Recent Developments and Biological Activities of N-Substituted Carbazole Derivatives: A Review
Abstract
:1. Introduction
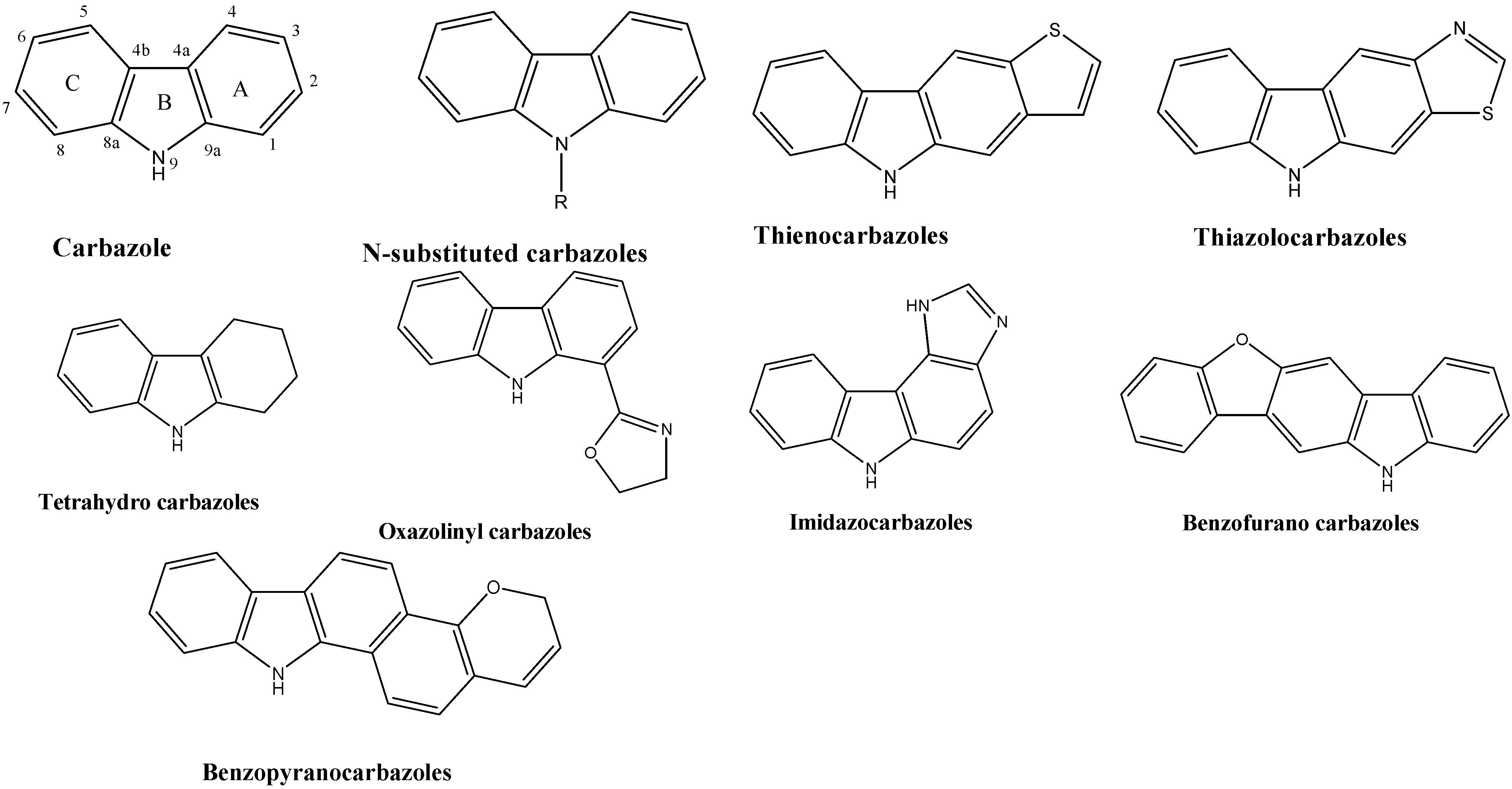
2. Biological Activities of N-Substituted Carbazoles
2.1. Antimicrobial Activity
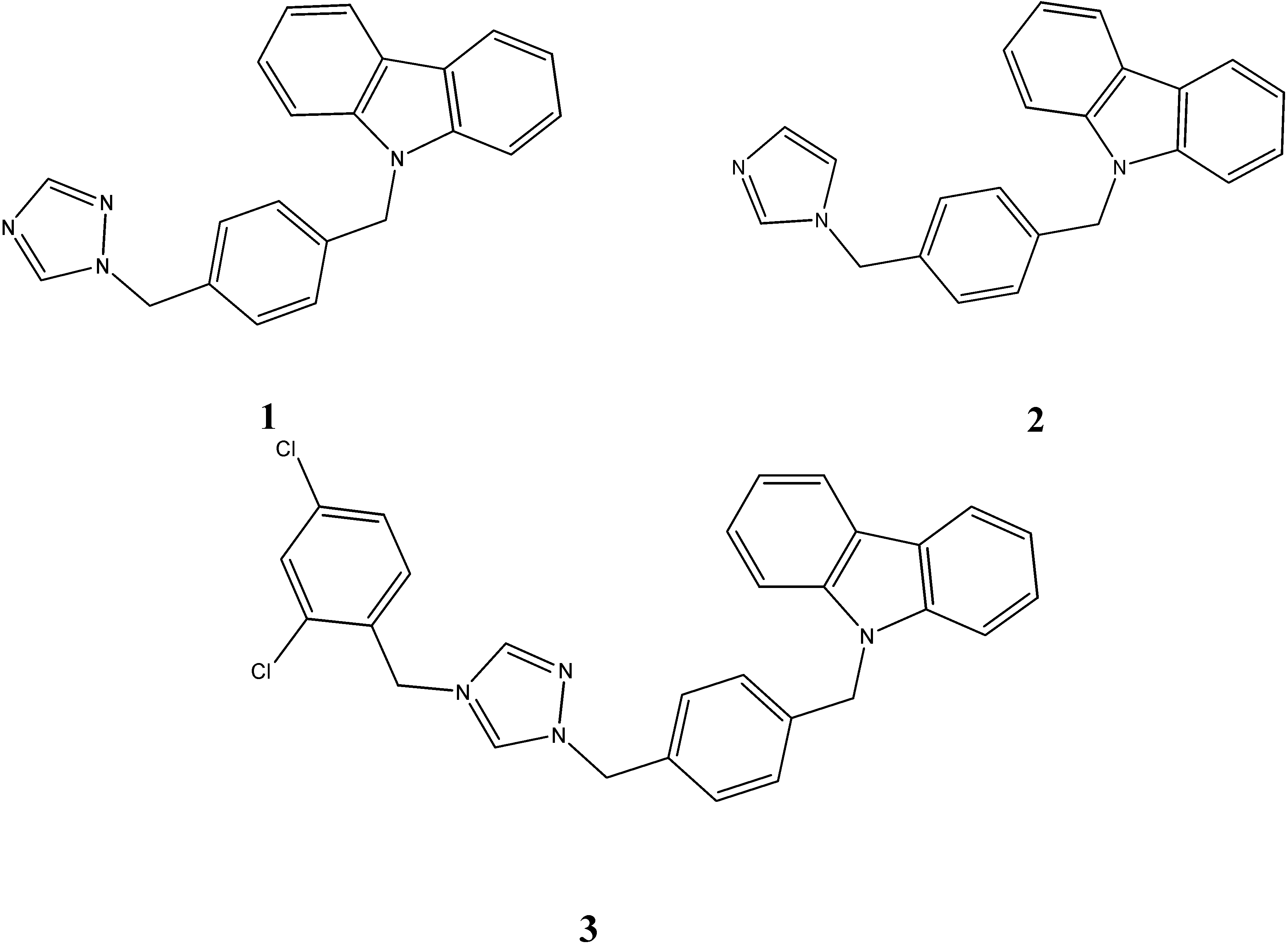
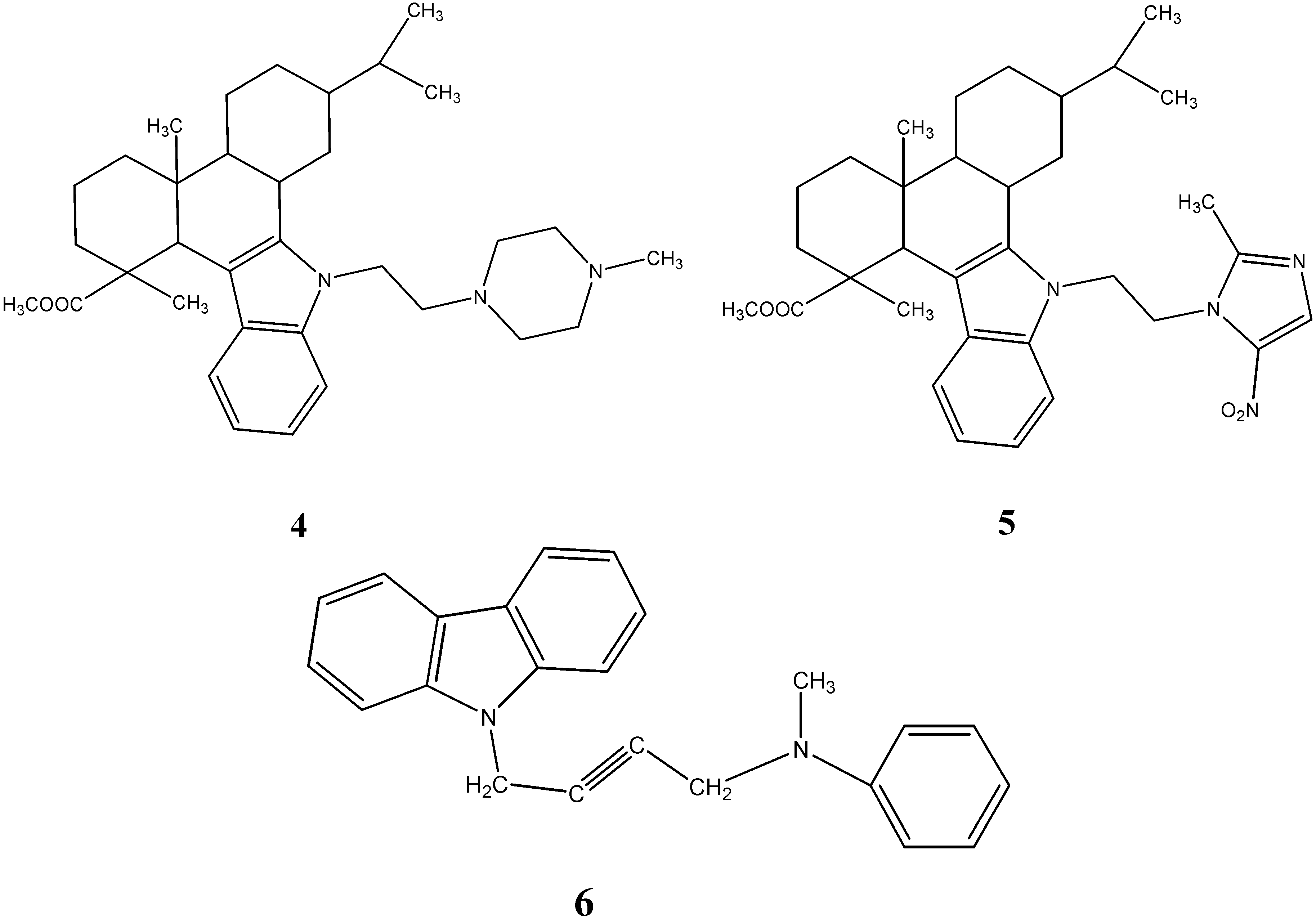
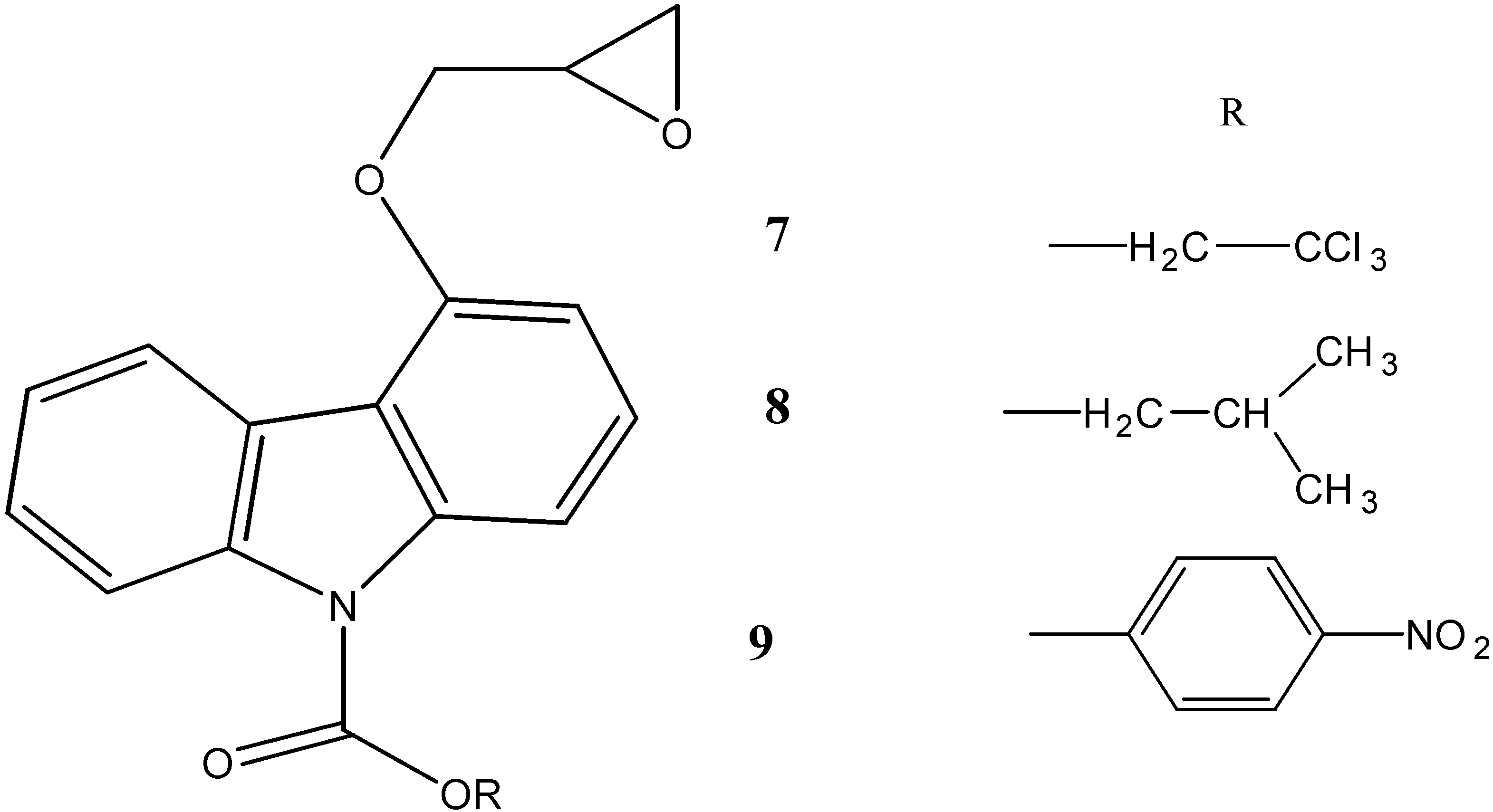
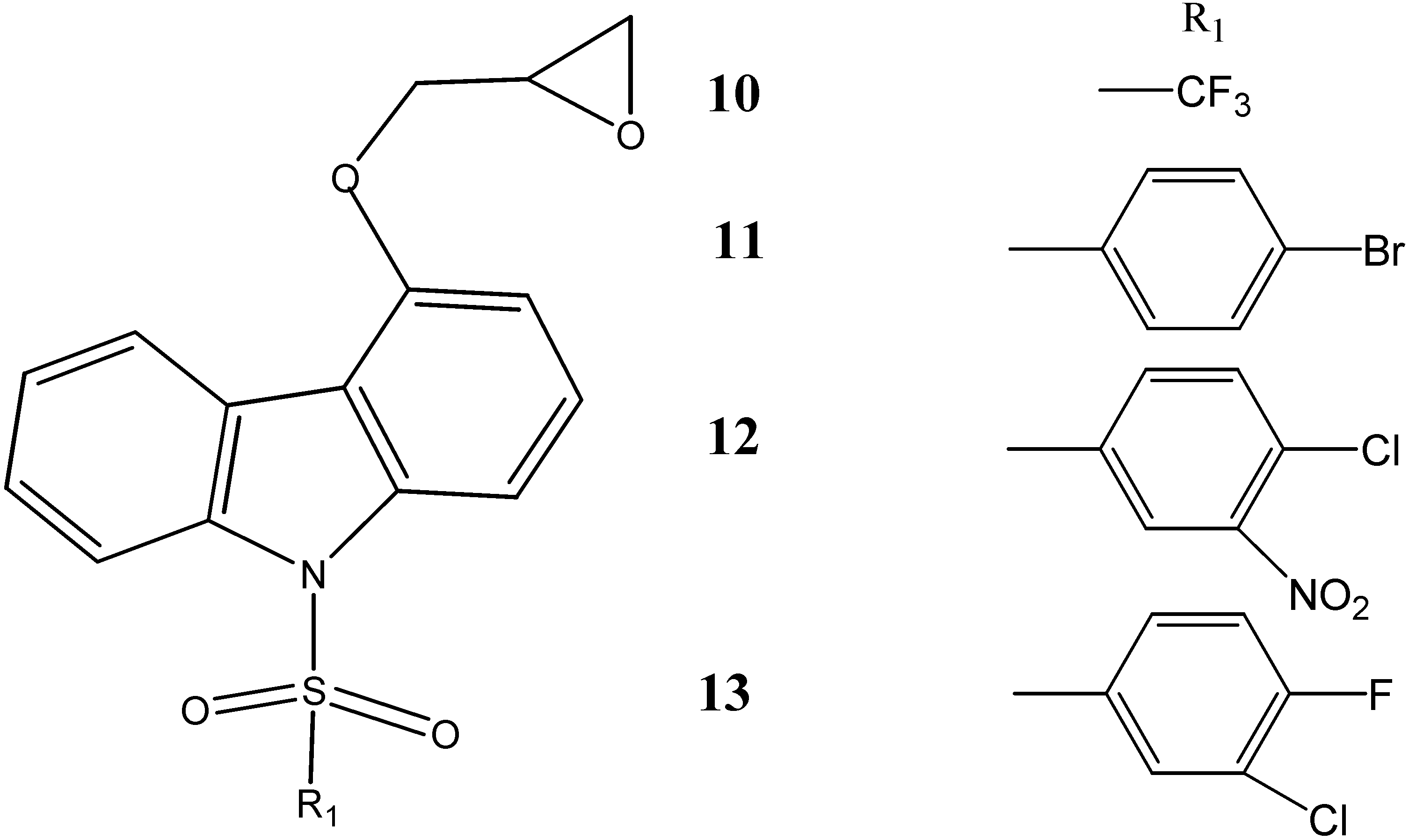
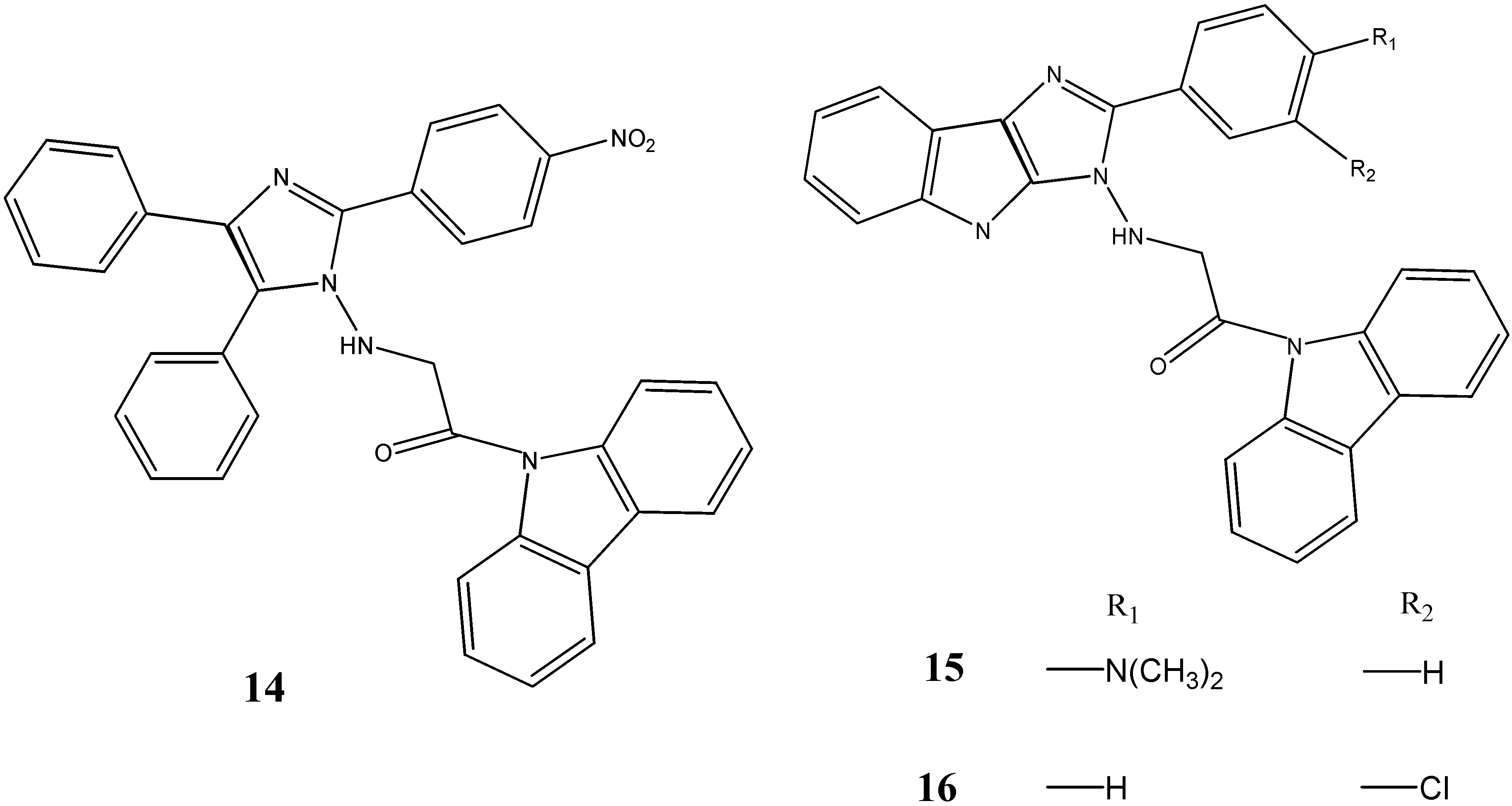
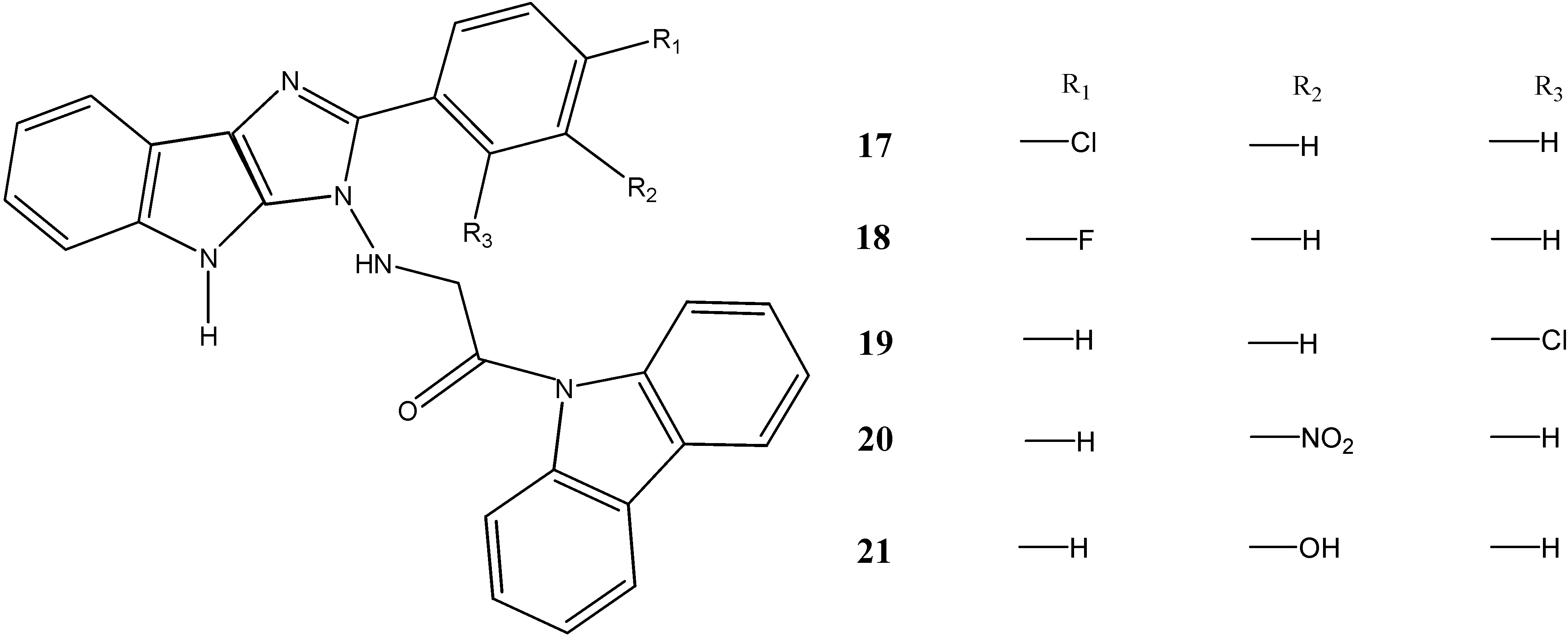
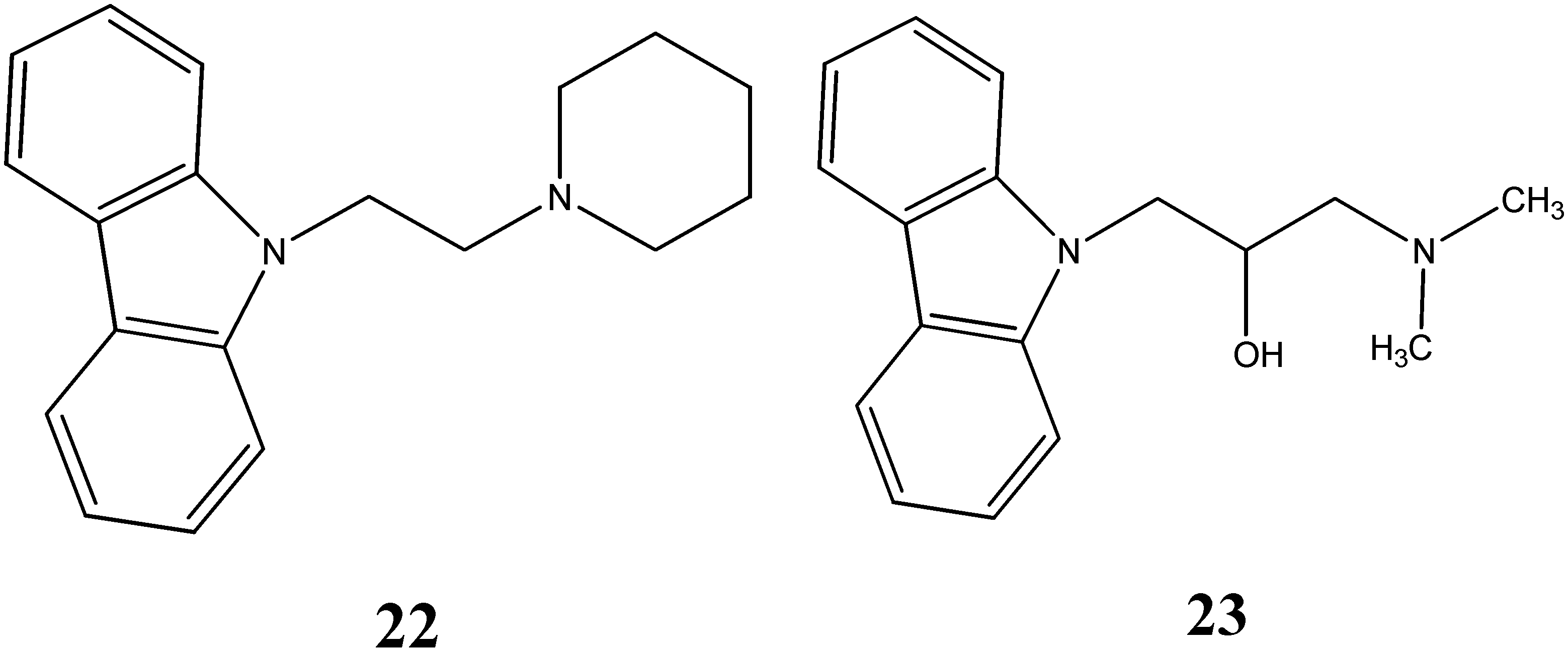
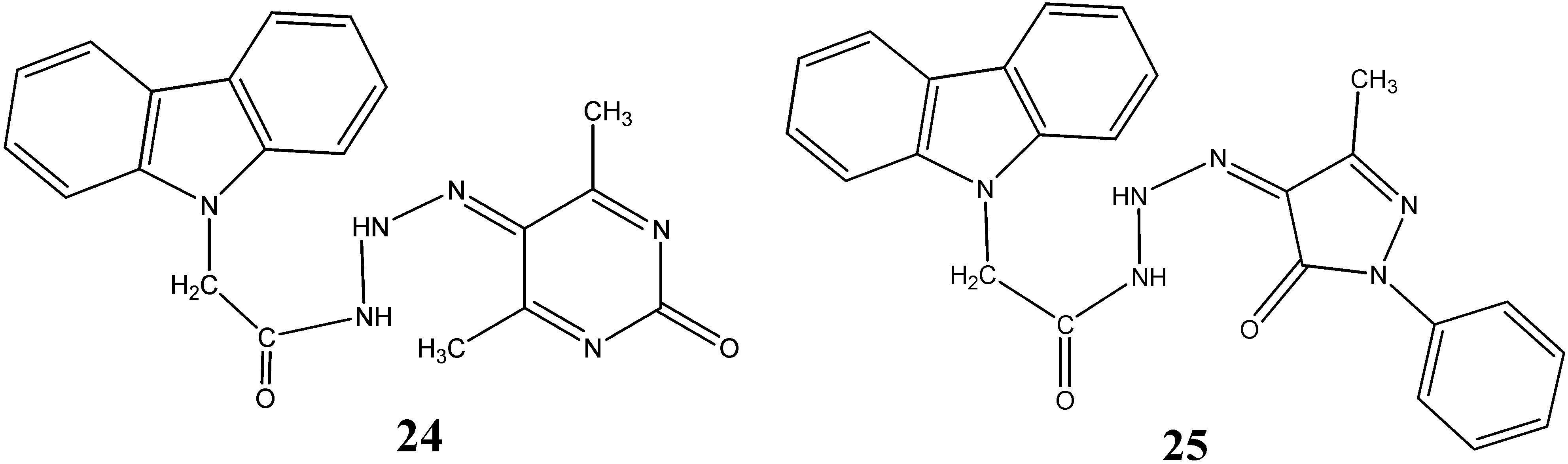
2.2. Anti-Cancer Activity
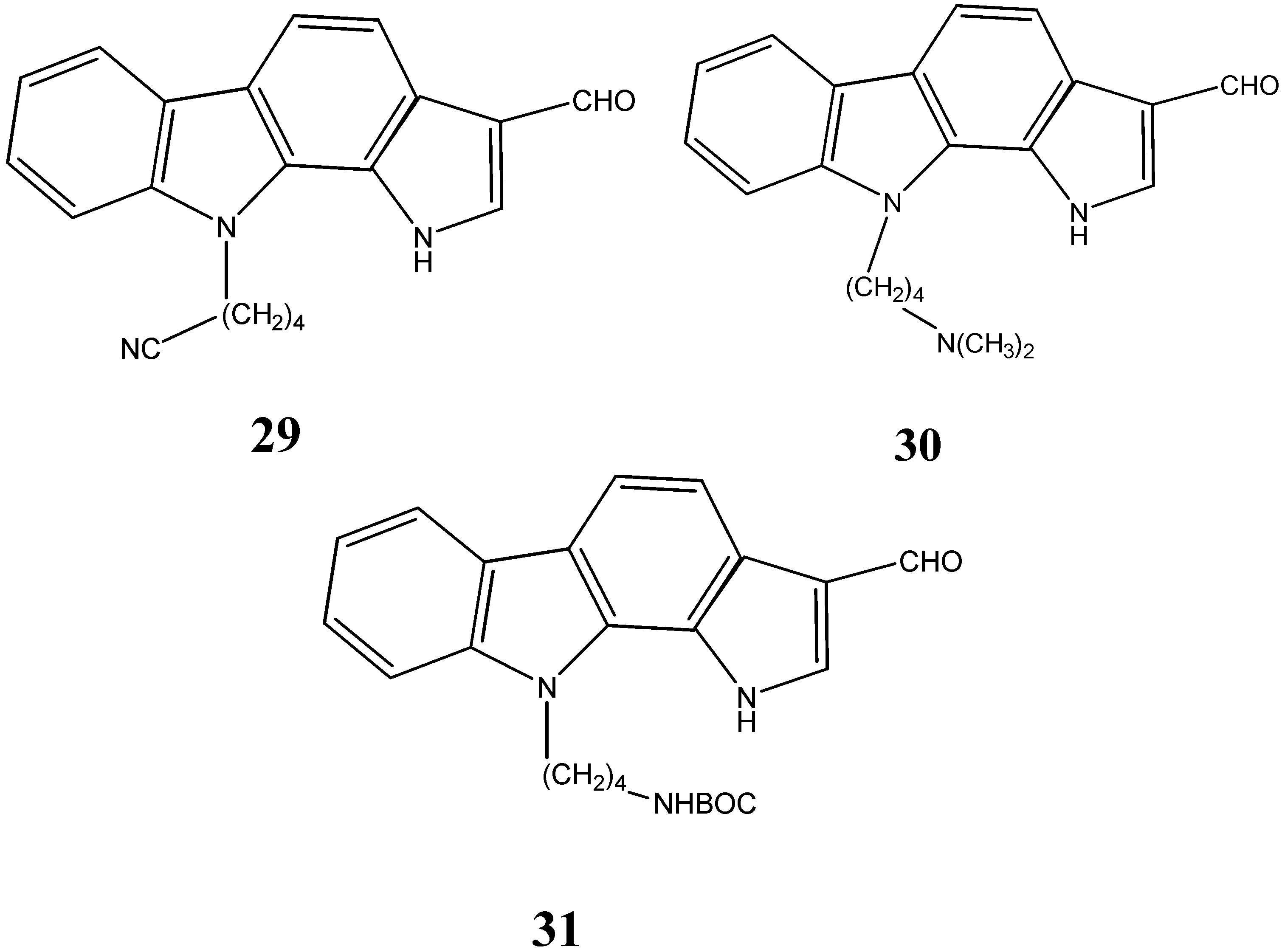
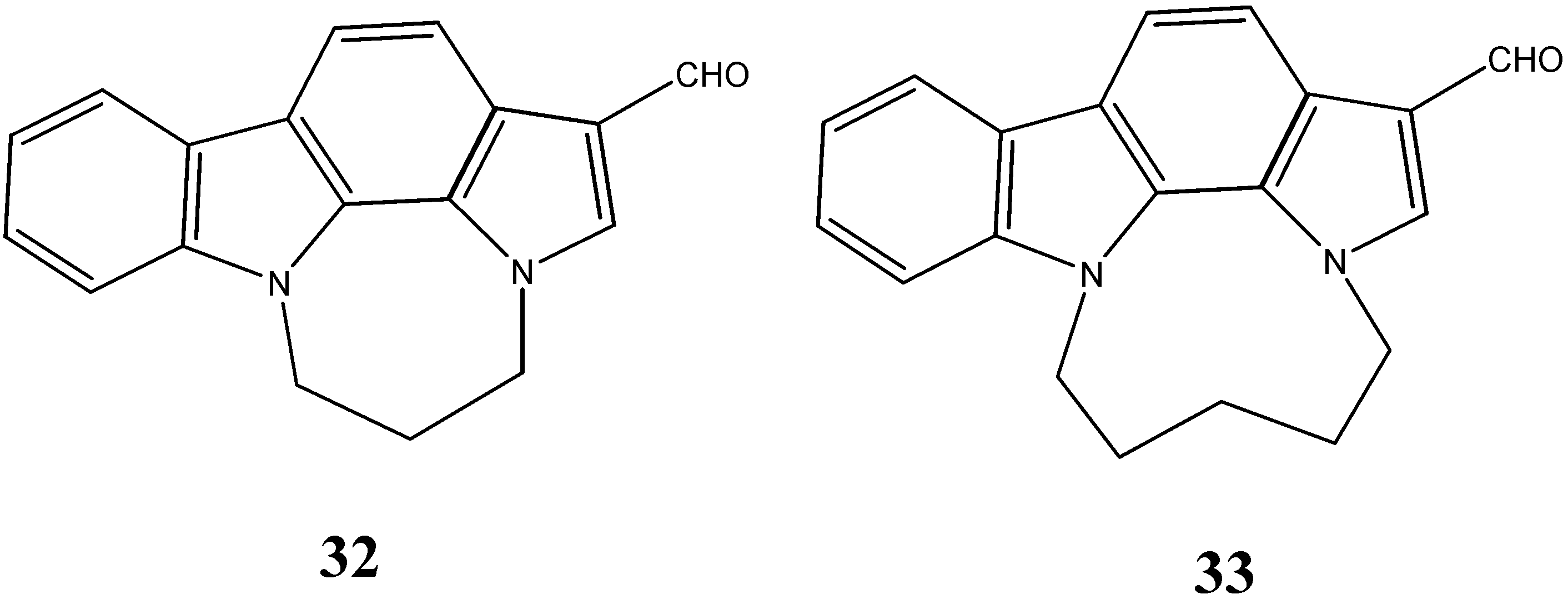
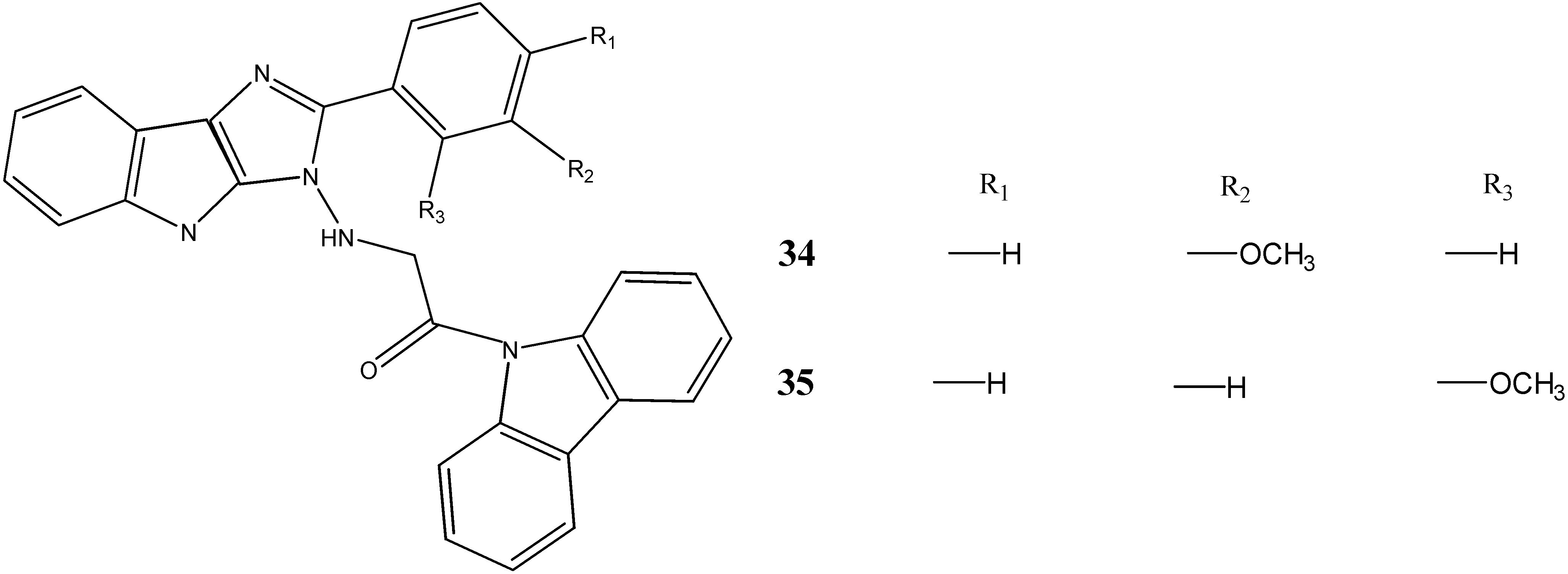
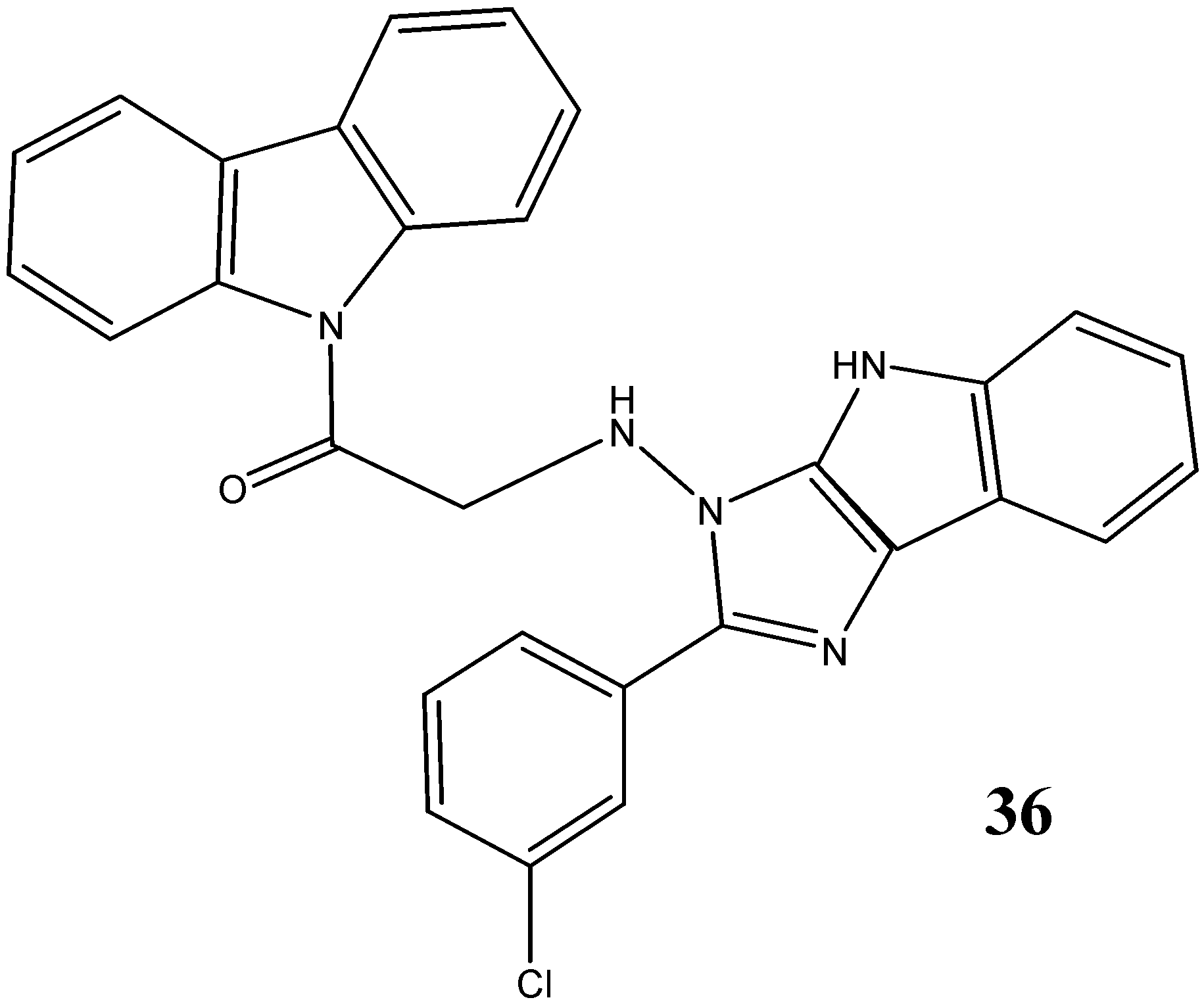
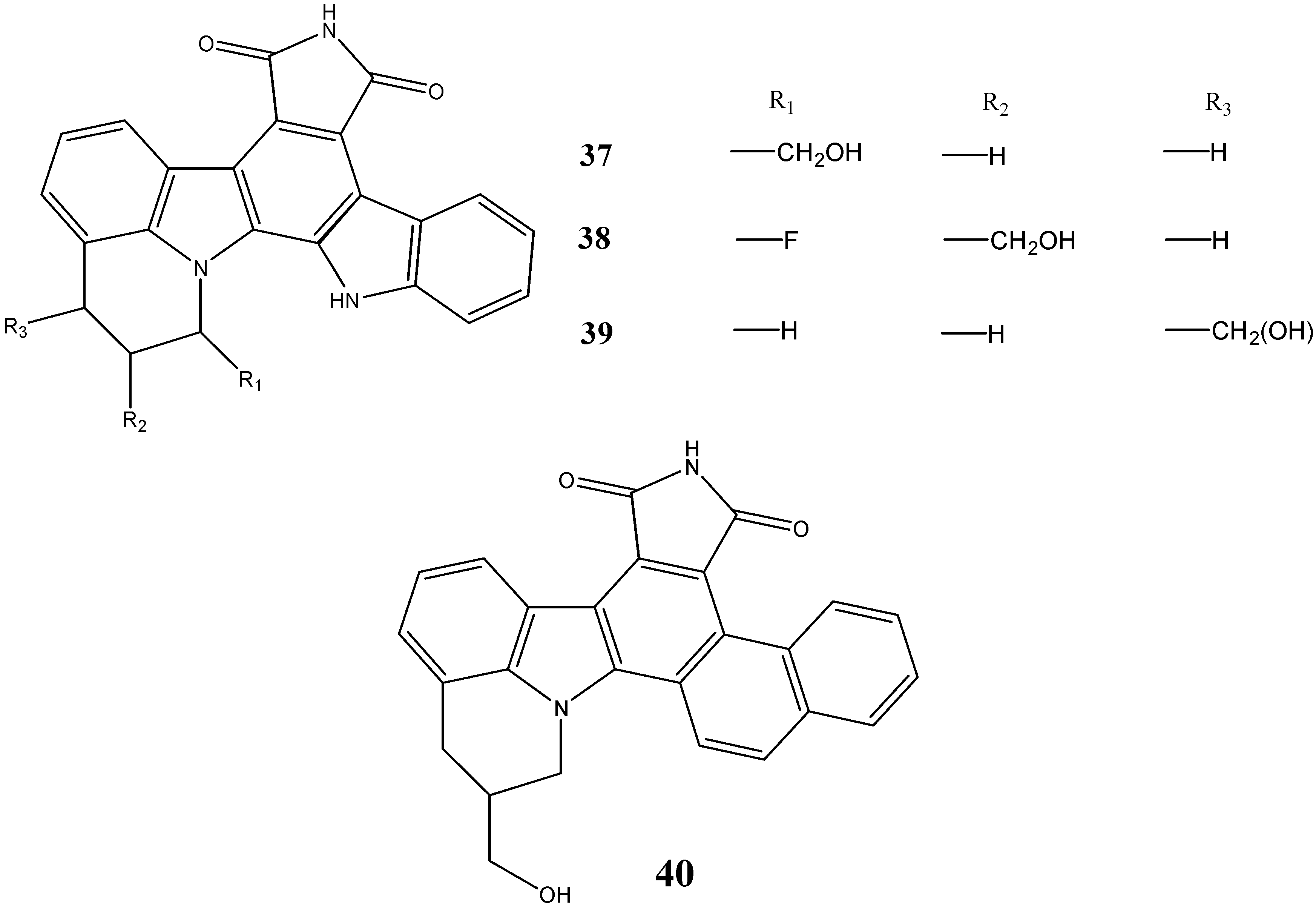
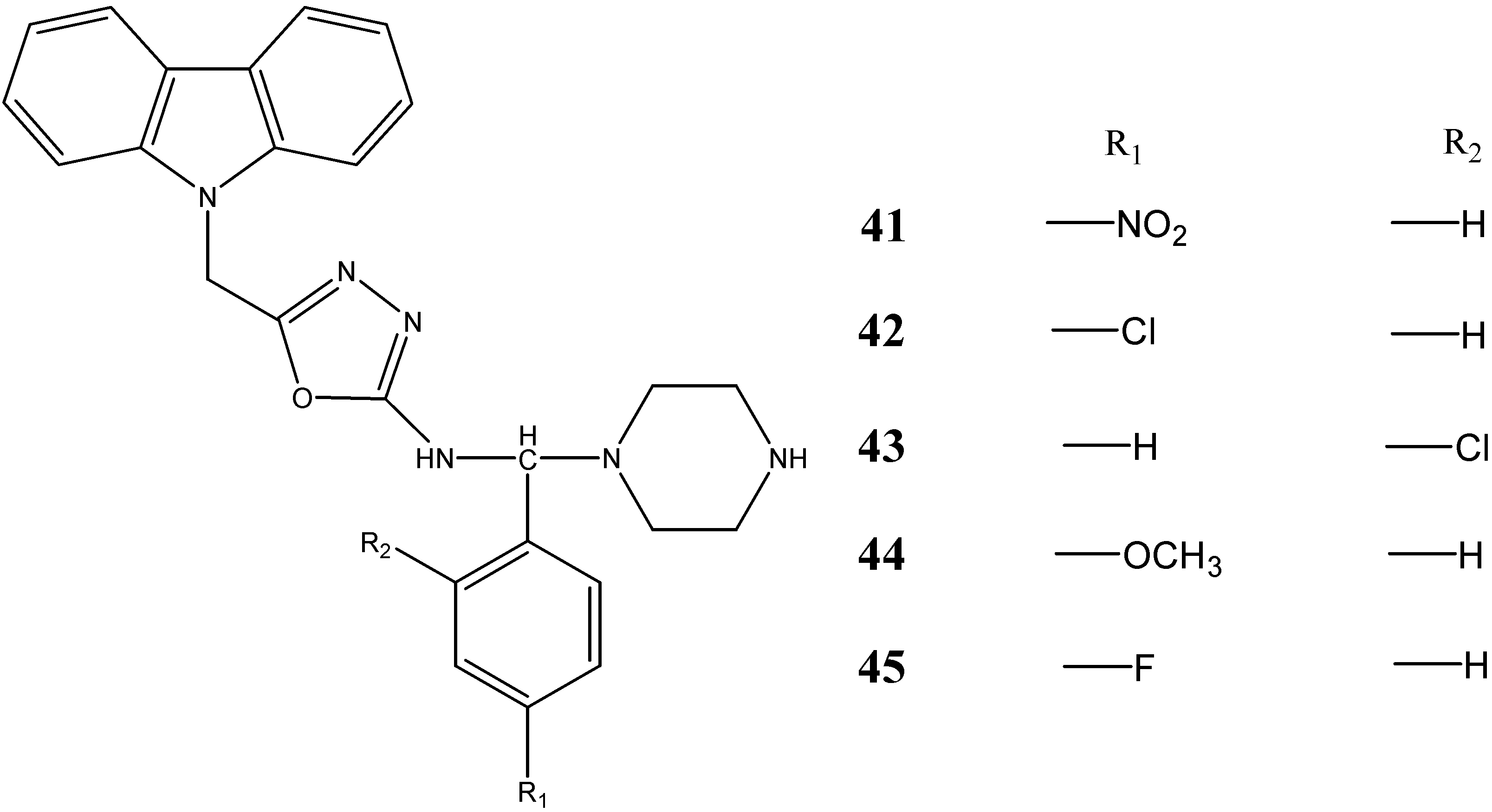
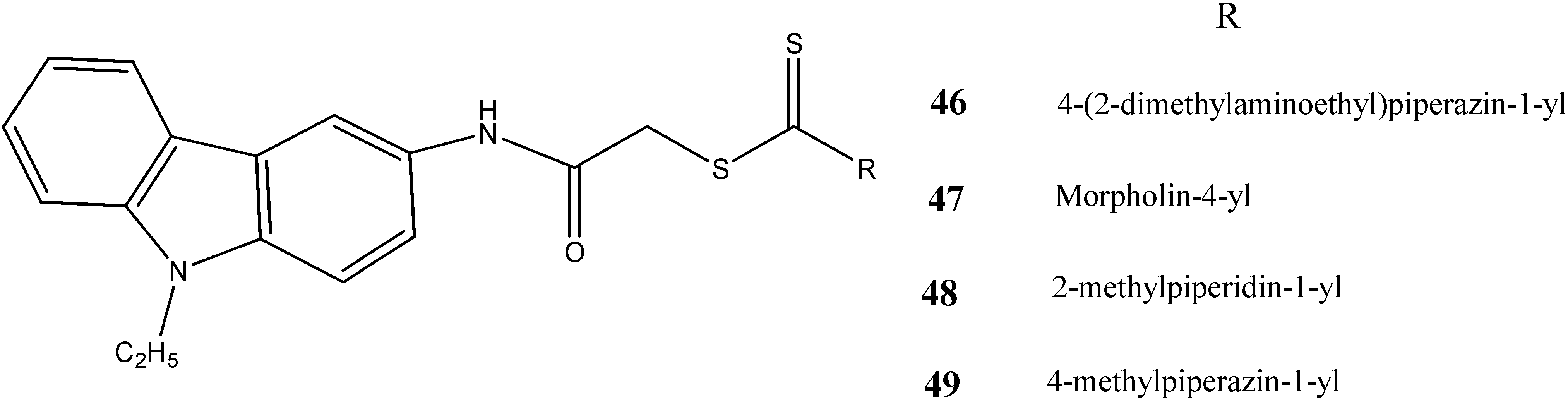
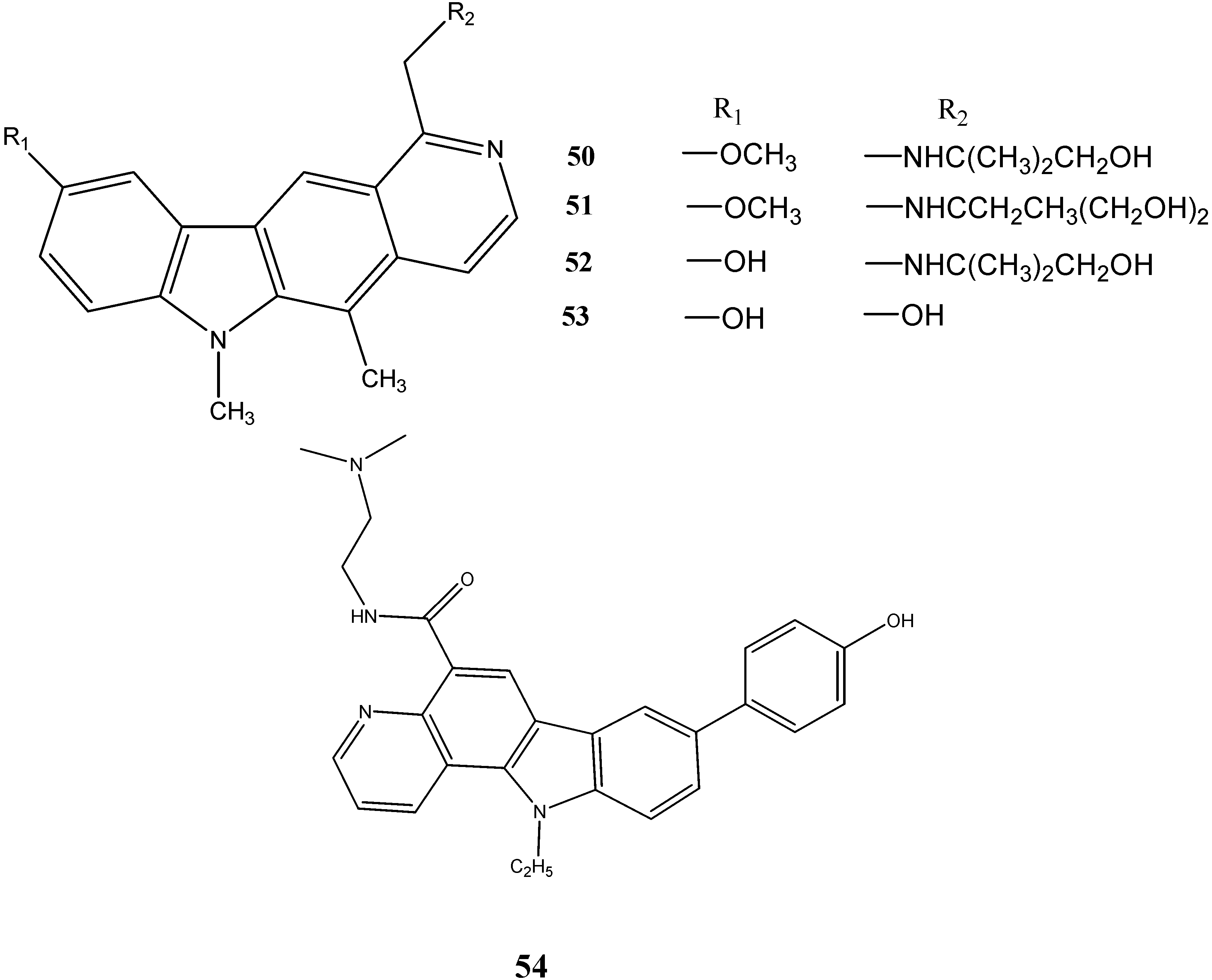
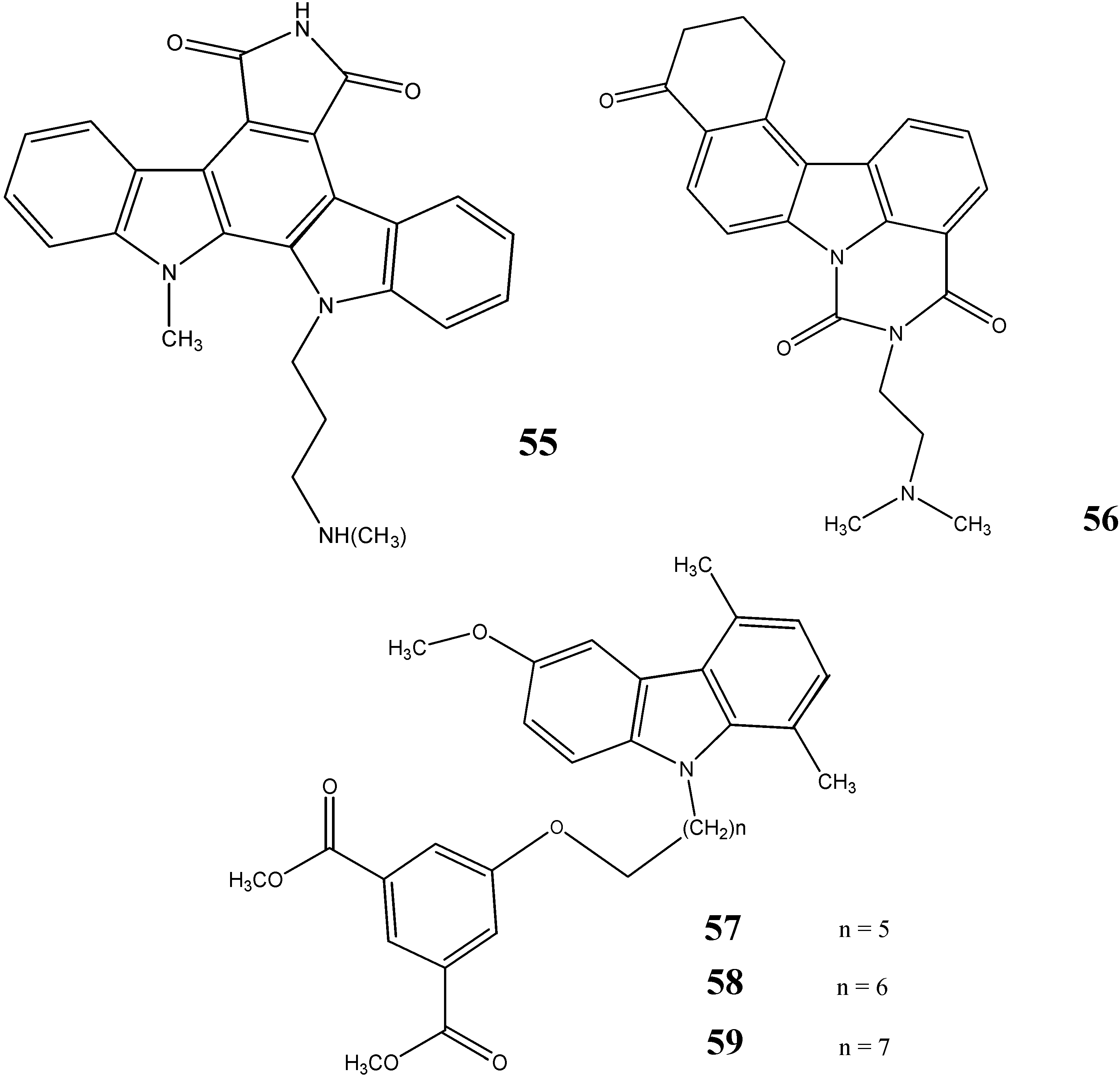
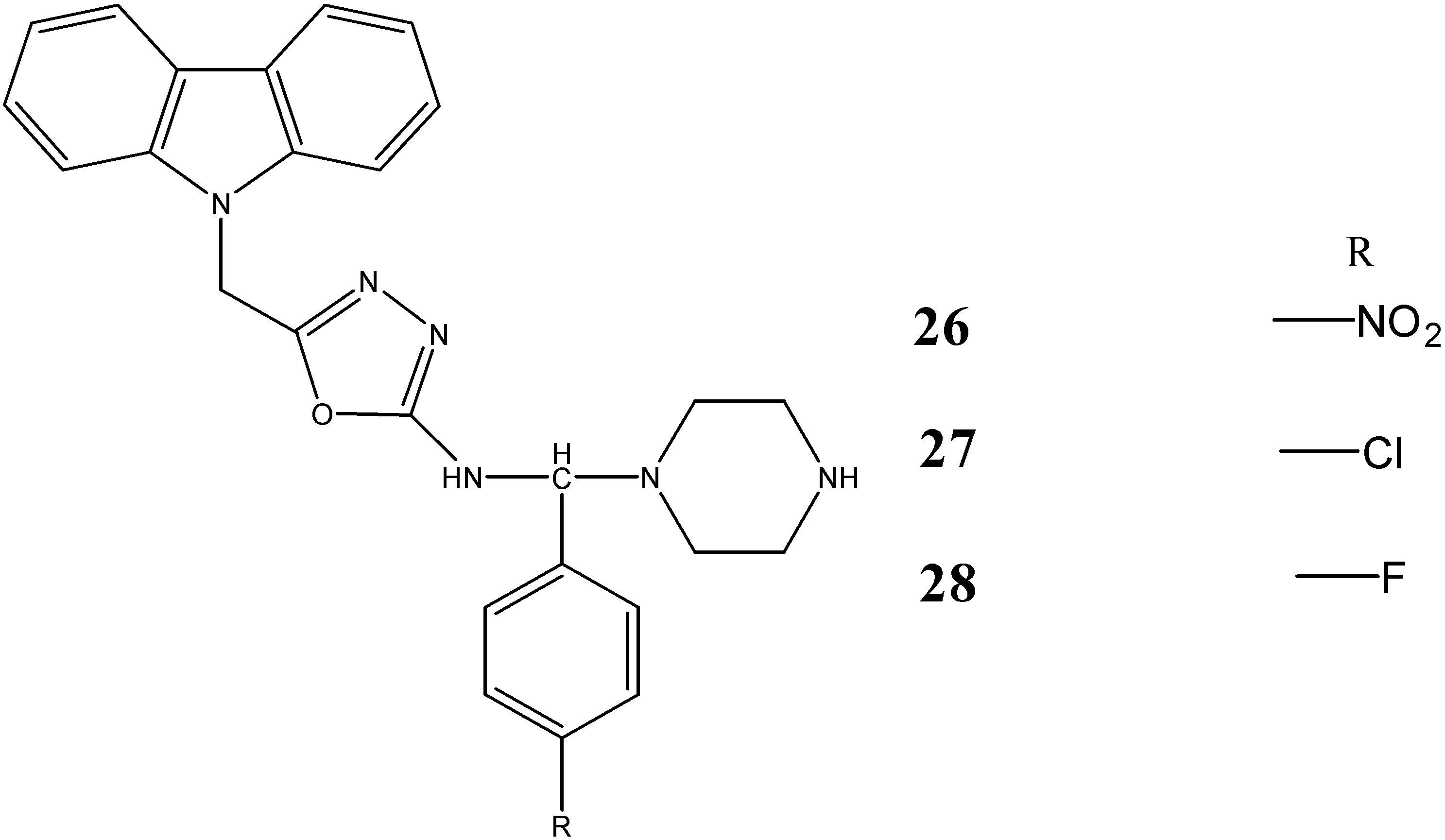
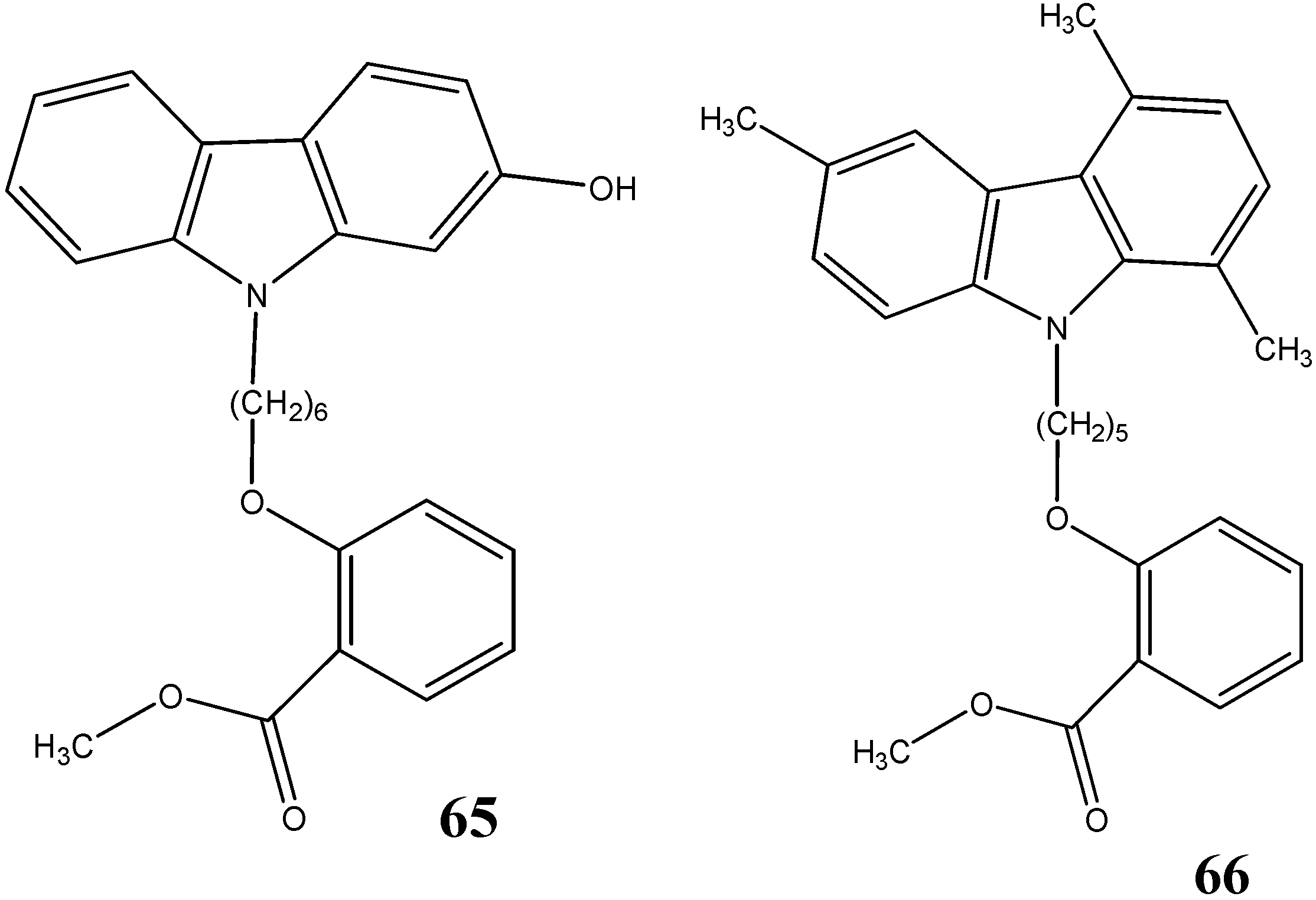
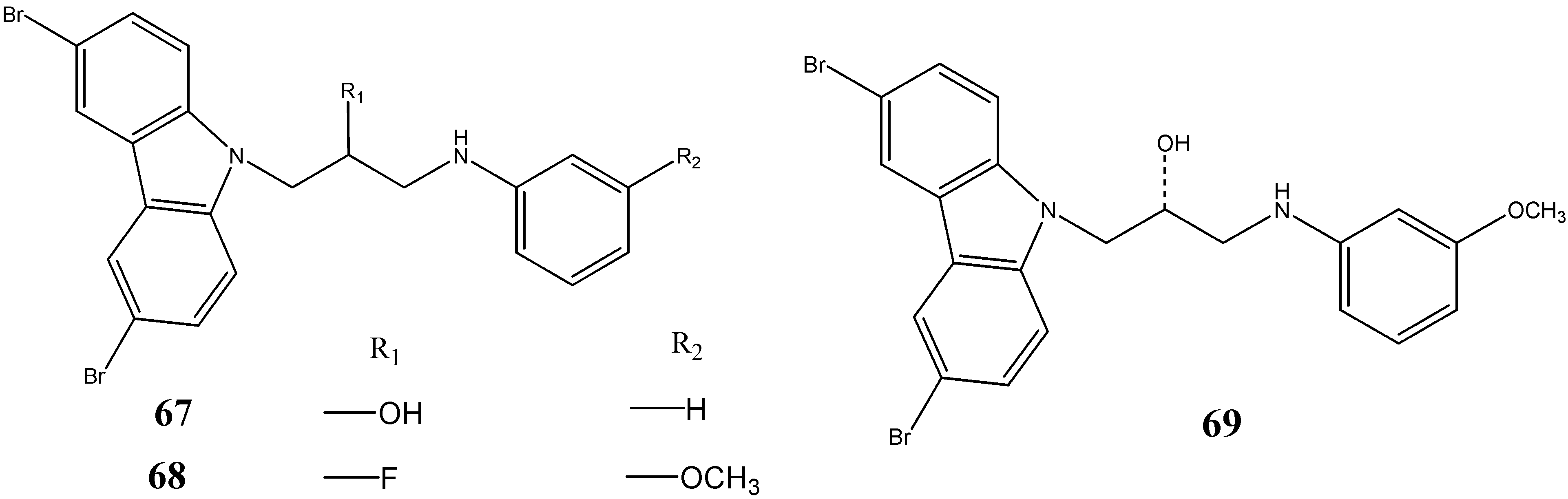
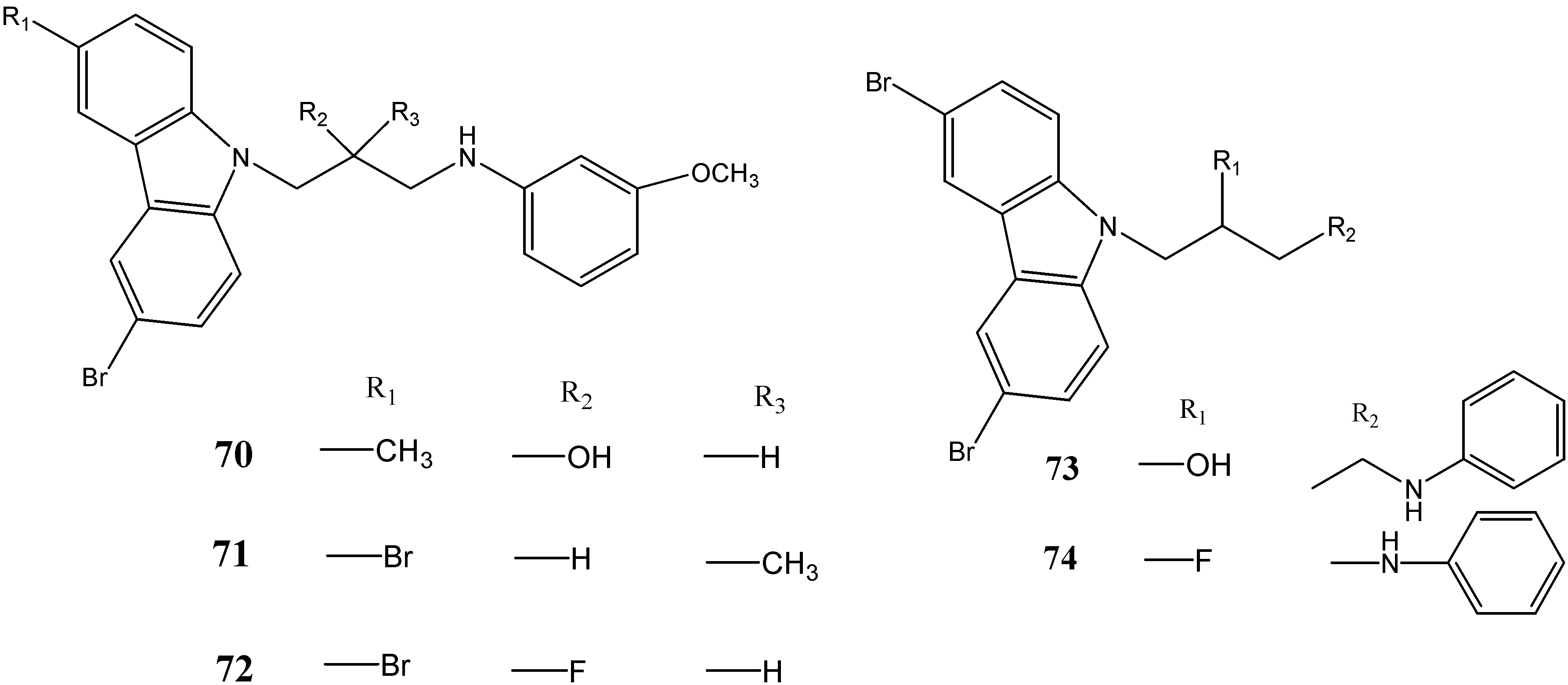
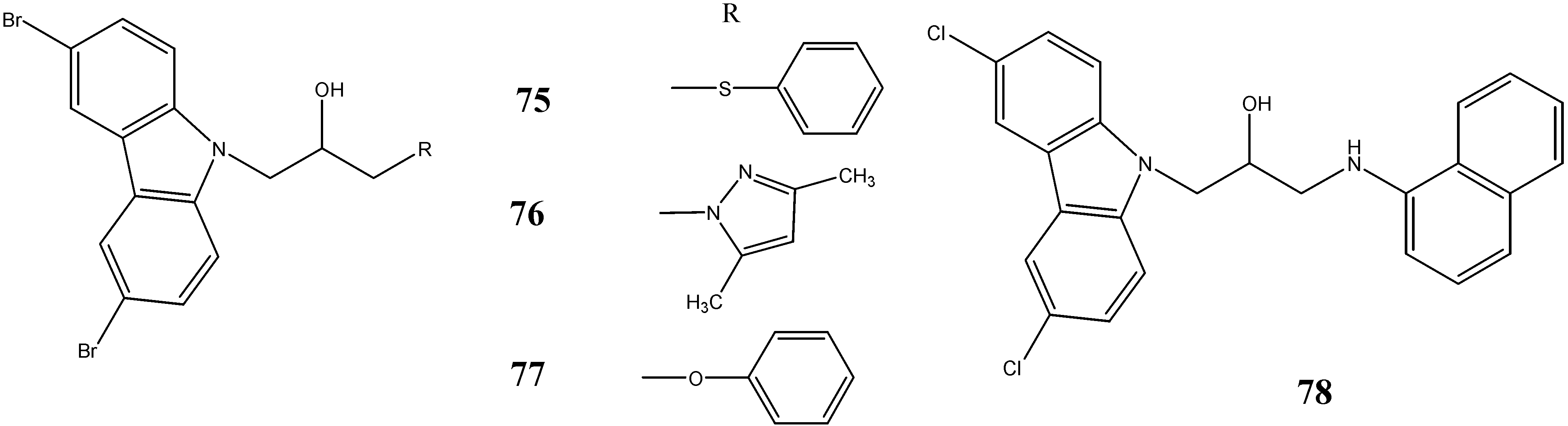
2.3. Neuroprotective Activity
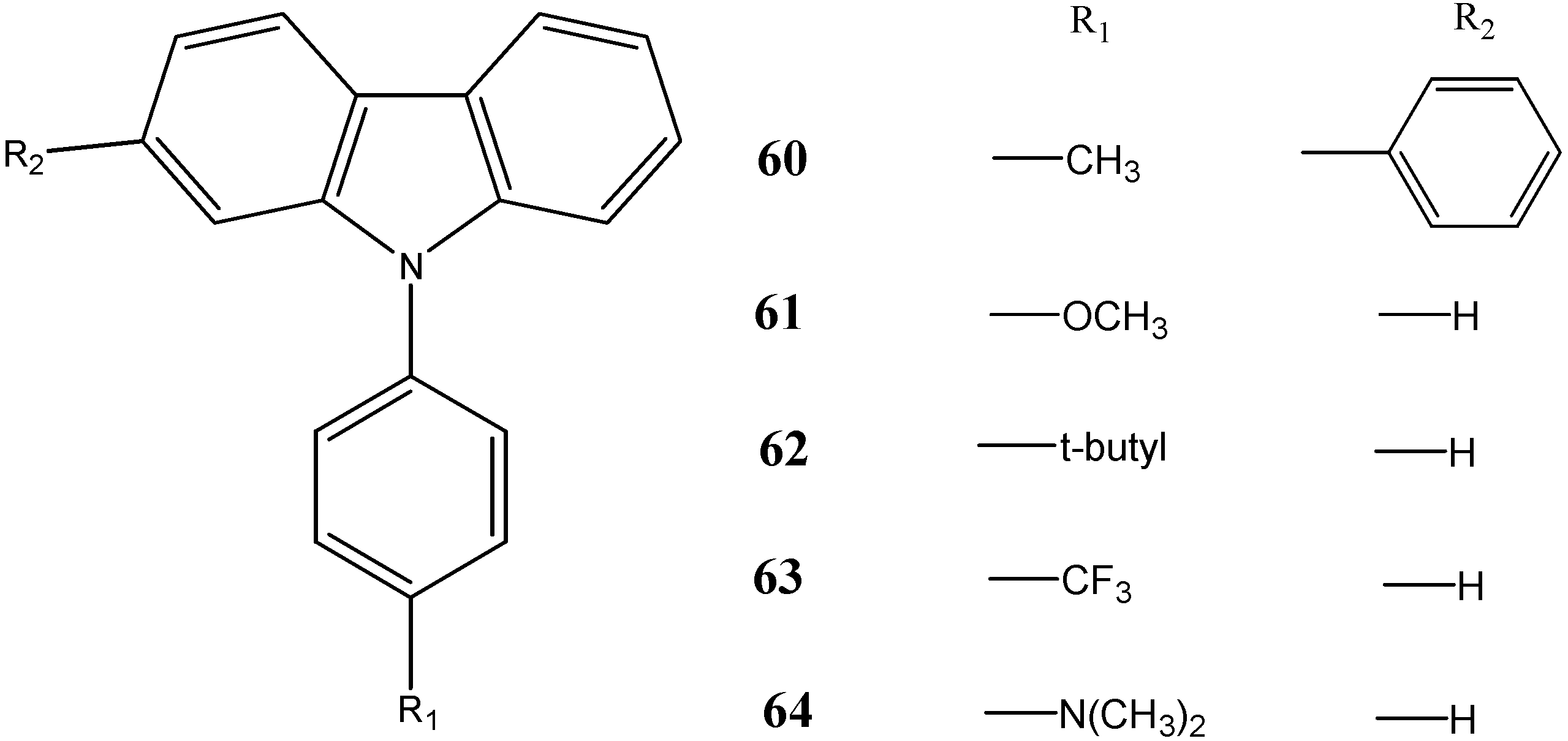
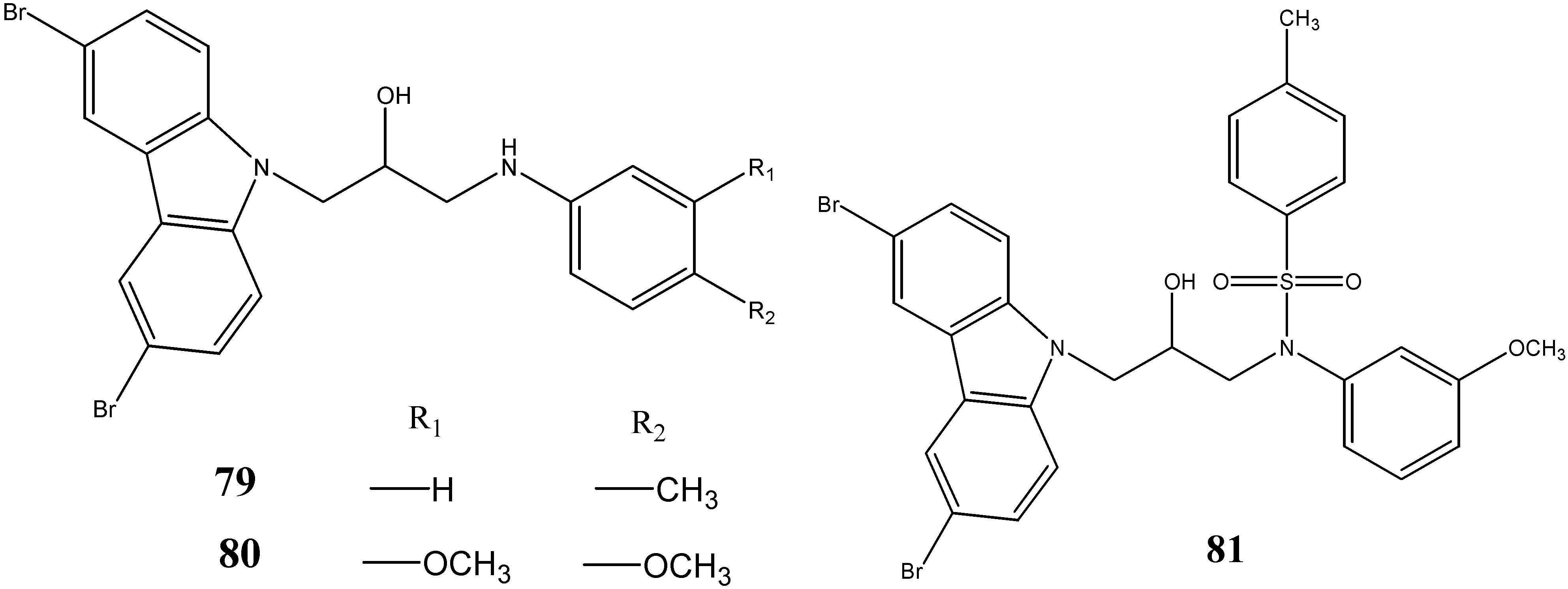
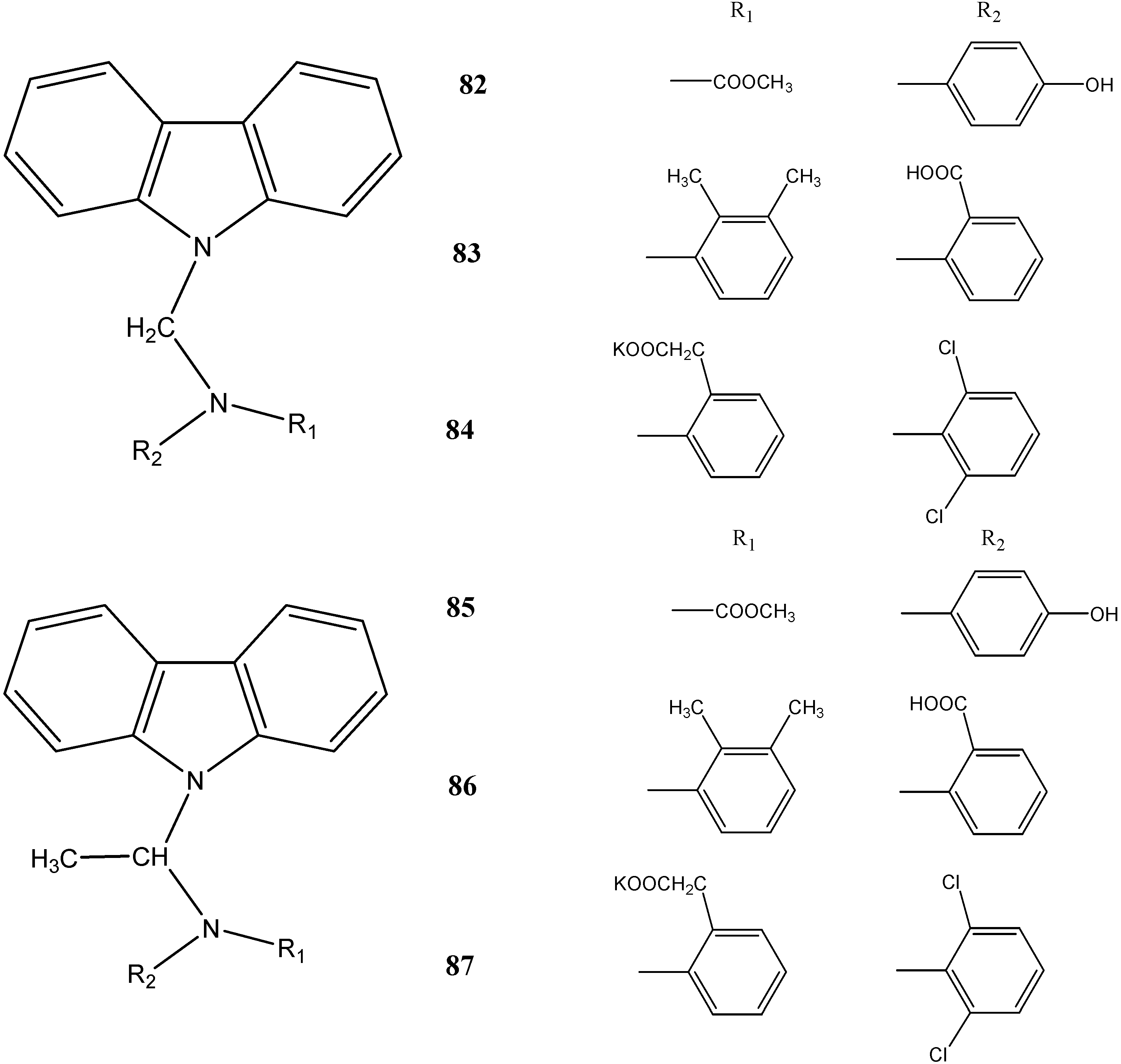
2.4. Anti-Epileptic and Antinociceptive Activities
| Treatment | Basel Reaction Time (s) before Treatment (Mean ± SEM) | Reaction Time (s) after Administration (Mean ± SEM) | |||
|---|---|---|---|---|---|
| 15 min | 30 min | 60 min | 120 min | ||
| Control | 4.1232 ± 0.2342 | 4.0228 ± 0.2322 | 4.1244 ± 0.2478 | 4.3244 ± 0.2286 | 4.3646 ± 0.2672 |
| Std a | 4.5120 ± 0.9012 | 8.800 ± 0.9031 ** | 10.22 ± 0.2642 ** | 11.48 ± 0.3476 ** | 13.22 ± 0.2974 ** |
| 82 | 4.9400 ± 0.6274 | 5.560 ± 0.6325 * | 6.48 ± 0.2224 * | 8.66 ± 0.2462 ** | 10.34 ± 0.2874 ** |
| 83 | 4.8819 ± 0.4654 | 5.042 ± 0.4761 * | 6.58 ± 0.2978 * | 8.44 ± 0.2536 ** | 10.48 ± 0.3576 ** |
| 84 | 4.7258 ± 0.4839 | 5.702 ± 0.4830 * | 7.46 ± 0.2564 ** | 9.26 ± 0.2978 ** | 11.22 ± 0.6428 ** |
| 85 | 4.5276 ± 0.5432 | 5.226 ± 0.5428 ** | 7.62 ± 0.2464 ** | 8.70 ± 0.3260 ** | 9.86 ± 0.2642 ** |
| 86 | 4.5276 ± 0.5324 | 5.406 ± 0.5428 * | 7.44 ± 0.4242 ** | 9.66 ± 0.2484 ** | 10.26 ± 0.2564 ** |
| 87 | 4.6863 ± 0.4912 | 5.863 ± 0.3726 * | 7.54 ± 0.4264 ** | 9.02 ± 0.2478 ** | 10.68 ± 0.2346 ** |
3. Conclusions
Conflicts of Interest
References
- Saini, M.S.; Kumar, A.; Dwivedi, J.; Singh, R. A review: Biological significances of heterocyclic compounds. Int. J. Pharm. Sci. Res. 2013, 4, 66–77. [Google Scholar]
- Rathavi, A.; Shukla, M.; Thakor, M.K. Synthesis and in vitro antibacterial and antifungal activity of trisubstituted S-triazines. J. Chem. Biol. Phys. Sci. Sec. A 2014, 4, 85–92. [Google Scholar]
- Dua, R.; Shrivastava, S.; Sonwane, S.K.; Srivastava, S.K. Pharmacological significance of synthetic heterocycles scaffold: A review. Adv. Biol. Res. 2011, 5, 120–144. [Google Scholar]
- Valverde, M.G.; Torroba, T. Sulfur-nitrogen heterocycles. Molecules 2005, 10, 318–320. [Google Scholar] [CrossRef]
- Nandy, B.C.; Gupta, A.K.; Mittal, A.; Vyas, V. Carbazole: It’s biological activity. J. Biomed. Pharm. Res. 2014, 3, 42–48. [Google Scholar]
- Knölker, H.J.; Reddy, K.R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002, 102, 4303–4428. [Google Scholar] [CrossRef] [PubMed]
- Knölker, H.J.; Fröhner, W.; Reddy, K.R. Iron-mediated synthesis of carbazomycin G and carbazomycin H, the first carbazole-1,4-quinol alkaloids from Streptoverticillium ehimense. Eur. J. Org. Chem. 2003, 4, 740–746. [Google Scholar] [CrossRef]
- Hagiwara, H.; Choshi, T.; Fujimoto, H.; Sugino, E.; Hibino, S. A novel total synthesis of antibiotic carbazole alkaloid carbazomycin G. Tetrahedron 2000, 56, 5807–5811. [Google Scholar] [CrossRef]
- Cuong, N.M.; Wilhelm, H.; Porzel, A.; Arnold, N.; Wessjohann, L. 1-O-Substituted derivatives of murrayafoline A and their antifungal. Nat. Prod. Res. 2008, 22, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, Z.; Issa, S.; Gentili, J.; Gratz, A.; Bollacke, A.; Kassack, M.; Jose, J.; Herfindal, L.; Gausdal, G.; Døskeland, S.O.; et al. Biologically active carbazole derivatives: Focus on oxazinocarbazoles and related compounds. J. Enzym. Inhib. Med. Chem. 2015, 30, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.D.; Kang, S.K.; Cheon, H.G.; Choi, J.K. Synthesis of tetrahydrocarbazole derivatives as potent β3-adrenoceptor agonists. Bull. Korean Chem. Soc. 2004, 25, 1784–1790. [Google Scholar]
- Asche, C.; Frank, W.; Albert, A.; Kucklaender, U. Synthesis, antitumour activity and structure-activity relationships of 5H-benzo[b]carbazoles. Bioorg. Med. Chem. 2005, 13, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Hajbi, Y.; Neagoie, C.; Biannic, B.; Chilloux, A.; Vedrenne, E.; Bladeyrou, B.; Bailly, C.; Mérour, J.Y.; Rosca, S.; Routier, S.; et al. Synthesis and biological activities of new furo[3,4-b]carbazoles: Potential topoisomerase II inhibitors. Eur. J. Med. Chem. 2010, 45, 5428–5437. [Google Scholar] [CrossRef] [PubMed]
- Tylinska, B.; Howorko, R.J.; Mastalarz, H.; Klopotowska, D.; Fillip, B.; Wietrzyk, J. Synthesis and structure-activity relationship analysis of new olivacine derivatives. Acta Pol. Pharm. Drug Res. 2010, 67, 495–502. [Google Scholar]
- Giraud, F.; Akué-Gédu, R.; Nauton, L.; Candelon, N.; Debiton, E.; Théry, V.; Anizon, F.; Moreau, P. Synthesis and biological activities of 4-substituted pyrrolo[2,3-a]carbazole Pim kinase inhibitors. Eur. J. Med. Chem. 2012, 56, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Akué-Gédu, R.; Letribot, B.; Saugues, E.; Debiton, E.; Anizon, F.; Moreau, P. Kinase inhibitory potencies and in vitro antiproliferative activities of N-10 substituted pyrrolo[2,3-a]carbazole derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 3807–3809. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Schaefer, G.; Heffron, T.P.; Shao, L.; Ye, X.; Sideris, S.; Malek, S.; Chan, E.; Merchant, M.; La, H.; et al. Noncovalent wild-typesparing inhibitors of EGFR T790M. Cancer Discov. 2013, 3, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Suzuki, T.; Nakada, M. Asymmetric catalysis of Nozaki−Hiyama allylation and methallylation with a new tridentate bis(oxazolinyl)carbazole Ligand. J. Am. Chem. Soc. 2003, 125, 1140–1141. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, G.H. Heterocyclic analogues of carbazole alkaloids. Curr. Org. Chem. 2001, 5, 507–518. [Google Scholar] [CrossRef]
- Achab, S.; Diker, K.; Potier, P. A short route to functionalized imidazo[4,5-c]carbazoles. Synthesis of the first example of the imidazo[4,5-c]β-carboline ring system. Tetrahedron Lett. 2001, 42, 8825–8828. [Google Scholar] [CrossRef]
- Chabane, H.; Lamazzi, C.; Thiery, V.; Guillaumet, G.; Besson, T. Synthesis of novel 2-cyanothiazolocarbazoles analogues of ellipticine. Tetrahedron Lett. 2002, 43, 2483–2486. [Google Scholar] [CrossRef]
- Oliveria, M.M.; Salvador, M.A.; Coelho, P.J.; Carvalho, L.M. New benzopyranocarbazoles: Synthesis and photochromic behavior. Tetrahedron 2005, 61, 1681–1691. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Nakano, K.; Nozaki, K. Synthesis, structures, and properties of unsymmetrical heteroacenes containing both pyrrole and furan rings. Org. Lett. 2008, 10, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.E.; Prasad, K.J.R. Synthesis and characterization of carbazole derivatives and their antimicrobial studies. Acta Pharm. 2006, 56, 79–86. [Google Scholar] [PubMed]
- Guillonneau, C.; Pierre, A.; Charton, Y.; Guilbard, N.; Berthier, L.K.; Leonce, S.; Michael, A.; Bisagni, E.; Atassi, G. Synthesis of 9-O-substituted derivatives of 9-hydroxy-5,6-dimethyl-6H- pyrido[4,3-b]carbazole-1-carboxylic acid (2-(Dimethylamino)ethyl)amide and their 10- and 11-methyl analogues with improved antitumor activity. J. Med. Chem. 1999, 42, 2191–2203. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, B.P.; Adsul, L.K.; Chavan, H.V.; Jalde, S.S.; Shringare, S.N.; Shaikh, R.; Meshram, R.J.; Gacche, R.N.; Masand, V. Synthesis, biological evaluation, and docking studies of 3-(substituted)-aryl-5-(9-methyl-3-carbazole)-1H-2-pyrazolines as potent anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. Lett. 2012, 22, 5839–5844. [Google Scholar] [CrossRef] [PubMed]
- Biamonte, M.A.; Wanner, J.; Roch, K.G.L. Recent advances in malaria drug discovery. Bioorg. Med. Chem. Lett. 2013, 23, 2829–2843. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Chiret, A.S.V.; Lancelot, J.C.; Sinicropi, M.S.; Garofalo, A.; Rault, S. Efficient and Simple Synthesis of 6-Aryl-1,4-dimethyl-9H-carbazoles. Molecules 2008, 13, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, M.; Ghosh, N.; Harigaya, Y. A clay-mediated, regioselective synthesis of 2-(aryl/alkyl)amino-thiazolo[4,5-c]carbazoles. Tetrahedron Lett. 2004, 45, 4955–4957. [Google Scholar] [CrossRef]
- Issa, S.; Walchshofer, N.; Kassab, I.; Termoss, H.; Chamat, S.; Geahchan, A.; Bouaziz, Z. Synthesis and antiproliferative activity of oxazinocarbazole and N,N-bis(carbazolylmethyl)amine derivatives. Eur. J. Med. Chem. 2010, 45, 2567–2577. [Google Scholar] [CrossRef] [PubMed]
- Danish, A.I.; Prasad, K.J.R. A one-pot synthesis of 1,2,4,5-tetraazaspiro [5.5]-6,7,8, 9-tetrahydrocarbazol-3-thiones and their antibacterial activities. Indian J. Heterocycl. Chem. 2006, 14, 19–22. [Google Scholar]
- Indumati, T.; Fronczek, F.R.; Prasad, K.J.R. Synthesis of 2-amino-8-chloro-4-phenyl-5,11-dihydro-6H-pyrido[2,3-a]carbazole-3-carbonitrile: Structural and biological evaluation. J. Mol. Struct. 2012, 1016, 134–139. [Google Scholar] [CrossRef]
- Kantevari, S.; Yempala, T.; Surineni, G.; Sridhar, B.; Sriram, D. Synthesis and antitubercular evaluation of novel dibenzo[b,d]furan and 9-methyl-9H-carbazole derived hexahydro-2H-pyrano[3,2-c]quinolines via Povarov reaction. Eur. J. Med. Chem. 2011, 46, 4827–4833. [Google Scholar] [CrossRef] [PubMed]
- Uttley, A.H.C.; Collins, C.H.; Naidoo, J.; George, R.C. Vancomycin-resistant enterococci. Lancet 1988, 1, 57–58. [Google Scholar] [CrossRef]
- Fox, R.; Neal, K.R.; Leen, C.L.S.; Ellis, M.E.; Mandal, B.K. Fluconazole resistant candida in AIDS. J. Infect. 1991, 22, 201–204. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Bale, M.; Buschelman, B. Selection of candidate quality control isolates and tentative quality control ranges for in vitro susceptibility testing of yeast isolates by National Committee for Clinical Laboratory Standards proposed standard methods. J. Clin. Microbiol. 1994, 32, 1650–1653. [Google Scholar] [PubMed]
- Gu, W.; Wang, S.F. Synthesis and antimicrobial activities of novel 1H-dibenzo[a,c]carbazoles from dehydroabietic acid. Eur. J. Med. Chem. 2010, 45, 4692–4696. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, G.; Hannan, A.; Akbar, E.; Usman, M.; Hamid, A.; Sadiq, Z.; Iqbal, M. Synthesis, antibacterial, and antifungal activities of novel pyridazino carbazoles. J. Chem. 2013, 2013, 818739. [Google Scholar] [CrossRef]
- Zhang, F.F.; Gan, L.L.; Zhou, G.H. Synthesis, anti-bacterial and antifungal activites of some carbazole derivatives. Bioorg. Med. Chem. 2010, 20, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Qiao, C.; Wang, S.F.; Hao, Y.; Miao, T.T. Synthesis and biological evaluation of novel N-substituted 1H-dibenzo[a,c]carbazole derivatives of dehydroabietic acid as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2014, 24, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Kaissy, W.W.N.A.; Tuama, S.H.F.; Majidi, S.M.H.A. Synthesis, characterization and evaluation of antimicrobial activity of some new acetylenic amine and 2- oxoazetidine of carbazole. Am. J. Sci. Ind. Res. 2013, 4, 389–398. [Google Scholar]
- Reddy, S.V.L.; Naresh, K.; Raju, C.N. New sulfonamide and carbamate derivatives of 4-(oxiran-2-ylmethoxy)-9Hcarbazole: Synthesis, characterization, antimicrobial and antioxidant activities. Der Pharm. Lett. 2013, 5, 221–231. [Google Scholar]
- Kumar, N.; Sharma, G.K.; Pathak, D. Microwave assisted and parallel synthesis of novel substituted carbazole derivatives of biological interest. Int. J. Pharm. Chem. Sci. 2013, 2, 273–282. [Google Scholar]
- Kaushik, K.; Kumar, N.; Pathak, D. Synthesis of some newer carbazole derivatives and evaluation for their pharmacological activity. Der Pharm. Sin. 2012, 3, 470–478. [Google Scholar]
- Segall, A.I.; Vitale, M.F.; Perez, V.L.; Pizzorno, M.T. HPLC analysis of 5H-benzo[a]carbazole with antifungal activity. J. Pharm. Biomed. Anal. 2003, 31, 1021–1026. [Google Scholar] [CrossRef]
- Thevissen, K.; Marchand, A.; Chaltin, P.; Meert, E.M.K.; Cammue, B.P.A. Antifungal carbazoles. Curr. Med. Chem. 2009, 16, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Salih, N.; Salimon, J.; Yousif, E. Synthesis and antimicrobial activities of 9H-carbazole derivatives. Arabian J. Chem. 2011, 2011. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, N.; Pathak, D. Synthesis, characterization and biological evaluation of some newer carbazole derivatives. J. Serb. Chem. Soc. 2014, 79, 125–132. [Google Scholar] [CrossRef]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef] [PubMed]
- Giraud, F.; Bourhis, M.; Nauton, L.; Théry, V.; Herfindal, L.; Døskeland, S.O.; Anizon, F.; Moreau, P. New N-1,N-10-bridged pyrrolo[2,3-a]carbazole-3-carbaldehydes: Synthesis and biological activities. Bioorg. Chem. 2014, 57, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Conner, S.E.; Zhou, X.; Chan, H.K.; Shih, C.; Engler, T.A.; Awar, R.S.A.; Brooks, H.B.; Watkins, S.A.; Spencer, C.D.; et al. Synthesis of 1,7-annulated indoles and their applications in the studies of cyclin dependent kinase inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 3057–3061. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, G.A.; Temel, H.E.; Yildirim, S.U.; Kaplancikli, Z.A.; Altintop, M.D.; Genc, L. Apoptotic effects of some carbazole derivatives on lung carcinoma and glioma cell lines. Med. Chem. Res. 2013, 22, 3751–3759. [Google Scholar] [CrossRef]
- Howorko, R.J.; Tylinska, B.; Biadun, B.; Gebarowski, T.; Gasiorowski, K. New pyridocarbazole derivatives. Synthesis and their in Vitro anticancer activity. Act. Pol. Pharm. Drug Res. 2013, 70, 823–832. [Google Scholar]
- Li, B.; Yue, Z.Z.; Feng, J.M.; He, Q.; Miao, Z.H.; Yang, C.H. Design and Synthesis of Pyrido[3,2-α]carbazole derivatives and their analogues as potent antitumor agents. Eur. J. Med. Chem. 2013, 66, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Eastman, A.; Gribble, G.W. Synthesis of bisindolylmaleimides related to GF109203x and their efficient conversion to the bioactive indolocarbazoles. Org. Biomol. Chem. 2006, 4, 3228–3234. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sugumi, H.; Yamaguchi, A.; Uenaka, T.; Kotake, Y.; Okada, T.; Kamata, J.; Niijima, J.; Nagasu, T.; Koyanagi, N.; et al. Antitumor activity of ER-37328, a novel carbazole topoisomerase II inhibitor. Mol. Cancer Ther. 2002, 1, 169–175. [Google Scholar] [PubMed]
- Saturnino, C.; Palladino, C.; Napoli, M.; Sinicropi, M.S.; Botta, A.; Sala, M.; Carcereri de Prati, A.; Novellino, E.; Suzuki, H. Synthesis and biological evaluation of new N-alkylcarbazole derivatives as STAT3 inhibitors: Preliminary study. Eur. J. Med. Chem. 2013, 60, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Khanum, F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012, 6, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Potential medicinal plants for CNS disorders: An overview. Phytother. Res. 2006, 20, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Doré, V.; Villemagne, V.L.; Bourgeat, P.; Fripp, J.; Acosta, O.; Chetélat, G.; Zhou, L.; Martins, R.; Ellis, K.A.; Masters, C.L.; et al. Cross-sectional and longitudinal analysis of the relationship between Aβ deposition, cortical thickness and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol. 2013, 70, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chen, M.; Li, M.; Luo, B.; Zhao, Y.; Huang, P.; Xue, F.; Rapposelli, S.; Pi, R.; Wen, S. Discovery of novel N-substituted carbazoles as neuroprotective agents with potent anti-oxidative activity. Eur. J. Med. Chem. 2013, 68, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, C.; Iacopetta, D.; Sinicropi, M.S.; Rosano, C.; Caruso, A.; Caporale, A.; Marra, N.; Marengo, B.; Pronzato, M.A.; Parisi, O.I.; et al. N-alkyl carbazole derivatives as new tools for Alzheimer’s disease: Preliminary studies. Molecules 2014, 19, 9307–1917. [Google Scholar] [CrossRef] [PubMed]
- Kamnasaran, D.; Muir, W.; Ferguson-Smith, M.; Cox, D. Disruption of the neuronal PAS3 gene in a family affected with schizophrenia. J. Med. Genet. 2003, 40, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Pickard, B.S.; Pieper, A.A.; Porteous, D.J.; Blackwood, D.H.; Muir, W.J. The NPAS3 gene—Emerging evidence for a role in psychiatric illness. Ann. Med. 2006, 38, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Erbel-Sieler, C.; Dudley, C.; Zhou, Y.; Wu, X.; Estill, S.J.; Han, T.; Diaz-Arrastia, R.; Brunskill, E.W.; Potter, S.S.; McKnight, S.L. Behavioral and regulatory abnormalities in mice deficient in NPAS1 and NPAS3 transcription factors. Proc. Natl. Acad. Sci. USA 2004, 101, 13648–13653. [Google Scholar] [CrossRef] [PubMed]
- Pieper, A.A.; Wu, X.; Han, T.W.; Estill, S.J.; Dang, W.; Wu, L.C.; Reece-Fincanon, S.; Dudley, C.A.; Richardson, J.A.; Brat, D.J.; et al. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 14052–14057. [Google Scholar] [CrossRef] [PubMed]
- Pieper, A.A.; Xie, S.; Capota, E.; Estill, S.J.; Zhong, J.; Long, J.M.; Becker, G.L.; Huntington, P.; Goldman, S.E.; Shen, C.H.; et al. Discovery of a Pro-neurogenic, Neuroprotective Chemical. Cell 2010, 142, 39–51. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, K.S.; Naidoo, J.; Liang, J.; Melito, L.; Williams, N.S.; Morlock, L.; Huntington, P.J.; Estill, S.J.; Longgood, J.; Becker, G.L.; et al. Development of proneurogenic, neuroprotective small molecules. J. Am. Chem. Soc. 2011, 133, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Cortés, H.D.J.; Xu, P.; Drawbridge, J.; Estill, S.J.; Huntington, P.; Tran, S.; Britt, J.; Tesla, R.; Morlock, L.; Naidoo, J.; et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. PNAS 2012, 109, 17010–17015. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Kong, S.Y.; Park, M.H.; Cho, Y.; Kim, S.E.; Shin, J.Y.; Jung, S.H.; Lee, J.; Farhanullah; Kim, H.J.; et al. Aminopropyl carbazole analogues as potent enhancers of neurogenesis. Bioorg. Med. Chem. 2013, 21, 7165–7174. [Google Scholar] [CrossRef] [PubMed]
- McCagh, J.; Fisk, J.E.; Baker, G.A. Epilepsy, psychosocial and cognitive functioning. Epilepsy Res. 2009, 86, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shindikar, A.V.; Khan, F.; Viswanathan, C.L. Design, synthesis and in vivo anticonvulsant screening in mice of novel phenylacetamides. Eur. J. Med. Chem. 2006, 41, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.J. Do we need any more new antiepileptic drugs? Epilepsy Res. 2001, 45, 3–6. [Google Scholar] [CrossRef]
- Bialer, M.; Johannessen, S.I.; Kupferberg, H.J.; Levy, R.Y.; Loiseau, P.; Perucca, E. Progress report on new antiepileptic drugs: A summary of the sixth Eilat conference (EILAT VI). Epilepsy Res. 2002, 51, 31–71. [Google Scholar] [CrossRef]
- Rajamanickam, V.; Rajasekarena, A.; Palanivelu, M.; Anandarajagopal, K.; Elahi, A.L.A.; Umaranib, N. Anti-nociceptive and anti-epileptic evaluation of N-Mannich bases of some substituted carbazoles. Int. J. Chem. Sci. 2008, 6, 1669–1675. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, M.; Bano, A.; Ijaz, A.S.; Chaudhary, B.A. Recent Developments and Biological Activities of N-Substituted Carbazole Derivatives: A Review. Molecules 2015, 20, 13496-13517. https://doi.org/10.3390/molecules200813496
Bashir M, Bano A, Ijaz AS, Chaudhary BA. Recent Developments and Biological Activities of N-Substituted Carbazole Derivatives: A Review. Molecules. 2015; 20(8):13496-13517. https://doi.org/10.3390/molecules200813496
Chicago/Turabian StyleBashir, Maryam, Afifa Bano, Abdul Subhan Ijaz, and Bashir Ahmad Chaudhary. 2015. "Recent Developments and Biological Activities of N-Substituted Carbazole Derivatives: A Review" Molecules 20, no. 8: 13496-13517. https://doi.org/10.3390/molecules200813496
APA StyleBashir, M., Bano, A., Ijaz, A. S., & Chaudhary, B. A. (2015). Recent Developments and Biological Activities of N-Substituted Carbazole Derivatives: A Review. Molecules, 20(8), 13496-13517. https://doi.org/10.3390/molecules200813496





