Novel Selective Butyrylcholinesterase Inhibitors Incorporating Antioxidant Functionalities as Potential Bimodal Therapeutics for Alzheimer’s Disease
Abstract
:1. Introduction
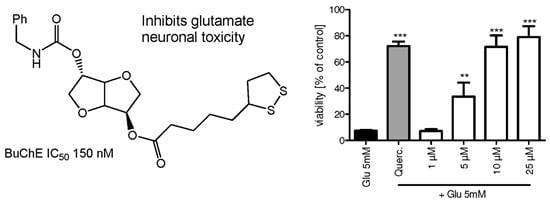
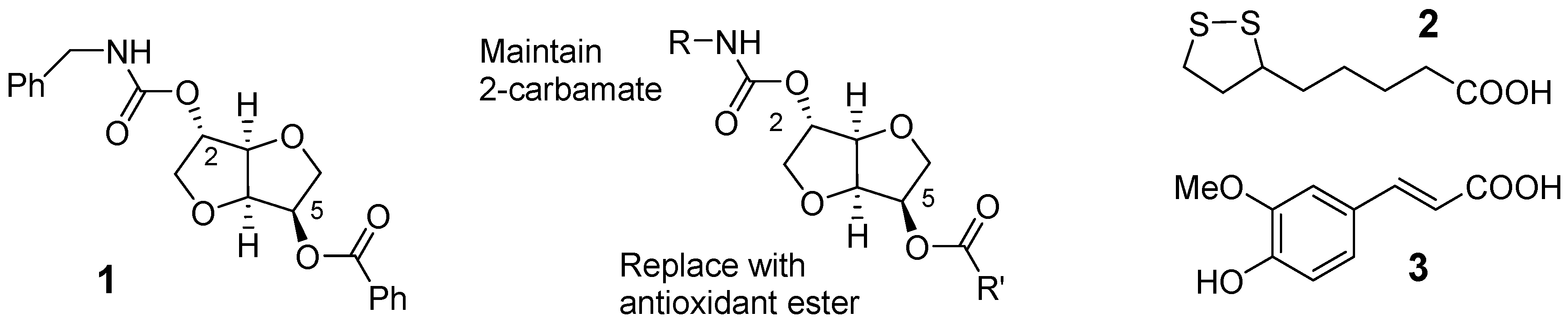
2. Results and Discussion
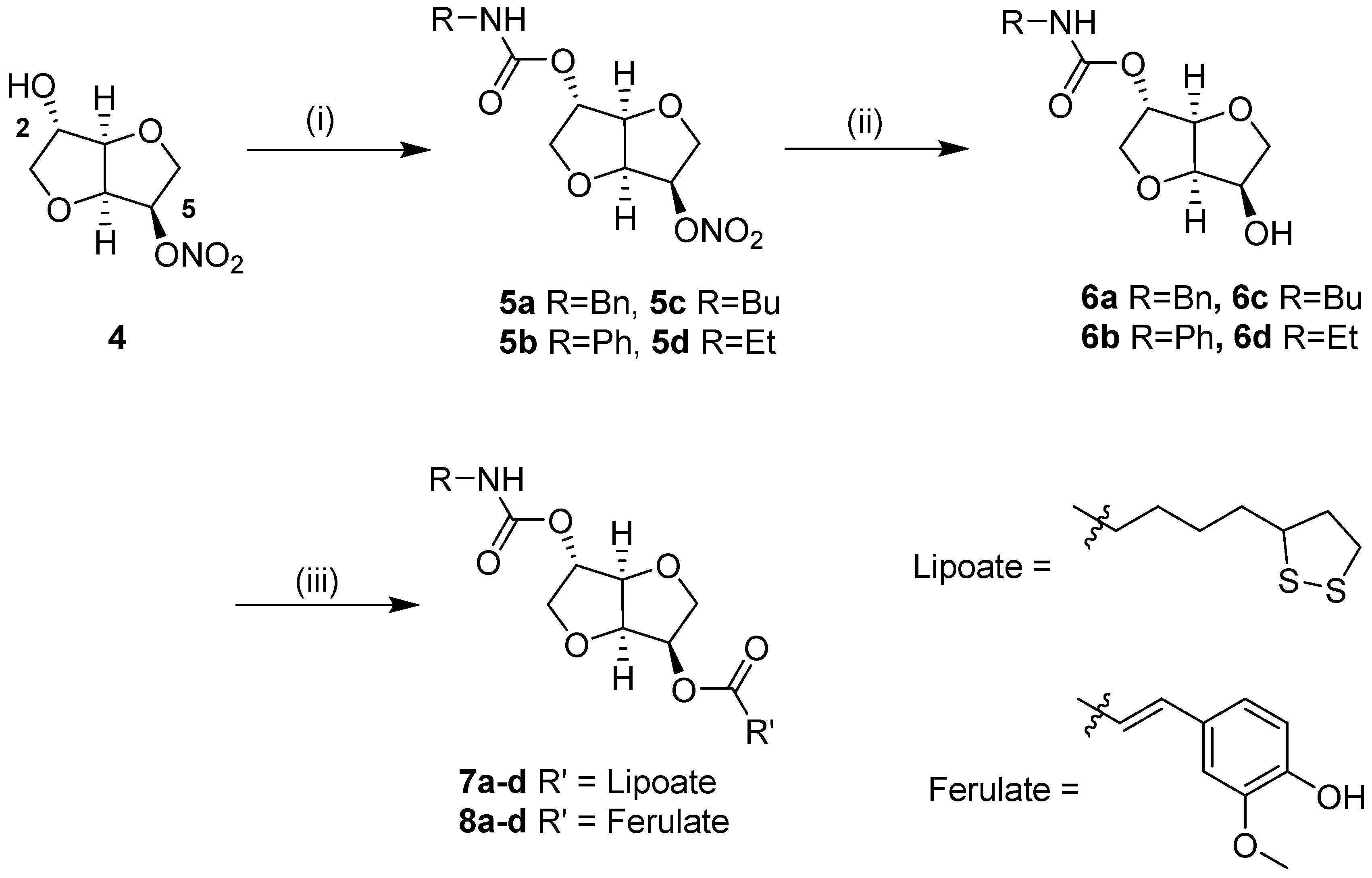
2.1. Synthesis of Isosorbide-Based Carbamate-Antioxidants
2.2. Cholinesterase Inhibition and Mechanism
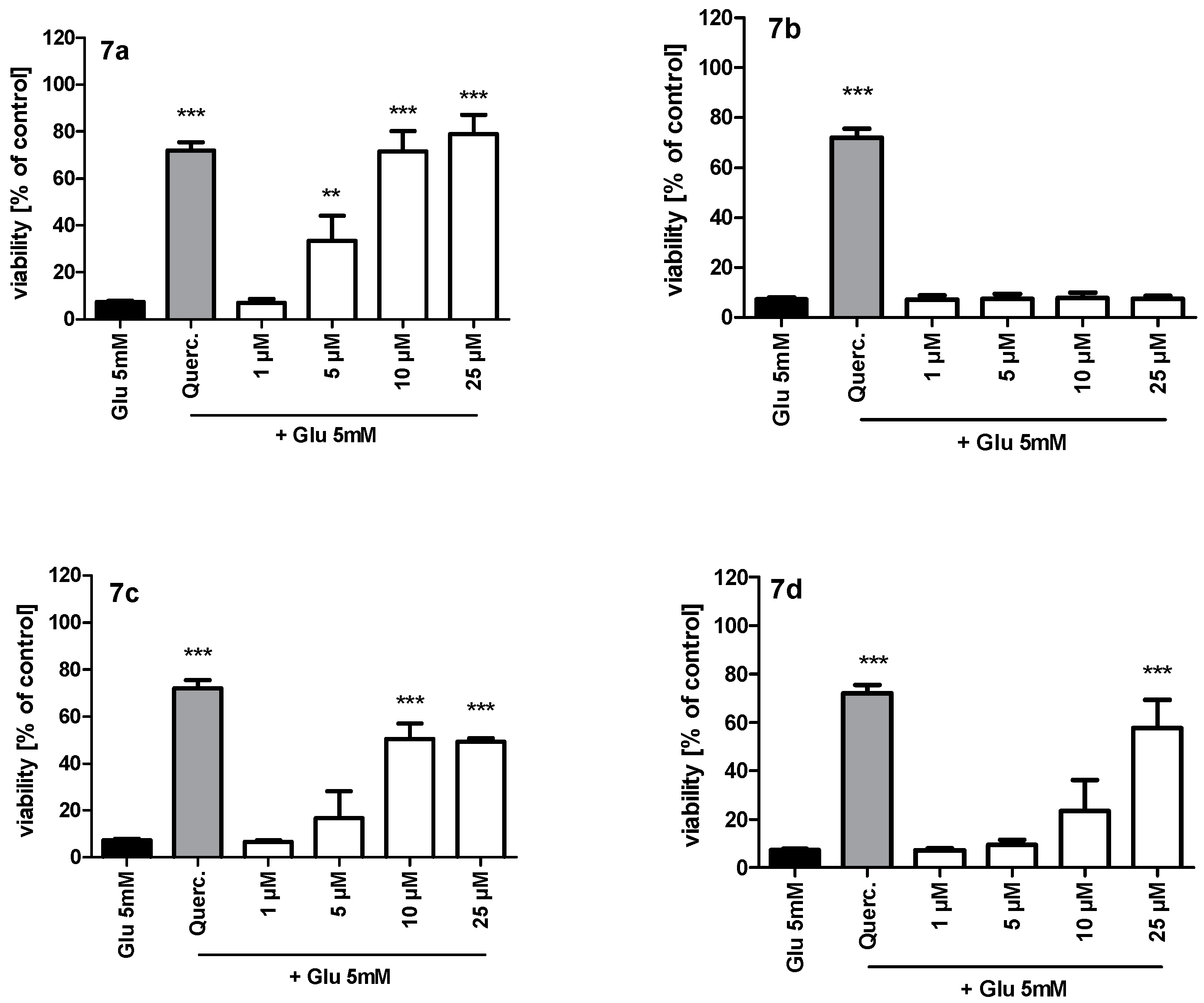
2.3. Neuroprotection and Antioxidant Activity
3. Materials and Methods
3.1. General Chemistry
3.1.1. General Procedure for Synthesis of Isosorbide-2-carbamates-5-nitrates
3.1.2. General Procedure for the Preparation of Isosorbide-2-carbamates (GP2)
3.1.3. General Procedure for Synthesis of Lipoic Acid Esters (GP3)
3.1.4. General Procedure for Synthesis of Carbamate Ferulates 8a–d (GP4)
3.2. Biochemical Studies
3.2.1. Ellman Assay for Measuring Inhibition of Cholinesterase Activity
3.2.2. Inhibitor Kinetics
3.2.3. MTT Assay and Neuroprotection of HT-22 Neurons
3.2.4. Statistics
3.2.5. ORAC Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bertram, L.; Tanzi, R.E. The genetic epidemiology of neurodegenerative disease. J. Clin. Investig. 2005, 115, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Dean, R.L., 3rd; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, W.J.; Feen, E.; Grossberg, G.T. The use of cholinesterase inhibitors across all sages of Alzheimer’s Disease. Drugs Aging 2015, 32, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.C.; Yu, J.T.; Wang, H.F.; Tan, M.S.; Meng, X.F.; Wang, C.; Jiang, T.; Zhu, X.C.; Tan, L. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease systematic review and meta-analysis. J. Alzheimers Dis. 2014, 41, 615–631. [Google Scholar] [PubMed]
- Brus, B.; Košak, U.; Turk, S.; Pišlar, A.; Coquelle, N.; Kos, J.; Stojan, J.; Colletier, J.P.; Gobec, S. Discovery, biological evaluation, and crystal structure of a novel nanomolar selective butyrylcholinesterase inhibitor. J. Med. Chem. 2014, 57, 8167–8179. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Kling, B.; Darras, F.H.; Heilmann, J.; Decker, M. Identification of a neuroprotective and selective butyrylcholinesterase inhibitor derived from the natural alkaloid evodiamine. Eur. J. Med. Chem. 2014, 81, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tikhonova, I.G.; Decker, M. Probing the mid-gorge of cholinesterases with spacer-modified bivalent quinazolinimines leads to highly potent and selective butyrylcholinesterase inhibitors. Bioorg. Med. Chem. 2011, 19, 1222–1235. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Strehaiano, M.; Siméon, N.; Bertrand, C.; Chatonnet, A. Learning performances and vulnerability to amyloid toxicity in the butyrylcholinesterase knockout mouse. Behav. Brain Res. 2016, 296, 351–360. [Google Scholar] [PubMed]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.M.; Guillozet, A.; Shaw, P.; Levey, A.; Duysen, E.G.; Lockridge, O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience 2002, 110, 627–639. [Google Scholar] [CrossRef]
- Montanari, S.; Bartolini, M.; Neviani, P.; Belluti, F.; Gobbi, S.; Pruccoli, L.; Tarozzi, A.; Falchi, F.; Andrisano, V.; Miszta, P.; et al. Multitarget Strategy to Address Alzheimer’s Disease: Design, Synthesis, Biological Evaluation, and Computational Studies of Coumarin-Based Derivatives. ChemMedChem 2015. [Google Scholar] [CrossRef] [PubMed]
- Decker, M. Recent advances in the development of hybrid molecules/designed multiple compounds with antiamnesic properties. Mini Rev. Med. Chem. 2007, 3, 221–229. [Google Scholar] [CrossRef]
- Koufaki, M.; Detsi, A.; Kiziridi, C. Multifunctional lipoic acid conjugates. Curr. Med. Chem. 2009, 16, 4728–4742. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.; Cash, A.D.; Smith, M.A. Alzheimer Disease and Oxidative Stress. J. Biomed. Biotechnol. 2002, 2, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Karunakaran, U.; Jeoung, N.H.; Jeon, J.H.; Lee, I.K. Physiological effect and therapeutic application of alpha lipoic acid. Curr. Med. Chem. 2014, 21, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- Decker, M.; Kraus, B.; Heilmann, J. Design, synthesis and pharmacological evaluation of hybrid molecules out of quinazolinimines and lipoic acid lead to highly potent and selective butyrylcholinesterase inhibitors with antioxidant properties. Bioorg. Med. Chem. 2008, 16, 4252–4261. [Google Scholar] [CrossRef] [PubMed]
- Rosini, M.; Simoni, E.; Bartolini, M.; Tarozzi, A.; Matera, R.; Milelli, A.; Hrelia, P.; Andrisano, V.; Bolognesi, M.L.; Melchiorre, C. Exploiting the lipoic acid structure in the search for novel multitarget ligands against Alzheimer’s disease. Eur. J. Med. Chem. 2011, 46, 5435–5442. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R. Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochim. Biophys. Acta 2012, 1822, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.V.; Tan, J.; Town, T. Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and Alzheimer-like pathology in transgenic mice. PLoS ONE 2013, 8, e55774. [Google Scholar]
- Yan, J.J.; Jung, J.S.; Kim, T.K.; Hasan, A.; Hong, C.W.; Nam, J.S.; Song, D.K. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol. Pharm. Bull. 2013, 36, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Benchekroun, M.; Bartolini, M.; Egea, J.; Romero, A.; Soriano, E.; Pudlo, M.; Luzet, V.; Andrisano, V.; Jimeno, M.L.; López, M.G.; et al. Novel tacrine-grafted Ugi adducts as multipotent anti-Alzheimer drugs: A synthetic renewal in tacrine-ferulic acid hybrids. ChemMedChem 2015, 10, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Carolan, C.G.; Dillon, G.P.; Gaynor, J.M.; Reidy, S.; Ryder, S.A.; Khan, D.; Marquez, J.F.; Gilmer, J.F. Isosorbide-2-carbamate Esters: Potent and Selective Butyrylcholinesterase Inhibitors. J. Med. Chem. 2008, 51, 6400–6409. [Google Scholar] [CrossRef] [PubMed]
- Dillon, G.P.; Gaynor, J.M.; Khan, D.; Carolan, C.G.; Ryder, S.A.; Marquez, J.F.; Reidy, S.; Gilmer, J.F. Isosorbide-based cholinesterase inhibitors; replacement of 5-ester groups leading to increased stability. Bioorg. Med. Chem. 2010, 18, 1045–1053. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carolan, C.G.; Dillon, G.P.; Khan, D.; Ryder, S.A.; Gaynor, J.M.; Reidy, S.; Marquez, J.F.; Jones, M.; Holland, V.; Gilmer, J.F. Isosorbide-2-benzyl carbamate-5-salicylate, a peripheral anionic site binding subnanomolar selective butyrylcholinesterase inhibitor. J. Med. Chem. 2010, 53, 1190–1199. [Google Scholar] [PubMed]
- Carolan, C.G.; Gaynor, J.M.; Dillon, G.P.; Khan, D.; Ryder, S.A.; Reidy, S.; Gilmer, J.F. Novel isosorbide di-ester compounds as inhibitors of acetylcholinesterase. Chem. Biol. Interact. 2008, 175, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Kling, B.; Bücherl, D.; Palatzky, P.; Kling, B.; Bücherl, D.; Palatzky, P.; Matysik, F.M.; Decker, M.; Wegener, J.; Heilmann, J. Flavonoids, flavonoid metabolites, and phenolic acids inhibit oxidative stress in the neuronal cell line HT-22 monitored by ECIS and MTT assay: A comparative study. J. Nat. Prod. 2014, 77, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wood, M.; Maher, P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J. Neurochem. 1998, 71, 95–105. [Google Scholar] [CrossRef]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Ohhmayer, S.; Brunner, G.; Heilmann, J. Natural and non-natural prenylated chalcones: Synthesis, cytotoxicity and anti-oxidative activity. Bioorg. Med. Chem. 2008, 16, 4286–4293. [Google Scholar] [PubMed]
- Fang, L.; Kraus, B.; Lehmann, J.; Heilmann, J.; Zhang, Y.; Decker, M. Design and synthesis of tacrine-ferulic acid hybrids as multi-potent anti-Alzheimer drug candidates. Bioorg. Med. Chem. Lett. 2008, 18, 2905–2909. [Google Scholar] [PubMed]
- Sample Availability: Not available.
| Cpd | R | R’ | BChE IC50 µM (pIC50 ± SEM) | AChE IC50 μM (%I at 100 μM) | Selectivity † |
|---|---|---|---|---|---|
| 7a | Bn | Lipoate | 0.15 (2.17 ± 0.03) | (30.75) | >667 |
| 7b | Ph | Lipoate | 13.30 (4.12 ± 0.11) | (18.75) | >7.5 |
| 7c | Bu | Lipoate | 0.17 (2.32 ± 0.04) | (68.70) | |
| 7d | Et | Lipoate | 5.71 (3.76 ± 0.92) | (84.10) | |
| 8a | Bn | Ferulate | 1.82 (3.21 ± 0.02) | (25.20) | >55 |
| 8b | Ph | Ferulate | 29.16 (4.42 ± 0.06) | 27.1 ± 11.3 μM | 0.92 |
| 8c | Bu | Ferulate | 0.43 (2.59 ± 0.05) | ND †† | |
| 8d | Et | Ferulate | 95.03 (1.98 ± 0.89) | ND | |
| 6a | Bn | H | 25.78 (1.41 ± 0.03) | 37.5 ± 7.4 μM | 1.45 |
| 6b | Ph | H | >100 μM | (25.30) | |
| 6c | Bu | H | 27.98 (1.45 ± 0.02) | (30.0) | 3.57 |
| 6d | Et | H | 46.86 (1.67 ± 0.03) | (22.0) | 2.13 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, M.; Wang, J.; Harmon, S.; Kling, B.; Heilmann, J.; Gilmer, J.F. Novel Selective Butyrylcholinesterase Inhibitors Incorporating Antioxidant Functionalities as Potential Bimodal Therapeutics for Alzheimer’s Disease. Molecules 2016, 21, 440. https://doi.org/10.3390/molecules21040440
Jones M, Wang J, Harmon S, Kling B, Heilmann J, Gilmer JF. Novel Selective Butyrylcholinesterase Inhibitors Incorporating Antioxidant Functionalities as Potential Bimodal Therapeutics for Alzheimer’s Disease. Molecules. 2016; 21(4):440. https://doi.org/10.3390/molecules21040440
Chicago/Turabian StyleJones, Mike, Jun Wang, Shona Harmon, Beata Kling, Jörg Heilmann, and John F. Gilmer. 2016. "Novel Selective Butyrylcholinesterase Inhibitors Incorporating Antioxidant Functionalities as Potential Bimodal Therapeutics for Alzheimer’s Disease" Molecules 21, no. 4: 440. https://doi.org/10.3390/molecules21040440
APA StyleJones, M., Wang, J., Harmon, S., Kling, B., Heilmann, J., & Gilmer, J. F. (2016). Novel Selective Butyrylcholinesterase Inhibitors Incorporating Antioxidant Functionalities as Potential Bimodal Therapeutics for Alzheimer’s Disease. Molecules, 21(4), 440. https://doi.org/10.3390/molecules21040440