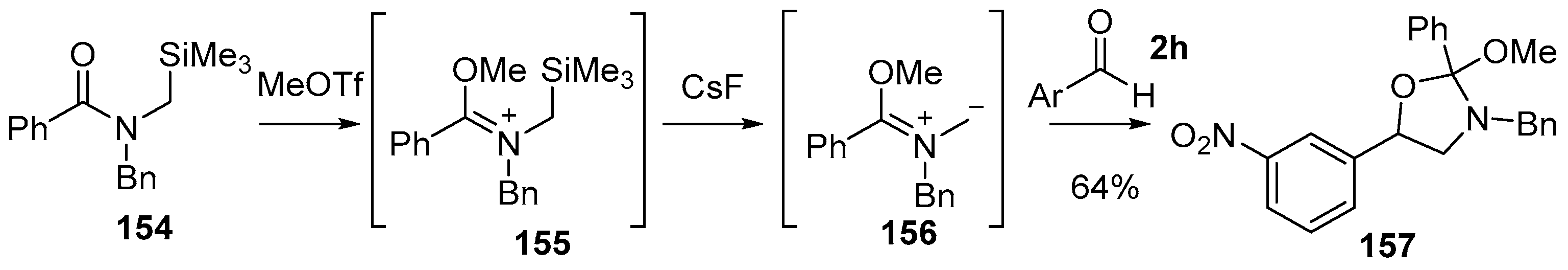
Padwa demonstrated that non-stabilized azomethine ylides formed by desilylation of N-(trimethylsilylmethyl)-immonium ions, a process initially developed by Vedejs [121], react with aldehydes 2 [122]. The reaction of N-(trimethylsilylmethyl)benzamide 154 with methyl triflate afforded the trimethylsilylmethyl iminium ion 155, which on treatment with CsF produced azomethine ylide 156, and in the presence of 3-nitrobenzaldehyde (2h) afforded cycloadduct 157 as the exclusive product, indicating a highly regioselective process (Scheme 38). The regioselectivity was determined by a combination of spectroscopic analyses and chemical transformation into a known derivative, however, no comment was made about the stereoselectivity. The exclusive formation of the isolated regioisomer was rationalised to be the result of the union of the larger azomethine ylide HOMO coefficient on the unsubstituted carbon of the ylide 156 with that of the larger dipolarophile LUMO coefficient on the carbon atom of the carbonyl group of the aldehyde 2h.
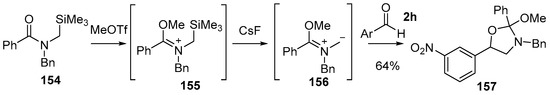
Scheme 38.
Cycloaddition of an azomethine ylide, generated from desilylation of a N-(trimethylsilylmethyl)-immonium ion, with 3-nitrobenzaldehyde (2h) (Ar = 3-NO2-C6H3).
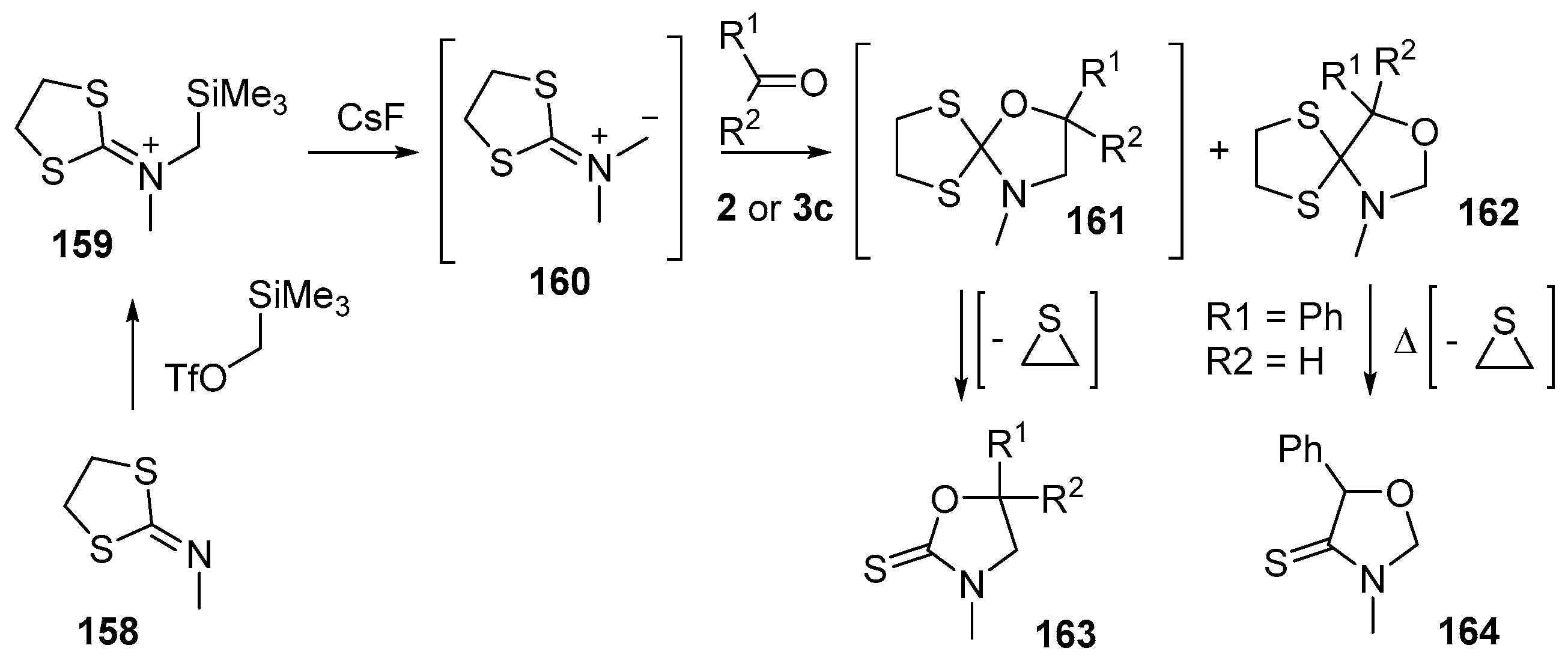
Dithiolane-isocyanate imminium methylides are interesting azomethine ylides that undergo efficient and regioselective cycloaddition to carbonyl compounds [123]. The azomethine ylide 160, produced from CsF-promoted desilylation of iminodithiolane salt 159 (in turn produced by the reaction of the readily available N-methylimino dithiolane (158) with trimethylsilylmethyl triflate), reacted with aldehydes 2 or benzophenone (3c) to give a mixture of regioisomeric cycloadducts 161 and 162 (Scheme 39, Table 21). While the minor adducts 162 could be isolated by silica chromatography, the major adducts 161 decomposed under these conditions, with loss of thiirane, to afford the isolated thiolactam 163. When the minor benzaldehyde cycloadduct 162a was heated in refluxing toluene for 48 h, it underwent an analogous decomposition to give thiolactam 164 in 48% yield. The preferred regioselectivity of the cycloaddition was thought to be a result of the union of the larger LUMO coefficient on the carbon of the carbonyl group with the larger HOMO coefficient on the unsaturated centre attached to the sulfur atoms of the dithiolane ring. Interestingly, it was noted that the cycloaddition only occurred with conjugated carbonyl compounds. For attempts at cycloaddition using simple non-conjugated aldehydes and ketones, e.g., acetone, cyclohexanone and butyraldehyde, no reaction was observed.
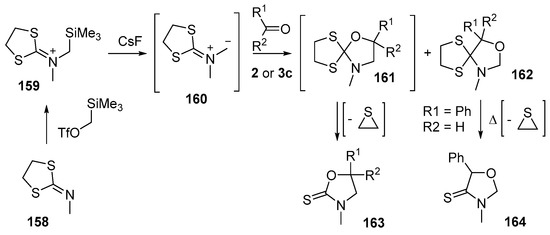
Scheme 39.
Cycloaddition of dithiolane-isocyanate immimium methylides 160 with aldehydes 2 and benzophenone (3c).

Table 21.
Cycloaddition of azomethine ylide 160 to aldehydes and a ketone (Scheme 39).
In a related study, reaction of azomethine ylide 160 with α,β-unsaturated ketones produced mainly cycloadducts of the carbon-carbon double bond, with small quantities (5%) of the ketone cycloadduct also isolated [124].
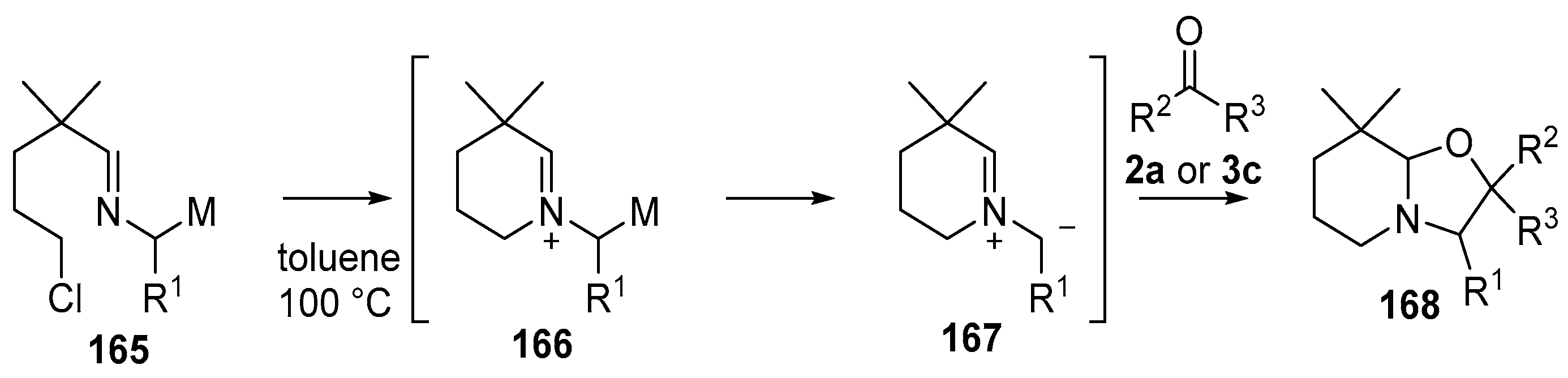
Non-stabilized azomethine ylides 167 were generated from (2-azaallyl)stannanes 165a or b or (2-azaallyl)silane 165c by an intramolecular N-alkylation/demetallation cascade [125,126]. The ylides 167 underwent reaction with a range of dipolarophiles including with benzaldehyde (2a) and benzophenone (3c) to afford bicyclic oxazolidines 168a–c (Scheme 40, Table 22).
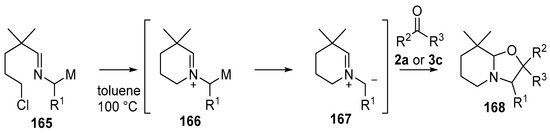
Scheme 40.
Cycloaddition of azomethine ylides, generated by an N-alkylation/demtallation ccascade, to aldehyde (2a) and benzophenone (3c).

Table 22.
Cycloaddition of azomethine ylides 167 to aldehyde 2a and ketone 3c (Scheme 40).
2.5.2. Desilylation of α-Substituted Methyl(trimethylsilylmethyl)amines
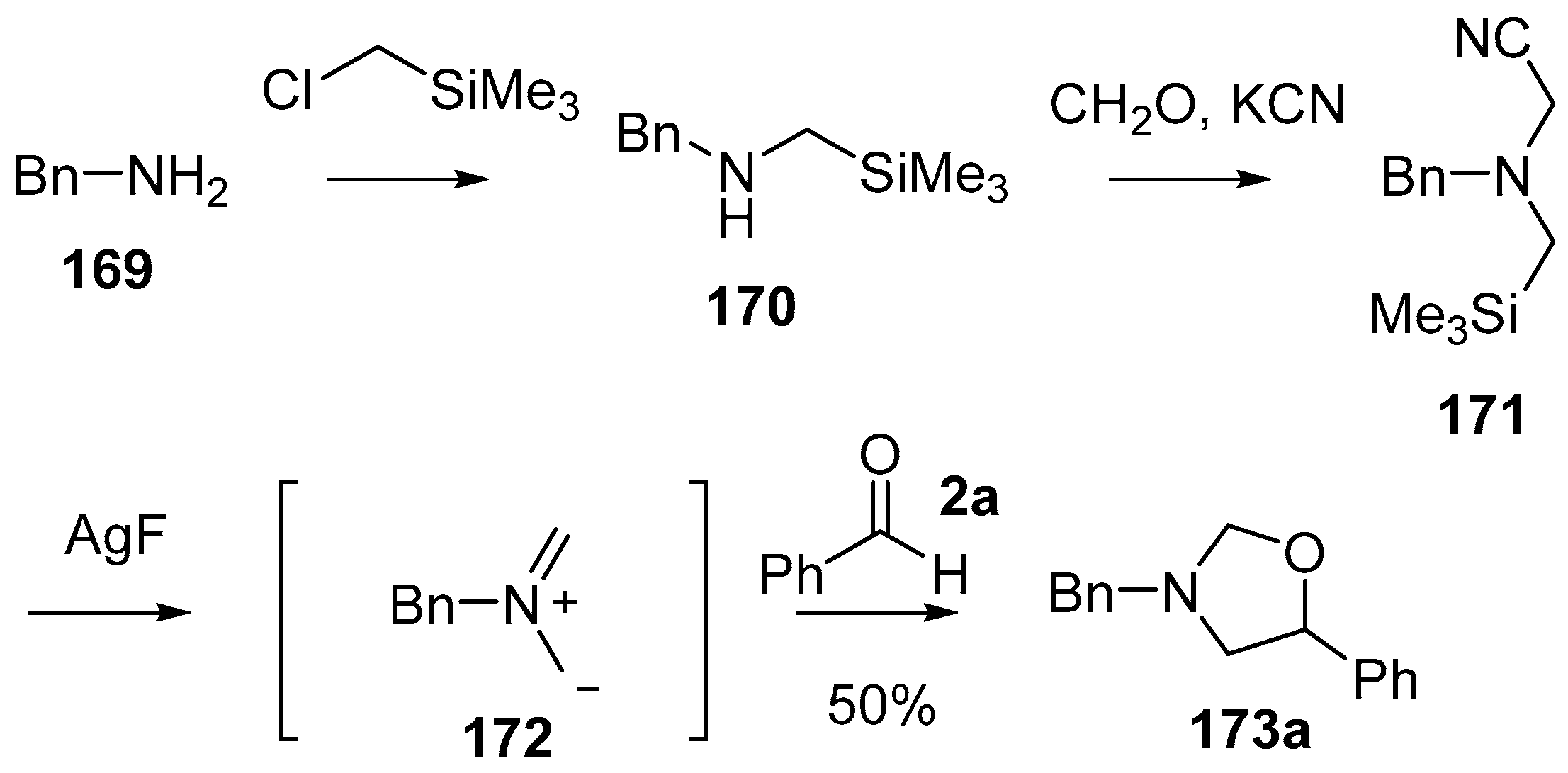
A series of α-substituted methyl(trimethylsilylmethyl)amine reagents were developed and shown to act as azomethine ylide precursors, adding to a range of dipolarophiles including carbonyl compounds. The first of these reagents to be explored in carbonyl cycloadditions was the cyanomethylamine reagent 171 [127] which was efficiently prepared from benzylamine (169) by firstly reacting with (chloromethyl)trimethylsilane and then formaldehyde and KCN (Scheme 41). Treatment of reagent 171 with AgF produced the non-stabilized azomethine ylide 172, which was trapped with a range of dipolarophiles including benzaldehyde (2a) which produced oxazolidine 173a, isolated in 50% yield (Scheme 41).
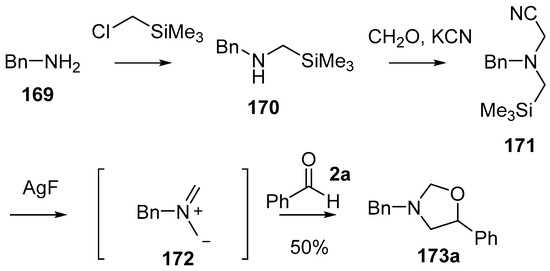
Scheme 41.
Cycloaddition of a non-stabilized ylide, derived from cyanomethylamine reagent 171, with benzaldehyde (2a).
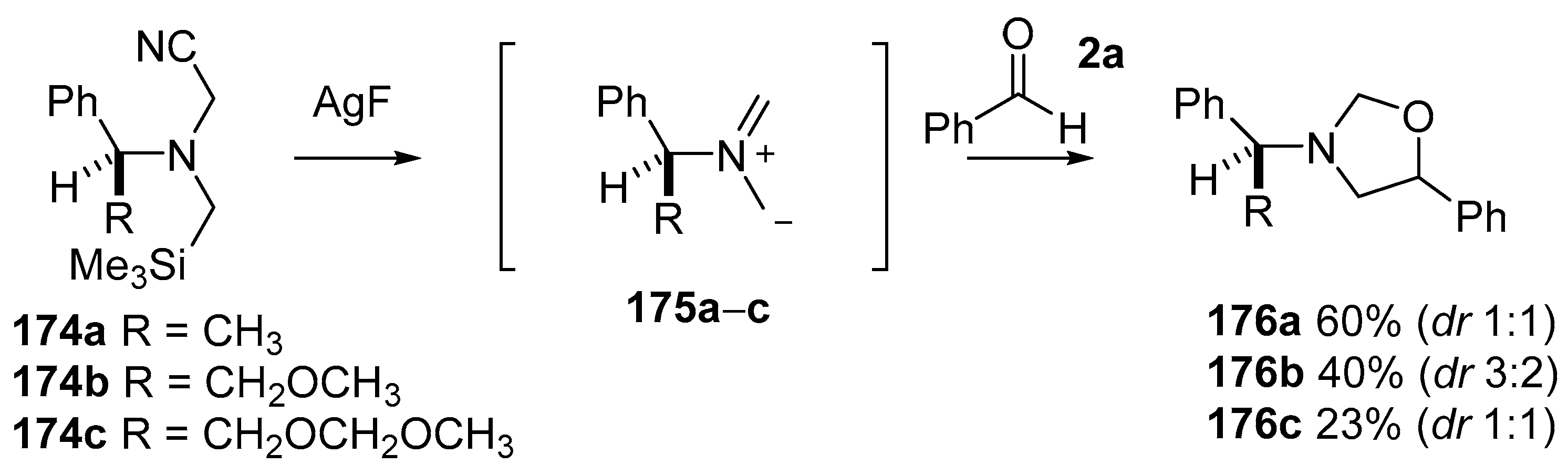
A series of chiral cyanoaminosilanes 174a–c were produced, using the same methods as used to prepare 171, to explore potential for asymmetric cycloaddition reactions [127]. Although cycloaddition reactions between the in situ produced ylides 175a–c and benzaldehyde (2a) did occur to give oxazolidines 176a–c respectively, little if any diastereoselectivity was observed for this carbonyl dipolarophile (Scheme 42). In contrast, improved and at times promising diastereoselectivity was observed for olefinic dipolarophiles.
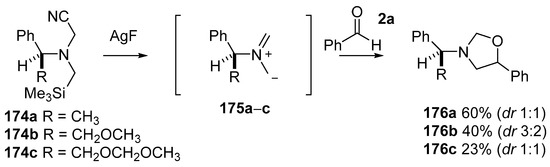
Scheme 42.
Cycloaddition of chiral non-stabilized ylides, derived from cyanomethylamine reagents 175, with benzaldehyde (2a).
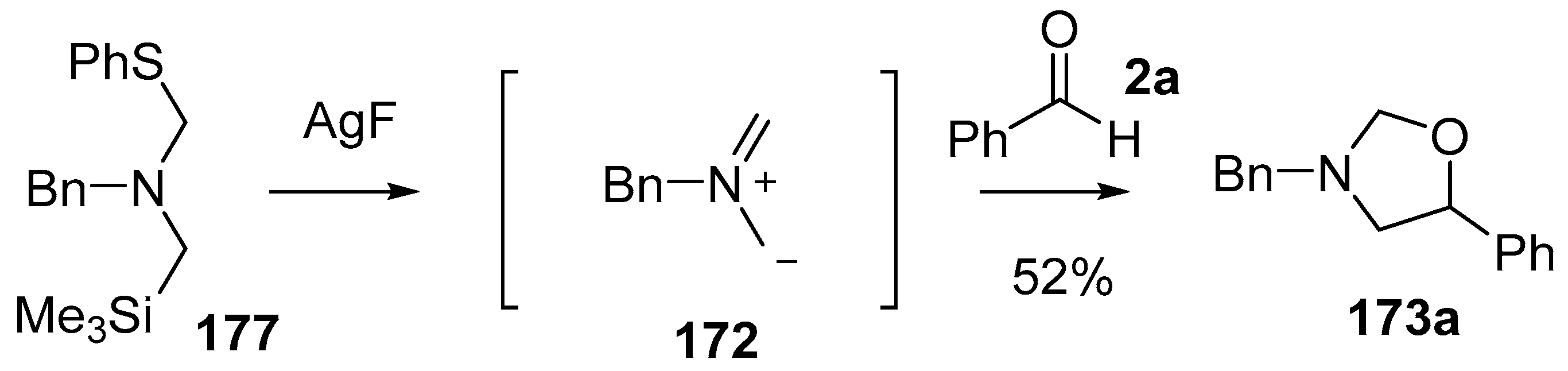
The related phenylthio-substituted amine 177 was produced from benzylamine by reacting with chloromethyltrimethylsilane followed by paraformaldehyde and thiophenol [128]. In a similar way to the cyanoaminosilane reagents 171 and 174, when treated with AgF, reagent 177 released non-stabilized azomethine ylide 172 and the ylide formed in this way showed utility in cycloaddition reactions with a range of dipolarophiles including benzaldehyde (2a), in which case oxazolidine 173a was produced in 52% yield (Scheme 43).
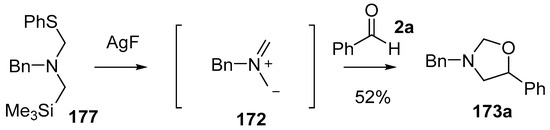
Scheme 43.
Cycloaddition of non-stabilized ylide 172, derived from phenylthiomethylamine reagent 177, with benzaldehyde (2a).
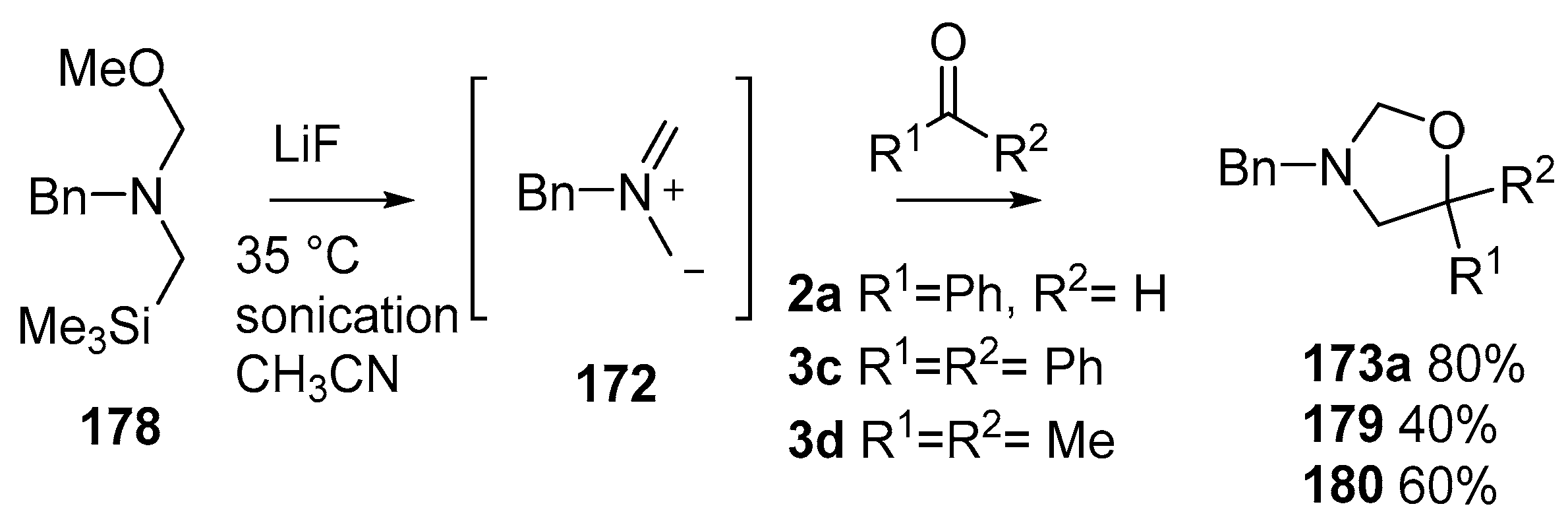
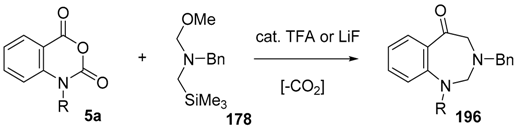
By far the most studied and utilized of the α-substituted methyl(trimethylsilylmethyl)amine reagents is the N-methoxymethyl-substituted reagent 178 [129,130,131], readily produced from benzylamine by alkylation with chloromethyltrimethylsilane followed by reaction with formaldehyde in the presence of methanol [132]. The reagent is now commercially available from various vendors. The advantages of this reagent include that the ylide 172 can be produced by treatment with LiF, without the use of Ag salts, by sonication at 35 °C in acetonitrile and when reacted with benzaldehyde (2a), cycloadduct 173a was obtained in a higher yield than that obtained with the previous reagents. When 178 was reacted in this way with ketones, benzophenone (3c) or acetone (3d), moderate yields of the corresponding cycloadducts 179 or 180 were obtained (Scheme 44).
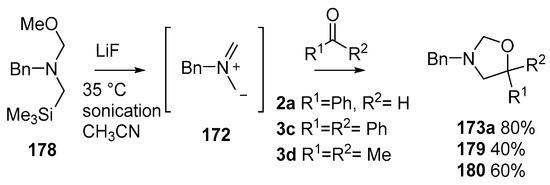
Scheme 44.
Cycloaddition of non-stabilized ylide 172, derived from methoxymethylamine reagent 178, with benzaldehyde (2a) and ketones 3c and 3d.
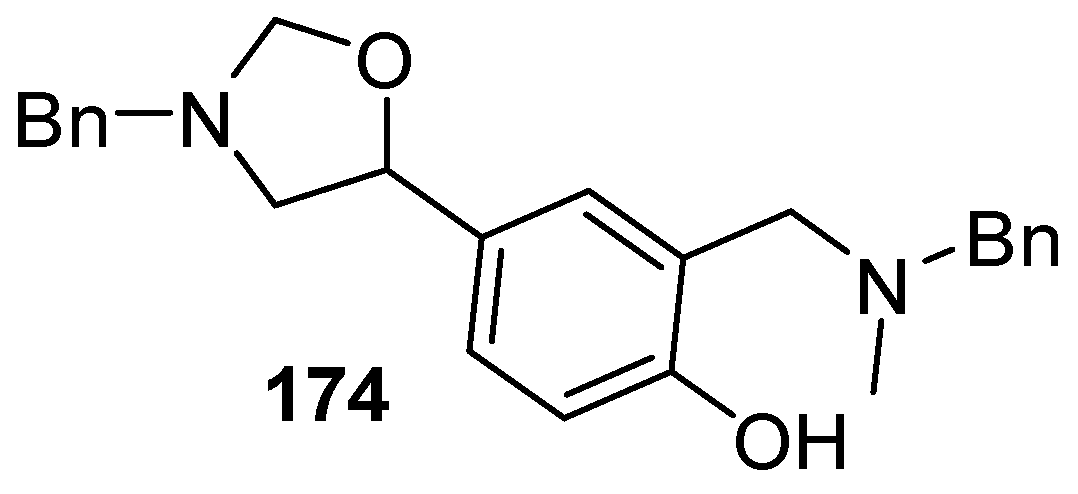
Recently, a thorough exploration of the reactions of reagent 178 with aromatic and heteroaromatic aldehydes 2 was reported [133]. Firstly, it was found that generating the ylide by trifluoroacetic acid catalysis [131] in CH2Cl2 at 25 °C in the presence of benzaldehyde (2a) afforded a 95% yield of the oxazolidine cycloadduct 173a (Scheme 45) (Table 23, entry 1), a higher yield than that reported using LiF to generate the ylide from reagent 178 [129]. The trifluoroacetic acid catalysis method was then applied to a range of aromatic aldehydes 2 (Table 23). High yields of cycloadducts 173 were obtained in the cases of benzaldehydes substituted with electron-withdrawing (entries 2–7), electron-donating (entries 8–11, 14), sterically demanding (entries 5 and 12), basic (entry 8) and acidic (entry 13) groups. Chemoselectivity was seen in the case of the 4-cyano example (entry 7) with only the product from cycloaddition to the aldehyde moiety being obtained. There were some limitations, in that 3-hydroxybenzaldehyde (2s) afforded complex mixtures and oxazolidine 173o could not be detected. Additionally, for 4-hydroxybenzaldehyde (2t), the main product was the bis adduct 174, isolated in 53% yield (Figure 2).
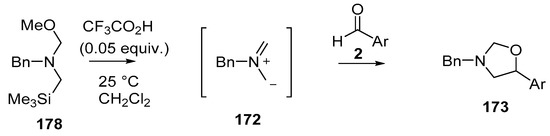
Scheme 45.
Cycloaddition of non-stabilized ylide 172, generated by CF3CO2H-catalyzed decomposition of methoxymethylamine reagent 178, with aromatic aldehydes 2.

Table 23.
Cycloaddition of azomethine ylide 172, formed from silylamine reagent 178, with aromatic aldehydes 2 (Scheme 45).
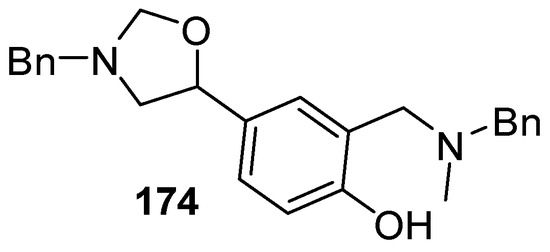
Figure 2.
The bis adduct isolated from the reaction of 4-hydroxybenzaldehyde (2t, Table 23, entry 16).
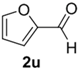
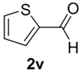
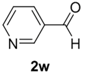
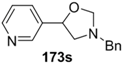
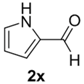
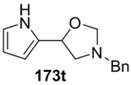
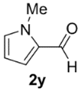
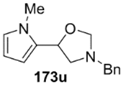
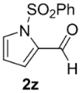
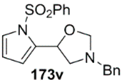
The cycloaddition of ylide 172, generated by trifluoroacetic acid catalyzed decomposition of silylamine reagent 178, to a range of heteroaromatic aldehydes 2 was also explored with the only limitation appearing to be electron-rich aromatic aldehydes (Table 24) [133]. 2-Furan-, 2-thiophene- and 3-pyridine carboxaldehydes (2u–w) all underwent efficient cycloaddition under these conditions (Entries 1–3). In contrast, experiments with pyrrole-2-carboxaldehyde (2x) and N-methylpyrrole-2-carboxaldehyde (2y) afforded complex intractable product mixtures with no sign of oxazolidine products (Entries 4 and 5). The lack of effective cycloaddition under these conditions was thought to be due to the electron-rich nature of the pyrrole leading to deactivation of the pyrrole via increasing the carbonyl LUMO energy with concomitant increase in the carbonyl LUMO-azomethine ylide HOMO energy gap. Consistent with this rationale, when the pyrrole contained the electron-withdrawing N-benzenesulfonyl group (compound 2z), which was expected to lower the carbonyl LUMO energy, a high yield of the oxazolidine product 173v was obtained.

Table 24.
Cycloaddition of azomethine ylide 172, formed from silylamine reagent 178, with heteroaromatic aldehydes 2 (Scheme 45).
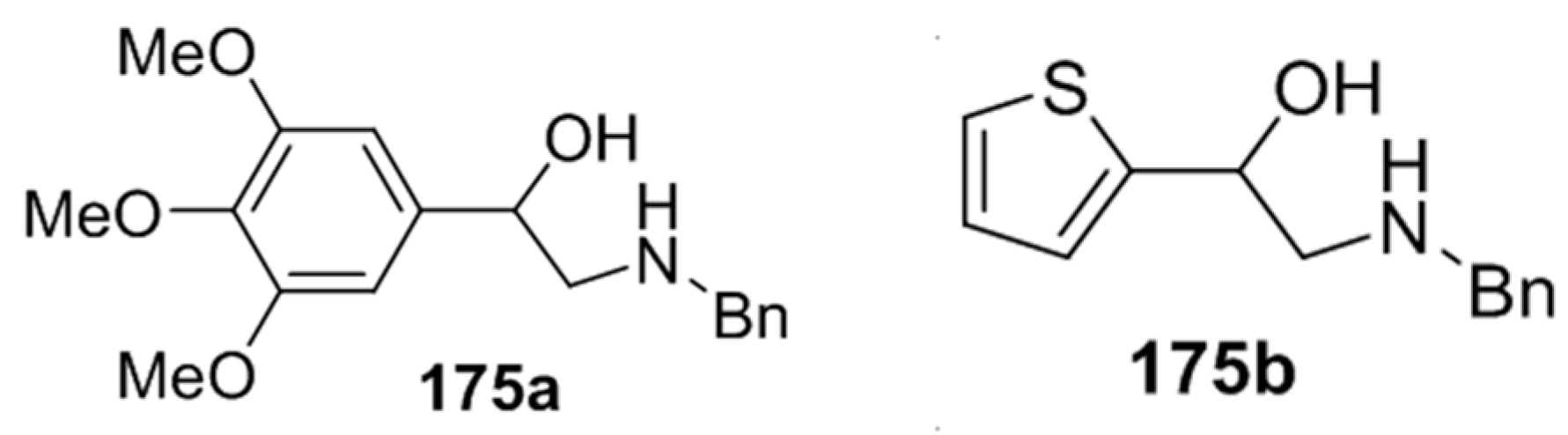
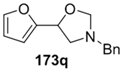
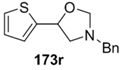
Recently, 5-aryloxazolidines 173, formed under the above conditions, were applied to the synthesis of β-hydroxy-β-arylethylamines [87]. The oxazolidines 173j and 173r underwent hydrazinolysis leading to the trimethoxyphenyl- and thiophenyl derivatives 175a and 175b, isolated in 68% and 71%, respectively (Figure 3).
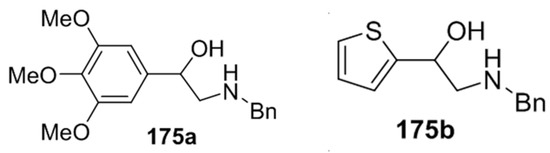
Figure 3.
Products from hydrazinolysis of oxazolidines 173j and 173r.
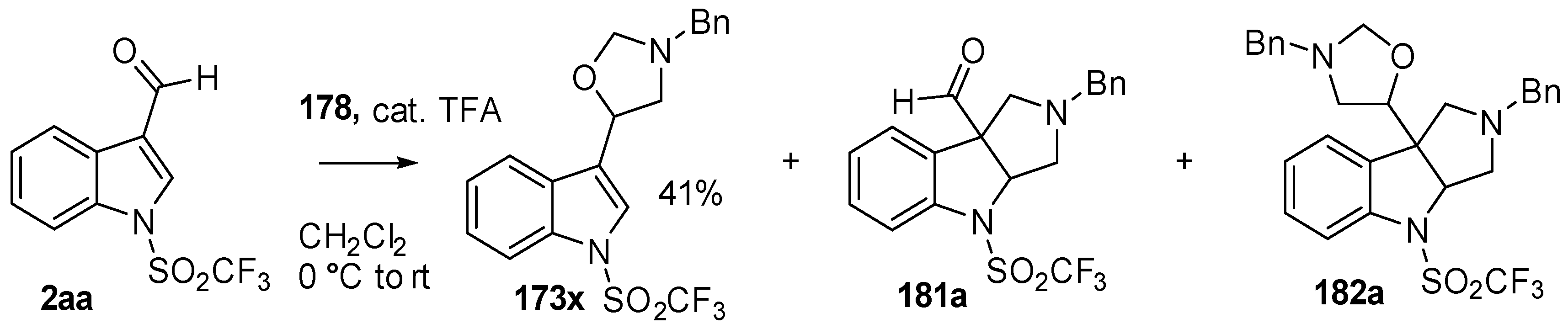
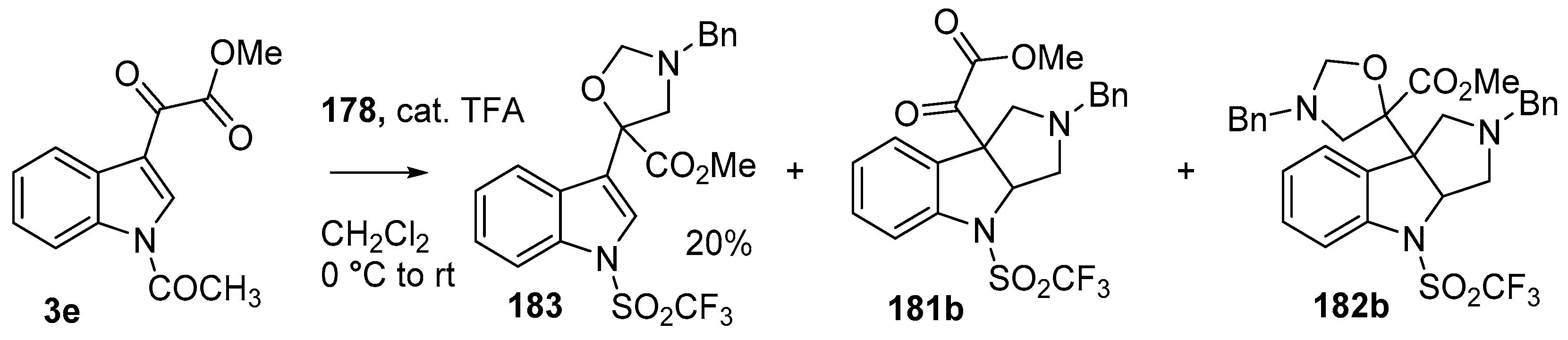
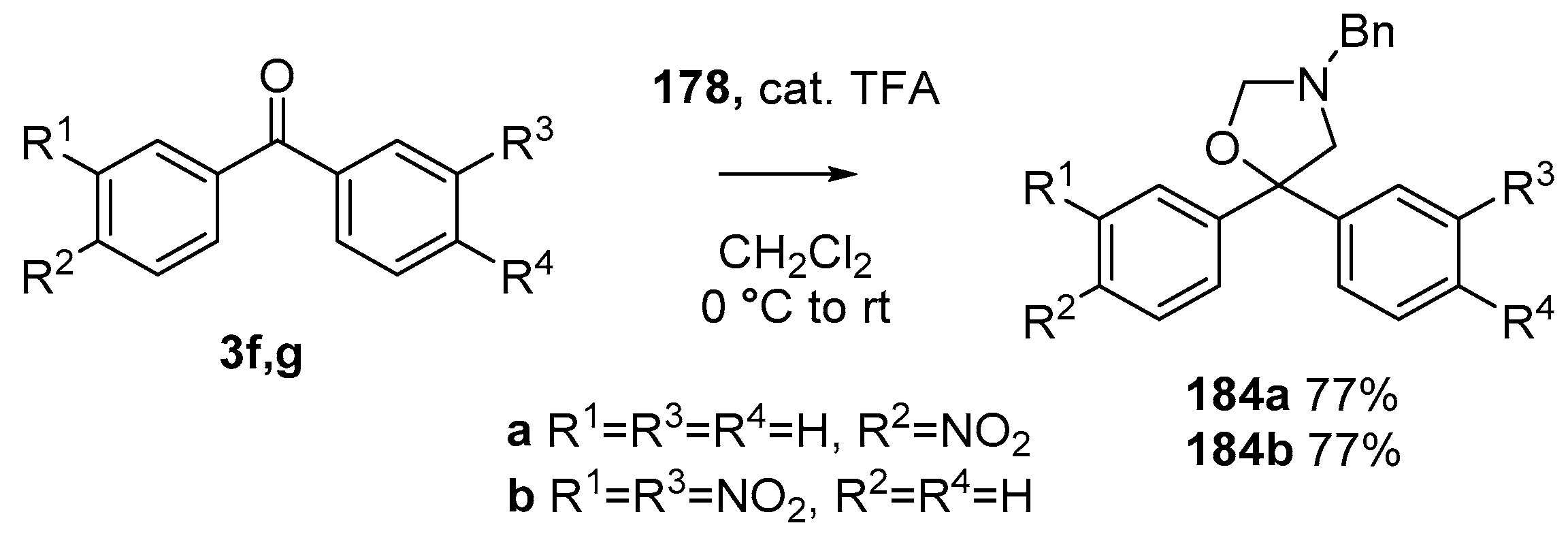
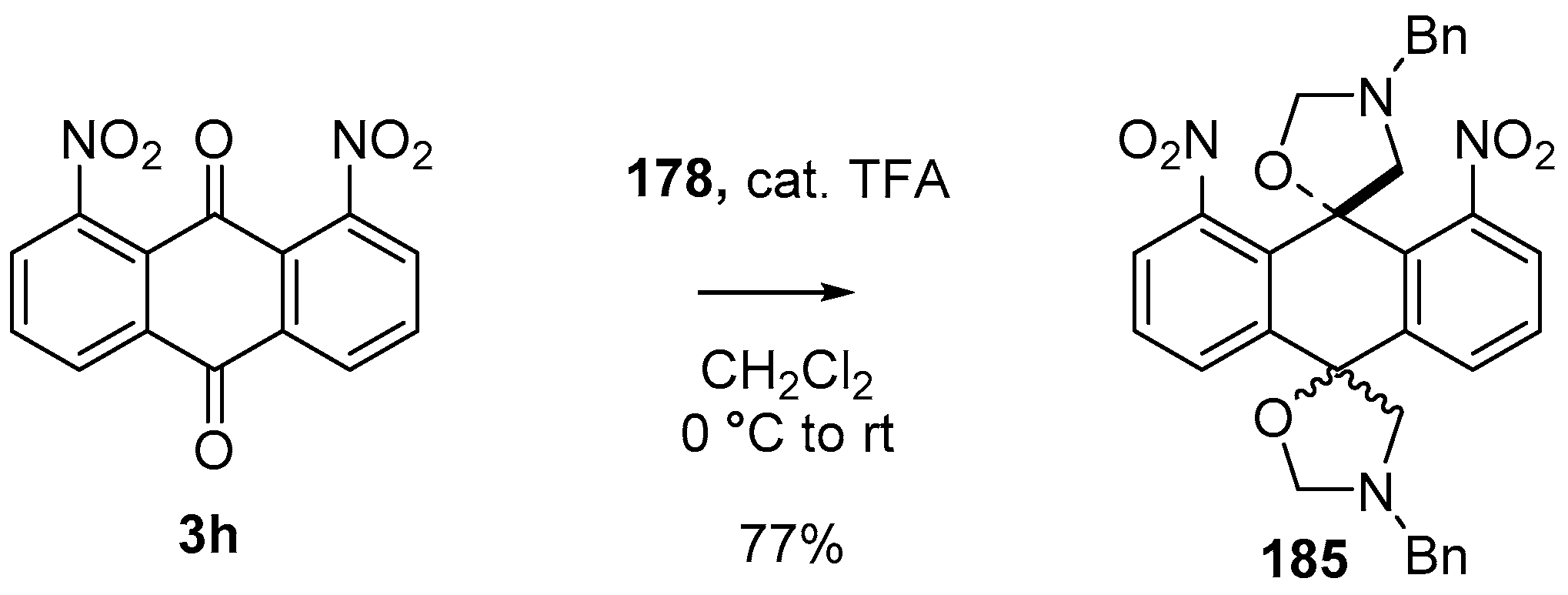
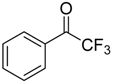
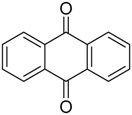
During a recent study into the dearomatization of electron-deficient arenes and heteroarenes by azomethine ylide cycloadditions, competing cycloadditions to aldehydes and ketones were occasionally observed [134]. The reaction of N-triflylindole-3-carboxaldehyde (2aa) with azomethine ylide 172, formed by trifluoroacetic acid-catalyzed decomposition of reagent 178, afforded a mixture of three products in a 6:2:2 ratio. The major product produced was the mono aldehyde adduct 173x (isolated in 41% yield) with the balance of the product being an inseparable mixture of mono indole C2-C3 adduct 181a and a bis-adduct 182a formed by addition to both carbonyl and indole C2-C3 double bonds (Scheme 46). Similarly, methyl N-acetyl-indole-3-pyruvate (3e) afforded a similar distribution of a mono ketone adduct 183 (70% of crude product, 20% isolated yield) along with a mixture of C2-C3 double bond adduct 181b and bis-adduct 182b (~30% of crude product) (Scheme 47). The reaction of the same ylide with 4-nitro- and 3,3′-dinitrobenzophenones (3f) and (3g) resulted in exclusively ketone adducts 184a and 184b both isolated in 77% yield (Scheme 48). In a further example, 3,3′-dinitroanthroquinone (3h) afforded bis ketone adduct 185 isolated in 66% yield as a 9:1 mixture of diastereoisomers (Scheme 49). These studies indicate the relatively greater reactivity of such aldehyde and ketone groups over the nitroarene in these particular systems.
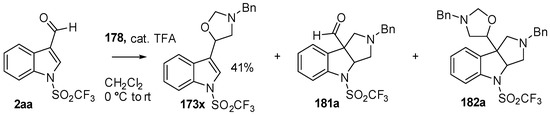
Scheme 46.
Cycloaddition of azomethine ylide 172, formed from silylamine reagent 178, with indole-3-carboxaldehyde 2aa.
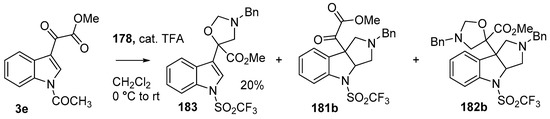
Scheme 47.
Cycloaddition of azomethine ylide 172, formed from silylamine reagent 178, with indole-3-pyruvate 3e.
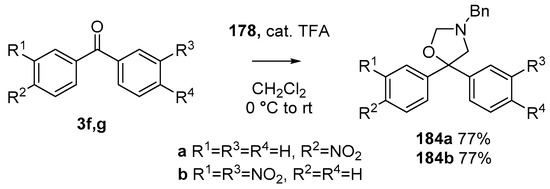
Scheme 48.
Cycloaddition of azomethine ylide 172, formed from silylamine reagent 178, with benzophenones 3f and 3g.
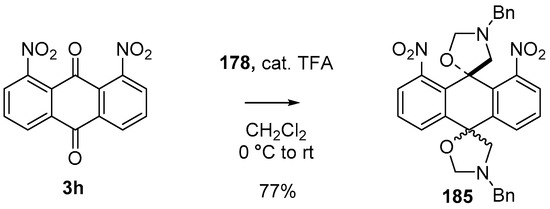
Scheme 49.
Cycloaddition of azomethine ylide 172, formed from silylamine reagent 178, with anthroquinone 3h.
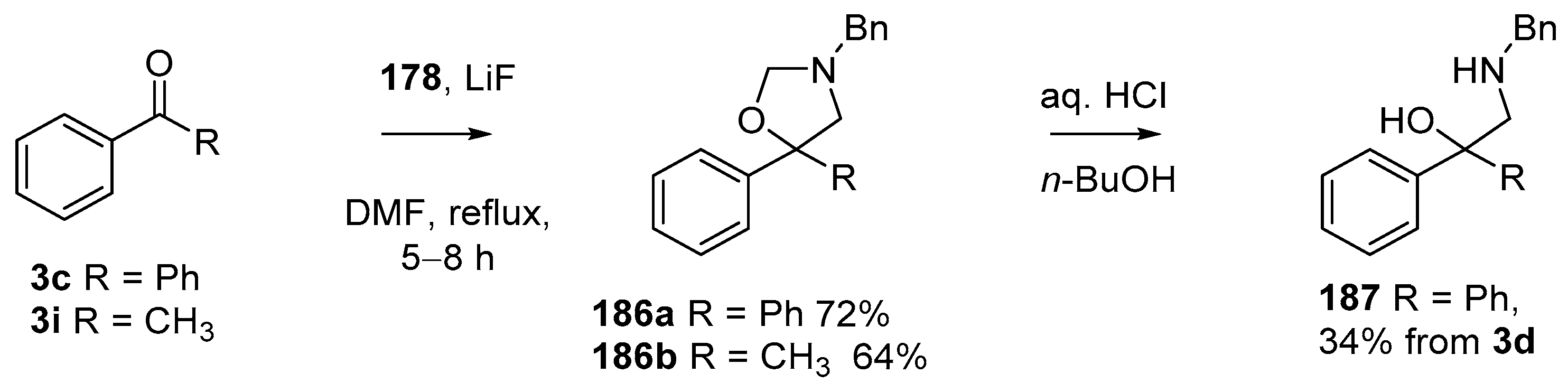
Using modified conditions (LiF, DMF, reflux), reagent 178 has been employed in azomethine ylide cycloadditions to benzophenone (3c) and acetophenone (3i), to produce good yields of the corresponding oxazolidines 186a and 186b, respectively (Scheme 50) [90]. Acid catalyzed hydrolysis of 186a provided access to 2-amino-1,1-diphenylethylalcohol derivative 187.
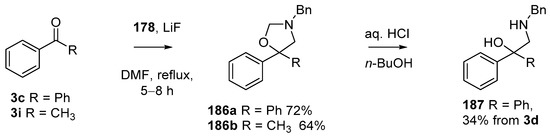
Scheme 50.
Cycloaddition of azomethine ylide 172, formed from silylamine reagent 178, with aromatic ketones 3c and 3i.
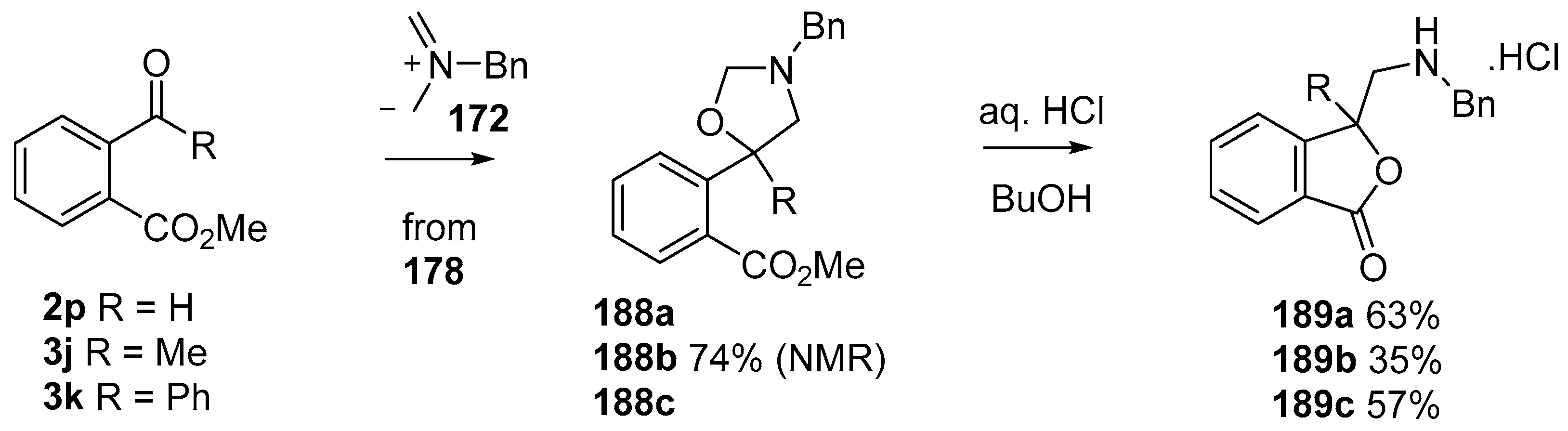
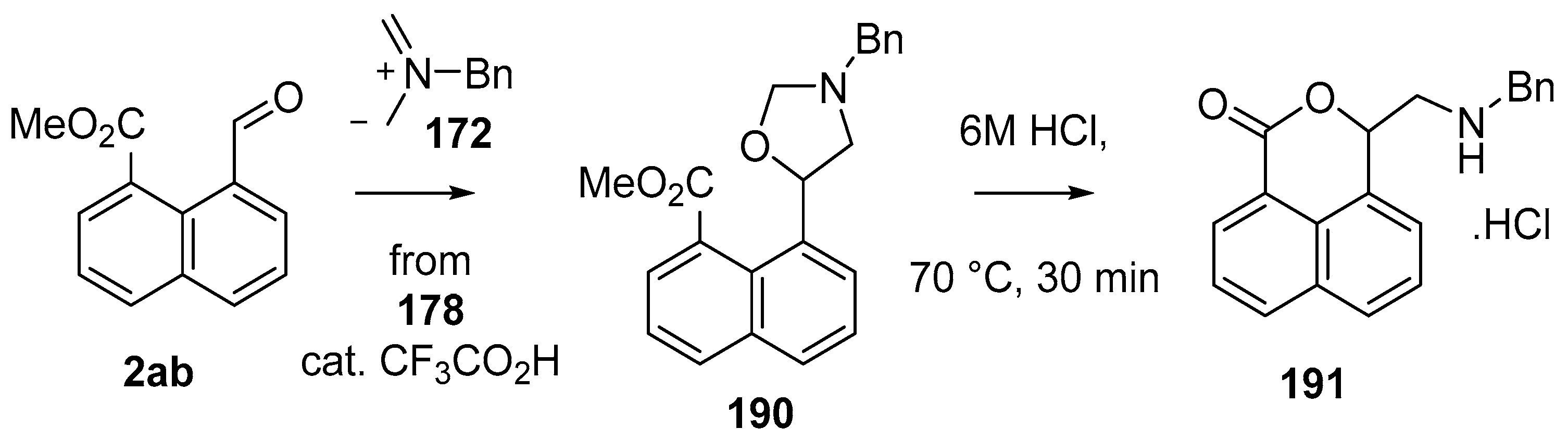
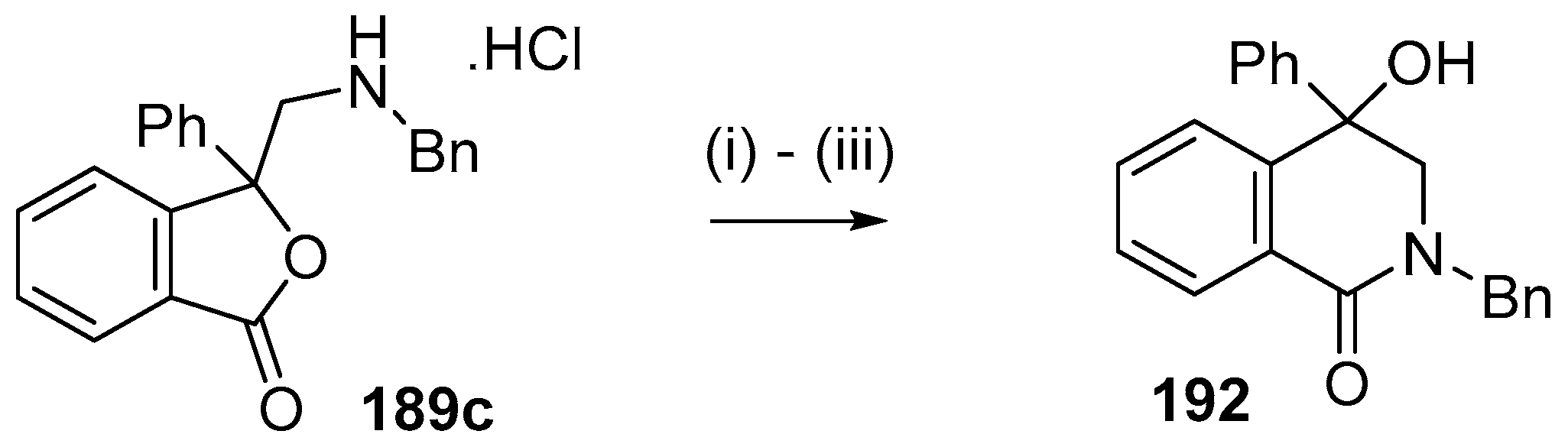
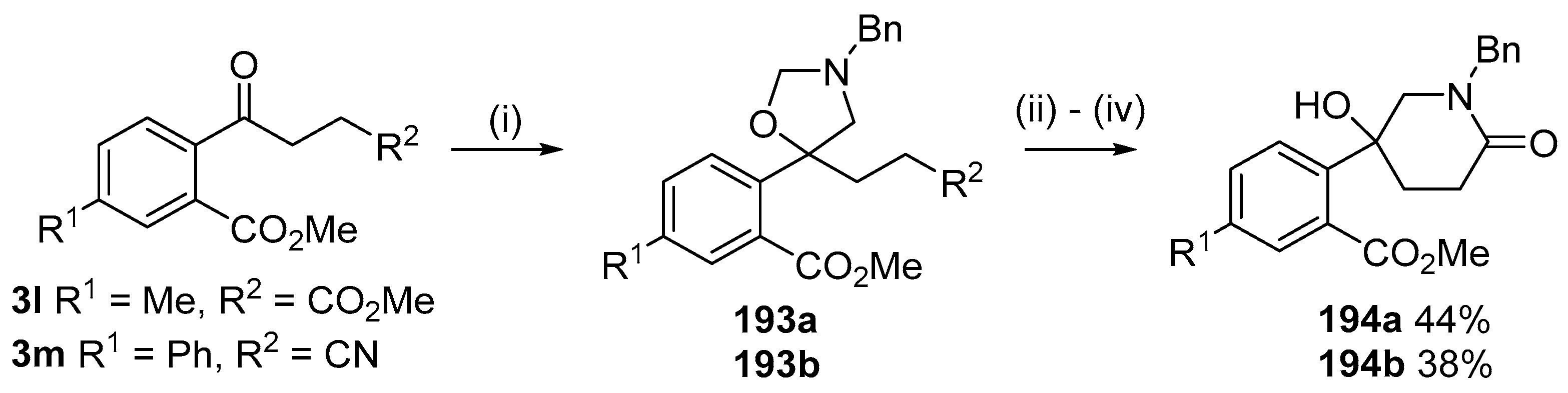
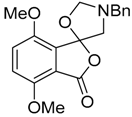
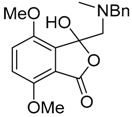
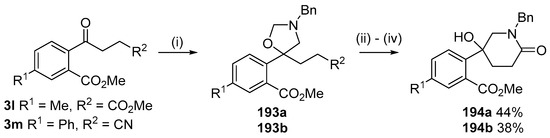
This alkylaminomethylation chemistry was applied to aryl aldehyde and aryl ketone derivatives to produce alkylaminomethylated species that could undergo further transformations to produce alkylaminomethylphthalides (Scheme 51) [91]. Methyl 2-formyl benzoate (2p) underwent cycloaddition reaction with azomethine ylide 172 generated by trifluoroacetic acid catalyzed decomposition of reagent 178 to give a quantitative yield of oxazolidine 188a which underwent hydrolysis and cyclization under acidic conditions at 70 °C to give phthalide 189a. The less reactive dipolarophile methyl ester of 2-acetylbenzoic acid (3j) gave low yields of the oxazoldine 188b under conditions involving trifluoroacetic acid catalysis; however, with the use of excess LiF in refluxing DMF a 74% yield of oxazoldine 188b was obtained. Acidic hydrolysis and heating at 90 °C afforded phthalide 189b in 35% yield from the ketone starting material. Similar treatment of the methyl ester of 2-benzoylbenzoic acid (3k) afforded phthalide 189c in 57% yield. In the case of the methyl ester of 8-formyl-naphthalene-1-carboxylic acid (2ab), the alkylaminomethyl lactone 191 was obtained in 40% yield (Scheme 52). Neutralisation of phthalide 189c followed by heating in refluxing butanol gave a 60% yield of the corresponding cyclized dihydroisoquinolinone 192 (Scheme 53). In related cycloaddition-hydrolysis-cyclization processes, the aryl ketone derivatives 3l and m were elaborated into the respective 4-aryl-4-hydroxypiperidone derivatives 194a and b (Scheme 54).
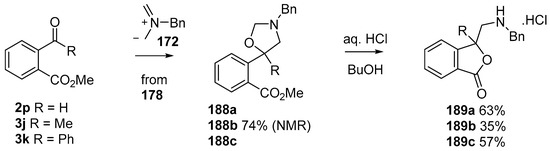
Scheme 51.
Cycloaddition of azomethine ylide 172, formed from silylamine reagent 178, with aromatic aldehyde 2p and aromatic ketones 3j and 3k.
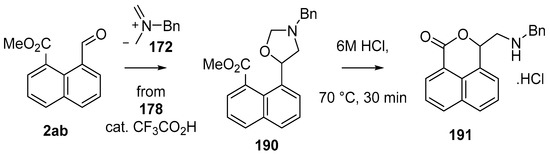
Scheme 52.
Cycloaddition of azomethine ylide 172, formed from silylamine reagent 178, with aromatic aldehyde 2ab.
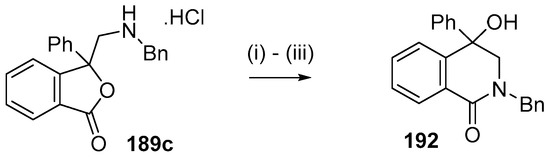
Scheme 53.
Lactamization of isobenzofuranone 189c. Reaction Conditions: (i) NaHCO3; (ii) NaOH; then NH4Cl; (iii) n-BuOH, reflux.
Scheme 54.
Cycloaddition of ylide 172 with aromatic ketones 3l and 3m followed by hydrolysis and condensation reactions to afford piperidones 194. Reaction Conditions: (i) 178 (2.5 equiv.), LiF, DMF, reflux, 5 h; (ii) aq. HCl, n-BuOH, 90 °C; (iii) NH4Cl;(iv) n-BuOH, reflux.
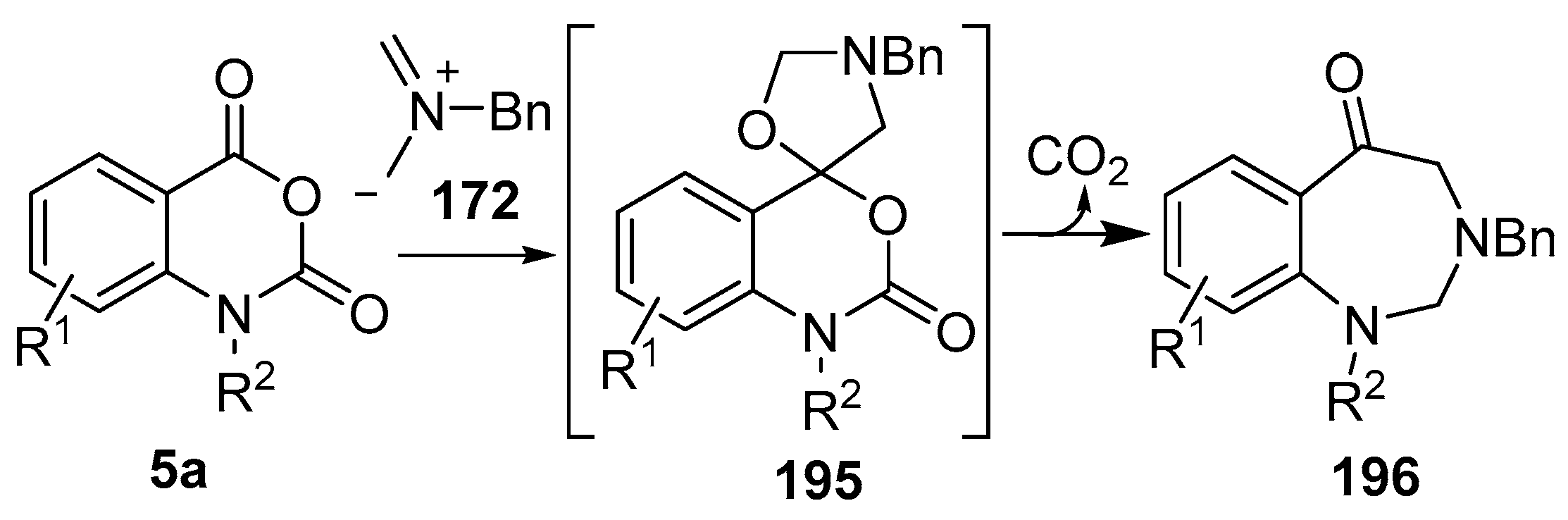
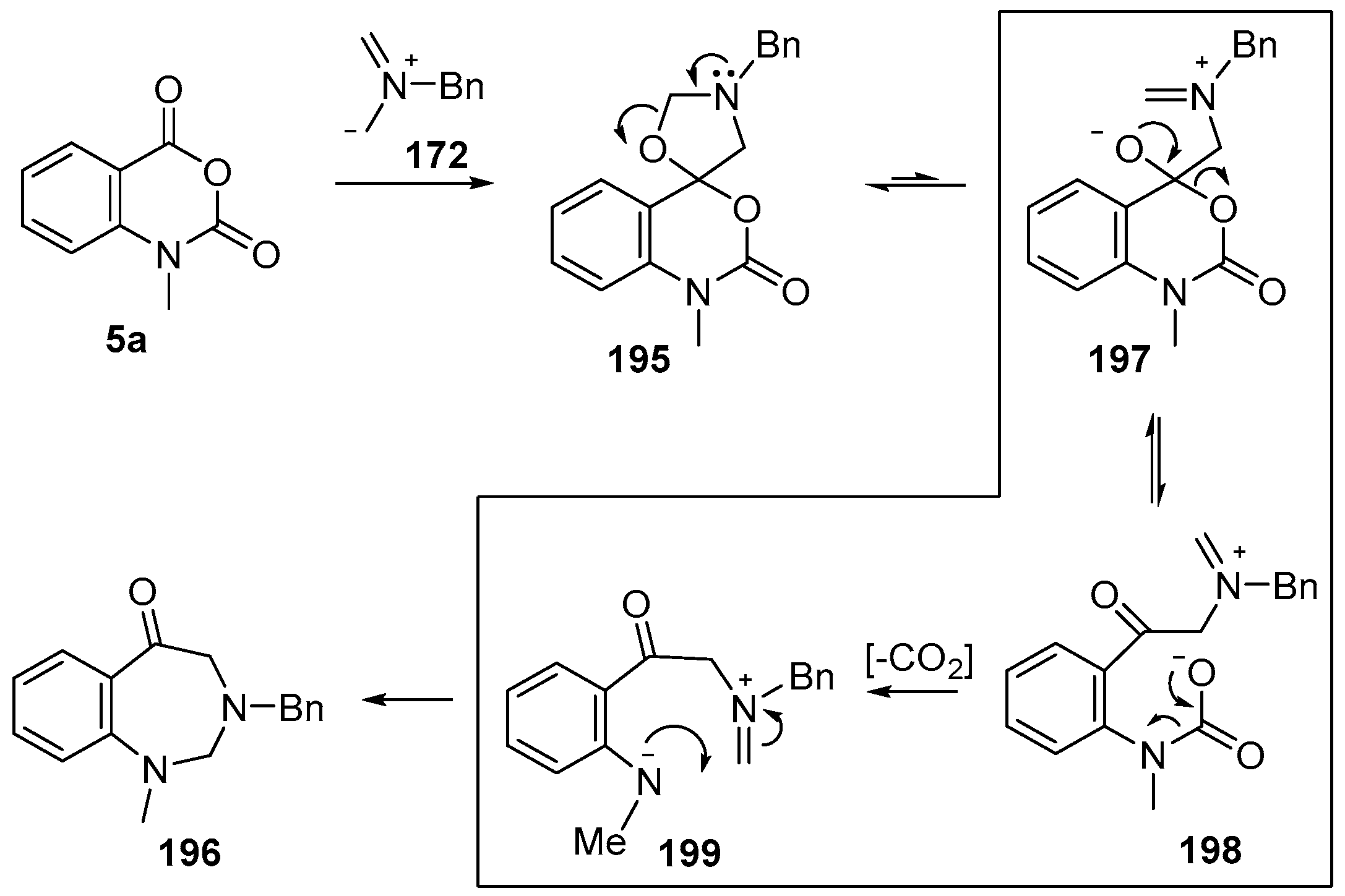
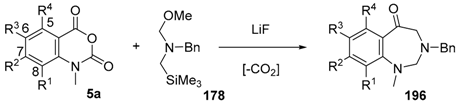
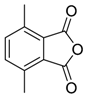
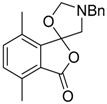
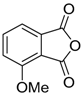
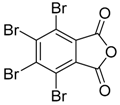
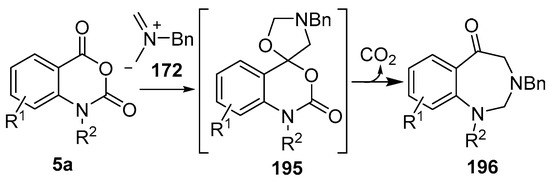
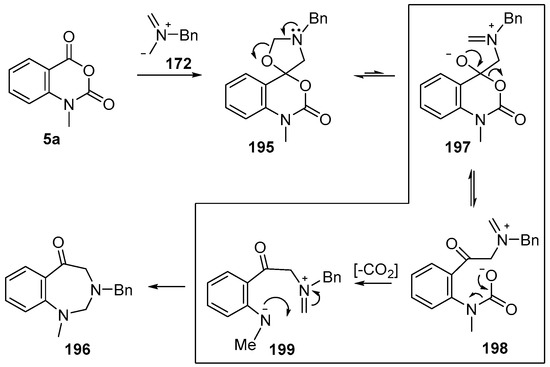
Recently it was reported that activated carboxyl systems can undergo 1,3-dipolar cycloaddition reactions [40]. A range of isatoic anhydrides 5a reacted with the azomethine ylide 172, generated from reagent 178 by trifluoroacetic acid-mediated catalysis or by treatment with LiF, to afford spiro-oxazolidine cycloadducts 195, that spontaneously extruded CO2 with structural rearrangement to give the isolated 1,3-benzodiazepin-5-one products 196 (Scheme 55). High yields of the benzodiazepinones 196 were obtained for a wide range of N- and benzo-substituted isatoic anhydrides except for cases with electron-releasing substituents ortho- or para-related to the dipolarophilic carboxyl group, in which case, starting material was returned (Table 25 and Table 26). The lack of reaction in these cases was thought to be due to stereoelectronic effects which resulted in raising the energy of the carbonyl LUMO energy and concomitant raising of the energy of the cycloaddition transition state. A plausible mechanism for the transformation of the isatoic anhydrides into the benzodiazepines was proposed involving a complex reaction cascade (Scheme 56). The initial cycloaddition gives the oxazolidine product (observable by NMR and IR spectroscopies) which undergoes step-wise oxazolidine ring-opening, decarboxylation and ring-closure.
Scheme 55.
Cycloaddition of azomethine ylide 172, formed from silylamine reagent 178, with isatoic anhydrides 5a, followed by conversion of the oxazolidines 195 to 1,3-benzodiazepin-5-ones 196.

Table 25.
Transformation of isatoic anhydride and N-functionalised isatoic anhydrides 5a into 1,3-benzodiazepin-5-ones 196.

Table 26.
Transformation of benzo-substituted N-methyl isatoic anhydrides 5a into 1,3-benzo-diazepin-5-ones 196 1.
Scheme 56.
Proposed mechanism for the conversion of isatoic anhydrides 5a into benzodiazepinones 196.
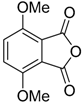
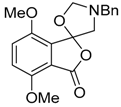
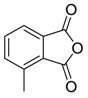
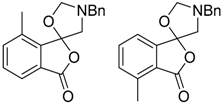
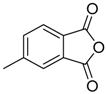
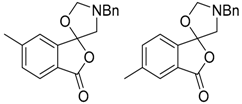
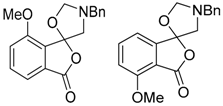
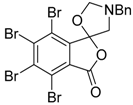
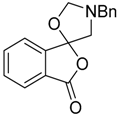
In a recent development, phthalic anhydrides 5b were reported to undergo cycloaddition with the azomethine ylide 172, formed from reagent 178 under conditions of trifluoroacetic acid catalysis [41]. The generated spiro-oxazolidine cycloadducts 200 proved to be more stable than the analogous cycloadducts generated from isatoic anhydrides 5a. Although the cycloadducts 200 decomposed during attempted purification on silica, they could be isolated in pure form after chromatography on Florosil™. The reaction appeared to be general with a range of substituted phthalic anhydrides reacting under these conditions and high yields of the cycloadduct products 200 were obtained (Table 27). In all cases only mono cycloaddition products were isolated. In the cases of the unsymmetrical phthalic anhydrides (Entries 4–6), inseparable mixtures of the two possible mono adducts were obtained, with varying degrees of regioselectivity. In the case, of 3-methoxyphthalic anhydride (Entry 6), a 70:30 ratio of the regioisomeric products were obtained, with the major product being that where the ylide adds to the carboxyl group distal to the methoxy group. This result was explained to be a combination of steric and electronic effects of the methoxy group on the adjacent carboxyl group having the effect of raising the transition state energy and lowering the rate of reactivity of the adjacent carboxyl group.

Table 27.
1,3-Dipolar cycloaddition reaction of phthalic anhydrides 5b with azomethine ylide 172, generated in situ from precursor 178 1.
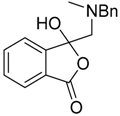
The reductive ring-opening of the spiro-oxazolidines 200 was also investigated [41]. Treatment of the oxazolidines with NaBH4 in methanol led to conversion, in reasonable yields, to 3-hydroxy-isobenzofuran-1-ones 201 (Table 28). The products were stable to chromatographic purification on silica which allowed for separation and characterization of the isomers (Entries 4 and 5). Additionally, the products appear to be mainly present as the depicted ring-closed form (rather than the tautomeric ring-opened keto-carboxylic acid form) in solution state, as assessed by NMR analyses and in solid state as shown by X-ray crystallographic analysis of a representative example. Lower yields obtained for two examples (Entries 7 and 8) were thought to be due to the electron-deficient nature of these systems resulting in over reduction side-reactions.

Table 28.
Reductive ring-opening of oxazolidines 200 with NaBH4 1.
2.5.3. Photochemistry of N-(silylmethyl)phthalimides and Related Reagents
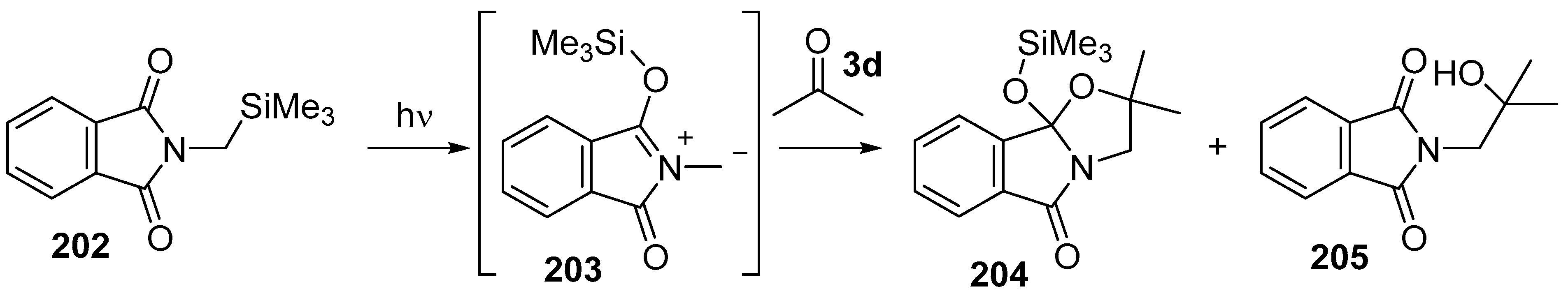
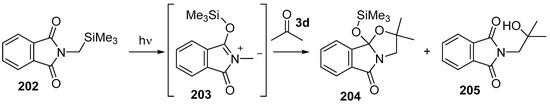
An investigation of the photochemistry of N-trimethylsilylmethyl-substituted phthalimides revealed a C- to O-trimethylsilyl transfer process that produced azomethine ylide intermediates [135,136]. Irradiation of an acetonitrile solution of N-trimethylsilylmethylphthalimide (202) with Pyrex-filtered light led to migration of the silyl group from carbon to oxygen to form azomethine ylide 203 (Scheme 57). When performed in the presence of dipolarophiles, cycloadditions proceeded, and in the case where the reaction was performed in acetone (3d), a single cycloadduct 204 was obtained in 84% yield together with an alcohol side-product 205. The alcohol side-product was presumed to be formed from hydrolysis of the cycloadduct in the reaction mixture and indeed the cycloadduct 204 could be transformed into the alcohol 205 by treatment with aqueous HCl.
Scheme 57.
Cycloaddition of azomethine ylide 203, generated by photochemical rearrangement of N-(silylmethyl)phthalimide (202), with acetone (3d).
2.5.4. Electrochemical Oxidation of bis(silylmethyl)amines
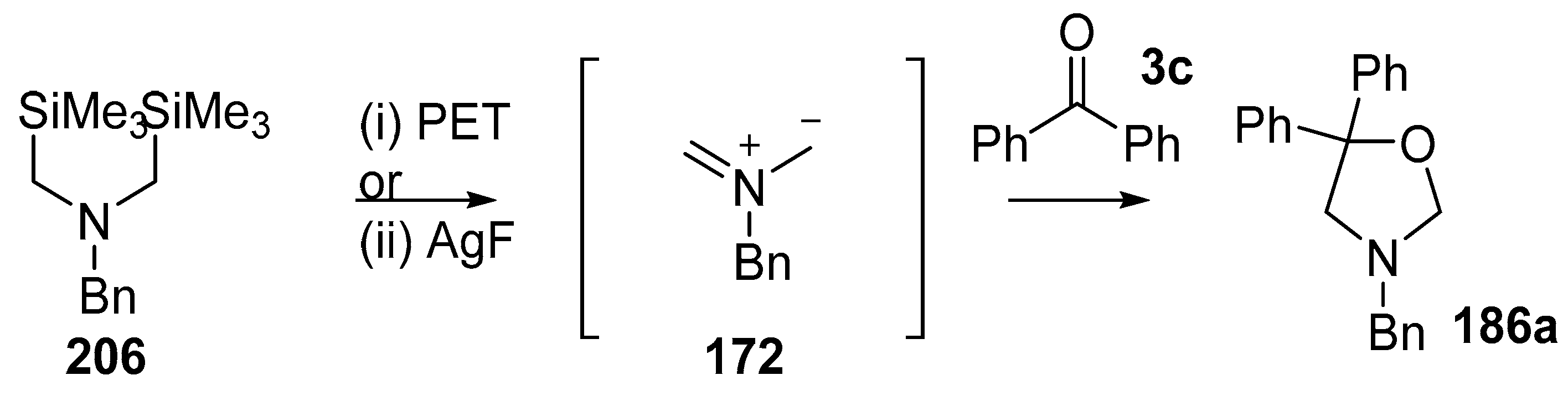
Double desilylation of bis(silylmethyl)amines using either photoelectron induced transfer (PET) or AgF were developed as processes for generating non-stabilised azomethine ylides [137]. In the case of bis(trimethylsilylmethyl)benzylamine (206) this process is an alternative method for generating ylide 172. Whilst most of the examples involved the use of alkene dipolarophiles, benzophenone (3c) was also studied resulting in cycloadduct 186a being isolated in 80% and 62% yields from PET and AgF processes, respectively (Scheme 58).
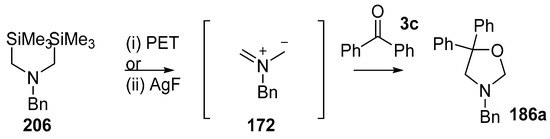
Scheme 58.
Cycloaddition of 172, formed by electrochemical oxidation of 206, with benzophenone 3c. Reaction conditions: (i) 206, 1,4-dicyanonaphthalene (0.20 equiv.), 3d (1.6 equiv.), 80%; (ii) 206, AgF (2 equiv.), 3d (1.2 equiv.), CH3CN, 62%.
2.6. Reactions of Ylides Formed from Addition of Carbenes and Carbenoids to Schiff Bases
The reaction of carbenes or carbenoid species with imines is a convenient way to generate azomethine ylides and has been applied to cycloadditions with a range of carbonyl dipolarophiles.
2.6.1. Diazoalkanes
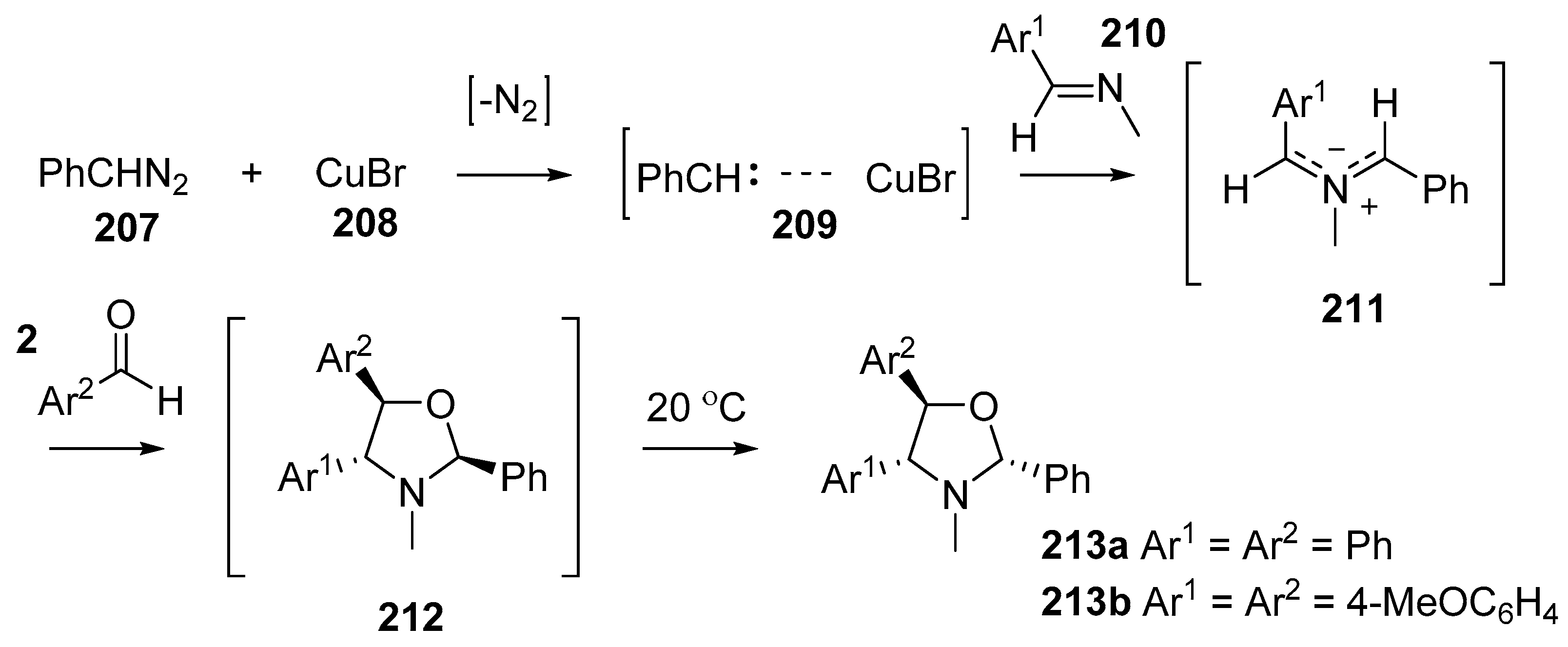
The catalytic decomposition of diazoalkanes in the presence of a Schiff’s base is a convenient source of azomethine ylides (Scheme 59) [138]. The copper(I) bromide-catalyzed decomposition of phenyldiazomethane 207 produces a carbene-Cu(I) complex 209. In the presence of a large excess of imine 210 a trans-azomethine ylide 211 is formed and this intermediate reacts with dipolarophiles to product five-membered heterocycles. With benzaldehyde (2a) or anisaldehyde (2c) as the dipolarophile, oxazolidines 212 were formed, however they proved to be unstable and on standing at room temperature for a few days transformed into the more thermodynamically stable isomers 213. Competition experiments were performed that indicated dimethyl maleate was a more reactive dipolarophile than benzaldehyde (2a).
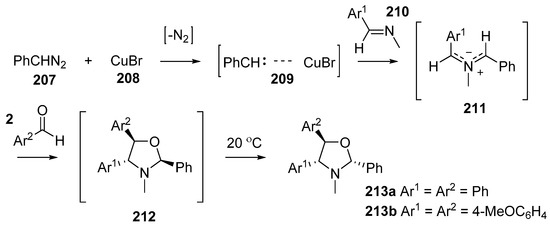
Scheme 59.
Cycloaddition of azomethines 211, generated from CuBr-catalyzed decomposition of phenyldiazomethane (207) in the presence of imines 210, with aromatic aldehydes 2.
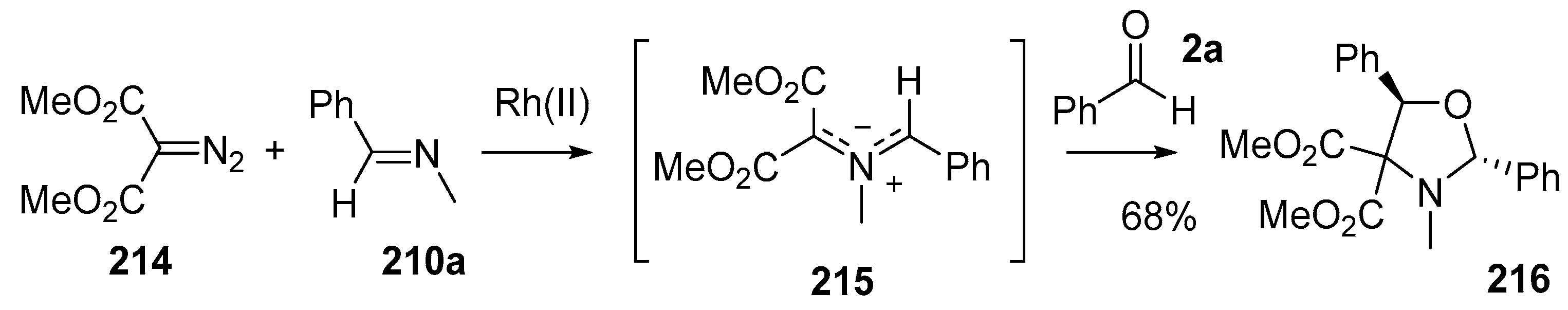
The Rh(II)-catalyzed decomposition of α-diazocarbonyls in the presence of imino π-bonds is also an elegant way to produce functionalized azomethine ylides [139]. Many variations of this process were explored, including the use of benzaldehyde (2a) as a dipolarophile. The Rh(I)-catalyzed decomposition of diazomalonate 214 in the presence of imine 210a provided stabilized azomethine ylide 215 which underwent cycloaddition to benzaldehyde (2a) to provide cycloadduct 216, isolated as a single diastereoisomer in 68% yield (Scheme 60).
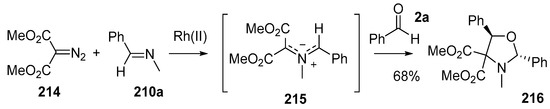
Scheme 60.
Cycloaddition of azomethine ylide 215, generated from Rh(II)-catalyzed decomposition of diazomalonate 214 in the presence of imine 210a, and benzaldehyde (2c).
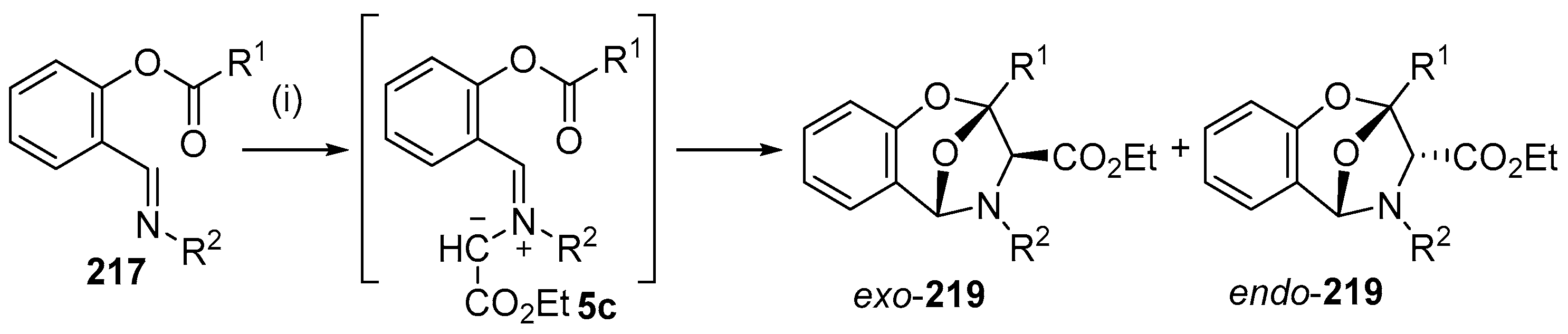
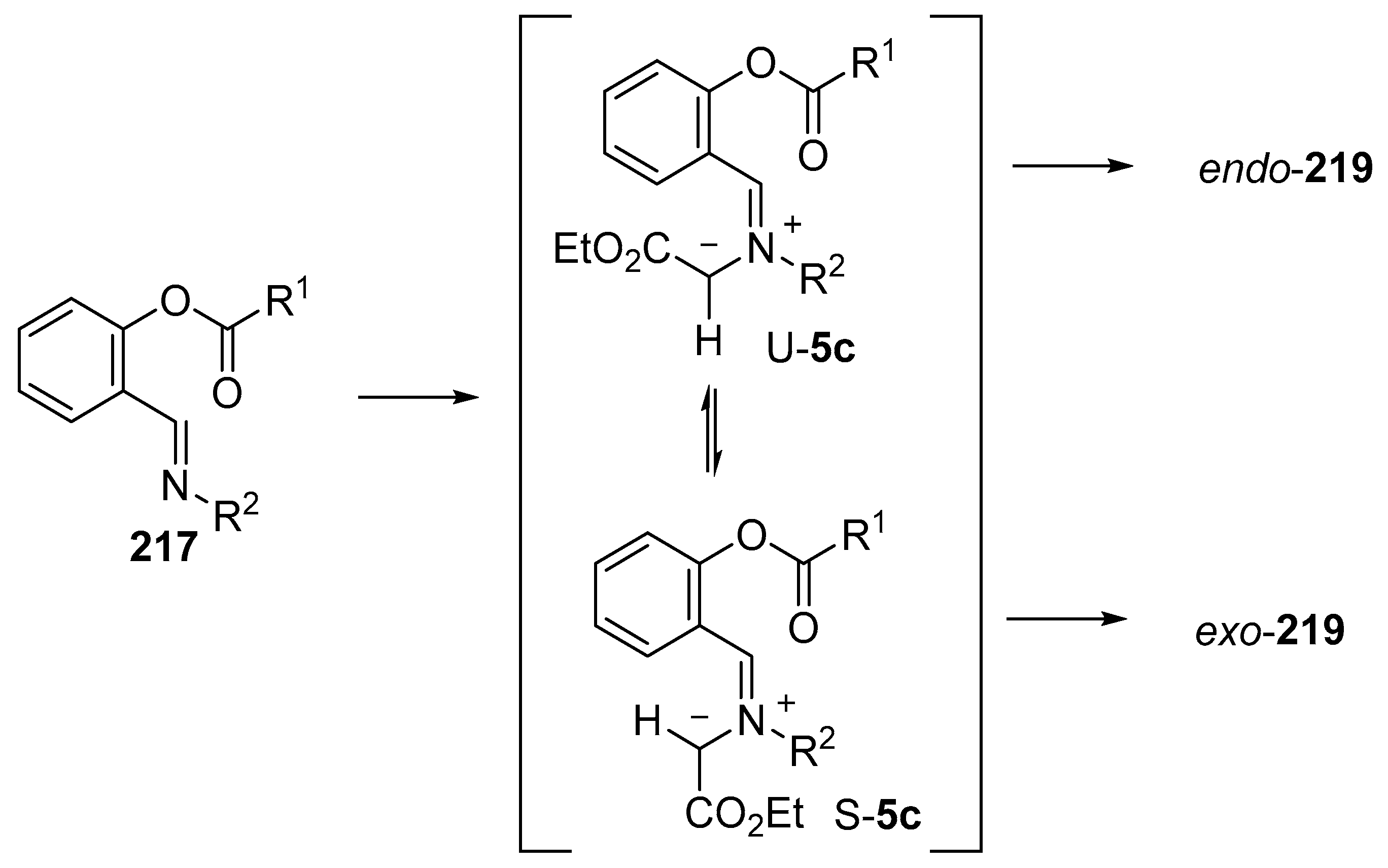
As an extension to studies on the intramolecular addition of azomethine ylides derived from imines to ester carbonyl moieties (see Section 2.6.2), the intramolecular reaction of imines of O-acylsalicylic aldehydes with metallocarbenes generated from diazocarbonyl compounds was recently investigated experimentally and computationally [140]. A range of Rh- and Cu-catalysts were tested with copper(II)trifluoroacetoacetate (Cu(tfacac)2) providing the highest yields (Scheme 61). The reaction of imine 217 with ethyl diazoacetate (218) catalyzed by 10 mol % of Cu(tfacac)2, in refluxing benzene, led to formation of azomethine ylide intermediate 5c which underwent intramolecular cycloaddition onto the ester carbonyl to produce both endo and exo isomers of 219, isolated in 13% and 14% yield, respectively. In refluxing CH2Cl2, endo-219 was obtained in 36% yield, whereas with the use of a stoichiometric amount of Cu(tfacac)2, a 41% yield of endo-219 resulted. The latter conditions were applied to a wide range of imines (Table 29). In order for cycloaddition to occur, an electron-withdrawing substituent was required on the imine aryl group (Entries 1–8). In these cases, where an electron-withdrawing substituent was also present on the benzoyl group, both endo- and exo-cycloadduct isomers were produced (Entries 6–8). For the subset of cases with an electron-donating substituent on the benzoyl group, exclusive endo stereochemistry resulted (Entries 1–5). A range of experimental and theoretical (DFT) calculations were performed that indicated that the change in stereochemistry of cycloaddition was due to a decrease in the barrier to cycloaddition and an increase in the barrier of U- to S-ylide interconversion with an electron-withdrawing group on the benzoyl group. The U-ylide leads to the endo-cycloadduct, whereas the S-ylide leads to the exo-cycloadduct (Scheme 62).
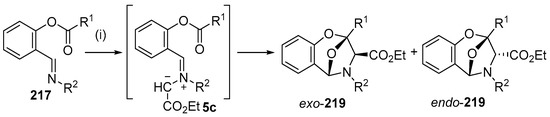
Scheme 61.
Intramolecular cycloaddition of azomethine ylide and ester carbonyl of intermediate 5c. Reagents and Conditions: (i) N2CCHCO2Et 218; Cu(tfacac)2, CH2Cl2, 40 °C.

Table 29.
Reaction of imines 217, ethyl diazoacetate (218) and Cu(tfacac)2 in CH2Cl2 at 40 °C (Scheme 61).
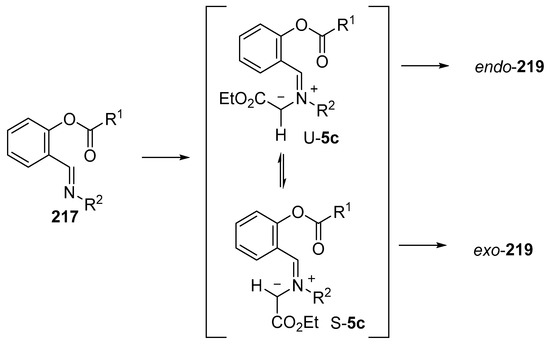
Scheme 62.
Mechanistic rationale for the formation of endo- and exo-cycloadducts 219.
2.6.2. Reaction with Ylides Formed by Interaction of Imines with Dihalocarbenes
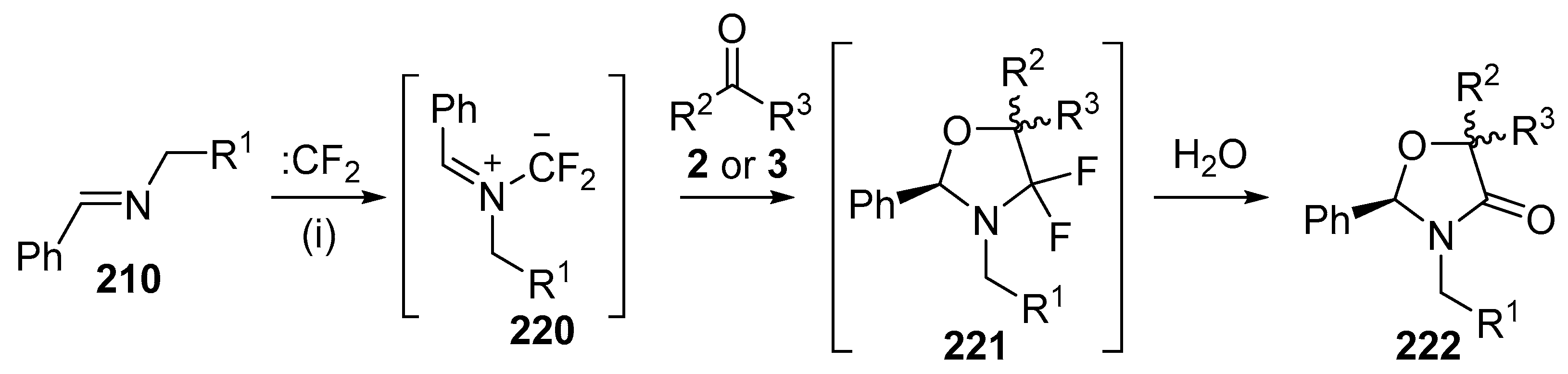
Azomethine ylides derived from the reaction of imines with difluoro- or dichlorocarbene also undergo cycloaddition with a range of carbonyl dipolarophiles. The first report of these processes demonstrated that ylides derived from difluorocarbene undergo regioselective cycloaddition with aldehydes and ketones to give oxazolidinone derivatives (Scheme 63, Table 30) [141]. Heating a mixture of the imine 210 (R1 = Ph), dibromodifluoromethane, Pb powder, tetrabutylammonium bromide and a twofold excess of benzaldehyde (2a) gave the oxazolidinone 222a in 39% as a ca. 5:3 mixture of diastereoisomers (Entry 1). Presumably, the oxazolidinone 222a was formed by hydrolysis of the cycloadduct 221a during silica chromatography. The reaction with other aldehydes provided moderate yields of oxazolidinones 222 (Entries 2–5), however, the experiments with acetone (3d) or acetophenone (3i) gave low or no yield of product (Entries 6 and 7).
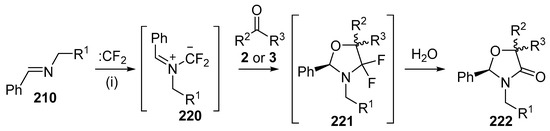
Scheme 63.
Cycloaddition of azomethine ylides 220 with aldehydes 2 or ketones 3. Reaction conditions: (i) imine 210, CBr2CF2, Pb powder, TBAF, carbonyl compound, CH2Cl2.

Table 30.
Reaction of benzaldehyde-derived imines 210 with difluorocarbene and aldehydes 2 or ketones 3, yielding oxazolidinones 222 (Scheme 63).
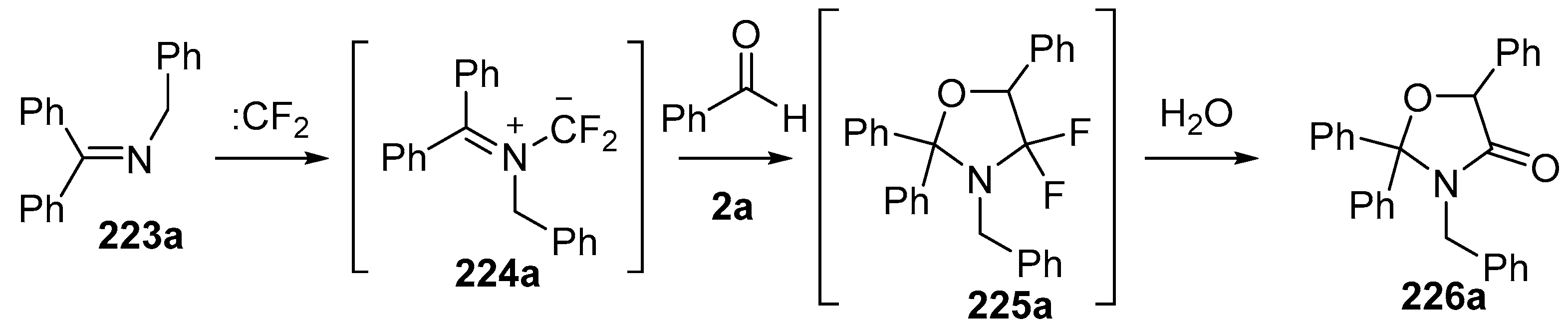
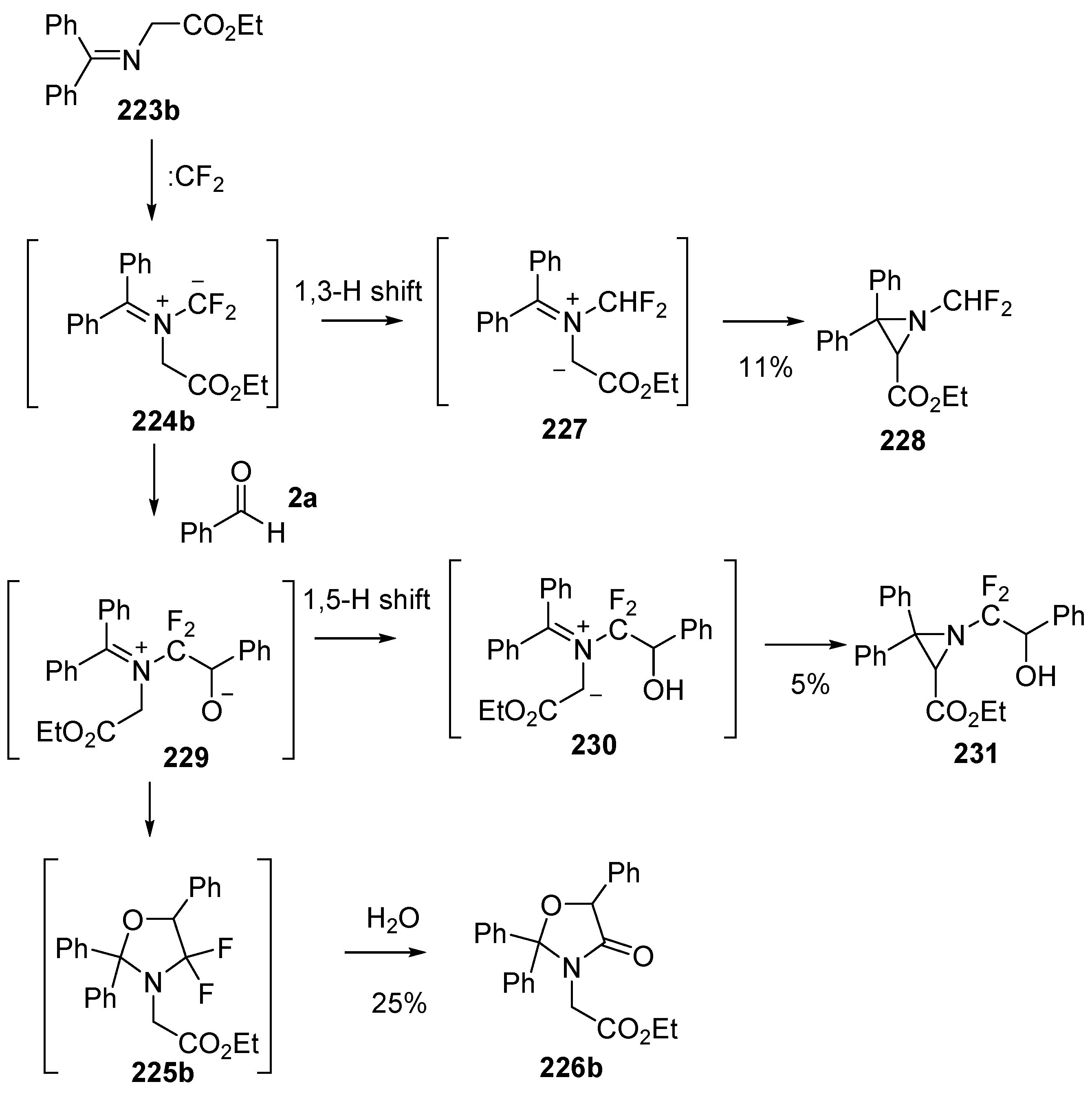
Dipolar cycloaddition reactions starting with benzophenone imines 223 and difluorocarbene were also studied [141]. Under the same conditions as above, imine 223a added to difluorocarbene to generate the azomethine ylide intermediate 224a, which underwent efficient cycloaddition to benzaldehyde (2a) to afford the cycloadduct 225a. The oxazolidine 225a hydrolysed on silica to give the oxazolidinone 226a which was isolated in 75% yield (Scheme 64). The reaction with the N-carboxymethyl ester 223b under these conditions with benzaldehyde (2a) as the dipolarophile resulted in formation of oxazolidinone 226b via ylide cycloaddition and hydrolysis along with aziridine side-products 228 and 231 (Scheme 65). The mechanism proposed for the formation of 228 involved a 1,3-H shift of the ylide intermediate 224a to give an alternative ylide 227 which ring-closed to afford the isolated aziridine 228. Alternatively, addition of benzaldehyde (2a) to the ylide 224b could give ring-opened adduct 229 which could undergo a rapid 1,5 H-shift to give another alternative ylide 230 which on ring closure would afford aziridine 231. The authors propose that ring-opened adduct 229 could ring-close to give oxazolidine 225b which when exposed to silica, underwent hydrolysis to afford the isolated oxazolidinone 226b. An alternative pathway whereby 224b undergoes 1,3-dipolar cycloaddition to afford oxazolidine 225b which then ring-opens to provide 229 eventually leading to 231, was not mentioned.
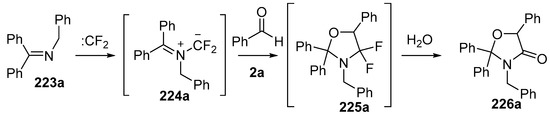
Scheme 64.
Cycloaddition of azomethine ylide 224a, generated from difluorocarbene and imine 223a, with benzaldehyde (2a).
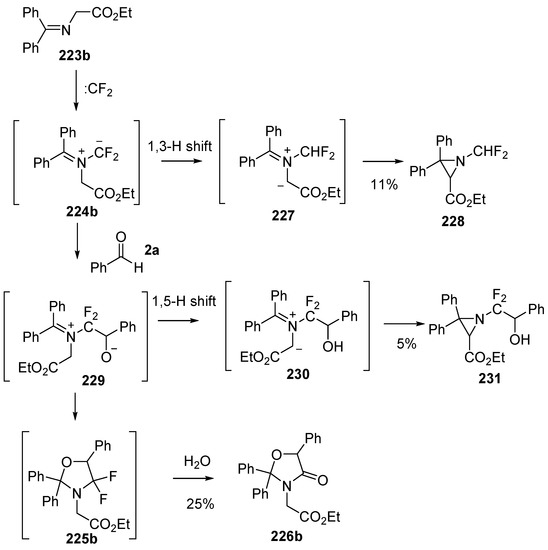
Scheme 65.
Cycloaddition of azomethine ylide 224b, generated from difluorocarbene and imine 223b, with benzaldehyde (2a).
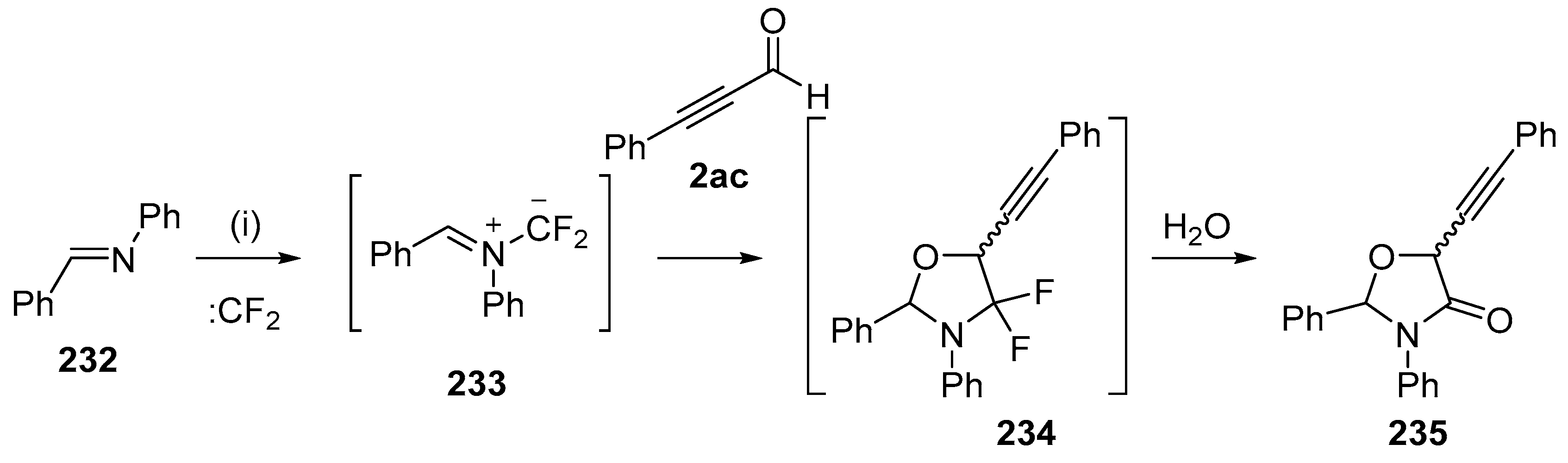
A later study of addition of the ylides formed by imines and difluorocarbene with alkynes revealed side reactions involving addition to alkynyl aldehydes [142]. The ylide 233, formed from N-phenylbenzaldehyde imine (232) and difluorocarbene, reacted with alkynyl aldehyde 2ac to form the cycloadduct 234. On silica chromatography, hydrolysis occurred and a mixture of oxazolidinones 235 was isolated in 25% yield as a 5:1 mixture of diastereoisomers (Scheme 66). In contrast, the corresponding alkynyl esters and alkynyl ketones resulted in selective addition to the alkyne triple bond.
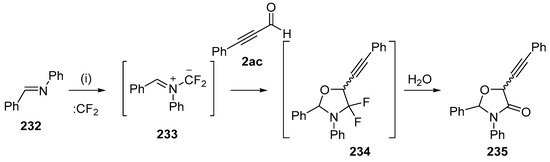
Scheme 66.
Regioselective cycloaddition of azomethine ylide 233 to the aldehyde of 2ac. Reaction conditions: (i) imine 232, CBr2CF2, Pb powder, TBAF, 2ac, CH2Cl2.
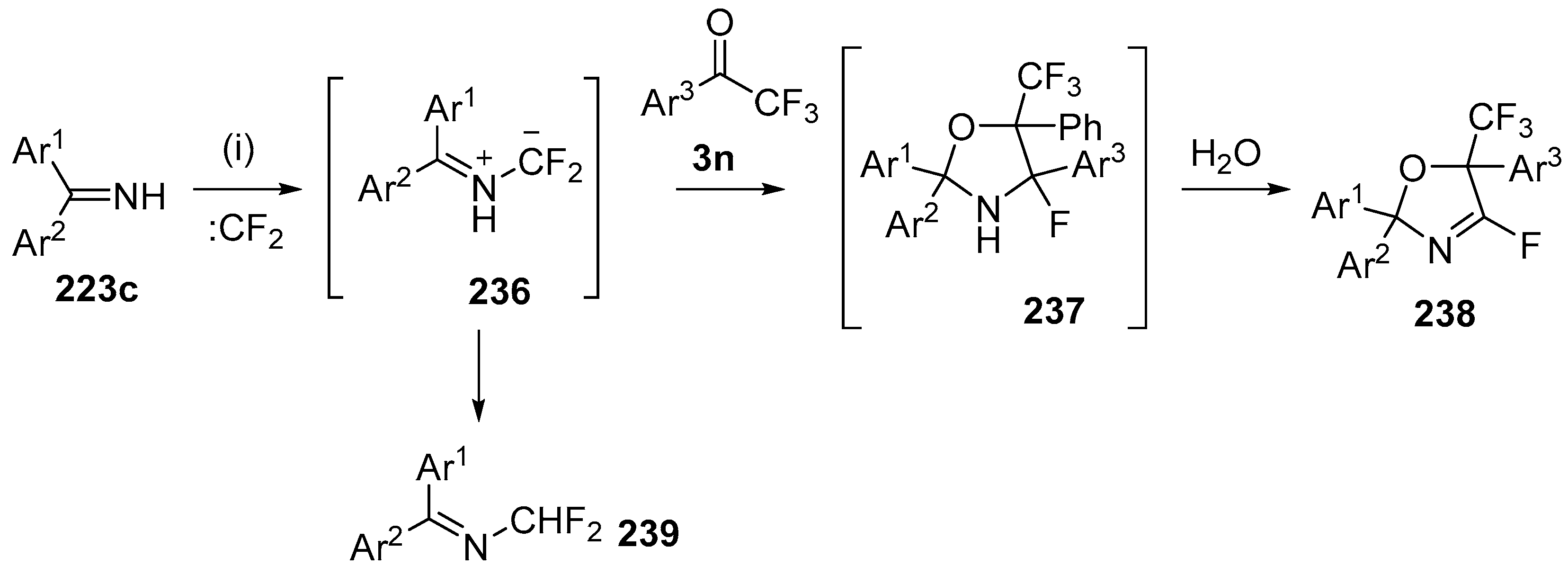
The cycloaddition of gem-difluorosubstituted NH-azomethine ylides with α,α,α-trifluoro-acetophenones has been reported to form 4-fluorooxazolidines [143]. The optimized procedure involved stirring a mixture of the imine 223c, with an excess of trifluoroacetophenone (3o), CBr2F2, Pb filings and tetrabutylammonium bromide, and resulted in isolation of the fluoro-oxazolines 238 after chromatography on silica (Scheme 67, Table 31). Presumably the imine 223c reacts with liberated difluorocarbene to afford the azomethine ylide 236 which undergoes cycloaddition with trifluoroacetophenones 3 to afford the cycloadducts 237. On exposure to silica, the cycloadducts 237 lose HF to afford the 2-fluorooxazolidine products 238. A side-product 239 was at times isolated, that was thought to be formed by the isomerization of the ylide 236. The optimized method was applicable to a range of substituted benzophenone imines 223c and substituted trifluoroacetophenones 3 (Table 31). Additionally the products appear to have potential utility, with 238 undergoing displacement of the fluoride with a range of N- and O-centred nucleophiles.
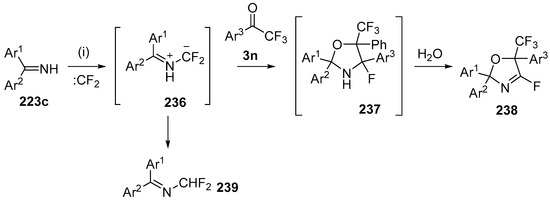
Scheme 67.
Cycloaddition of azomethine ylides 236 to trifluoroacetophenones 3n. Reagants and Conditions: (i): imine 223c, Pb (3 equiv.), Bu4NBr (3 equiv.), CF2Br2 (3 equiv.), Ar1COCF3 3 (3 equiv.), CH2Cl2, ultrasound.

Table 31.
The reaction of imines 223c, difluorocarbene and trifluoromethylketones 3 (Scheme 67).
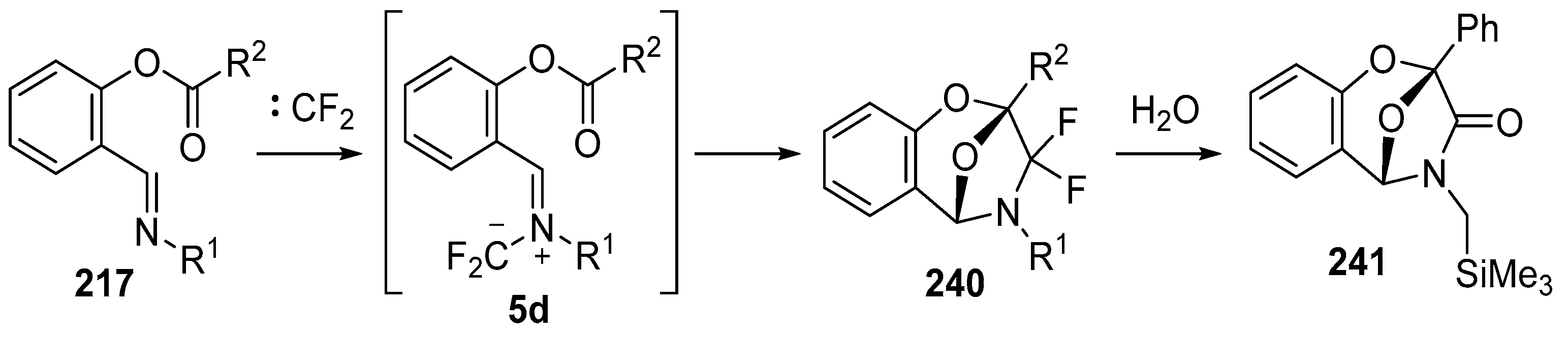
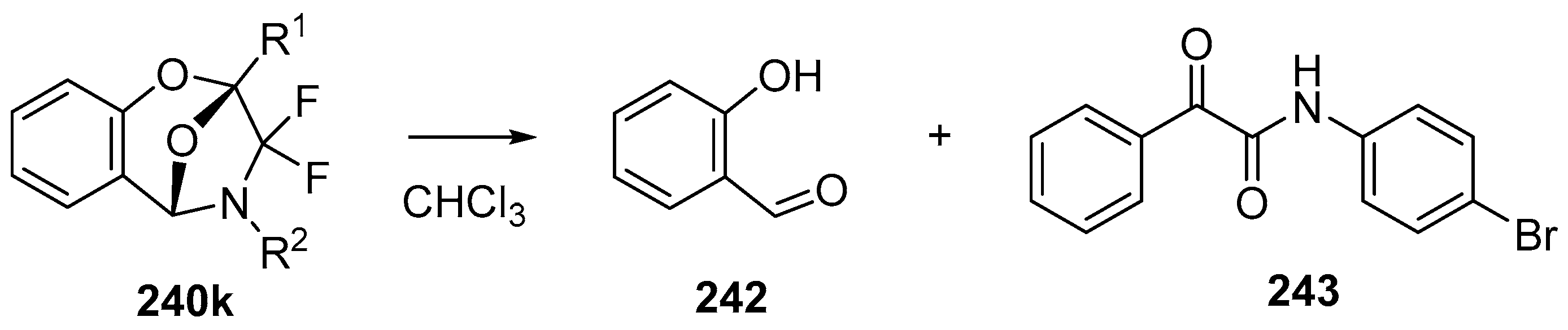
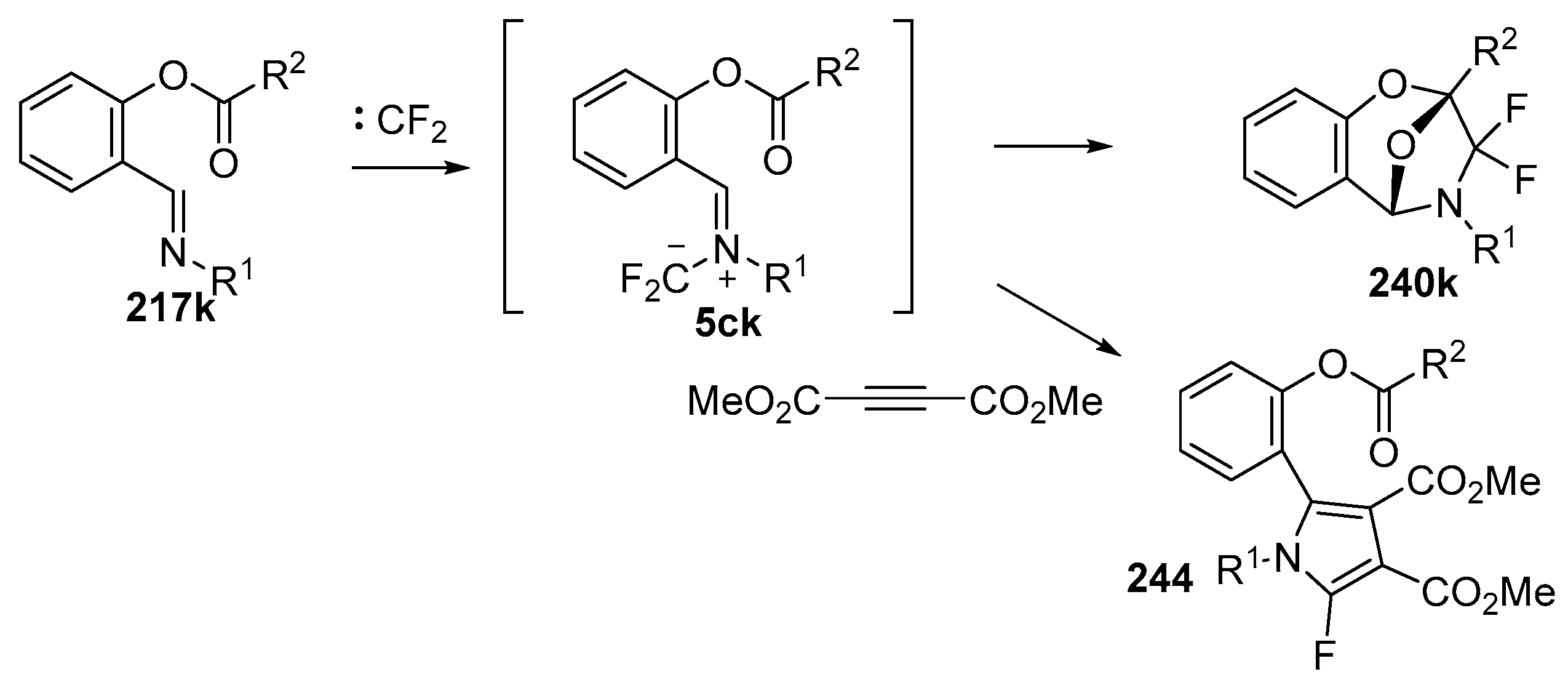
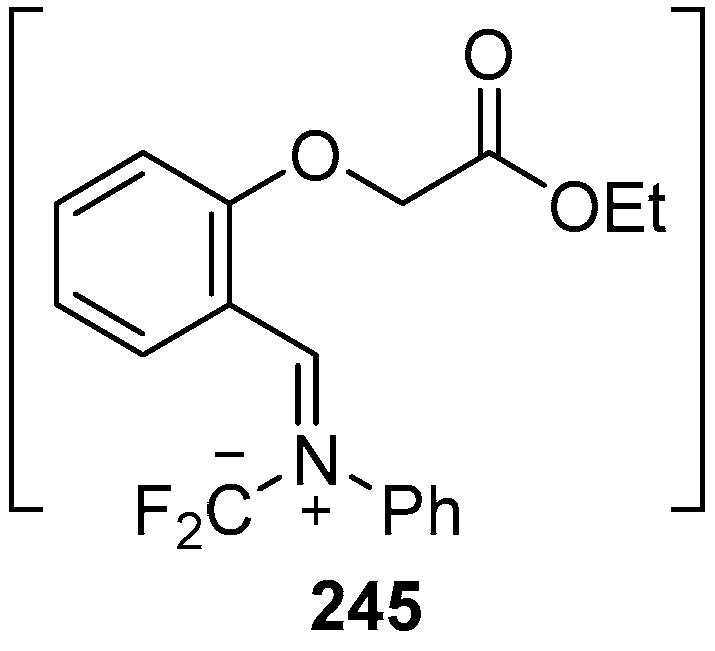
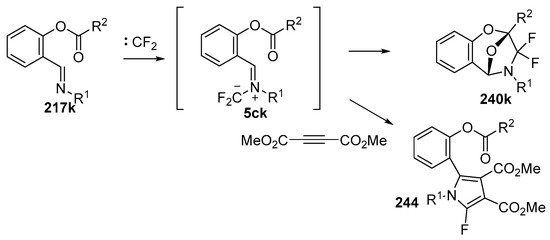
In 2002, the first 1,3-dipolar cycloaddition of an azomethine ylide to an ester carbonyl was reported [144,145]. Azomethine ylides 5d, generated by the reaction of difluorocarbene with aryl and alkyl imines of O-acylated salicylaldehydes 217, underwent intramolecular 1,3-dipolar cycloaddition onto the ester carbonyl to give cycloadducts 240 (Scheme 68). This process proved to be quite general for a range of N-aryl substituted imines as well as N-alkylimines (Table 32). The cycloadducts 240a–m were stable enough to be isolated and characterized whereas the cycloadduct 240n, from the N-(trimethylsilylmethyl) imine, underwent rapid hydrolysis during chromatographic purification on silica, affording lactam 241 in 67% yield. Interestingly, the cycloadducts 240 degraded on longer term storage in chloroform, yielding, for example, from 240k after 3.5 months, salicylaldehyde (242) and phenylglyoxamide 243 (Scheme 69). In the cases of imines 217f–i there is an internal competition between cycloaddition of the ylide to the ester carbonyl and cycloaddition to the olefin. Only cycloaddition to the ester carbonyl was observed indicating a much faster rate of cycloaddition to the ester than these types of olefins. An external competition reaction was performed by running the reaction of 217k in the presence of a reactive dipolarophile, dimethyl acetylenedicarboxylate, and resulted in roughly equimolar amounts of intramolecular cycloadduct 240k and the pyrrole 244 resulting from intermolecular cycloaddition of the ylide with the alkyne (Scheme 70). Additionally the ylide 245 (Figure 4) did not undergo intramolecular cycloaddition, indicating the requirement of the close proximity of the ester group to the ylide for efficient ester carbonyl cycloaddition to occur.
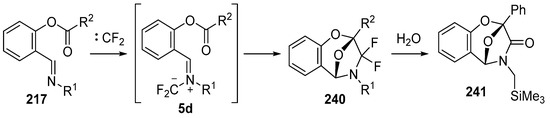
Scheme 68.
Intramolecular cycloaddition of the azomethine ylide and ester moieties of intermediate 5d.

Table 32.
Intramolecular cycloaddition of ylide and carbonyl esters within 5d yielding epoxybenzodiazepines 240 (Scheme 68) 1.
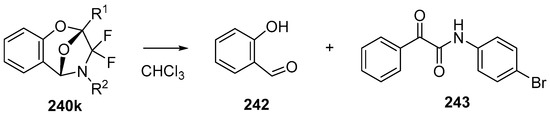
Scheme 69.
Decomposition of cycloadduct 240k in chloroform.
Scheme 70.
Intramolecular versus intermolecule competition reactions of azomethine ylides 5ck.
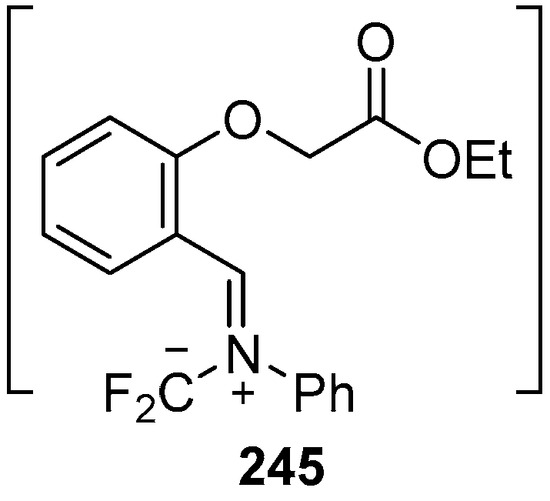
Figure 4.
Analogous azomethine ylide that fails to undergo intramolecular cycloaddition.
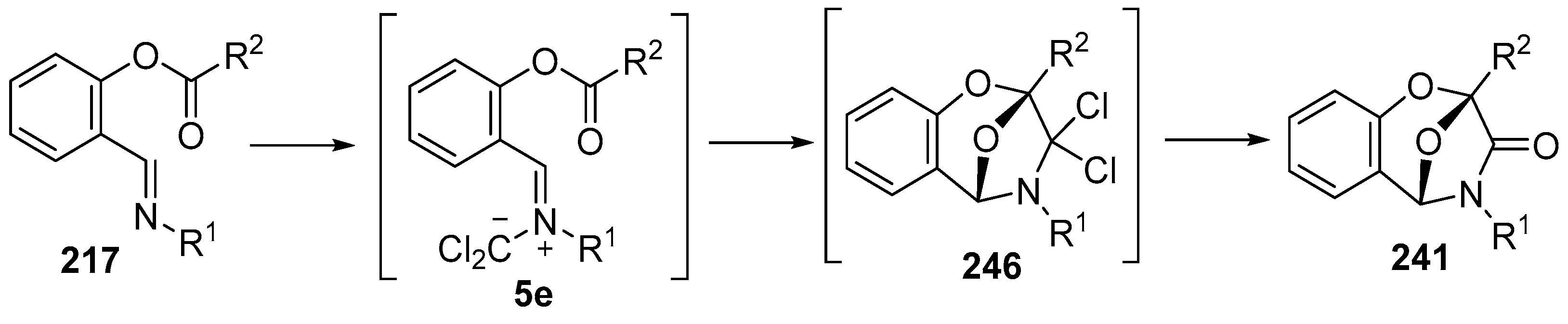
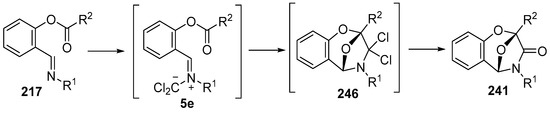
The reaction of analogous ylides derived from imines and dichlorocarbene has also been explored [146]. Although N-benzylidene anilines generally react with dichlorocarbene to give aziridines, the salicylaldehyde imines 217 reacted with dichlorocarbene, generated under a number of different conditions, to produce 2,5-epoxy-1,4-benzoxepin-3-ones 241, isolated in high yields (Scheme 71, Table 33). The proposed mechanism involves addition of the dichlorocarbene to the imine 217 to produce 5e which undergoes intramolecular cycloaddition of the ylide onto the ester carbonyl to give adduct 246 which then hydrolyses on work up or chromatographic purification, providing the isolated lactams 241.
Scheme 71.
Intramolecular cycloadditions of the azomethine ylide 5e.

Table 33.
Intramolecular cycloaddition of dichlorocarbene derived ylides and ester carbonyl 5e yielding epoxybenzodiazepinones 241 (Scheme 71) 1.
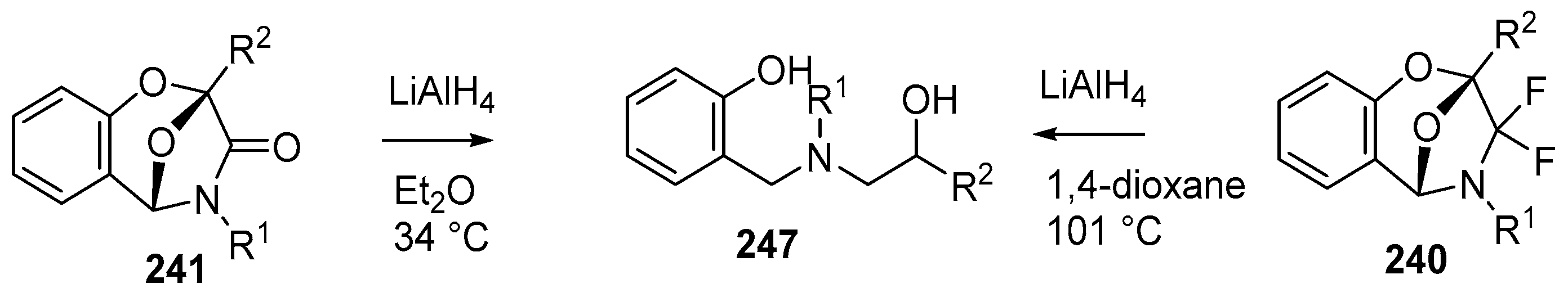
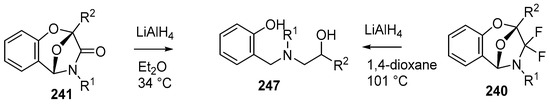
The chemistry of the bicyclic systems was also briefly explored. Reduction of 241b, o or i with LiAlH4 in refluxing ether afforded the respective aminoalcohols 247b, o or i (Scheme 72). The analogous reduction of the difluorinated cycloadducts 240a and m, required more forcing conditions and was achieved in refluxing dioxane (Scheme 72, Table 34).
Scheme 72.
Reduction of cycloadducts 240 and 241.

Table 34.
Reduction of cycloadducts 241 and 240 with LiAlH4 (Scheme 72).
2.7. Miscellaneous Reactions
2.7.1. Reactions with Ylides Formed by Reaction of Isocyanides, Alkylboranes and Aldehydes
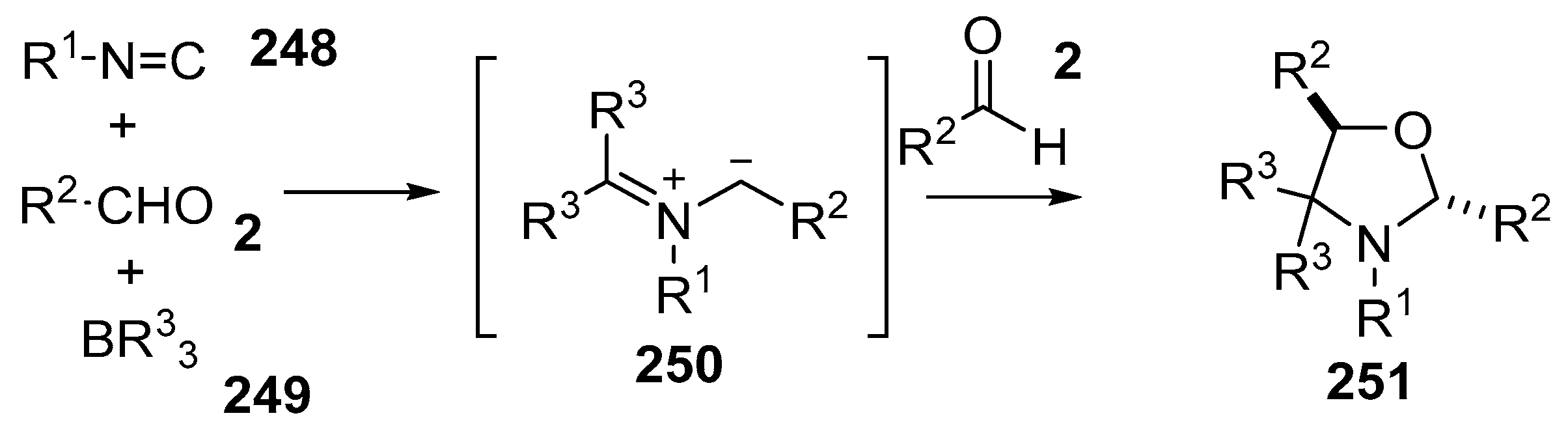
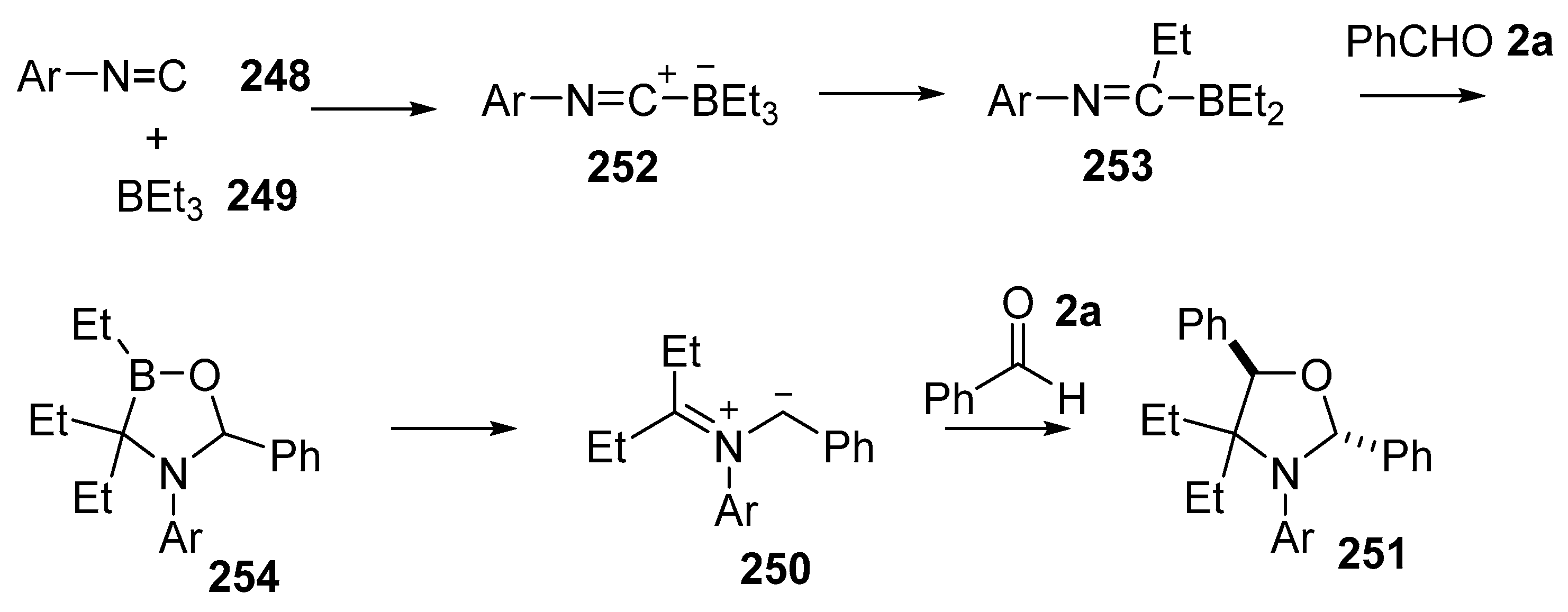
A multicomponent reaction of aldehydes 2, isocyanides 248 and trialkylboron reagents 249 resulted in formation of azomethine ylides 250 which underwent further reaction with aldehydes 2 to yield oxazolidines 251 in an efficient manner, with the aldehyde group playing dual roles in the formation of the ylide as well as the dipolarophile (Scheme 73) [147]. The reaction generally worked for aryl isocyanides and aryl carboxaldehydes, however failed in the case of alkyl and alkenyl isocyanides and sterically hindered aldehydes (Table 35).
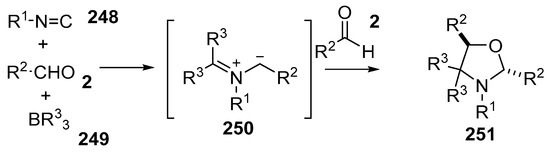
Scheme 73.
Cycloaddition of azomethine ylides, generated by the reaction of aldehydes 2, isocyanides 248 and organoboron compounds 249.

Table 35.
Multicomponent reaction of aldehydes 2, isocyanides 248 and trialkylboron reagents 249 (Scheme 73).
The mechanism that was proposed for this transformation involves a complex series of steps (Scheme 74). The process is initiated by addition of the isocyanide 248 to the trialkylborane 249 to give adduct 252. Alkyl migration would then give iminoborane 253. Further interaction of 253 with benzaldehyde 2a with concomitant alkyl migration gives an oxazaborolidine intermediate 254. Electrocyclic ring-opening of 254 affords the non-stabilized azomethine ylide 250. Dipolar cycloaddition with further aldehyde 2a would then provide for the trans-oxazolidine 251.
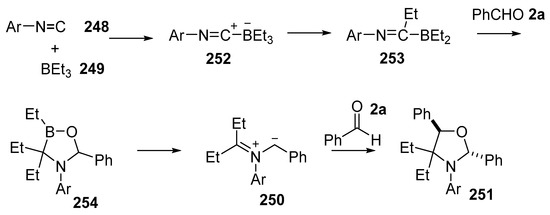
Scheme 74.
Proposed mechanism for the formation of oxazolidines 251.
2.7.2. Reactions of Dicyanoazomethine Ylides
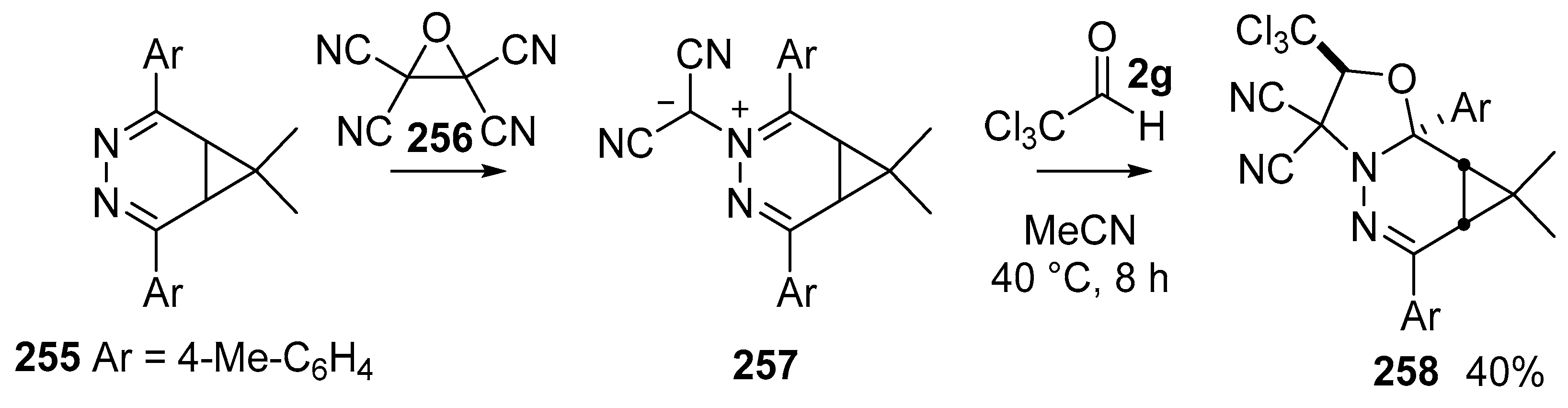
The stable dicyanoazomethine ylides 257, derived from reaction of 3,4-diazanorcaradienes 255 with tetracyanoethylene oxide 256 [148], undergo reaction with a range of dipolarophiles [149]. The carbonyl dipolarophile trichloroacetaldehyde (2g) undergoes efficient cycloaddition with ylide 257 to give cycloadduct 258 isolated as a single endo stereoisomer in 40% yield (Scheme 75).
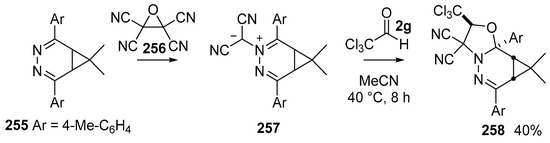
Scheme 75.
Cycloaddition of azomethine ylides 257 with trichloroacetaldehyde 2g.
3. Conclusions
The 1,3-dipolar cycloaddition reaction of azomethine ylides and carbonyl dipolarophiles is a general method for producing oxazolidines. In terms of scope, many ylides have been demonstrated to react with carbonyl groups including an array of stabilized and non-stabilized ylides and many more ylide systems could be explored. Aldehydes including formaldehyde are the most studied group of carbonyl dipolarophiles studied, with ketones are also being well represented. There are few examples of ketenes, even though these systems appear quite reactive. Activated carboxyl systems such as isataoic anhydrides and phthalic anhydrides have been shown to react with non-stabilized azomethine ylides and the interesting reactivity of the spiro-fused oxazolidine products deserves further exploration. An intramolecular cycloaddition of azomethine ylides and esters (the latter group is typically unreactive in dipolar cycloadditions) provides for bicyclic systems and also warrants further exploration.
Acknowledgments
The authors thank CSIRO Manufacturing for supporting the research.
Author Contributions
The authors contributed equally to the research and authorship of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huisgen, R. 1,3-Dipolar cycloaddition. Past and Future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maruoka, K. Recent advances of catalytic asymmetric 1,3-dipolar cycloadditions. Chem. Rev. 2015, 115, 5366–5412. [Google Scholar] [CrossRef] [PubMed]
- Gothelf, K.V.; Jorgensen, K.A. Asymmetric 1,3-dipolar cycloaddition reactions. Chem. Rev. 1998, 98, 863–910. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Tang, Y. Intermolecular 1,3-dipolar cycloadditions of alkenes, alkynes and allenes. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4, pp. 1342–1383. [Google Scholar]
- Menon, R.S.; Nair, V. Intramolecular 1,3-dipolar cycloadditions of alkenes, alkynes and allenes. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4, pp. 1281–1341. [Google Scholar]
- Padwa, A. (Ed.) 1,3-Dipolar Cycloaddition Chemistry; Wiley-Interscience: New York, NY, USA, 1984; Volumes 1–2.
- Padwa, A.; Pearson, W.H. (Eds.) Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. In The Chemistry of Heterocyclic Compounds; Wiley: New York, NY, USA, 2003; Volume 59.
- Izquierdo, C.; Esteban, F.; Ruano, J.L.G.; Fraile, A.; Alemán, J. Asymmetric Synthesis of 1,2-Diamines bearing Tetrasubstituted Centers from Nonstabilized Azomethine Ylides and N-Sulfinylketimines under Brønsted Acid Catalysis. Org. Lett. 2016, 18, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Seela, F. Circular DNA by “Bis-Click” Ligation: Template-Independent Intramolecular Circularization of Oligonucleotides with Terminal Alkynyl Groups Utilizing Bifunctional Azides. Chem. Eur. J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, S.A.; Li, X.; He, S.; Yu, M.; Wu, M.; Vederas, J.C. Synthesis of Tridecaptin–Antibiotic Conjugates with in Vivo Activity against Gram-Negative Bacteria. J. Med. Chem. 2015, 58, 9779–9785. [Google Scholar] [CrossRef] [PubMed]
- Nakhla, M.C.; Lee, C.-W.; Wood, J.L. Chemoselective Intramolecular Carbonyl Ylide Formation through Electronically Differentiated Malonate Diesters. Org. Lett. 2015, 17, 5760–5763. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, J.; Zuilhof, H.; Wennekes, T. Versatile Scope of a Masked Aldehyde Nitrone in 1,3-Dipolar Cycloadditions. Org. Lett. 2015, 17, 5550–5553. [Google Scholar] [CrossRef] [PubMed]
- Haberhauer, G.; Gleiter, R.; Woitschetzki, S. anti-Diradical Formation in 1,3-Dipolar Cycloadditions of Nitrile Oxides to Acetylenes. J. Org. Chem. 2015, 80, 12321–12332. [Google Scholar] [CrossRef] [PubMed]
- Hatano, J.; Okuro, K.; Aida, T. Photoinduced Bioorthogonal 1,3-Dipolar Poly-cycloaddition Promoted by Oxyanionic Substrates for Spatiotemporal Operation of Molecular Glues. Angew. Chem. Int. Ed. 2016, 55, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Brioche, J.; Meyer, C.; Cossy, J. Synthesis of 2-Aminoindolizines by 1,3-Dipolar Cycloaddition of Pyridinium Ylides with Electron-Deficient Ynamides. Org. Lett. 2015, 17, 2800–2803. [Google Scholar] [CrossRef] [PubMed]
- Lown, J.W. Azomethine Ylides. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, NY, USA, 1984; ISBN 047108364X. ISBN 9780471083641. Volume 1, p. 653. [Google Scholar]
- Harwood, L.M.; Vickers, R.J. Azomethine Ylides. In The Chemistry of Heterocyclic Compounds, Volume 59. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Padwa, A., Pearson, W.H., Eds.; Wiley: New York, NY, USA, 2003; Volume 59, Ch. 3; pp. 169–252. [Google Scholar]
- Pandey, G.; Banerjee, P.; Gadre, S.R. Construction of Enantiopure Pyrrolidine Ring System via Asymmetric [3 + 2]-Cycloaddition of Azomethine Ylides. Chem. Rev. 2006, 106, 4484–4517. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R.; Niklas, K. The Chemistry of an Isolable Azomethine Ylide. Heterocycles 1984, 22, 21–26. [Google Scholar] [CrossRef]
- Song, G.; Chen, D.; Su, Y.; Han, K.; Pan, C.-L.; Jia, A.; Li, X. Isolation of Azomethine Ylides and Their Complexes: Iridium(III)-Mediated Cyclization of Nitrone Substrates Containing Alkynes. Angew. Chem. Int. Ed. 2011, 50, 7791–7796. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Han, H.S.; Shin, J.; Yoo, E.J. Multicomponent [5 + 2] Cycloaddition Reaction for the Synthesis of 1,4-Diazepines: Isolation and Reactivity of Azomethine Ylides. J. Am. Chem. Soc. 2014, 136, 11606–11609. [Google Scholar] [CrossRef] [PubMed]
- Coldham, I.; Hufton, R. Intramolecular Dipolar Cycloaddition Reactions of Azomethine Ylides. Chem. Rev. 2005, 105, 2765–2810. [Google Scholar] [CrossRef] [PubMed]
- Husinec, S.; Savic, V. Chiral catalysts in the stereoselective synthesis of pyrrolidine derivatives via metallo-azomethine ylides. Tetrahedron Asymmetry 2005, 16, 2047–2061. [Google Scholar] [CrossRef]
- Najera, C.; Sansano, J.M. Catalytic Enantioselective 1,3-Dipolar Cycloaddition Reaction of Azomethine Ylides and Alkenes: The Direct Strategy to Prepare Enantioenriched Highly Substituted Proline Derivatives. Angew. Chem. Int. Ed. Engl. 2005, 44, 6272–6276. [Google Scholar] [CrossRef] [PubMed]
- Pearson, W.H.; Stoy, P. Cycloadditions of Nonstabilized 2-Azaallyllithiums (2-Azaallyl Anions) and Azomethine Ylides with Alkenes: [3 + 2] Approaches to Pyrrolidines and Application to Alkaloid Total Synthesis. Synlett 2003, 903–921. [Google Scholar] [CrossRef]
- Koumbis, A.E.; Gallos, J.K. 1,3-Dipolar Cycloadditions in the Synthesis of Carbohydrate Mimics. Part 3: Azides, Diazo Compounds and Other Dipoles. Curr. Org. Chem. 2003, 7, 771–797. [Google Scholar] [CrossRef]
- Maggini, M.; Scorrano, G.; Prato, M. Addition of azomethine ylides to C60: Synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 1993, 115, 9798–9799. [Google Scholar] [CrossRef]
- Prato, M.; Maggini, M. Fulleropyrrolidines: A Family of Full-Fledged Fullerene Derivatives. Acc. Chem. Res. 1998, 31, 519–526. [Google Scholar] [CrossRef]
- Ruiz, A.; Morera-Boado, C.; Almagro, L.; Coro, J.; Maroto, E.E.; Herranz, M.A.; Filippone, S.; Molero, D.; Martinez-Alvarez, R.; de la Garcia Vega, J.M.; et al. Dumbbell-Type Fullerene-Steroid Hybrids: A Join Experimental and Theoretical Investigation for Conformational, Configurational, and Circular Dichroism Assignments. J. Org. Chem. 2014, 79, 3473–3486. [Google Scholar] [CrossRef] [PubMed]
- Aroua, S.; Schweizer, W.B.; Yamakoshi, Y. C60 Pyrrolidine Bis-carboxylic Acid Derivative as a Versatile Precursor for Biocompatible Fullerene. Org. Lett. 2014, 16, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Maroto, E.E.; Filippone, S.; Suarez, M.; Martinez-Alvarez, R.; de Cozar, A.; Cossio, F.P.; Martin, N. Stereodivergent Synthesis of Chiral Fullerenes by [3 + 2] Cycloadditions to C60. J. Am. Chem. Soc. 2014, 136, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Eberbach, W. Azomethine Ylides. Sci. Synth. 2004, 27, 441–498. [Google Scholar] [CrossRef]
- Najera, C.; Sansano, J.M. Azomethine Ylides in Organic Synthesis. Curr. Org. Chem. 2003, 7, 1105–1150. [Google Scholar] [CrossRef]
- Tsuge, O.; Kanemasa, S. Recent Advances in Azomethine Ylide Chemistry. Adv. Heterocycl. Chem. 1989, 45, 231–349. [Google Scholar]
- Lown, J.W.; Dallas, G.; Maloney, T.W. Reaction of 3-aroylaziridines with aryl isothiocyanates. Can. J. Chem. 1969, 47, 3557–3567. [Google Scholar] [CrossRef]
- Lown, J.W.; Moser, J.P. 1,3-Dipolar cycloaddition of nitrosonaphthols to 3-aroylazindines. Novel syntheses of substituted naphtho[1,2-d]oxazoles and naphtho[2,1-d]oxazoles. Can. J. Chem. 1970, 48, 2227–2233. [Google Scholar]
- Huisgen, R.; Martin-Ramos, V.; Scheer, W. Cycloadditions of an aziridine via azomethine ylide to heteromultiple bonds. Tetrahedron Lett. 1971, 12, 477–480. [Google Scholar] [CrossRef]
- Heine, H.W.; Henzel, R.P. Aziridines. XXI. 1,4-Diazabicyclo[4.1.0]hept-4-enes and 1,1a-dihydro-1,2-diarylazirino[1,2-a]quinoxalines. J. Org. Chem. 1969, 34, 171–175. [Google Scholar] [CrossRef]
- Ryan, J.H. 1,3-Dipolar cycloaddition reactions of azomethine ylides with aromatic dipolarophiles. ARKIVOC 2015, 2015, 160–183. [Google Scholar]
- D’Souza, A.M.; Spiccia, N.; Basutto, J.; Jokisz, P.; Wong, L.S.-M.; Meyer, A.G.; Holmes, A.B.; White, J.M.; Ryan, J.H. 1,3-Dipolar cycloaddition—Decarboxylation reactions of an azomethine ylide with isatoic anhydrides: Formation of novel benzodiazepinones. Org. Lett. 2011, 13, 486–489. [Google Scholar] [PubMed]
- Santos, H.; Distiller, A.; D’Souza, A.M.; Arnoux, Q.; White, J.M.; Meyer, A.G.; Ryan, J.H. 1,3-Dipolar cycloaddition reactions of phthalic anhydrides with an azomethine ylide. Org. Chem. Front. 2015, 2, 705–712. [Google Scholar] [CrossRef]
- Moshkin, V.S.; Sosnovskikh, V.Y. A one-pot synthesis of tetrahydroisoquinolin-4-ols via a novel acid-catalyzed rearrangement of 5-aryloxazolidines. Tetrahedron Lett. 2013, 54, 2455–2457. [Google Scholar] [CrossRef]
- Davis, C.B.; Camp, B.J.; Read, J.C. The vasoactive potential of halostachine, an alkaloid of tall fescue (Festuca arundinaceae, Schreb) in cattle. Vet. Hum. Toxicol. 1983, 25, 408–411. [Google Scholar] [PubMed]
- Bowman, W.C.; Nott, M.W. Actions of sympathomimetic am.ines and their antagonists on skeletal muscle. Pharmacol. Rev. 1969, 21, 27–72. [Google Scholar]
- Antoniu, S. For treating asthma and chronic obstructive pulmonary disease. Expert Opin. Investig. Drugs 2014, 23, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Keck, J.; Engelhardt, G. Neue oxazolidine. DE 2213271, 27 August 1973. [Google Scholar]
- Jordan, D.B.; Livingston, R.S.; Bisaha, J.J.; Duncan, K.E.; Pember, S.O.; Picollelli, M.A.; Schwartz, R.S.; Sternberg, J.A.; Tang, X.-S. Oxazolidinones: A new chemical class of fungicides and inhibitors of mitochondrial cytochrome bc1 function. Pestic. Sci. 1999, 55, 213–215. [Google Scholar] [CrossRef]
- Hooper, A.J.; Burnett, J.R. Anacetrapib, a cholesteryl ester transfer protein inhibitor. Expert Opin. Investig. Drugs 2012, 21, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R. Neues über 1,3-Cycloadditionen. Helv. Chim. Acta 1967, 50, 2421–2439. [Google Scholar] [CrossRef]
- Brunn, E.; Huisgen, R. Cycloadditions of aziridines to azo compounds. Tetrahedron Lett. 1971, 12, 473–476. [Google Scholar] [CrossRef]
- Texier, F.; Carrié, R. Cycloadditions d’ylures d’azométhines à quelques aldéhydes et imines. Syntheses d’oxazolidines et d’imidazolidines. C. R. Acad. Sci. Paris Ser. C 1969, 269, 709–712. [Google Scholar]
- Texier, F.; Carrié, R. Addition d’un ylure d’azométhine à quelques dipolarophiles hétéroatomiques. Bull. Soc. Chim. Fr. 1973, 3437–3442. [Google Scholar]
- Texier, F.; Carrié, R.; Jaz, J. Cycloaddition of an aziridine to ketens. J. Chem. Soc. Chem. Commun. 1972, 199–200. [Google Scholar] [CrossRef]
- Dallas, G.; Lown, J.W.; Moser, J.P. The reaction of 2-aroylaziridines with aldehydes to form oxazolidines. J. Chem. Soc. D 1970, 278–279. [Google Scholar] [CrossRef]
- Dallas, G.; Lown, J.W.; Moser, J.P. The reaction of 2-arenoylaziridines with aldehydes to form oxazolidines. J. Chem. Soc. C 1970, 2383–2394. [Google Scholar] [CrossRef]
- Lown, J.W.; Smalley, R.K.; Dallas, G. The addition of azomethine ylids to diphenylcyclopropenone: Synthesis of novel 4-oxazolines. Chem. Commun. (Lond.) 1968, 1543–1545. [Google Scholar] [CrossRef]
- Lown, J.W.; Smalley, R.K.; Dallas, G.; Maloney, T.W. Reaction of 3-aroylaziridines with diphenylcyclopropenone. Synthesis and properties of novel 4-aroyl-4-oxazolines. Can. J. Chem. 1970, 48, 89–101. [Google Scholar] [CrossRef]
- Lown, J.W.; Akhtar, M.H. Thermal Decomposition of Stereoisomeric 3-Aroyl- and 3-Carbomethoxyaziridines in Acetonitrile. Can. J. Chem. 1972, 50, 2236–2248. [Google Scholar] [CrossRef]
- Schirmeister, T. Aziridine-2,2-dicarboxylates: Synthesis, Reactions, and Photochromism. Liebigs Ann. 1997, 1997, 1895–1899. [Google Scholar] [CrossRef]
- Zbruyev, A.I.; Yaremenko, F.G.; Chebanov, V.A.; Desenko, S.M.; Shishkin, O.V.; Lukinova, E.V.; Knyazeva, I.V. Synthesis and study of new 2-aryl-1-(4-nitrophenyl)-1,1a-dihydroazireno[1,2-a]quinoxaline derivatives. Russ. Chem. Bull. 2006, 55, 362–368. [Google Scholar] [CrossRef]
- Danielsson, J.; Toom, L.; Somfai, P. 1,3-Dipolar Cycloaddition of Azomethine Ylides to Aldehydes: Synthesis of anti α-Amino-β-Hydroxy Esters. Eur. J. Org. Chem. 2011, 607–613. [Google Scholar] [CrossRef]
- Wu, X.; Li, L.; Zhang, J. Nickel(II)-catalyzed diastereoselective [3 + 2] cycloaddition of N.-tosyl-aziridines and aldehydes via selective carbon-carbon bond cleavage. Chem. Commun. 2011, 47, 7824–7826. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, J.; Lu, P.; Wang, Y. Diasteroselective synthesis of oxazolidines and imidazolidines via the Lewis acid catalyzed C–C cleavage of aziridines. Tetrahedron 2011, 67, 9609–9617. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J. Lewis Acid-catalyzed [3 + 2] Cycloaddition of Alkynes with N.-Tosyl-aziridines via Carbon–Carbon Bond Cleavage: Synthesis of Highly Substituted 3-Pyrrolines. Org. Lett. 2011, 13, 5940–5943. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, G.P. Evidence for an azomethine ylide intermediate in the carbonyl-assisted decarboxylation of sarcosine. Novel synthesis of dl-phenylephrine hydrochloride. J. Org. Chem. 1970, 35, 2069–2072. [Google Scholar] [CrossRef]
- Forte, M.; Orsini, F.; Pelizzoni, F. Synthesis of Pyrrolizidines and 1-Oxapyrrolizidines from Proline. Gazz. Chim. Ital. 1985, 115, 569–571. [Google Scholar]
- Orsini, F.; Pelizzoni, F.; Forte, M.; Destro, R.; Gariboldi, P. 1,3-Dipolar cycloadditions of azomethine ylides with aromatic aldehydes. Syntheses of 1-oxapyrrolizidines and 1,3-oxazolidines. Tetrahedron 1988, 44, 519–541. [Google Scholar] [CrossRef]
- Gayen, B.; Banerji, A. Simple and efficient routes to substituted oxazolidine and spiro-oxindole systems by one-pot synthetic strategies. Monatsh. Chem. 2014, 145, 1953–1965. [Google Scholar] [CrossRef]
- Moshkin, V.S.; Sosnovskikh, V.Y. A novel synthesis of 2-alkyl(aryl)pyrrolidines from proline via 2,3-diphenylhexahydropyrrolo[2,1-b]oxazoles. Mendeleev. Commun. 2015, 25, 440–442. [Google Scholar] [CrossRef]
- Aly, M.F.; Ardill, H.; Grigg, R.; Leong-Ling, S.; Rajviroongit, S.; Surendrakumar, S. The reaction of secondary α-amino acids with carbonyl compounds. Properties of the intermediate azomethine ylides. Oxazolidine formation versus 1.4-prototropy. Tetrahedron Lett. 1987, 28, 6077–6080. [Google Scholar] [CrossRef]
- Grigg, R.; Surendrakumar, S.; Thianpatanagul, S.; Vipond, D. X=Y-ZH systems as potential 1,3-dipoles. Part 11. Stereochemistry of 1,3-dipoles generated by the decarboxylative route to azomethine ylides. J. Chem. Soc. Perkin Trans. 1 1988, 2693–2701. [Google Scholar] [CrossRef]
- Kanemasa, S.; Sakamoto, K.; Tsuge, O. Nonstabilized azomethine ylides generated by decarboxylative condensation of α-amino acids. Structural variation, reactivity, and stereoselectivity. Bull. Chem. Soc. Jpn. 1989, 62, 1960–1968. [Google Scholar] [CrossRef]
- Arany, A.; Bendell, D.W.; Groundwater, P.; Garnett, I.; Nyerges, M. 1,7-Electrocyclisation of non-stabilised α,β:γ,δ-unsaturated azomethine ylides. J. Chem. Soc. Perkin Trans. 1 1999, 2605–2608. [Google Scholar] [CrossRef]
- Dambruoso, P.; Massi, A.; Dondoni, A. Efficiency in Isotetronic Acid Synthesis via a Diamine-Acid Couple Catalyzed Ethyl Pyruvate Homoaldol Reaction. Org. Lett. 2005, 7, 4657–4660. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, H.; Iranpoor, N.; Ghaderi, A.; Ghavami, M. Cerium(IV) oxide as a neutral catalyst for aldehyde-induced decarboxylative coupling of l-proline with triethyl phosphite and nitromethane. Tetrahedron Lett. 2012, 53, 5515–5518. [Google Scholar] [CrossRef]
- Ning, F.; Anderson, R.J.; Hibbs, D.E.; Groundwater, P.W. A new, one-step synthesis of 1-heteroaryl-2-alkylaminoethanols. Tetrahedron Lett. 2010, 51, 843–845. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Q.; Zhang, J.; Song, G. Catalyst-free synthesis of N-β-hydroxyethyl pyrroles and indoles via a domino [3 + 2] cycloaddition and ring-opening aromatization process. Tetrahedron Lett. 2015, 56, 2913–2916. [Google Scholar] [CrossRef]
- Grigg, R.; Thianpatanagul, S. Decarboxylative transamination. Mechanism and applications to the synthesis of heterocyclic compounds. J. Chem. Soc. Chem. Commun. 1984, 180–181. [Google Scholar] [CrossRef]
- Grigg, R.; Idle, J.; McMeekin, P.; Vipond, D. The decarboxylative route to azomethine ylides. Mechanism of 1,3-dipole formation. J. Chem. Soc. Chem. Commun. 1987, 49–51. [Google Scholar] [CrossRef]
- Tsuge, O.; Kanemasa, S.; Ohe, M.; Takenaka, S. Simple Generation of Nonstabilized Azomethine Ylides through Decarboxylative Condensation of α-Amino Acids with Carbonyl Compounds via 5-Oxazolidinone Intermediates. Bull. Chem. Soc. Jpn. 1987, 60, 4079–4089. [Google Scholar] [CrossRef]
- Joucla, M.; Mortier, J. Parent and N-substituted azomethine ylides from α-amino acids and formaldehyde. An easy access to 2,5-unsubstituted pyrrolidines. Evidence for oxazolidin-5-ones as direct precursor of these reactive intermediates. Bull. Soc. Chim. Fr. 1988, 125, 579–583. [Google Scholar]
- Andrews, M.D.; Brown, G.A.; Charmant, J.P.H.; Peakman, T.M.; Rebello, A.; Walsh, K.E.; Gallagher, T.; Hales, N.J. Aldehydes and ketones as dipolarophiles: Application to the synthesis of oxapenams. Chem. Commun. 1999, 249–250. [Google Scholar] [CrossRef]
- Nyerges, M.; Fejes, I.; Virányi, A.; Groundwater, P.W.; Töke, L. A New and Convenient Synthesis of 1-Aryl-2-dimethylaminoethanols. Synthesis 2001, 1479–1482. [Google Scholar] [CrossRef]
- Rudas, M.; Fejes, I.; Nyerges, M.; Szöllõsy, Á.; Tõke, L.; Groundwater, P.W. Substituent effects on the 4π + 2π cycloadditions of 4H-pyran-4-one derivatives. J. Chem. Soc. Perkin Trans. 1 1999, 1167–1172. [Google Scholar] [CrossRef]
- Tóth, J.; Blaskó, G.; Dancsó, A.; Tőke, L.; Nyerges, M. Synthesis of New Quinoline Derivatives. Synth. Commun. 2006, 36, 3581–3589. [Google Scholar] [CrossRef]
- Korotaev, V.Y.; Barkov, A.Y.; Moshkin, V.S.; Matochkina, E.G.; Kodess, M.I.; Sosnovskikh, V.Y. Highly diastereoselective 1,3-dipolar cycloaddition of nonstabilized azomethine ylides to 3-nitro-2-trihalomethyl-2H-chromenes: Synthesis of 1-benzopyrano[3,4-c]pyrrolidines. Tetrahedron 2013, 69, 8602–8608. [Google Scholar] [CrossRef]
- Moshkin, V.S.; Sosnovskikh, V.Y. Reaction of 5-aryloxazolidines with arylmagnesium bromides as a new route to N.-benzyl-β-hydroxyphenethylamines as starting materials for the preparation of 4-aryl-1,2,3,4-tetrahydroisoquinolines. Tetrahedron Lett. 2013, 54, 2699–2702. [Google Scholar] [CrossRef]
- Moshkin, V.S.; Sosnovskikh, V.Y. A one-pot synthesis of 4-aryl-2-methyl-1,2,3,4-tetrahydro-γ-carbolines from 5-aryloxazolidines and indoles via a Mannich/Friedel–Crafts sequence. Tetrahedron Lett. 2014, 55, 6121–6124. [Google Scholar] [CrossRef]
- Moshkin, V.S.; Sosnovskikh, V.Y. A simple two-step synthesis of 2-(alkylamino)-1-arylethanols, including racemic adrenaline, from aromatic aldehydes via 5-aryloxazolidines. Tetrahedron Lett. 2013, 54, 5869–5872. [Google Scholar] [CrossRef]
- Moshkin, V.S.; Buev, E.M.; Sosnovskikh, V.Y. Nonstabilized azomethine ylides as reagents for alkylaminomethylation of aromatic ketones via 5-aryloxazolidines. Tetrahedron Lett. 2015, 56, 5278–5281. [Google Scholar] [CrossRef]
- Buev, E.M.; Moshkin, V.S.; Sosnovskikh, V.Y. Synthesis of (alkylaminomethyl)lactones and hydroxypiperidones using alkylaminomethylation methodology. Tetrahedron Lett. 2015, 56, 6590–6592. [Google Scholar] [CrossRef]
- Sosnovskikh, V.Y.; Kornev, M.Y.; Moshkin, V.S. 3-Cyanochromones in [3 + 2] cycloadditions with an azomethine ylide derived from sarcosine and formaldehyde. A short synthesis of 1-benzopyrano[2,3-c:3,4-c′]dipyrrolidines. Tetrahedron Lett. 2014, 55, 212–214. [Google Scholar] [CrossRef]
- Sosnovskikh, V.Y.; Kornev, M.Y.; Moshkin, V.S.; Buev, E.M. Substituted chromones in [3 + 2] cycloadditions with nonstabilized azomethine ylides: Synthesis of 1-benzopyrano[2,3-c]pyrrolidines and 1-benzopyrano[2,3-c:3,4-c′]dipyrrolidines. Tetrahedron 2014, 70, 9253–9261. [Google Scholar] [CrossRef]
- Nyerges, M.; Fejes, I.; Virányi, A.; Groundwater, P.W.; Tőke, L. Synthesis of indazole-N.-oxides via the 1,7-electrocyclisation of azomethine ylides. Tetrahedron Lett. 2001, 42, 5081–5083. [Google Scholar] [CrossRef]
- Nyerges, M.; Virányi, A.; Zhang, W.; Groundwater, P.W.; Blaskó, G.; Tőke, L. Synthesis of indazole-N-oxides via the 1,7-electrocyclization of azomethine ylides. Tetrahedron 2004, 60, 9937–9944. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Y.; Tan, C.; Chen, H. Cycloaddition of porphyrins to isatin: Synthesis of novel spiro porphyrin derivatives. Synth. Commun. 2006, 36, 2655–2659. [Google Scholar] [CrossRef]
- Sousa, R.M.S.; Pinto, D.C.G.A.; Silva, A.M.S.; Vaz Serra, V.; Barros, A.I.R.N.A.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Flavone-Nitrogen Heterocycle Conjugate Formation by 1,3-Dipolar Cycloadditions. Eur. J. Org. Chem. 2012, 132–143. [Google Scholar] [CrossRef]
- Lubineau, A.; Bouchain, G.; Queneau, Y. Reactivity of the carbonyl group in water. Generation of azomethine ylides from aqueous formaldehyde: Michael addition versus dipolar trapping. J. Chem. Soc. Perkin Trans. 1 1995, 2433–2437. [Google Scholar] [CrossRef]
- Orsini, F.; Pelizzoni, F.; Forte, M.; Sisti, M.; Merati, F.; Gariboldi, P. 1,3-Dipolar cycloadditions of azomethine ylides with dipolarophiles. II. Syntheses of pyrrolizidines. J. Heterocycl. Chem. 1988, 25, 1665–1673. [Google Scholar] [CrossRef]
- Kanemasa, S.; Doi, K.; Wada, E. Intramolecular Cycloaddition of the Azomethine Ylides Derived from α-Amino Acids or Esters and 5-Oxo-6-heptenals or 4-Oxo-5-hexenals. Bull. Chem. Soc. Jpn. 1990, 63, 2866–2871. [Google Scholar] [CrossRef]
- Purushothaman, S.; Raghunathan, R. Stereoselective synthesis of oxazolidine, hexahydropyrrolo [2,1-b] oxazole, and tetrahydro-2H-oxazolo [3,2-c] thiazole grafted macrocycles through intramolecular 1,3-dipolar cycloaddition reaction. Tetrahedron Lett. 2009, 50, 6848–6850. [Google Scholar] [CrossRef]
- Ardill, H.; Grigg, R.; Sridharan, V.; Surendrakumar, S.; Thianpatanagul, S.; Kanajun, S. Iminium ion route to azomethine yields from primary and secondary amines. J. Chem. Soc. Chem. Commun. 1986, 602–604. [Google Scholar] [CrossRef]
- Ardill, H.; Fontaine, X.L.R.; Grigg, R.; Henderson, D.; Montgomery, J.; Sridharan, V.; Surendrakumar, S. X = Y-ZH compounds as potential 13-dipoles. Part 29. The iminium ion route to azomethine ylides. Reaction of cyclic secondary amines with mono- and bi- functional aldehydes. Tetrahedron 1990, 46, 6449–6466. [Google Scholar] [CrossRef]
- Seashore-Ludlow, B.; Torssell, S.; Somfai, P. Addition of Azomethine Ylides to Aldehydes: Mechanistic Dichotomy of Differentially Substituted α-Imino Esters. Eur. J. Org. Chem. 2010, 3927–3933. [Google Scholar] [CrossRef]
- Bowman, R.K.; Johnson, J.S. Lewis Acid Catalyzed Dipolar Cycloadditions of an Activated Imidate. J. Org. Chem. 2004, 69, 8537–8540. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Bagdi, A.K.; Mishra, S.; Hajra, A. Functionalization of an sp3 C-H bond via a redox-neutral domino reaction: Diastereoselective synthesis of hexahydropyrrolo[2,1-b]oxazoles. Chem. Commun. 2014, 50, 2951–2953. [Google Scholar] [CrossRef] [PubMed]
- Pavan Kumar, C.S.; Harsha, K.B.; Mantelingu, K.; Rangappa, K.S. Diastereoselective synthesis of fused oxazolidines and highly substituted 1H-pyrrolo [2,1-c][1,4] oxazines via C-H functionalization. RSC Adv. 2015, 5, 61664–61670. [Google Scholar] [CrossRef]
- Joucla, M.; Mortier, J. Flash vacuum thermolysis of oxazolidines: A new way to reactive azomethine ylides. Regio and stereospecific synthesis of substituted pyrrolidines. Tetrahedron Lett. 1987, 28, 2973–2974. [Google Scholar] [CrossRef]
- Joucla, M.; Mortier, J.; Hamelin, J.; Toupet, L. 1,3-Dipolar cyclo addition reactions of azomethine ylides generated in situ through condensation of secondary α-amino esters with benzaldehyde. Bull. Soc. Chim. Fr. 1988, 125, 143–150. [Google Scholar]
- Mortier, J.; Joucla, M.; Bureau, R. Formation d’ylures d’azométhine par thermolyse d’oxazolidines. Etude de la réaction en solution et en phase vapeur. Bull. Soc. Chim. Fr. 1993, 130, 584–596. [Google Scholar]
- Felluga, F.; Pitacco, G.; Visintin, C.; Valentin, E. Synthesis of polysubstituted pyrrolizidines from proline derivatives and conjugated nitroolefins. Helv. Chim. Acta 1997, 80, 1457–1472. [Google Scholar] [CrossRef]
- Trávnícek, M.; Potácek, M. Pyrrolidines and 1,3-oxazolidines formation influenced by change from classical conditions to microwave irradiation. ARKIVOC 2001, 156–163. [Google Scholar] [CrossRef]
- Harwood, L.M.; Macro, J.; Watkin, D.; Williams, C.E.; Wong, L.F. Asymmetric cycloadditions of aldehydes to stabilised azomethine ylids: Enantiocontrolled construction of β-hydroxy-α-amino acid derivatives. Tetrahedron Asymmetry 1992, 3, 1127–1130. [Google Scholar] [CrossRef]
- Alker, D.; Hamblett, G.; Harwood, L.M.; Robertson, S.M.; Watkin, D.J.; Williams, C.E. Application of enantiopure templated azomethine ylids to β-hydroxy-α-amino acid synthesis. Tetrahedron 1998, 54, 6089–6098. [Google Scholar] [CrossRef]
- Bonin, M.; Chauveau, A.; Micouin, L. Asymmetric 1,3-Dipolar Cycloadditions of Cyclic Stabilized Ylides Derived from Chiral 1,2-Amino Alcohols. Synlett 2006, 2349–2363. [Google Scholar] [CrossRef]
- Harwood, L.M.; Robertson, S.M. Double diastereocontrol in the synthesis of enantiomerically pure polyoxamic acid. Chem. Commun. 1998, 2641–2642. [Google Scholar] [CrossRef]
- Aldous, D.J.; Drew, M.G.B.; Draffin, W.N.; Hamelin, E.M.-N.; Harwood, L.M.; Thurairatnam, S. 1,3-Dipolar Cycloadditions to Unsymmetrical Ketone-Derived Chiral Stabilized Azomethine Ylides: Strategies for the Synthesis of Highly Substituted Amino Acids. Synthesis 2005, 3271–3278. [Google Scholar] [CrossRef]
- Brome, V.A.; Harwood, L.M.; Osborn, H.M. Preparation of enantiopure long chain threo-2-amino-3-hydroxyesters via chiral morpholinone-derived azomethine ylids. Can. J. Chem. 2006, 84, 1448–1455. [Google Scholar] [CrossRef]
- Alezra, V.; Bonin, M.; Chiaroni, A.; Micouin, L.; Riche, C.; Husson, H.-P. Asymmetric functionalization of a chiral non-racemic oxazolidine ester enolate. A new route towards the preparation of quaternary serine esters. Tetrahedron Lett. 2000, 41, 1737–1740. [Google Scholar] [CrossRef]
- Castelló, L.M.; Nájera, C.; Sansano, J.M. Domino 1,3-Dipolar Cycloadditions of N-Alkyl-α-Amino Esters with Paraformaldehyde: A Direct Access to α-Hydroxymethyl α-Amino Acids. Synthesis 2014, 46, 967–971. [Google Scholar] [CrossRef]
- Vedejs, E.; Martinez, G.R. Methylides from trimethylsilylmethylsulfonium, -ammonium, -immonium, and -phosphonium salts. J. Am. Chem. Soc. 1979, 101, 6452–6454. [Google Scholar] [CrossRef]
- Padwa, A.; Haffmanns, G.; Tomas, M. Generation of Azomethine Ylides via the Desilylation Reaction of Immonium Salts. J. Org. Chem. 1984, 49, 3314–3322. [Google Scholar] [CrossRef]
- Fishwick, C.W.G.; Foster, R.J.; Carr, R.E. Hetero-1,3-dipolar cycloadditions of dithiolane-isocyanate imminium methylides: A novel route to 1,3-oxazolidine- and thiazolidine-2-thiones. Tetrahedron Lett. 1996, 37, 711–714. [Google Scholar] [CrossRef]
- Fishwick, C.W.G.; Foster, R.J.; Carr, R.E. A short dipolar cycloaddition approach to γ-lactam alkaloids from cynometra hankei. Tetrahedron Lett. 1996, 37, 3915–3918. [Google Scholar] [CrossRef]
- Pearson, W.H.; Mi, Y. The generation and cycloaddition of 2-azaallyl anions and azomethine ylides from a common precursor. A novel synthesis of indolizidines and other heterocycles. Tetrahedron Lett. 1997, 38, 5441–5444. [Google Scholar] [CrossRef]
- Pearson, W.H.; Stoy, P.; Mi, Y. A Three-Component, One-Pot Synthesis of Indolizidines and Related Heterocycles via the [3 + 2] Cycloaddition of Nonstabilized Azomethine Ylides. J. Org. Chem. 2004, 69, 1919–1939. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Chen, Y.-Y.; Chiacchio, U.; Dent, W. Diastereofacial selectivity in azomethine ylide cycloaddition reactions derived from chiral α-cyanoaminosilanes. Tetrahedron 1985, 41, 3529–3535. [Google Scholar] [CrossRef]
- Padwa, A.; Dent, W.; Nimmesgern, H.; Venkatramanan, M.K.; Wong, G.S.K. Utilization of Phenylthio Substituted Amines for the Synthesis of Pyrrolidines. Chem. Ber. 1986, 119, 818–828. [Google Scholar] [CrossRef]
- Padwa, A.; Dent, W. On the Use of N-[(T rimethylsilyl)methyl]amino Ethers as Capped Azomethine Ylide Equivalents. J. Org. Chem. 1987, 52, 235–244. [Google Scholar] [CrossRef]
- Hosomi, A.; Sakata, Y.; Sakurai, H. N-(Trimethylsilylmethyl)aminomethyl ethers as azomethine ylide synthons. A new and convenient access to pyrrolidine derivatives. Chem. Lett. 1984, 7, 1117–1120. [Google Scholar] [CrossRef]
- Terao, Y.; Kotaki, H.; Imai, N.; Achiwa, K. Trifluoroacetic acid-catalysed 1,3-cycloaddition of the simplest iminium ylide leading to 3- or 3,4-substituted pyrrolidines and 2,5-dihydropyrroles. Chem. Pharm. Bull. 1985, 33, 2762–2766. [Google Scholar] [CrossRef]
- Padwa, A.; Dent, W. N-Benzyl-N-methoxymethyl-N-(trimethylsilyl)methylamine as an Azomethine Ylide Equivalent: 2,6-dioxo-1-phenyl-4-benzyl-1,4-diazabicyclo[3.3.0]octane. Org. Synth. 1989, 67, 133–137. [Google Scholar]
- Ryan, J.H.; Spiccia, N.; Wong, L.S.-M.; Holmes, A.B. Synthesis of 5-Aryloxazolidines via 1,3-Dipolar Cycloaddition Reaction of a Non-Stabilized Azomethine Ylide with Aromatic Aldehydes. Aust. J. Chem. 2007, 60, 898–904. [Google Scholar] [CrossRef]
- Lee, S.; Diab, S.; Queval, P.; Sebban, M.; Chataigner, I.; Piettre, S.R. Aromatic C=C Bonds as Dipolarophiles: Facile Reactions of Uncomplexed Electron-Deficient Benzene Derivatives and Other Aromatic Rings with a Non-Stabilized Azomethine Ylide. Chem. Eur. J. 2013, 19, 7181–7192. [Google Scholar] [CrossRef] [PubMed]
- Yoon, U.C.; Kim, D.U.; Kim, J.C.; Lee, J.G. A novel azomethine ylid forming photoreaction of N-trimethylsilylmethylphthalimides. Tetrahedron Lett. 1993, 34, 5859–5862. [Google Scholar]
- Yoon, U.C.; Kim, D.U.; Lee, C.W.; Choi, Y.S.; Lee, Y.-J.; Ammon, H.L.; Mariano, P.S. Novel and efficient azomethine ylide forming photoreactions of N-(silylmethyl)phthalimides and related acid and alcohol derivatives. J. Am. Chem. Soc. 1995, 117, 2698–2710. [Google Scholar] [CrossRef]
- Pandey, G.; Lakshmaiah, G.; Gadre, S.R. Sequential two-electron oxidation of α,α′-disilylmethylamines to generate non-stabilised azomethine ylide: An ideal approach for the construction of substituted and fused pyrrolidine ring systems. Ind. J. Chem. 1996, 35, 91–98. [Google Scholar]
- Bartnik, R.; Mlostoń, G. Aziridines-IV Catalytic decomposition of phenyldiazomethane in Schiff’s bases. Tetrahedron 1984, 40, 2569–2576. [Google Scholar] [CrossRef]
- Padwa, A.; Dean, D.C.; Osterhout, M.H.; Precedo, L.; Semones, M.A. Synthesis of functionalized azomethine ylides via Rh(II)-ccatalysed cyclisation of α-diazocarbonyls onto imino π-bonds. J. Org. Chem. 1994, 59, 5347–5357. [Google Scholar] [CrossRef]
- Kadina, A.P.; Khlebnikov, A.F.; Novikov, M.S.; Pérez, P.J.; Yufit, D.S. Intramolecular cycloaddition of azomethine ylides, from imines of O.-acylsalicylic aldehyde and ethyl diazoacetate, to ester carbonyl—Experimental and DFT computational study. Org. Biomol. Chem. 2012, 10, 5582–5591. [Google Scholar] [CrossRef] [PubMed]
- Novikov, M.S.; Khlebnikov, A.F.; Krebs, A.; Kostikov, R.R. Unprecedented 1,3-dipolar cycloaddition of azomethine ylides derived from difluorocarbene and imines to carbonyl compounds. Synthesis of oxazolidine derivatives. Eur. J. Org. Chem. 1998, 1998, 133–137. [Google Scholar] [CrossRef]
- Novikov, M.S.; Khlebnikov, A.F.; Sidorina, E.S.; Kostikov, R.R. 1,3-Dipolar cycloaddition of azomethine ylides derived from imines and difluorocarbene to alkynes: A new active Pb-mediated approach to 2-fluoropyrrole derivatives. J. Chem. Soc. Perkin Trans. 1 2000, 231–237. [Google Scholar] [CrossRef]
- Khistiaev, K.A.; Novikov, M.S.; Khlebnikov, A.F.; Magull, J. gem-Difluorosubstituted NH-azomethine ylides in the synthesis of 4-fluorooxazolines via the three-component reaction of imines, trifuoroacetophenones and CF2Br2. Tetrahedron Lett. 2008, 49, 1237–1240. [Google Scholar] [CrossRef]
- Novikov, M.S.; Voznyi, I.V.; Khlebnikov, A.F.; Kopf, J.; Kostikov, R.R. Unprecedented 1,3-dipolar cycloaddition of azomethine ylides to ester carbonyl. J. Chem. Soc. Perkin Trans. 1 2002, 1628–1630. [Google Scholar] [CrossRef]
- Voznyi, I.V.; Novikov, M.S.; Khlebnikov, A.F.; Kopf, J.; Kostikov, R.R. Intramolecular 1,3-Dipolar cycloaddition to ester carbonyl of azomethinylides prepared from aldimines and difluorocarbene. Russ. J. Org. Chem. 2004, 40, 199–205. [Google Scholar] [CrossRef]
- Voznyi, I.V.; Novikov, M.S.; Khlebnikov, A.F.; Kostikov, R.R. Azomethine ylides derived from dichlorocarbene and O-acylsalicylaldehyde anils in the synthesis of 2,5-epoxy-2,3,4,5-tetrahydro-1,4-benzoxazepin-2-ones and 2-aminoethanols. Russ. Chem. Bull. 2004, 53, 1087–1091. [Google Scholar] [CrossRef]
- Kielland, N.; Catti, F.; Bello, D.; Isambert, N.; Soteras, I.; Luque, F.J.; Lavilla, R. Boron-based dipolar multicomponent reactions: Simple generation of substituted aziridines, oxazolidines and pyrrolidines. Chem. Eur. J. 2010, 16, 7904–7915. [Google Scholar] [CrossRef] [PubMed]
- Böhm, T.; Elender, K.; Riebel, P.; Troll, T.; Weber, A.; Sauer, J. 3,4-Diazanorcaradienes and 4,5-dihydropyridazines as precursors for new stable azomethine ylides. Tetrahedron 1999, 55, 9515–9534. [Google Scholar] [CrossRef]
- Elender, K.; Nöth, H.; Riebel, P.; Weber, A.; Sauer, J. 1,3-Dipolar Cycloaddition Reactions of Stable Bicyclic and Monocyclic Azomethine Ylides: Preparative Aspects. Tetrahedron 2000, 56, 5443–5463. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).