Abstract
A series of novel palladium(ii) acetylacetonato complexes bearing mesoionic carbenes (MICs) have been synthesized and characterized. The synthesis of the complexes of type (MIC)Pd(acac)I (MIC = 1-mesityl-3-methyl-4-phenyl-1,2,3-triazol-5-ylidene (1), 1,4-(2,4,6-methyl)-phenyl-3-methyl-1,2,3-triazol-5-ylidene (2), 1,4-(2,6-diisopropyl)-phenyl-3-methyl-1,2,3-triazol-5-ylidene (3); acac = acetylacetonato) via direct metalation starting from the corresponding triazolium iodides and palladium(ii) acetylacetonate is described herein. All complexes were characterized by 1H- and 13C-NMR spectroscopy and high resolution mass spectrometry. Additionally, two of the complexes were characterized by single crystal X-ray crystallography confirming a square-planar coordination geometry of the palladium(ii) center. A delocalized bonding situation was observed within the triazolylidene rings as well as for the acac ligand respectively. Complex 2 was found to be an efficient pre-catalyst for the Suzuki-Miyaura cross coupling reaction between aryl-bromides or -chlorides with phenylboronic acid.
1. Introduction
N-heterocyclic carbenes (NHCs) are a very attractive class of compounds. Because of their easy accessibility and their high complex stability, these ligands have been used extensively in organometallic chemistry in the last decades [1,2,3,4,5,6]. They are mainly applicable in homogeneous catalysis [7,8] but also in other fields of chemistry such as material science [9,10], bioorganometallic chemistry [10,11,12] or metallosupramolecular chemistry [13,14,15]. A special kind of NHCs are mesoionic carbene (MIC), which have received high popularity in recent years. Apart from imidazol-5-ylidenes, 1,2,3-triazol-5-ylidenes are the most noted MICs found in the literature [16,17,18,19,20,21,22]. Their precursors, the 1,2,3-triazoles, are easily accessible via the copper(i) catalyzed azide-alkyne cycloaddition (CuAAC) reaction [23,24,25,26,27]; subsequent alkylation results in the corresponding 1,2,3-triazolium salts, which can be converted into mesoionic carbenes [6,22,28]. Metal complexes of 1,2,3-triazol-5-ylidenes are used as catalysts in a variety of catalytic transformations [16,17,18,19,20,21]. For example, copper(i) complexes have been found to be potent catalysts for the “click” reaction [29,30,31,32,33,34], while cyclization reactions can be performed using gold(i) 1,2,3-triazol-5-ylidene complexes as catalysts [14,28,35,36,37,38,39,40,41]. Iridium(iii) complexes were shown to be efficient catalysts in water oxidation reactions [9,42,43,44,45] as well as other oxidation reactions and transfer hydrogenations [46,47,48,49,50,51], which are also catalyzed by ruthenium(ii) and osmium(ii) complexes [46,47,49,50,52,53,54,55,56,57,58]. Furthermore, 1,2,3-triazol-5-ylidenes and their metal complexes have also been investigated for their photochemical [59,60,61,62,63,64] and redox properties [60,65].
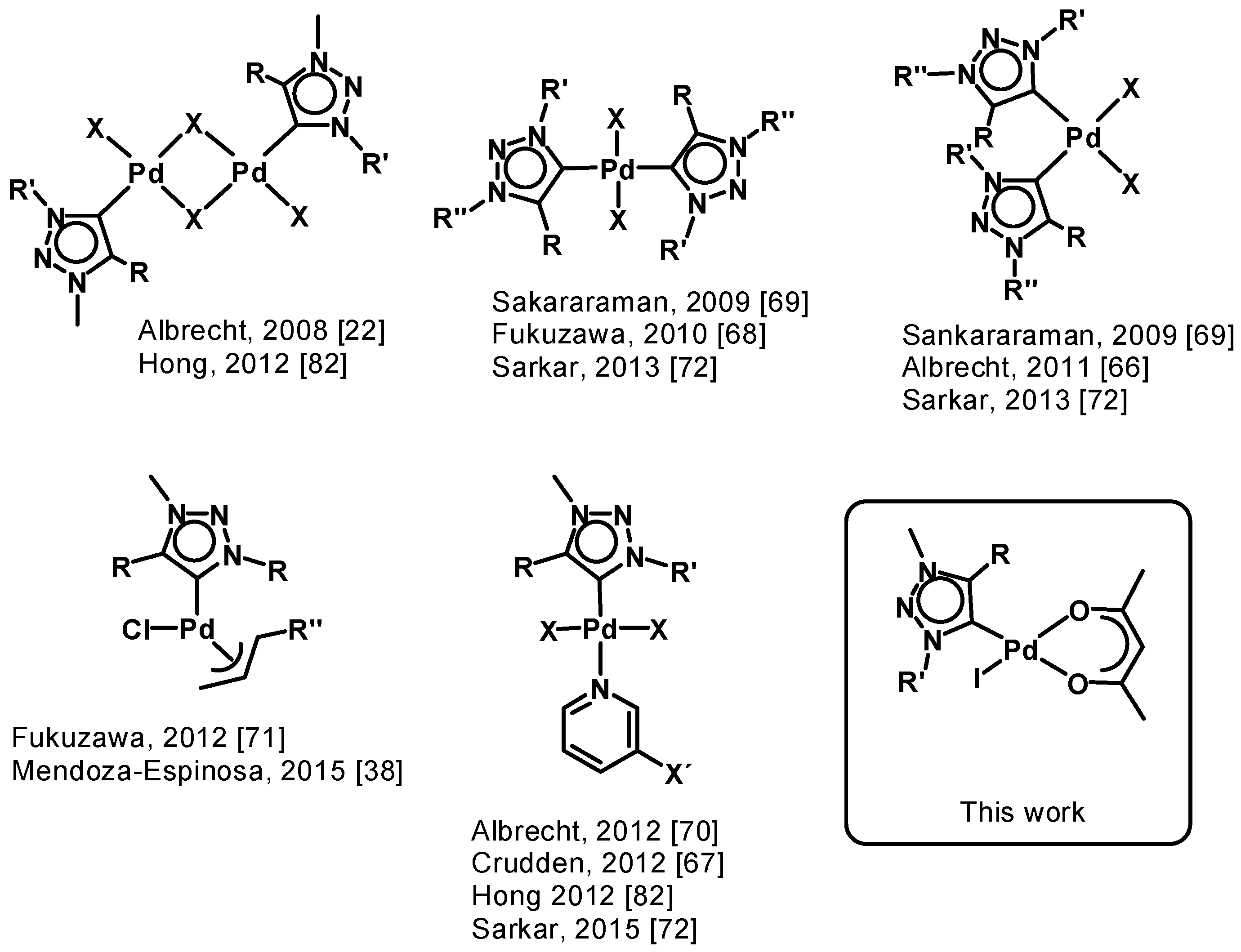
Especially palladium(ii) 1,2,3-triazol-5-ylidene complexes obtained a lot of attention since the discovery of 1,2,3-triazol-5-ylidene complexes [22]. Various types of palladium(ii) complexes show diversities in the coordination fashion and have different additional ligands (Figure 1) [22,38,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82]. Almost all palladium(ii) complexes were used as catalysts in a variety of cross coupling reactions. Mainly, they are used as pre-catalysts in reactions like the Suzuki-Miyaura cross coupling reaction [68,69,70,71,72,73,74,75,80,81,82,83] or α-arylation and α-methylation reactions [38,75,80].
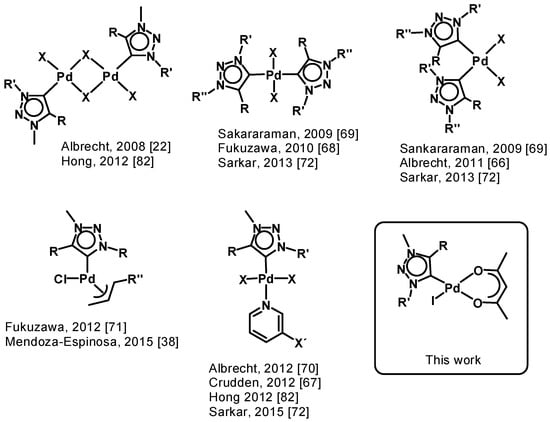
Figure 1.
Overview of selected palladium(ii) complexes with 1,2,3-triazol-5-ylidene ligands.
We have been interested in the development of a novel coordination motif of palladium(ii) 1,2,3-triazol-5-ylidenes complexes, in which κ2-acetylacetonate is coordinated as additional chelating ligand to the palladium(ii) center. For normal N-heterocyclic carbenes (NHC), these kind of palladium(ii) complexes, but with chlorido instead of iodido as ligand, were first reported in 2005 [84]. These NHC complexes showed high catalytic activity in the Buchwald–Hartwig amination and in the arylation of α-ketones [85,86].
Herein we report on the synthesis of a series of novel palladium(ii) acetylacetonato complexes bearing mesoionic carbenes. The complexes have been characterized by 1H- and 13C-NMR spectroscopy, mass spectrometry and single crystal X-ray crystallography. Their catalytic activity in the Suzuki-Miyaura cross coupling reaction are presented as well.
2. Results and Discussion
2.1. Synthesis and Characterization of the Palladium(ii) Complexes
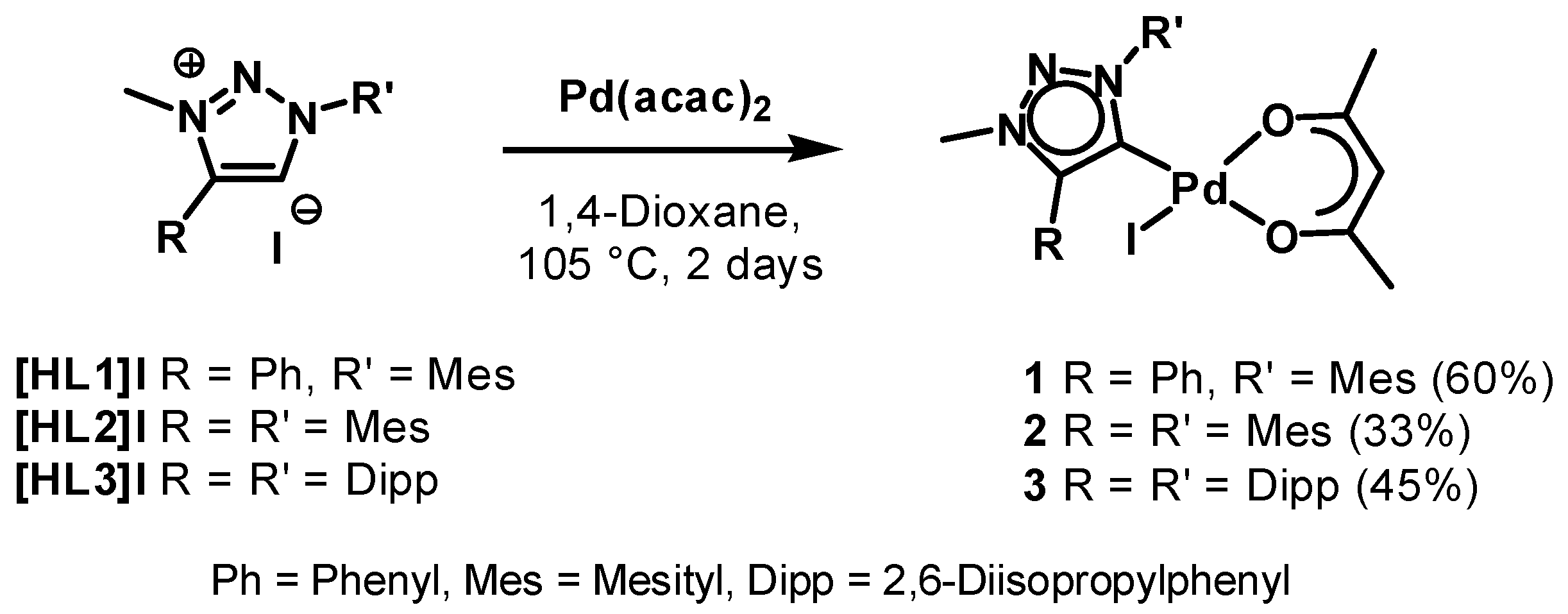
The triazolium salts [HL1]I [70,87], [HL2]I [68] and [HL3]I [32] were synthesized according to procedures reported in the literature. The complexes 1–3 were synthesized in a one-pot reaction starting from the corresponding triazolium salts via direct metalation by use of [Pd(acac)2] under inert conditions (Scheme 1). The route used here is similar as for the preparation of related nNHC complexes [85], but for the triazolylidene complexes the iodides of the triazolium salts were used because of synthetic ease. One of the acac ligands on [Pd(acac)2] acts as an internal base for the deprotonation of the triazolium salts. The coordinated iodides on the palladium centers originate from the corresponding triazolium salts (Scheme 1). The identity and purity of the compounds were unambiguously proven by mass spectrometry, 1H- and 13C-NMR spectroscopy. All complexes were isolated as yellow solids after precipitation or as yellow crystalline solids after recrystallization from a mixture of dichloromethane and hexane. In contrast to the achieved moderate to good yields of these MIC complexes, the yields obtained for the nNHC complexes have been nearly quantitative [85], even under aerobic conditions [86]. The reason for this might be the higher acidity of the imidazolium-2H [88,89,90] in comparison with the acidity of the triazolium-5H [19,91]. While the solubility of complexes 1–3 is very high in THF, diethyl ether, toluene or chlorinated solvents, the complexes were only moderately to sparingly soluble in hexane or pentane.
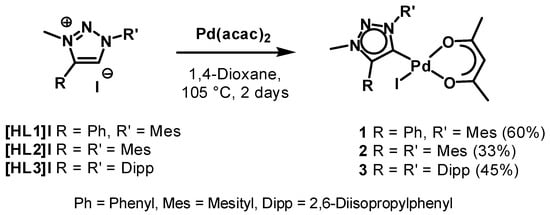
Scheme 1.
Synthesis of the palladium(ii) MIC complexes.
The first indication for the formation of the palladium(ii) MIC complexes in solution was the disappearance of the triazolium-5H in the 1H-NMR spectra and the appearance of the single proton of the bound acac ligand at 5.14, 5.12 and 5.13 ppm for 1, 2 and 3 respectively. Further proof was given by 13C-NMR spectroscopy based on the signals of the carbene carbons at 151.1 and 150.2 ppm for 2 and 3 respectively. Unfortunately, the signal corresponding to the carbene carbon for 1 was not resolved in its 13C-NMR spectrum. Two signals of the quaternary carbons of the bound acac ligand was observable for each complex in the range of 183–187 ppm (see Figures S1–S3). For the methyl-group of the acac ligand two separate signals could be observed in the 1H-NMR as well as in the 13C-NMR spectra. Additional characterization of the complexes by ESI-MS showed molecular peaks corresponding to the molecular mass with loss of the iodide (see ESI).
2.2. Structural Characterization
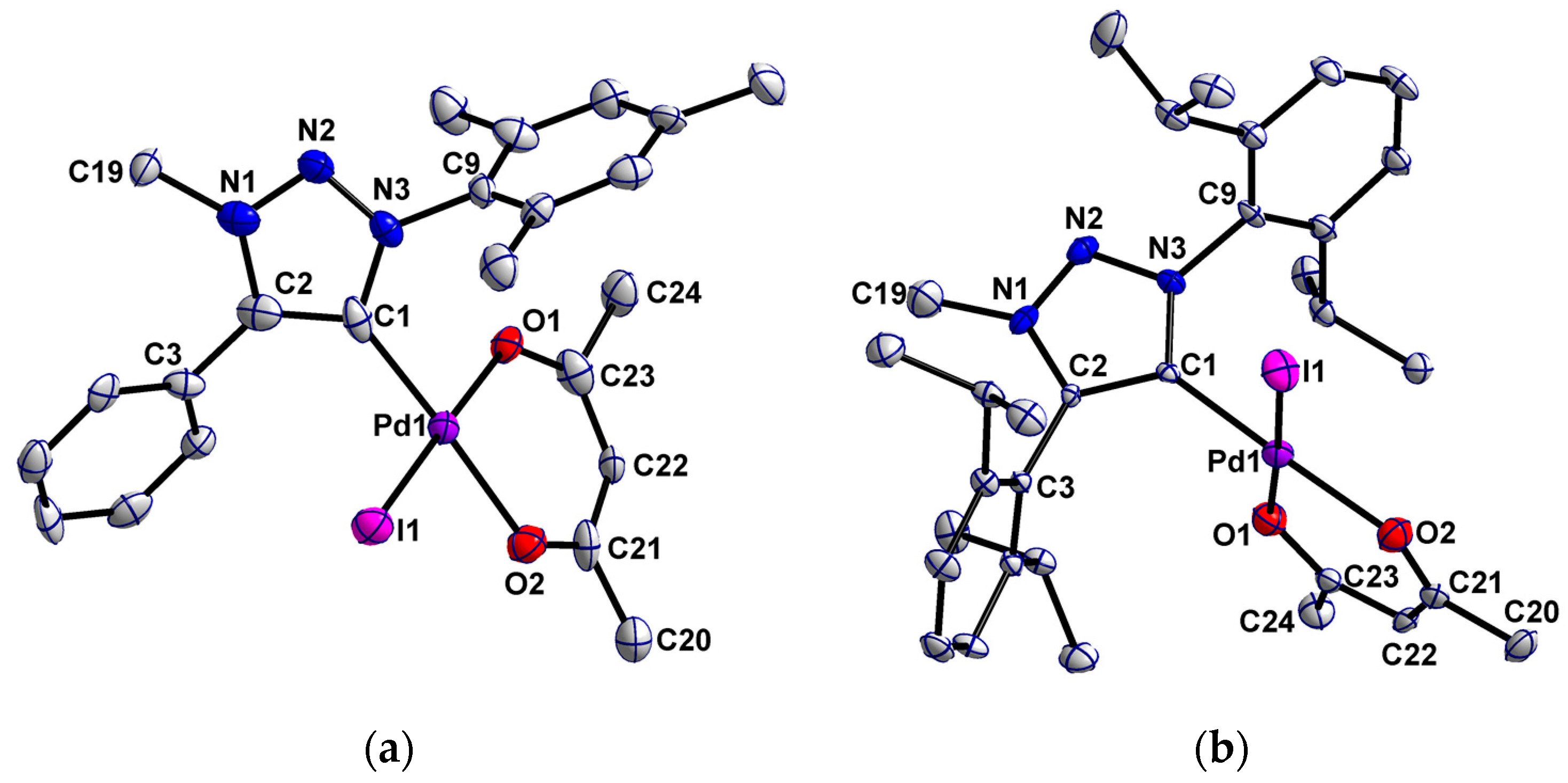
The molecular structures of palladium(ii) complexes 1 and 3 in crystal could be confirmed by single crystal X-ray crystallography (Figure 2). Single crystals were grown by slow diffusion of hexane onto a concentrated solution in dichloromethane. Crystallographic details for these complexes are given in Table S1. Important bond length and angles are depicted in Table 1.
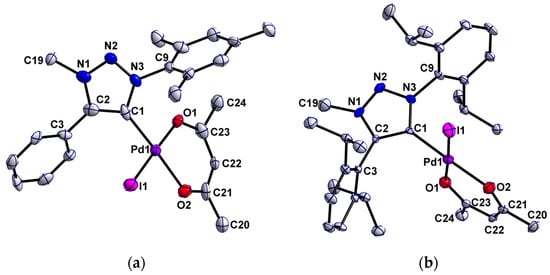
Figure 2.
Perspective view of complexes 1 (a) and 3 (b). Ellipsoids are drawn at the 50% probability level. Hydrogen atoms have been omitted for clarity.

Table 1.
Selected bond length (Å) and angles (°).
Complex 1 crystallized as yellow needles in the orthorhombic space group Pcba, while complex 3 crystallized as yellow plates in the monoclinic space group P21/n (see Table S1). The C–C, C–N and N–N bond lengths and the corresponding angles within the five-membered triazolylidene rings indicate a delocalized bonding situation in those rings for both complexes (Table 1). The aryl-substituents are twisted with respect to the triazolylidene ring. In complex 1 the dihedral angles between the aryl-substituents and the triazolylidene ring are 46.7° and 47.5°. The Dipp-substituents in complex 3 are almost perpendicular to the triazolyldiene rings with dihedral angles of 86.0° and 71.1°. The large twists observed in the aforementioned case is likely due to the higher steric demand of the Dipp substituents (Table 1). Within the acac ligand, the bond length and angles are also indicative of a delocalized bonding situation.
Both palladium(ii) MIC complexes display an almost square-planar coordination geometry, in which the angles between palladium and the different ligands are in between 88.7(2)° and 91.9(3)°. The square-planar geometry is typical for palladium(ii) centers with a d8 electronic configuration. The related nNHC complex showed a slightly higher deviation from the perfect square-planar geometry [84]. In complex 1 the triazolylidene ring is twisted about 57.1° with respect to the acac-Pd plane, while in complex 3 the triazolylidene ring is rotated about 82.7° resulting from the higher steric demand of the Dipp substituents (Table 1).
The distances between the palladium atoms and the carbene carbon atoms are almost similar in both complexes with 1.98 (1) Å and 1.957 (5) Å respectively, which is in accordance with other palladium(ii) triazolylidene complexes [65,75,80,92]. The Pd-C bond length of 1.969 (2) Å for the related nNHC complex lies in between the Pd-C distances of the MIC complexes reported here [84]. The Pd-I distances of 2.561 (1) Å and 2.560 (1) Å are a bit lower compared to Pd-I bond distances in other palladium(ii) MIC complexes [65,75,80,92]. While the acac ligand in 1 is unsymmetrically bound to the Pd center (2.027 (7) Å and 2.060 (7) Å) in 3 the bonding situation was found to be more symmetric with Pd-O1 and Pd-O2 being 2.054 (4) Å and 2.046 (4) Å respectively. For the related nNHC complex the Pd-O bond length are shorter with 2.044 (1) Å and 2.036 (2) Å compared to the MIC complexes [84].
2.3. Catalysis
Palladium(ii) NHC complexes in general are known to be effective catalysts in various transformations and therefore they play a central role in organic chemistry [3,7,93,94]. Different bonds such as C–X (X = halide) can be activated and new C–C or C–N bonds can be built. Also, many examples for catalytically active palladium(ii) MIC complexes in cross coupling reactions are known in the literature. The main application was reported in Suzuki–Miyaura coupling reactions [68,69,70,71,72,73,74,75,80,81,83], Mizoroki-Heck reactions [67] or Hiyama coupling reactions [77,78]. Other reactions like α-arylation or α-methylation [38,75,80] and the Buchwald–Hartwig reaction [79] were presented as well.
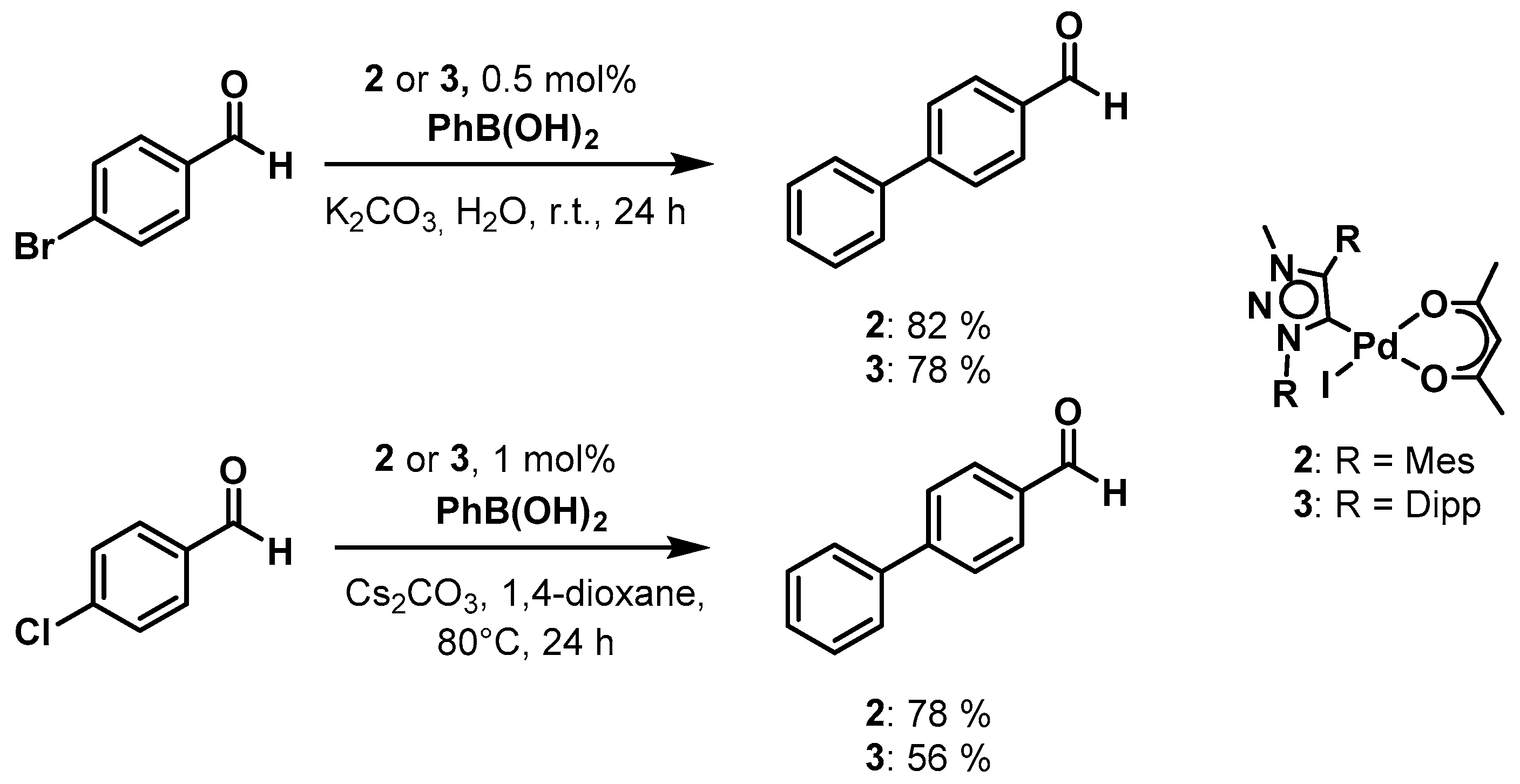
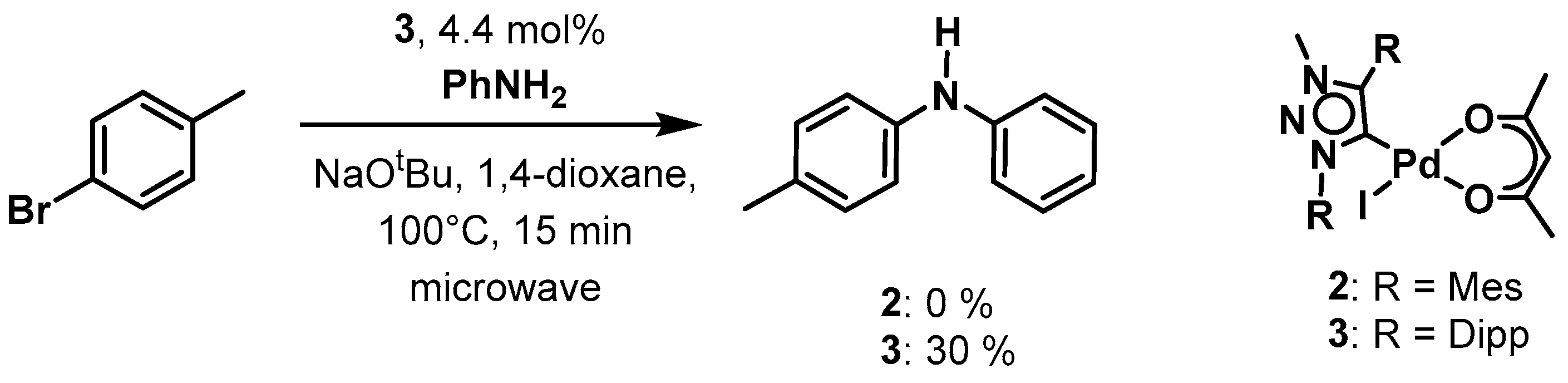
To gain an insight into the catalytic activity of our novel palladium(ii) MIC complexes we have chosen the Suzuki-Miyaura cross coupling and the Buchwald-Hartwig amination reactions as examples and complexes 2 and 3 as pre-catalysts. Both complexes show good to excellent activities in the Suzuki-Miyaura cross-coupling reaction between aryl bromides and chlorides with phenylboronic acid (Scheme 2).
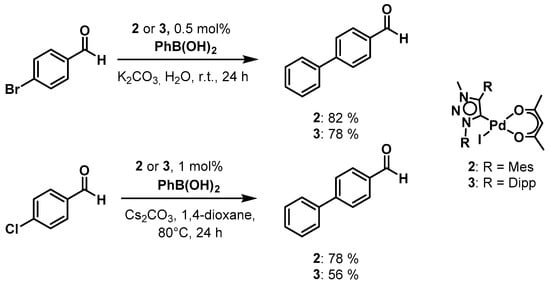
Scheme 2.
Palladium(ii) mesoionic carbene (MIC) complexes 2 and 3 as pre-catalysts in the Suzuki-Miyaura cross coupling reaction.
For 4-bromobenzaldehyde, the reaction could be performed in the environment-friendly solvent water at room temperature under air, using potassium carbonate as base. With a catalyst loading of only 0.5 mol % of 2 a conversion of 82% could be obtained within 24 h. For complex 3, the conversion was fairly similar at 78%. In the case of 4-chlororbenzaldehyde, which tends to be less easy to activate, the reaction conditions had to be changed. Therefore, we used 1,4-dioxane as the solvent under an Ar atmosphere, cesium carbonate as a base and the reaction temperature was increased to 80°. Applying these conditions, we were able to achieve a conversion of 78% within 24 h with catalyst loadings of 1 mol % of 2. For 3, the conversion was 56% under the same conditions. Additionally, we investigated the Buchwald-Hartwig amination reaction with both 2 and 3 as pre-catalysts under microwave irradiation conditions. This was necessary as the Buchwald-Hartwig amination is intrinsically a more difficult reaction to perform in comparison to the Suzuki-Miyaura cross-coupling reaction. Unfortunately, with complex 2, no conversion was observed under the used conditions. With complex 3, however, we were able to observe 30% conversions to product by using 4.4% catalyst loading under only 15 min of microwave irradiation (Scheme 3). The investigation of these two classes of catalytic reactions shows that the substituents on the triazolylidene ligands do have a direct or an indirect influence on the catalytic activities of the resulting palladium complexes. In the Suzuki-Miyaura cross-coupling reaction, the activity of the complexes presented here seems to be better than those of the corresponding PEPPSI complexes containing triazolylidene ligands, in which case a catalyst loading of 2.5 mol % (in comparison to 0.5 mol %) was necessary with similar substrates [80]. These results indicate a positive effect of the use of the acac containing complexes for these kinds of catalysis.
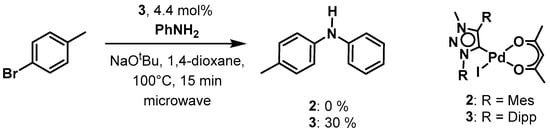
Scheme 3.
Palladium(ii) MIC complex 3 as pre-catalyst in the Buchwald-Hartwig amination.
3. Materials and Methods
3.1. General Procedures, Materials and Instrumentations
The triazolium salts [HL1]I [70,87], [HL2]I [68] and [HL3]I [32] were prepared as described previously in the literature. Commercially available chemicals were used as purchased, unless otherwise noted. The solvents used for synthesis and catalysis were dried and distilled under inert gas and degassed by common techniques prior to use, unless otherwise noticed. 1H- and 13C-NMR spectra were recorded on a Jeol ECS 400 or Jeol ECZ 400R spectrometer (Joel, Munich, Germany) using the chemical shift of the solvent as an internal standard. Multiplets are reported as follows: singlet (s), duplet (d), triplet (t) quartet (q), quintet (quint), and combinations thereof. Mass spectrometry was performed on an Agilent 6210 ESI-TOF (Agilent, Walbronn, Germany).
3.2. X-ray Crystallography
X-Ray data were collected on a Bruker Smart AXS diffractometer (Bruker, Karlsruhe, Germany). Data were collected at 140(2) K (see Table S1) using graphite-monochromated Mo Kα radiation (λα = 0.71073 Å). The strategy for the data collection was evaluated by using the Smart software (Bruker, AXS Inc., Madison, WI, USA). The data were collected by the standard ‘ωscan techniques’ and were scaled and reduced using Saint+ and SADABS software. The structures were solved by direct methods using SHELXS-97 or SHELXS_2014/7 and refined by full matrix least-squares, refining on F2. Non-hydrogen atoms were refined anisotropically [95,96,97,98,99,100,101]. If it is noted, bond length and angles were measured with Diamond Crystal Version 4.0.2 (Crystal Impact GbR, Bonn, Germany) and Molecular Structure Visualization Version 3.1 (Crystal Impact GbR). CCDC 965893 and 1015507 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk)
3.3. Synthesis
3.3.1. General Procedure for the Preparation of the Palladium(ii) 1,2,3-Triazolylidene Complexes 1–3
The corresponding triazolium salt [HL1]I, [HL2]I or [HL3]I (0.2 mmol, 1 eq.) and palladium(ii) acetylacetonate (60.9 mg, 0.2 mmol, 1 eq.) were dissolved in absolute 1,4-dioxane (3 mL) and stirred for 48 h at 105 °C. The reaction mixture was filtered over Celite and volatiles were removed under vacuum. The residue was re-resolved in dichloromethane (2 mL) and precipitated with diethyl ether (50 mL) to remove unreacted triazolium salt. The filtrate was reduced and precipitated again with hexane (50 mL) to remove the unreacted Pd(acac)2, which is soluble in hexane. The yellow precipitate was collected by filtration and washed with hexane. Recrystallization from a mixture of dichloromethane and hexane (1:4) gave the complexes as crystalline yellow solids. Single crystals suitable for X-ray diffraction analysis were obtained by slow diffusion of hexane onto a concentrated solution of the complex in dichloromethane.
Palladium(ii) complex 1: The product was obtained as yellow solid in a yield of 60% (73.1 mg, 0.12 mmol). 1H-NMR (401 MHz, CDCl3, 23 °C) δ = 7.96–7.91 (m, 2H, Ar-H), 7.61–7.50 (m, 3H, Ar-H), 7.04–6.98 (m, 2H, Ar-H), 5.14 (s, 1H, CH of acac), 4.11 (s, 3H, NCH3), 2.37 (s, 3H, CH3), 2.29 (s, 3H, CH3), 2.16 (s, 3H, CH3), 1.85 (s, 3H, CH3 of acac), 1.76 (s, 3H, CH3 of acac) ppm. 13C-NMR (101 MHz, CDCl3, 24 °C) δ = 186.6, 183.6 (CO, acac), 145.6, 141.4, 140.5, 135.6, 135.3, 130.8, 130.1, 129.0, 127.3 (all Aryl-C), 99.1 (CH, acac), 37.8 (NCH3), 27.6, 26.3, 21.4 (all Alkyl-C) ppm. HRMS (ESI): calcd for [C23H26IN3O2Pd] [M − I]+ m/z 482.1060, found 482.1065.
Palladium(ii) complex 2: The product was obtained as yellow solid in a yield of 33% (43.0 mg, 0.066 mmol). 1H-NMR (401 MHz, CDCl3, 22 °C) δ = 7.01 (s, 4H, Aryl-H), 5.12 (s, 1H, CH of acac), 3.80 (s, 3H, NCH3), 2.37 (p-d, 6H, CH3), 2.27–2.19 (m, 12H, CH3), 1.81 (s, 3H, CH3 of acac), 1.76 (s, 3H, CH3 of acac) ppm. 13C-NMR (126 MHz, CDCl3, 21 °C) δ = 186.4, 183.2 (CO, acac), 151.1 (Carbene-C), 147.8, 144.5, 141.5, 140.5, 140.4, 139.1, 135.6, 135.5, 122.9 (all Aryl-C), 99.0 (CH, acac), 36.5 (NCH3), 29.8, 27.6, 26.2, 21.5, 21.4 (all Alkyl-C) ppm. HRMS (ESI): calcd for [C26H32IN3O2Pd] [M − I]+ m/z 524.1529, found 524.1518.
Palladium(ii) complex 3: The product was obtained as yellow solid in a yield of 45% (66.2 mg, 0.09 mmol). 1H-NMR (400 MHz, CDCl3, 20 °C) δ = 7.53 (t, J = 7.8 Hz, 2H, Aryl-H), 7.34 (d, J = 7.8 Hz, 4H, Aryl-H), 5.13 (s, 1H, CH of acac), 3.78 (s, 3H, NCH3), 2.99 (hept, J = 6.9 Hz, 2H, CH), 2.92–2.82 (m, 2H, CH), 1.86 (s, 3H, CH3 of acac), 1.81 (s, 3H, CH3 of acac), 1.36–1.32 (m, 12H, CH3), 1.07 (dd, J = 10.8, 6.9 Hz, 12H, CH3) ppm. 13C-NMR (101 MHz, CDCl3, 22 °C) δ = 186.0, 183.5 (CO, acac), 150.2 (Carbene-C), 146.1, 143.5, 143.3, 136.1, 131.5, 131.3, 124.2, 124.2, 123.4 (all Aryl-C), 98.8 (CH, acac), 37.5 (NCH3), 30.9, 29.1, 27.7, 27.5, 26.0, 25.1, 24.9, 22.9 (all Alkyl-C) ppm. HRMS (ESI): calcd for [C32H44IN3O2Pd] [M − I]+ m/z 608.2468, found 608.2471.
3.3.2. Suzuki-Miyaura Cross Coupling Reactions
The 4-bromobenzaldeyhde (92.5 mg, 0.5 mmol, 1 eq.), phenylboronic acid (73.2 mg, 0.6 mmol, 1.2 eq.) and potassium carbonate (103.9 mg, 0.75 mmol, 1.5 eq.) were mixed in the presence of water (1.5 mL) under air. Then complexes 2 or 3 (0.5 mol %) was added. The mixture was stirred at room temperature for 24 h. For the aryl-chloride, 4-chlorobenzaldeyhde (28.1.5 mg, 0.2 mmol, 1 eq.), phenylboronic acid (20.3 mg, 0.24 mmol, 1.2 eq.) and cesium carbonate (130.3 mg, 0.4 mmol, 2 eq.) were mixed in presence of absolute 1,4-dioxane (1 mL) under nitrogen atmosphere. After that, complexes 2 or 3 (1 mol %) was added. The mixture was stirred at 80 °C for 24 h. The crude reaction mixtures were poured into dichloromethane respectively and extracted with water five times. Finally, the combined aqueous layers were extracted one last time with dichloromethane. The combined organic layers were dried over sodium sulfate, filtered and evaporated to dryness. Afterwards, yields were determined via 1H-NMR spectroscopy with the help of the aldehyde protons.
3.3.3. Buchwald-Hartwig Amination
4-Bromobenzene (34.2 mg, 0.2 mmol, 1 eq.), aniline (21.9 μL, 22.4 mg, 0.24 mmol, 1.2 eq.), NaOtBu (23.1 mg, 0.24 mmol, 1.2 eq.) and the complexes 2 or 3 (4.4 mol %) were mixed in the presence of absolute 1,4-dioxane (2 mL). The reaction mixture was stirred at 100 °C for 15 min in the microwave. The reaction mixture was cooled to room temperature, and the solvent was removed under vacuum. Afterwards, yields were determined via 1H-NMR spectroscopy with the help of the protons of the methyl-group of the product with 1,3,5-tribromobenzene as internal standard.
4. Conclusions
In conclusion, we have presented here the first examples of three palladium(ii) MIC complexes of the type (MIC)Pd(acac)I. Additional to the MIC, the chelating bidentate ligand κ2-acetylacetonate is coordinated to the palladium(ii) center. The synthesis and characterization of all complexes by mass spectrometry, 1H- and 13C-NMR spectroscopy is described herein. The molecular structure in crystal was verified for two of the three palladium(ii) complexes by single crystal X-ray crystallography. Structural characterization revealed the expected square-planar coordination fashion of the palladium(ii) center. Due to the popular application of palladium(ii) MIC complexes as pre-catalysts in cross coupling reactions, we utilized one of our new complexes as a pre-catalyst in the Suzuki–Miyaura cross coupling reaction. First attempts showed that these kind of complexes are excellent pre-catalysts for the cross coupling of phenylboronic acid with an aryl-bromide as well as an aryl-chloride. The inclusion of the acac ligand on the Pd-MIC unit opens up new possibilities for the synthesis of novel palladium complexes with mesoionic carbene ligands. Changing the donor properties of the acac ligand is conceivable, as is its substitution with other chelating anionic ligands. Such new motifs might open up additional opportunities and perspectives in the chemistry of the metal complexes of MIC ligands.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/11/1561/s1.
Acknowledgments
The Deutsche Forschungsgemeinschaft /DFG) and the Fonds der Chemischen Industrie (FCI) are kindly acknowledged for the financial support of this work. We thank Samir Patra for preliminary work on the Buchwald-Hartwig amination reactions.
Author Contributions
L. Hettmanczyk and B. Sarkar designed the project. Experiments and data analysis was performed by L. Hettmanczyk and B. Schmid. Crystal data were recorded and solved by S. Hohloch. Manuscript was written by L. Hettmanczyk and B. Sarkar.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Frémont, P.; Marion, N.; Nolan, S.P. Carbenes: Synthesis, properties, and organometallic chemistry. Coord. Chem. Rev. 2009, 253, 862–892. [Google Scholar] [CrossRef]
- Egbert, J.D.; Cazin, C.S.J.; Nolan, S.P. Copper N-heterocyclic carbene complexes in catalysis. Catal. Sci. Technol. 2013, 3, 912–926. [Google Scholar] [CrossRef]
- Kantchev, E.A.; O’Brien, C.J.; Organ, M.G. Palladium complexes of N-heterocyclic carbenes as catalysts for cross-coupling reactions—A synthetic chemist’s perspective. Angew. Chem. Int. Ed. 2007, 46, 2768–2813. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.C.; Enders, D. Merging organocatalysis and gold catalysis–a critical evaluation of the underlying concepts. Eur. J. Chem. 2012, 18, 10212–10225. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.E.; Jahnke, M.C. Heterocyclic carbenes: Synthesis and coordination chemistry. Angew. Chem. Int. Ed. 2008, 47, 3122–3172. [Google Scholar] [CrossRef] [PubMed]
- Guisado-Barrios, G.; Bouffard, J.; Donnadieu, B.; Bertrand, G. Crystalline 1H-1,2,3-triazol-5-ylidenes: New stable mesoionic carbenes (MICs). Angew. Chem. Int. Ed. 2010, 49, 4759–4762. [Google Scholar] [CrossRef] [PubMed]
- Díez-González, S.; Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.A. N-heterocyclic carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Lalrempuia, R.; McDaniel, N.D.; Müller-Bunz, H.; Bernhard, S.; Albrecht, M. Water oxidation catalyzed by strong carbene-type donor-ligand complexes of iridium. Angew. Chem. Int. Ed. 2010, 49, 9765–9768. [Google Scholar] [CrossRef] [PubMed]
- Mercs, L.; Albrecht, M. Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal, luminescent, and functional materials applications. Chem. Soc. Rev. 2010, 39, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Sivaram, H.; Tan, J.; Huynh, H.V. Syntheses, characterizations, and a preliminary comparative cytotoxicity study of gold(I) and gold(III) complexes bearing benzimidazole- and pyrazole-derived N-heterocyclic carbenes. Organometallics 2012, 31, 5875–5883. [Google Scholar] [CrossRef]
- Hickey, J.L.; Ruhayel, R.A.; Barnard, P.J.; Baker, M.V.; Berners-Price, S.J.; Filipovska, A. Mitochondria-targeted chemotherapeutics: The rational design of gold(I) N-heterocyclic carbene complexes that are selectively toxic to cancer cells and target protein selenols in preference to thiols. J. Am. Chem. Soc. 2008, 130, 12570–12571. [Google Scholar] [CrossRef] [PubMed]
- Maity, R.; Rit, A.; Schulte to Brinke, C.; Daniliuc, C.G.; Hahn, F.E. Metal center dependent coordination modes of a tricarbene ligand. Chem. Commun. 2013, 49, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Mejuto, C.; Guisado-Barrios, G.; Gusev, D.; Peris, E. First homoleptic MIC and heteroleptic NHC-MIC coordination cages from 1,3,5-triphenylbenzene-bridged tris-MIC and tris-NHC ligands. Chem. Commun. 2015, 51, 13914–13917. [Google Scholar] [CrossRef] [PubMed]
- Schmidtendorf, M.; Pape, T.; Hahn, F.E. Stepwise preparation of a molecular square from NR,NR- and NH,O-substituted dicarbene building blocks. Angew. Chem. Int. Ed. 2012, 51, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.D.; Lee, A.-L.; Kilpin, K.J. 1,3,4-Trisubstituted-1,2,3-triazol-5-ylidene ‘click’ carbene ligands: Synthesis, catalysis and self- assembly. Aust. J. Chem. 2011, 64, 1118–1132. [Google Scholar] [CrossRef]
- Schweinfurth, D.; Deibel, N.; Weisser, F.; Sarkar, B. Mit Klick zu neuen Liganden. Nachr. Chem. 2011, 937–941. [Google Scholar] [CrossRef]
- Crabtree, R.H. Abnormal, mesoionic and remote N-heterocyclic carbene complexes. Coord. Chem. Rev. 2013, 257, 755–766. [Google Scholar] [CrossRef]
- Donnelly, K.F.; Petronilho, A.; Albrecht, M. Application of 1,2,3-triazolylidenes as versatile NHC-type ligands: Synthesis, properties, and application in catalysis and beyond. Chem. Commun. 2013, 49, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua, J.M.; Fratila, R.M.; Monasterio, Z.; Pérez-Esnaola, N.; Andreieff, E.; Irastorza, A.; Sagartzazu-Aizpurua, M. Triazolium cations: From the “click” pool to multipurpose applications. New J. Chem. 2014, 38, 474–480. [Google Scholar] [CrossRef]
- Schulze, B.; Schubert, U.S. Beyond click chemistry–supramolecular interactions of 1,2,3-triazoles. Chem. Soc. Rev. 2014, 43, 2522–2571. [Google Scholar] [CrossRef] [PubMed]
- Mathew, P.; Neels, A.; Albrecht, M. 1,2,3-Triazolylidenes as versatile abnormal carbene ligands for late transition metals. J. Am. Chem. Soc. 2008, 130, 13534–13535. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R.; Knorr, R.; Möbius, L.; Szeimies, G. 1.3-Dipolare Cycloadditionen, XXIII. Einige Beobachtungen zur Addition organischer Azide an CC-Dreifachbindungen. Chem. Ber. 1965, 98, 4014–4021. [Google Scholar] [CrossRef]
- Huisgen, R.; Szeimies, G.; Möbius, L. 1.3-Dipolare Cycloadditionen, XXXII. Kinetik der Additionen organischer Azide an CC-Mehrfachbindungen. Chem. Ber. 1967, 100, 2494–2507. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Kilpin, K.J.; Paul, U.S.; Lee, A.L.; Crowley, J.D. Gold(I) “click” 1,2,3-triazolylidenes: Synthesis, self-assembly and catalysis. Chem. Commun. 2011, 47, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Hohloch, S.; Su, C.-Y.; Sarkar, B. Copper(I) complexes of normal and abnormal carbenes and their use as catalysts for the Huisgen [3+2] cycloaddition between azides and alkynes. Eur. J. Inorg. Chem. 2011, 3067–3075. [Google Scholar] [CrossRef]
- Hohloch, S.; Sarkar, B.; Nauton, L.; Cisnetti, F.; Gautier, A. Are Cu(I)-mesoionic NHC carbenes associated with nitrogen additives the best Cu-carbene catalysts for the azide–alkyne click reaction in solution? A case study. Tetrahedron Lett. 2013, 54, 1808–1812. [Google Scholar] [CrossRef]
- Hohloch, S.; Scheiffele, D.; Sarkar, B. Activating azides and alkynes for the click reaction with [Cu(aNHC)2I] or [Cu(aNHC)2]+(aNHC = triazole-derived abnormal carbenes): Structural characterization and catalytic properties. Eur. J. Inorg. Chem. 2013, 3956–3965. [Google Scholar] [CrossRef]
- Hohloch, S.; Duecker, F.L.; van der Meer, M.; Sarkar, B. Copper(I) complexes of mesoionic carbene: Structural characterization and catalytic hydrosilylation reactions. Molecules 2015, 20, 7379–7395. [Google Scholar] [CrossRef] [PubMed]
- Hohloch, S.; Suntrup, L.; Sarkar, B. Exploring potential cooperative effects in dicopper(I)-di-mesoionic carbene complexes: applications in click catalysis. Inorg. Chem. Front. 2016, 3, 67–77. [Google Scholar] [CrossRef]
- Bidal, Y.D.; Lesieur, M.; Melaimi, M.; Nahra, F.; Cordes, D.B.; Athukorala Arachchige, K.S.; Slawin, A.M.Z.; Bertrand, G.; Cazin, C.S.J. Copper(I) complexes bearing carbenes beyond classical N-heterocyclic carbenes: Synthesis and catalytic activity in “click chemistry”. Adv. Synth. Catal. 2015, 357, 3155–3161. [Google Scholar] [CrossRef]
- Canseco-Gonzalez, D.; Petronilho, A.; Müller-Bunz, H.; Ohmatsu, K.; Ooi, T.; Albrecht, M. Carbene transfer from triazolylidene gold complexes as a potent strategy for inducing high catalytic activity. J. Am. Chem. Soc. 2013, 135, 13193–13203. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.R.; Young, P.C.; Lucas, N.T.; Lee, A.L.; Crowley, J.D. Gold(I) and palladium(II) complexes of 1,3,4-trisubstituted 1,2,3-triazol-5-ylidene “click” carbenes: Systematic study of the electronic and steric influence on catalytic activity. Organometallics 2013, 32, 7065–7076. [Google Scholar] [CrossRef] [PubMed]
- Hettmanczyk, L.; Manck, S.; Hoyer, C.; Hohloch, S.; Sarkar, B. Heterobimetallic complexes with redox-active mesoionic carbenes as metalloligands: Electrochemical properties, electronic structures and catalysis. Chem. Commun. 2015, 51, 10949–10952. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Espinosa, D.; González-Olvera, R.; Negrón-Silva, G.E.; Angeles-Beltrán, D.; Suárez-Castillo, O.R.; Álvarez-Hernández, A.; Santillan, R. Phenoxy-linked mesoionic triazol-5-ylidenes as platforms for multinuclear transition metal complexes. Organometallics 2015, 34, 4529–4542. [Google Scholar] [CrossRef]
- Mendoza-Espinosa, D.; González-Olvera, R.; Osornio, C.; Negrón-Silva, G.E.; Santillan, R. Versatile O- and S-functionalized 1,2,3-triazoliums: Ionic liquids for the Baylis–Hillman reaction and ligand precursors for stable MIC-transition metal complexes. New J. Chem. 2015, 39, 1587–1591. [Google Scholar] [CrossRef]
- Tolentino, D.R.; Liqun, J.; Melaimi, M.; Bertrand, G. Mesoionic carbene-gold(I) catalyzed bis-hydrohydrazination of alkynes with parent hydrazine. Chem. Asian J. 2015, 10, 2139–2142. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, R.; Fructos, M.R.; Müller-Bunz, H.; Gossage, R.A.; Perez, P.J.; Albrecht, M. Synthesis and catalytic applications of 1,2,3-triazolylidene gold(I) complexes in silver-free oxazoline syntheses and C-H bond activation. Dalton Trans. 2016. [Google Scholar] [CrossRef] [PubMed]
- Petronilho, A.; Rahman, M.; Woods, J.A.; Al-Sayyed, H.; Müller-Bunz, H.; Don MacElroy, J.M.; Bernhard, S.; Albrecht, M. Photolytic water oxidation catalyzed by a molecular carbene iridium complex. Dalton Trans. 2012, 41, 13074–13080. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, S.; English, N.J.; MacElroy, J.M.D. Density functional theory calculations of catalytic mechanistic pathways for the formation of O2 involving triazolylidene iridium complexes. New J. Chem. 2014, 38, 4060–4070. [Google Scholar] [CrossRef]
- Petronilho, A.; Woods, J.A.; Bernhard, S.; Albrecht, M. Bimetallic iridium-carbene complexes with mesoionic triazolylidene ligands for water oxidation catalysis. Eur. J. Inorg. Chem. 2014, 2014, 708–714. [Google Scholar] [CrossRef]
- Woods, J.A.; Lalrempuia, R.; Petronilho, A.; McDaniel, N.D.; Müller-Bunz, H.; Albrecht, M.; Bernhard, S. Carbene iridium complexes for efficient water oxidation: Scope and mechanistic insights. Energy Environ. Sci. 2014, 7, 2316–2328. [Google Scholar] [CrossRef]
- Hohloch, S.; Suntrup, L.; Sarkar, B. Arene–ruthenium(II) and −iridium(III) complexes with “click”-based pyridyl-triazoles, bis-triazoles, and chelating abnormal carbenes: Applications in catalytic transfer hydrogenation of nitrobenzene. Organometallics 2013, 32, 7376–7385. [Google Scholar] [CrossRef]
- Hohloch, S.; Hettmanczyk, L.; Sarkar, B. Introducing potential hemilability into “click” triazoles and triazolylidenes: Synthesis and characterization of d6-metal complexes and oxidation catalysis. Eur. J. Inorg. Chem. 2014, 3164–3171. [Google Scholar] [CrossRef]
- Maity, R.; Hohloch, S.; Su, C.Y.; van der Meer, M.; Sarkar, B. Cyclometalated mono- and dinuclear Ir(III) complexes with “click”-derived triazoles and mesoionic carbenes. Chem. Eur. J. 2014, 20, 9952–9961. [Google Scholar] [CrossRef] [PubMed]
- Bolje, A.; Hohloch, S.; van der Meer, M.; Kosmrlj, J.; Sarkar, B. Ru(II), Os(II), and Ir(III) complexes with chelating pyridyl-mesoionic carbene ligands: Structural characterization and applications in transfer hydrogenation catalysis. Chem. Eur. J. 2015, 21, 6756–6764. [Google Scholar] [CrossRef] [PubMed]
- Bolje, A.; Hohloch, S.; Kosmrlj, J.; Sarkar, B. RuII, IrIII and OsII mesoionic carbene complexes: Efficient catalysts for transfer hydrogenation of selected functionalities. Dalton Trans. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hohloch, S.; Kaiser, S.; Dücker, F.L.; Bolje, A.; Maity, R.; Kosmrlj, J.; Sarkar, B. Catalytic oxygenation of sp3 “C-H” bonds with Ir(III) complexes of chelating triazoles and mesoionic carbenes. Dalton Trans. 2015, 44, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Bernet, L.; Lalrempuia, R.; Ghattas, W.; Müller-Bunz, H.; Vigara, L.; Llobet, A.; Albrecht, M. Tunable single-site ruthenium catalysts for efficient water oxidation. Chem. Commun. 2011, 47, 8058–8060. [Google Scholar] [CrossRef] [PubMed]
- Prades, A.; Peris, E.; Albrecht, M. Oxidations and oxidative couplings catalyzed by triazolylidene ruthenium complexes. Organometallics 2011, 30, 1162–1167. [Google Scholar] [CrossRef]
- Canseco-Gonzalez, D.; Albrecht, M. Wingtip substituents tailor the catalytic activity of ruthenium triazolylidene complexes in base-free alcohol oxidation. Dalton Trans. 2013, 42, 7424–7432. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Inomata, S.; Fukuzawa, S.-I. Position-selective intramolecular aromatic C-H bond activation of 1,2,3-triazol-5-ylidene (tzNHC) ligands in (p-cymene)ruthenium(II) complexes. Dalton Trans. 2013, 42, 2362–2365. [Google Scholar] [CrossRef] [PubMed]
- Bagh, B.; McKinty, A.M.; Lough, A.J.; Stephan, D.W. 1,2,3-Triazolylidene ruthenium(II)(eta(6)-arene) complexes: Synthesis, metallation and reactivity. Dalton Trans. 2014, 43, 12807–13146. [Google Scholar] [CrossRef] [PubMed]
- Bolje, A.; Hohloch, S.; Urankar, D.; Pevec, A.; Gazvoda, M.; Sarkar, B.; Košmrlj, J. Exploring the scope of pyridyl- and picolyl-functionalized 1,2,3-triazol-5-ylidenes in bidentate coordination to ruthenium(II) cymene chloride complexes. Organometallics 2014, 33, 2588–2598. [Google Scholar] [CrossRef]
- Delgado-Rebollo, M.; Canseco-Gonzalez, D.; Hollering, M.; Müller-Bunz, H.; Albrecht, M. Synthesis and catalytic alcohol oxidation and ketone transfer hydrogenation activity of donor-functionalized mesoionic triazolylidene ruthenium(II) complexes. Dalton Trans. 2014, 43, 4462–4473. [Google Scholar] [CrossRef] [PubMed]
- Sinn, S.; Schulze, B.; Friebe, C.; Brown, D.G.; Jager, M.; Altuntas, E.; Kubel, J.; Guntner, O.; Berlinguette, C.P.; Dietzek, B.; Schubert, U.S. Physicochemical analysis of ruthenium(II) sensitizers of 1,2,3-triazole-derived mesoionic carbene and cyclometalating ligands. Inorg. Chem. 2014, 53, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Leigh, V.; Ghattas, W.; Lalrempuia, R.; Müller-Bunz, H.; Pryce, M.T.; Albrecht, M. Synthesis, photo-, and electrochemistry of ruthenium bis(bipyridine) complexes comprising a N-heterocyclic carbene ligand. Inorg. Chem. 2013, 52, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Sinn, S.; Schulze, B.; Friebe, C.; Brown, D.G.; Jager, M.; Kubel, J.; Dietzek, B.; Berlinguette, C.P.; Schubert, U.S. A heteroleptic bis(tridentate) ruthenium(II) platform featuring an anionic 1,2,3-triazolate-based ligand for application in the dye-sensitized solar cell. Inorg. Chem. 2014, 53, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kjaer, K.S.; Fredin, L.A.; Chabera, P.; Harlang, T.; Canton, S.E.; Lidin, S.; Zhang, J.; Lomoth, R.; Bergquist, K.E.; et al. A heteroleptic ferrous complex with mesoionic bis(1,2,3-triazol-5-ylidene) ligands: Taming the MLCT excited state of iron(II). Chem. Eur. J. 2015, 21, 3628–3639. [Google Scholar] [CrossRef] [PubMed]
- Naziruddin, A.R.; Lee, C.S.; Lin, W.J.; Sun, B.J.; Chao, K.H.; Chang, A.H.; Hwang, W.S. Platinum complexes bearing normal and mesoionic N-heterocyclic carbene based pincer ligands: Syntheses, structures, and photo-functional attributes. Dalton Trans. 2016, 45, 5848–5859. [Google Scholar] [CrossRef] [PubMed]
- Soellner, J.; Tenne, M.; Wagenblast, G.; Strassner, T. Phosphorescent platinum(II) complexes with mesoionic 1H-1,2,3-triazolylidene ligands. Chem. Eur.J. 2016, 22, 9914–9918. [Google Scholar] [CrossRef] [PubMed]
- Maity, R.; van der Meer, M.; Sarkar, B. Redox-active multinuclear Pd(II) complexes with bis- and tris-mesoionic carbenes. Dalton Trans. 2015, 44, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Poulain, A.; Canseco-Gonzalez, D.; Hynes-Roche, R.; Müller-Bunz, H.; Schuster, O.; Stoeckli-Evans, H.; Neels, A.; Albrecht, M. Synthesis and tunability of abnormal 1,2,3-triazolylidene palladium and rhodium complexes. Organometallics 2011, 30, 1021–1029. [Google Scholar] [CrossRef]
- Keske, E.C.; Zenkina, O.V.; Wang, R.; Crudden, C.M. Synthesis and structure of palladium 1,2,3-triazol-5-ylidene mesoionic carbene PEPPSI complexes and their catalytic applications in the Mizoroki–Heck reaction. Organometallics 2012, 31, 6215–6221. [Google Scholar] [CrossRef]
- Nakamura, T.; Ogata, K.; Fukuzawa, S.-I. Synthesis of dichlorobis(1,4-dimesityl-1H-1,2,3-triazol-5-ylidene)palladium [PdCl2(TMes)2] and its application to Suzuki–Miyaura coupling reaction. Chem. Lett. 2010, 39, 920–922. [Google Scholar] [CrossRef]
- Karthikeyan, T.; Sankararaman, S. Palladium complexes with abnormal N-heterocyclic carbene ligands derived from 1,2,3-triazolium ions and their application in Suzuki coupling. Tetrahedron Lett. 2009, 50, 5834–5837. [Google Scholar] [CrossRef]
- Canseco-Gonzalez, D.; Gniewek, A.; Szulmanowicz, M.; Müller-Bunz, H.; Trzeciak, A.M.; Albrecht, M. PEPPSI-type palladium complexes containing basic 1,2,3-triazolylidene ligands and their role in Suzuki-Miyaura catalysis. Chem. Eur. J. 2012, 18, 6055–6062. [Google Scholar] [CrossRef] [PubMed]
- Terashima, T.; Inomata, S.; Ogata, K.; Fukuzawa, S.-I. Synthetic, structural, and catalytic studies of well-defined allyl 1,2,3-triazol-5-ylidene (tzNHC) palladium complexes. Eur. J. Inorg. Chem. 2012, 2012, 1387–1393. [Google Scholar] [CrossRef]
- Hohloch, S.; Frey, W.; Su, C.Y.; Sarkar, B. Abnormal carbenes derived from the 1,5-cycloaddition product between azides and alkynes: structural characterization of Pd(II) complexes and their catalytic properties. Dalton Trans. 2013, 42, 11355–11358. [Google Scholar] [CrossRef] [PubMed]
- Shaik, J.B.; Ramkumar, V.; Varghese, B.; Sankararaman, S. Synthesis and structure of trans-bis(1,4-dimesityl-3-methyl-1,2,3-triazol-5-ylidene)palladium(II) dichloride and diacetate. Suzuki-Miyaura coupling of polybromoarenes with high catalytic turnover efficiencies. Beilstein J. Org. Chem. 2013, 9, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Guchhait, S.; Ghosh, K.; Sureshbabu, B.; Ramkumar, V.; Sankararaman, S. C2-Symmetric normal and mesoionic bis-N-heterocyclic carbenes with biphenyl backbone. A comparison of bis(1,2,3-triazol-5-ylidene) and bis(imidazol-2-ylidene) ligands. J. Organomet. Chem. 2014, 768, 68–74. [Google Scholar] [CrossRef]
- Maity, R.; Mekic, A.; van der Meer, M.; Verma, A.; Sarkar, B. Triply cyclometalated trinuclear iridium(III) and trinuclear palladium(II) complexes with a tri-mesoionic carbene ligand. Chem. Commun. 2015, 51, 15106–15109. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Huynh, H.V. Hetero-dicarbene complexes of palladium(II): Syntheses and catalytic activities. Organometallics 2014, 33, 6033–6043. [Google Scholar] [CrossRef]
- Mitsui, T.; Sugihara, M.; Tokoro, Y.; Fukuzawa, S.-I. Synthesis of adamantyl substituted 1,2,3-triazol-5-ylidene ligands and their PEPPSI-type palladium complexes. Tetrahedron 2015, 71, 1509–1514. [Google Scholar] [CrossRef]
- Modak, S.; Gangwar, M.K.; Nageswar Rao, M.; Madasu, M.; Kalita, A.; Dorcet, V.; Shejale, M.A.; Butcher, R.J.; Ghosh, P. Fluoride-free Hiyama coupling by palladium abnormal N-heterocyclic carbene complexes. Dalton Trans. 2015, 44, 17617–17628. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Espinosa, D.; González-Olvera, R.; Osornio, C.; Negrón-Silva, G.E.; Álvarez-Hernández, A.; Bautista-Hernández, C.I.; Suárez-Castillo, O.R. Structural diversity of phenoxy functionalized triazol-5-ylidene palladium(II) complexes and their application in C–N bond formation. J. Organomet. Chem. 2016, 803, 142–149. [Google Scholar] [CrossRef]
- Maity, R.; Verma, A.; van der Meer, M.; Hohloch, S.; Sarkar, B. Palladium complexes bearing mesoionic carbene ligands: Applications in α-arylation, α-methylation and Suzuki-Miyaura coupling reactions. Eur. J. Inorg. Chem. 2016, 111–117. [Google Scholar] [CrossRef]
- Mohan, A.; Ramkumar, V.; Sankararaman, S. Synthesis and structures of (−) menthyl and (+) neomenthyl substituted enantio pure bis(1,2,3-triazol-5-ylidene)PdI2 complexes and PEPPSI type (1,2,3-triazol-5-ylidene) (pyridine)PdI2complexes. Comparison of catalytic activities for C–C coupling. J. Organomet. Chem. 2015, 799–800, 115–121. [Google Scholar] [CrossRef]
- Huang, J.; Hong, J.-T.; Hong, S.H. Suzuki–Miyaura cross-coupling reaction catalyzed by PEPPSI-type 1,4-di(2,6-diisopropylphenyl)-1,2,3-triazol-5-ylidene (tzIPr) palladium complex. Eur. J. Org. Chem. 2012, 6630–6635. [Google Scholar] [CrossRef]
- Sureshbabu, B.; Ramkumar, V.; Sankararaman, S. A mild and efficient method for the synthesis of structurally diverse 1,2,3-triazolylidene palladium(II) diiodo complexes. Comparison of catalytic activities for Suzuki–Miyaura coupling. J. Organomet. Chem. 2015, 799–800, 232–238. [Google Scholar] [CrossRef]
- Navarro, O.; Marion, N.; Scott, N.M.; González, J.; Amoroso, D.; Bell, A.; Nolan, S.P. Synthesis of novel (NHC)Pd(acac)Cl complexes (acac = acetylacetonate) and their activity in cross-coupling reactions. Tetrahedron 2005, 61, 9716–9722. [Google Scholar] [CrossRef]
- Marion, N.; Ecarnot, E.C.; Navarro, O.; Amoroso, D.; Bell, A.; Nolan, S.P. (IPr)Pd(acac)Cl: An easily synthesized, efficient, and versatile precatalyst for C-N and C-C bond formation. J. Org. Chem. 2006, 71, 3816–3821. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.; de Frémont, P.; Puijk, I.M.; Ecarnot, E.C.; Amoroso, D.; Bell, A.; Nolan, S.P. N-heterocyclic carbene–palladium complexes [(NHC)Pd(acac)Cl]: Improved synthesis and catalytic activity in large-scale cross-coupling reactions. Adv. Synth. Catal. 2007, 349, 2380–2384. [Google Scholar] [CrossRef]
- Klein, J.E.M.N.; Holzwarth, M.S.; Hohloch, S.; Sarkar, B.; Plietker, B. Redox-active triazolium-derived ligands in nucleophilic Fe-catalysis–reactivity profile and development of a regioselective O-allylation. Eur. J. Org. Chem. 2013, 6310–6316. [Google Scholar] [CrossRef]
- Chu, Y.; Deng, H.; Cheng, J.-P. An acidity scale of 1,3-dialkylimidazolium salts in dimethyl sulfoxide solution. J. Org. Chem. 2007, 72, 7790–7793. [Google Scholar] [CrossRef] [PubMed]
- Higgins, E.M.; Sherwood, J.A.; Lindsay, A.G.; Armstrong, J.; Massey, R.S.; Alder, R.W.; O’Donoghue, A.C. pKas of the conjugate acids of N-heterocyclic carbenes in water. Chem. Commun. 2011, 47, 1559–1561. [Google Scholar] [CrossRef] [PubMed]
- Dröge, T.; Glorius, F. The measure of all rings—N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2010, 49, 6940–6952. [Google Scholar] [CrossRef] [PubMed]
- Bouffard, J.; Keitz, B.K.; Tonner, R.; Lavallo, V.; Guisado-Barrios, G.; Frenking, G.; Grubbs, R.H.; Bertrand, G. Synthesis of highly sTable 1,3-diaryl-1H-1,2,3-triazol-5-ylidenes and their applications in ruthenium-catalyzed olefin metathesis. Organometallics 2011, 30, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Heckenroth, M.; Kluser, E.; Neels, A.; Albrecht, M. Palladation of diimidazolium salts at the C4 position: access to remarkably electron-rich palladium(II) centers. Dalton Trans. 2008, 6242–6249. [Google Scholar] [CrossRef] [PubMed]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.; Nolan, S.P. Well-defined N-heterocyclic carbenes–palladium(II) precatalysts for cross-coupling reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXS-97; Program for Crystal Structure Solution and Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- SAINT+. Data Integration Engine, Version 8.27b©, Bruker AXS Inc.: Madison, WI, USA, 1997–2012.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Bruker. APEX2, Bruker AXS Inc.: Madison, WI, USA, 2012.
- Sheldrick, G.M. SHELXL Version 2014/7, Program for crystal structure solution and refinement; University of Göttingen: Göttingen, Germany, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Sheldrick, G.M. SADABS, Ver. 2008/1; Program for Empirical Absorption Correction; University of Göttingen: Göttingen, Germany, 2008. [Google Scholar]
- Sample Availability: Samples of the compounds 1–3 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).