Abstract
The efficient organocatalytic synthesis of heterocyclic systems of biological relevance is a subject of growing interest. We have found that the pyrrolidine/benzoic acid-catalyzed reaction of α-substituted propenals such as methacrolein, 2-benzylpropenal and 2-(n-hexyl)propenal with activated hydrazines takes place in very good yields (83%–99.6%) under very mild conditions to afford 4-substituted pyrazolidin-3-ols (as diastereomer mixtures); subsequent oxidation with PCC affords the corresponding-4-substituted-3-pyrazolidinones in essentially quantitative yields. In a similar way, 4-substituted isoxazolidinones are obtained with N-Cbz-hydroxylamine as a reagent. The use of chiral diarylprolinol trimethylsilyl ethers as catalysts allows the synthesis of several of these compounds in optically active form, in some cases with excellent enantioselectivity (up to 96:4 er). A preliminary evaluation of the biological activity shows that some of these compounds exhibit interesting antibacterial and antifungal activities.
1. Introduction
The pyrazolidinone (pyrazolidin-3-one) moiety is an important structural motif that can be found both in natural products and in synthetic compounds with interesting pharmacological activity (analgesic, antibiotic, anticonvulsant, and with inhibitory cyclooxygenase, lipoxygenase, and γ-aminobutyrate transferase activity, among others) [1,2,3,4,5,6,7,8,9,10]. On the other hand, the selective oxidation of pyrazolidinones affords the corresponding pyrazolones (pyrazol-5-ones), a class of compounds that also exhibits very relevant pharmacological properties [11,12,13,14,15,16,17,18]. Figure 1 shows structures of some biologically active pyrazolidinones and pyrazolones.
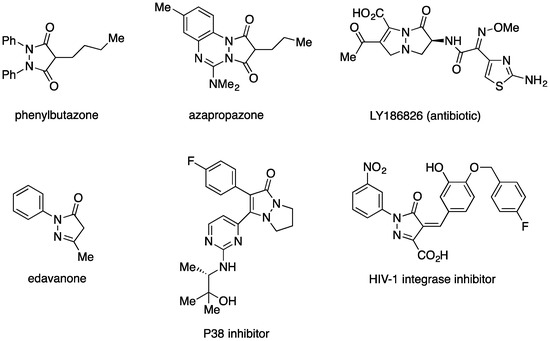
Figure 1.
Selected biologically active pyrazolidinones and pyrazolones.
It is therefore not surprising that efficient and selective syntheses of pyrazolidinone derivatives have been actively pursued [19]. Recently, organocatalytic approaches to these compounds, based either on the amine-catalyzed [20,21] or on the carbene-catalyzed [22] reaction of α,β-unsaturated aldehydes with hydrazine derivatives and subsequent oxidation of the intermediate pyrazolidinols, have been described, enabling their preparation in optically active form [23,24,25]. Although these approaches are highly interesting, all of them suffer from the common limitation that they have been applied only to β-substituted enals, so that they can exclusively lead to 5-substituted-3-pyrazolidinones.
In the course of our studies on the organocatalytic synthesis of α,β-disubstituted-β-amino acids we had found that the pyrrolidine-promoted aza-Michael addition/cyclization of N-protected hydroxylamines to acyclic α,β-unsaturated, α,β-disubstituted enals took place in several instances with excellent yields and good diastereoselectivities [26], so that we set out to investigate if the secondary amine-catalyzed reaction of these branched enals with substituted hydrazines, followed by oxidation of the resulting pyrazolidin-3-ols, would provide a general route to 4,5-disubstituted-3-pyrazolidinones (Scheme 1).
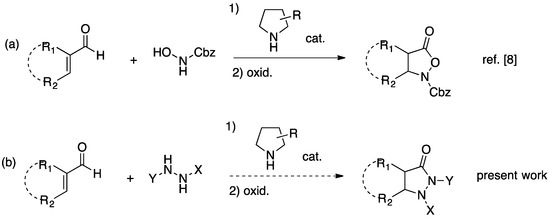
Scheme 1.
Background (a) and goals (b) of the present work.
2. Results and Discussion
2.1. Organocatalytic Synthesis of 4-Substituted Pyrazolidinones and Isoxazolidinones
We began our research by examining the pyrrolidine-catalyzed addition/cyclization of cyclopentene-1-carboxaldehyde (1) with 1-Boc-2-(4-nitrobenzenesulfonyl)hydrazine (2a) [20] using benzoic acid as a co-catalyst. After some experiments, we found that by using 40 mol % of both pyrrolidine and benzoic acid the expected bicyclic pyrazolidin-3-ol 3 was obtained in excellent yield (87% isolated yield after chromatographic purification) and as a single isomer (dr > 30:1), after three days in toluene at room temperature (r.t.) (Scheme 2).
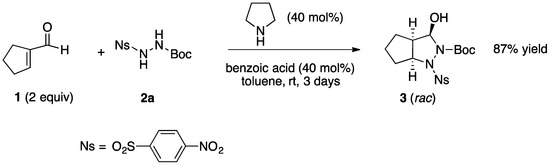
Scheme 2.
Synthesis of the bicyclic pyrazolidinol 3.
Next, we tried the same reaction conditions with acyclic α,β-substituted enals. While the reaction with tiglic aldeyhyde ((E)-2-methylbutenal, 4) led to the formation of the pyrazolidinol 5 (76% yield, 6:1 dr), we found that (E)-2-methyl-3-phenylbutenal 6 was completely unreactive, no product being detected after 7 days of stirring at r.t. (Scheme 3).
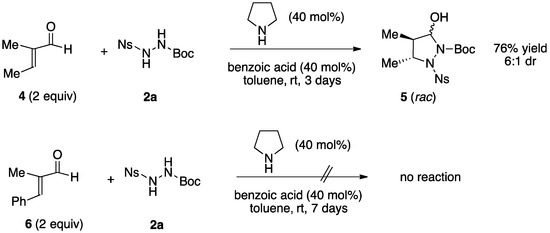
Scheme 3.
Reaction of hydrazine 2a with the acyclic α,β-disubstituted enals 4 and 6.
Contrary to what we had observed for the corresponding isoxazolidinols [26], attempted oxidation of both 3 and 5, either with pyridinium chlorochromate (PCC) or with pyridinium dichromate (PDC), failed to cleanly give the desired pyrazolidinones, so we decided to reduce the steric hindrance of the intermediate pyrazolidinols by suppressing the β-substituent in the starting enal.
We were pleased to find that the pyrrolidine/benzoic acid-catalyzed reaction of the α-substituted enals methacrolein (7a), 2-benzylpropenal (7b), or 2-(n-hexyl)propenal (7c) with the activated hydrazines 2a and 1,2-bis(p-toluenesulfonyl)hydrazine (2b) took place in very good yields (83%–99.6%), affording the 4-substituted pyrazolidin-3-ols 8aa–8bc as diastereomer mixtures; without further purification, oxidation with PCC was performed to afford the corresponding-4-substituted-3-pyrazolidinones 9aa–9bc in essentially quantitative yields (Scheme 4 and Table 1).
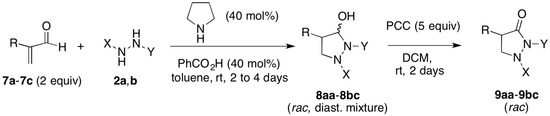
Scheme 4.
Organocatalytic synthesis of pyrazolidin-3-ones from α-substituted enals.

Table 1.
Pyrrolidine-catalyzed synthesis of pyrazolidin-3-ols 8aa–8bc from α-substituted enals 7a–7c and hydrazines 2a and 2b, and oxidation to the corresponding pyrazolidin-3-ones 9aa–9bc.
As it can be seen, the aza-Michael addition/cyclization step took place in excellent yield and with high diastereoselectivity, irrespective of the nature of the hydrazine (2a or 2b) and of the acrolein substituent (methyl, benzyl, or n-hexyl). It should be noted however that when the more hindered α-isopropyl acrylaldehyde (3-methyl-2-methylenebutyraldehyde (7d)) was used under the same conditions, no reaction with hydrazine 2a was observed after 7 days at rt. In our hands, N,N’-bis(tert-butoxycarbonyl)hydrazine (2c), reported to give good yields of pyrazolidin-3-ols upon pyrrolidine-catalyzed reaction with cinnamaldehyde derivatives [21], failed to react with the α-substituted enals 1, 4, and 7a–7d.
In the light of these results, we explored the possible use of the α-substituted acroleins 7 as starting materials for the synthesis of 4-substituted isoxazolidin-5-ones, potentially interesting compounds both from the biological point of view and as advanced intermediates towards α-substituted-β-amino acids [26], by the amine-catalyzed reaction with a suitable protected hydroxylamine. To our satisfaction, we found that in fact α-substituted acroleins 7, with the sole exception of the isopropyl derivative 7d, reacted smoothly with N-Cbz-hydroxylamine 10 (Cbz = benzyloxycarbonyl) to afford the isoxazolidinols 11 in good yields (78%–83% isolated yields) as diastereomer mixtures. Subsequent oxidation of these compounds with PCC took place uneventfully, providing the target 4-substituted isoxazolidin-5-ones 12 (Scheme 5 and Table 2).
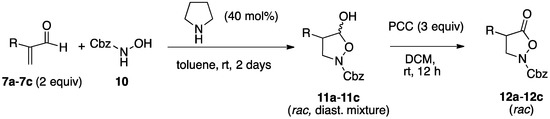
Scheme 5.
Organocatalytic synthesis of isoxazolidin-5-ones from α-substituted enals.

Table 2.
Pyrrolidine-catalyzed synthesis of isoxazolidin-5-ols 11a–11c from α-substituted enals 7a–7c and N-Cbz-hydroxylamine 10, and oxidation to the corresponding isoxazolidin-5-ones 12a–12c.
Previous studies from our group [26] and those of Córdova [27,28] and Vicario [20] had shown that the use of chiral diarylprolinol-silyl ethers as catalysts led to high enantioselectivities in the aza-Michael/cyclization reactions of β-substituted [20,27,28] and of α,β-disubstituted [26] enals with hydroxylamines or hydrazines. However, α-branched vinyl carbonyls remain a very challenging substrate for this type of reaction and only one successful example of aminocatalytic asymmetric aza-Michael addition to α-substituted vinyl ketones has been reported so far [29,30]. Bearing these precedents in mind, we proceeded to examine the performance of the Jørgensen-Hayashi catalysts 13 and 14 (Figure 2) in the reaction between aldehydes 7a–7c and hydrazines 2a and 2b (Table 3), using a 20 mol % of the catalyst together with a 20 mol % of benzoic acid co-catalyst in toluene at r.t. (typical reaction time 3 days). The stereoisomeric mixture of pyrazolidinols 8 was then submitted to oxidation with PCC as before, and the enantiomeric purity of the corresponding pyrazolidinones 9 was determined by chiral HPLC analysis. After 7 days of stirring at rt, no product was observed in the attempted reactions of aldehyde 7c with hydrazine 2a, either when using 13 or 14 as catalysts. On the other hand, we could not find satisfactory HPLC conditions for the separation of the enantiomers of 9bb, so that the enantioselective catalysis for the reaction of aldehyde 7b with hydrazine 2b was not attempted.
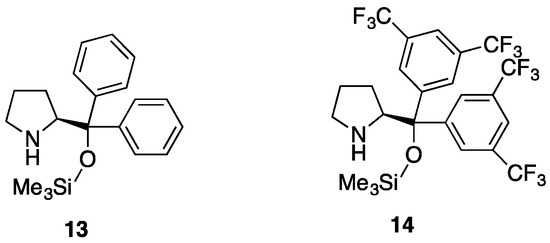
Figure 2.
Jørgensen-Hayashi catalysts 13 ((S)-diphenylprolinol trimethylsilyl ether) and 14 ((S)-bis(3,5-trifluoromethylphenyl)prolinol trimethylsilyl ether) used in the enantioselective synthesis of pyrazolidinones 9.

Table 3.
Enantioselective synthesis of pyrazolidin-3-ones 9.
As it can be seen in Table 3, the enantioselectivity of the reaction depends strongly both in the nature of the reactants and of the catalyst. The highest enantiomeric purities were obtained for the less hindered methyl-substituted enal 7a, either with hydrazine 2a (entry 2, 90:10 er, catalyst 14) or with hydrazine 2b (entry 7, 96:4 er, catalyst 13). In any case, these preliminary results demonstrate for the first time the feasibility of the asymmetric synthesis of 4-substituted-3-pyrazolidinones by the organocatalytic aza-Michael/cyclization of activated hydrazines to α-substituted acroleines.
A simplified mechanistic proposal for the formation of enantiomerically enriched 4-substituted pyrazolidinols 8aa–8ac is depicted in Scheme 6. While in the formation of the first carbon-nitrogen bond by the aza-Michael reaction of the chiral iminium intermediate A (formed from the α-substituted enal 7a–c and the chiral pyrrolidine catalyst 13 or 14) with the hydrazine 2a no new chiral center is formed, the protonation of the intermediate enamine B can take place on the two diastereotopic faces of the C–C double bond. When using the trifluoromethyl-substituted catalyst 14, we assume (based on our previous studies on the asymmetric organocatalytic synthesis of isoxazolidines) [26] that when R = Me protonation will take place under kinetic control from the face opposite to the bulky pyrrolidine substituent, leading to the second iminium intermediate C as the major diastereomer. Fast, irreversible hydrolysis and cyclization of this intermediate would predominantly give the (4R) enantiomer of 8aa. When the steric bulk of the R substituent is increased (7b or 7c), the formation of the diastereomeric iminium ion C’ will be relatively favoured (in this intermediate the steric interactions of the α-substituent R with the pyrrolidine substituent are minimized), resulting of the competitive formation of the (4S) enantiomer of the pyrazolidinol 8ab or 8ac.
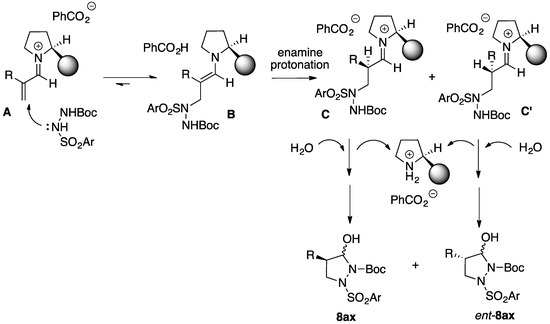
Scheme 6.
Mechanistic proposal for the Michael addition-cyclization sequence leading to the formation of enantiomerically enriched 4-substituted pyrazolidinols.
When the asymmetric synthesis of isoxazolidinones 12a–12c was attempted by means of the use of the chiral catalysts 13 and 14 in the reactions between enals 7a–7c and N-Cbz-hydroxylamine 10, the results were much less satisfactory, since after oxidation of the intermediate isoxazolidinols 11a–11c to the corresponding isoxazolidinones 12, we were not able to find suitable HPLC conditions for the determination of the enantiomeric purity of 12a; on the other hand, 12b was obtained in essentially racemic form with both catalysts, and scalemic 12c could be prepared but only in low enantiomeric purity (57:43 er) when using 14 as the chiral catalyst (see details in the Experimental Section).
2.2. Primary Antimicrobial Screening
Finally, and in the framework of the Community for Open Antimicrobial Drug Discovery (CO-ADD) program [31], the new heterocyclic systems synthesized by us (both in racemic and when possible in enantiomerically enriched form) were submitted to a primary antimicrobial screening study by whole cell growth inhibition assays. The inhibition of growth was measured against five bacteria: Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Staphylococcus aureus, and two fungi: Candida albicans and Cryptococcus neoformans. The results of the study are summarized in Table 4, which shows the active compounds found for each microbial strain.

Table 4.
Active compounds 1 in the primary antimicrobial screening (bacterial and fungal).
Among the samples tested, five showed antimicrobial activity and have been selected for hit confirmation. It is worth noting that antifungal activity was found only for isoxazolidinones (12a and 12b) and for isoxazolidinol 11c, and that the pyrazolidinol 8aa, both in racemic and in enantiomerically enriched form, was active against Staphylococcus aureus. The fact that neither the corresponding pyrazolidinone 9aa nor the 4-methyl-1,2-bis-(p-toluenesulfonyl)pyrazolidin-3-ol 8ba show any growth inhibition for the same bacterial strain strongly suggests that 4-methyl-1-(4-nitrobenzenesulfonyl)-4,5-dihydro-1H-pyrazole, resulting from acid-catalyzed deprotection/ dehydration of 8aa [20], could be the biologically active species.
3. Experimental Section
3.1. General Information
Reactions were generally performed at room temperature, either in round-bottomed flasks or in loosely stoppered glass vials, with magnetic stirring and open to the air. Commercially available reagents, catalysts, and solvents were used as received with the exception of dichloromethane, which was distilled from calcium hydride under nitrogen. Aldehydes 1 [32] and 7b–7d [33,34] were prepared according to literature procedures. Yields refer to products isolated after chromatographic purification. Reactions were monitored both by 1H-NMR and by thin-layer chromatography, carried out on silica gel plates Merck 60 F254 (Sigma-Aldrich Química SL, Madrid, Spain), and compounds were visualized by irradiation with UV light and/or treatment with a solution of KMnO4 as developing agent followed by heating. Flash column chromatography was performed using silica gel Merck 60 (particle size: 0.040–0.063 mm). 1H (400 MHz) and 13C (100.6 MHz) NMR spectra were recorded with a Mercury 400 spectrometer (Varian Inc., Palo Alto, CA, USA). Chemical shifts (δ) are given in ppm relative to tetramethylsilane (TMS), and coupling constants (J) are given in Hz. The spectra were recorded in CDCl3 as solvent at room temperature. TMS served as an internal standard (δ = 0.00 ppm) for 1H-NMR spectra, and CDCl3 (δ = 77.0 ppm) for 13C-NMR spectra. Data are reported as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad signal. Where appropriate, 2D techniques (COSY, NOESY) were also used to assist in structure elucidation. High-resolution mass spectra (HRMS) were recorded with a MicrOTOF spectrometer (Bruker, Billerica, MA, USA) by the Unitat d’Espectrometria de Masses, CCiT-UB. Specific rotations were determined at room temperature with a 241 MC polarimeter (Perkin-Elmer, Waltham, MA, USA). Chiral HPLC analyses were performed with a LC Series 20 apparatus (Shimadzu Corp., Kyoto, Japan) with an M20 diode array UV/Vis detector, using Chiralpak® IC, IA and IB columns (Daicel Corporation, Tokio-Osaka, Japan). The homogeneity of the peaks corresponding to the two enantiomers of the product was thoroughly checked by comparison of the UV spectra.
3.2. General Procedures for the Preparation of Pyrazolidines
3.2.1. Racemic Pyrazolidinols from Enals and Hydrazines
An ordinary glass vial equipped with a magnetic stirring bar was charged with pyrrolidine (1.42 mL, 14 mg, 0.20 mmol, 40 mol %), PhCOOH (24 mg, 0.20 mmol, 40 mol %) and toluene (2 mL). The hydrazine 2a or 2b (0.50 mmol, 1 eq) and the α,β-unsaturated aldehyde (1.0 mmol, 2 eq) were added sequentially. Stirring was maintained at room temperature until the reaction was complete (TLC and or 1H-NMR monitoring, 24 h–4 days) and the crude reaction mixture was diluted with 10 mL of ethyl acetate. The organic phase was washed first with 10% w/w aqueous sodium bicarbonate solution (10 mL), then with brine (10 mL), and dried over MgSO4. Filtration and evaporation of solvents under reduced pressure afforded the crude reaction product that was purified by column chromatography (silica gel; hexane/ethyl acetate mixtures of increasing polarity) to afford the intermediate pyrazolidinol as a diastereomer mixture.
3.2.2. Asymmetric Synthesis of Pyrazolidinols from Enals and Hydrazines
An ordinary glass vial equipped with a magnetic stirring bar was charged with the chiral prolinol silyl ether 13 or 14 (0.05 mmol, 20 mol %), PhCOOH (6 mg, 0.05 mmol, 20 mol %) and toluene (1 mL). The hydrazine 2a or 2b (0.24 mmol, 1 eq) and the α,β-unsaturated aldehyde (0.48 mmol, 2 eq) were added sequentially. The stirring was maintained at room temperature until the reaction was complete (TLC and or 1H-NMR monitoring, 3–6 days) and the crude reaction mixture was diluted with 10 mL of ethyl acetate. The organic phase was washed first with 10% w/w aqueous sodium bicarbonate solution (10 mL), then with brine (10 mL), and dried over MgSO4. Filtration and evaporation of solvents under reduced pressure afforded the crude reaction product that was purified by column chromatography (silica gel; hexane/ethyl acetate mixtures of increasing polarity) to afford the intermediate pyrazolidinol as a diastereomer mixture.
3.2.3. Pyrazolidinones by Oxidation of Pyrazolidinols
The pyrazolidinol diastereomer mixture (0.15 mmol) and pyridinium chlorochromate PCC (158 mg, 0.73 mmol, 5 eq) were added sequentially to a stirred suspension of activated 4 Å molecular sieves (300 mg) in anhydrous dichloromethane (2 mL), and the reaction mixture was stirred at room temperature until completion (24–48 h). After the addition of diethyl ether (10 mL) to precipitate the chromium salts, the solution was decanted and filtered through a short pad of Celite® eluting with a 1:10 mixture of ethyl acetate and diethyl ether). After removal of the solvents in vacuo, the reaction product was purified by column chromatography (silica gel, hexane/ethyl acetate mixtures) to give the desired pyrazolidinone.
3.3. General Procedures for the Preparation of Isoxazolidines
3.3.1. Racemic Isoxazolidinols from Enals and Hydroxylamine 10
Pyrrolidine (from a 10 mg/mL solution in toluene; 1.48 mL, 0.21 mmol, 40 mol %) and N-Cbz-hydroxylamine 10 (87 mg, 0.52 mmol, 1 eq) were added sequentially to a magnetically stirred solution of the α,β-unsaturated aldehyde 7a, 7b, or 7c (1.04 mmol, 2 eq) in toluene (2 mL), and the resulting solution was stirred at room temperature. The progress of the reaction was monitored both by 1H-NMR spectroscopy and by TLC. When the starting hydroxylamine 10 was not detected (2 days), toluene and pyrrolidine were removed in vacuo, and the crude residue was directly purified by column chromatography (silica gel; hexane/ethyl acetate mixtures) to give the corresponding isoxazolidinol as a diastereomer mixture.
3.3.2. Asymmetric Synthesis of Isoxazolidinols from Enals and Hydroxylamine 10
The chiral prolinol silyl ether 13 or 14 (0.104 mmol, 20 mol %) and N-Cbz-hydroxylamine 10 (87 mg, 0.52 mmol, 1 eq) were added sequentially to a magnetically stirred solution of the α,β-unsaturated aldehyde 7a, 7b, or 7c (1.04 mmol, 2 eq) in toluene (2 mL), and the resulting solution was stirred at room temperature. The progress of the reaction was monitored both by 1H-NMR spectroscopy and by TLC. When the starting hydroxylamine 10 was not detected (3 days), toluene was removed in vacuo, and the crude residue was directly purified by column chromatography (silica gel; hexane/ethyl acetate mixtures) to give the corresponding isoxazolidinol as a diastereomer mixture.
3.3.3. Isoxazolidinones by Oxidation of Isoxazolidinols
The isoxazolidinol diastereomer mixture (0.126 mmol) and pyridinium chlorochromate PCC (82 mg, 0.38 mmol, 5 eq) were added sequentially to a stirred suspension of activated 4Å molecular sieves (150 mg) in anhydrous dichloromethane (2 mL), and the reaction mixture was stirred overnight at room temperature. After the addition of diethyl ether (5 mL) to precipitate the chromium salts, the solution was decanted and filtered through a short pad of Celite® eluting with a 4:1 mixture of hexane and ethyl acetate. After removal of the solvents in vacuo, the reaction product was purified by column chromatography (silica gel, hexane/ethyl acetate mixtures) to give the desired isoxazolidinone.
3.4. Characterization of the Products
tert-Butyl (3RS,3aRS,6aSR)-3-hydroxy-1-((4-nitrophenyl)sulfonyl)hexahydrocyclopenta[c]pyrazole-2(1H)-carboxylate, 3. Obtained in 87% yield (450 mg, 1.09 mmol) according to Procedure 3.2.1. Colorless solid, m.p. 83–85 °C (from 1:9 chloroform/hexanes). 1H-NMR (CDCl3): δ = 1.24 (s, 9H), 1.47–1.54 (m, 3H), 1.67–1.75 (m, 1H), 1.77–1.83 (m, 1H), 1.86–1.95 (m, 1H), 2.72–2.77 (m, 1H), 3.38 (br d, 1H, OH), 4.73–4.78 (m, 1H), 5.42 (m, 1H), 8.11 (d, J = 8.0 Hz, 2H), 8.29 (d, J = 8.0 Hz, 2H) ppm. 13C-NMR (CDCl3): δ = 24.5, 27.8, 30.5, 33.7, 53.6, 62.2, 65.5, 83.0, 91.9, 123.7, 131.1, 141.8, 150.7 ppm. HRMS (ESI): Calculated for C17H27N4O7S [M + NH4]+ = 431.1595; found, 431.1592.
tert-Butyl 3,4-trans-5-hydroxy-3,4-dimethyl-2-((4-nitrophenyl)sulfonyl)-pyrazolidine-1-carboxylate, 5. Obtained as a 6:1 diastereomer mixture in 76% yield (380 mg, 0.95 mmol) according to Procedure 3.2.1. Colorless solid, m.p. 71–73 °C (major isomer, 1:9 chloroform/hexanes). 1H-NMR (CDCl3): δ = 1.00 (d, J = 7.0 Hz, 3H), 1.24 (s, 9H), 1.36 (d, J = 7.0 Hz, 3H), 1.80–1.87 (m, 1H), 2.86 (br d, 1H, OH), 3.72–3.79 (m, 1H), 5.46–5.48 (m, 1H), 8.07 (d, J = 8.0 Hz, 2H), 8.28 (d, J = 8.0 Hz, 2H) ppm. 13C-NMR (CDCl3): δ = 11.1, 20.7, 27.81, 27.83, 47.4, 62.2, 83.2, 86.6, 123.6, 130.9, 141.8, 150.6, 154.7 ppm. HRMS (ESI): Calculated for C16H24N3O7S [M + H]+ = 402.1337; found, 402.1340.
tert-Butyl 5-hydroxy-4-methyl-2-((4-nitrophenyl)sulfonyl)-pyrazolidine-1-carboxylate, 8aa. The racemic compound was obtained as a 4:1 diastereomer mixture in 93% yield (180 mg, 0.47 mmol) according to Procedure 3.2.1. Colorless solid, m.p. 111–114 °C (major isomer, 1:9 chloroform/hexanes). 1H-NMR (CDCl3): δ = 1.09 (d, J = 7.0 Hz, 3H), 1.35 (s, 9H), 2.25–2.30 (m, 1H), 3.03–3.09 (m, 1H), 3.22 (br d, 1H, OH), 3.29–3.36 (m, 1H), 3.83–3.87 (m, 1H), 5.47 (m, 1H), 8.12 (d, J = 8.0 Hz, 2H), 8.34 (d, J = 8.0 Hz, 2H) ppm. 13C-NMR (CDCl3): δ = 15.0, 27.9, 43.0, 55.3, 83.2, 91.9, 123.9, 131.0, 141.8, 150.8, 154.5 ppm. HRMS (ESI): Calculated for C15H21N3NaO7S [M + Na]+ = 410.0992; found, 410.1000. When using 14 as a catalyst and following Procedure 3.2.2, scalemic 8aa was obtained in 98.6% yield (3:1 diastereomer mixture). Colorless solid, m.p. = 135–137 °C (major isomer, 1:9 chloroform/hexanes).
(−)-tert-Butyl 4-methyl-2-((4-nitrophenyl)sulfonyl)-pyrazolidin-5-one-1-carboxylate, 9aa. Oxidation of 8aa obtained by catalyst 14 gave (−)-9aa (90:10 er) in 94% yield (101 mg, 0.26 mmol) according to Procedure 3.2.3. Colorless solid, m.p. 131–134 °C (from 1:9 chloroform/hexanes). 1H-NMR (CDCl3): δ = 1.16 (d, J = 7.0 Hz, 3H), 1.47 (s, 9H), 2.54–2.64 (m, 1H), 3.38–3.45 (m, 1H), 4.40–4.45 (m, 1H), 8.18 (d, J = 8.0 Hz, 2H), 8.41 (d, J = 8.0 Hz, 2H) ppm. 13C-NMR (CDCl3): δ = 13.4, 27.7, 36.8, 53.7, 85.6, 124.4, 130.7, 141.3, 151.4, 173.4 ppm. HRMS (ESI): Calculated for C15H19N3NaO7S [M + Na]+ = 408.0836; found, 408.0847. [α] = −56.0 (1.23, DCM). Conditions for the HPLC analysis: Chiralpak® IC column, 90:10 hexane/2-propanol, 1 μL/min, 25 °C, λ = 254 nm. tR (major enant.) = 58.1 min, tR (minor enant.) = 84.4 min.
tert-Butyl 4-benzyl-5-hydroxy-2-((4-nitrophenyl)sulfonyl)-pyrazolidine-1-carboxylate, 8ab. The racemic compound was obtained in 83% yield (165 mg, 0.36 mmol, >20:1 dr) according to procedure 3.2.1. Colorless solid, m.p. 122–126 °C (major isomer, recrystallized from 1:2:7 dichloromethane/chloroform/hexanes). 1H-NMR (CDCl3): δ = 1.36 (s, 9H), 2.53–2.58 (m, 2H), 3.14–3.19 (m, 1H), 3.24 (br d, 1H, OH), 4.01–4.06 (m, 1H), 5.39–5.41 (m, 1H), 7.09 (d, J = 7.8 Hz, 2H), 7.20–7.33 (m, 3H), 8.13 (d, J = 8.0 Hz, 2H), 8.34 (d, J = 8.0 Hz, 2H) ppm. 13C-NMR (CDCl3): δ = 27.9, 36.7, 50.1, 53.7, 83.3, 89.9, 123.8, 126.9, 128.4, 128.8, 131.0, 137.8, 141.7, 150.8, 154.3 ppm. HRMS (ESI): Calculated for C21H25N3NaO7S [M + Na]+ = 486.1305; found, 486.1298. When using 13 as a catalyst and following Procedure 3.2.2, scalemic 8ab was obtained in 77% yield. Colorless solid, m.p. = 132–136 °C (major isomer, 1:2:7 dichloromethane/chloroform/hexanes).
(−)-tert-Butyl 4-benzyl-2-((4-nitrophenyl)sulfonyl)-pyrazolidin-5-one-1-carboxylate, 9ab. Oxidation of 8ab obtained by catalyst 13 gave (−)-9ab (44:56 er) in 98% yield (108 mg, 0.23 mmol) according to Procedure 3.2.3. Colorless solid, m.p. 115–118 °C (recrystallized from 1:2:7 dichloromethane/chloroform/hexanes). 1H-NMR (CDCl3): δ = 1.46 (s, 9H), 2.61–2.67 (m, 1H), 2.86–2.92 (m, 1H), 3.17–3.21 (m, 1H), 3.46–3.52 (m, 1H), 4.16–4.21 (m, 1H), 7.07 (d, J = 6.7 Hz, 2H), 7.25–7.32 (m, 3H), 8.13 (d, J = 7.8 Hz, 2H), 8.37 (d, J = 7.8 Hz, 2H) ppm. 13C-NMR (CDCl3): δ = 27.7, 34.8, 43.6, 51.7, 85.7, 124.3, 127.2, 128.5, 130.7, 136.7, 141.2, 147.5, 151.4, 172.1 ppm. HRMS (ESI): Calculated for C42H50N7O14S2 [2M + NH4]+ = 940.2852; found, 940.2847. [α] = −11.4 (1.19, DCM). Conditions for the HPLC analysis: Chiralpak® IC column, 90:10 hexane/2-propanol, 1 μL/min, 25 °C, λ = 254 nm. tR (minor enant.) = 62.4 min, tR (major enant.) = 73.2 min.
tert-Butyl 4-hexyl-5-hydroxy-2-((4-nitrophenyl)sulfonyl)-pyrazolidine-1-carboxylate, 8ac. Obtained in 99.6% yield as a diastereoisomer mixture (227 mg, 1.00 mmol, 5:1 dr) according to Procedure 3.2.1. Colorless solid, m.p. 83–85 °C. 1H-NMR (CDCl3, major isomer): δ = 0.86 (t, J = 7Hz, 3H), 1.25–1.32 (m, 8H), 1.33 (s, 9H), 1.49–1.56 (m, 2H), 2.15–2.19 (m, 1H), 3.01–3.07 (m, 1H), 3.25 (br d, 1H, OH), 4.19–4.24 (m, 1H), 5.25–5.28 (m, 1H), 8.17 (d, J = 8.0 Hz, 2H), 8.36 (d, J = 8.0 Hz, 2H) ppm. HRMS (ESI): Calculated for C40H62N6NaO14S2 [2M + Na]+ = 937.3658; found, 937.3654.
tert-Butyl 4-hexyl-2-((4-nitrophenyl)sulfonyl)-pyrazolidin-5-one-1-carboxylate, 9ac. Obtained in 99% yield (150 mg, 0.33 mmol) according to Procedure 3.2.3. Colorless solid, m.p. 123–126 °C. 1H-NMR (CDCl3): δ = 0.87 (t, J = 6.5 Hz, 3H), 1.24–1.30 (m, 9H), 1.47 (s, 9H), 1.78–1.83 (m, 1H), 2.40–2.47 (m, 1H), 3.42–3.49 (m, 1H), 4.37–4.42 (m, 1H), 8.19 (d, J = 7.8 Hz, 2H), 8.42 (d, J = 7.8 Hz, 2H) ppm. 13C-NMR (CDCl3): δ = 13.9, 22.5, 26.8, 27.7, 28.8, 29.1, 31.4, 41.8, 52.6, 124.4, 130.7, 141.3, 147.7, 151.3, 172.9 ppm. HRMS (ESI): Calculated for C20H29N3NaO7S [M + Na]+ = 478.1618; found, 478.1627.
4-Methyl-1,2-bis-(4-toluenesulfonyl)-pyrazolidin-3-ol, 8ba. The racemic compound was obtained as a 8:1 diastereomer mixture in 88% yield (75 mg, 0.18 mmol) according to Procedure 3.2.1. Yellow solid, m.p. 131–134 °C. 1H-NMR (CDCl3, major isomer): δ = 0.86 (d, J = 7.0 Hz, 3H), 2.17–2.25 (m, 2H), 2.45 (s, 3H), 2.46 (s, 3H), 3.33–3.40 (m, 1H), 4.04–4.11 (m, 1H), 5.21–5.22 (m, 1H), 7.29–7.33 (m, 4H), 7.70 (d, J = 8.0 Hz, 2 H), 7.82 (d, J = 8.0 Hz, 2 H) ppm. HRMS (ESI): Calculated for C36H44N4NaO10S4 [2M + Na]+ = 843.1832; found, 843.1832. When using 13 as a catalyst and following Procedure 3.2.2, scalemic 8ba was obtained in 89.4% yield (8:1 diastereomer mixture).
(−)-4-Methyl-1,2-bis-(4-toluenesulfonyl)-pyrazolidin-3-one, 9ba. Oxidation of 8ba obtained by catalyst 13 gave (−)-9ba (96:4 er) in 98% yield (58 mg, 0.14 mmol) according to Procedure 3.2.3. Colorless solid, m.p. 156–160 °C (recrystallized from 1:2:7 dichloromethane/chloroform/hexanes). 1H-NMR (CDCl3): δ = 0.76 (d, J = 7.0 Hz, 3H), 2.12–2.23 (m, 1H), 2.46 (s, 6H), 2.83–2.90 (m, 1H), 4.28–4.33 (m, 1H), 7.34–7.36 (m, 4H), 7.89 (d, J = 8.1 Hz, 2H), 7.89 (d, J = 8.1 Hz, 2H) ppm. 13C-NMR (CDCl3): δ = 15.3, 21.8, 34.7, 65.8, 127.0, 128.3, 128.7, 128.8, 129.6, 129.8, 130.1, 146.0, 146.2, 176.5 ppm. HRMS (ESI): Calculated for C36H40N4NaO10S2 [2M + Na]+ = 839.1519; found, 839.1516. [α] = −64.7 (0.67, DCM). Conditions for the HPLC analysis: Chiralpak® IA column, 90:10 hexane/2-propanol, 1 μL/min, 25 °C, λ = 254 nm. tR (major enant.) = 33.6 min, tR (minor enant.) = 51.5 min.
4-Benzyl-1,2-bis-(4-toluenesulfonyl)-pyrazolidin-3-ol, 8bb. The racemic compound was obtained as a diastereomer mixture (>9:1 dr) in 85% yield (85 mg, 0.18 mmol) according to Procedure 3.2.1. Yellow solid, m.p. 110–113 °C. 1H-NMR (CDCl3, major isomer): δ = 2.14–2.20 (m, 1H), 2.35–2.42 (m, 2H), 2.46 (s, 3H), 2.47 (s, 3H), 2.82–2.87 (m, 1H), 3.45–3.47 (br d, 1H, OH), 3.89–3.94 (m, 1H), 5.36–5.40 (m, 1H), 7.28–7.31 (m, 5H), 7.67 (d, J = 8.0 Hz, 2H), 7.77 (d, J = 8.0 Hz, 2H) ppm. HRMS (ESI): Calculated for C48H56N5O10S4 [2M + NH4]+ = 990.2805; found, 990.2805. When using 13 or 14 as catalysts and following Procedure 3.2.2, scalemic 8bb was obtained in the same yield and diastereomeric ratio.
4-Benzyl-1,2-bis-(4-toluenesulfonyl)-pyrazolidin-3-one, 9bb. Oxidation of 8bb according to Procedure 3.2.3 gave 9bb in 97% yield (58 mg, 0.12 mmol). Yellow solid, m.p. 156–160 °C. 1H-NMR (CDCl3): δ = 2.01–2.07 (m, 1H), 2.45–2.47 (m, 1H), 2.47 (s, 3H), 2.48 (s, 3H), 2.90–2.95 (m, 1H), 2.98–3.01 (m, 1H), 4.05–4.011 (m, 1H), 6.81 (m, 1H), 7.20 (m, 3H), 7.34 (d, J = 6 Hz, 4H), 7.73 (d, J = 7.6 Hz, 2H), 7.86 (d, J = 7.6 Hz, 2H) ppm. 13C-NMR (CDCl3): δ = 21.79, 21.82, 34.7, 42.7, 54.0, 127.0, 128.3, 128.7, 128.8, 129.6, 129.8, 130.1, 131.2, 134.67, 134.73, 146.0, 146.2, 176.5 ppm. HRMS (ESI): Calculated for C24H28N3NaO5S2 [M + NH4]+ = 502.1465; found, 502.1466. No satisfactory conditions for the chiral HPLC analysis of this compound could be found.
4-Hexyl-1,2-bis-(4-toluenesulfonyl)-pyrazolidin-3-ol, 8bc. The racemic compound was obtained as a diastereomer mixture (10:1 dr) in 98% yield (97 mg, 0.20 mmol) according to Procedure 3.2.1. Yellow solid, m.p. 78–80 °C. 1H-NMR (CDCl3, major isomer): δ = 0.87 (t, J = 7.0 Hz, 3H), 0.95–1.00 (m, 1H), 1.13–1.18 (m, 6H), 1.22–1.27 (m, 3H), 2.01–2.14 (m, 1H), 2.44 (s, 3H), 2.45 (s, 3H), 2.85–2.87 (m, 1H), 3.35–3.37 (br d, 1H, OH), 4.08–4.13 (m, 1H), 5.24–5.28 (m, 1H), 7.27–7.32 (m, 4H), 7.68 (d, J = 8.0 Hz, 2H), 7.81 (d, J = 8.0 Hz, 2H) ppm. HRMS (ESI): Calculated for C23H32N2NaO5S2 [M + Na]+ = 503.1642; found, 503.1645. When using 13 as a catalyst and following Procedure 3.2.2, scalemic 8bc was obtained in 93% yield (104 mg, 0.23 mmol; 10:1 dr).
(−)-4-Hexyl-1,2-bis-(4-toluenesulfonyl)-pyrazolidin-3-one, 9bc. Oxidation of 8bc (obtained by catalyst 13) according to Procedure 3.2.3 gave (−)-9bc (78:22 er) in 98% yield (58 mg, 0.12 mmol). Colourless solid, m.p. 160–164 °C. 1H-NMR (CDCl3): δ = 0.83–0.86 (m, 5H), 1.05–1.12 (m, 4H), 1.19–1.25 (m, 3H), 1.40–1.52 (m, 1H), 2.08–2.11 (m, 1H), 2.45 (s, 3H), 2.46 (s, 3H), 2.93–3.00 (m, 1H), 4.24–4.29 (m, 1H), 7.33–7.36 (m, 4H), 7.78 (d, J = 8.0 Hz, 2H), 7.88 (d, J = 8.0 Hz, 2H) ppm. 13C-NMR (CDCl3): δ = 13.9, 21.7, 21.8, 22.4, 26.2, 28.6, 28.7, 31.3, 41.0, 54.1, 128.7, 129.6, 129.8, 130.1, 131.3, 134.8, 145.9, 146.2, 177.5 ppm. HRMS (ESI): Calculated for C23H30N2NaO5S2 [M + Na]+ = 501.1488; found, 501.1491. [α] = −8.0 (1.01, DCM). Conditions for the HPLC analysis: Chiralpak® IB column, 93:7 hexane/2-propanol, 1 μL/min, 25 °C, λ = 220 nm. tR (major enant.) = 21.5 min, tR (minor enant.) = 27.6 min.
Benzyl 5-hydroxy-4-methyl-isoxazolidine-2-carboxylate, 11a. The racemic compound was obtained as a 2:1 diastereomer mixture in 83% yield (102 mg, 0.43 mmol) according to Procedure 3.3.1. Colorless oil. 1H-NMR (CDCl3, major isomer): δ = 1.09 (d, J = 7 Hz, 3H), 2.60–2.67 (m, 1H), 3.61–3.64 (m, 1H), 4.26–4.29 (m, 1H), 5.16 (br s, 2H), 5.34–5.35 (m, 1H), 7.32–7.35 (m, 5H) ppm. 1H-NMR (CDCl3, minor isomer): δ = 1.13 (d, J = 7.0 Hz, 3H), 2.55–2.59 (m, 1H), 3.78–3.82 (m, 1H), 4.14–4.17 (m, 1H), 5.22 (br s, 2H), 5.55–5.56 (m, 1H), 7.37–7.39 (m, 5H) ppm. 13C-NMR (CDCl3): δ = 9.4, 14.7, 40.4, 44.1, 67.8, 68.1, 73.5, 74.9, 82.1, 88.0, 128.29, 128.34, 128.43, 128.46, 128.5, 128.6, 135.38, 135.44, 141.8, 155.3, 159.0 ppm. When using 13 or 14 as catalysts and following Procedure 3.3.2, scalemic 11a was obtained in similar yield and diastereomer ratio.
Benzyl 4-methyl-5-oxo-isoxazolidine-2-carboxylate, 12a. Oxidation of 11a gave 12a in 98% yield (50 mg, 0.22 mmol) according to Procedure 3.3.3. Colorless oil. 1H-NMR (CDCl3): δ = 1.29 (d, J = 6.8 Hz, 3H), 3.01–3.11 (m, 1H), 3.96–4.01 (m, 1H), 4.54–4.58 (m, 1H), 5.33 (br s, 2H), 7.33–7.45 (m, 5H) ppm.13C-NMR (CDCl3): δ = 12.2, 39.4, 68.8, 73.9, 98.0, 128.54, 128.6, 134.5, 147.7, 169.9 ppm. HRMS (ESI): Calculated for C12H14NO4 [M + H]+ = 236.0917; found, 236.0916. No satisfactory conditions could be found for the chiral HPLC analysis of this compound.
Benzyl 4-benzyl-5-hydroxy-isoxazolidine-2-carboxylate, 11b. The racemic compound was obtained as a 3:1 diastereomer mixture in 79% yield (128 mg, 0.41 mmol) according to Procedure 3.3.1. Colorless oil. 1H-NMR (CDCl3, major isomer): δ = 2.74–2.78 (m, 2H), 3.02–3.06 (m, 1H), 3.57–3.61 (m, 1H), 3.77–3.80 (m, 1H), 4.19–4.23 (m, 1H), 5.25 (br s, 2H), 5.47–5.48 (m, 1H), 7.31–7.41 (m, 10H) ppm. 1H-NMR (CDCl3, minor isomer): δ = 2.59–2.68 (m, 2H), 2.94–2.98 (m, 1H), 3.01–3.27 (m, 1H), 3.67–3.71 (m, 1H), 4.01–4.05 (m, 1H), 5.36 (br s, 2H), 5.56–5.57 (m, 1H), 7.13–7.30 (m, 10H) ppm. 13C-NMR (CDCl3): δ = 23.5, 31.2, 35.8, 48.0, 48.8, 51.0, 68.1, 71.9, 72.9, 81.3, 86.1, 97.2, 101.4, 126.5, 126.7, 128.1, 128.2, 128.4, 128.5, 128.6, 128.7, 135.5, 135.7, 138.2, 138.9, 155.2, 158.8 ppm.
Benzyl 4-benzyl-5-oxo-isoxazolidine-2-carboxylate, 12b. Oxidation of 11b gave 12b in 99% yield (68 mg, 0.22 mmol) according to Procedure 3.3.3. Colorless oil. 1H-NMR (CDCl3): δ = 2.78–2.84 (m, 1H), 3.24–3.28 (m, 2H), 4.07–4.11 (m, 1H), 4.35–4.39 (m, 1H), 5.33 (br s, 2H), 7.15–7.43 (m, 10H) ppm.13C-NMR (CDCl3): δ = 30.7, 43.0, 65.7, 68.8, 123.9, 125.3, 125.4, 125.5, 125.7, 131.3, 134.0, 144.4, 165.4 ppm. HRMS (ESI): Calculated for C18H21N2O4 [M + NH4]+ = 329.1496; found, 329.1494.
Benzyl 4-hexyl-5-hydroxy-isoxazolidine-2-carboxylate, 11c. The racemic compound was obtained as a 3:1 diastereomer mixture in 78% yield (120 mg, 0.39 mmol) according to Procedure 3.3.1. Colorless oil. 1H-NMR (CDCl3, major isomer): δ = 0.88 (d, J = 7.0 Hz, 3H), 1.28–1.31 (m, 9H), 1.34–1.38 (m, 1H), 2.45–2.55 (m, 2H), 3.62–3.66 (m, 1H), 4.26–4.28 (m, 1H), 5.22 (br s, 2H), 5.39–5.40 (m, 1H), 7.37–7.40 (m, 5H) ppm. 1H-NMR (CDCl3, minor isomer): δ = 0.87 (d, J = 7.0 Hz, 3H), 1.34–1.38 (m, 9H), 3.15–3.19 (m, 2H), 3.81–3.86 (m, 1H), 4.02–4.25 (m, 1H), 5.33 (br s, 2H), 5.39–5.40 (m, 1H), 7.32–7.36 (m, 5H) ppm. When using 14 as a catalyst and following Procedure 3.3.2, scalemic 11c was obtained in 87% yield (201 mg, 0.65 mmol) and in a 3:1 diastereomer ratio.
(−)-Benzyl 4-hexyl-5-oxo-isoxazolidine-2-carboxylate, 12c. Oxidation of 11c obtained with catalyst 14 gave (−)-12c (57:43 er) in 87% yield (87 mg, 0.28 mmol) according to Procedure 3.3.3. Colorless oil. 1H-NMR (CDCl3): δ = 0.88 (d, J = 7.0 Hz, 3H), 1.28–1.40 (m, 8H), 1.50–1.58 (m, 1H), 1.88–1.93 (m, 1H), 2.92–2.99 (m, 1H), 4.04–4.08 (m, 1H), 4.52–4.56 (m, 1H), 5.33 (br s, 2H), 7.33–7.45 (m, 5H) ppm. 13C-NMR (CDCl3): δ = 14.0, 22.5, 27.0, 28.1, 29.0, 31.5, 44.5, 68.8, 72.6, 128.5, 128.6, 128.7, 134.6, 147.7, 169.5 ppm. HRMS (ESI): Calculated for C34H46N2NaO8 [2M + Na]+ = 633.3146; found, 633.3148. [α] = −4.1 (0.41, DCM). Conditions for the HPLC analysis: Chiralpak® IB column, 90:10 hexane/2-propanol, 1 μL/min, 25 °C, λ = 254 nm. tR (minor enant.) = 13.6 min, tR (major enant.) = 16.3 min.
3.5. Methods for the Biological Activity Studies
3.5.1. Sample Preparation
Samples were provided as dry material, and were made to 10 mg/mL in DMSO solution and stored at −20 °C. An aliquot of each sample was diluted to 320 μg/mL in water, and plated in 384-well polypropylene plates. 5 μL was plated in duplicate into a 384-well non-binding surface plate for each strain or cell type assayed against. Once cells were added this gave a final compound concentration range of 32 μg/mL. The final DMSO concentration was 0.3%.
3.5.2. Antimicrobial Assay
Procedure
All bacteria were cultured in Cation-adjusted Müller Hinton broth (CAMBH) at 37 °C overnight. A sample of each culture was then diluted 40-fold in fresh broth and incubated at 37 °C for 1.5–3 h. The resulting mid-log phase cultures were diluted (CFU/mL measured by OD600), and then 45 μL was added to each well of the compound containing plates, giving a cell density of 5 × 105 CFU/mL and the nominated final compound concentration. All the plates were covered and incubated at 37 °C for 18 h without shaking.
Analysis
Inhibition of bacterial growth was determined measuring absorbance at 600 nm (OD600), using a M1000 Pro monochromator plate reader (Tecan, Männedorf, Switzerland). The percentage of growth inhibition was calculated for each well, using negative control (media only) and positive control (bacteria without inhibitors) on the same plate as references. The significance of the inhibition values was determined by Z-scores, calculated using the average and standard deviation of the sample wells (no controls) on the same plate. Samples with inhibition value above 80% and Z-Score above 2.5 (n = 2 on different plates) for either replicate were classed as actives.
3.5.3. Antifungal Assay
Procedure
Fungi strains were cultured for 3 days on Yeast Extract-Peptone Dextrose (YPD) agar at 30 °C. A yeast suspension of 1 × 106 to 5 × 106 cells/mL (as determined by OD350) was prepared from five colonies. These stock suspensions were diluted with Yeast Nitrogen Base (YNB) broth to a final concentration of 2.5 × 103 CFU/mL. Then, 45 μL of the fungi suspension was added to each well of the compound-containing plates, giving a final concentration of 32 μg/mL for the tested samples. Plates were covered and incubated at 35 °C for 24 h without shaking.
Analysis
Growth inhibition of C. albicans was determined measuring absorbance at 530 nm (OD530), while the growth inhibition of C. neoformans was determined measuring the difference in absorbance between 600 and 570 nm (OD600–570), after the addition of resazurin (0.001% final concentration) and incubation at 35 °C for additional 2 h. The absorbance was measured using a Biotek Synergy HTX plate reader. The percentage of growth inhibition was calculated for each well, using negative control (media only) and positive control (fungi without inhibitors) on the same plate as references. The significance of the inhibition values was determined by Z-scores, calculated using the average and standard deviation of the sample wells (no controls) on the same plate. Samples with inhibition value above 80% and Z-Score above 2.5 (n = 2 on different plates) for either replicate were classed as actives.
3.5.4. Antibiotic Standards Preparation and Quality Control
Colistin and vancomycin were used as positive bacterial inhibitor standards for Gram-negative and Gram-positive bacteria, respectively. Fluconazole was used as a positive fungal inhibitor standard both for C. albicans and C. neoformans.
The antibiotics were provided in four concentrations, with two above and two below its MIC value, and plated into the first 8 wells of column 23 of the 384-well NBS plates. The quality control (QC) of the assays was determined by the antimicrobial controls and the Z’-factor (using positive and negative controls). Each plate was deemed to fulfill the quality criteria (pass QC), if the Z’-factor was above 0.4, and the antimicrobial standards showed full range of activity, with full growth inhibition at their highest concentration, and no growth inhibition at their lowest concentration.
3.5.5. Materials
Both the compound preparation plate and the assay plates were purchased from Corning (Corning, NY, USA). CAMHB from Bacto Laboratories (Mount Pritchard, Australia) was used as growth media for bacteria; culture agar and growth media for fungi were purchased from Becton Dickinson (Franklin Lakes, NJ, USA). Resazurin was provided by Sigma Aldrich (Sydney, Australia).
3.5.6. Microbial Strains
| Escherichia coli | ATCC 25922 (FDA control strain) |
| Klebsiella pneumoniae | ATCC 700603 (MDR) |
| Acinetobacter baumannii | ATCC 19606 (type strain) |
| Pseudomonas aeruginosa | ATCC 27853 (Quality control strain) |
| Staphylococcus aureus | ATCC 43300 (MRSA) |
| Candida albicans | ATCC 90028 (CLSI reference) |
| Cryptococcus neoformans | ATCC 208821 (H99—Type strain) |
4. Conclusions
The pyrrolidine-catalyzed reaction of the α-substituted enals methacrolein (7a), 2-benzylpropenal (7b), and 2-(n-hexyl)propenal (7c) with the activated hydrazines 1-Boc-2-(4-nitrobenzenesulfonyl)hydrazine (2a) and 1,2-bis(p-toluenesulfonyl)hydrazine (2b) takes place in very good yields (83%–99.6%) in toluene at room temperature and in the presence of benzoic acid as a co-catalyst, to afford the 4-substituted pyrazolidin-3-ols 8aa–8bc (generally as diastereomer mixtures); oxidation of these compounds with PCC leads to the corresponding-4-substituted-3-pyrazolidinones 9aa–9bc in essentially quantitative yields. In the same conditions α-substituted acroleins 7a–7c react smoothly with N-Cbz-hydroxylamine 10 to afford the isoxazolidinols 11 in good yields (78%–83% isolated yields) as diastereomer mixtures. Subsequent oxidation with PCC gives rise to the 4-substituted isoxazolidin-5-ones 12a–12c. The use of chiral diarylprolinol trimethylsilyl ethers 13 and 14 as catalysts allows the synthesis of several of these compounds in optically active form, in some cases with excellent enantioselectivity (up to 96:4 er). The 4-substituted pyrazolidinols, pyrazolidinones, isoxazolidinols and isoxazolidinones (both in racemic and when possible in enantiomerically enriched form) have been submitted to a primary antimicrobial screening study (antibacterial and antifungal) by whole cell growth inhibition assays. Antifungal activity has been found for the isoxazolidinones 12a and 12b, and for isoxazolidinol 11c, and the 4-methyl-pyrazolidinol 8aa, both in racemic and in enantiomerically enriched form, is highly active against Staphylococcus aureus.
Supplementary Materials
The following are available online at: http://www.mdpi.com/1420-3049/21/12/1655/s1, NMR spectra and chiral HPLC traces for compounds 3, 5, 8aa–8bc, 9aa–9bc, 11a–11c and 12a–12c (31 pages).
Acknowledgments
A.M. is grateful to MINECO, Spain (Project CTQ2013-47401-C2-1-P) for financial support. T.Y., M.H. and R.M. are grateful to the Ministry of Higher Education and Scientific Research of Algeria for funding and for a residential program fellowship 2014–2016 (to T.Y.). We gratefully acknowledge support from the Wellcome Trust Charity (UK) and The University of Queenland (Australia) for the primary antimicrobial screening assays as part of the Co-ADD program.
Author Contributions
A.M. conceived and designed the experiments; T.Y. and M.H. performed the chemical experiments; A.E. performed the biological inhibition assays; A.M., T.Y. and R.M. analyzed the data; A.M. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Mixich, G. Isolierung, Struktur und Synthese des Metaboliten von Azapropazon-dihydrat. Helv. Chim. Acta 1972, 55, 1031–1038. [Google Scholar] [CrossRef]
- Bennett, G.B.; Houlihan, W.J.; Mason, R.B.; Roach, J.B., Jr. Synthesis and biological activity of a series of 1-aryl-3-pyrazolidinones. J. Med. Chem. 1976, 19, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Jungheim, L.N.; Sigmund, S. 1,3-Dipolar cycloaddition reactions of pyrazolidinium ylides with acetylenes. Synthesis of a new class of antibacterial agents. J. Org. Chem. 1987, 52, 4007–4013. [Google Scholar] [CrossRef]
- Marchand-Baynaert, J.; Ghosez, L. Non-β-Lactam Analogs of Penicillins and Cephalosporins. In Recent Progress in the Chemical Synthesis of Antibiotics; Lukacs, G., Ohno, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 726–794. [Google Scholar]
- Hadjipavlou-Litina, D. Review, reevaluation, and new results in quantitative structure-activity studies of anticonvulsants. Med. Res. Rev. 1998, 18, 91–119. [Google Scholar] [CrossRef]
- Coulognier, E.; Cartier, D.; Labia, R. Synthesis of pyrazolidinone antibacterial agents. Bioorg. Med. Chem. Lett. 1999, 9, 2205–2206. [Google Scholar] [CrossRef]
- Cusan, C.; Spalluto, G.; Prato, M.; Adams, M.; Bodensieck, A.; Bauer, R.; Tubaro, A.; Bernardi, P.; Da Ros, T. Synthesis and biological evaluation of new phenidone analogues as potential dual cyclooxygenase (COX-1 and COX-2) and human lipoxygenase (5-LOX) inhibitors. Farmaco 2005, 60, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.H.; Krueger, W.A.; Dieterich, H.-J.; Nohé, B. Activity of the lipoxygenase inhibitor 1-phenyl-3-pyrazolidinone (phenidone) and derivatives on the inhibition of adhesion molecule expression on human umbilical vascular endothelial cells. Biol. Targets. Ther. 2008, 2, 151–160. [Google Scholar] [CrossRef]
- Sachdeva, H.; Dwivedi, D.; Goyal, P. Green Chemical Synthesis and Analgesic Activity of Fluorinated Thiazolidinone, Pyrazolidinone, and Dioixanedione Derivatives. Org. Chem. Int. 2013, 2013, 976032. [Google Scholar] [CrossRef]
- Radhika, C.; Venkatesham, A.; Anantha Krishna Chaitanya, D. Synthesis and Biological activity of new 5-Methyl-3-Oxo-N2-[5′-Carbonyl-(4′-Aryl-6′-methyl)-1′,2′,3′,4′-tetrahydropyrimidine-2′-one]pyrazolidinones. J. Adv. Pharm. Educ. Res. 2014, 4, 66–70. [Google Scholar]
- Varvounis, G. Pyrazol-5-ones. Part IV: Synthesis and Applications. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press Inc.: London, UK, 2009; Volume 98, pp. 143–223. [Google Scholar]
- Brogden, R.N. Pyrazolone derivatives. Drugs 1986, 32, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.W.; Zeng, L.H.; Wu, J.; Fung, K.P. Myocardial protection of MCI-186 in rabbit ischemia-reperfusion. Life Sci. 2002, 71, 2249–2255. [Google Scholar] [CrossRef]
- Lange, J.H.M.; Kruse, G.G. Recent Advances in CB1 Receptor Antagonists. Curr. Opin. Drug Discov. Dev. 2004, 7, 498–506. [Google Scholar]
- Ali, M.A.; Shaharyar, M. Discovery of novel phenoxyacetic acid derivatives as antimycobacterial agents. Bioorg. Med. Chem. 2007, 15, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Janin, Y.L. Antituberculosis drugs: Ten years of research. Bioorg. Med. Chem. 2007, 15, 2479–2513. [Google Scholar] [CrossRef] [PubMed]
- Pégurier, C.; Collart, P.; Danhaive, P.; Defays, S.; Gillard, M.; Gilson, F.; Kogej, T.; Pasau, P.; van Houtvin, N.; van Thuyne, M. Pyrazolone methylamino piperidine derivatives as novel CCR3 antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 4228–4231. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.S.; Castellano, E.E.; Ellena, J.; García-Tasende, M.S.; Peses-Paralle, M.L.; Sánchez, A.; Sánchez-González, A.; Sordo, J.; Touceda, A. New Pd(II) and Pt(II) complexes with N,S-chelated pyrazolonate ligands: Molecular and supramolecular structure and preliminary study of their in vitro antitumoral activity. J. Inorg. Biochem. 2008, 102, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Groselj, U.; Svete, J. Recent advances in the synthesis of polysubstituted 3-pyrazolidinones. Arkivoc 2015, 6, 175–205. [Google Scholar]
- Fernández, M.; Reyes, E.; Vicario, J.L.; Badía, L.; Carrillo, L. Organocatalytic Enantioselective Synthesis of Pyrazolidines, Pyrazolines and Pyrazolidinones. Adv. Synth. Catal. 2012, 354, 371–376. [Google Scholar] [CrossRef]
- Geng, Z.-G.; Chen, J.; Li, N.; Huang, X.-F.; Zhang, Y.; Zhang, Y.-W.; Wang, X.-W. Organocatalytic cascade aza-Michael/hemiacetal reaction between disubstituted hydrazines and α,β-unsaturated aldehydes: Highly diastereo- and enantioselective synthesis of pyrazolidine derivatives. Beilstein J. Org. Chem. 2012, 8, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, B.; Zhang, Y.; Jeret, M.; Wang, H.; Zheng, P.; Yang, S.; Song, A.-B.; Chi, Y.R. Enantioselective Nucleophilic β-Carbon-Atom Amination of Enals: Carbene-Catalyzed Formal [3 + 2] Reactions. Angew. Chem. Int. Ed. 2016, 55, 12280–12284. [Google Scholar] [CrossRef] [PubMed]
- Companyó, X.; Zea, A.; Alba, A.-N.R.; Mazzanti, A.; Moyano, A.; Rios, R. Organocatalytic synthesis of spiro compounds via a cascade Michael-Michael-aldol reaction. Chem. Commun. 2010, 46, 6953–6955. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, A.; Calbet, T.; Font-Bardía, M.; Moyano, A.; Rios, R. Organocatalytic enantioselective pyrazol-3-one addition to maleimides: Reactivity and stereochemical course. Org. Biomol. Chem. 2012, 10, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Bao, X.; Qu, J.; Wang, B. Asymmetric tandem Michael addition/oxidation of pyrazolones with p-benzoquinone catalyzed by cinchona alkaloids. Tetrahedron Asymmetry 2015, 26, 1382–1387. [Google Scholar] [CrossRef]
- Pou, A.; Moyano, A. Stereoselective Organocatalytic Approach to α,β-Disubstituted-β-amino Acids: A Short Enantioselective Synthesis of Cispentacin. Eur. J. Org. Chem. 2013, 2013, 3103–3111. [Google Scholar] [CrossRef]
- Ibrahem, I.; Rios, R.; Vesely, J.; Zhao, G.-L.; Córdova, A. Organocatalytic asymmetric 5-hydroxyisoxazolidinone synthesis: A highly enantioselective route to β-amino acids. Chem. Commun. 2007, 8, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.-L.; Lin, A.; Korotvicka, A.; Deiana, L.; Kullberg, M.; Córdova, A. Asymmetric Synthesis of Maraviroc (UK-427,857). Adv. Synth. Catal. 2010, 352, 2291–2298. [Google Scholar] [CrossRef]
- Fu, N.; Zhang, L.; Luo, S.; Cheng, J.-P. Chiral primary amine catalyzed asymmetric conjugate additions of azoles to α-substituted vinyl ketones. Org. Chem. Front. 2014, 1, 68–72. [Google Scholar] [CrossRef]
- Fu, N.; Zhang, L.; Luo, S.; Cheng, J.-P. Asymmetric Sulfa-Michael Addition to α-Substituted Vinyl Ketones Catalyzed by Chiral Primary Amine. Org. Lett. 2014, 16, 4626–4629. [Google Scholar] [CrossRef] [PubMed]
- Community for Open Antimicrobial Drug Discovery. Available online: http://www.co-add.org (accessed on 21 October 2016).
- Meyers, M.J.; Arhancet, G.B.; Hockerman, S.L.; Chen, X.; Long, S.A.; Mahoney, M.W.; Rico, J.R.; Garland, D.J.; Blinn, J.R.; Collins, J.T.; et al. Discovery of (3S,3aR)-2-(3-chloro-4-cyanophenyl)-3-cyclopentyl-3,3a,4,5-tetrahydro-2H-benzo[g]indazole-7-carboxylic acid (PF-3882845), an orally efficacious mineralocorticoid receptor (MR) antagonist for hypertension and nephropathy. J. Med. Chem. 2010, 53, 5979–6002. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, A.; Pihko, P.M. Mild Organocatalytic α-Methylenation of Aldehydes. J. Org. Chem. 2006, 71, 2538–2541. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, A.; Pihko, P.M. Rapid Organocatalytic Aldehyde-Aldehyde Condensation Reactions. Eur. J. Org. Chem. 2007, 4205–4216. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3, 5, 8aa–8bc, 9aa–9bc, 11a–11c and 12a–12c are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).