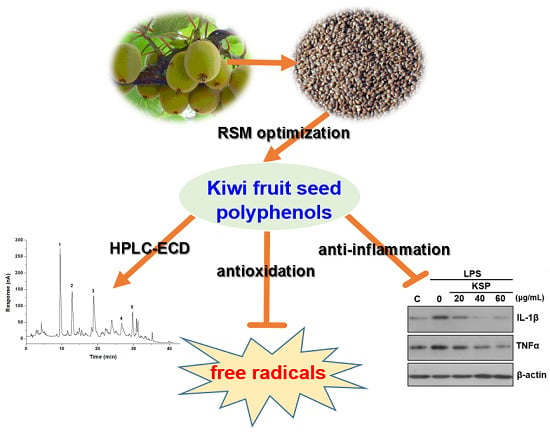
Extraction Optimization of Polyphenols from Waste Kiwi Fruit Seeds (Actinidia chinensis Planch.) and Evaluation of Its Antioxidant and Anti-Inflammatory Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fitting Model
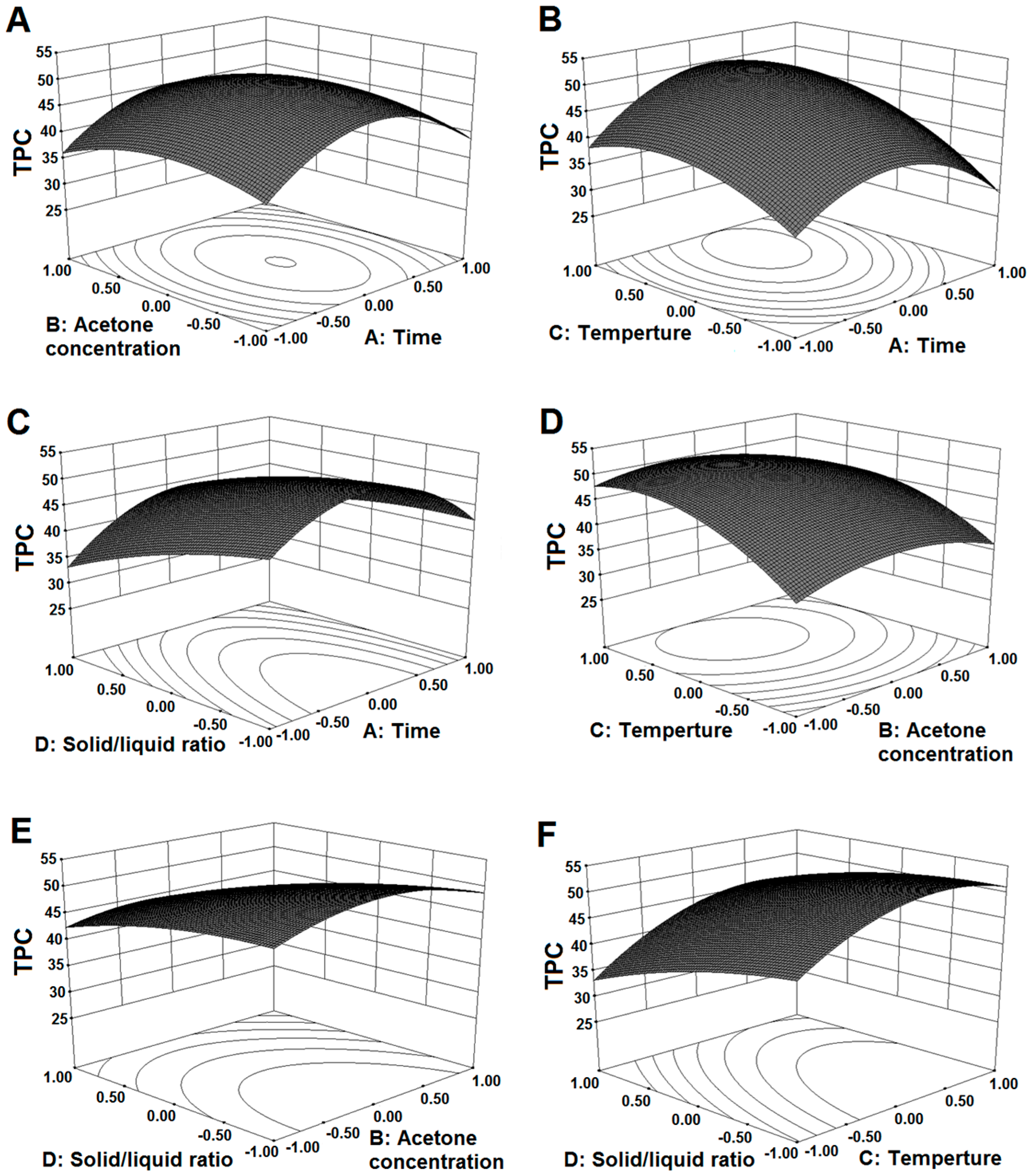
2.2. Influence of Extraction Parameters on TPC
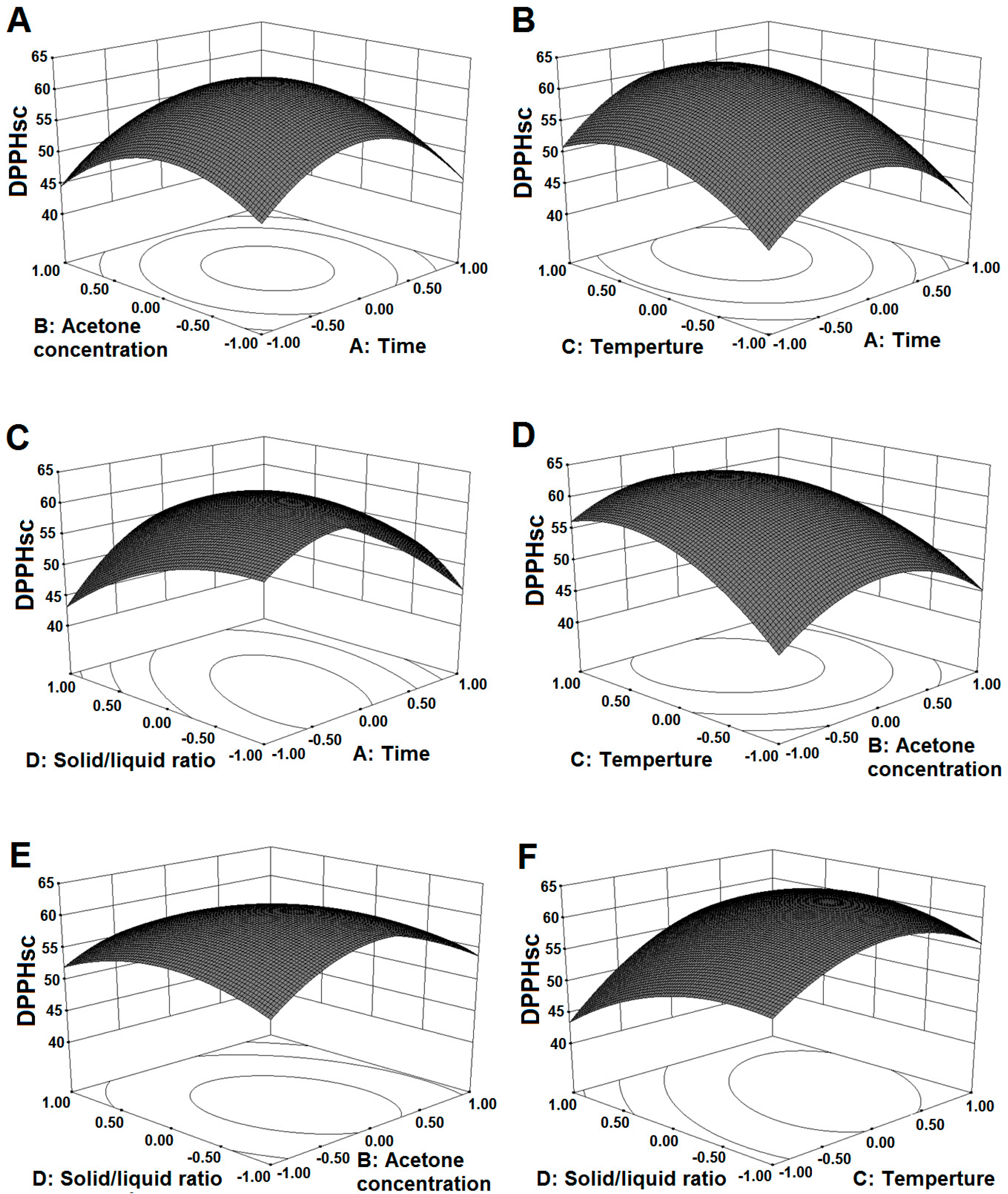
2.3. Influ ence of Extraction Parameters on DPPHsc
2.4. Optimization of Extraction Conditions for TPC and DPPHsc
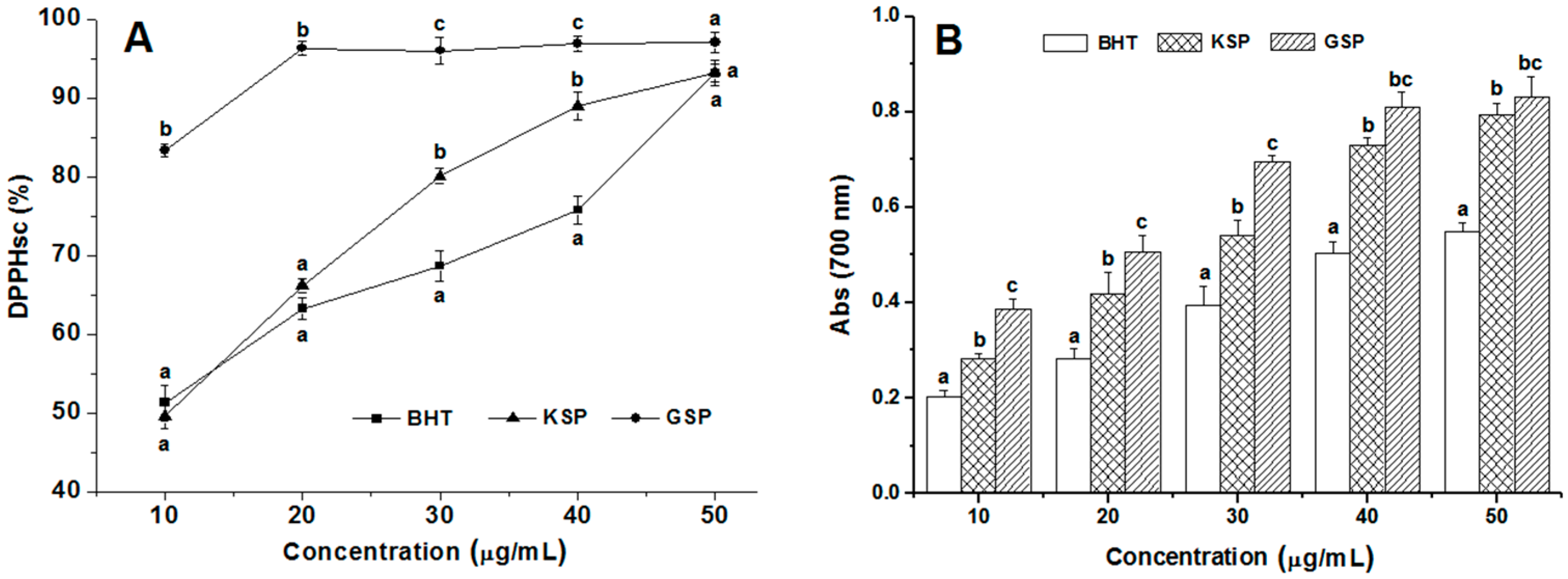
2.5. Antioxidant Activities of KSP
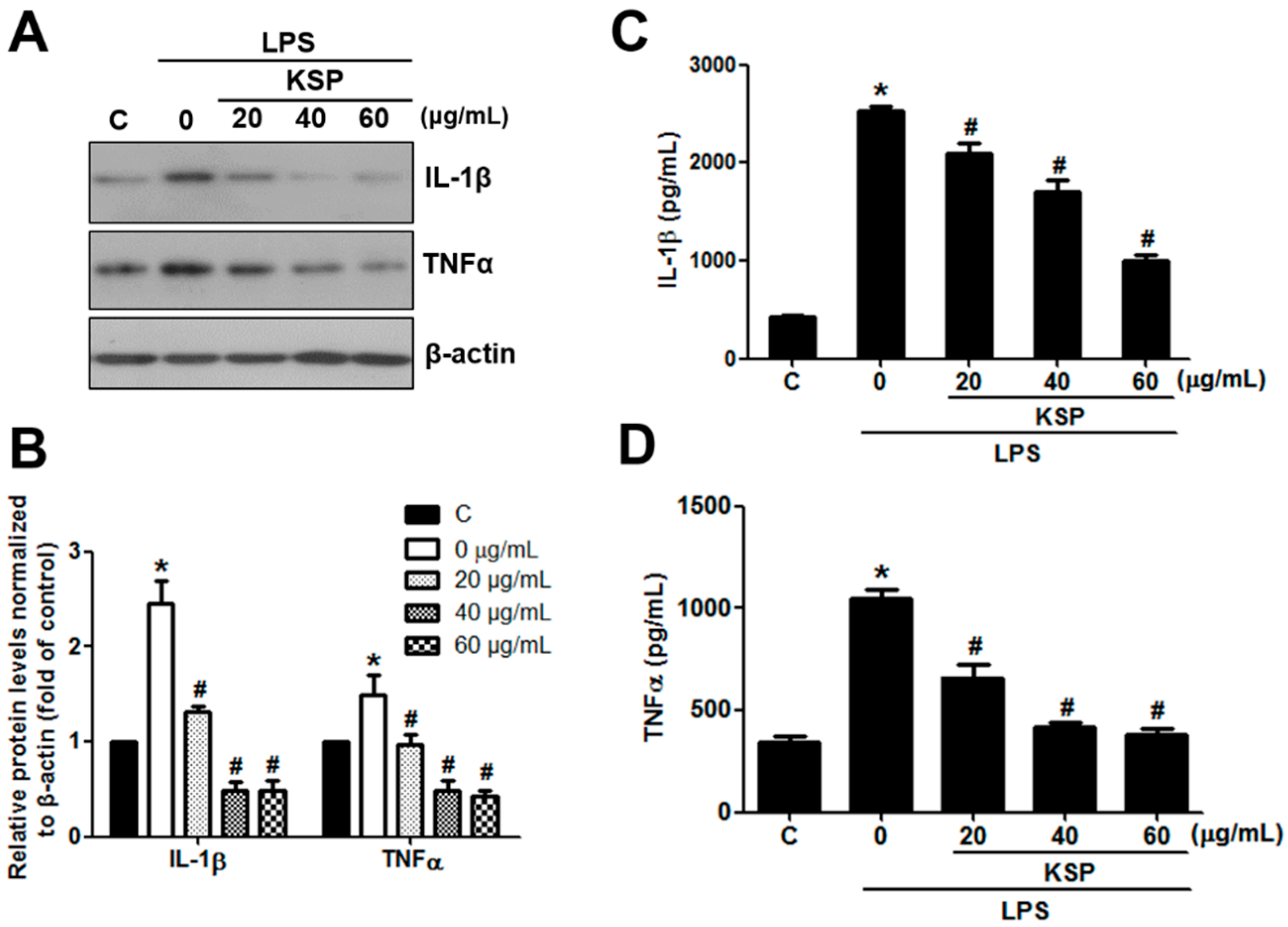
2.6. Anti-Inflammatory Activities of KSP in LPS-Stimulated RAW 264.7 Cells
2.7. Identification of Phenolic Compounds in KSP
3. Materials and Methods
3.1. Materials
3.2. Polyphenols Extraction
3.3. Experimental Design
3.4. TPC Assay
3.5. DPPHsc Assay
3.6. FRAP Assay
3.7. Determination of IL-1β and TNFα
3.8. HPLC-ECD Conditions
3.9. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BHT | Butylated Hydroxytoluene |
| CCD | Central Composite Design |
| DPPH | 2,2-Diphenyl-1-Picrylhyclrazyl Radical |
| FRAP | Ferric Reducing Antioxidant Power |
| GAE | Gallic Acid Equivalents |
| GSP | Grape Seed Polyphenols |
| HPLC-ECD | High Performance Liquid Chromatography-Electrochemical Detector |
| IL-1β | Interleukin-1β |
| KSP | Kiwi Fruit Seed Polyphenols |
| LPS | Lipopolysaccharides |
| RSM | Response Surface Methodology |
| TPC | Total Phenolic Content |
| TNFα | Tumor Necrosis Factor-Alpha |
References
- Gong, Y.; Hou, Z.; Gao, Y.; Xue, Y.; Liu, X.; Liu, G. Optimization of extraction parameters of bioactive components from defatted marigold (Tagetes erecta L.) residue using response surface methodology. Food Bioprod. Process. 2012, 90, 9–16. [Google Scholar] [CrossRef]
- Biglari, F.; AlKarkhi, A.F.; Easa, A.M. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food Chem. 2008, 107, 1636–1641. [Google Scholar] [CrossRef]
- Gan, C.-Y.; Latiff, A.A. Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 2011, 124, 1277–1283. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [PubMed]
- Darvesh, A.S.; Carroll, R.T.; Bishayee, A.; Geldenhuys, W.J.; van der Schyf, C.J. Oxidative stress and Alzheimer’s disease: Dietary polyphenols as potential therapeutic agents. Expert. Rev. Neurother. 2010, 10, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.J.; Hirst, J.J.; Durham, P.L. Dietary grape seed polyphenols repress neuron and glia activation in trigeminal ganglion and trigeminal nucleus caudalis. Mol. Pain 2010, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, S.; Anuradha, C. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin & metformin in a rat model. Indian J. Med. Res. 2009, 129, 401–408. [Google Scholar] [PubMed]
- Schieber, A.; Stintzing, F.; Carle, R. By-products of plant food processing as a source of functional compounds—recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- LIsa, M.; Holcapek, M.; Bohac, M. Statistical evaluation of triacylglycerol composition in plant oils based on high-performance liquid chromatography—Atmospheric pressure chemical ionization mass spectrometry data. J. Agric. Food Chem. 2009, 57, 6888–6898. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Sun, T.; Cao, W.; Fan, D.; Cheng, N.; Wang, B.; Gao, H.; Yang, H. Extraction optimization and functional properties of proteins from kiwi fruit (Actinidia chinensis Planch.) seeds. Int. J. Food Prop. 2014, 17, 1612–1625. [Google Scholar] [CrossRef]
- Rajaei, A.; Barzegar, M.; Hamidi, Z.; Sahari, M. Optimization of extraction conditions of phenolic compounds from pistachio (Pistachia vera) green hull through response surface method. J. Agric. Sci. Technol. 2010, 12, 605–615. [Google Scholar]
- Prasad, K.N.; Hassan, F.A.; Yang, B.; Kong, K.W.; Ramanan, R.N.; Azlan, A.; Ismail, A. Response surface optimisation for the extraction of phenolic compounds and antioxidant capacities of underutilised Mangifera pajang Kosterm. peels. Food Chem. 2011, 128, 1121–1127. [Google Scholar] [CrossRef]
- Hismath, I.; Wan Aida, W.; Ho, C. Optimization of extraction conditions for phenolic compounds from neem (Azadirachta indica) leaves. Int. Food Res. J. 2011, 18, 931–939. [Google Scholar]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Singh, A.; Kuila, A.; Yadav, G.; Banerjee, R. Process optimization for the extraction of polyphenols from okara. Food Technol. Biotechnol. 2011, 49, 322–328. [Google Scholar]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.; Zhao, J.; Wang, Q. Phenolic, flavonoid, and lutein ester content and antioxidant activity of 11 cultivars of Chinese marigold. J. Agric. Food Chem. 2007, 55, 8478–8484. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines IL-6 and TNF-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15, 120–122. [Google Scholar] [PubMed]
- Dostert, C.; Pétrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiao, L.; Yuan, Y.; Luo, X.; Jiang, M.; Ni, J.; Wang, N. Procyanidin B2 inhibits NLRP3 inflammasome activation in human vascular endothelial cells. Biochem. Pharmacol. 2014, 92, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.Y.; Hu, M.M.; Xin, Y.F.; Gang, C. Resveratrol alleviates vascular inflammatory injury by inhibiting inflammasome activation in rats with hypercholesterolemia and vitamin D2 treatment. Inflamm. Res. 2015, 64, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.Z.; Liu, W.; Luo, Y.; Okamoto, M.; Qu, D.; Dunn, J.H.; Fujita, M. Green tea polyphenol epigallocatechin-3-gallate suppresses melanoma growth by inhibiting inflammasome and IL-1β secretion. Biochem. Biophys. Res. Commun. 2011, 414, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Eremina, A.; Reshetnyak, V.Y.; Vezirishvili, M. HPLC determination of polyphenols in dry grape stem extract. Pharm. Chem. J. 2004, 38, 143–145. [Google Scholar] [CrossRef]
- Wijngaard, H.; Brunton, N. The optimization of extraction of antioxidants from apple pomace by pressurized liquids. J. Agric. Food Chem. 2009, 57, 10625–10631. [Google Scholar] [CrossRef] [PubMed]
- Gođevac, D.; Tešević, V.; Veličković, M.; Vujisić, L.; Vajs, V.; Milosavljević, S. Polyphenolic compounds in seeds from some grape cultivars grown in Serbia. J. Serbian Chem. Soc. 2010, 75, 1641–1652. [Google Scholar] [CrossRef]
- Spilioti, E.; Jaakkola, M.; Rolonen, T.; Lipponen, M.; Virtanen, V.; Chinou, I.; Kassi, E.; Karabournioti, S.; Moutsatsou, P. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PLoS ONE 2014, 9, e94860. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.K.; Kang, L.-S. Application of response surface method as an experimental design to optimize coagulation tests. Environ. Eng. Res. 2010, 15, 63–70. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar] [PubMed]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Sample Availability: Not available.

| Independent Variables | Symbol | Coded Variables Levels a | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Time (min) | X1 | 70 | 75 | 80 | 85 | 90 |
| Acetone concentration (v/v) | X2 | 50 | 55 | 60 | 65 | 70 |
| Temperature (°C) | X3 | 30 | 35 | 40 | 45 | 50 |
| Solid/liquid ratio (w/v) | X4 | 1:06 | 1:09 | 1:12 | 1:15 | 1:18 |
| Std | Run | Variables a | TPC (mg GAE/g DW) | DPPHsc (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x4 | Experimental | Predicted | Experimental | Predicted | ||
| Ye1 | Yp1 | Ye2 | Yp2 | ||||||
| 27 | 1 | 80(0) | 60(0) | 40(0) | 1:15(0) | 53.73 | 49.99 | 62.75 | 61.77 |
| 28 | 2 | 80(0) | 60(0) | 40(0) | 1:15(0) | 48.49 | 49.99 | 64.86 | 61.77 |
| 9 | 3 | 75(−1) | 55(−1) | 35(−1) | 1:18(1) | 20.50 | 23.30 | 26.87 | 27.00 |
| 25 | 4 | 80(0) | 60(0) | 40(0) | 1:15(0) | 49.64 | 49.99 | 63.89 | 61.77 |
| 3 | 5 | 75(−1) | 65(1) | 35(−1) | 1:12(−1) | 34.83 | 34.64 | 43.65 | 44.52 |
| 8 | 6 | 85(1) | 65(1) | 45(1) | 1:12(−1) | 37.28 | 36.99 | 35.92 | 35.56 |
| 30 | 7 | 80(0) | 60(0) | 40(0) | 1:15(0) | 47.25 | 49.99 | 61.12 | 61.77 |
| 10 | 8 | 85(1) | 55(−1) | 35(−1) | 1:18(1) | 22.49 | 21.68 | 29.78 | 31.12 |
| 23 | 9 | 80(0) | 60(0) | 40(0) | 1:09(−2) | 55.10 | 49.72 | 48.21 | 51.43 |
| 7 | 10 | 75(−1) | 65(1) | 45(1) | 1:12(−1) | 39.12 | 37.19 | 49.26 | 42.64 |
| 16 | 11 | 85(1) | 65(1) | 45(1) | 1:18(1) | 35.39 | 33.98 | 42.62 | 41.28 |
| 6 | 12 | 85(1) | 55(−1) | 45(1) | 1:12(−1) | 40.48 | 39.91 | 36.65 | 36.56 |
| 26 | 13 | 80(0) | 60(0) | 40(0) | 1:15(0) | 52.28 | 49.99 | 61.29 | 61.77 |
| 22 | 14 | 80(0) | 60(0) | 50(2) | 1:15(0) | 37.02 | 41.16 | 43.32 | 47.71 |
| 14 | 15 | 85(1) | 55(−1) | 45(1) | 1:15(1) | 39.48 | 42.18 | 50.61 | 49.52 |
| 29 | 16 | 80(0) | 60(0) | 40(0) | 1:15(0) | 48.53 | 49.99 | 53.51 | 61.77 |
| 19 | 17 | 80(0) | 50(−2) | 40(0) | 1:15(0) | 37.76 | 37.69 | 36.13 | 36.97 |
| 31 | 18 | 80(0) | 60(0) | 40(0) | 1:15(0) | 50.13 | 49.99 | 64.96 | 61.77 |
| 11 | 19 | 75(−1) | 65(1) | 35(−1) | 1:18(1) | 21.94 | 19.78 | 27.83 | 22.64 |
| 17 | 20 | 70(−2) | 60(0) | 40(0) | 1:15(0) | 11.11 | 15.91 | 19.53 | 26.53 |
| 24 | 21 | 80(0) | 60(0) | 40(0) | 1:21(2) | 31.53 | 37.13 | 40.23 | 42.51 |
| 13 | 22 | 75(−1) | 55(−1) | 45(1) | 1:18(1) | 34.73 | 34.50 | 45.29 | 41.80 |
| 5 | 23 | 75(−1) | 55(−1) | 45(1) | 1:12(−1) | 36.06 | 40.10 | 46.27 | 45.80 |
| 1 | 24 | 75(−1) | 55(−1) | 35(−1) | 1:12(−1) | 34.21 | 32.88 | 45.58 | 41.64 |
| 20 | 25 | 80(0) | 70(2) | 40(0) | 1:15(0) | 30.90 | 31.26 | 26.89 | 31.61 |
| 15 | 26 | 75(−1) | 65(1) | 45(1) | 1:18(1) | 26.98 | 26.31 | 32.15 | 31.40 |
| 18 | 27 | 90(2) | 60(0) | 40(0) | 1:15(0) | 18.65 | 14.09 | 25.01 | 23.57 |
| 2 | 28 | 85(1) | 55(−1) | 35(−1) | 1:12(−1) | 22.42 | 23.38 | 28.27 | 28.80 |
| 21 | 29 | 80(0) | 60(0) | 30(−2) | 1:15(0) | 22.01 | 18.10 | 22.01 | 31.19 |
| 12 | 30 | 85(1) | 65(1) | 35(−1) | 1:18(1) | 19.67 | 18.15 | 28.63 | 28.92 |
| 4 | 31 | 85(1) | 65(1) | 35(−1) | 1:12(−1) | 27.65 | 25.14 | 35.62 | 33.84 |
| Regression Coefficient | Polynomial Equation | R2 | Adjusted R2 | p Value | Lack of Fit (p Value) |
|---|---|---|---|---|---|
| TPC (Y1) | 50.01 + 0.48X1 − 0.88X2 + 4.83X3 − 4.08X4 − 0.14X1X2 + 2.19X1X3 + 1.83X1X4 − 1.03X2X3 − 1.18X2X4 + 1.13X3X4 − 8.73X12 − 3.87X22 − 5.07X32 − 1.62X42 | 0.9654 | 0.9351 | <0.0001 | 0.1764 |
| DPPHsc (Y2) | 61.77 − 0.74X1 − 1.34X2 + 4.13X3 − 2.23X4 + 0.54X1X2 + 0.90X1X3 + 4.24X1X4 − 1.51X2X3 − 1.81X2X4 + 2.66X3X4 − 9.18X12 − 6.87X22 − 5.58X32 − 3.70X42 | 0.9420 | 0.8913 | <0.0001 | 0.3545 |
| Compounds | Retention Time (tR) (min) | Amount Detected (mg·g−1 KSP) |
|---|---|---|
| protocatechuic acid | 9.51 | 96.80 ± 1.93 |
| p-hydroxybenzoic acid | 12.82 | 186.32 ± 3.05 |
| caffeic acid | 18.89 | 40.40 ± 1.73 |
| p-coumaric acid | 26.69 | 81.65 ± 2.25 |
| ferulic acid | 29.71 | 19.64 ± 1.12 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Liu, Q.; Zhang, C.; Cao, W.; Fan, D.; Yang, H. Extraction Optimization of Polyphenols from Waste Kiwi Fruit Seeds (Actinidia chinensis Planch.) and Evaluation of Its Antioxidant and Anti-Inflammatory Properties. Molecules 2016, 21, 832. https://doi.org/10.3390/molecules21070832
Deng J, Liu Q, Zhang C, Cao W, Fan D, Yang H. Extraction Optimization of Polyphenols from Waste Kiwi Fruit Seeds (Actinidia chinensis Planch.) and Evaluation of Its Antioxidant and Anti-Inflammatory Properties. Molecules. 2016; 21(7):832. https://doi.org/10.3390/molecules21070832
Chicago/Turabian StyleDeng, Jianjun, Qingqing Liu, Chao Zhang, Wei Cao, Daidi Fan, and Haixia Yang. 2016. "Extraction Optimization of Polyphenols from Waste Kiwi Fruit Seeds (Actinidia chinensis Planch.) and Evaluation of Its Antioxidant and Anti-Inflammatory Properties" Molecules 21, no. 7: 832. https://doi.org/10.3390/molecules21070832
APA StyleDeng, J., Liu, Q., Zhang, C., Cao, W., Fan, D., & Yang, H. (2016). Extraction Optimization of Polyphenols from Waste Kiwi Fruit Seeds (Actinidia chinensis Planch.) and Evaluation of Its Antioxidant and Anti-Inflammatory Properties. Molecules, 21(7), 832. https://doi.org/10.3390/molecules21070832