Oxidatively Locked [Co2L3]6+ Cylinders Derived from Bis(bidentate) 2-Pyridyl-1,2,3-triazole “Click” Ligands: Synthesis, Stability, and Antimicrobial Studies
Abstract
:1. Introduction
2. Results
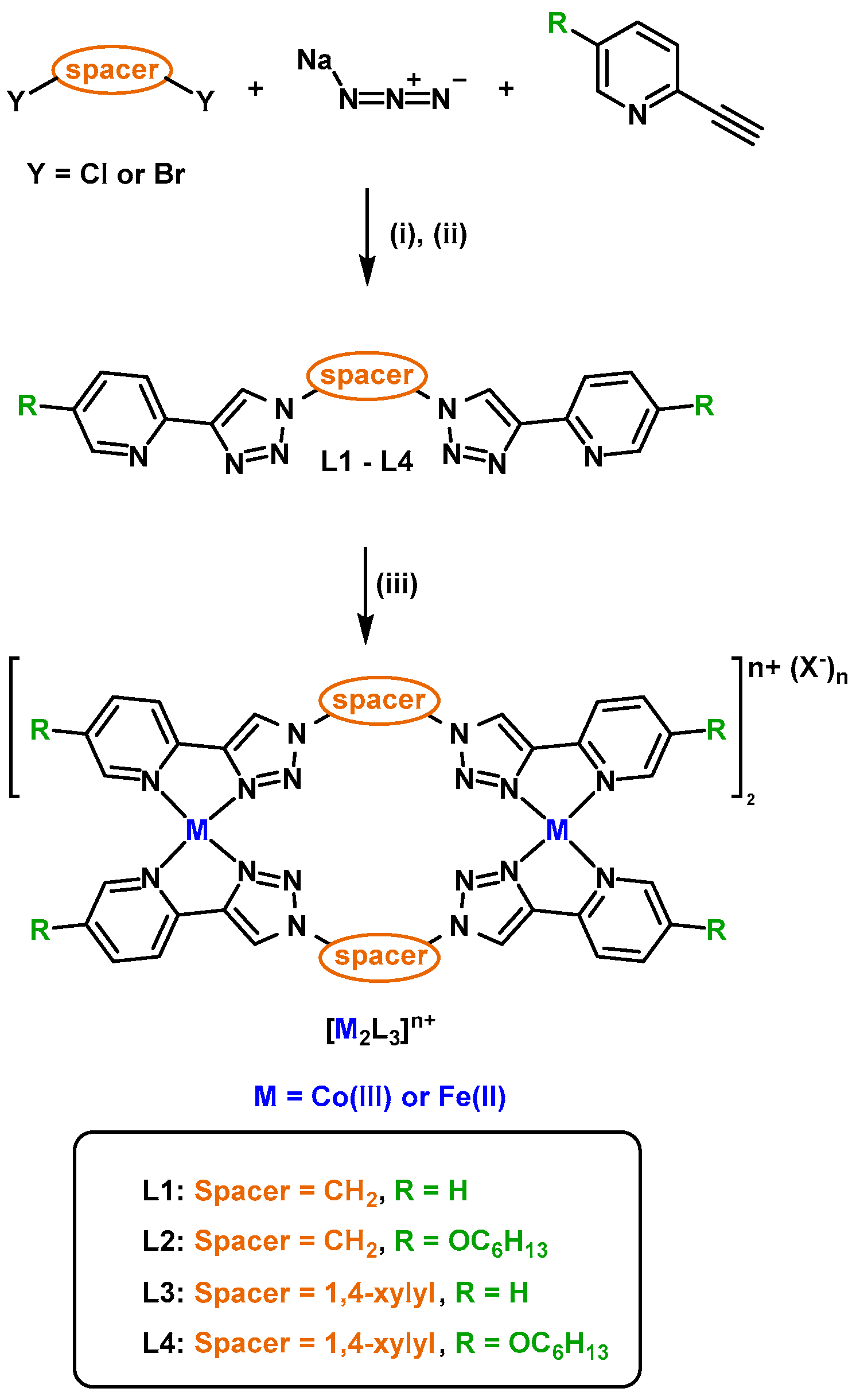
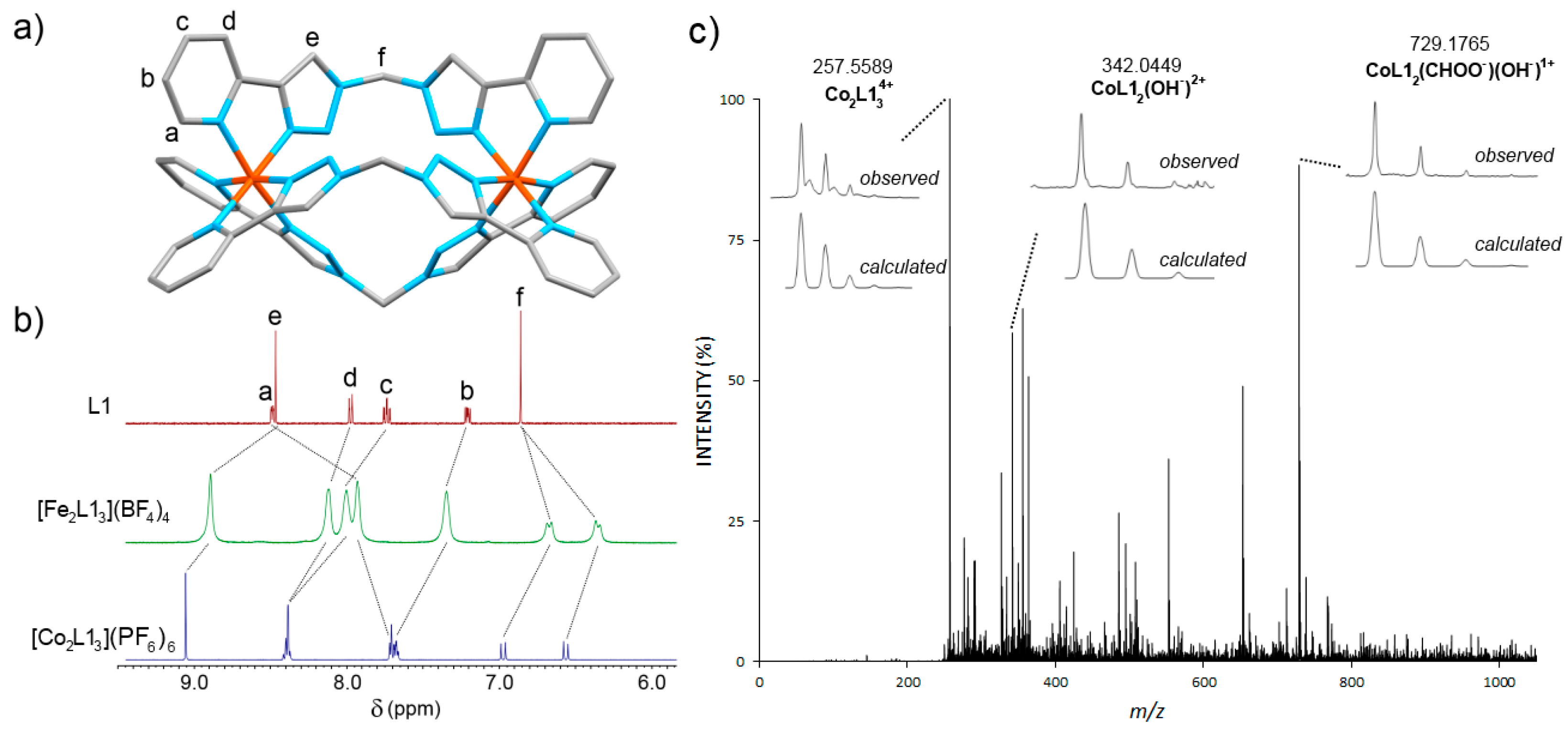
2.1. Cylinder Design and Synthesis
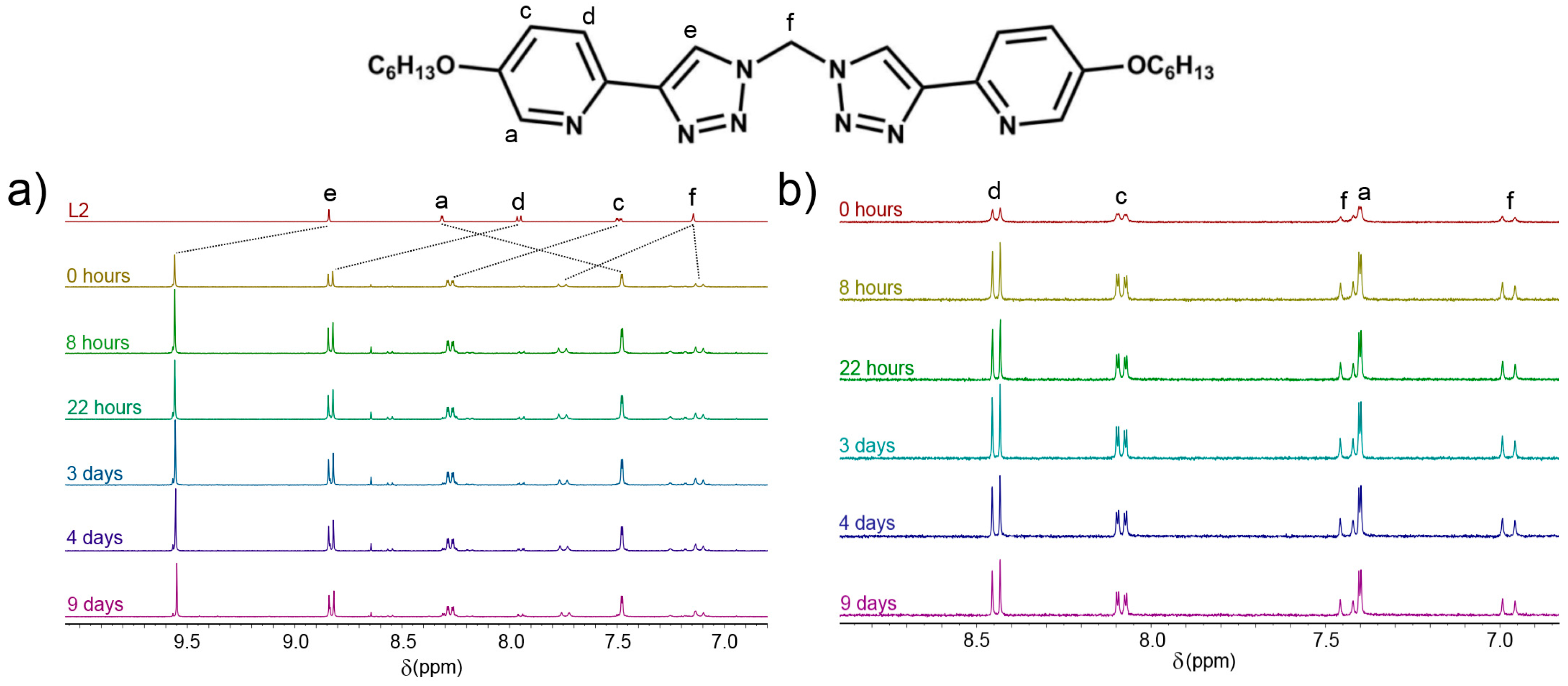
2.2. Stability Studies
2.3. Antimicrobial Activity
3. Conclusions
4. Experimental
4.1. General
4.2. General Synthetic Procedure for [Co2L3](OTf)6 Compounds
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Cook, T.R.; Vajpayee, V.; Lee, M.H.; Stang, P.J.; Chi, K.-W. Biomedical and Biochemical Applications of Self-Assembled Metallacycles and Metallacages. Acc. Chem. Res. 2013, 46, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- Schoentjes, B.; Lehn, J.-M. Interaction of Double-Helical Polynuclear Copper(I) complexes with double-stranded DNA. Helv. Chim. Acta 1995, 78, 1–12. [Google Scholar] [CrossRef]
- Albrecht, M. “Let’s Twist Again”Double-Stranded, Triple-Stranded, and Circular Helicates. Chem. Rev. 2001, 101, 3457–3498. [Google Scholar] [CrossRef] [PubMed]
- Hannon, M.J.; Painting, C.L.; Jackson, A.; Hamblin, J.; Errington, W. An inexpensive approach to supramolecular architecture. Chem. Commun. 1997, 1807–1808. [Google Scholar] [CrossRef]
- Moldrheim, E.; Hannon, M.J.; Meistermann, I.; Rodger, A.; Sletten, E. Interaction between a DNA oligonucleotide and a dinuclear iron(II) supramolecular cylinder; an NMR and molecular dynamics study. J. Biol. Inorg. Chem. 2002, 7, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Boer, D.R.; Kerckhoffs, J.M.C.A.; Parajo, Y.; Pascu, M.; Usón, I.; Lincoln, P.; Hannon, M.J.; Coll, M. Self-Assembly of Functionalizable Two-Component 3D DNA Arrays through the Induced Formation of DNA Three-Way-Junction Branch Points by Supramolecular Cylinders. Angew. Chem. Int. Ed. 2010, 49, 2336–2339. [Google Scholar] [CrossRef] [PubMed]
- Cerasino, L.; Hannon, M.J.; Sletten, E. DNA Three-Way Junction with a Dinuclear Iron(II) Supramolecular Helicate at the Center: A NMR Structural Study. Inorg. Chem. 2007, 46, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- Malina, J.; Hannon, M.J.; Brabec, V. Recognition of DNA Three-Way Junctions by Metallosupramolecular Cylinders: Gel Electrophoresis Studies. Chem. Eur. J. 2007, 13, 3871–3877. [Google Scholar] [CrossRef] [PubMed]
- Oleksi, A.; Blanco, A.G.; Boer, R.; Usón, I.; Aymamí, J.; Rodger, A.; Hannon, M.J.; Coll, M. Molecular Recognition of a Three-Way DNA Junction by a Metallosupramolecular Helicate. Angew. Chem. Int. Ed. 2006, 45, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Phongtongpasuk, S.; Paulus, S.; Schnabl, J.; Sigel, R.K.O.; Spingler, B.; Hannon, M.J.; Freisinger, E. Binding of a Designed Anti-Cancer Drug to the Central Cavity of an RNA Three-Way Junction. Angew. Chem. Int. Ed. 2013, 52, 11513–11516. [Google Scholar] [CrossRef] [PubMed]
- Pascu, G.I.; Hotze, A.C.G.; Sanchez-Cano, C.; Kariuki, B.M.; Hannon, M.J. Dinuclear ruthenium(II) triple-stranded helicates: Luminescent supramolecular cylinders that bind and coil DNA and exhibit activity against cancer cell lines. Angew. Chem. Int. Ed. 2007, 46, 4374–4378. [Google Scholar] [CrossRef] [PubMed]
- Hotze, A.C.G.; Hodges, N.J.; Hayden, R.E.; Sanchez-Cano, C.; Paines, C.; Male, N.; Tse, M.-K.; Bunce, C.M.; Chipman, J.K.; Hannon, M.J. Supramolecular Iron Cylinder with Unprecedented DNA Binding Is a Potent Cytostatic and Apoptotic Agent without Exhibiting Genotoxicity. Chem. Biol. 2008, 15, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.D.; Rodger, A.; Hannon, M.J.; Bolhuis, A. Antimicrobial activity of an iron triple helicate. Int. J. Antimicrob. Agents 2009, 33, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Brabec, V.; Howson, S.E.; Kaner, R.A.; Lord, R.M.; Malina, J.; Phillips, R.M.; Abdallah, Q.M.A.; McGowan, P.C.; Rodger, A.; Scott, P. Metallohelices with activity against cisplatin-resistant cancer cells; does the mechanism involve DNA binding? Chem. Sci. 2013, 4, 4407–4416. [Google Scholar] [CrossRef]
- Faulkner, A.D.; Kaner, R.A.; Abdallah, Q.M.A.; Clarkson, G.; Fox, D.J.; Gurnani, P.; Howson, S.E.; Phillips, R.M.; Roper, D.I.; Simpson, D.H.; et al. Asymmetric triplex metallohelices with high and selective activity against cancer cells. Nat. Chem. 2014, 6, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Kaner, R.A.; Allison, S.J.; Faulkner, A.D.; Phillips, R.M.; Roper, D.I.; Shepherd, S.L.; Simpson, D.H.; Waterfield, N.R.; Scott, P. Anticancer metallohelices: Nanomolar potency and high selectivity. Chem. Sci. 2016, 7, 951–958. [Google Scholar] [CrossRef]
- Li, M.; Howson, S.E.; Dong, K.; Gao, N.; Ren, J.; Scott, P.; Qu, X. Chiral Metallohelical Complexes Enantioselectively Target Amyloid β for Treating Alzheimer’s Disease. J. Am. Chem. Soc. 2014, 136, 11655–11663. [Google Scholar] [CrossRef] [PubMed]
- Howson, S.E.; Bolhuis, A.; Brabec, V.; Clarkson, G.J.; Malina, J.; Rodger, A.; Scott, P. Optically pure, water-stable metallo-helical ‘flexicate’ assemblies with antibiotic activity. Nat. Chem. 2012, 4, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Lewis, J.E.M.; Cameron, S.A.; Moratti, S.C.; Crowley, J.D. A multi-component CuAAC ‘click’ approach to an exo functionalised pyridyl-1,2,3-triazole macrocycle: Synthesis, characterisation, Cu(I) and Ag(I) complexes. Supramol. Chem. 2012, 24, 492–498. [Google Scholar] [CrossRef]
- Schweinfurth, D.; Deibel, N.; Weisser, F.; Sarkar, B. Mit Klick zu neuen Liganden. Nachr. Chem. 2011, 59, 937–941. [Google Scholar] [CrossRef]
- Struthers, H.; Mindt, T.L.; Schibli, R. Metal chelating systems synthesized using the Copper(I) catalyzed azide-alkyne cycloaddition. Dalton Trans. 2010, 39, 675–696. [Google Scholar] [CrossRef] [PubMed]
- Schulze, B.; Schubert, U.S. Beyond click chemistry—Supramolecular interactions of 1,2,3-triazoles. Chem. Soc. Rev. 2014, 43, 2522–2571. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.D.; McMorran, D.A. “Click-triazole” coordination chemistry: Exploiting 1,4-disubstituted-1,2,3-triazoles as ligands. Top. Heterocycl. Chem. 2012, 28, 31–83. [Google Scholar]
- Preston, D.; Tucker, R.A.J.; Garden, A.L.; Crowley, J.D. Heterometallic [MnPtn(L)2n]x+ Macrocycles from Dichloromethane-Derived Bis-2-pyridyl-1,2,3-triazole Ligands. Inorg. Chem. 2016, 55, 8928–8934. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, U.R.; Fronczek, F.R.; Maverick, A.W. Cyclic pyridyltriazole-Cu(II) dimers as supramolecular hosts. Dalton Trans. 2013, 42, 14064–14067. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, U.R.; Fronczek, F.R.; Maverick, A.W. Reduction of carbon dioxide to oxalate by a binuclear copper complex. Nat. Commun. 2014, 5, 5883. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.-Q.; Kai, D.; Ke, K.L.; Lin, M.; Jiang, L.; Jiang, Y.; Young, D.J.; Loh, X.J.; Li, X.; Hor, T.S.A. A Triazolyl-Pyridine-Supported CuI Dimer: Tunable Luminescence and Fabrication of Composite Fibers. ChemPlusChem 2015, 80, 1235–1240. [Google Scholar] [CrossRef]
- Stevenson, K.A.; Melan, C.F.C.; Fleischel, O.; Wang, R.; Petitjean, A. Solid-State Self-Assembly of Triazolylpyridine-Based Helicates and Mesocate: Control of the Metal-Metal Distances. Cryst. Growth Des. 2012, 12, 5169–5173. [Google Scholar] [CrossRef]
- Wu, N.; Melan, C.F.C.; Stevenson, K.A.; Fleischel, O.; Guo, H.; Habib, F.; Holmberg, R.J.; Murugesu, M.; Mosey, N.J.; Nierengarten, H.; et al. Systematic study of the synthesis and coordination of 2-(1,2,3-triazol-4-yl)-pyridine to Fe(II), Ni(II) and Zn(II); ion-induced folding into helicates, mesocates and larger architectures, and application to magnetism and self-selection. Dalton Trans. 2015, 44, 14991–15005. [Google Scholar] [CrossRef] [PubMed]
- Vellas, S.K.; Lewis, J.E.M.; Shankar, M.; Sagatova, A.; Tyndall, J.D.A.; Monk, B.C.; Fitchett, C.M.; Hanton, L.R.; Crowley, J.D. [Fe2L3]4 cylinders derived from bis(bidentate) 2-pyridyl-1,2,3-triazole “click” ligands: Synthesis, structures and exploration of biological activity. Molecules 2013, 18, 6383–6407. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Lo, W.K.C.; Brooks, H.J.L.; Crowley, J.D. Synthesis, structure, stability and antimicrobial activity of a ruthenium(II) helicate derived from a bis-bidentate “click” pyridyl-1,2,3-triazole ligand. Inorg. Chim. Acta 2015, 425, 1–6. [Google Scholar] [CrossRef]
- McNeill, S.M.; Preston, D.; Lewis, J.E.M.; Robert, A.; Knerr-Rupp, K.; Graham, D.O.; Wright, J.R.; Giles, G.I.; Crowley, J.D. Biologically active [Pd2L4]4+ quadruply-stranded helicates: Stability and cytotoxicity. Dalton Trans. 2015, 44, 11129–11136. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.J.; Nichol, G.S.; Lusby, P.J. Orthogonal Selection and Fixing of Coordination Self-Assembly Pathways for Robust Metallo-organic Ensemble Construction. J. Am. Chem. Soc. 2016, 138, 9308–9315. [Google Scholar] [CrossRef] [PubMed]
- Symmers, P.R.; Burke, M.J.; August, D.P.; Thomson, P.I.T.; Nichol, G.S.; Warren, M.R.; Campbell, C.J.; Lusby, P.J. Non-equilibrium cobalt(III) “click” capsules. Chem. Sci. 2015, 6, 756–760. [Google Scholar] [CrossRef]
- Maghami, M.; Farzaneh, F.; Simpson, J.; Ghiasi, M.; Azarkish, M. Synthesis, crystal structure, antibacterial activity and theoretical studies on a novel mononuclear Cobalt(II) complex based on 2,4,6-tris(2-pyridyl)-1,3,5-triazine ligand. J. Mol. Struct. 2015, 1093, 24–32. [Google Scholar] [CrossRef]
- Dimiza, F.; Papadopoulos, A.N.; Tangoulis, V.; Psycharis, V.; Raptopoulou, C.P.; Kessissoglou, D.P.; Psomas, G. Biological evaluation of non-steroidal anti-inflammatory drugs-Cobalt(II) complexes. Dalton Trans. 2010, 39, 4517–4528. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Böttcher, A.; Quezada, C.M.; Meade, T.J.; Gray, H.B. Inhibition of thermolysin and human α-thrombin by Cobalt(III) Schiff base complexes. Bioorg. Med. Chem. 1999, 7, 815–819. [Google Scholar] [CrossRef]
- Ott, I.; Gust, R. Non Platinum Metal Complexes as Anti-cancer Drugs. Arch. Pharm. 2007, 340, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Katiyar, A.; Singh, S. Synthesis, characterization of some transition metal(II) complexes of acetone p-amino acetophenone salicyloyl hydrazone and their anti microbial activity. BioMetals 2008, 21, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Maccari, R.; Ottanà, R.; Bottari, B.; Rotondo, E.; Vigorita, M.G. In vitro advanced antimycobacterial screening of Cobalt(II) and Copper(II) complexes of fluorinated isonicotinoylhydrazones. Bioorg. Med. Chem. Lett. 2004, 14, 5731–5733. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Ji, L.-N. 27Co Cobalt Complexes As Potential Pharmaceutical Agents. In Metallotherapeutic Drugs and Metal-Based Diagnostic Agents; John Wiley & Sons, Ltd.: Chichester, UK, 2005; pp. 201–218. [Google Scholar]
- Crowley, J.D.; Bandeen, P.H. A multicomponent CuAAC “click” approach to a library of hybrid polydentate 2-pyridyl-1,2,3-triazole ligands: New building blocks for the generation of metallosupramolecular architectures. Dalton Trans. 2010, 39, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Charbonniere, L.J.; Bernardinelli, G.; Piguet, C.; Sargeson, A.M.; Williams, A.F. Synthesis, structure and resolution of a dinuclear Co triple helix. J. Chem. Soc. Chem. Commun. 1994, 1419–1420. [Google Scholar] [CrossRef]
- Leigh, D.A.; Lusby, P.J.; Slawin, A.M.Z.; Walker, D.B. Half-rotation in a kinetically locked [2]catenane induced by transition metal ion substitution. Chem. Commun. 2012, 48, 5826–5828. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Yamada, Y.; Akine, S.; Sugimoto, K.; Tanaka, K. Kinetically “locked” metallomacrocycle. Dalton Trans. 2016, 45, 3831–3837. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.S.; Constable, E.C.; Housecroft, C.E.; Kulicke, K.J.; Tao, Y. When electron exchange is chemical exchange-assignment of 1H NMR spectra of paramagnetic Cobalt(II)-2,2′:6′,2″-terpyridine complexes. Dalton Trans. 2005, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Harris, K.; Housecroft, C.E.; Neuburger, M. When is a metallopolymer not a metallopolymer? When it is a metallomacrocycle. Dalton Trans. 2011, 40, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Harris, K.; Housecroft, C.E.; Neuburger, M.; Zampese, J.A. Turning {M(tpy)2}n+ embraces and CH···π interactions on and off in homoleptic cobalt(II) and cobalt(III) bis(2,2′:6′,2″-terpyridine) complexes. CrystEngComm 2010, 12, 2949–2961. [Google Scholar] [CrossRef]
- Small crystals of low quality were generated from a reaction mixture containing the [Co2(Lpytrz)3]6+ complex. The quality of the X-ray data was very modest but we present the structure obtained in the Supplementary Materials.
- Albrecht, M. How do they know? influencing the relative stereochemistry of the complex units of dinuclear triple-stranded helicate-type complexes. Chem. Eur. J. 2000, 6, 3485–3489. [Google Scholar] [CrossRef]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium complexes as antimicrobial agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Gasser, G.; Metzler-Nolte, N. Small organometallic compounds as antibacterial agents. Dalton Trans. 2012, 41, 6350–6358. [Google Scholar] [CrossRef] [PubMed]
- Kilah, N.L.; Meggers, E. Sixty Years Young: The Diverse Biological Activities of Metal Polypyridyl Complexes Pioneered by Francis P. Dwyer. Aust. J. Chem. 2012, 65, 1325–1332. [Google Scholar] [CrossRef]
- Kumar, S.V.; Lo, W.K.C.; Brooks, H.J.L.; Hanton, L.R.; Crowley, J.D. Antimicrobial Properties of Mono- and Di-fac-rhenium Tricarbonyl 2-Pyridyl-1,2,3-triazole Complexes. Aust. J. Chem. 2016, 69, 489–498. [Google Scholar] [CrossRef]
- Li, F.; Mulyana, Y.; Feterl, M.; Warner, J.M.; Collins, J.G.; Keene, F.R. The antimicrobial activity of inert oligonuclear polypyridylruthenium(II) complexes against pathogenic bacteria, including MRSA. Dalton Trans. 2011, 40, 5032–5038. [Google Scholar] [CrossRef] [PubMed]
- Pandrala, M.; Li, F.; Feterl, M.; Mulyana, Y.; Warner, J.M.; Wallace, L.; Keene, F.R.; Collins, J.G. Chlorido-containing ruthenium(II) and iridium(III) complexes as antimicrobial agents. Dalton Trans. 2013, 42, 4686–4694. [Google Scholar] [CrossRef] [PubMed]
- Pandrala, M.; Li, F.; Wallace, L.; Steel, P.J.; Moore, B.; Autschbach, J.; Collins, J.G.; Keene, F.R. Iridium(III) Complexes Containing 1,10-Phenanthroline and Derivatives: Synthetic, Stereochemical, and Structural Studies, and their Antimicrobial Activity. Aust. J. Chem. 2013, 66, 1065–1073. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors upon request.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasdev, R.A.S.; Preston, D.; Scottwell, S.Ø.; Brooks, H.J.L.; Crowley, J.D.; Schramm, M.P. Oxidatively Locked [Co2L3]6+ Cylinders Derived from Bis(bidentate) 2-Pyridyl-1,2,3-triazole “Click” Ligands: Synthesis, Stability, and Antimicrobial Studies. Molecules 2016, 21, 1548. https://doi.org/10.3390/molecules21111548
Vasdev RAS, Preston D, Scottwell SØ, Brooks HJL, Crowley JD, Schramm MP. Oxidatively Locked [Co2L3]6+ Cylinders Derived from Bis(bidentate) 2-Pyridyl-1,2,3-triazole “Click” Ligands: Synthesis, Stability, and Antimicrobial Studies. Molecules. 2016; 21(11):1548. https://doi.org/10.3390/molecules21111548
Chicago/Turabian StyleVasdev, Roan A. S., Dan Preston, Synøve Ø. Scottwell, Heather J. L. Brooks, James D. Crowley, and Michael P. Schramm. 2016. "Oxidatively Locked [Co2L3]6+ Cylinders Derived from Bis(bidentate) 2-Pyridyl-1,2,3-triazole “Click” Ligands: Synthesis, Stability, and Antimicrobial Studies" Molecules 21, no. 11: 1548. https://doi.org/10.3390/molecules21111548
APA StyleVasdev, R. A. S., Preston, D., Scottwell, S. Ø., Brooks, H. J. L., Crowley, J. D., & Schramm, M. P. (2016). Oxidatively Locked [Co2L3]6+ Cylinders Derived from Bis(bidentate) 2-Pyridyl-1,2,3-triazole “Click” Ligands: Synthesis, Stability, and Antimicrobial Studies. Molecules, 21(11), 1548. https://doi.org/10.3390/molecules21111548





