Labdane Diterpenes from the Fruits of Sinopodophyllum emodi
Abstract
:1. Introduction
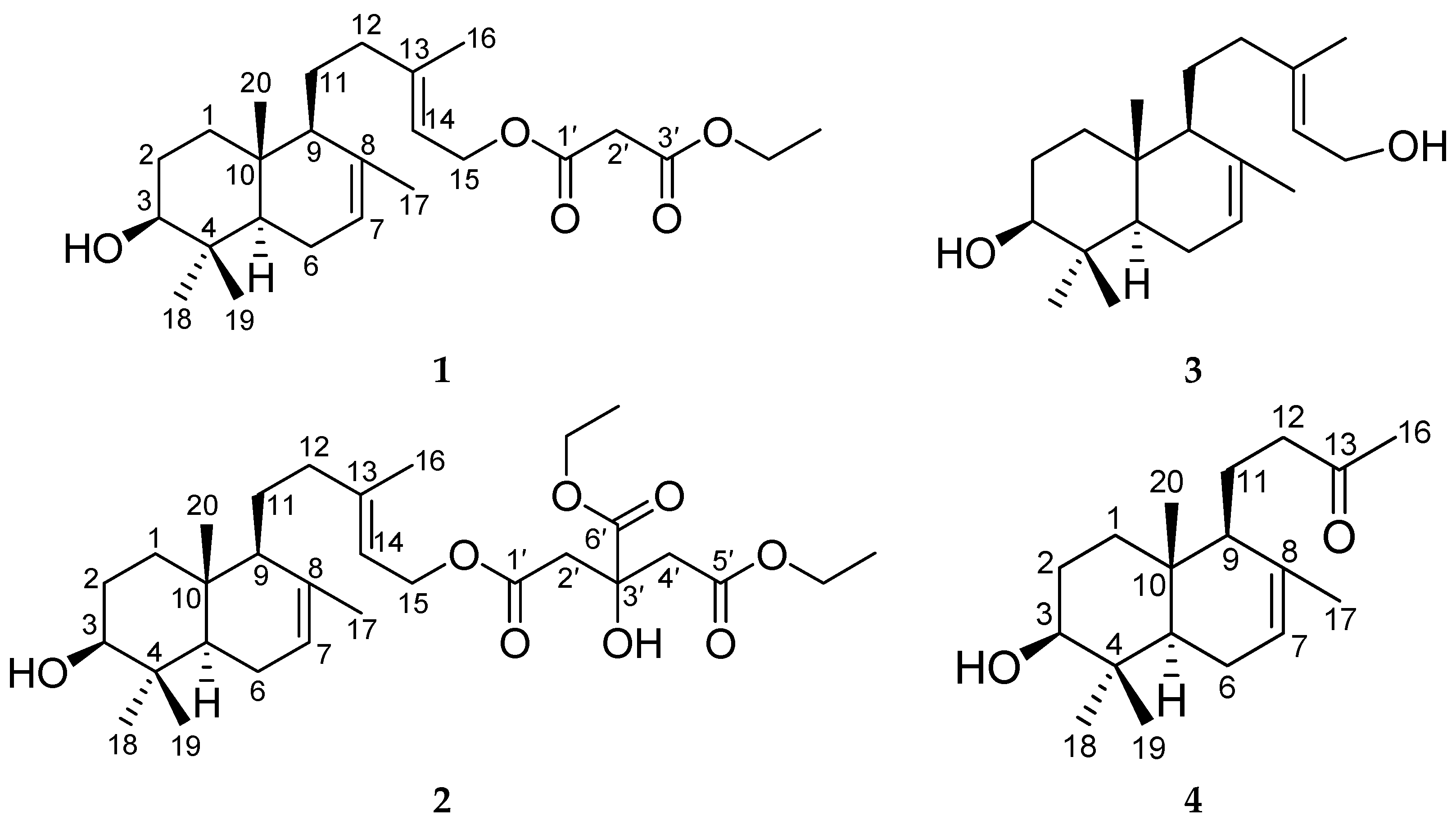
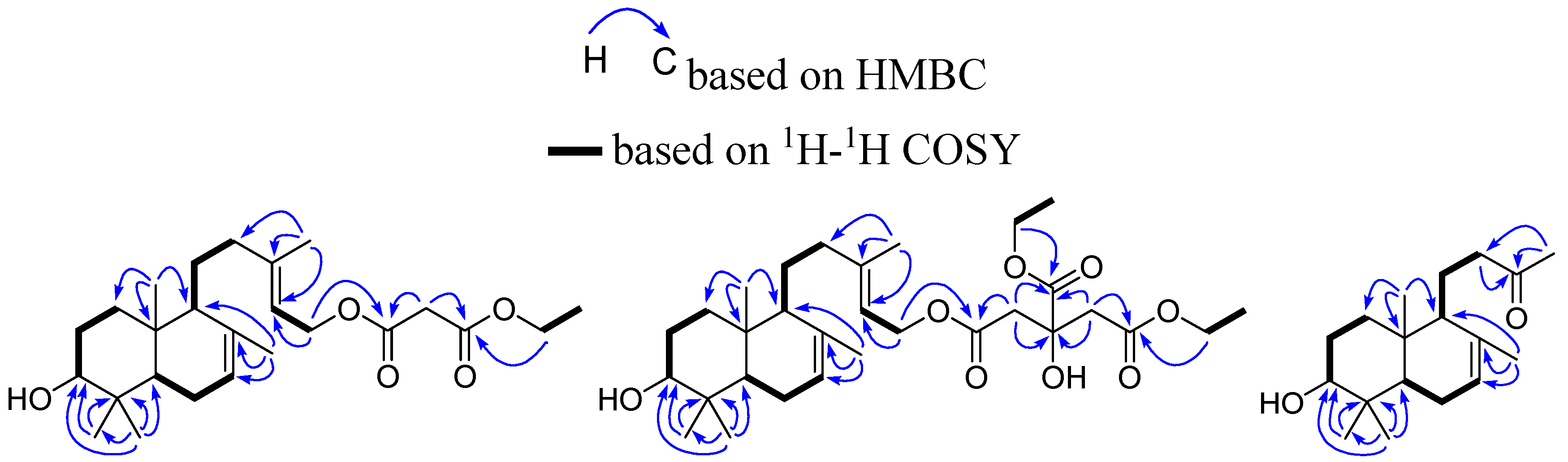
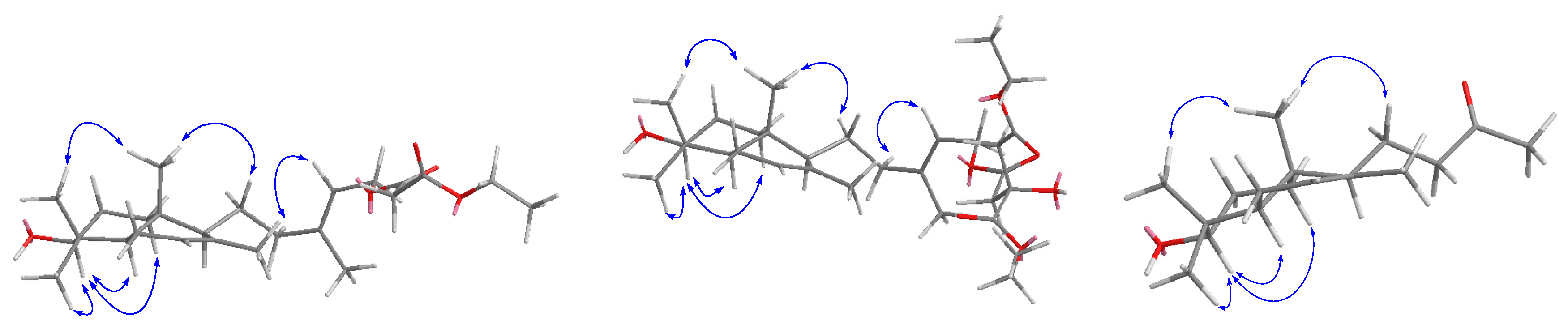
2. Results and Discussion
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic and Physical Data
3.5. Alkali Hydrolysis
3.6. Cytotoxicity Asssay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, Y.J.; Sun, Y.S.; Chen, H.; Hao, Z.Y.; Wang, J.M.; Guan, Y.B.; Zhang, Y.L.; Feng, W.S.; Zheng, X.K. Isolation of two new prenylated flavonoids from Sinopodophyllum emodi fruit by silica gel column and high-speed counter-current chromatography. J. Chromatogr. B 2014, 969, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Cao, W.; Nagatsu, A.; Ogihara, Y. Three new glycosides from Sinopodophyllum emodi (Wall.) Ying. Chem. Pharm. Bull. 2001, 49, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Zhu, Y.Y.; Chen, S.Y.; Ogihara, Y. Lignan glucoside from Sinopodophyllum emodi and its cytotoxic activity. Chin. Chem. Lett. 2011, 22, 181–184. [Google Scholar] [CrossRef]
- Yang, X.Z.; Shao, H.; Zhang, L.Q.; Zhou, C.; Xuan, Q.; Yang, C.Y. Present situation of studies on resources of podophyllotoxin. Chin. Tradit. Herbal Drugs 2001, 32, 1042–1044. [Google Scholar]
- Shi, X.L.; Li, X.W.; Liu, J.B.; Zhou, H.Y.; Zhang, H.Q.; Jin, Y.R. Lignan extraction from the roots of Sinopodophyllum emodi Wall by matrix solid phase dispersion. Chromatographia 2010, 72, 713–717. [Google Scholar] [CrossRef]
- Kong, Y.; Xiao, J.J.; Meng, S.C.; Dong, X.M.; Ge, Y.W.; Wang, R.F.; Shang, M.Y.; Cai, S.Q. A new cytotoxic flavonoid from the fruit of Sinopodophyllum hexandrum. Fitoterapia 2010, 81, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Huang, J.; Nagatsu, A.; Ogihara, Y. Two new podophyllotoxin glucosides from Sinopodophyllum emodi (Wall.) Ying. Chem. Pharm. Bull. 2001, 49, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Nagatsu, A.; Hatano, K.; Shirai, N.; Kato, S.; Ogihara, Y. New lignin glycosides from Chinese medicinal plant, Sinopodophillum emodi. Chem. Pharm. Bull. 2003, 51, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Pei, L.X.; Wang, K.B.; Sun, Y.S.; Wang, J.M.; Zhang, Y.L.; Gao, M.L.; Ji, B.Y. Preparative isolation of two prenylated biflavonoids from the roots and rhizomes of Sinopodophyllum emodi by Sephadex LH-20 column and high-speed counter-current chromatography. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Sun, Y.J.; Li, Z.L.; Chen, H.; Liu, X.Q.; Zhou, W.; Hua, H.M. Three cytotoxic arytetralin lignans from Sinopodophyllum emodi. Bioorg. Med. Chem. Lett. 2011, 21, 3794–3797. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Hao, Z.Y.; Si, J.G.; Wang, Y.; Zhang, Y.L.; Wang, J.M.; Gao, M.L.; Chen, H. Prenylated flavonoids from the fruits of Sinopodophyllum emodi and their cytotoxic activities. RSC Adv. 2015, 5, 582736–582742. [Google Scholar] [CrossRef]
- Sun, Y.J.; Li, Z.L.; Chen, H.; Liu, X.Q.; Zhou, W.; Hua, H.M. Four new cytotoxic tetrahydrofuranoid lignans from Sinopodophyllum emodi. Planta Med. 2012, 78, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Urones, J.G.; Marcos, I.S.; Oliva, I.M.; Garrido, N.M.; Hagget, J.; Humphreys, V.M. Labdane diterpenes from Halimium viscosum. Phytochemistry 1995, 38, 663–666. [Google Scholar] [CrossRef]
- Lu, L.Q.; Cui, H.Y.; Zhang, L.C.; Liu, Q.; Qin, B. Chemical constituents from the aerial parts of Stellera chamaejasme L. Nat. Prod. Res. Dev. 2014, 26, 53–55. [Google Scholar]
- Ferracini, V.L.; Roewer, I.; Gao, F.; Mabry, T.J. Ent-Labdane diterpenoids, tremetone and chromene derivatives and flavonoids from Ophryosporus heptanthus. Phytochemistry 1989, 28, 1463–1465. [Google Scholar] [CrossRef]
- Zdero, C.; Bohlmann, F. Macrolide diterpenes and other ent-labdanes from Corymbium villosum. Phytochemistry 1988, 27, 227–231. [Google Scholar] [CrossRef]
- Toki, M.; Ooi, T.; Kusumi, T. Sesterterpenoids and diterpenoids of the wax excreted by a scale insect, Ceroplastes pseudoceriferus. J. Nat. Prod. 1999, 62, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, K.K.; Sarada, A.; Saraswathy, A.; Connolly, J.D. Sempervirenic acid, a diterpene acid from Solidago sempervirens. Phytochemistry 1983, 22, 1042–1043. [Google Scholar] [CrossRef]
- Cui, C.W.; Sun, C.L.; Chen, Q.C.; Zou, X.H.; Huang, X.M.; Chen, H.F. Preliminary study on chemical constituents seperated from Cayratia japonica. Chin. J. Chin. Mater. Med. 2012, 37, 2906–2909. [Google Scholar]
- Sun, Y.J.; Zhou, W.; Chen, H.; Li, Z.L.; Hua, H.M. Isolation and identification of flavonoids from the roots and rhizomes of Sinopodophyllum emodi. J. Shenyang Pharm. Univ. 2012, 29, 185–189. [Google Scholar]
- Sun, Y.J.; Li, Z.L.; Chen, H.; Zhou, W.; Hua, H.M. Study on chemical constituents from the roots and rhizomes of Sinopodophyllum emodi. J. Chin. Med. Mat. 2012, 35, 1607–1609. [Google Scholar]
- Sun, Y.J.; Zhou, W.; Chen, H.; Li, Z.L.; Hua, H.M. Phenols from roots and rhizomes of Sinopodophyllum emodi. Chin. Tradit. Herbal Drugs 2012, 43, 226–229. [Google Scholar]
- Jiangsu New Medical College. Chinese Traditional Medicine Dictionary; Shanghai Science and Technology Publishing House: Shanghai, China, 1977; pp. 1791–1792. [Google Scholar]
- Sample Availability: Samples of the compounds 1–4 are available from the authors.
| No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 1.07 (1H, td, 13.2, 4.2) | 1.06 (1H, td, 13.1, 4.5) | 1.06 (1H, td, 13.2, 4.4) | 1.10 (1H, td, 13.3, 4.0) |
| 1.84 (1H, dt, 13.2, 3.4) | 1.84 (1H, dt, 13.1, 3.4) | 1.84 (1H, dt, 13.2, 3.4) | 1.91 (1H, dt, 13.3, 3.6) | |
| 2 | 1.60 (2H, m) | 1.60 (2H, m) | 1.60 (2H, m) | 1.60 (2H, m) |
| 3 | 3.22 (1H, dd, 11.2, 4.5) | 3.22 (1H, dd, 11.0, 4.8) | 3.20 (1H, dd, 11.2, 4.5) | 3.21 (1H, dd, 11.4, 4.4) |
| 5 | 1.16 (1H, dd, 10.9, 6.1) | 1.17 (1H, dd, 10.9, 6.1) | 1.15 (1H, dd, 10.9, 6.1) | 1.15 (1H, dd, 10.9, 6.1) |
| 6 | 1.95 (2H, m) | 1.95 (2H, m) | 1.95 (2H, m) | 1.95 (2H, m) |
| 7 | 5.38 (1H, br.s) | 5.38 (1H, br.s) | 5.37 (1H, br.s) | 5.39 (1H, br.s) |
| 9 | 1.57 (1H, m) | 1.58 (1H, m) | 1.58 (1H, m) | 1.57 (1H, m) |
| 11 | 1.47 (1H, m) | 1.50 (1H, m) | 1.50 (1H, m) | 1.78 (1H, m) |
| 1.25 (1H, m) | 1.25 (1H, m) | 1.28 (1H, m) | 1.45 (1H, m) | |
| 12 | 1.95 (1H, m) | 1.95 (1H, m) | 1.95 (1H, m) | 2.40 (1H, m) |
| 2.22 (1H, m) | 2.20 (1H, m) | 2.20 (1H, m) | 2.63 (1H, m) | |
| 14 | 5.32 (1H, t, 7.2) | 5.32 (1H, t, 7.5) | 5.40 (1H, t, 7.0) | |
| 15 | 4.64 (2H, d, 7.2) | 4.58 (2H, d, 7.5) | 4.13 (2H, d, 7.0) | |
| 16 | 1.70 (3H, s) | 1.68 (3H, s) | 1.67 (3H, s) | 2.11 (3H, s) |
| 17 | 1.67 (3H, s) | 1.68 (3H, s) | 1.66 (3H, s) | 1.64 (3H, s) |
| 18 | 0.95 (3H, s) | 0.95 (3H, s) | 0.94 (3H, s) | 0.94 (3H, s) |
| 19 | 0.83 (3H, s) | 0.83 (3H, s) | 0.83 (3H, s) | 0.83 (3H, s) |
| 20 | 0.74 (3H, s) | 0.73 (3H, s) | 0.73 (3H, s) | 0.76 (3H, s) |
| 2′ | 3.22 (2H, s) | 2.76 (1H, d, 15.6) | ||
| 2.87 (1H, d, 15.6) | ||||
| 4′ | 2.76 (1H, d, 15.6) | |||
| 2.87 (1H, d, 15.6) | ||||
| CH2 | 4.18 (2H, q, 7.2) | 4.12 (2H, q, 7.2) | ||
| CH3 | 1.26 (3H, t, 7.2) | 1.28 (3H, t, 7.2) | ||
| CH2 | 4.26 (2H, q, 7.2) | |||
| CH3 | 1.26 (3H, t, 7.2) |
| No. | 1 | 2 | 3 | 4 | No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37.2 | 37.2 | 37.1 | 37.4 | 16 | 16.6 | 16.6 | 16.5 | 30.0 |
| 2 | 27.3 | 27.4 | 27.3 | 27.4 | 17 | 21.9 | 21.9 | 21.9 | 22.0 |
| 3 | 79.1 | 79.1 | 79.1 | 79.1 | 18 | 27.9 | 27.9 | 27.8 | 27.9 |
| 4 | 38.6 | 38.6 | 38.6 | 38.7 | 19 | 15.0 | 15.0 | 15.0 | 15.1 |
| 5 | 49.5 | 49.5 | 49.5 | 49.5 | 20 | 13.6 | 13.6 | 13.6 | 13.6 |
| 6 | 23.4 | 23.4 | 23.4 | 23.4 | 1′ | 166.7 | 169.80 | ||
| 7 | 122.3 | 122.3 | 122.1 | 122.9 | 2′ | 41.7 | 43.2 | ||
| 8 | 135.0 | 135.0 | 135.1 | 134.3 | 3′ | 166.6 | 73.2 | ||
| 9 | 54.3 | 54.2 | 54.3 | 54.3 | 4′ | 43.3 | |||
| 10 | 36.6 | 36.6 | 36.6 | 36.7 | 5′ | 169.76 | |||
| 11 | 25.5 | 25.3 | 25.5 | 20.8 | 6′ | 173.4 | |||
| 12 | 41.9 | 41.8 | 41.9 | 45.7 | CH2 | 62.3 | 62.3 | ||
| 13 | 143.3 | 143.2 | 140.0 | 208.7 | CH3 | 14.1 | 14.0 | ||
| 14 | 117.9 | 118.0 | 123.5 | CH2 | 61.0 | ||||
| 15 | 61.5 | 61.8 | 59.4 | CH3 | 14.1 |
| Compound | MCF-7 | HepG2 | Compound | MCF-7 | HepG2 |
|---|---|---|---|---|---|
| 1 | 74.6 ± 5.5 | 63.5 ± 6.2 | 3 | 5.73 ± 0.46 | 3.85 ± 0.29 |
| 2 | 88.3 ± 7.1 | 75.2 ± 6.8 | 4 | 50.2 ± 5.0 | 39.1 ± 4.7 |
| 5-Fluorouracil | 6.74 ± 0.52 | 5.18 ± 0.40 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-J.; Gao, M.-L.; Zhang, Y.-L.; Wang, J.-M.; Wu, Y.; Wang, Y.; Liu, T. Labdane Diterpenes from the Fruits of Sinopodophyllum emodi. Molecules 2016, 21, 434. https://doi.org/10.3390/molecules21040434
Sun Y-J, Gao M-L, Zhang Y-L, Wang J-M, Wu Y, Wang Y, Liu T. Labdane Diterpenes from the Fruits of Sinopodophyllum emodi. Molecules. 2016; 21(4):434. https://doi.org/10.3390/molecules21040434
Chicago/Turabian StyleSun, Yan-Jun, Mei-Ling Gao, Yan-Li Zhang, Jun-Min Wang, Ya Wu, Yu Wang, and Tao Liu. 2016. "Labdane Diterpenes from the Fruits of Sinopodophyllum emodi" Molecules 21, no. 4: 434. https://doi.org/10.3390/molecules21040434
APA StyleSun, Y.-J., Gao, M.-L., Zhang, Y.-L., Wang, J.-M., Wu, Y., Wang, Y., & Liu, T. (2016). Labdane Diterpenes from the Fruits of Sinopodophyllum emodi. Molecules, 21(4), 434. https://doi.org/10.3390/molecules21040434





