Silymarin-Loaded Nanoparticles Based on Stearic Acid-Modified Bletilla striata Polysaccharide for Hepatic Targeting
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Silymarin-Loaded Nanoparticles
2.2. In Vitro Release Study
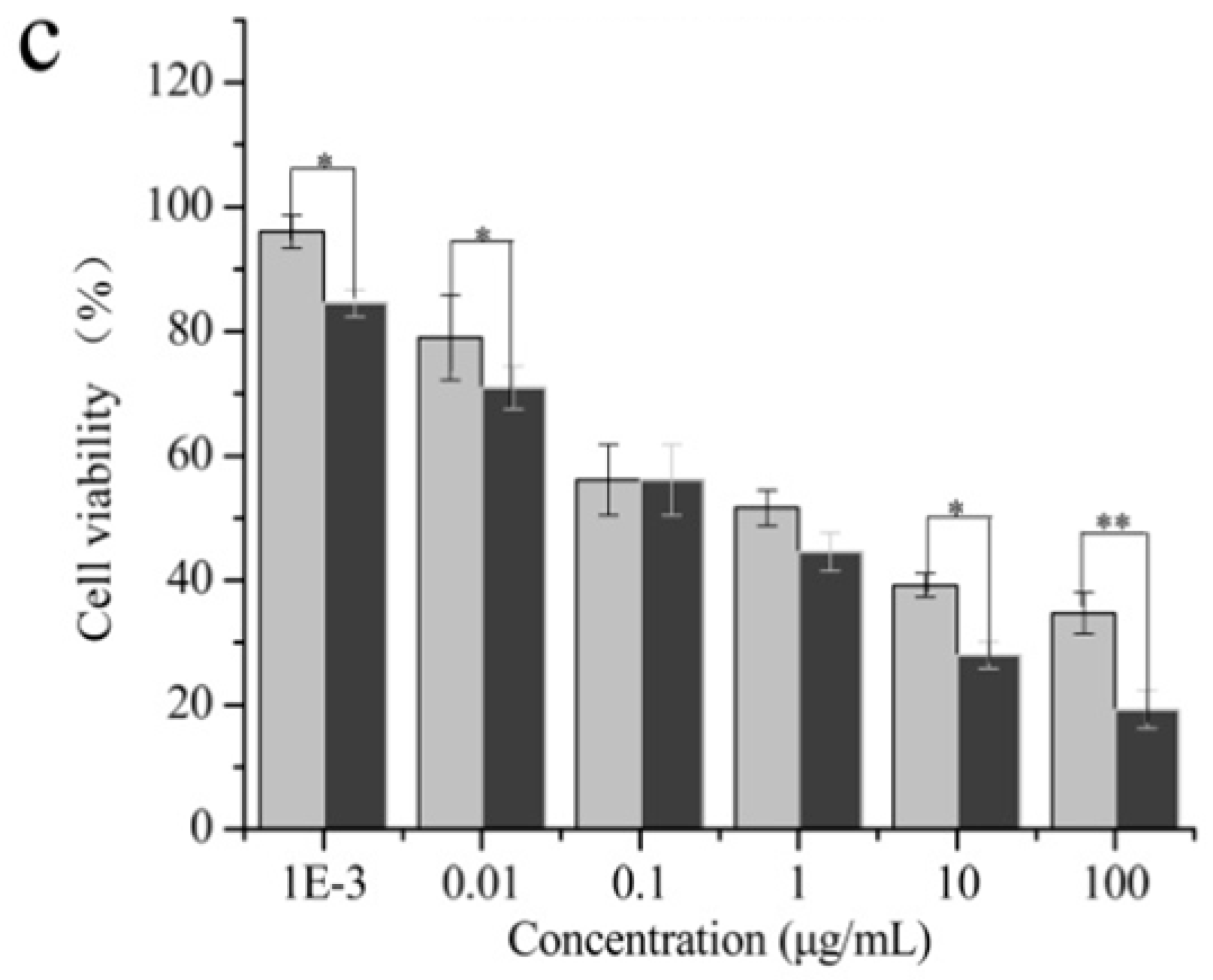
2.3. Cytotoxicity Test
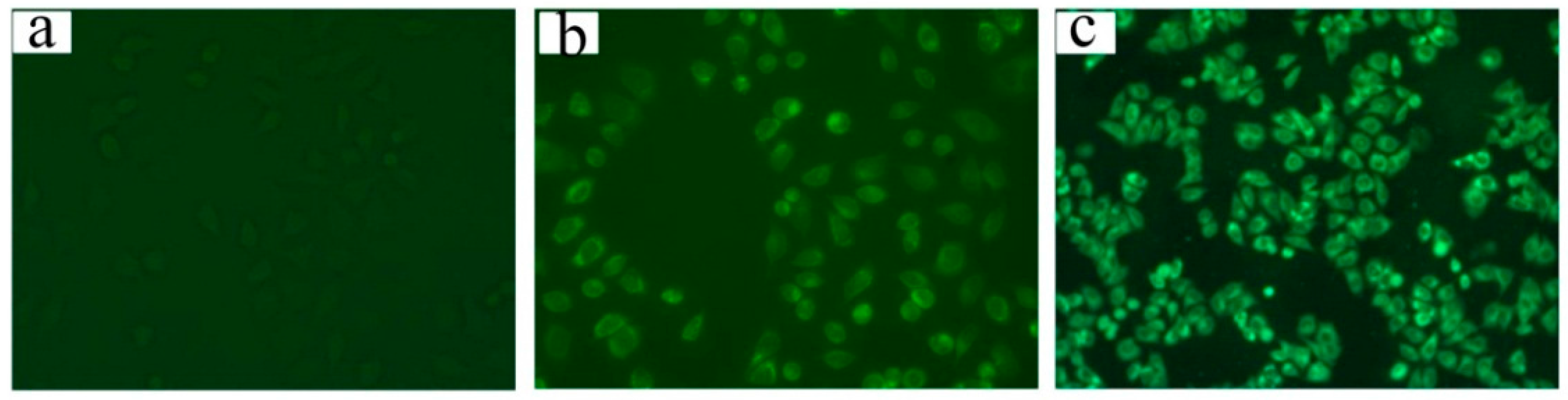
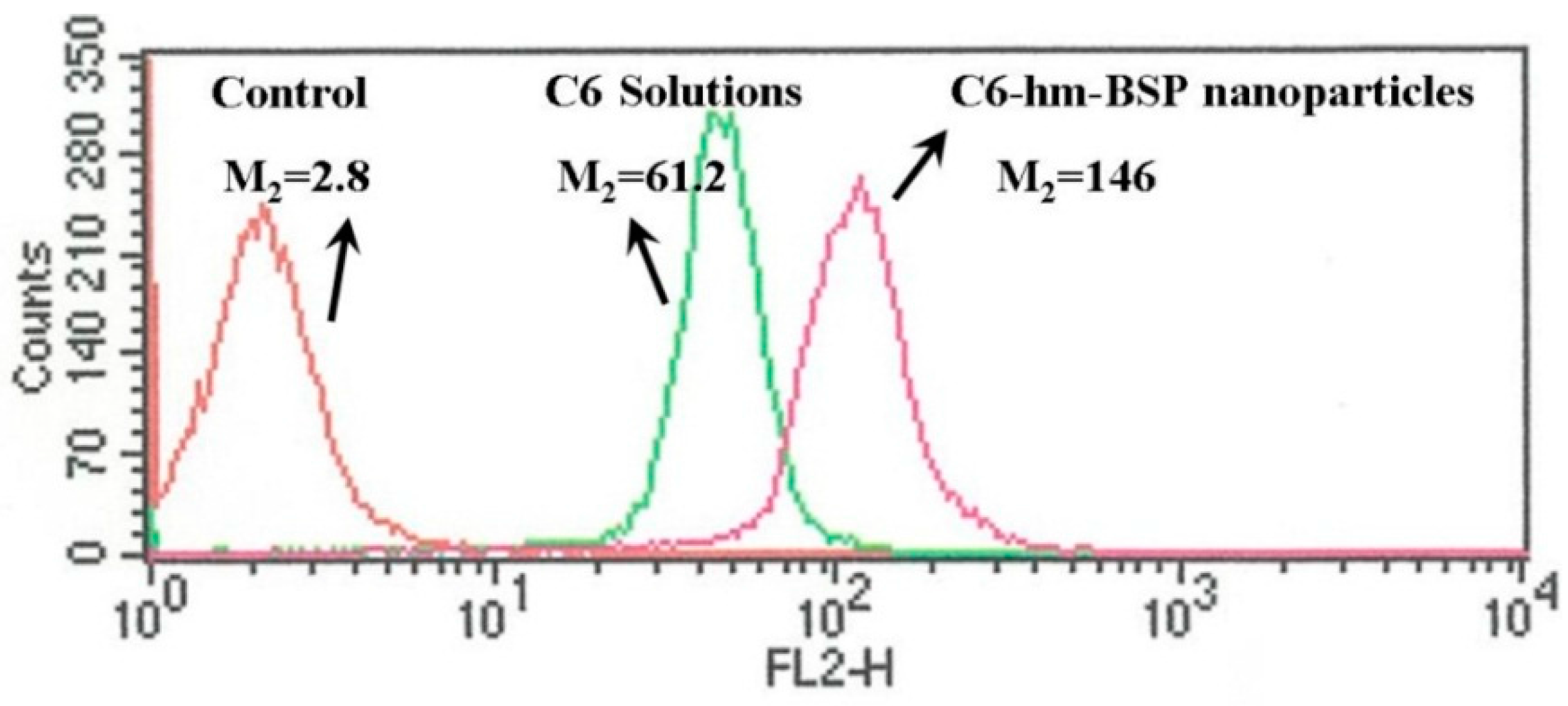
2.4. Cell Uptake and Flow Cytometry Study
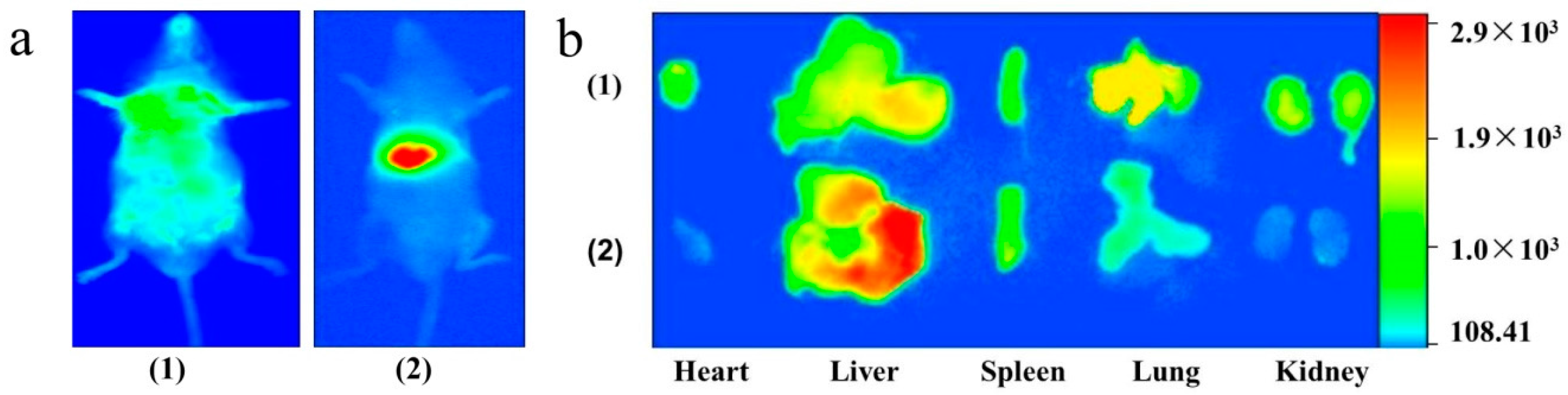
2.5. Tissue Distribution Study
3. Experimental Section
3.1. Materials
3.2. Synthesis of hm-BSP
3.3. Preparation of Silymarin-Loaded Nanoparticles
3.4. Characterization of Silymarin-Loaded Nanoparticles
3.5. In Vitro Dissolution Study
3.6. Cytotoxicity Test
3.7. Cell Uptake and Flow Cytometry Study
3.8. Tissue Distribution Study
3.9. Data Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luper, S.A. Review of Plants Used in the Treatment of Liver Disease: Part 1. Altern. Med. Rev. 1998, 3, 410–421. [Google Scholar] [PubMed]
- Draz, E.I.; Abdin, A.A.; Sarhan, N.I.; Gabr, T.A. Neurotrophic and antioxidant effects ofsilymarin comparable to 4-methylcatechol in protection against gentamicin-induced ototoxicity in guinea pigs. Pharmacol. Rep. 2015, 67, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.M.; Arbid, M.S. Estimation of the novel antipyretic, anti-inflammatory, antinociceptive and antihyperlipidemic effects of silymarin in Albino rats and mice. Asian Pac. J. Trop. Biomed. 2015, 5, 619–623. [Google Scholar] [CrossRef]
- Gharagozloo, M.; Karimi, M.; Amirghofran, Z. Immunomodulatory effects of silymarin in patients with β-thalassemia major. Int. Immunopharmacol. 2013, 16, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.; Subramanya, G.; Uprichard, S.; Hammouda, F.; Saleh, M. Antioxidant and Anti-Hepatitis C Viral Activities of Commercial Milk Thistle Food Supplements. Antioxidants 2013, 2, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Kohli, K.; Ali, M. Reassessing bioavailability of silymarin. Altern. Med. Rev. 2011, 16, 239–249. [Google Scholar] [PubMed]
- Rutter, K.; Scherzer, T.M.; Beinhardt, S.; Kerschner, H.; Stattermayer, A.F.; Hofer, H.; Popow-Kraupp, T.; Steindl-Munda, P.; Ferenci, P. Intravenous silibinin as “rescue treatment” for on-treatment non-respondets to pegylated interferon/ribavirin combination thetapy. Antivir. Ther. 2011, 16, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, E.; Kechagia, I.A.; Tzimas, S.; Balafas, E.; Kostomitsopoulos, N.; Archontaki, H.; Dokoumetzidis, A.; Valsami, G. Serum and tissue pharmacokinetics of silibinin after per os and i.v. administration to mice as a HP-β-CD lyophilized product. Int. J. Pharm. 2015, 493, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Viitala, T.; Ibrahim, H.M.; Abu-Elyazid, S.K.; Samy, A.; Kassem, A.; Yliperttula, M. Silymarin loaded liposomes for hepatic targeting: In vitro evaluationand HepG2 drug uptake. Eur. J. Pharm. Sci. 2013, 50, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Lai, T.C.; Tomoda, K.; Kwon, G.S. Polymeric Micelles for Multi-Drug Delivery in Cancer. AAPS PharmSciTech 2015, 16, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S. Polymeric micelles: Nanocarriers for cancer-targeted drug delivery. AAPS PharmSciTech 2014, 15, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Mao, S. Amphiphilic polymeric micellesas the nanocarrier for peroraldeliveryof poorly soluble anticancer drugs. Expert Opin. Drug Deliv. 2012, 9, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, Y.; Yang, W.; Li, X.; Liu, L.; Zhou, Z.; Wang, Y.; Li, R.; Zhang, Q. Preparation and characterization of self-assembled nanoparticles of 6-O-cholesterol-modified chitosan for drug delivery. Carbohydr. Polym. 2011, 8, 1244–1251. [Google Scholar] [CrossRef]
- Platt, V.M.; Szoka, F.C., Jr. Anticancer Therapeutics: Targeting Macromolecules and anocarriers to Hyaluronan or CD44, a Hyaluronan Receptor. Mol. Pharm. 2008, 5, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, S.; Hong, T.; Zhang, Y.; Sui, H.; Zhang, X.; Ma, Y. Synthesis, self-assembly, and in vitro toxicity of fatty acids-modified Bletilla striata polysaccharide. Aritif. Cells Nanamed. Biotechnol. 2016, 11. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, D.; Li, Z.; Duan, C.; Wang, Y.; Feng, F.; Wang, F.; Liu, Y.; Zhang, Q. Nanostructured lipid carriers for parenteral delivery of silybin: Biodistributionand pharmacokinetic studies. Colloids Surf. B Biointerfaces 2010, 80, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Lian, R.; Lu, Y.; Qi, J.; Tan, Y.; Niu, M.; Guan, P.; Hu, F.; Wu, W. Silymarin Glyceryl Monooleate/Poloxamer 407 Liquid Crystalline Matrices: Physical Characterization and Enhanced Oral Bioavailability. AAPS PharmSciTech 2011, 12, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, M.; Ma, L.; Li, H.; Huang, L. Synthesis and characterization of biotin modified cholesteryl pullulanas a novel anticancer drug carrier. Carbohydr. Polym. 2014, 99, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, X.; Yang, J.; Wang, Y.; Chen, R.; Wu, J.; Liu, Y.; Zhang, N. Self-assembled nanoparticles of methotrexate conjugated O-carboxymethylchitosan: Preparation, characterization and drug release behavior in vitro. Carbohydr. Polym. 2011, 86, 1665–1670. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Wang, H.; Liu, L.; Wang, W.; Wang, C.; Wang, Q.; Liu, W. The biocompatibility of fatty acid modified dextran-agmatine bioconjugate gene delivery vector. Biomaterials 2012, 33, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Sallustio, S.; Galantini, L.; Gente, G.; Masci, G.; la Mesa, C. Hydrophobically Modified Pullulans: Characterization and Physicochemical Properties. J. Phys. Chem. 2004, 108, 18876–18883. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Zhang, P.; He, Z. Amphiphilic Polysaccharide-Hydrophobicized Graft Polymeric Micelles for Drug Delivery Nanosystems. Curr. Med. Chem. 2011, 18, 2638–2648. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.Q.; Yang, F.L.; Hu, F.Q.; Du, Y.Z.; Yuan, H.; Yu, H.Y. Core-modified chitosan-based polymeric micelles for controlled release of doxorubicin. Int. J. Pharm. 2008, 352, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Radjadian, T.; Rezazadeh, S.H.; Huseini, H.F. Analysis of silymarin components in the seed extracts of some milk thistle ecotypes from Iran by HPLC. Iran. J. Sci. Technol. A 2008, 32, 141–146. [Google Scholar]
- Usman, M.; Ahmad, M.; Dayo, A.; Madni, A.; Ali, L.; Yousuf, M.; Khan, M.A.; Munir, A.; Sohail, M.; Mahmood, A. Effect of β-Glucuronidase on Extraction Efficiency of Silymarin from Human Plasma Samples Using Validated HPLC-UV Analysis. Trop. J. Pharm. Res. 2012, 11, 84–90. [Google Scholar] [CrossRef]
- Shakeel, F.; Anwer, M.K. Dissolution thermodynamics and solubility of silymarin in PEG 400-water mixtures at different temperatures. Drug Dev. Ind. Pharm. 2015, 41, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, S.; Fan, R.; Yu, M.; Li, F.; Zhu, C.; Gan, Y. Dual-functional liposome for tumor targeting and overcoming multidrug resistance in hepatocellular carcinoma cells. Biomaterials 2012, 33, 7103–7114. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the nanoparticles are available from the authors.
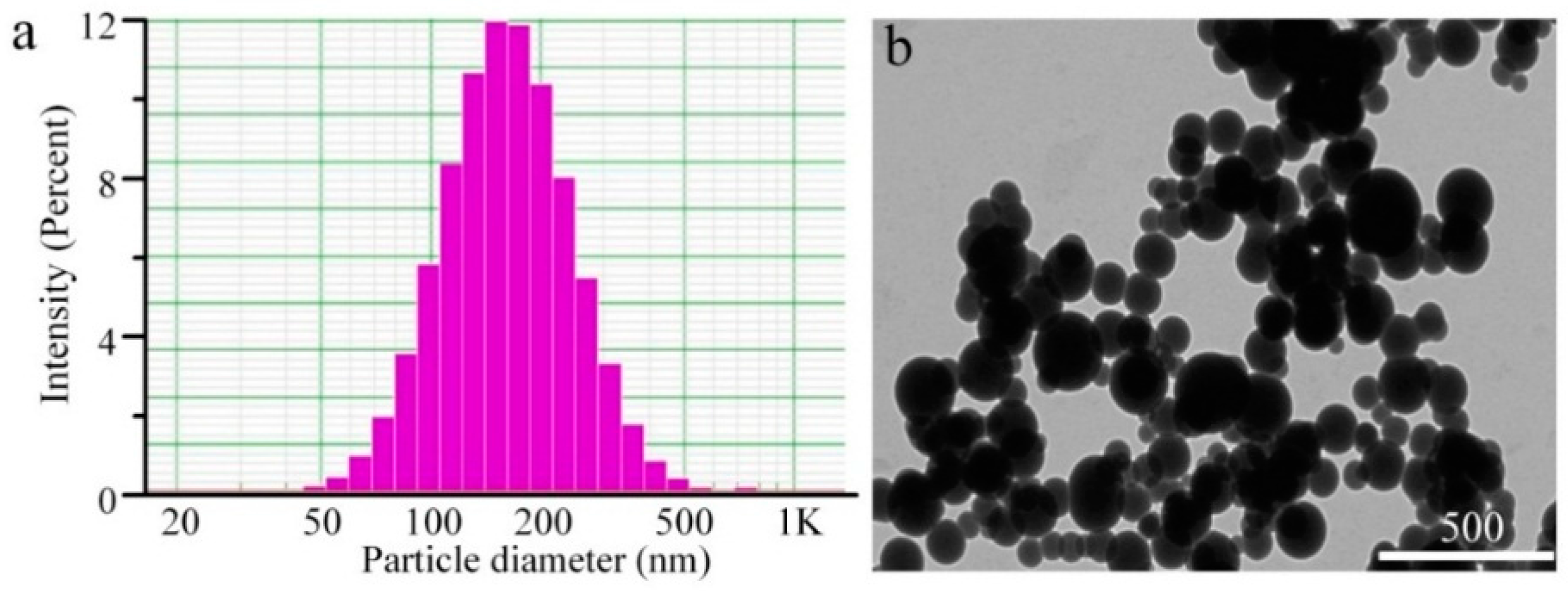
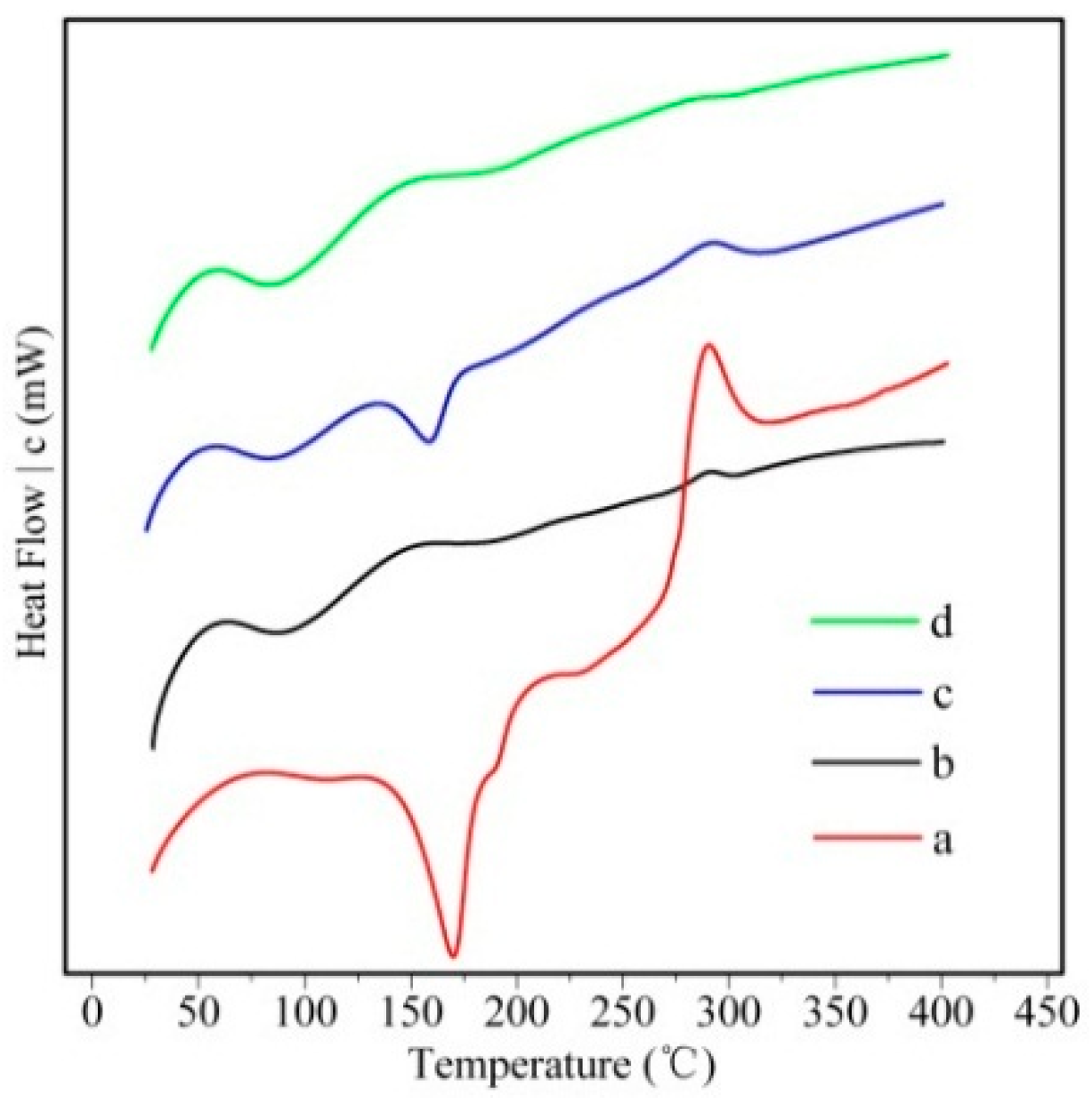
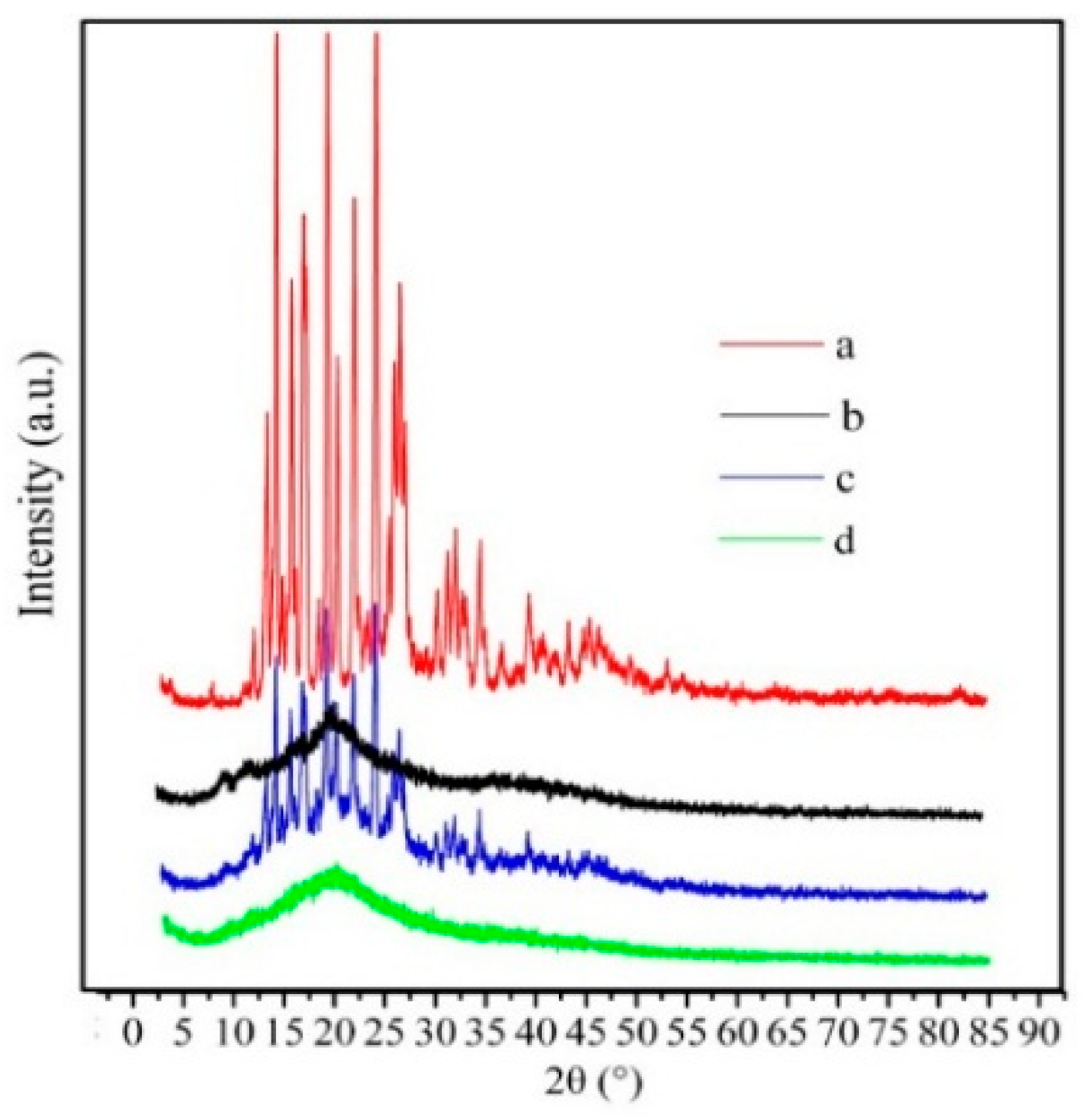

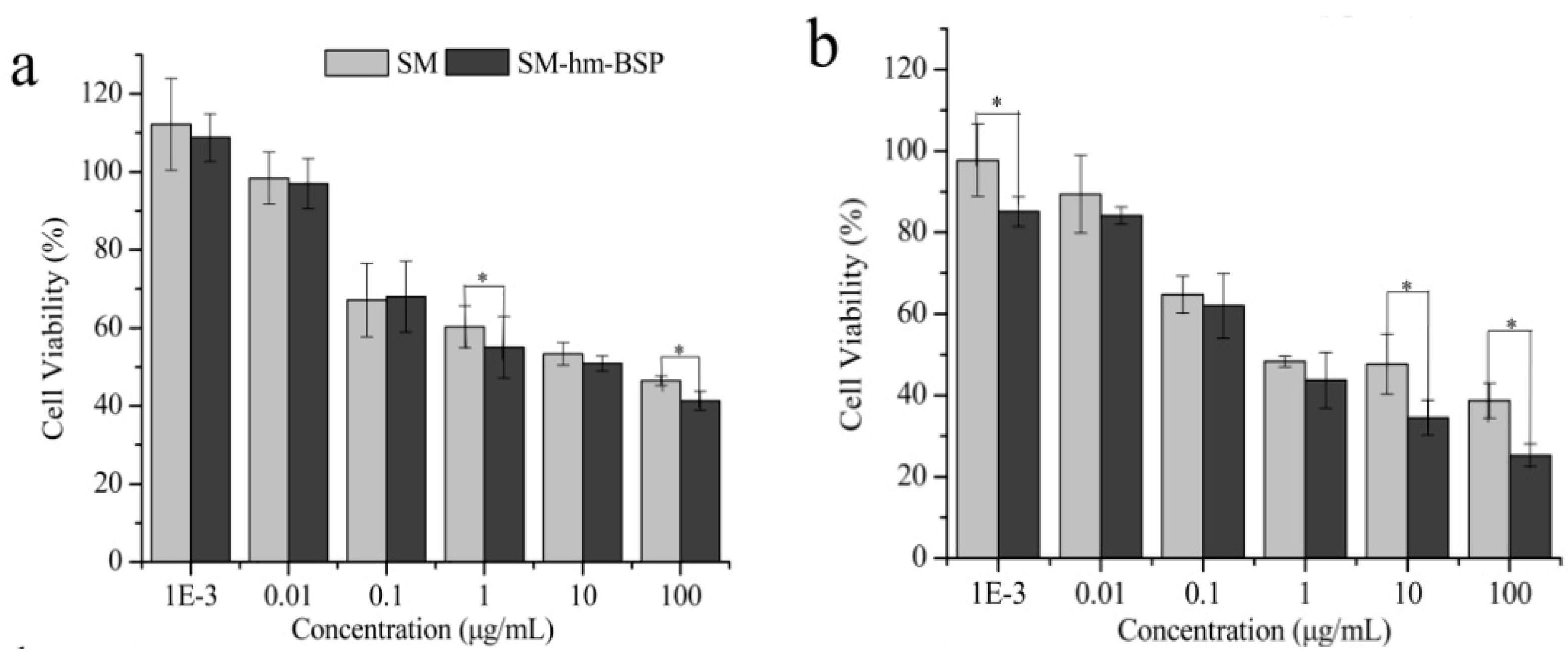
| Sample | Diameter (nm) | PDI | Zeta (mV) a | EE (%) | DL (%) |
|---|---|---|---|---|---|
| SM-hm-BSP | 200.83 ± 8.10 | 0.25 ± 0.04 | −0.36 ± 0.93 | 78.86 ± 0.66 | 7.31 ± 0.05 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; He, S.; Ma, X.; Hong, T.; Li, Z.; Park, K.; Wang, W. Silymarin-Loaded Nanoparticles Based on Stearic Acid-Modified Bletilla striata Polysaccharide for Hepatic Targeting. Molecules 2016, 21, 265. https://doi.org/10.3390/molecules21030265
Ma Y, He S, Ma X, Hong T, Li Z, Park K, Wang W. Silymarin-Loaded Nanoparticles Based on Stearic Acid-Modified Bletilla striata Polysaccharide for Hepatic Targeting. Molecules. 2016; 21(3):265. https://doi.org/10.3390/molecules21030265
Chicago/Turabian StyleMa, Yanni, Shaolong He, Xueqin Ma, Tongtong Hong, Zhifang Li, Kinam Park, and Wenping Wang. 2016. "Silymarin-Loaded Nanoparticles Based on Stearic Acid-Modified Bletilla striata Polysaccharide for Hepatic Targeting" Molecules 21, no. 3: 265. https://doi.org/10.3390/molecules21030265
APA StyleMa, Y., He, S., Ma, X., Hong, T., Li, Z., Park, K., & Wang, W. (2016). Silymarin-Loaded Nanoparticles Based on Stearic Acid-Modified Bletilla striata Polysaccharide for Hepatic Targeting. Molecules, 21(3), 265. https://doi.org/10.3390/molecules21030265





