Pharmacogenomics of Scopoletin in Tumor Cells
Abstract
:1. Introduction
2. Results
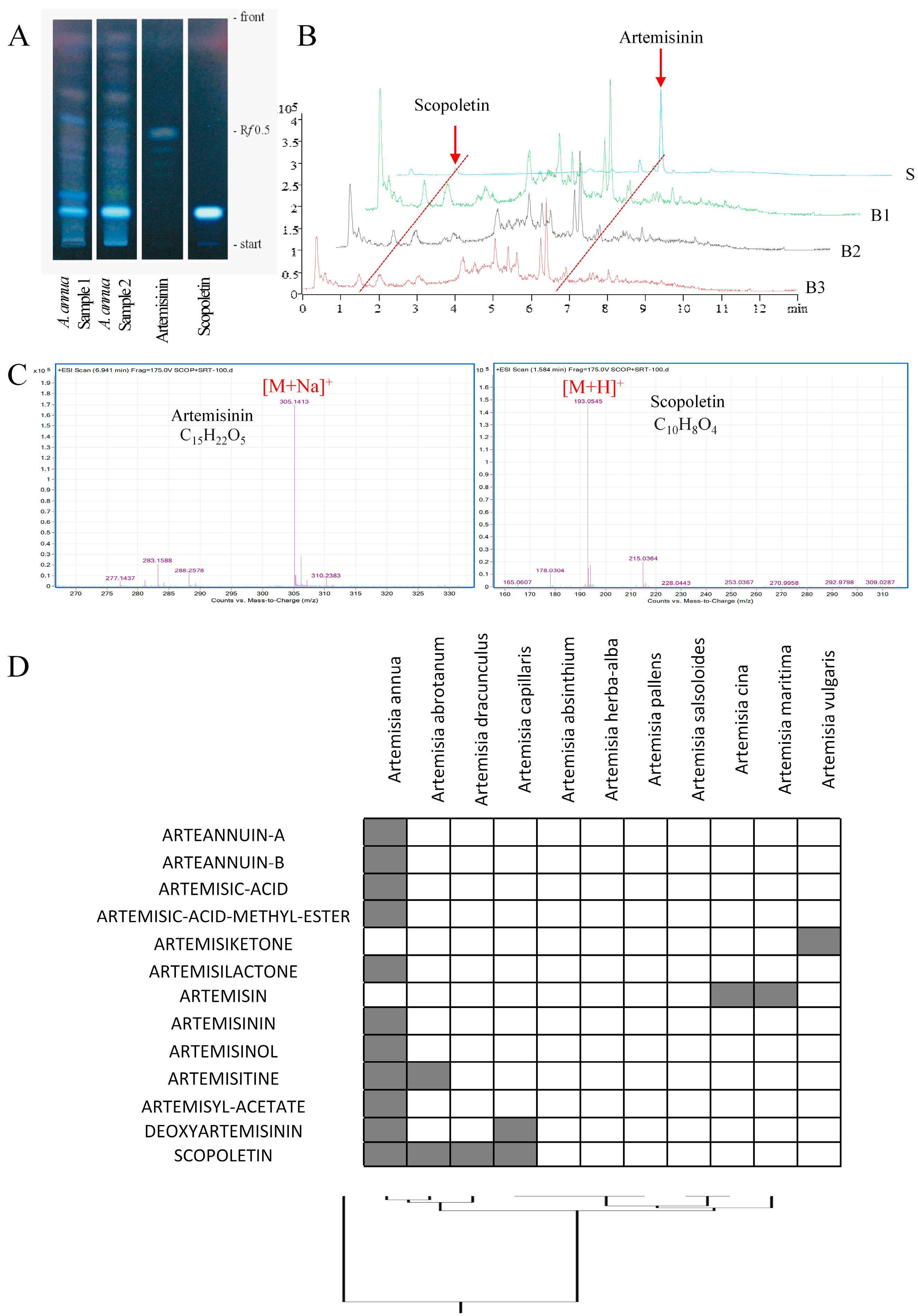
2.1. Detection of Scopoletin in Artemisia annua
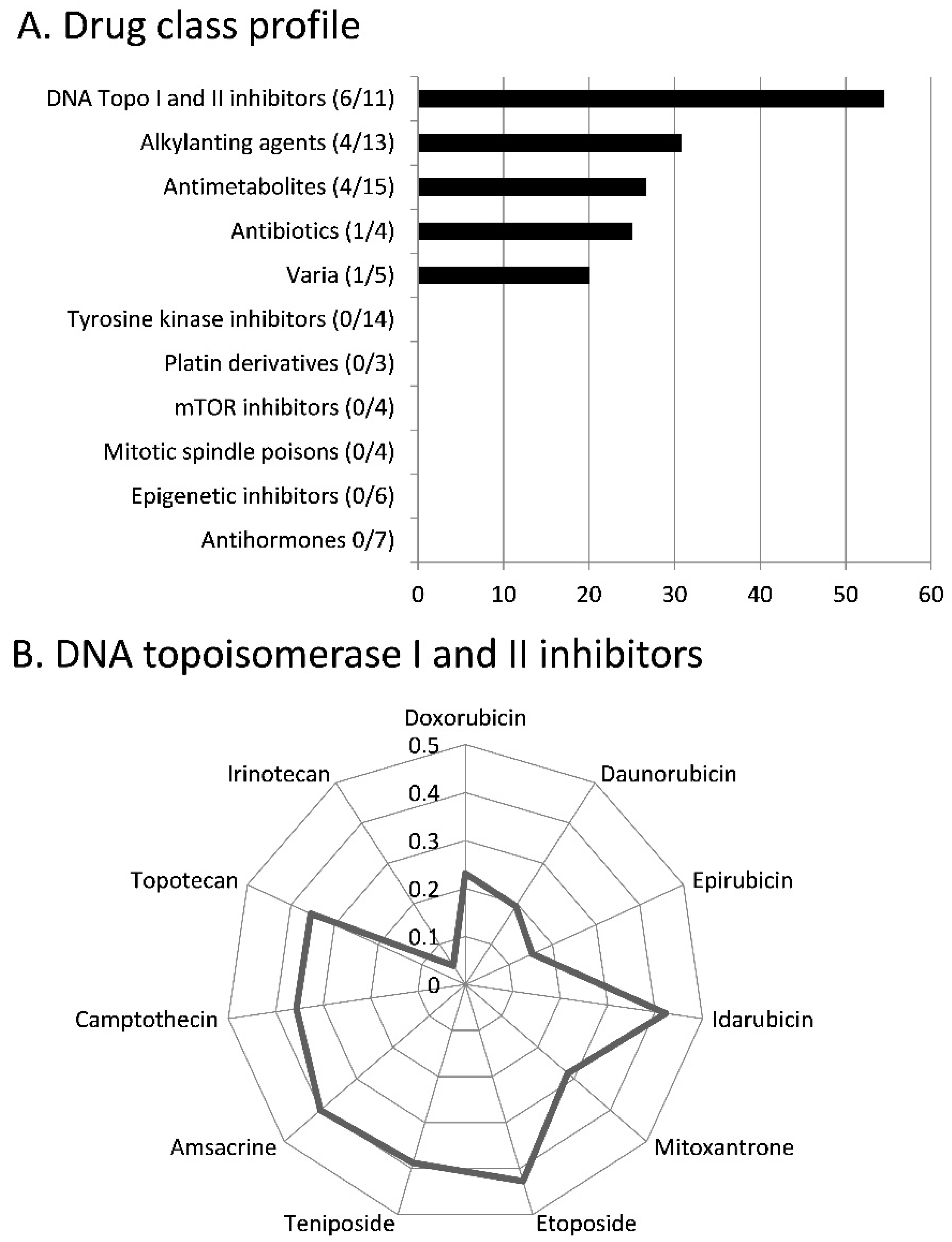
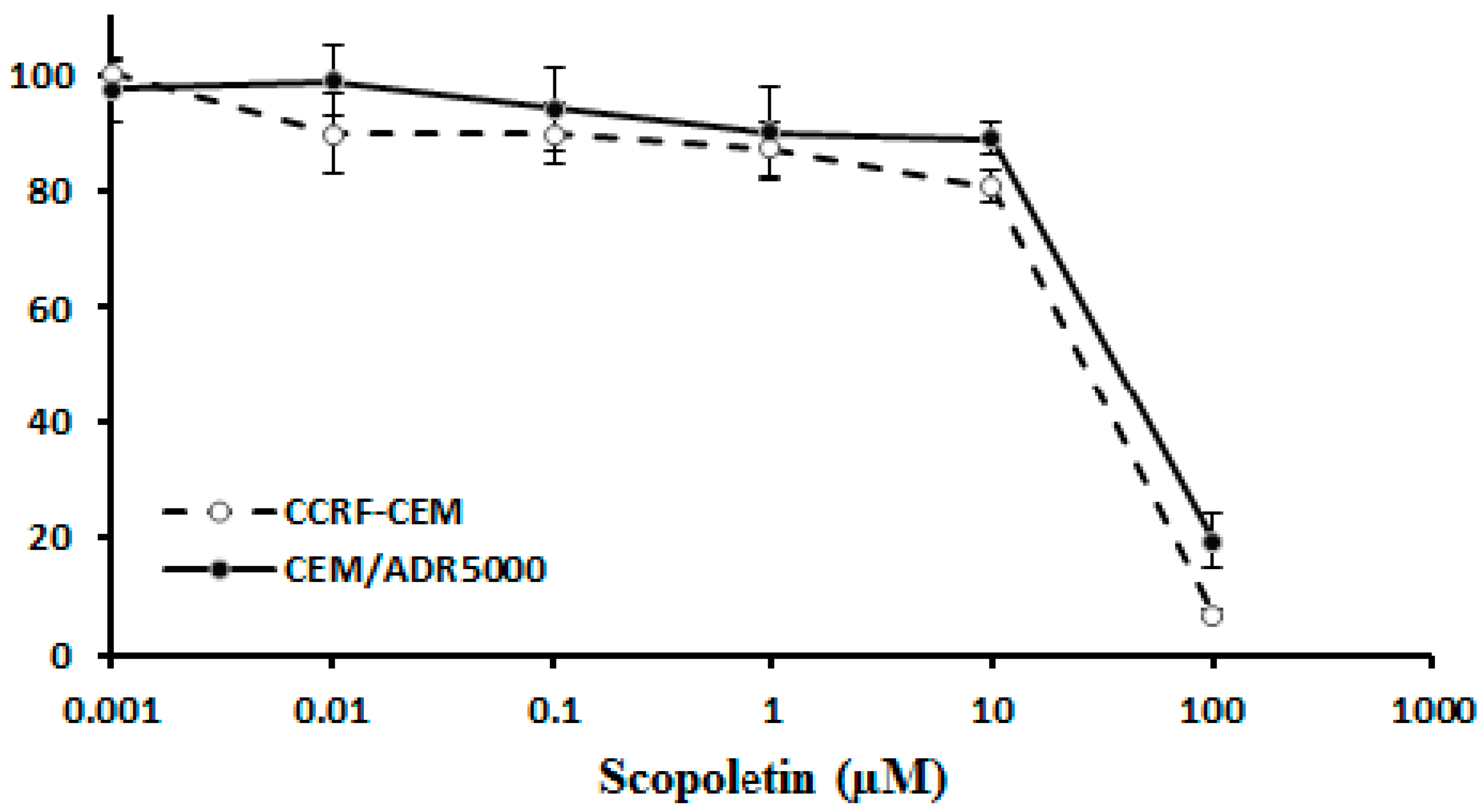
2.2. Cross-Resistance of Scopoletin to Established Anticancer Drugs
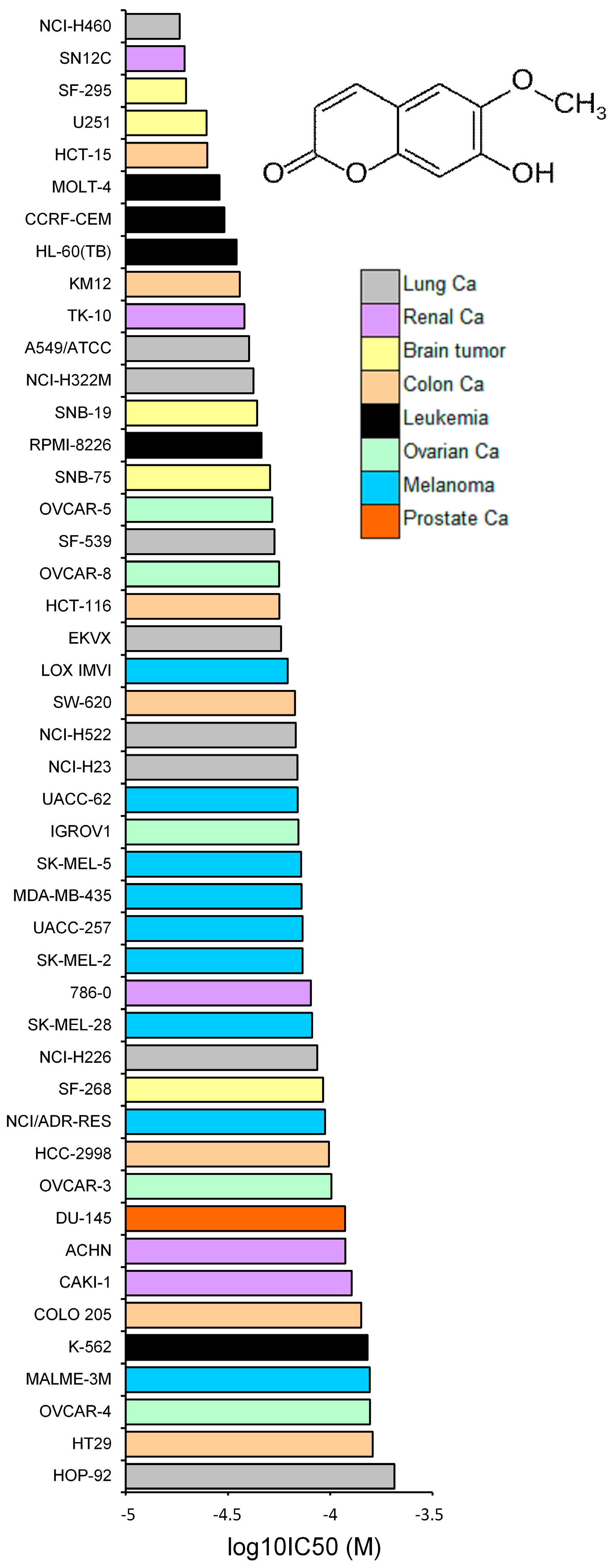
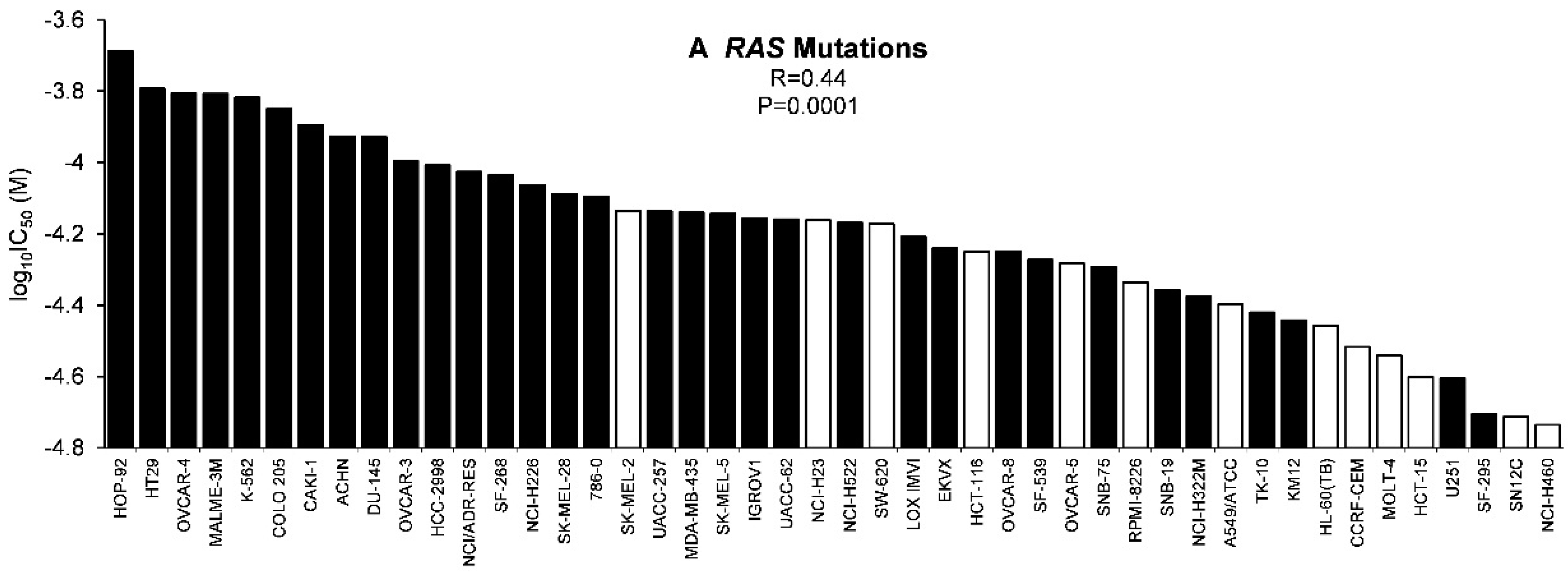
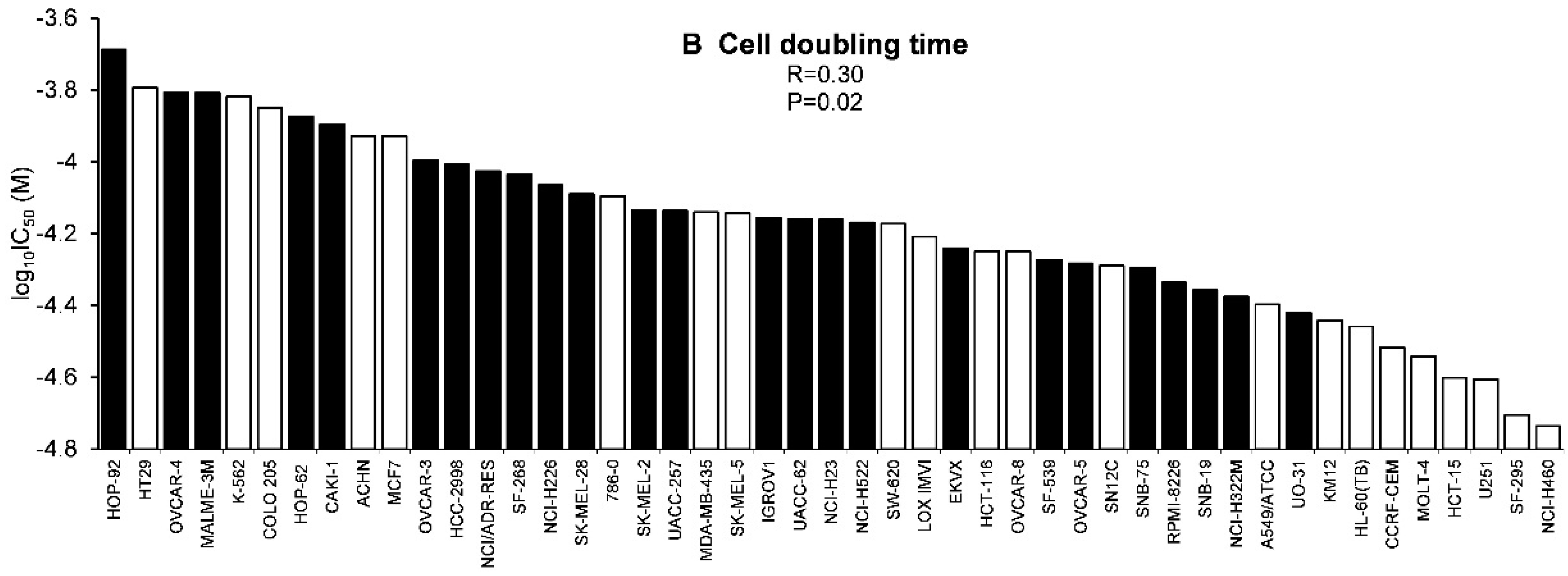
2.3. Tumor-Type Dependent Response towards Scopoletin
2.4. Role of Classical Drug Resistance Mechanisms for Scopoletin
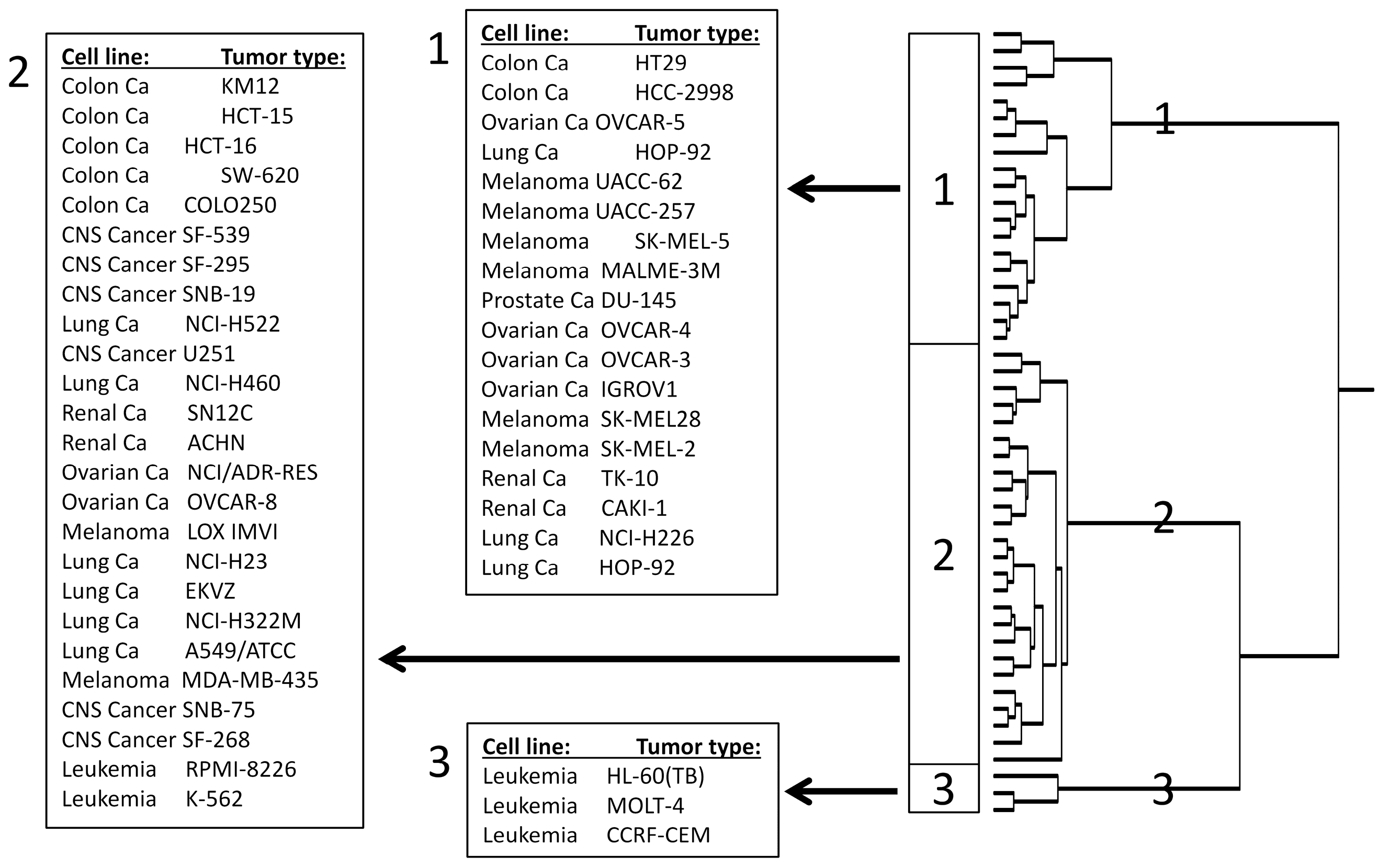
2.5. COMPARE and Hierarchical Cluster Analyses of mRNA Microarray Data
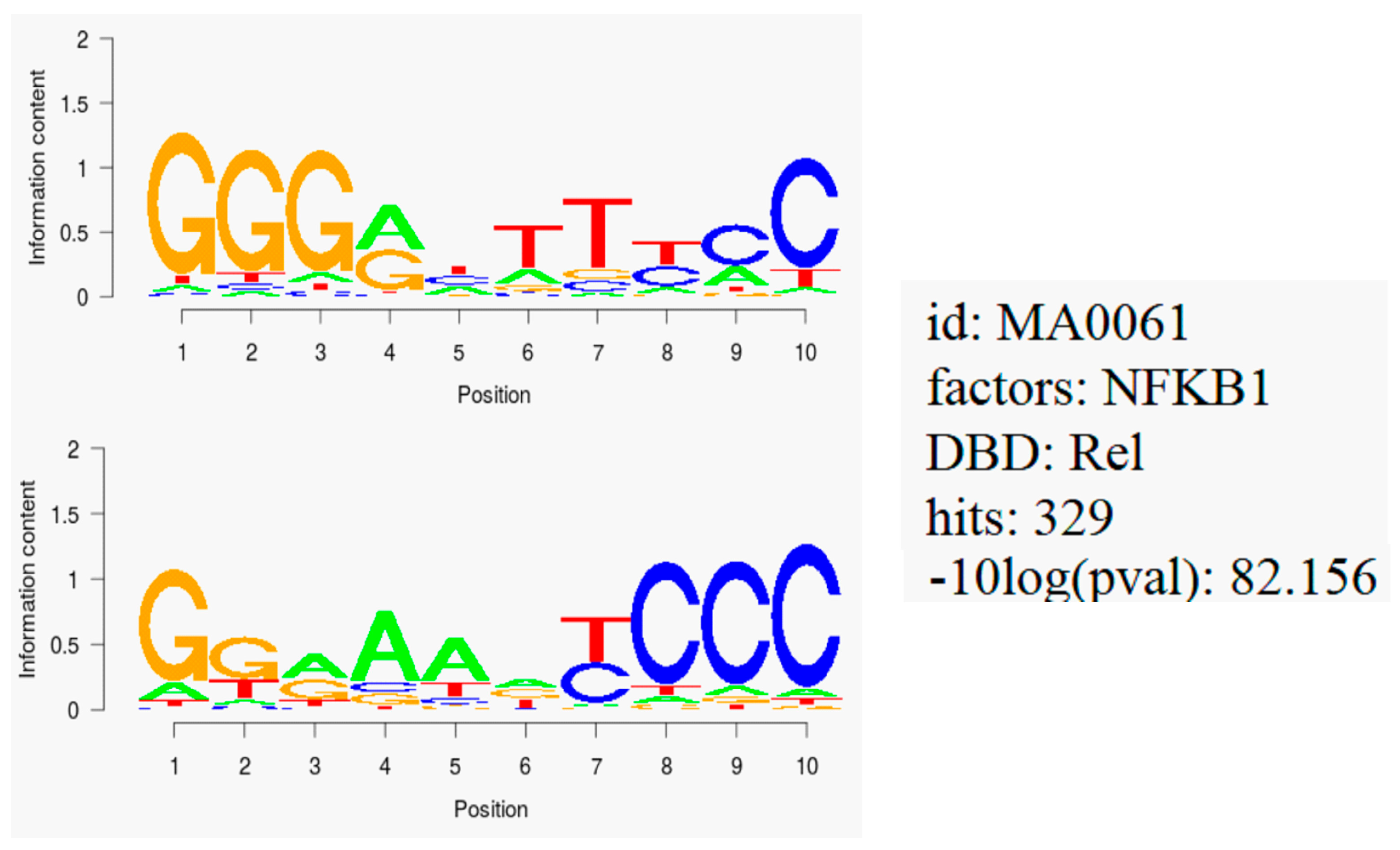
2.6. NF-κB Motif Analysis
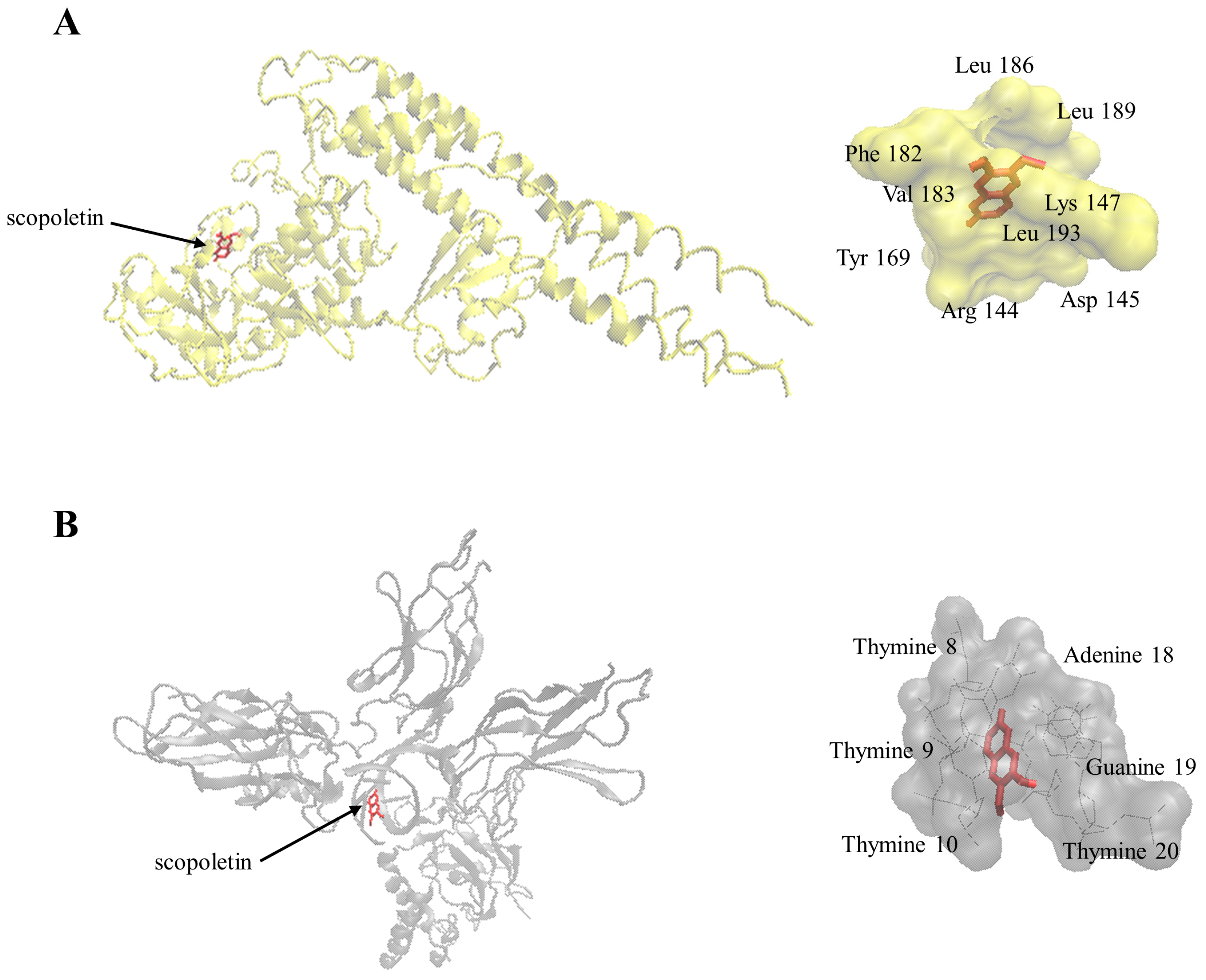
2.7. Molecular Docking
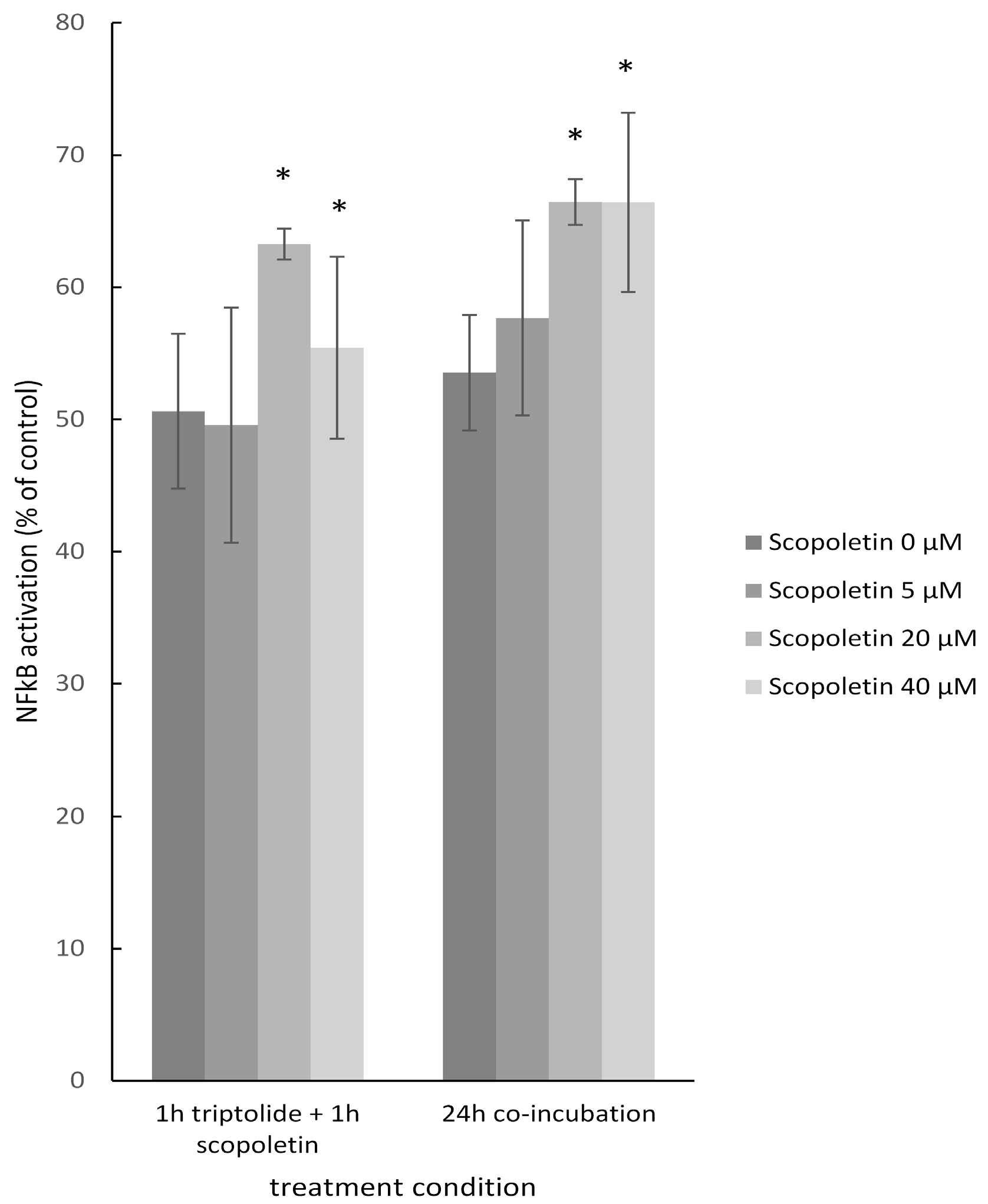
2.8. NF-κB Reporter Assay
3. Discussion
3.1. Chemoprofiling
3.2. Cross-resistance and Drug Resistance Mechanisms
3.3. COMPARE and Hierarchical Cluster Analyses of mRNA Microarray Data
3.4. NF-κB as Mechanism of Therapy Resistance
3.5. Mutagenic and Genotoxic Potential of Scopletin
4. Material and Methods
4.1. Chemicals and Extracts
4.2. Thin Layer Chromatography
4.3. Preparation of Standard Solutions and the A. annua Methanol Extract
4.4. Ultra-High Pressure Liquid Chromatography (UHPLC)
4.5. Cell Lines
4.6. Resazurin Assay
4.7. DNA Analyses with the NCI Cell Line Panel
4.8. mRNA Analyses with the NCI Cell Line Panel
4.9. Protein Analyses with the NCI Cell Line Panel
4.10. Other Analyses with the NCI Cell Line Panel
4.10.1. Rhodamine 123 Efflux Assay
4.10.2. Cell Doubling Times
4.11. Statistical Analysis
4.12. Binding Motif Analysis
4.13. Molecular Docking
4.14. NF-κB Reporter Assay
Author Contributions
Conflicts of Interest
Abbreviations
References
- Wahl, O.; Oswald, M.; Tretzel, L.; Herres, E.; Arend, J.; Efferth, T. Inhibition of tumor angiogenesis by antibodies, synthetic small molecules and natural products. Curr. Med. Chem. 2011, 18, 3136–3155. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Grassmann, R. Impact of viral oncogenesis on responses to anti-cancer drugs and irradiation. Crit. Rev. Oncog. 2000, 11, 165–187. [Google Scholar] [PubMed]
- Efferth, T.; Volm, M. Pharmacogenetics for individualized cancer chemotherapy. Pharmacol. Ther. 2005, 107, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. The human atp-binding cassette transporter genes: From the bench to the bedside. Curr. Mol. Med. 2001, 1, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Gillet, J.P.; Efferth, T.; Remacle, J. Chemotherapy-induced resistance by atp-binding cassette transporter genes. Biochim. Biophys. Acta 2007, 1775, 237–262. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Bustamante, L.; Haynes, R.K.; Staines, H.M. Artemisinins: Their growing importance in medicine. Trends Pharmacol. Sci. 2008, 29, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.; Marschall, M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Zacchino, S.; Georgiev, M.I.; Liu, L.; Wagner, H.; Panossian, A. Nobel prize for artemisinin brings phytotherapy into the spotlight. Phytomedicine 2015, 22, A1–A3. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, F.W.; Saeed, M.E.; Schwarzkopf, J.; Efferth, T. Activity of artemisia annua and artemisinin derivatives, in prostate carcinoma. Phytomedicine 2015, 22, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Ooko, E.; Saeed, M.E.; Kadioglu, O.; Sarvi, S.; Colak, M.; Elmasaoudi, K.; Janah, R.; Greten, H.J.; Efferth, T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine 2015, 22, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Yakasai, A.M.; Hamza, M.; Dalhat, M.M.; Bello, M.; Gadanya, M.A.; Yaqub, Z.M.; Ibrahim, D.A.; Hassan-Hanga, F. Adherence to artemisinin-based combination therapy for the treatment of uncomplicated malaria: A systematic review and meta-analysis. J. Trop. Med. 2015, 2015, 189232. [Google Scholar] [CrossRef] [PubMed]
- Breuer, E.; Efferth, T. Treatment of iron-loaded veterinary sarcoma by artemisia annua. Nat. Prod. Bioprospect. 2014, 4, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Zhang, L.; Fu, X.L.; Chen, K.; Qian, B.C. Effect of scopoletin on pc3 cell proliferation and apoptosis. Acta Pharmacol. Sin. 2001, 22, 929–933. [Google Scholar] [PubMed]
- Adams, M.; Efferth, T.; Bauer, R. Activity-guided isolation of scopoletin and isoscopoletin, the inhibitory active principles towards ccrf-cem leukaemia cells and multi-drug resistant cem/adr5000 cells, from artemisia argyi. Planta Med. 2006, 72, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Herrmann, F.; Tahrani, A.; Wink, M. Cytotoxic activity of secondary metabolites derived from artemisia annua l. Towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine 2011, 18, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida, L.D., Jr.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Kim, M.M. Scopoletin has a potential activity for anti-aging via autophagy in human lung fibroblasts. Phytomedicine 2015, 22, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Resistance to Targeted abc Transporters in Cancer; Springer: New York, NY, USA, 2014. [Google Scholar]
- U.S. Department of Agriculture, A.R.S., Dr. Duke’s Phytochemical and Ethnobotanical Databases. Available online: http://phytochem.nal.usda.gov/ (accessed on 13 April 2016).
- Dr. Duke’s Phytochemical and Ethnobotanical Databases. Available online: http://dx.doi.org/10.15482/USDA.ADC/1239279 (accessed on 13 April 2016).
- Breuninger, L.M.; Paul, S.; Gaughan, K.; Miki, T.; Chan, A.; Aaronson, S.A.; Kruh, G.D. Expression of multidrug resistance-associated protein in nih/3t3 cells confers multidrug resistance associated with increased drug efflux and altered intracellular drug distribution. Cancer Res. 1995, 55, 5342–5347. [Google Scholar] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Deeken, J.F.; Robey, R.W.; Shukla, S.; Steadman, K.; Chakraborty, A.R.; Poonkuzhali, B.; Schuetz, E.G.; Holbeck, S.; Ambudkar, S.V.; Bates, S.E. Identification of compounds that correlate with abcg2 transporter function in the national cancer institute anticancer drug screen. Mol. Pharmacol. 2009, 76, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Sztiller-Sikorska, M.; Koprowska, K.; Majchrzak, K.; Hartman, M.; Czyz, M. Natural compounds’ activity against cancer stem-like or fast-cycling melanoma cells. PLoS ONE 2014, 9, e90783. [Google Scholar]
- Elfawal, M.A.; Towler, M.J.; Reich, N.G.; Weathers, P.J.; Rich, S.M. Dried whole-plant artemisia annua slows evolution of malaria drug resistance and overcomes resistance to artemisinin. Proc. Natl. Acad. Sci. USA 2015, 112, 821–826. [Google Scholar] [CrossRef] [PubMed]
- El-Deiry, W.S. Role of oncogenes in resistance and killing by cancer therapeutic agents. Curr. Opin. Oncol. 1997, 9, 79–87. [Google Scholar] [CrossRef] [PubMed]
- El-Deiry, W.S. The role of p53 in chemosensitivity and radiosensitivity. Oncogene 2003, 22, 7486–7495. [Google Scholar] [CrossRef] [PubMed]
- Shetzer, Y.; Solomon, H.; Koifman, G.; Molchadsky, A.; Horesh, S.; Rotter, V. The paradigm of mutant p53-expressing cancer stem cells and drug resistance. Carcinogenesis 2014, 35, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Konkimalla, V.B.; Wang, Y.F.; Sauerbrey, A.; Meinhardt, S.; Zintl, F.; Mattern, J.; Volm, M. Prediction of broad spectrum resistance of tumors towards anticancer drugs. Clin. Cancer Res. 2008, 14, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: http://www.ncbi.nlm.nih.gov/omim (accessed on 13 April 2016).
- Bioinformatics and Biological Computing Unit. Available online: http://bioinfo.weizmann.ac.il/cards/index.html (accessed on 13 April 2016).
- Kim, E.K.; Kwon, K.B.; Shin, B.C.; Seo, E.A.; Lee, Y.R.; Kim, J.S.; Park, J.W.; Park, B.H.; Ryu, D.G. Scopoletin induces apoptosis in human promyeloleukemic cells, accompanied by activations of nuclear factor kappab and caspase-3. Life Sci. 2005, 77, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Lee, B.H.; Jeong, H.J.; An, H.J.; Park, S.J.; Kim, H.R.; Ko, S.G.; Um, J.Y.; Hong, S.H.; Kim, H.M. Use of scopoletin to inhibit the production of inflammatory cytokines through inhibition of the ikappab/nf-kappab signal cascade in the human mast cell line hmc-1. Eur. J. Pharmacol. 2007, 555, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Yinjun, L.; Jie, J.; Yungui, W. Triptolide inhibits transcription factor nf-kappab and induces apoptosis of multiple myeloma cells. Leuk Res. 2005, 29, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Duan, W.; Liu, B.; Gong, P.; Ding, Y.; Wu, X. Anti-inflammatory effects of triptolide by inhibiting the nf-kappab signalling pathway in lps-induced acute lung injury in a murine model. Mol. Med. Rep. 2014, 10, 447–452. [Google Scholar] [PubMed]
- Kadioglu, O.; Efferth, T. Pharmacogenomic characterization of cytotoxic compounds from salvia officinalis in cancer cells. J. Nat. Prod. 2015, 78, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.Y.; Ni, M.Y.; Zhong, Y.R.; Li, L.N.; Cui, S.L.; Zhang, M.Q.; Wang, X.Z.; Liang, X.T. Studies on the constituents of artemisia annua l. (author’s transl). Yao Xue Xue Bao 1981, 16, 366–370. [Google Scholar] [PubMed]
- Huang, L.; Liu, J.F.; Liu, L.X.; Li, D.F.; Zhang, Y.; Nui, H.Z.; Song, H.Y.; Zhang, C.Y. Antipyretic and anti-inflammatory effects of artemisia annua l. Zhongguo Zhong Yao Za Zhi 1993, 18, 44–48, 63–64. [Google Scholar] [PubMed]
- Luo, S.Q.; Yuan, L.; Wu, Y.K.; Huang, J.G. Effect of fertilization on phenolic components and antioxidant activities of artemisia annua. Zhongguo Zhong Yao Za Zhi 2013, 38, 1493–1499. [Google Scholar] [PubMed]
- Yang, Y.F.; Xu, W.; Song, W.; Ye, M.; Yang, X.W. Transport of twelve coumarins from angelicae pubescentis radix across a mdck-phamdr cell monolayer-an in vitro model for blood-brain barrier permeability. Molecules 2015, 20, 11719–11732. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, P.; Tuccillo, F.M.; Borrelli, A.; Schiattarella, A.; Buonaguro, F.M. Cdk/ccn and cdki alterations for cancer prognosis and therapeutic predictivity. Biomed. Res. Int. 2014, 2014, 361020. [Google Scholar] [CrossRef] [PubMed]
- Shcherba, M.; Liang, Y.; Fernandes, D.; Perez-Soler, R.; Cheng, H. Cell cycle inhibitors for the treatment of nsclc. Expert Opin. Pharmacother. 2014, 15, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist 2002, 7 (Suppl. 4), 2–8. [Google Scholar] [CrossRef] [PubMed]
- Lui, V.W.; Grandis, J.R. Egfr-mediated cell cycle regulation. Anticancer Res. 2002, 22, 1–11. [Google Scholar] [PubMed]
- Lim, S.L.; Goh, Y.M.; Noordin, M.M.; Rahman, H.S.; Othman, H.H.; Abu Bakar, N.A.; Mohamed, S. Morinda citrifolia edible leaf extract enhanced immune response against lung cancer. Food Funct. 2016, 2, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J. Wnt signaling pathway in non-small cell lung cancer. J. Natl. Cancer Inst. 2014, 106, djt356. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.A.; Hernandez, A.; Mark Evers, B. The role of nf-kappab/ikappab proteins in cancer: Implications for novel treatment strategies. Surg. Oncol. 1999, 8, 143–153. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Krystof, V.; Uldrijan, S. Cyclin-dependent kinase inhibitors as anticancer drugs. Curr. Drug Targets 2010, 11, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sandhu, S.; McArthur, G. Cell cycle control as a promising target in melanoma. Curr. Opin. Oncol. 2015, 27, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Klempner, S.J.; Myers, A.P.; Cantley, L.C. What a tangled web we weave: Emerging resistance mechanisms to inhibition of the phosphoinositide 3-kinase pathway. Cancer Discov. 2013, 3, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Niederst, M.J.; Engelman, J.A. Bypass mechanisms of resistance to receptor tyrosine kinase inhibition in lung cancer. Sci. Signal. 2013, 6, re6. [Google Scholar] [CrossRef] [PubMed]
- Namani, A.; Li, Y.; Wang, X.J.; Tang, X. Modulation of nrf2 signaling pathway by nuclear receptors: Implications for cancer. Biochim. Biophys. Acta 2014, 1843, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Jiang, H.; Gao, X.; Hafner, M.S.; Wong, H.; Divine, G.; Chapman, R.A.; Dulchavsky, S.A.; Gautam, S.C. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/apo2l by inhibiting nuclear factor-kappab through suppression of ikappabalpha phosphorylation. Mol. Cancer Ther. 2004, 3, 803–812. [Google Scholar] [PubMed]
- Bednarski, B.K.; Ding, X.; Coombe, K.; Baldwin, A.S.; Kim, H.J. Active roles for inhibitory kappab kinases alpha and beta in nuclear factor-kappab-mediated chemoresistance to doxorubicin. Mol. Cancer Ther. 2008, 7, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, X.; Bai, L.; Liang, X.; Zhuang, J.; Lin, Y. Blockage of nf-kappab by ikkbeta- or rela-sirna rather than the nf-kappab super-suppressor ikappabalpha mutant potentiates adriamycin-induced cytotoxicity in lung cancer cells. J. Cell. Biochem. 2008, 105, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Kim, C.G.; Lim, Y.; Shin, S.Y.; Lee, Y.H. Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the pi3k/akt/nf kappa b pathway. Cancer Lett. 2008, 259, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lounnas, N.; Frelin, C.; Gonthier, N.; Colosetti, P.; Sirvent, A.; Cassuto, J.P.; Berthier, F.; Sirvent, N.; Rousselot, P.; Dreano, M.; et al. Nf-kappab inhibition triggers death of imatinib-sensitive and imatinib-resistant chronic myeloid leukemia cells including t315i bcr-abl mutants. Int. J. Cancer 2009, 125, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Tracey, L.; Streck, C.J.; Du, Z.; Williams, R.F.; Pfeffer, L.M.; Nathwani, A.C.; Davidoff, A.M. Nf-kappab activation mediates resistance to ifn beta in mll-rearranged acute lymphoblastic leukemia. Leukemia 2010, 24, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Roelandts, R. Mutagenicity and carcinogenicity of methoxsalen plus uv-a. Arch. Dermatol. 1984, 120, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Roelandts, R. Carcinogenic risks of phototherapy and photochemotherapy. Hautarzt 1987, 38, 388–394. [Google Scholar] [PubMed]
- Lake, B.G. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Romert, L.; Jansson, T.; Curvall, M.; Jenssen, D. Screening for agents inhibiting the mutagenicity of extracts and constituents of tobacco products. Mutat. Res. 1994, 322, 97–110. [Google Scholar] [CrossRef]
- Wagner, H.; Bauer, R.; Melchart, D.; Xiao, P.-G.; Staudinger, A. Chromatographic Fingerprint Analysis of Herbal Medicines. Thin-Layer and High Performance Liquid Chromatography of Chinese Drugs; Springer: Heidelberg, Germany, 2011. [Google Scholar]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Rubinstein, L.V.; Shoemaker, R.H.; Paull, K.D.; Simon, R.M.; Tosini, S.; Skehan, P.; Scudiero, D.A.; Monks, A.; Boyd, M.R. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J. Natl. Cancer Inst. 1990, 82, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Staunton, J.E.; Slonim, D.K.; Coller, H.A.; Tamayo, P.; Angelo, M.J.; Park, J.; Scherf, U.; Lee, J.K.; Reinhold, W.O.; Weinstein, J.N.; et al. Chemosensitivity prediction by transcriptional profiling. Proc. Natl. Acad. Sci. USA 2001, 98, 10787–10792. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Fabry, U.; Osieka, R. Apoptosis and resistance to daunorubicin in human leukemic cells. Leukemia 1997, 11, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Scherf, U.; Ross, D.T.; Waltham, M.; Smith, L.H.; Lee, J.K.; Tanabe, L.; Kohn, K.W.; Reinhold, W.C.; Myers, T.G.; Andrews, D.T.; et al. A gene expression database for the molecular pharmacology of cancer. Nat. Genet. 2000, 24, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Paull, K.D.; Shoemaker, R.H.; Hodes, L.; Monks, A.; Scudiero, D.A.; Rubinstein, L.; Plowman, J.; Boyd, M.R. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: Development of mean graph and compare algorithm. J. Natl. Cancer Inst. 1989, 81, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Wosikowski, K.; Schuurhuis, D.; Johnson, K.; Paull, K.D.; Myers, T.G.; Weinstein, J.N.; Bates, S.E. Identification of epidermal growth factor receptor and c-erbb2 pathway inhibitors by correlation with gene expression patterns. J. Natl. Cancer Inst. 1997, 89, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ortiz, J.A.; Taing, L.; Meyer, C.A.; Lee, B.; Zhang, Y.; Shin, H.; Wong, S.S.; Ma, J.; Lei, Y.; et al. Cistrome: An integrative platform for transcriptional regulation studies. Genome Biol. 2011, 12, R83. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- The Protein Data Bank. Available online: http://www.rcsb.org/pdb (accessed on 13 April 2016).
- Sample Availability: Samples of the compounds are available from the authors.
| Scopoletin | Control Drug | ||
|---|---|---|---|
| (log10 IC50, M) | (log10 IC50, M) | ||
| ABCB1 Expression | Daunorubicin | ||
| 7q21 (Chromosomal | R-value | 0.099 | * 0.597 |
| Locus of ABCB1 Gene) | p-value | 0.262 | * 4.82 × 10−6 |
| ABCB1 Expression | R-value | 0.071 | * 0.684 |
| (Microarray) | p-value | 0.317 | * 1.57 × 10−8 |
| ABCB1 Expression | R-value | −0.146 | * 0.579 |
| (RT-PCR) | p-value | 0.167 | * 4.19 × 10−6 |
| Rhodamine 123 | R-value | −0.017 | * 0.544 |
| Accumulation | p-value | 0.453 | * 1.51 × 10−5 |
| ABCB5 Expression | Maytansine | ||
| ABCB5 Expression | R-value | 0.094 | * 0.454 |
| (Microarray) | p-value | 0.265 | * 6.67 × 10−4 |
| ABCB5 Expression | R-value | 0.024 | * 0.402 |
| (RT-PCR) | p-value | 0.438 | * 0.0034 |
| ABCC1 Expression | Vinblastine | ||
| DNA Gene | R-value | 0.003 | * 0.429 |
| Copy Number | p-value | 0.493 | * 0.001 |
| ABCC1 Expression | R-value | −0.199 | * 0.399 |
| (Microarray) | p-value | 0.090 | * 0.002 |
| ABCC1 Expression | R-value | −0.129 | 0.299 |
| (RT-PCR) | p-value | 0.181 | * 0.036 |
| ABCG2 Expression | Pancratistatin | ||
| ABCG2 Expression | R-value | −0.311 | * 0.323 |
| (Microarray) | p-value | 0.017 | * 0.006 |
| ABCG2 Expression | R-value | −0.310 | * 0.346 |
| (Western Blot) | p-value | 0.017 | * 0.004 |
| EGFR Expression | Erlotinib | ||
| EGFR Gene | R-value | −0.123 | −0.245 |
| Copy Number | p-value | 0.205 | * 0.029 |
| EGFR Expression | R-value | −0.095 | * −0.458 |
| (Microarray) | p-value | 0.262 | * 1.15 × 10−4 |
| EGFR Expression | R-value | 0.056 | * 0.409 |
| (RNAse Protection) | p-value | 0.358 | * 7.08 × 10−4 |
| EGFR Expression | R-value | −0.096 | * −0.376 |
| (Protein Array) | p-value | 0.260 | * 0.001 |
| TP53 Mutation | 5-Fluorouracil | ||
| TP53 Mutation | R-value | 0.070 | * −0.502 |
| (cDNA Sequencing) | p-value | 0.323 | * 3.50 × 10−5 |
| TP53 Function | R-value | −0.026 | * −0.436 |
| (Yeast Functional Assay) | p-value | 0.434 | * 5.49 × 10−4 |
| COMPARE Coefficent | Experimental ID | GenBank Accession | Gene Symbol | Name | Function |
|---|---|---|---|---|---|
| −0.542 | GC37742 | M25753 | CCNB1 | Cyclin B1 | G2/M cell cycle transition |
| −0.496 | GC31070 | X70944 | SFPQ | Splicing factor proline/glutamine-rich | DNA- and RNA binding protein |
| −0.476 | GC32832 | D29012 | PSMB6 | Proteasome (prosome, macropain) subunit, β type, 6 | Proteasome component |
| −0.472 | GC27340 | U16954 | MLLT11 | Myeloid/lymphoid or mixed-lineage leukemia (trithorax homologue, Drosophila); translocated to, 11 | Translocation partner in leukemia |
| −0.47 | GC35737 | AF007140 | ILF3 | Interleukin enhancer binding factor 3, 90 kDa | Posttranscriptional gene expression regulator |
| −0.462 | GC29921 | X92106 | BLMH | Bleomycin hydrolase | Hydrolase involved in pulmonary fibrosis |
| −0.455 | GC36916 | AJ223349 | HIRIP3 | HIRA interacting protein 3 | Histone-binding protein |
| −0.448 | GC27809 | X94232 | MAPRE2 | Microtubule-associated protein, RP/EB family, member 2 | Signal transduction, mitotic regulator? |
| −0.442 | GC32318 | X65550 | MKI67 | Antigen identified by monoclonal antibody Ki-67 | Cell proliferation |
| −0.442 | GC30069 | S60415 | CACNB2 | Calcium channel, voltage-dependent, β 2 subunit | Calcium channel |
| −0.438 | GC37769 | AF020043 | SMC3 | Structural maintenance of chromosomes 3 | Chromosome cohesion during cell cycle. |
| −0.438 | GC34877 | D64142 | H1FX | H1 histone family, member X | Nucleosome condensation |
| −0.437 | GC31579 | U62961 | OXCT1 | 3-oxoacid CoA transferase 1 | Extrahepatic ketone body catabolism |
| −0.434 | GC31159 | U37426 | KIF11 | Kinesin family member 11 | Prevents centrosome migration and arrest cells in mitosis |
| −0.434 | GC36274 | AF106941 | ARRB2 | Arrestin, β 2 | Desensitization of G-protein-coupled receptors |
| −0.433 | GC32940 | M94630 | HNRNPD | Heterogeneous nuclear ribonucleoprotein D (AU-rich element RNA binding protein 1, 37kDa) | Binds with high affinity to oncogene and cytokine RNA molecules |
| −0.432 | GC35688 | U04810 | TROAP | Trophinin associated protein (tastin) | Cell adhesion |
| −0.429 | GC33612 | L25876 | CDKN3 | Cyclin-dependent kinase inhibitor 3 | CDK1and CDK2 inhibitor |
| −0.428 | GC35573 | U20979 | CHAF1A | Chromatin assembly factor 1, subunit A (p150) | Chromatin assembly in DNA replication and DNA repair. |
| −0.428 | GC27963 | W84438 | MTERFD2 | MTERF domain containing 2 | |
| 0.442 | GC31059 | AI192108 | RHEB | Ras homologue enriched in brain | Signal transduction |
| 0.44 | GC30387 | U59305 | CDC42BPA | CDC42 binding protein kinase α (DMPK-like) RNA | Signal transduction |
| 0.434 | GC28965 | AI984786 | BCAP29 | B-cell receptor-associated protein 29 | Membrane protein transport from endoplasmic reticulum to Golgi |
| 0.425 | GC30348 | AI935420 | ARHGAP12 | Rho GTPase activating protein 12 | GTPase activator for the Rho-type GTPases |
| 0.423 | GC34805 | AF027516 | TGOLN2 | Trans-Golgi network protein 2 | Membrane traffic to and from trans-Golgi network |
| 0.423 | GC37323 | D87120 | FAM3C | Family with sequence similarity 3, member C | Epithelial to mesenchymal transition |
| 0.421 | GC27420 | X16832 | CTSH | Cathepsin H | Lysosomal cysteine proteinase |
| 0.411 | GC30285 | M98343 | CTTN | Cortactin | Organization of actin cytoskeleton and cell shape |
| 0.409 | GC37560 | Z29074 | KRT9 | Keratin 9 | Cytoskeletal element |
| 0.405 | GC39430 | AL050220 | KLK13 | Kallikrein-related peptidase 13 | steroid-regulated breast cancer marker |
| 0.405 | GC31424 | AI953179 | TSPAN5 | Tetraspanin 5 | Member of the transmembrane 4 superfamily |
| 0.4 | GC36737 | AI741756 | ATP6V1H | ATPase, H+ transporting, lysosomal 50/57kDa, V1 subunit H | Couples ATPase activity to proton flow. |
| 0.4 | GC38134 | M62982 | ALOX12 | Arachidonate 12-lipoxygenase RNA | Inflammation, carcinogenesis, membrane remodeling |
| 0.399 | GC32483 | M32313 | SRD5A1 | Steroid-5-α-reductase, α polypeptide 1 (3-oxo-5 α-steroid delta 4-dehydrogenase α1) | Conversion of testosterone to dihydrotestosterone |
| 0.398 | GC29054 | AJ133115 | TSC22D4 | TSC22 domain family, member 4 | DNA binding transcription factor |
| 0.396 | GC30751 | D12763 | IL1RL1 | Interleukin 1 receptor-like 1 | Interleukin receptor |
| 0.389 | GC28792 | U32315 | STX3 | Syntaxin 3 | Docking of synaptic vesicles |
| 0.389 | GC33870 | D15049 | PTPRH | Protein tyrosine phosphatase, receptor type, H | Contact inhibition of cell growth and motility |
| 0.386 | GC29420 | Z50022 | PTTG1IP | Pituitary tumor-transforming 1 interacting protein | PTTG1 nuclear translocation |
| 0.384 | GC28855 | U49837 | CSRP3 | Cysteine and glycine-rich protein 3 (cardiac LIM protein) | Cell differentiation |
| Partition | Cluster 1 | Cluster 2 | Cluster 3 | |
|---|---|---|---|---|
| sensitive | <−4.160 M | 2 | 19 | 3 |
| resistant | >−4.160 M | 17 | 6 | 0 |
| Protein | Binding Energy (kcal/mol) | Predicted Inhibition Constant (µM) | Number of Residues Involved in Hydrophobic Interactions | Residues Involved in Hydrogen Bond |
|---|---|---|---|---|
| I-κB kinase | −7.45 ± 0.00 | 3.45 ± 0.01 | 7 | Arg144, Lys147 |
| NF-κB-DNA complex | −7.41 ± 0.00 | 3.69 ± 0.00 | 4 | adenine18, guanine19 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, E.-J.; Saeed, M.; Law, B.Y.K.; Wu, A.G.; Kadioglu, O.; Greten, H.J.; Efferth, T. Pharmacogenomics of Scopoletin in Tumor Cells. Molecules 2016, 21, 496. https://doi.org/10.3390/molecules21040496
Seo E-J, Saeed M, Law BYK, Wu AG, Kadioglu O, Greten HJ, Efferth T. Pharmacogenomics of Scopoletin in Tumor Cells. Molecules. 2016; 21(4):496. https://doi.org/10.3390/molecules21040496
Chicago/Turabian StyleSeo, Ean-Jeong, Mohamed Saeed, Betty Yuen Kwan Law, An Guo Wu, Onat Kadioglu, Henry Johannes Greten, and Thomas Efferth. 2016. "Pharmacogenomics of Scopoletin in Tumor Cells" Molecules 21, no. 4: 496. https://doi.org/10.3390/molecules21040496
APA StyleSeo, E.-J., Saeed, M., Law, B. Y. K., Wu, A. G., Kadioglu, O., Greten, H. J., & Efferth, T. (2016). Pharmacogenomics of Scopoletin in Tumor Cells. Molecules, 21(4), 496. https://doi.org/10.3390/molecules21040496








