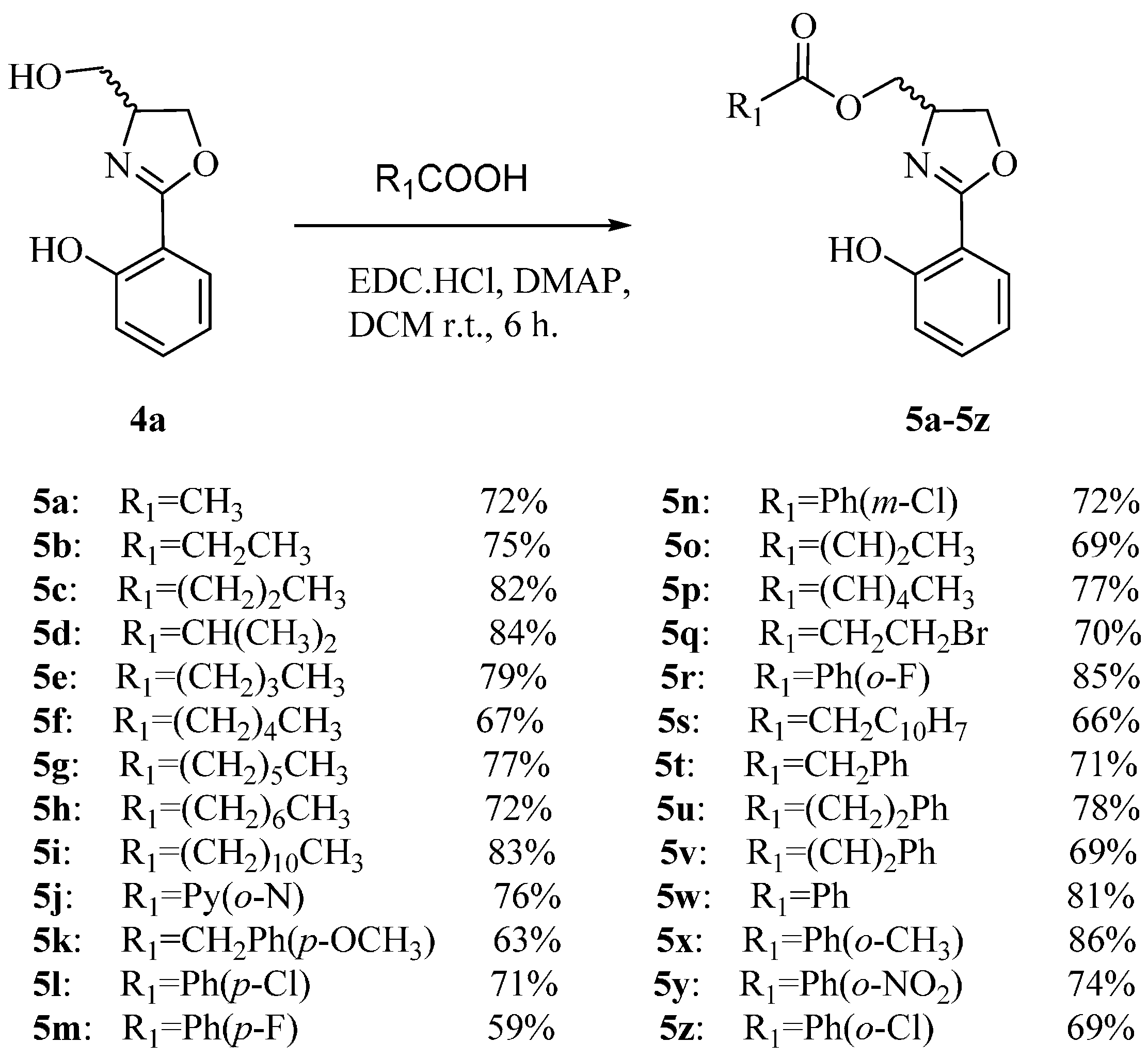General unless otherwise noted, all reagents and solvents were purchased from commercial suppliers and used without further purification, which were of analytical reagent (AR) grade. TLC was performed on GF254 silica gel plates (Qingdao Haiyang Co., Ltd., Qingdao, Shandong, China). Column chromatography was carried out with silica gel (Qingdao Haiyang Co., Ltd.); all compounds were eluted with petroleum ether and ethyl acetate in sequence. Melting point (m.p.) was determined on a Yanagimoto apparatus (uncorrected). 1H-NMR and 13C-NMR spectra were performed on a Bruker-Avance-500 spectrometer (Bruker Daltonics Inc., Bremen, Germany) with DMSO-d6 or CDCl3 as solvent and SiMe4 (tetramethylsilane) as the internal standard. MS were recorded on an electrospray ionization (ESI) conditions by using a Thermo LCQ Fleet instrument (Thermo Fisher Scientific Co., Waltham, MA, USA). HR-MS were recorded on an Agilent 1290-6224 instrument (Agilent, Santa Clara, CA, USA).
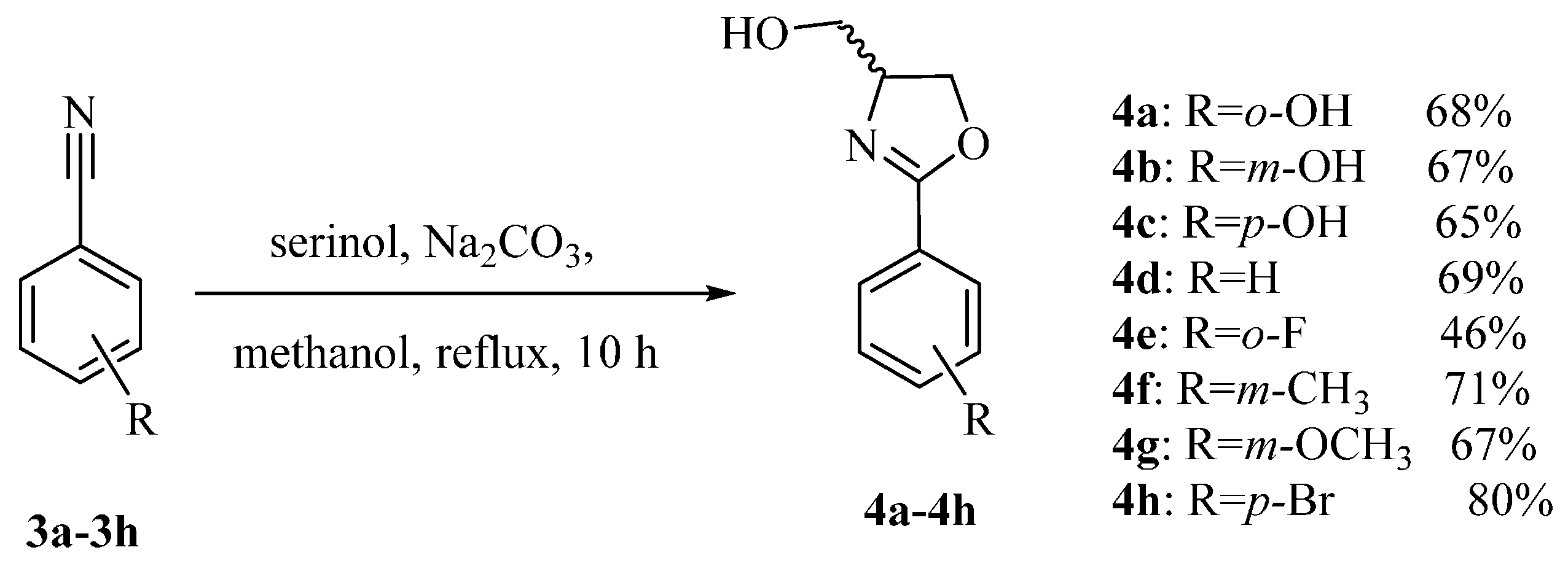
3.1.1. Synthesis of 4a–4h
The solution of substituted benzonitrile (100 mmol), serinol (54.660 g, 600 mmol), and Na2CO3 (10.599 g, 100 mmol) in anhydrous methanol (100 mL) was heated to reflux for 10 h. After the reaction solution was cooled to room temperature, the solvent was removed under vacuum, the resulting residue was then diluted with anhydrous CH2Cl2 (30 mL). The organic fractions were successively washed with an aqueous saturated solution of NH4Cl (30 mL × 2), brine (30 mL × 3), and dried over anhydrous Na2SO4. The solvent was got rid of under vacuum to afford a scarlet residue which was purified by flash column chromatography using petroleum ether/ethyl acetate (8:1, v/v) as the eluent.
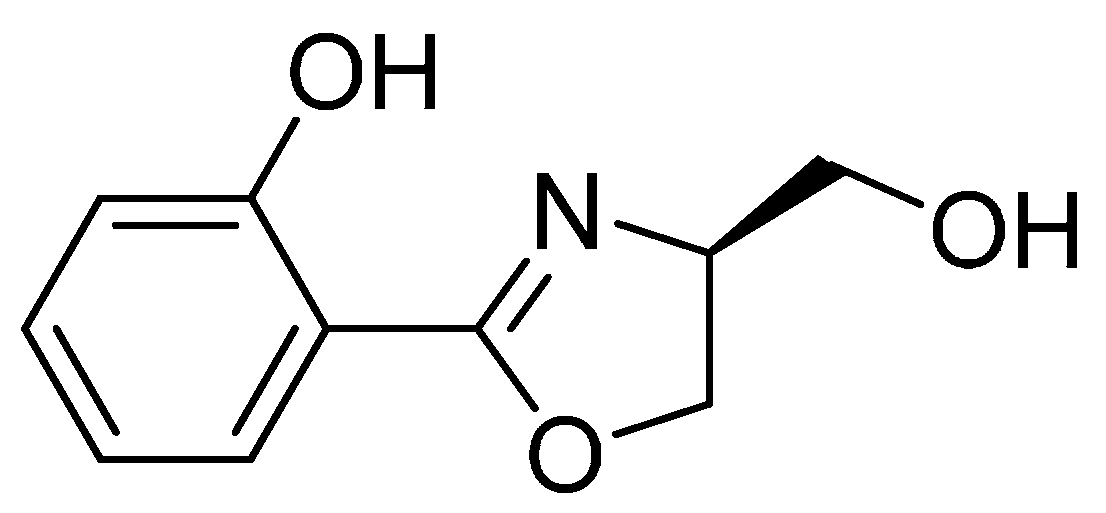
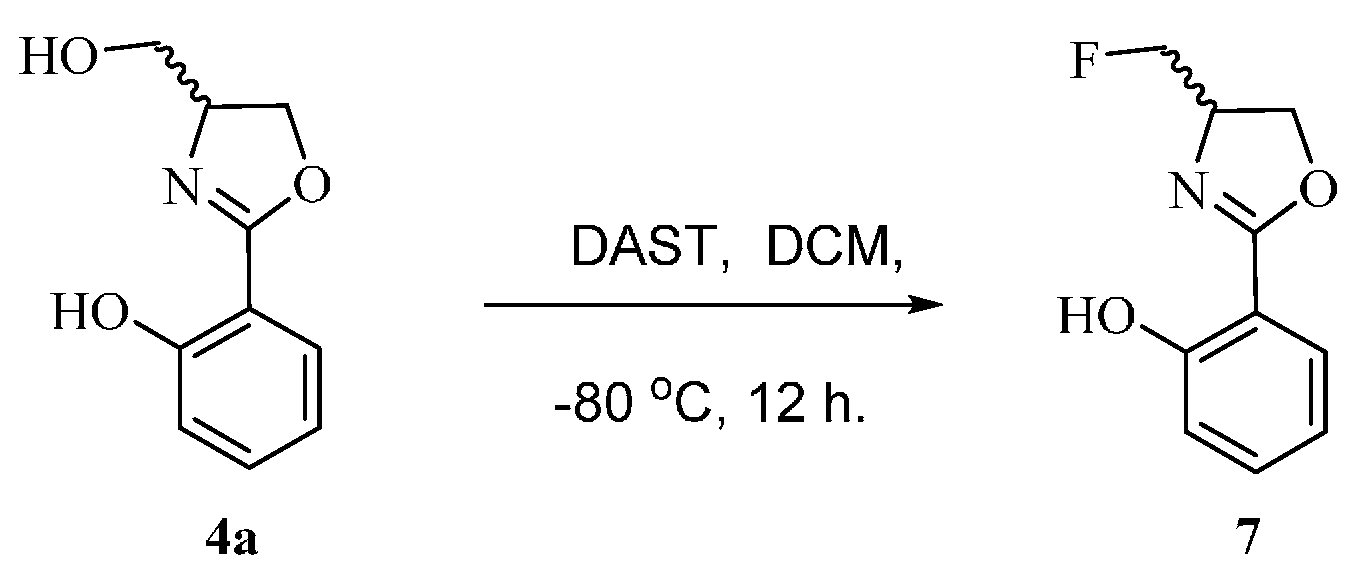
2-(4-(Hydroxymethyl)-4,5-dihydrooxazol-2-yl)phenol (4a) Yield: 68%–78%; white solids; m.p. 78–80 °C; ESI-MS m/z 194.07 [M + H]+; HR-MS m/z: Calcd for C10H12NO3: 194.0817 [M + H]+; found 194.0803 [M + H]+; 1H-NMR (CDCl3, 500 MHz) δ 7.66 (dd, J = 1.5, 7.5 Hz, 1H), 7.39 (m, 1H), 7.01 (d, J = 8.0 Hz, 1H), 6.89 (m, 1H), 4.50 (m, 2H), 4.36 (t, J = 6.0 Hz, 1H), 3.89 (dd, J = 3.5, 11.5 Hz, 1H), 3.71 (dd, J = 3.5, 11.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.93, 159.78, 133.68, 128.22, 118.81, 116.75, 110.40, 68.58, 66.87, 63.95.
3-(4-(Hydroxymethyl)-4,5-dihydrooxazol-2-yl)phenol (4b) Yield: 67%; white solids; m.p. 91–93 °C; ESI-MS m/z 194.17 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.48 (m, 1H), 7.44 (m, 1H), 7.28 (m, 1H), 7.03 (m, 1H), 4.52 (dd, J = 3.5, 9.5 Hz, 1H), 4.42 (m, 1H), 4.36 (t, J = 7.5 Hz, 1H), 3.95 (dd, J = 3.5, 11.5 Hz, 1H), 3.67 (m, 1H); 13C-NMR (125 MHz, CDCl3) δ 165.41, 159.86, 128.98, 127.49, 120.81, 118.42, 113.19, 69.27, 68.11, 64.58.
4-(4-(Hydroxymethyl)-4,5-dihydrooxazol-2-yl)phenol (4c) Yield: 65%; white solids; m.p. 192–194 °C; ESI-MS m/z 194.02 [M + H]+; 1H-NMR (500 MHz, DMSO-d6) δ 10.03 (br.s., 1H), 7.69 (d, J = 8.8 Hz, 2H), 6.81 (d, J = 8.5 Hz, 2H), 4.79 (t, J = 5.5 Hz, 1H), 4.37 (t, J = 8.2 Hz, 1H), 4.19 (t, J = 6.0 Hz, 1H), 3.62 (m, 1H), 3.41 (td, J = 5.5, 10.8 Hz, 1H); 13C-NMR (125 MHz, DMSO-d6) δ 163.25, 160.66, 130.17, 118.3, 115.65, 69.95, 68.43, 63.74.
(2-Phenyl-4,5-dihydrooxazol-4-yl)methanol (4d) Yield: 69%; white solids; m.p. 82–84 °C; ESI-MS m/z 178.12 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.98 (m, 2H), 7.47 (m, 1H), 7.38 (m, 2H), 4.43 (m, 1H), 4.34 (m, 1H), 4.49 (m, 1H), 3.98 (dd, J = 3.5, 11.5 Hz, 1H), 3.67 (dd, J = 4.0, 11.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 165.61, 131.57, 128.37, 128.31, 127.21, 69.29, 68.08, 63.94.
(2-(2-Fluorophenyl)-4,5-dihydrooxazol-4-yl)methanol (4e) Yield: 46%; yellow oil; ESI-MS m/z 196.16 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.88 (td, J = 1.5, 7.5 Hz, 1H), 7.47 (m, 1H), 7.19 (m, 2H), 4.50 (m, 2H), 4.34 (m, 1H), 3.85 (dd, J = 3.0, 11.5 Hz, 1H), 3.71 (dd, J = 4.0, 11.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 162.28, 162.21, 162.17, 160.22, 133.16, 133.09, 131.19, 124.01, 123.98, 116.80, 116.63, 69.06, 68.42, 64.16.
(2-(m-Tolyl)-4,5-dihydrooxazol-4-yl)methanol (4f) Yield: 71%; white solids; m.p. 146–148 °C; ESI-MS m/z 192.12 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.71 (s, 1H), 7.68 (m, 2H), 7.28 (m, 2H), 4.49 (dd, J = 7.5, 9.5 Hz, 1H), 4.44 (m, 1H), 4.34 (m, 1H), 3.96 (dd, J = 3.5, 11.5 Hz, 1H), 3.68 (dd, J = 4.0, 11.5 Hz, 1H), 2.36 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 165.73, 138.05, 132.31, 128.92, 128.23, 127.16, 125.44, 69.26, 68.07, 64.06, 21.23.
(2-(3-Methoxyphenyl)-4,5-dihydrooxazol-4-yl)methanol (4g) Yield: 67%; white solids; m.p. 123–125 °C; ESI-MS m/z 208.03 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.49 (m, 1H), 7.42 (m, 1H), 7.29 (m, 1H), 7.02 (m, 1H), 4.50 (dd, J = 3.5, 9.5 Hz, 1H), 4.44 (m, 1H), 4.35 (t, J = 7.5 Hz, 1H), 3.97 (dd, J = 3.5, 11.5 Hz, 1H), 3.83 (s, 3H), 3.68 (m, 1H); 13C-NMR (125 MHz, CDCl3) δ 165.47, 159.46, 129.38, 128.53, 120.83, 118.34, 112.69, 69.34, 68.12, 64.03, 55.40.
(2-(4-Bromophenyl)-4,5-dihydrooxazol-4-yl)methanol (4h) Yield: 80%; yellow oil; ESI-MS m/z 255.01:257.13 (1:1), [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.74 (m, 2H), 7.51 (m, 2H), 4.51 (dd, J = 7.5, 9.5 Hz, 1H), 4.41 (m, 1H), 4.36 (m, 1H), 3.98 (dd, J = 3.5, 11.5 Hz, 1H), 3.68 (dd, J = 4.0, 11.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 175.12, 164.74, 131.59, 129.85, 126.30, 126.21, 69.44, 68.19, 63.85.
3.1.2. Synthesis of 5a–5z
A solution of compound 4a (193 mg, 1.0 mmol), carboxylic acid (1.2 mmol), EDC·HCl (288 mg, 1.5 mmol), and DMAP (6 mg, 5%) in anhydrous CH2Cl2 (8 mL) is stirred at room temperature for 6 h. Then, the reaction solution was quenched with saturated aqueous NaHCO3 (50 mL), concentrated under reduced pressure until approximately 30 mL remained. The organic layer was separated with CH2Cl2 (30 mL × 3) and washed sequentially with water (30 mL × 2), brine (30 mL × 3), and dried over anhydrous Na2SO4. The solvent is concentrated under reduced pressure, and purified by flash column chromatography using petroleum ether/ethyl acetate (10:1, v/v) as the eluent.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl acetate (5a) Yield: 72%; yellow oil; ESI-MS m/z 236.12 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 2.0, 9.0 Hz, 1H), 7.41 (m, 1H), 7.02 (dd, J = 1.0, 8.5 Hz, 1H), 6.90 (m, 1H), 4.65 (m, 1H), 4.51 (dd, J = 8.5, 9.5 Hz, 1H), 4.31 (m, 2H), 4.21 (dd, J = 5.5, 21.5 Hz, 1H), 2.07 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 170.83, 166.74, 159.95, 133.75, 128.18, 118.72, 116.88, 110.29, 69.14, 65.43, 64.21, 20.78.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl propionate (5b) Yield: 75%; orange oil; ESI-MS m/z 250.17 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 1.5, 8.0 Hz, 1H), 7.40(m, 1H), 7.02(dd, J = 1.0, 7.5 Hz, 1H), 6.89 (m, 1H), 4.65 (m, 1H), 4.51 (dd, J = 1.5, 8.0 Hz, 1H), 4.32 (m, 2H), 4.23 (dd, J = 5.5, 11.0 Hz, 1H), 2.34 (q, J = 7.5 Hz, 2H), 1.12 (m, 3H); 13C-NMR (125 MHz, CDCl3) δ 174.25, 166.71, 159.95, 133.72, 128.16, 118.71, 116.87, 110.31, 69.13, 65.25, 64.27, 27.44, 9.05.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl butyrate (5c) Yield: 82%; yellow oil; ESI-MS m/z 264.09 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.88 (s, 1H, Ar-OH), 7.66 (dd, J = 2.0, 8.0 Hz, 1H), 7.40 (m, 1H), 7.02 (dd, J = 1.0, 8.5 Hz, 1H), 6.89 (m, 1H), 4.65 (m, 1H), 4.50 (dd, J = 9.0, 10.0 Hz, 1H), 4.31 (m, 2H), 4.24 (dd, J = 4.5, 9.5 Hz, 1H), 2.29 (t, J = 7.0 Hz, 2H), 1.66 (m, 2H), 0.91 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 173.47, 166.71, 159.96, 133.74, 128.17, 118.73, 116.87, 110.32, 69.13, 65.25, 64.27, 36.13, 18.45, 13.60.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl isobutyrate (5d) Yield: 84%; yellow oil; ESI-MS m/z 264.13 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 2.0, 8.0 Hz, 1H), 7.40 (m, 1H), 7.01(dd, J = 0.5, 8.5 Hz, 1H), 6.89 (m, 1H), 4.65 (m, 1H), 4.50 (m, 1H), 4.30 (m, 1H), 4.27 (m, 1H), 2.59 (m, 1H), 1.13 (t, J = 8.0 Hz, 6H); 13C-NMR (125 MHz, CDCl3) δ 176.85, 166.70, 159.95, 133.71, 128.14, 118.70, 116.86, 110.30, 69.06, 65.08, 64.32, 33.93, 18.91, 18.87, 1.03.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl pentanoate (5e) Yield: 79%; orange oil; ESI-MS m/z 278.09 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.60 (br. s, 1H, Ar-OH), 7.65 (d, J = 7.5 Hz, 1H), 7.39 (t, J = 7.5 Hz, 1H), 7.02 (d, J = 8.0 Hz, 1H), 6.88 (t, J = 7.0 Hz, 1H), 4.62 (m, 1H), 4.48 (t, J = 9.0 Hz, 1H), 4.27 (m, 3H), 2.32 (m, 2H), 1.57 (m, 2H), 1.32 (m, 2H), 0.86 (t, J = 6.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 173.66, 166.70, 159.94, 133.71, 128.15, 118.71, 116.86, 110.30, 69.09, 65.14, 64.27, 33.88, 26.98, 22.19, 13.63.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl hexanoate (5f) Yield: 67%; orange oil; ESI-MS m/z 292.17 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.88(br. s, 1H, Ar-OH), 7.66 (dd, J = 2.0, 8.0 Hz, 1H), 7.40 (m, 1H), 7.02 (dd, J = 1.0, 8.5 Hz, 1H), 6.89 (m, 1H), 4.63 (m, 1H), 4.50 (dd, J = 9.0, 10.0 Hz, 1H), 4.30 (m, 3H), 2.30 (t, J = 7.5 Hz, 2H), 1.61 (m, 2H), 1.29(m, 4H), 0.85 (t, J = 7.0 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 173.67, 166.70, 159.94, 133.70, 128.15, 118.71, 116.86, 110.32, 69.10, 65.14, 64.29, 34.14, 31.22, 24.60, 22.25, 13.84.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl heptanoate (5g) Yield: 77%; pale yellow oil; ESI-MS m/z 306.13 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.87 (br. s, 1H, Ar-OH), 7.66 (d, J = 7.0 Hz, 1H), 7.38 (t, J = 6.5 Hz, 1H), 7.02 (d, J = 9.0 Hz, 1H), 6.88 (t, J = 6.5 Hz, 1H), 4.61 (m, 1H), 4.48 (t, J = 8.0 Hz, 1H), 4.28 (m, 3H), 2.30 (t, J = 6.5 Hz, 2H), 1.57 (t, J = 6.5 Hz, 2H), 1.25 (m, 6H), 0.86 (t, J = 6.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 173.65, 166.70, 159.95, 133.71, 128.15, 118.70, 116.86, 110.30, 69.10, 65.12, 64.28, 34.18, 31.39, 28.75, 24.88, 22.42, 14.01.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl octanoate (5h) Yield: 72%; yellow oil; ESI-MS m/z 320.17 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.84 (br. s, 1H, Ar-OH), 7.66 (dd, J = 1.5, 9.0 Hz, 1H), 7.40 (m, 1H), 7.02 (dd, J = 1.0, 8.5 Hz, 1H), 6.89 (m, 1H), 4.64 (m, 1H), 4.50 (dd, J = 8.5, 9.5 Hz, 1H), 4.30 (m, 3H), 2.30 (t, J = 6.5 Hz, 2H), 1.60 (m, 2H), 1.29 (m, 8H), 0.87 (t, J = 7.0 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 173.65, 166.69, 159.94, 133.71, 128.15, 118.70, 116.86, 110.31, 69.10, 65.12, 64.28, 34.18, 31.60, 29.04, 28.87, 24.92, 22.59, 14.06.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl dodecanoate (5i) Yield: 83%; pale yellow oil; ESI-MS m/z 376.22 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 1.5, 9.0 Hz, 1H), 7.40 (m, 1H), 7.02 (dd, J = 0.5, 8.5 Hz, 1H), 6.89 (m, 1H), 4.64 (m, 1H), 4.50 (dd, J = 8.5, 9.5 Hz, 1H), 4.30 (m, 3H), 2.30 (t, J = 6.5 Hz, 2H), 1.60 (m, 2H), 1.31 (m, 16H), 0.88 (t, J = 7.0 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 173.64, 166.71, 159.97, 133.71, 128.16, 118.70, 116.88, 110.31, 69.11, 65.12, 64.29, 34.18, 31.92, 29.60, 29.42, 29.34, 29.22, 29.09, 24.93, 22.69, 14.12.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl isonicotinate (5j) Yield: 76%; white solid; m.p. 111 °C; ESI-MS m/z 299.12 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.83 (br. s, 1H, Ar-OH), 8.76 (dd, J = 1.5, 5.0 Hz, 2H), 7.79 (dd, J = 1.5, 4.5 Hz, 2H), 7.69 (dd, J = 1.5, 8.0 Hz, 1H), 7.42 (m, 1H), 7.02 (dd, J = 0.5, 8.5 Hz, 1H), 6.91 (m, 1H), 4.80 (m, 1H), 4.59 (m, 3H), 4.39 (dd, J = 6.5, 8.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 167.02, 164.89, 159.95, 150.68, 136.79, 133.93, 128.18, 122.83, 118.85, 116.93, 110.12, 68.98, 66.59, 64.18.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl 2-(4-methoxyphenyl)acetate (5k) Yield: 63%; colorless solid; m.p. 105–106 °C; ESI-MS m/z 342.21 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.64 (dd, J = 1.5, 8.0 Hz, 1H), 7.41 (m, 1H), 7.14 (m, 2H), 7.03 (dd, J = 1.0, 8.5 Hz, 1H), 6.90 (m, 1H), 6.78 (m, 2H), 4.61 (m, 1H), 4.43 (dd, J = 8.5, 9.5 Hz, 1H), 4.27 (dd, J = 3.0, 4.5 Hz, 2H), 4.19 (dd, J = 7.5, 8.5 Hz, 1H), 3.76 (s, 3H), 3.55 (s, 2H); 13C-NMR (125 MHz, CDCl3) δ 171.63, 166.70, 159.95, 158.72, 133.70, 130.17, 128.18, 125.62, 118.69, 116.85, 114.00, 110.30, 68.87, 65.43, 64.20, 55.22, 40.39.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl 4-chlorobenzoate (5l) Yield: 71%; white solid; m.p. 106 °C; ESI-MS m/z 332.06:334.11 (3:1), [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.93 (m, 2H), 7.68 (dd, J = 1.5, 7.5 Hz, 1H), 7.42 (m, 3H), 7.02 (dd, J = 0.5, 8.0 Hz, 1H), 6.91 (m, 1H), 4.78 (m, 1H), 4.57 (m, 2H), 4.47 (dd, J = 4.5, 9.5 Hz, 1H), 4.39 (dd, J = 7.0, 8.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.90, 165.48, 159.96, 139.78, 133.84, 131.05, 128.84, 128.16, 128.04, 118.80, 116.91, 110.21, 69.12, 66.11, 64.29.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl 4-fluorobenzoate (5m) Yield: 59%; white solid; m.p. 85–87 °C; ESI-MS m/z 316.12 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 8.02 (m, 2H), 7.68 (dd, J = 1.5, 8.0 Hz, 1H), 7.41 (m, 1H), 7.10 (m, 2H), 7.03 (dd, J = 1.0, 8.5 Hz, 1H), 6.91 (m, 1H), 4.78 (m, 1H), 4.57 (m, 2H), 4.47 (dd, J = 4.5, 9.5 Hz, 1H), 4.39 (dd, J = 7.0, 9.0 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.95, 166.88, 165.36, 164.93, 159.98, 133.82, 132.29, 132.21, 128.17, 125.85, 118.78, 116.91, 115.75, 115.58, 110.24, 69.14, 66.02, 64.33.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl 3-chlorobenzoate (
5n) Yield: 72%; white solid; m.p. 87–89 °C; ESI-MS
m/z 332.11:333.06 (3:1), [M + H]
+;
1H-NMR (500 MHz, CDCl
3) δ 7.79 (m, 2H), 7.70 (dd,
J = 2.0, 8.0 Hz, 1H), 7.40 (m, 1H), 7.36 (m, 1H), 7.30 (m, 1H), 7.01 (dd,
J = 0.5, 8.5 Hz, 1H), 6.90 (m, 1H), 4.78 (m, 1H), 4.56 (m, 2H), 4.44 (m, 2H);
13C-NMR (125 MHz, CDCl
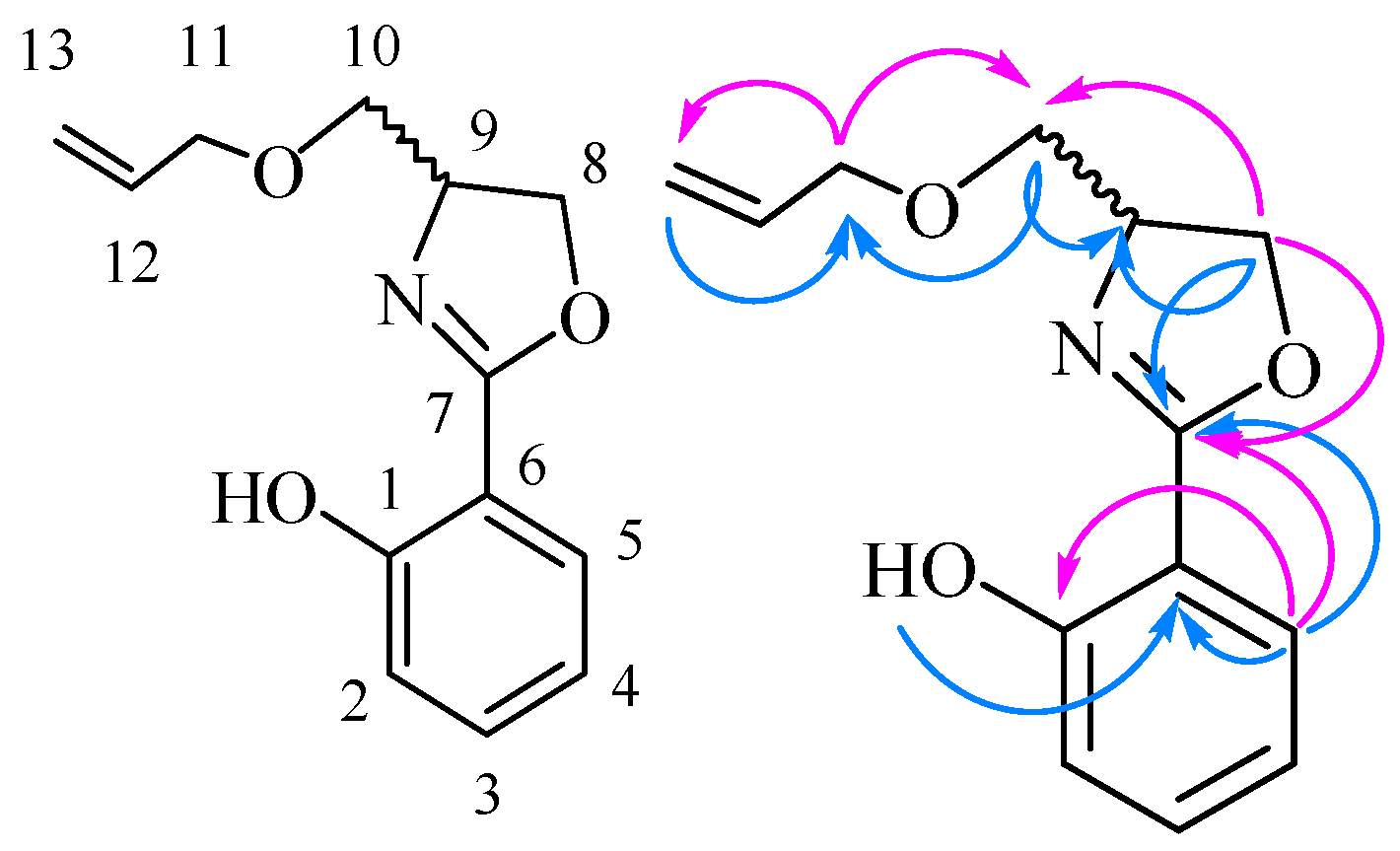
3) δ 166.81, 166.45, 160.29, 138.20, 134.10, 133.72, 130.22, 129.45, 128.37, 128.27, 126.86, 118.83, 116.39, 110.38, 69.27, 65.69, 64.54. The structure of
5n was also confirmed by 2D NMR data and can be seen in
Table 3 and
Figure 2.
Figure 2.
The main HMBC of compound 5n.
Figure 2.
The main HMBC of compound 5n.
Table 3.
HMBC data of compound 5n.
Table 3.
HMBC data of compound 5n.
| 13C-NMR | 1H-NMR |
|---|
| C No. | δC | HMBC Correlations | H No. | δH | HMBC Correlations |
|---|
| 1-C | 159.94, C | 2-H, 3-H, 5-H | Ar-OH | - | - |
| 2-C | 116.89, CH | 3-H, 4-H | 2-H | 7.02, dd, J = 0.5, 9.0 Hz, 1H | 1-C, 4-C, 6-C, 7-C |
| 3-C | 133.83, CH | 4-H, 5-H | 3-H | 7.41–7.38, m, 1H | 1-C, 2-C, 5-C |
| 4-C | 118.81, CH | 2-H | 4-H | 6.90–6.87, m, 1H | 2-C, 3-C, 5-C, 6-C |
| 5-C | 128.20, CH | 3-H, 4-H | 5-H | 7.69, dd, J = 1.5, 8.0 Hz, 1H | 1-C, 3-C, 7-C |
| 6-C | 110.21, C | 2-H, 4-H | 6-H | - | - |
| 7-C | 166.95, C | 2-H, 4-H, 5-H, 8-H, 9-H | 7-H | - | - |
| 8-C | 66.33, CH2 | 9-H, 10-H | 8-H | 4.57–4.54, m, 1H; 4.39, dd, J = 7.0, 9.0 Hz, 1H | 7-C, 9-C, 10-C |
| 9-C | 64.26, CH | 8-H, 10-H | 9-H | 4.78–4.73, m, 1H; | 7-C, 8-C |
| 10-C | 69.19, CH2 | 8-H | 10-H | 4.54–4.52, m, 1H; 4.47, dd, J = 5.5, 11.5 Hz, 1H | 8-C, 9-C, 11-C |
| 11-C | 165.13, C | 10-H, 13-H, 16-H, 17-H | 11-H | - | - |
| 12-C | 134.64, C | 13-H, 16-H, 17-H | 12-H | - | - |
| 13-C | 129.74, CH | 15-H, 16-H, 17-H | 13-H | 7.95, t, J = 1.5 Hz, 1H | 11-C, 12-C, 14-C, 15-C, 17-C |
| 14-C | 131.33, C | 13-H, 15-H, 16-H | 14-H | - | - |
| 15-C | 133.29, CH | 13-H, 16-H, 17-H | 15-H | 7.53, dq, J = 1.5, 8.5 Hz, 1H | 13-C, 14-C, 16-C, 17-C |
| 16-C | 129.80, CH | 15-H, 17-H | 16-H | 7.37, t, J = 8.0 Hz, 1H | 11-C, 12-C, 14-C, 15-C, 17-C |
| 17-C | 127.77, CH | 13-H, 15-H, 16-H | 17-H | 7.87, dt, J = 1.5, 7.5 Hz, 1H | 11-C, 12-C, 13-C, 15-C, 16-C |
(E)-(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl but-2-enoate (5o) Yield: 69%; pale yellow solid; m.p. 106 °C; ESI-MS m/z 262.08 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.89 (br. s, 1H, Ar-OH), 7.66 (dd, J = 1.5, 7.5 Hz, 1H), 7.40 (m, 1H), 7.02 (m, 2H), 6.89 (m, 1H), 5.86 (dq, J = 1.5, 15.5 Hz, 1H), 4.67 (m, 1H), 4.51 (dd, J = 9.0, 10.0 Hz, 1H), 4.38 (dd, J = 4.5, 11.0 Hz, 1H), 4.30 (dd, J = 7.0, 8.5 Hz, 1H), 4.26 (dd, J = 4.5, 9.5 Hz, 1H), 1.87 (dd, J = 2.0, 7.0 Hz, 3H). 13C-NMR (125 MHz, CDCl3) δ 166.69, 166.22, 159.95, 145.77, 133.69, 128.16, 122.02, 118.70, 116.86, 110.34, 69.18, 65.14, 64.32, 18.04.
(2E,4E)-(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl hexa-2,4-dienoate (5p) Yield: 77%; dark green solid; m.p. 67–69 °C; ESI-MS m/z 288.10 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.90 (br. s, 1H, Ar-OH), 7.67 (dd, J = 1.5, 8.0 Hz, 1H), 7.40 (m, 1H), 7.26 (dd, J = 10.5, 15.5 Hz, 1H), 7.02 (dd, J = 1.0, 8.5 Hz, 1H), 6.89 (m, 1H), 6.20 (m, 2H), 5.77 (d, J = 15.5 Hz, 1H), 4.68 (m, 1H), 4.52 (m, 1H), 4.40 (m, 1H), 4.30 (dd, J = 7.0, 8.5 Hz, 1H), 4.26 (dd, J = 4.5, 9.5 Hz, 1H), 1.85 (m, 3H). 13C-NMR (125 MHz, CDCl3) δ 166.99, 166.70, 159.97, 145.97, 140.13, 133.68, 129.65, 128.16, 118.68, 118.03, 116.87, 110.35, 69.25, 65.24, 64.35, 18.67.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl 3-bromopropanoate (5q) Yield: 70%; pale yellow solid; m.p. 113–114 °C; ESI-MS m/z 328.17:330.21 (1:1), [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 1.5, 8.0 Hz, 1H), 7.39 (m, 1H), 7.00 (dd, J = 1.0, 7.5 Hz, 1H), 6.89 (m, 1H), 4.67 (m, 1H), 4.56 (dd, J = 1.5, 8.0 Hz, 1H), 4.32 (m, 2H), 4.20 (dd, J = 5.5, 11.0 Hz, 1H), 3.66 (t, 2H), 2.38 (q, J = 7.5 Hz, 2H); 13C-NMR (125 MHz, CDCl3) δ 174.31, 166.60, 159.88, 133.71, 128.16, 118.71, 116.57, 110.19, 69.03, 65.77, 64.37, 37.49, 27.01.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl 2-fluorobenzoate (5r) Yield: 85%; white solid; m.p. 83–84 °C; ESI-MS m/z 316.07 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.79 (dd, J = 1.5, 8.0 Hz, 1H), 7.67 (dd, J = 1.5, 8.0 Hz, 1H), 7.42 (m, 3H), 7.29 (m, 1H), 7.02 (dd, J = 0.5, 8.0 Hz, 1H), 6.90 (m, 1H), 4.79 (m, 1H), 4.57 (m, 2H), 4.49 (dd, J = 4.5, 9.5 Hz, 1H), 4.42 (dd, J = 7.0, 8.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.92, 165.59, 159.94, 133.79, 133.77, 132.86, 131.67, 131.16, 129.57, 128.18, 126.67, 118.75, 116.88, 110.32, 69.17, 66.37, 64.20.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl 2-(naphthalen-2-yl)acetate (5s) Yield: 66%; white solid; m.p. 80 °C; ESI-MS m/z 362.17 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.94 (m, 1H), 7.83 (dd, J = 2.5, 7.5 Hz, 1H), 7.75 (m, 1H), 7.57 (dd, J = 2.0, 8.0 Hz, 1H), 7.48 (m, 2H), 7.39 (m, 1H), 7.34 (m, 2H), 7.01 (dd, J = 0.5, 8.0 Hz, 1H), 6.89 (m, 1H), 4.53 (m, 1H), 4.29 (dd, J = 4.0, 11.0 Hz, 1H), 4.24 (m, 2H), 4.06 (s, 2H), 3.98 (dd, J = 7.5, 9.0 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 171.30, 166.63, 159.91, 133.75, 133.67, 131.91, 130.09, 128.75, 128.23, 128.16, 127.99, 126.40, 125.79, 125.42, 123.51, 118.64, 116.83, 110.22, 68.68, 65.46, 64.07, 39.05.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl 2-phenylacetate (5t) Yield: 71%; white solid; m.p. 106 °C; ESI-MS m/z 312.07 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.64 (dd, J = 1.5, 7.5 Hz, 1H), 7.41 (m, 1H), 7.25 (m, 5H), 7.02 (dd, J = 0.5, 8.0 Hz, 1H), 6.90 (m, 1H), 4.61 (m, 1H), 4.42 (dd, J = 8.5, 9.5 Hz, 1H), 4.30 (m, 2H), 4.18 (dd, J = 7.5, 9.0 Hz, 1H), 3.61 (s, 2H); 13C-NMR (125 MHz, CDCl3) δ 171.31, 166.70, 159.95, 133.72, 133.57, 129.13, 128.57, 128.21, 127.17, 118.69, 116.86, 110.29, 68.84, 65.50, 64.17, 41.30.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl 3-phenylpropanoate (5u) Yield: 78%; pale yellow oil; ESI-MS m/z 326.07 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.64 (dd, J = 1.5, 7.5 Hz, 1H), 7.41 (m, 1H), 7.24 (m, 5H), 7.02 (dd, J = 0.5, 8.0 Hz, 1H), 6.92 (m, 1H), 4.60 (m, 1H), 4.42 (dd, J = 8.5, 9.5 Hz, 1H), 4.30 (m, 2H), 4.04 (dd, J = 7.5, 9.0 Hz, 1H), 3.61 (t, J = 7.5 Hz, 2H), 2.83 (t, J = 7.5 Hz, 2H); 13C-NMR (125 MHz, CDCl3) δ 171.31, 166.70, 159.95, 133.72, 133.57, 129.13, 128.57, 128.21, 127.17, 118.69, 116.86, 110.29, 68.84, 65.50, 64.17, 41.30, 34.12.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl cinnamate (5v) Yield: 69%; white solid; m.p. 85 °C; ESI-MS m/z 324.22 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 1.5, 7.5 Hz, 1H), 7.40 (m, 1H), 7.28 (m, 2H), 7.21 (m, 3H), 7.02 (dd, J = 0.5, 8.0 Hz, 1H), 6.89 (m, 1H), 4.58 (m, 1H), 4.41 (dd, J = 9.0, 10.0 Hz, 1H), 4.30 (dd, J = 4.5, 11.5 Hz, 1H), 4.20 (dd, J = 4.5, 9.5 Hz, 1H), 4.16 (dd, J = 7.0, 8.5 Hz, 1H), 2.92 (t, J = 7.5 Hz, 2H), 2.65 (t, J = 7.5 Hz, 2H); 13C-NMR (125 MHz, CDCl3) δ 172.65, 166.73, 159.95, 140.19, 133.74, 128.52, 128.23, 128.17, 126.35, 118.72, 116.88, 110.28, 69.08, 65.35, 64.17, 35.62, 30.86.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl benzoate (5w) Yield: 81%; white solid; m.p. 88 °C; ESI-MS m/z 298.21 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.86 (br. s, 1H, Ar-OH), 7.99 (dd, J = 1.0, 8.0 Hz, 2H), 7.69 (dd, J = 1.5, 8.0 Hz, 1H), 7.57 (m, 1H), 7.43 (m, 3H), 7.02 (dd, J = 0.5, 8.0 Hz, 1H), 6.90 (m, 1H), 4.79 (m, 1H), 4.57 (m, 2H), 4.46 (dd, J = 4.5, 9.5 Hz, 1H), 4.40 (dd, J = 7.0, 8.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.87, 166.32, 159.99, 133.77, 133.25, 129.68, 129.64, 128.46, 128.18, 118.74, 116.89, 110.30, 99.98, 69.23, 65.92, 64.37.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl 2-methylbenzoate (5x) Yield: 86%; white solid; m.p. 63 °C; ESI-MS m/z 312.10 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.86 (dd, J = 1.5, 7.5 Hz, 1H), 7.67 (dd, J = 1.5, 8.0 Hz, 1H), 7.40 (m, 2H), 7.22 (m, 2H), 7.02 (dd, J = 1.0, 7.5 Hz, 1H), 6.90 (m, 1H), 4.78 (m, 1H), 4.56 (m, 2H), 4.46 (dd, J = 4.5, 9.5 Hz, 1H), 4.38 (dd, J = 7.0, 8.5 Hz, 1H), 2.55 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 167.33, 166.81, 159.98, 140.36, 133.74, 132.29, 131.77, 130.76, 129.03, 128.17, 125.80, 118.73, 116.87, 110.34, 69.15, 65.76, 64.39, 21.78.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl 2-nitrobenzoate (5y) Yield: 74%; pale yellow crystal; m.p. 108–109 °C; ESI-MS m/z 343.12 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.80 (br. s, 1H, Ar-OH), 7.91 (dd, J = 1.5, 7.5 Hz, 1H), 7.73 (dd, J = 2.0, 7.5 Hz, 1H), 7.67 (m, 3H), 7.40 (m, 1H), 7.02 (dd, J = 1.0, 8.5 Hz, 1H), 6.89 (m, 1H), 4.75 (m, 1H), 4.58 (m, 2H), 4.44 (dd, J = 6.5, 11.0 Hz, 1H), 4.34 (dd, J = 6.5, 9.0 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 167.07, 165.17, 159.95, 133.79, 133.02, 132.02, 130.07, 128.28, 127.12, 123.94, 118.74, 116.87, 110.30, 69.31, 67.12, 63.91.
(2-(2-Hydroxyphenyl)-4,5-dihydrooxazol-4-yl)methyl 2-chlorobenzoate (5z) Yield: 69%; white needle crystal; m.p. 91 °C; ESI-MS m/z 332.12: 333.10 (1:1), [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.85 (br. s, 1H, Ar-OH), 7.79 (dd, J = 1.0, 7.5 Hz, 1H), 7.67 (dd, J = 2.0, 8.0 Hz, 1H), 7.42 (m, 3H), 7.29 (m, 1H), 7.02 (dd, J = 0.5, 8.5 Hz, 1H), 6.89 (m, 1H), 4.79 (m, 1H), 4.57 (m, 2H), 4.49 (dd, J = 4.5, 9.5 Hz, 1H), 4.42 (dd, J = 7.5, 9.0 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.92, 165.57, 159.97, 133.79, 133.77, 132.84, 131.66, 131.15, 129.60, 128.19, 126.67, 118.74, 116.88, 110.33, 69.19, 66.37, 64.21.
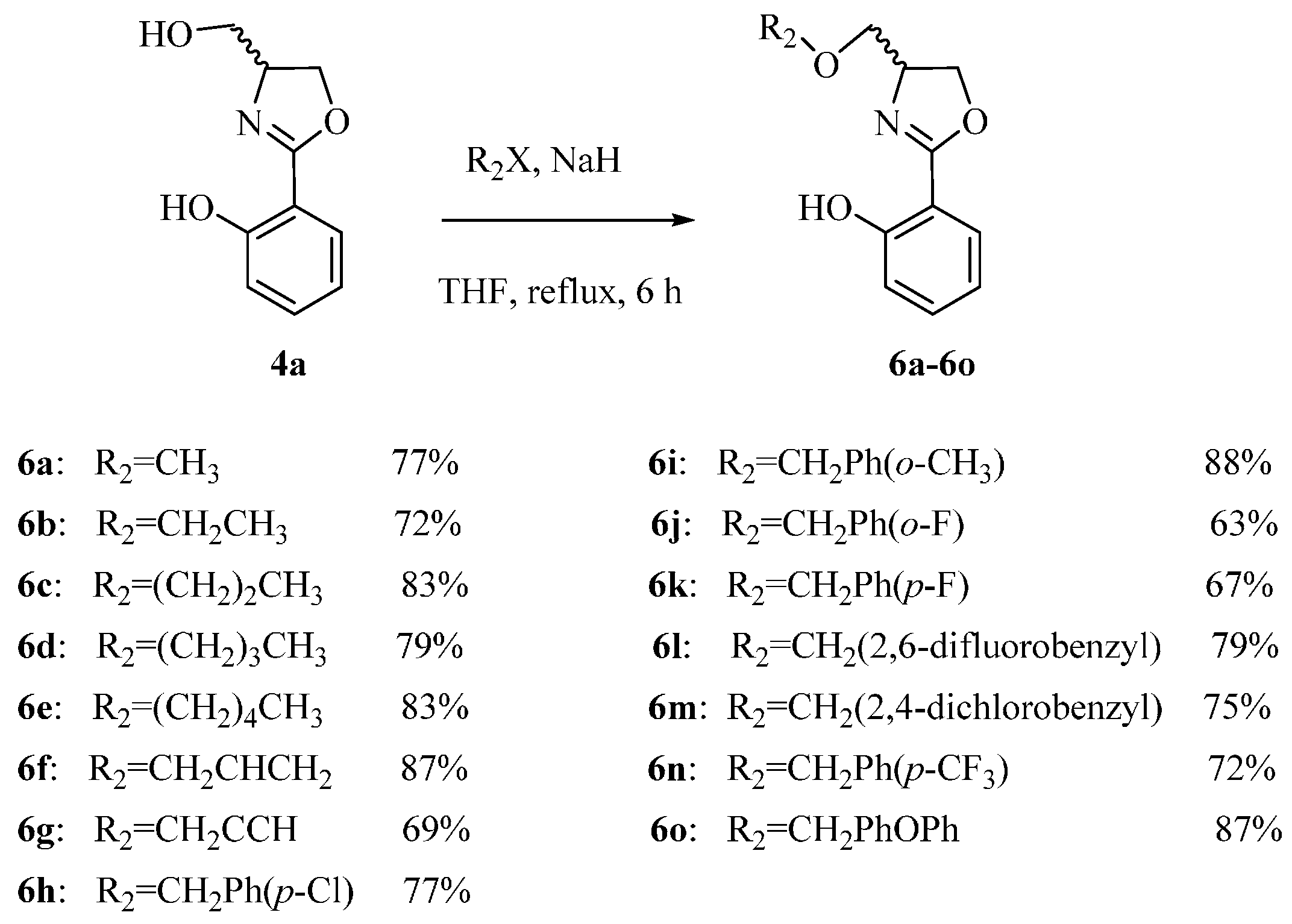
3.1.3. Synthesis of 6a–6o
A solution of compound 4a (193 mg, 1.0 mmol) and halides (1.1 mmol), NaH (192 mg, 8.0 mmol) in anhydrous THF (8 mL) is heated to reflux for 6 h. After cooling, the solution is quenched with water (95.0 μL, 8.0 mmol), concentrated under reduced pressure until approximately 5 mL remained, and diluted with CH2Cl2 (50 mL). Then the mixture is separated with CH2Cl2 (30 mL × 3) and subsequently washed with an aqueous saturated solution of NH4Cl (30 mL × 2), brine (30 mL × 3), and dried over anhydrous Na2SO4. The solvent is concentrated under reduced pressure, and purified by flash column chromatography using petroleum ether/ethyl acetate (12:1, v/v) as the eluent.
2-(4-(Methoxymethyl)-4,5-dihydrooxazol-2-yl)phenol (6a) Yield: 77%; pale yellow oil; ESI-MS m/z 208.09 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 1.5, 8.0 Hz, 1H), 7.39 (m, 1H), 7.01 (dd, J = 1.0, 8.5 Hz, 1H), 6.88 (m, 1H), 4.56 (m, 1H), 4.48 (dd, J = 8.0, 9.0 Hz, 1H), 4.33 (dd, J = 7.5, 8.5 Hz, 1H), 3.67 (dd, J = 4.0, 9.5 Hz, 1H), 3.46 (dd, J = 6.5, 9.5 Hz, 1H), 3.40 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 166.36, 159.92, 137.59, 134.79, 133.46, 129.16, 128.13, 127.90, 118.61, 116.75, 110.60, 73.41, 71.71, 69.98, 65.25, 21.16.
2-(4-(Ethoxymethyl)-4,5-dihydrooxazol-2-yl)phenol (6b) Yield: 72%; pale yellow oil; ESI-MS m/z 222.10 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.71 (dd, J = 2.0, 9.0 Hz, 1H), 7.43 (m, 1H), 7.06(dd, J = 0.5, 9.0 Hz, 1H), 6.93 (m, 1H), 4.60 (m, 1H), 4.53 (m, 1H), 4.37 (dd, J = 7.0, 9.0 Hz, 1H), 3.78 (dd, J = 4.5, 9.5 Hz, 1H), 3.64 (m, 2H), 3.51 (dd, J = 7.0, 9.5 Hz, 1H), 1.24 (t, J = 3.0 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 166.33, 159.93, 133.46, 128.14, 118.63, 116.76, 110.65, 72.45, 70.07, 67.04, 65.29, 15.10.
2-(4-(Propoxymethyl)-4,5-dihydrooxazol-2-yl)phenol (6c) Yield: 83%; brown oil; ESI-MS m/z 236.17 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 1.5, 9.0 Hz, 1H), 7.38 (m, 1H), 7.01 (dd, J = 0.5, 9.0 Hz, 1H), 6.88 (m, 1H), 4.56 (m, 1H), 4.48 (m, 1H), 4.33 (dd, J = 7.0, 8.0 Hz, 1H), 3.72 (dd, J = 4.5, 10.0 Hz, 1H), 3.49 (m, 3H), 1.16 (m, 2H), 0.90 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 166.33, 159.94, 133.44, 128.12, 118.61, 116.75, 110.65, 73.39, 72.60, 70.04, 65.30, 22.79, 10.48.
2-(4-(Butoxymethyl)-4,5-dihydrooxazol-2-yl)phenol (6d) Yield: 79%; yellow oil; ESI-MS m/z 250.10 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 1.5, 7.5 Hz, 1H), 7.38 (m, 1H), 7.01 (dd, J = 0.5, 8.5 Hz, 1H), 6.88 (m, 1H), 4.55 (m, 1H), 4.48 (m, 1H), 4.33 (dd, J = 7.0, 8.0 Hz, 1H), 3.72 (dd, J = 4.0, 9.5 Hz, 1H), 3.53 (m, 3H), 1.57 (m, 2H), 1.38 (m, 2H), 0.90 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 166.32, 159.94, 133.44, 128.12, 118.61, 116.75, 110.66, 72.65, 71.53, 70.05, 65.30, 31.67, 19.26, 13.86.
2-(4-((Pentyloxy)methyl)-4,5-dihydrooxazol-2-yl)phenol (6e) Yield: 83%; yellow oil; ESI-MS m/z 264.17 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 2.0, 9.0 Hz, 1H), 7.38 (m, 1H), 7.01(dd, J = 1.0, 8.5 Hz, 1H), 6.88 (m, 1H), 4.55 (m, 1H), 4.48 (m, 1H), 4.33 (dd, J = 6.5, 8.0 Hz, 1H), 3.72 (dd, J = 4.5, 9.5 Hz, 1H), 3.52 (m, 3H), 1.58 (m, 2H), 1.32 (m, 4H), 0.87 (t, J = 7.0 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 166.33, 159.94, 133.44, 128.12, 118.61, 116.75, 110.66, 72.63, 71.86, 70.04, 65.30, 29.28, 28.27, 22.48, 14.00.
2-(4-((Allyloxy)methyl)-4,5-dihydrooxazol-2-yl)phenol (
6f) Yield: 87%; yellow oil; ESI-MS
m/z 234.12 [M + H]
+;
1H-NMR (500 MHz, CDCl
3) δ 11.99 (br. s, 1H, Ar-OH), 7.66 (dd,
J = 1.5, 6.5 Hz, 1H), 7.38 (m, 1H), 7.01 (dd,
J = 1.0, 8.5 Hz, 1H), 6.88 (m, 1H), 5.92 (m, 1H), 5.29 (dq,
J = 1.5, 17.0 Hz, 1H), 5.21 (dq,
J = 1.5, 10.5 Hz, 1H), 4.57 (m, 1H), 4.49 (m, 1H), 4.35 (dd,
J = 7.0, 9.0 Hz, 1H), 4.04 (dq,
J = 1.5, 5.5 Hz, 2H), 3.73 (dd,
J = 4.5, 9.5 Hz, 1H), 3.49 (dd,
J = 7.0, 9.5 Hz, 1H);
13C-NMR (125 MHz, CDCl
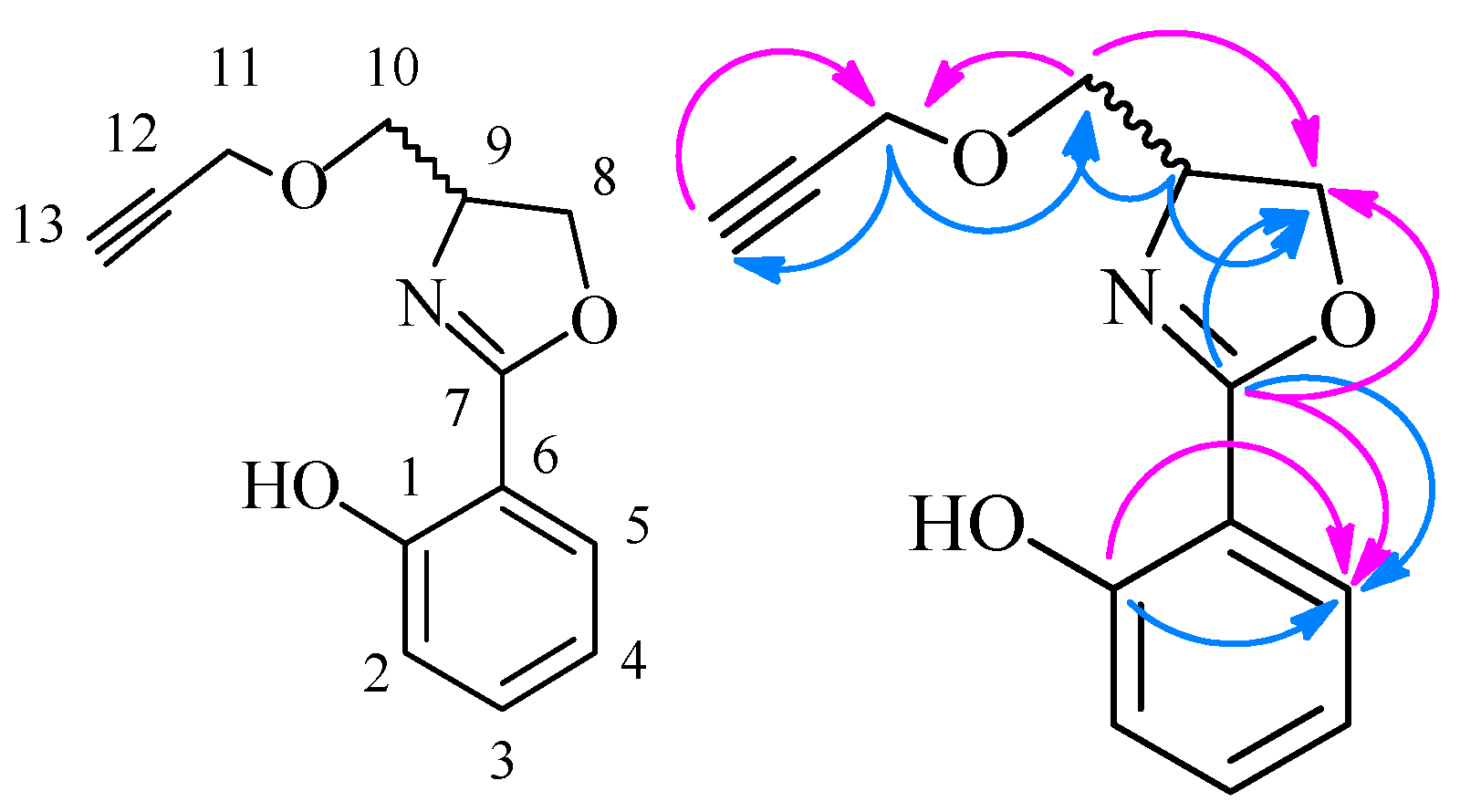
3) δ 166.39, 159.92, 134.37, 133.48, 128.14, 118.63, 117.42, 116.75, 110.61, 72.46, 71.92, 69.94, 65.25. The structure of
6f was also confirmed by 2D-NMR data and can be seen in
Table 4 and
Figure 3.
Figure 3.
The main HMBC of compound 6f.
Figure 3.
The main HMBC of compound 6f.
Table 4.
HMBC data of compound 6f.
Table 4.
HMBC data of compound 6f.
| 13C-NMR | 1H-NMR |
|---|
| C No. | δC | HMBC Correlations | H No. | δH | HMBC Correlations |
|---|
| 1-C | 159.67, C | Ar-OH, 2-H, 3-H, 5-H | Ar-OH | 12.22, s, 1H | 1-C, 2-C, 6-C |
| 2-C | 116.93, CH | Ar-OH, 4-H | 2-H | 7.65, dd, J = 1.5, 80 Hz, 1H | 1-C, 3-C, 7-C |
| 3-C | 134.23, CH | 2-H, 4-H | 3-H | 7.49–7.46, m, 1H | 1-C, 5-C, 6-C |
| 4-C | 119.41, CH | 5-H | 4-H | 6.97–6.94, m, 1H | 1-C, 2-C, 3-C, 5-C, 6-C |
| 5-C | 128.28, CH | 3-H, 4-H | 5-H | 7.02, dd, J = 0.5, 8.5 Hz, 1H | 1-C, 4-C, 6-C, 7-C |
| 6-C | 110.57, C | Ar-OH, 4-H, 5-H | 6-H | - | - |
| 7-C | 165.84, C | 2-H, 5-H, 8-H, 9-H | 7-H | - | - |
| 8-C | 71.56, CH2 | 9-H | 8-H | 4.57–4.54, m, 1H; 4.32–4.30, m, 1H | 7-C, 9-C, 10-C |
| 9-C | 65.19, CH | 8-H, 10-H | 9-H | 4.62–4.58, m, 1H | 7-C, 10-C |
| 10-C | 71.80, CH2 | 8-H, 11-H | 10-H | 3.64–3.57, m, 2H | 9-C, 11-C |
| 11-C | 69.71, CH2 | 10-H | 11-H | 4.03–4.02, m, 2H | 10-C, 12-C, 13-C |
| 12-C | 135.49, CH | 11-H, 13-H | 12-H | 5.93–5.86, m, 1H | 10-C |
| 13-C | 117.06, CH2 | 11-H | 13-H | 5.28–5.24, ddd, J = 1.5, 3.5, 17.5 Hz, 1H; 5.17–5.15, m, 1H | 10-C, 12-C |
2-(4-((Prop-2-yn-1-yloxy)methyl)-4,5-dihydrooxazol-2-yl)phenol (6g) Yield: 69%; yellow oil; ESI-MS m/z 232.10; [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.98 (br. s, 1H, Ar-OH), 7.66 (dd, J = 1.5, 6.5 Hz, 1H), 7.37 (m, 1H), 7.03 (dd, J = 1.0, 8.5 Hz, 1H), 6.88 (m, 1H), 4.51 (d, J = 4.5 Hz, 2H), 4.49 (m, 1H), 4.11 (s, 2H), 3.73 (dd, J = 4.5, 9.5 Hz, 1H), 3.49 (dd, J = 7.0, 9.5 Hz, 1H), 3.35 (s, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.39, 159.92, 134.37, 133.48, 128.14, 118.63, 117.42, 116.75, 110.61, 72.46, 71.92, 69.94, 65.25.
2-(4-(((4-Chlorobenzyl)oxy)methyl)-4,5-dihydrooxazol-2-yl)phenol (6h) Yield: 77%; pale pink oil; ESI-MS m/z 318.09: 320.02 (3:1), [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 1.5, 8.0 Hz, 1H), 7.39 (m, 1H), 7.31 (dt, J = 2.0, 7.5 Hz, 2H), 7.24 (m, 2H), 7.01 (dd, J = 0.5, 9.0 Hz, 1H), 6.88 (m, 1H), 4.57(m, 1H), 4.52 (d, J = 4.0 Hz, 2H), 4.49 (m, 1H), 4.33 (dd, J = 7.0, 8.0 Hz, 1H), 3.73 (dd, J = 4.0, 9.5 Hz, 1H), 3.53 (dd, J = 6.5, 9.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.45, 159.91, 136.38, 133.58, 133.54, 128.98, 128.64, 128.15, 118.67, 116.77, 110.53, 72.73, 71.93, 69.73, 65.24.
2-(4-(((2-Methylbenzyl)oxy)methyl)-4,5-dihydrooxazol-2-yl)phenol (6i) Yield: 88%; pale yellow oil; ESI-MS m/z 298.10 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 2.0, 9.0 Hz, 1H), 7.38 (m, 1H), 7.29 (m, 1H), 7.21 (m, 3H), 7.01 (dd, J = 0.5, 8.0 Hz, 1H), 6.88 (m, 1H), 4.58 (m, 3H), 4.48 (dd, J = 8.5, 9.5 Hz, 1H), 4.32 (dd, J = 7.0, 8.0 Hz, 1H), 3.77 (dd, J = 4.0, 9.5 Hz, 1H), 3.53 (dd, J = 6.5, 9.5 Hz, 1H), 2.32 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 166.39, 159.93, 136.85, 135.67, 133.47, 130.36, 128.78, 128.13, 128.08, 125.81, 118.62, 116.75, 110.59, 72.07, 72.01, 69.93, 65.25, 18.79.
2-(4-(((2-Fluorobenzyl)oxy)methyl)-4,5-dihydrooxazol-2-yl)phenol (
6j) Yield: 63%; pale yellow oil; ESI-MS
m/z 302.11 [M + H]
+;
1H-NMR (500 MHz, CDCl
3) δ 11.98 (br. s, 1H, Ar-OH), 7.65 (dd,
J = 1.5, 6.5 Hz, 1H), 7.38 (m, 2H), 7.29 (m, 1H), 7.14 (m, 1H), 7.06 (m, 1H), 7.01 (dd,
J = 0.5, 8.0 Hz, 1H), 6.88 (m, 1H), 4.66 (m, 2H), 4.59 (m, 1H), 4.48 (m, 1H), 4.35 (dd,
J = 7.0, 8.0Hz, 1H), 3.79 (dd,
J = 4.0, 9.5 Hz, 1H), 3.55 (dd,
J = 7.0, 9.5 Hz, 1H);
13C-NMR (125 MHz, CDCl
3) δ 166.46, 161.80, 159.94, 133.50, 130.12, 129.65, 128.15, 124.18, 118.63, 116.77, 115.41, 115.24, 110.58, 72.15, 69.89, 67.04, 65.17. The structure of
6j was also confirmed by 2D NMR data and can be seen in
Table 5 and
Figure 4.
Figure 4.
The main HMBC of compound 6j.
Figure 4.
The main HMBC of compound 6j.
Table 5.
HMBC data of compound 6j.
Table 5.
HMBC data of compound 6j.
| 13C-NMR | 1H-NMR |
|---|
| C No. | δC | HMBC Correlations | H No. | δH | HMBC Correlations |
|---|
| 1-C | 159.64, C | 2-H, 3-H, 5-H | Ar-OH | 12.17, s, 1H | - |
| 2-C | 116.93, CH | 4-H | 2-H | 7.65, dd, J = 1.5, 8.0 Hz, 1H | 1-C, 3-C, 7-C |
| 3-C | 134.27, CH | 2-H, 4-H | 3-H | 7.49–7.46, m, 1H | 1-C, 5-C |
| 4-C | 119.42, CH | 5-H | 4-H | 6.98–6.95, m, 1H | 1-C, 2-C, 3-C, 5-C, 6-C |
| 5-C | 128.30, CH | 3-H, 4-H | 5-H | 7.02–7.01, m, 1H | 1-C, 4-C, 6-C, 7-C |
| 6-C | 110.52, C | 5-H | 6-H | - | - |
| 7-C | 165.90, C | 2-H, 5-H, 8-H, 9-H | 7-H | - | - |
| 8-C | 69.59, CH2 | 10-H | 8-H | 4.58–4.55, m, 1H; 4.31–4.28, m, 1H | 7-C, 9-C, 10-C |
| 9-C | 64.91, CH | 8-H, 10-H | 9-H | 4.63–4.59, m, 1H | 7-C, 10-C |
| 10-C | 71.12, CH2 | 8-H, 9-H, 11-H | 10-H | 3.67–3.66, m, 2H | 8-C, 9-C, 11-C |
| 11-C | 58.38, CH2 | 10-H, 13-H | 11-H | 4.23–4.22, m, 2H | 10-C, 12-C, 13-C |
| 12-C | 80.55, C | 11-H | 12-H | - | - |
| 13-C | 77.95, CH | 11-H | 13-H | 3.49–3.48, m, 1H | 11-C |
2-(4-(((4-Fluorobenzyl)oxy)methyl)-4,5-dihydrooxazol-2-yl)phenol (6k) Yield: 67%; yellow oil; ESI-MS m/z 302.18 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 1.5, 8.0 Hz, 1H), 7.38 (m, 1H), 7.28 (m, 2H), 7.04 (m, 3H), 6.88 (m, 1H), 4.57 (m, 1H), 4.52 (m, 2H), 4.47 (m, 1H), 4.33 (dd, J = 7.5, 8.5 Hz, 1H), 3.73 (dd, J = 4.5, 5.0 Hz, 1H), 3.52 (dd, J = 7.0, 9.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.44, 163.41, 161.46, 159.92, 133.64, 133.62, 133.53, 129.49, 129.43, 128.15, 118.67, 116.77, 115.43, 115.26, 110.56, 72.82, 71.85, 69.80, 65.24.
2-(4-(((2,6-Difluorobenzyl)oxy)methyl)-4,5-dihydrooxazol-2-yl)phenol (6l) Yield: 79%; pale yellow oil; ESI-MS m/z 320.08 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 11.96(br. s, 1H, Ar-OH), 7.64 (dd, J = 1.5, 8.0 Hz, 1H), 7.38 (m, 1H), 7.31 (m, 1H), 7.00 (dd, J = 0.5, 8.0 Hz, 1H), 6.92 (m, 2H), 6.87 (m, 1H), 4.69 (m, 2H), 4.56 (m, 1H), 4.46 (m, 1H), 4.30 (dd, J = 7.5, 8.5 Hz, 1H), 3.81 (dd, J = 4.0, 9.0 Hz, 1H), 3.51 (dd, J = 7.5, 9.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.47, 162.92, 160.93, 159.91, 133.48, 130.40, 128.14, 118.62, 116.75, 111.45, 111.25, 110.59, 72.09, 70.02, 65.06, 60.60.
2-(4-(((2,4-Dichlorobenzyl)oxy)methyl)-4,5-dihydrooxazol-2-yl)phenol (6m) Yield: 75%; colourless oil; ESI-MS m/z 352.14:354.23:353.12 (9:6:1), [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 1.5, 8.0 Hz, 1H), 7.39 (m, 1H), 7.35 (m, 1H), 7.01 (m, 1H), 6.88 (m, 2H), 6.82 (m, 1H), 4.61 (m, 2H), 4.56 (m, 1H), 4.48 (m, 1H), 4.33 (dd, J = 7.0, 8.0 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.49, 159.92, 133.55, 131.11, 131.07, 131.04, 130.99, 128.15, 118.67, 116.78, 111.41, 111.25, 111.22, 110.53, 104.01, 103.81, 103.60, 72.10, 69.76, 66.58, 66.55, 65.16.
2-(4-(((4-(Trifluoromethyl)benzyl)oxy)methyl)-4,5-dihydrooxazol-2-yl)phenol (6n) Yield: 72%; yellow oil; ESI-MS m/z 352.10 [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 1.5, 9.0 Hz, 1H), 7.60 (d, J = 9.0 Hz, 2H), 7.42 (d, J = 9.0 Hz, 2H), 7.40 (m, 1H), 7.02 (d, J = 8.5 Hz, 1H), 4.65 (dd, J = 7.5, 12.5 Hz, 2H), 4.59 (m, 1H), 4.50 (m, 1H), 4.37 (dd, J = 7.0, 8.0 Hz, 1H), 3.77 (dd, J = 4.5, 9.0 Hz, 1H), 3.59 (dd, J = 6.5, 9.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.53, 159.92, 141.99, 133.59, 128.16, 127.56, 125.44, 125.41, 118.71, 116.79, 110.50, 72.72, 72.19, 69.64, 65.24.
2-(4-(((4-(Benzyloxy)benzyl)oxy)methyl)-4,5-dihydrooxazol-2-yl)phenol (6o) Yield: 87%; brown oil; ESI-MS m/z 390.19; [M + H]+; 1H-NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 1.5, 8.0 Hz, 1H), 7.39 (m, 1H), 7.31 (dt, J = 2.0, 7.5 Hz, 2H), 7.24 (m, 2H), 7.10 (m, 7H), 5.22 (s, 2H), 4.52 (s, 2H), 4.49 (m, 1H), 4.13 (d, J = 6.5 Hz, 2H), 3.73 (dd, J = 4.0, 9.5 Hz, 1H), 3.56 (dd, J = 6.5, 9.5 Hz, 1H); 13C-NMR (125 MHz, CDCl3) δ 166.45, 159.91, 136.38, 133.58, 133.54, 128.98, 128.64, 128.15, 126.07, 126.01, 125.34, 123.44, 118.67, 116.77, 110.53, 72.73, 71.93, 70.88, 69.73, 65.24.