Docetaxel-Loaded Self-Assembly Stearic Acid-Modified Bletilla striata Polysaccharide Micelles and Their Anticancer Effect: Preparation, Characterization, Cellular Uptake and In Vitro Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. 1H Nuclear Magnetic Resonance (1H-NMR) Analysis
2.2. Particle Size and Zeta Potential
2.3. Encapsulation Efficiency and Loading Capacity
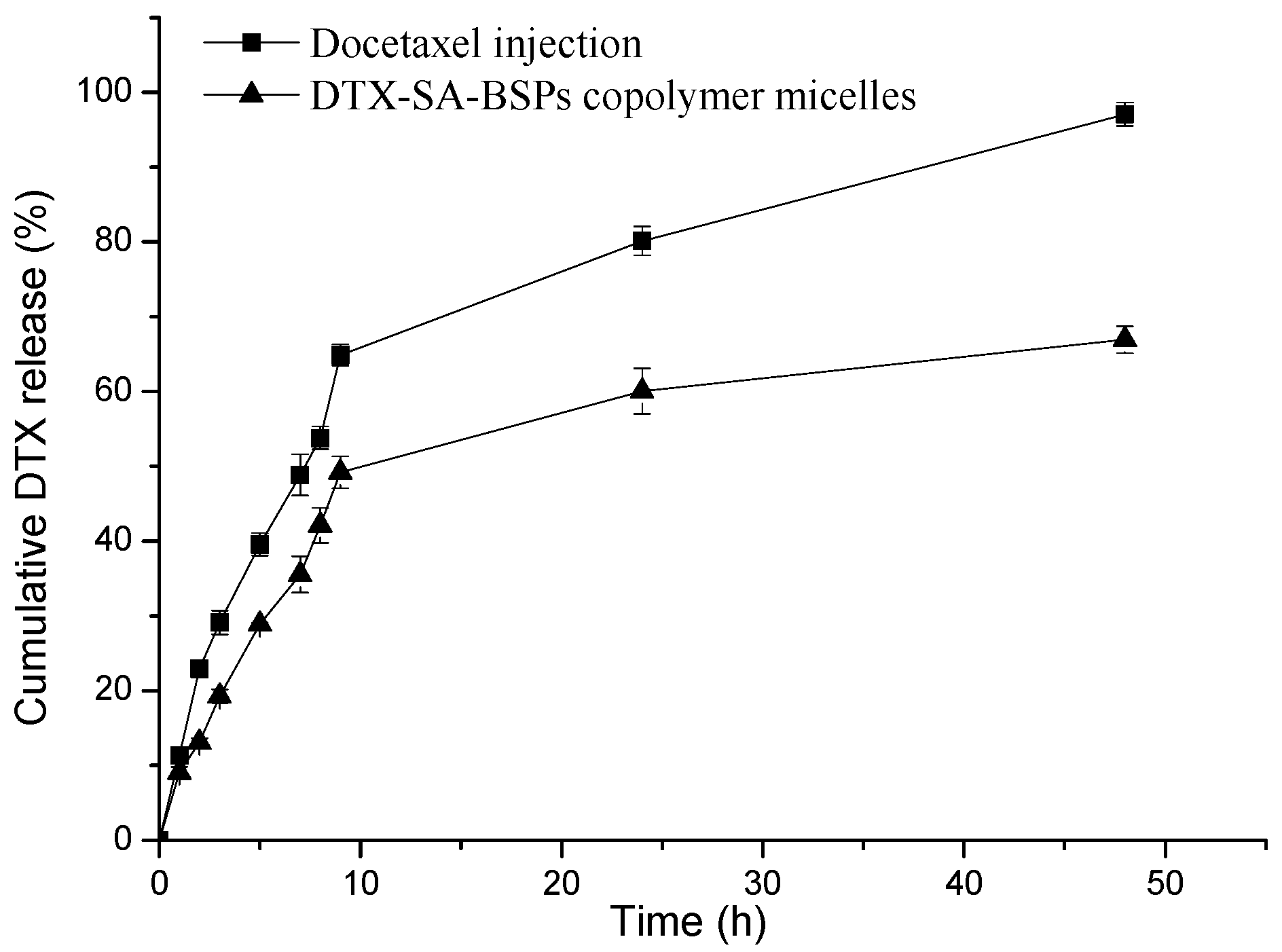
2.4. In Vitro Drug Release Study
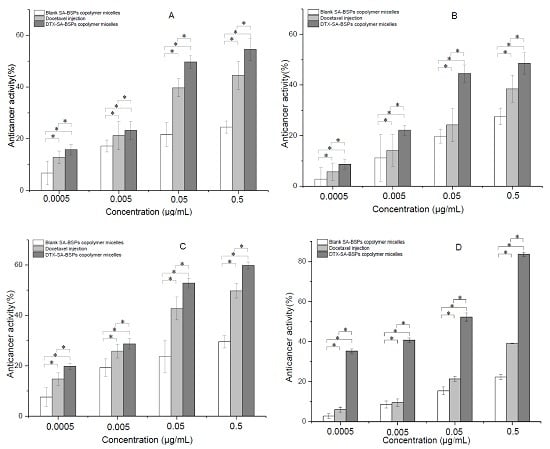
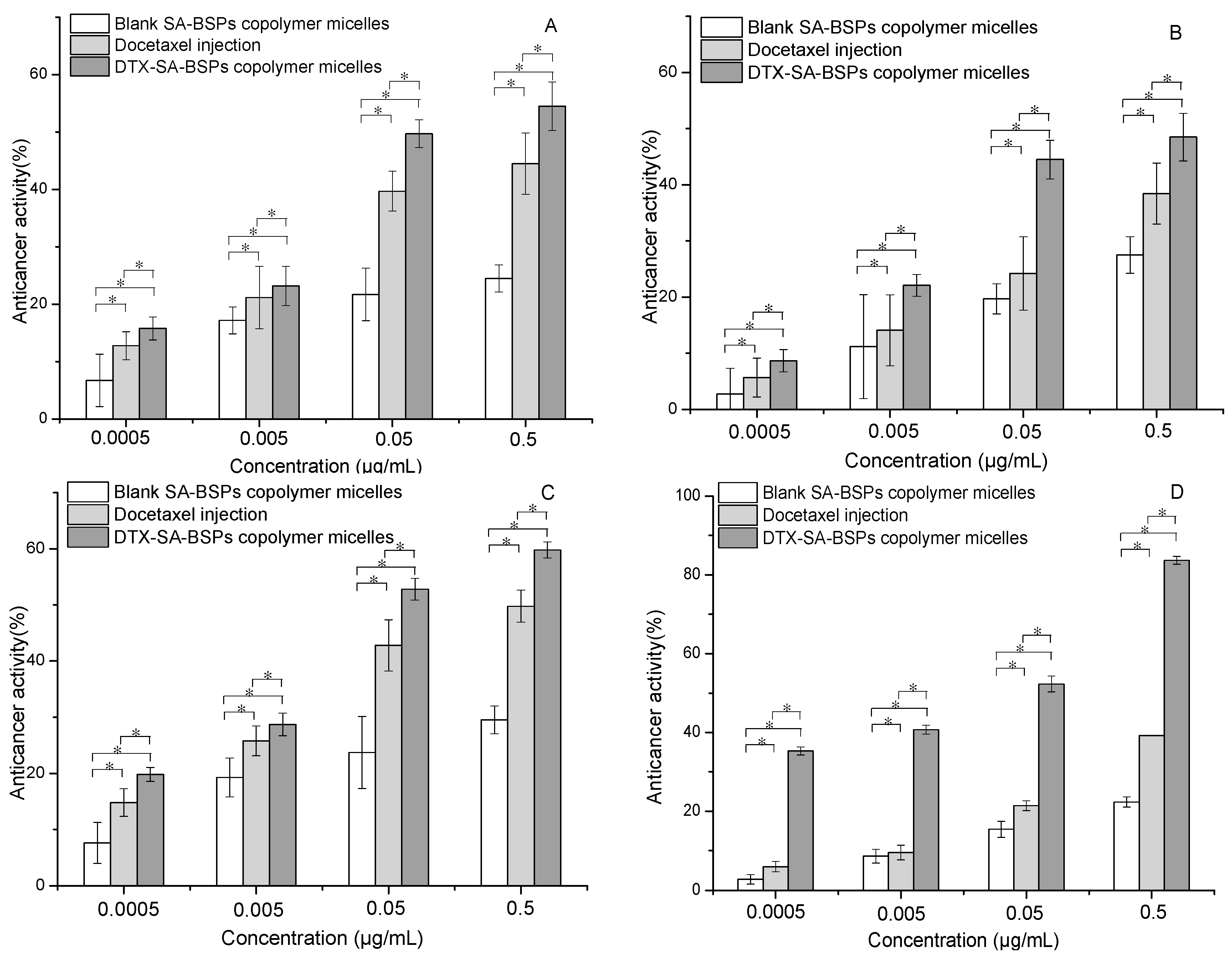
2.5. In Vitro Anticancer Activity
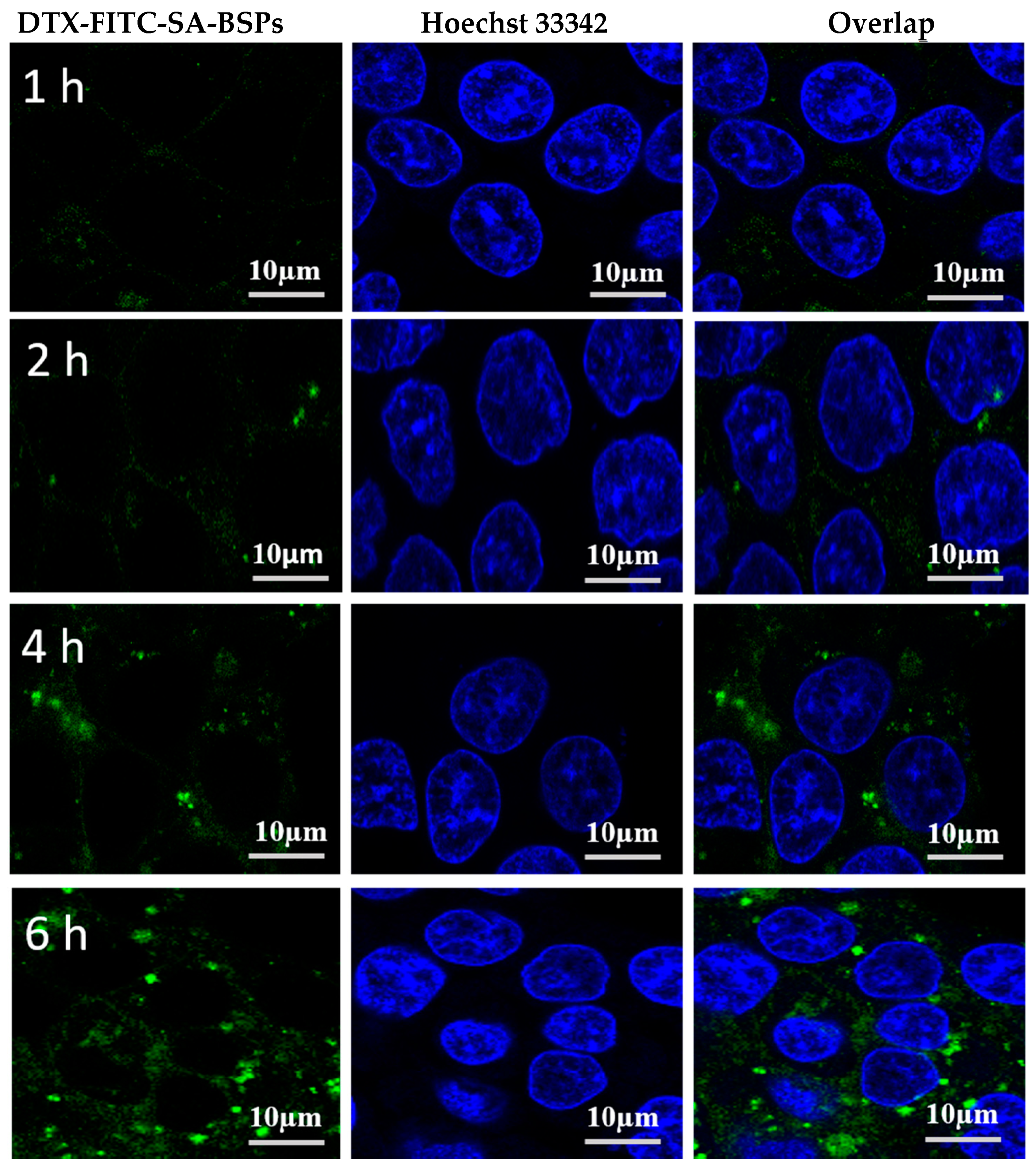
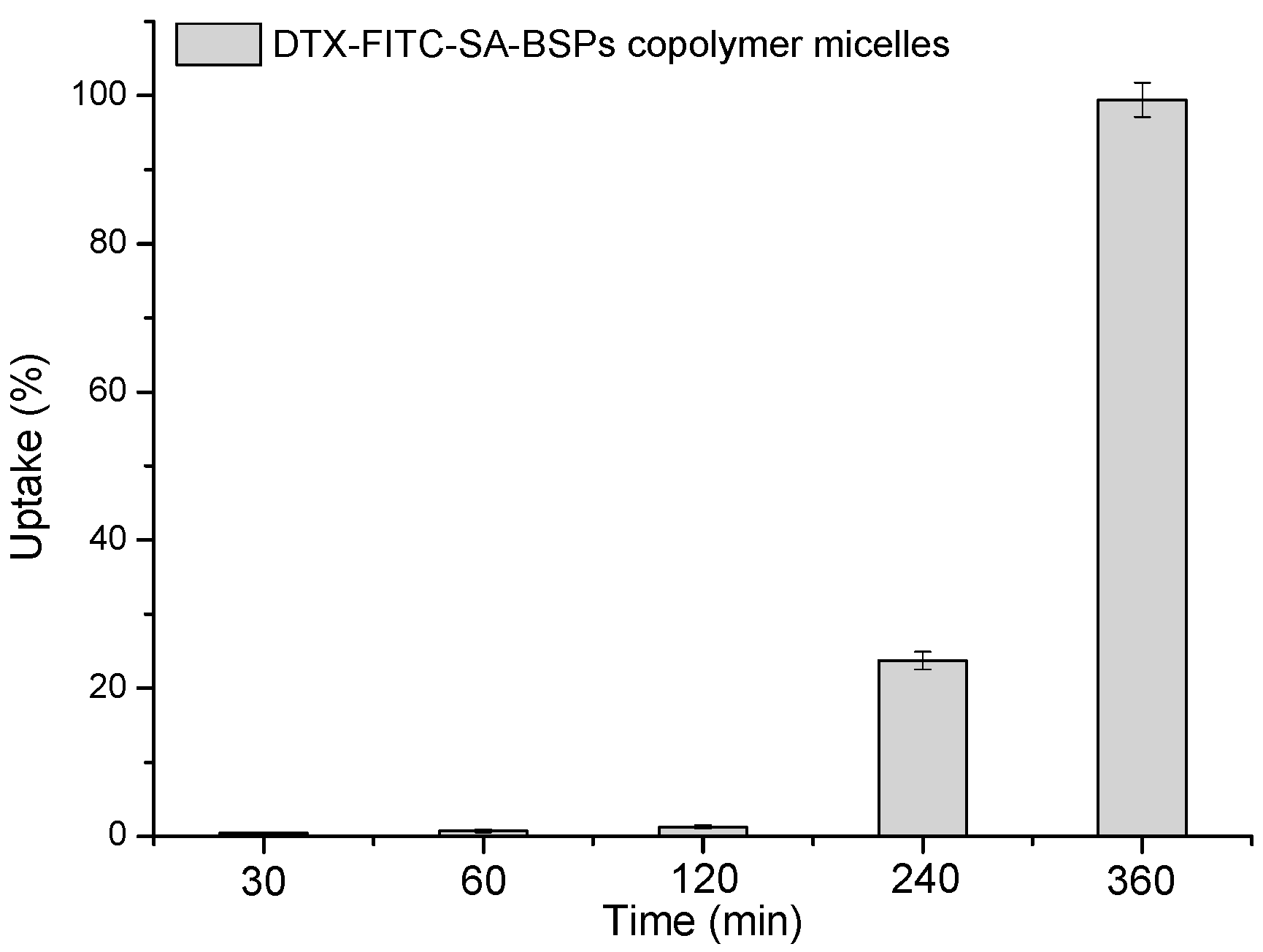
2.6. Cellular Uptake
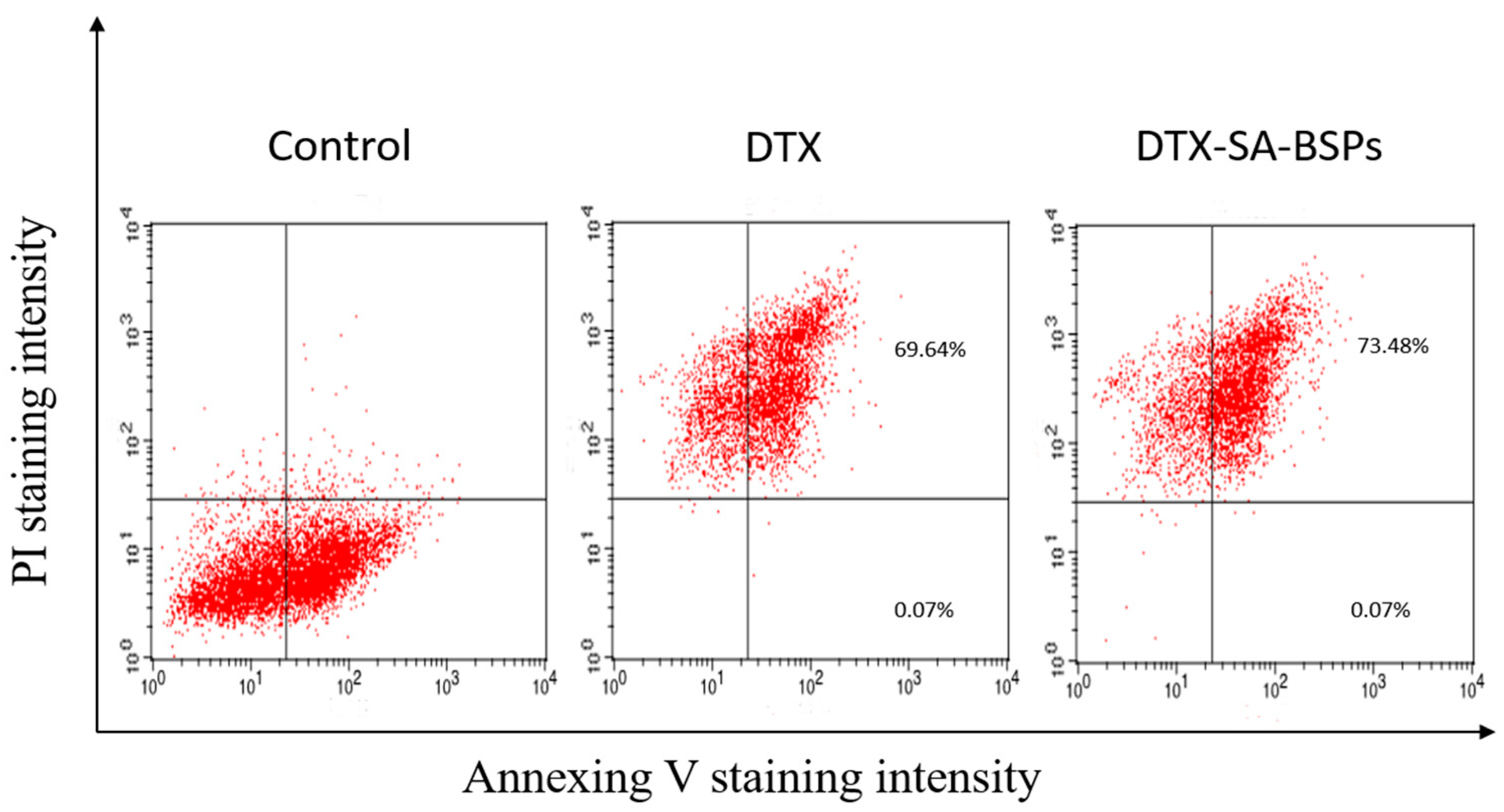
2.7. Determining Apoptosis
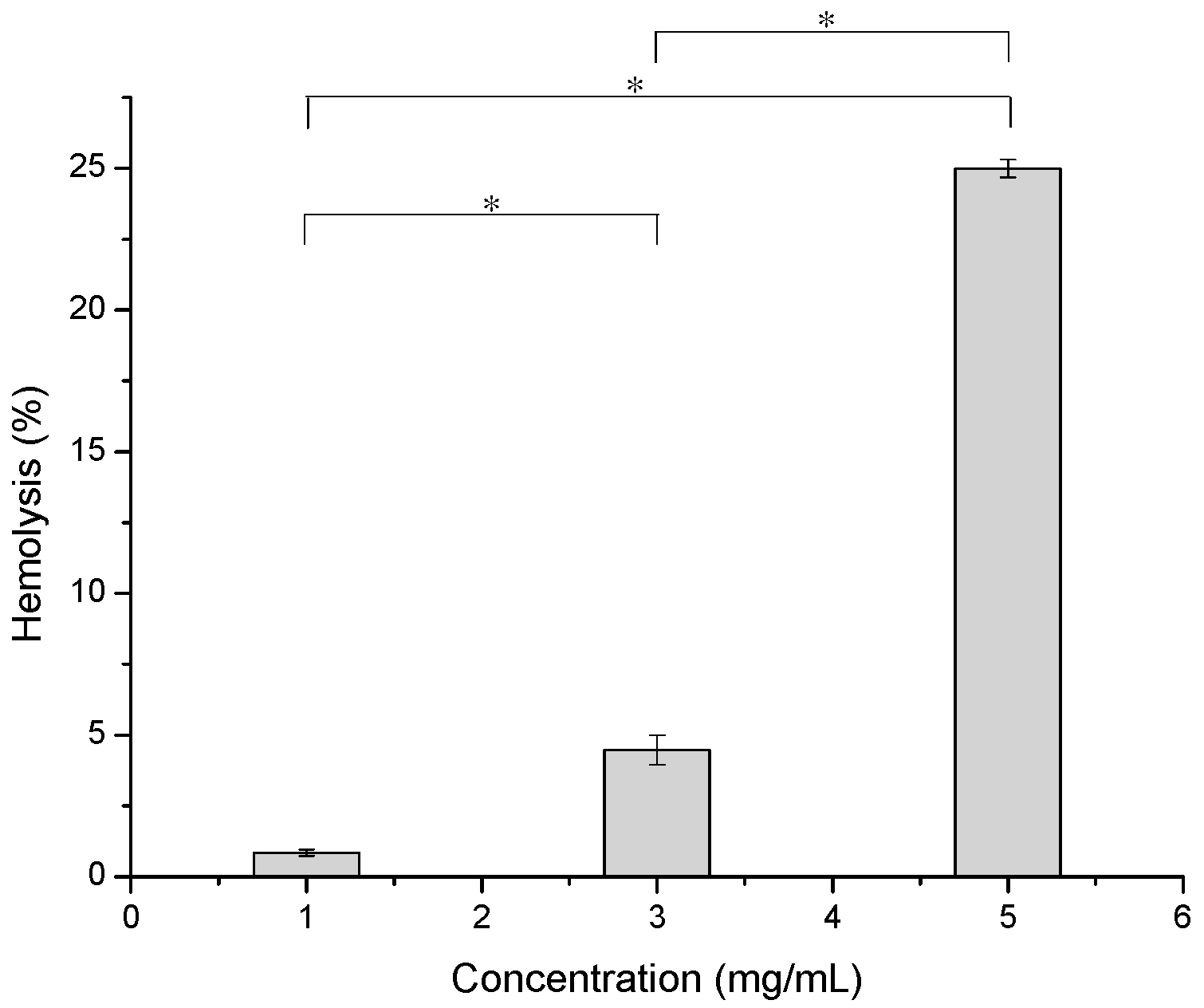
2.8. In Vitro Hemolysis Assay
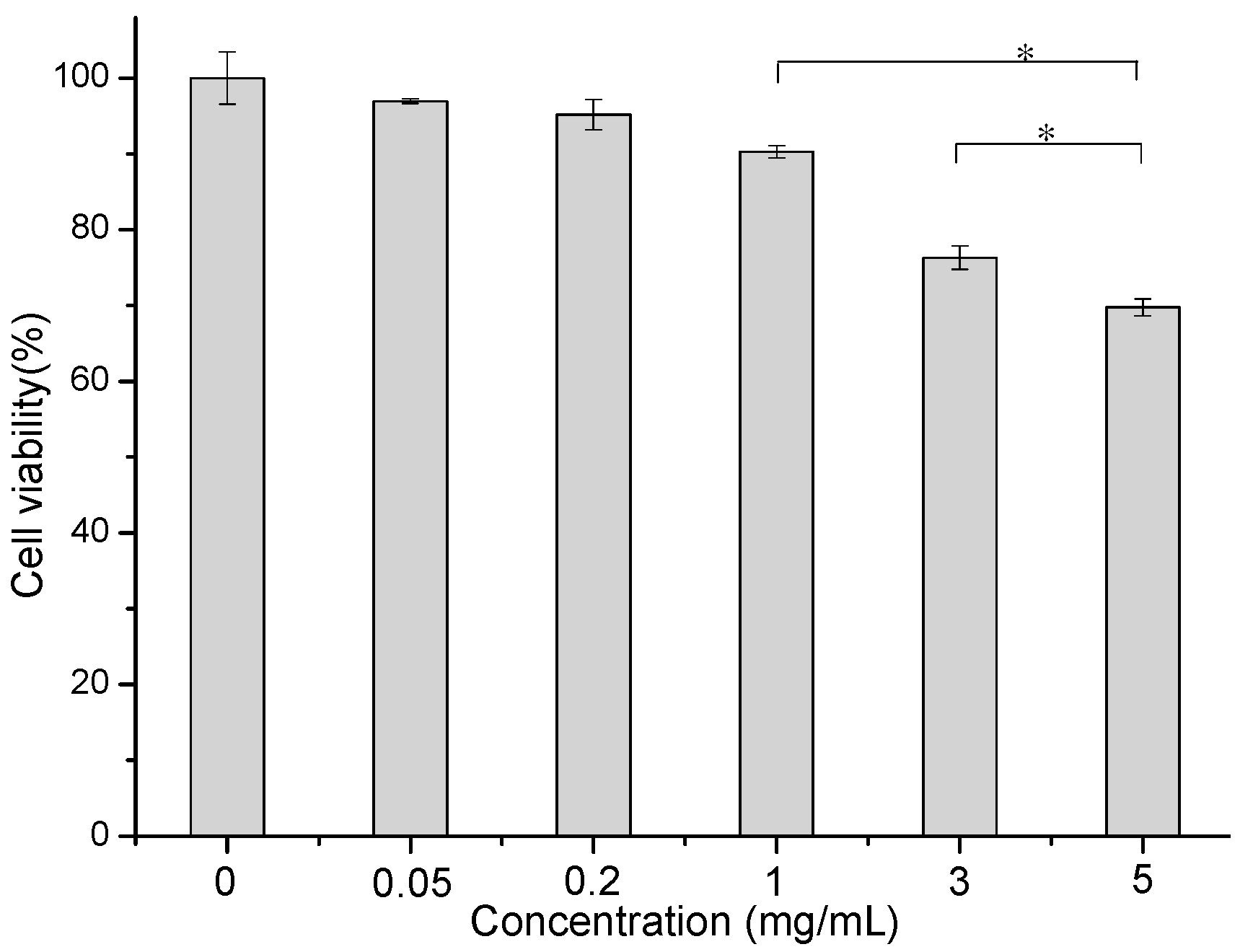
2.9. Toxicity of SA-BSPs Study
3. Experimental Section
3.1. Materials and Reagents
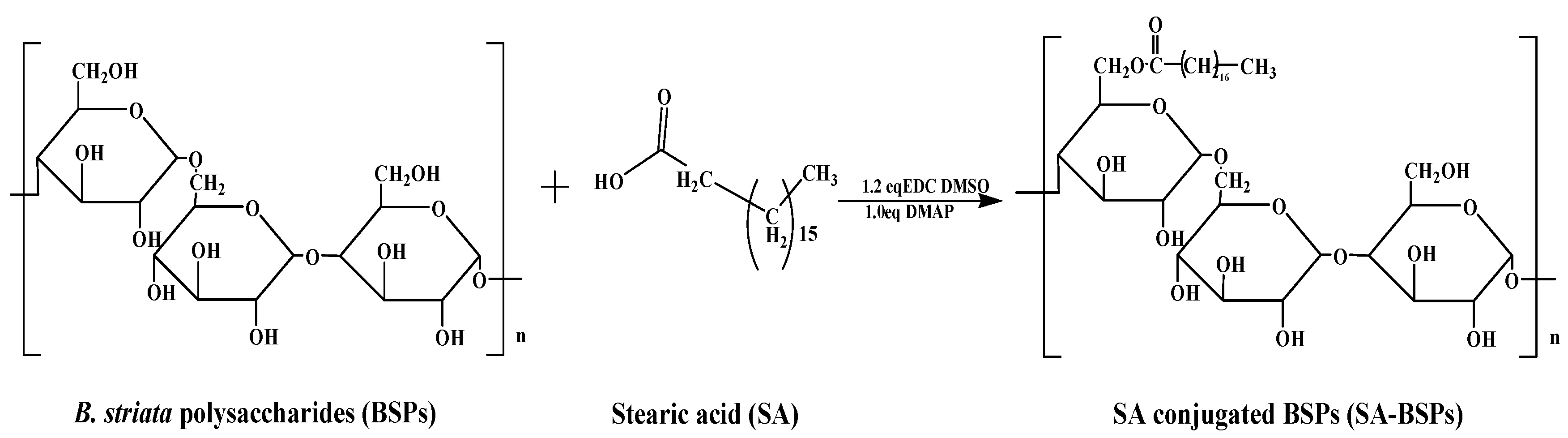
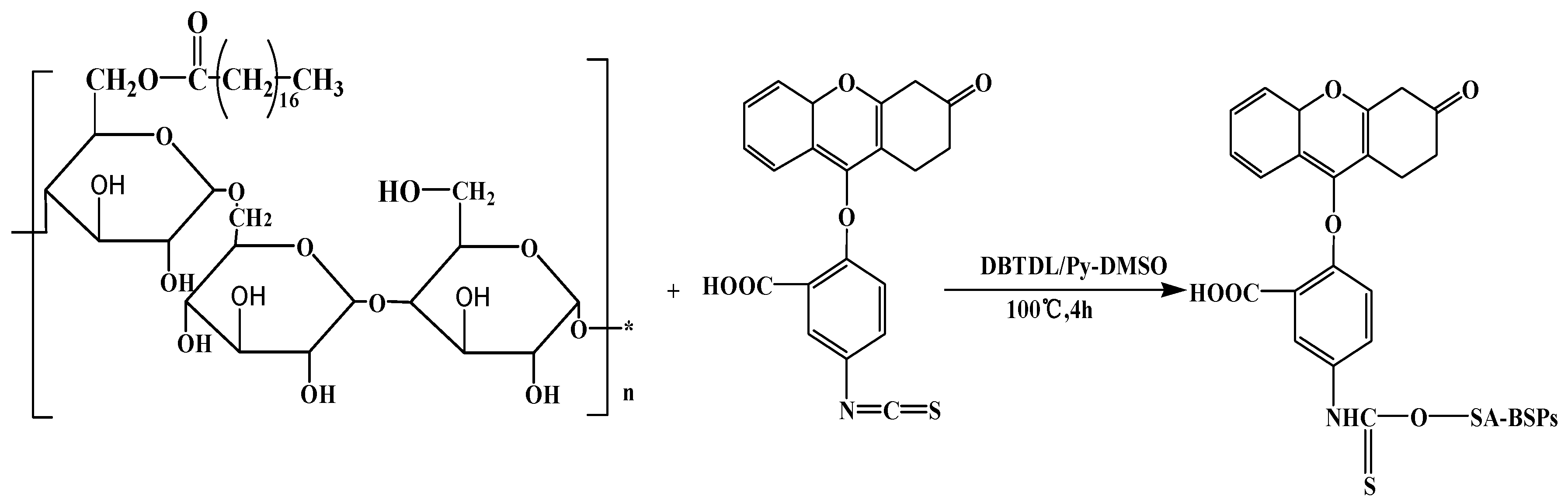
3.2. Synthesis of SA-BSPs and FITC-SA-BSPs Copolymer
3.3. 1H Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
3.4. Preparation of DTX-SA-BSPs and DTX-FITC-SA-BSPs Copolymer Micelles
3.5. Particle Size and Zeta Potential
3.6. Encapsulation Efficiency and Loading Capacity
3.7. In Vitro Drug Release Study
3.8. In Vitro Anticancer Activity
3.9. Cellular Uptake In Vitro
3.10. Confocal Laser Scanning Microscopy Observation
3.11. Determining Apoptosis
3.12. Hemolysis Assay
3.13. Toxicity of SA-BSPs Study
4. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Kataok, K.; Harad, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Savić, R.; Eisenberg, A.; Maysinger, D. Block copolymer micelles as delivery vehicles of hydrophobic drugs: Micelle-cell interactions. J. Drug Target. 2006, 14, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Bae, Y.; Nishiyama, N.; Miyata, K.; Oba, M.; Kataoka, K. Transfection study using multicellular tumor spheroids for screening non-viral polymeric gene vectors with low cytotoxicity and high transfection efficiencies. J. Control. Release 2007, 121, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Milane, J.J.; Vlerken, L.V.; Devalapally, H.; Shenoy, D.; Komareddy, S.; Bhavsar, M.; Amiji, M. Multi-functional nanocarriers for targeted delivery of drugs and genes. J. Control. Release 2008, 130, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Wei, X.; Wang, X.; Wang, Y.; Guo, G.; Mao, Y.; Luo, F.; Qian, Z. Biodegradable self-assembled PEG-PCL-PEG micelles for hydrophobic honokiol delivery: I. Preparation and characterization. Nanotechnology 2010, 21, 215103. [Google Scholar] [CrossRef] [PubMed]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langerl, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Gref, R.; Luck, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Muller, R.H. Stealth corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Yin, J.; Chen, Y.; Zhang, Z.H.; Han, X. Stimuli-responsive block copolymer-based assemblies for cargo delivery and theranostic applications. Polymers 2016, 8. [Google Scholar] [CrossRef]
- Truong, N.P.; Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Polymeric filomicelles and nanoworms: Two decades of synthesis and application. Polym. Chem. 2016, 7, 4295–4312. [Google Scholar] [CrossRef]
- Truong, N.P.; Whittaker, M.R.; Anastasaki, A.; Haddleton, D.M.; Quinn, J.F.; Davis, T.P. Facile production of nanoaggregates with tuneable morphologies from thermoresponsive P(DEGMA-co-HPMA). Polym. Chem. 2016, 7, 430–440. [Google Scholar] [CrossRef]
- Truong, N.P.; Quinn, J.F.; Anastasaki, A.; Haddleton, D.M.; Whittaker, M.R.; Davis, T.P. Facile access to thermoresponsive filomicelles with tuneable cores. Chem. Commun. 2016, 52, 4497–4500. [Google Scholar] [CrossRef] [PubMed]
- Alhaique, F.; Matricardi, P.; di Meo, C.; Coviello, T.; Montanari, E. Polysaccharide-based self-assembling nanohydrogels: An overview on 25-years research on pullulan. J. Drug Deliv. Sci. Technol. 2015, 30, 300–309. [Google Scholar] [CrossRef]
- Kumar, M.R.; Muzzarelli, R.; Muzzarelli, C.; Sashiwa, H.; Domb, A. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan: Pharmaceutical characterization and drug delivery. Drug Deliv. 2005, 12, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Chen, F.; Aoyama, A.; Date, H. Nanoparticle of cholesterol-bearing pullulan as a carrier of anticancer drugs. Eur. J. Cancer Suppl. 2008, 6, 139. [Google Scholar] [CrossRef]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. eXPRESS Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Xiong, Y.; Qi, J.; Yao, P. Amphiphilic cholic-acid-modified dextran sulfate and its application for the controlled delivery of superoxide dismutase. Macromol. Biosci. 2012, 12, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wardwell, P.R.; Bader, R.A. Polysaccharide-based micelles for drug delivery. Pharmaceutics 2013, 5, 329–352. [Google Scholar] [CrossRef] [PubMed]
- China Pharmacopoeia Committee. Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2010; Part I; p. 95. [Google Scholar]
- Baveja, S.K.; Rao, K.V.; Arora, J. Examination of natural gums and mucilages as sustaining materials in tablet dosage forms. Indian J. Pharm. Sci. 1989, 51, 115–119. [Google Scholar]
- Suvakanta, D.; Narsimh, M.P.; Pulak, D.; Joshabir, C.; Biswajit, D. Optimization and characterization of purified polysaccharide from Musa sapientum L. as a pharmaceutical excipient. Food Chem. 2014, 149, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Xin, L.; Feng, G.; Zheng, C.; Liang, H.M.; Liu, X.; Xiong, B. Feasibility of a polysaccharide isolated from Bletilla striata used as a gene vector administered through an interventional pathway. World Chin. J. Digestol. 2009, 17, 1832–1835. [Google Scholar]
- Li, W.; Du, D.; Feng, G. Preparation of Bletilla striata microspheres and experimental study on embolization of hepatic artery in pigs. Acta Univ. Med. Tongji 1999, 28, 62–64. [Google Scholar]
- Li, W.; Du, D.; Feng, G. Pharmacokinetics of 5-Fu bletilla microspheres following renal arterial embolization in rabbits. Acta Univ. Med. Tangji 2001, 30, 501–502. [Google Scholar]
- Lee, K.; Jo, W.; Kwon, L.; Kim, Y.; Jeong, S. Structural determination and interior polarity of self-aggregates prepared from deoxycholic acid-modified chitosan in water. Macromolecules 1998, 31, 378–383. [Google Scholar] [CrossRef]
- Belder, A.; Granath, K. Preparation and properties of fluorescein-labelled dextrans. Carbohydr. Res. 1973, 30, 375–378. [Google Scholar] [CrossRef]
- Olsson, Y.; Svensjö, E.; Arfors, K.-E.; Hultström, D. Fluorescein labelled dextrans as tracers for vascular permeability studies in the nervous system. Acta Neuropathol. 1975, 33, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Sutthasupa, S.; Sanda, F. Synthesis of diblock copolymers of indomethacin/aspartic acid conjugated norbornenes and characterization of their self-assembled nanostructures as drug carriers. Eur. Polym. J. 2016, 85, 211–224. [Google Scholar] [CrossRef]
- Song, J.; Wang, L.; Han, F. The retrospective analysis of rhizoma bletillae used as an antitumor medicine. Inf. Tradit. Chin. Med. 2013, 30, 148–150. [Google Scholar]
- Moreira, J.N.; Gaspar, R.; Allen, T.M. Targeting Stealth liposomes in a murine model of human small cell lung cancer. Biochim. Biophys. Acta 2001, 1515, 167–176. [Google Scholar]
- Wong, H.L.; Rauth, A.M.; Bendayan, R.; Manias, J.L.; Ramaswamy, M.; Liu, Z.; Erhan, S.Z.; Wu, X.Y. A new polymer-lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm. Res. 2006, 23, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Xue, H.Y.; Babakhanian, K.; Wu, X.Y. A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. J. Pharmacol. Exp. Ther. 2006, 317, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.A.; Castile, J.; Smith, A.; Adams, G.G.; Harding, S.E. The effect of prolonged storage at different temperatures on the particle size distribution of tripolyphosphate (TPP)-chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1430–1434. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting Apoptosis Pathways in Cancer Therapy. CA Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J. Hemocompatibility testing for nanomedicines and biologicals: Predictive assays for complement mediated infusion reactions. Eur. J. Nanomed. 2012, 4, 33–52. [Google Scholar] [CrossRef]
- Cho, J.; Reip, V.; Hitchins, V.M.; Goering, P.L.; Malinauskas, R.A. Physicochemical characterization and in vitro hemolysis evaluation of silver nanoparticles. Toxicol. Sci. 2011, 123, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Niu, Y.; Zhao, N.; Mao, C.; Xu, F. A biocleavable pullulan-based vector via ATRP for liver cell-targeting gene delivery. Biomaterials 2014, 35, 3873–3884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Pan, Q.; Lin, Z.; Zhang, Q. Determination fo Docetaxel in Human Plasma by HPLC. Chin. Pharm. 2012, 23, 1286–1288. [Google Scholar]
- Kong, L.; Yua, L.; Feng, T.; Yin, X.; Liu, T.; Dong, L. Physicochemical characterization of the polysaccharide from Bletilla striata: Effect of drying method. Carbohydr. Polym. 2015, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.I.; Kim, S.H.; Jung, T.Y.; Kim, I.Y.; Kang, S.S.; Jin, Y.H.; Ryu, H.H.; Sun, H.S.; Jin, S.; Kim, K.K.; et al. Polyion complex micelles composed of all-trans retinoic acid and poly(ethylene glycol)-grafted-chitosan. J. Pharm. Sci. 2006, 95, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.T.; Shen, L.H.; Liu, Z.W.; Lu, J. Acetylation of β-cyclodextrin in ionic liquid green solvent. J. Mater. Sci. 2009, 44, 1813–1820. [Google Scholar] [CrossRef]
- Guan, Q.; Zhang, G.; Sun, S.; Fan, H.; Sun, C.; Zhang, S. Enhanced Oral Bioavailability of Pueraria Flavones by a Novel Solid Self-microemulsifying Drug Delivery System (SMEDDS) Dropping Pills. Biol. Pharm. Bull. 2016, 39, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Yanasarn, N.; Sloat, B.R.; Cui, Z. Nanoparticles engineered from lecithin-in-water emulsions as a potential delivery system for docetaxel. Int. J. Pharm. 2009, 379, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chae, S.Y.; Son, S.; Lee, M.; Jang, M.-K.; Nah, J.-W. Deoxycholic acid-conjugated chitosan oligosaccharide nanoparticles for efficient gene carrier. J. Control. Release 2005, 109, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Dong, X.; Zhou, L.; Xiao, H.; Ho, P.-Y.; Wong, M.-S.; Wanga, Y. Doxorubicin-loaded biodegradable self-assembly zein nanoparticle and its anti-cancer effect: Preparation, in vitro evaluation, and cellular uptake. Colloids Surf. B Biointerfaces 2016, 140, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, J.; Wang, L.; Tian, Z. Growth inhibition of human hepatocellular carcinoma cells by blocking STAT3 activation with decoy-ODN. Cancer Lett. 2008, 262, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds (BSPs, SA-BSPs and FITC-SA-BSPs) are available from the authors.
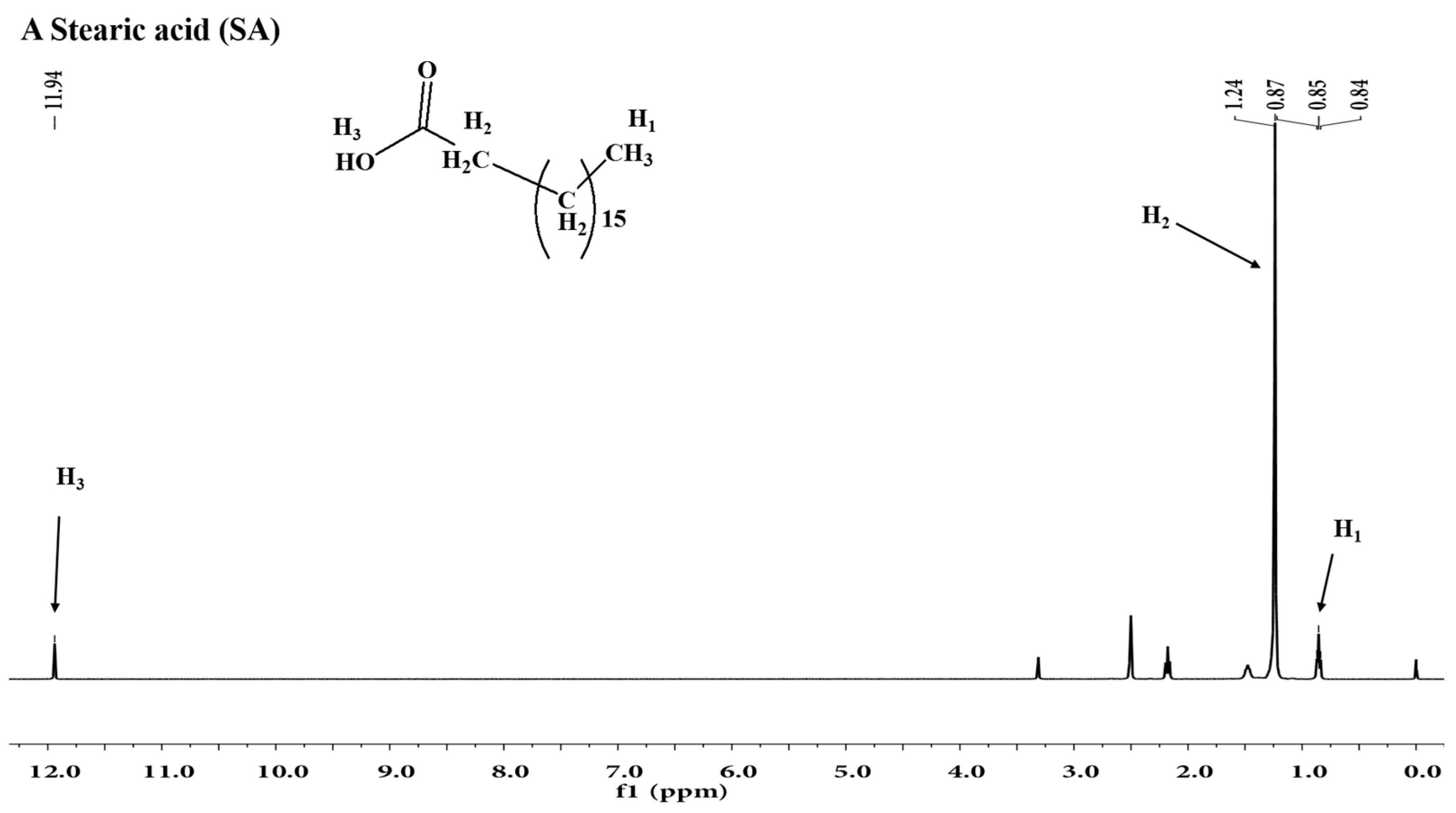
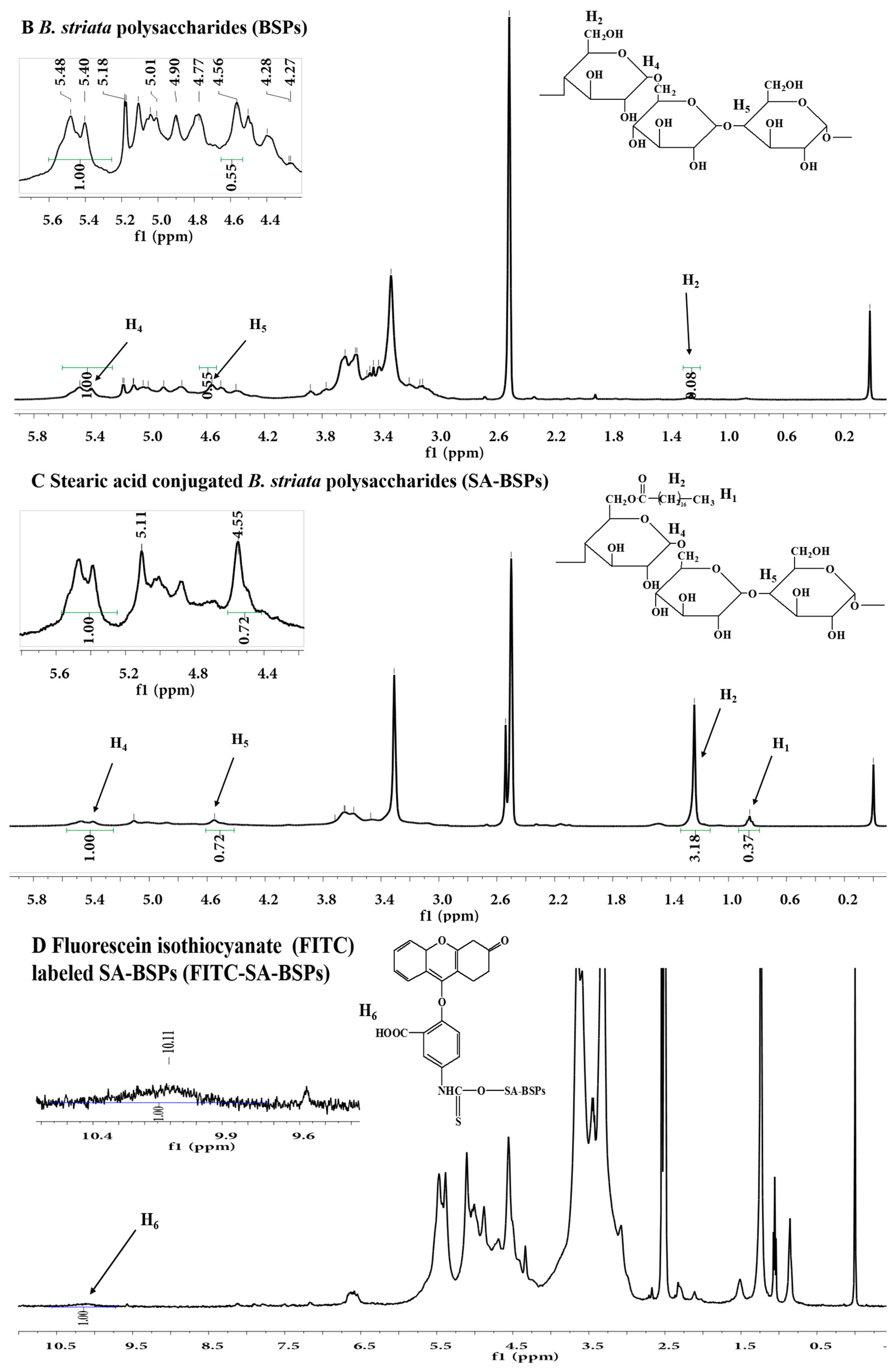
| Sample | Aδ5.43 | Aδ4.55 | Aδ1.24 | Aδ0.85 |
|---|---|---|---|---|
| SA-BSPs | 0.94 | 0.64 | 2.85 | 0.33 |
| Drug/Carrier (w/w) | EE (%) | LC (%) | Average Diameter (nm) | Zeta Potential (mV) |
|---|---|---|---|---|
| 0:10 | - | - | 96.27 ± 1.21 | −35.66 ± 0.28 |
| 1:10 | 78.7 ± 0.12 | 7.87 ± 0.18 | 96.54 ± 5.27 | −35.46 ± 0.10 |
| 1:9 | 89.8 ± 0.19 | 9.98 ± 0.16 | 99.21 ± 3.83 | −34.76 ± 0.22 |
| 1:7 | 88.3 ± 0.16 | 12.5 ± 0.20 | 121.61 ± 9.81 | −28.37 ± 0.12 |
| 1:6 | 86.6 ± 0.17 | 14.8 ± 0.13 | 125.30 ± 1.89 | −26.92 ± 0.18 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Q.; Sun, D.; Zhang, G.; Sun, C.; Wang, M.; Ji, D.; Yang, W. Docetaxel-Loaded Self-Assembly Stearic Acid-Modified Bletilla striata Polysaccharide Micelles and Their Anticancer Effect: Preparation, Characterization, Cellular Uptake and In Vitro Evaluation. Molecules 2016, 21, 1641. https://doi.org/10.3390/molecules21121641
Guan Q, Sun D, Zhang G, Sun C, Wang M, Ji D, Yang W. Docetaxel-Loaded Self-Assembly Stearic Acid-Modified Bletilla striata Polysaccharide Micelles and Their Anticancer Effect: Preparation, Characterization, Cellular Uptake and In Vitro Evaluation. Molecules. 2016; 21(12):1641. https://doi.org/10.3390/molecules21121641
Chicago/Turabian StyleGuan, Qingxiang, Dandan Sun, Guangyuan Zhang, Cheng Sun, Miao Wang, Danyang Ji, and Wei Yang. 2016. "Docetaxel-Loaded Self-Assembly Stearic Acid-Modified Bletilla striata Polysaccharide Micelles and Their Anticancer Effect: Preparation, Characterization, Cellular Uptake and In Vitro Evaluation" Molecules 21, no. 12: 1641. https://doi.org/10.3390/molecules21121641
APA StyleGuan, Q., Sun, D., Zhang, G., Sun, C., Wang, M., Ji, D., & Yang, W. (2016). Docetaxel-Loaded Self-Assembly Stearic Acid-Modified Bletilla striata Polysaccharide Micelles and Their Anticancer Effect: Preparation, Characterization, Cellular Uptake and In Vitro Evaluation. Molecules, 21(12), 1641. https://doi.org/10.3390/molecules21121641