Synthesis and Biological Evaluation of Resveratrol Derivatives as Melanogenesis Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
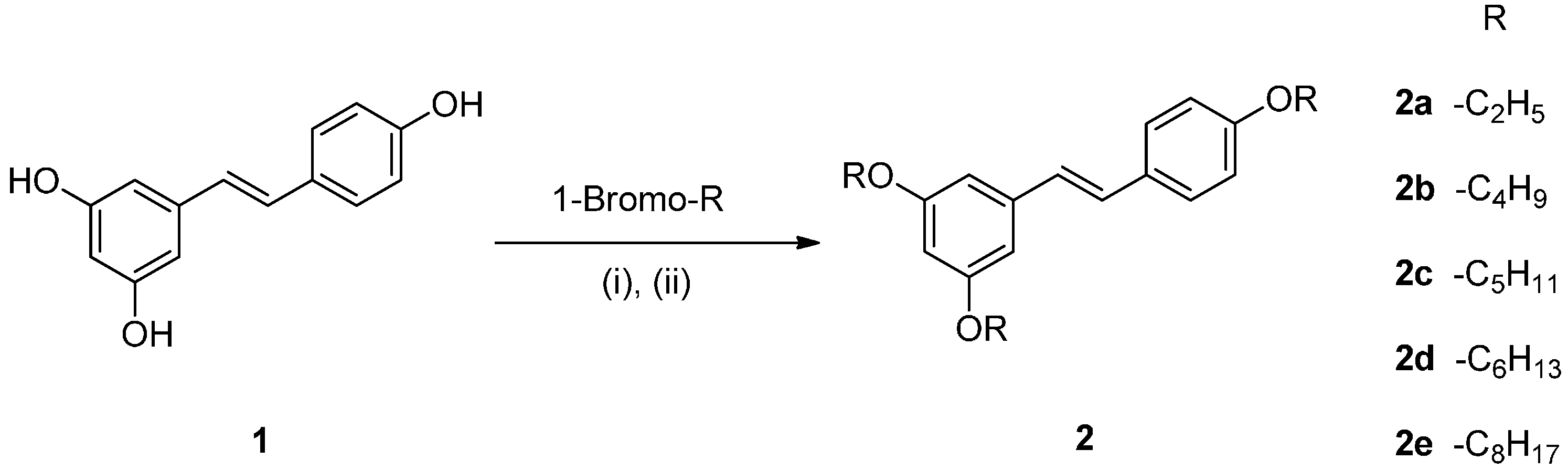
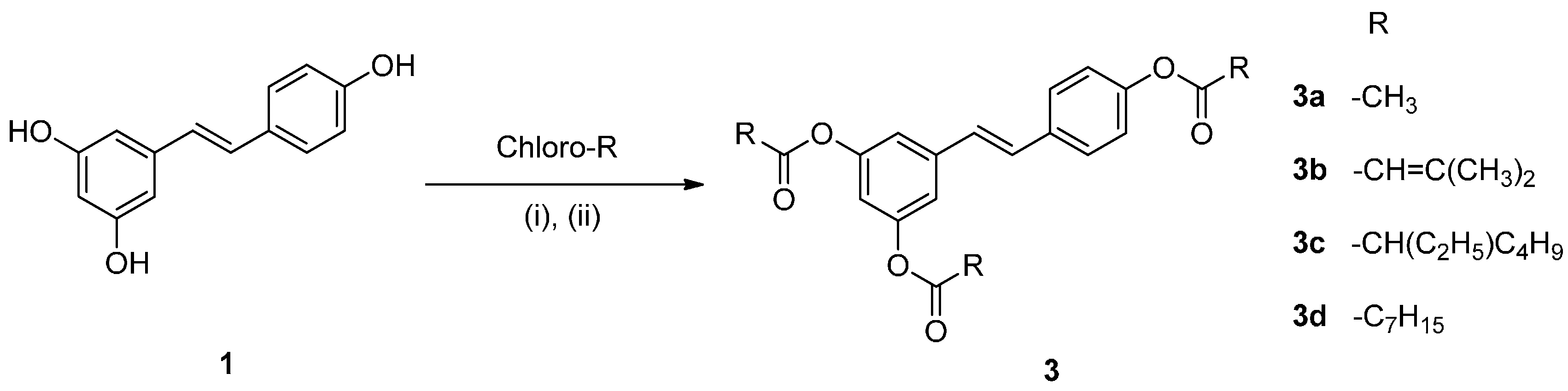
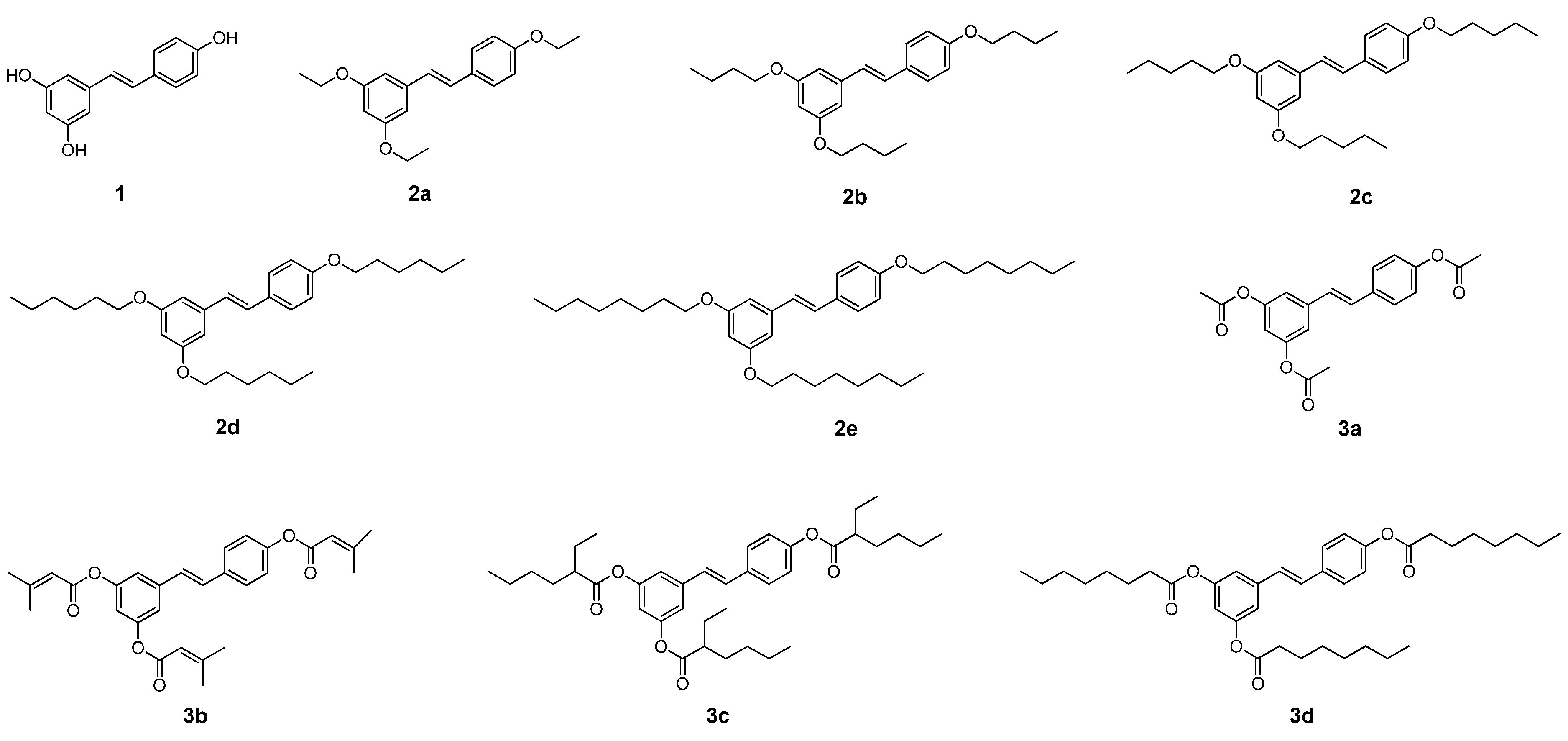
2.2. Effect on Melanogenesis
2.2.1. Effect on Melanin Content in B16F10 Melanoma Cells
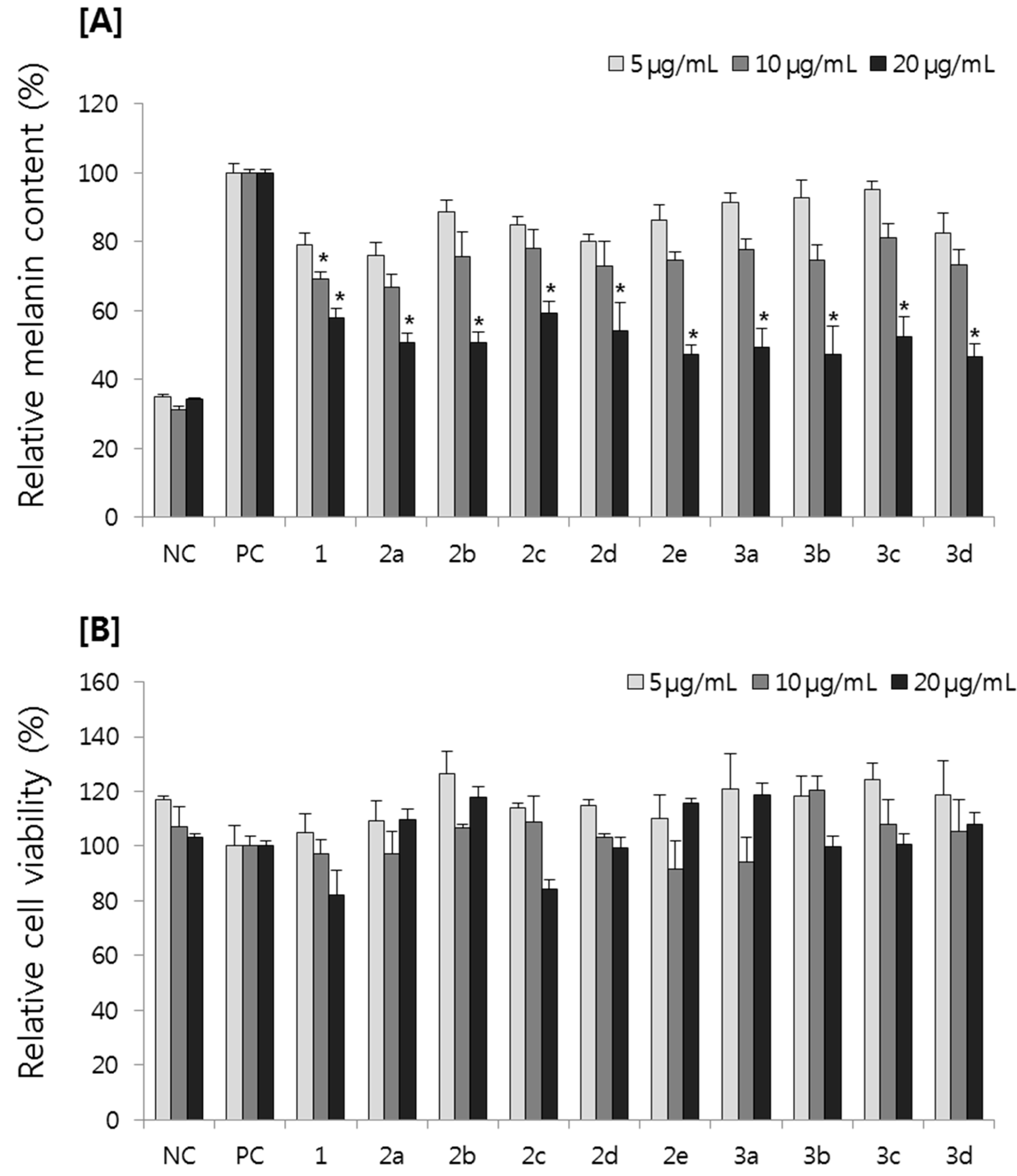
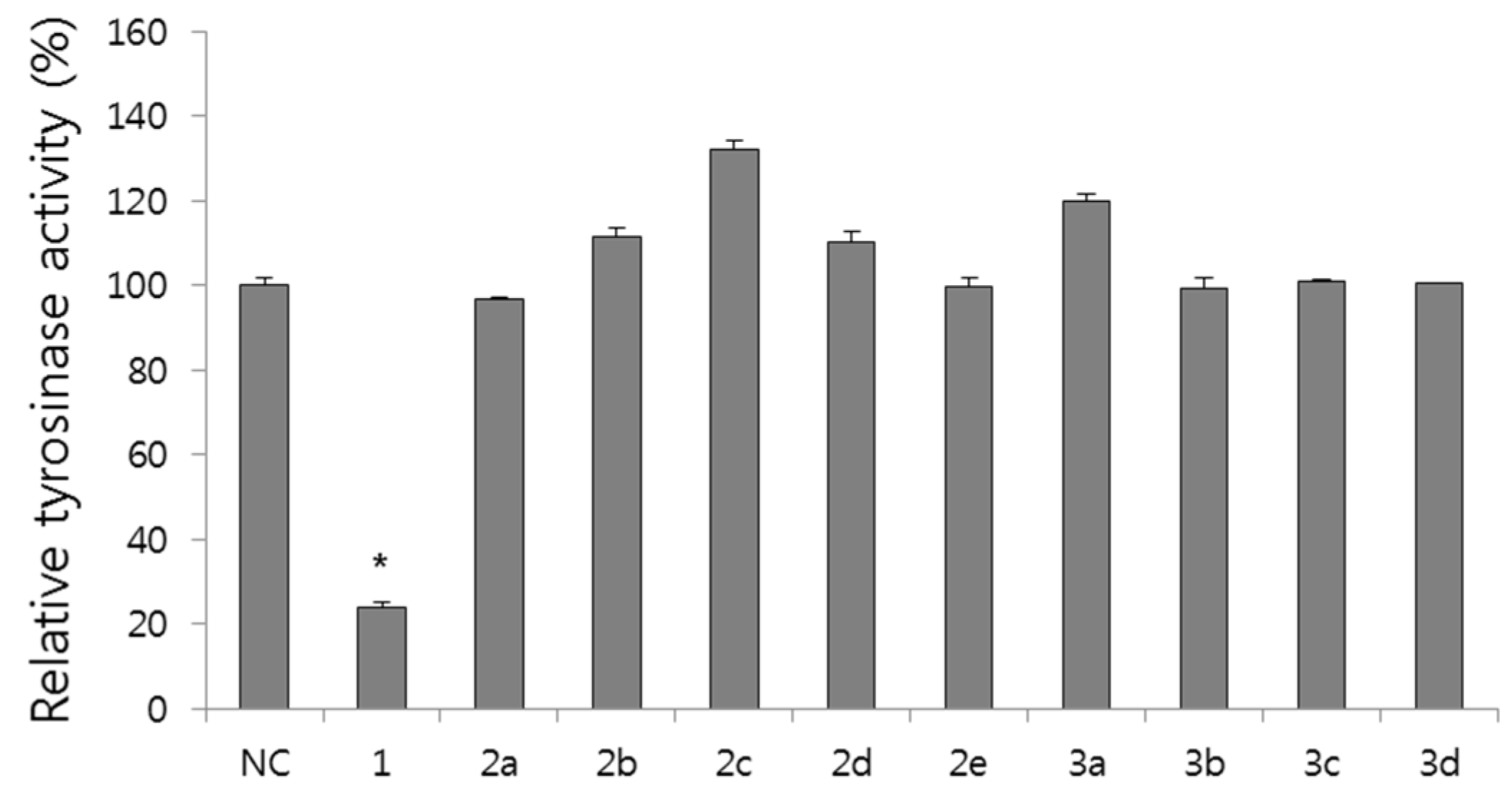
2.2.2. Effect on Tyrosinase Activity
2.2.3. Effect on Melanin Synthesis in B16F10 Melanoma Cells
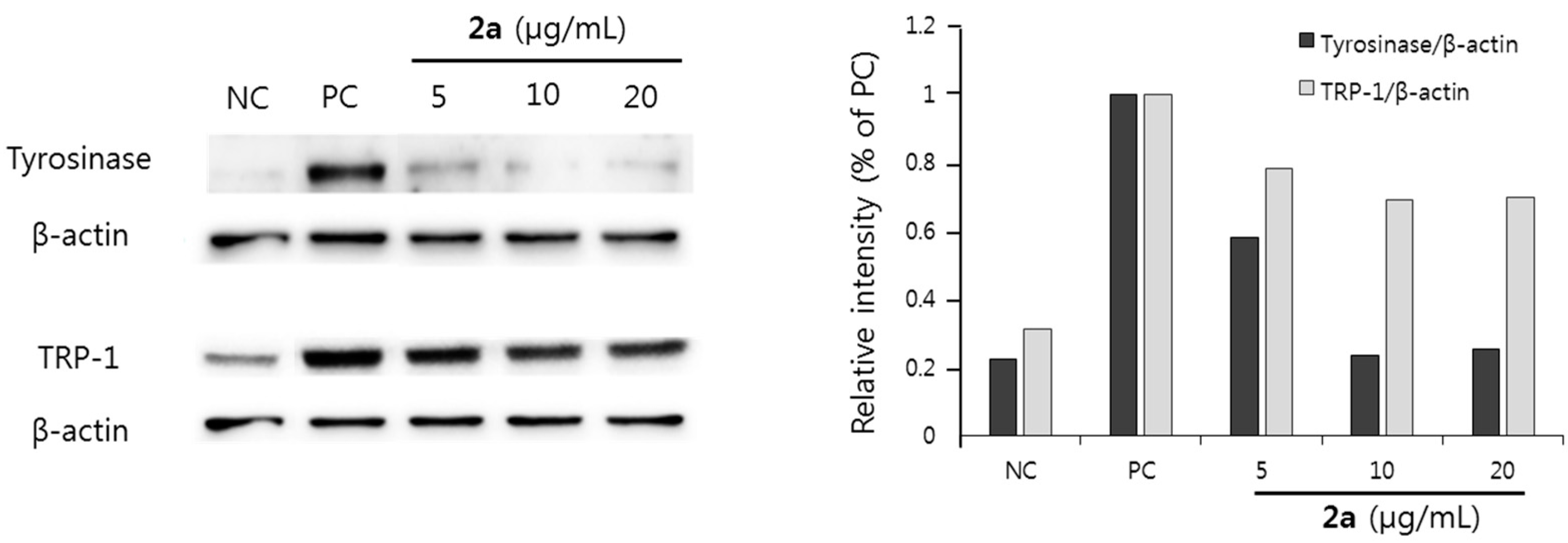
2.3. Discussion
3. Experimental Section
3.1. General Information
3.2. Synthesis of Resveratrol Derivatives
3.3. Evaluation of Anti-Melanogenesis Activity
3.3.1. Assessment of Tyrosinase Activity
3.3.2. Cell Culture
3.3.3. Measurement of Cellular Melanin Contents
3.3.4. Cell Viability
3.3.5. Western Blot Analysis
3.3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rees, J.L. Genetics of hair and skin color. Annu. Rev. Genet. 2003, 37, 67–90. [Google Scholar] [CrossRef] [PubMed]
- Prota, G. Progress in the chemistry of melanins and related metabolites. Med. Res. Rev. 1988, 8, 525–556. [Google Scholar] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Uyana, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.W.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [PubMed]
- Mayer, A.M. Polyphenol oxidases in plant: Recent progress. Phytochemistry 1987, 26, 11–20. [Google Scholar]
- Seo, S.Y.; Sharma, V.K.; Sharma, N. Mushroom tyrosinase: Recent prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Aromatic-turmerone inhibits α-MSH and IBMX-induced melanogenesis by inactivating CREB and MITF signaling pathways. Arch. Dermatol. Res. 2011, 303, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, S.; Matsuda, H.; Oda, Y.; Nakamura, S.; Xu, F.; Yoshikawa, M. Melanogenesis inhibitors from the desert plant Anastatica hierochuntica in B16 melanoma cells. Bioorg. Med. Chem. 2010, 18, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Fernandez-Quintela, A.; Arias, N.; Portillo, M.P. Resveratrol: Anti-obesity mechanisms of action. Molecules 2014, 19, 18632–18655. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.; Louis, X.L.; Thandapilly, S.J.; Movahed, A.; Zieroth, S.; Netticadan, T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014, 95, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Jorgensen, J.O.; Jessen, M.; Richelsen, B.; Pedersen, S.B. Ann. N. Y. Acad. Sci. 2013, 1290, 74–82. [PubMed]
- Farris, P.; Krutmann, J.; Li, Y.H.; McDaniel, D.; Krol, Y. Resveratrol: A unique antioxidant offering a multi-mechanistic approach for treating aging skin. J. Drugs Dermatol. 2013, 12, 1389–1394. [Google Scholar] [PubMed]
- Sattoka, H.; Kubo, I. Resveratrol as a kcat type inhibitor for tyrosinase: Potentiated melanogenesis inhibitor. Bioorg. Med. Chem. 2012, 20, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.A.; Cook, A.L.; Roberts, D.W.; Leonard, J.H.; Sturm, R.A. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J. Investig. Dermatol. 2007, 127, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Davidov-Pardo, G.; McClements, D.J. Nutraceutical delivery systems: Resveratrol encapsulation in grape seed oil nanoemulsions formed by spontaneous emulsification. Food Chem. 2015, 167, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Davidov-Pardo, G.; Pérez-Ciordia, S.; Marı́n-Arroyo, M.R.; McClements, D.J. Improving resveratrol bioaccessibility using biopolymer nanoparticles and complexes: Impact of protein-carbohydrate maillard conjugation. J. Agric. Food Chem. 2015, 63, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Chalal, M.; Klinjuer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial activity of resveratrol analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Houillé, B.; Papon, N.; Boudesocque, L.; Bourdeaue, E.; Besseau, S.; Courdavault, V.; Enguehard-Gueiffier, C.; Delanoue, G.; Guérin, L.; Bouchara, J.P.; et al. Antifungal activity of resveratrol derivatives against Candida species. J. Nat. Prod. 2014, 77, 1658–1662. [Google Scholar]
- Lee, S.H.; Liu, Q.; Hwang, B.Y.; Lee, M.K. Inhibitory effects of stilbene derivatives from Parthenocissus tricuspidata on adipocyte differentiation and pancreatic lipase. Nat. Prod. Comm. 2013, 8, 1439–1441. [Google Scholar]
- Silva, F.; Figueiras, A.; Gallardo, E.; Nerin, C.; Domingues, F.C. Strategies to improve the solubility and stability of stilbene antioxidants: A comparative study between cyclodextrins and bile acids. Food Chem. 2012, 145, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Mastromarino, P.; Masci, A.; D’Erme, M.; Mosca, L. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, J.H.; Suh, H.J.; Lee, I.C.; Koh, J.; Yoo, Y.C. Effect of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch. Dermatol. Res. 2014, 306, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Yun, J.; Lee, C.K.; Lee, H.; Min, K.R.; Kim, Y. Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J. Biol. Chem. 2002, 277, 16340–16344. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Seok, J.K.; An, S.M.; Baek, J.H.; Koh, J.S.; Boo, Y.C. A study of the human skin-whitening effects of resveratryl triacetate. Arch. Dermatol. Res. 2015, 307, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.C.; de Carvalho, G.S.; Rocha, P.R.; da Silva, T.R.; da Silva, A.D.; Raposo, N.R. Inhibitory effects of resveratrol analogs on mushroom tyrosinase activity. Molecules 2012, 17, 11816–11825. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.M.; Ha, Y.M.; Kim, J.A.; Chung, K.W.; Uehara, Y.; Lee, K.J.; Chun, P.; Byun, Y.; Chung, H.Y.; Moon, H.R. Synthesis of novel azo-resveratrol, azo-oxyresveratrol and their derivatives as potent tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 7451–7455. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Rubio, L.; Touriño, S.; Martí, M.; Barba, C.; Fernández-Campos, F.; Coderch, L.; Parra, J.L. Antioxidative effects and percutaneous absorption of five polyphenols. Free Radic. Biol. Med. 2014, 75, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Kim, C.; Jo, Y.H.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Synthesis and Biological Evaluation of Resveratrol Derivatives as Melanogenesis Inhibitors. Molecules 2015, 20, 16933-16945. https://doi.org/10.3390/molecules200916933
Liu Q, Kim C, Jo YH, Kim SB, Hwang BY, Lee MK. Synthesis and Biological Evaluation of Resveratrol Derivatives as Melanogenesis Inhibitors. Molecules. 2015; 20(9):16933-16945. https://doi.org/10.3390/molecules200916933
Chicago/Turabian StyleLiu, Qing, CheongTaek Kim, Yang Hee Jo, Seon Beom Kim, Bang Yeon Hwang, and Mi Kyeong Lee. 2015. "Synthesis and Biological Evaluation of Resveratrol Derivatives as Melanogenesis Inhibitors" Molecules 20, no. 9: 16933-16945. https://doi.org/10.3390/molecules200916933
APA StyleLiu, Q., Kim, C., Jo, Y. H., Kim, S. B., Hwang, B. Y., & Lee, M. K. (2015). Synthesis and Biological Evaluation of Resveratrol Derivatives as Melanogenesis Inhibitors. Molecules, 20(9), 16933-16945. https://doi.org/10.3390/molecules200916933





