Abstract
This study describes some characteristics of the Rubiaceae family pertaining to the occurrence and distribution of secondary metabolites in the main genera of this family. It reports the review of phytochemical studies addressing all species of Rubiaceae, published between 1990 and 2014. Iridoids, anthraquinones, triterpenes, indole alkaloids as well as other varying alkaloid subclasses, have shown to be the most common. These compounds have been mostly isolated from the genera Uncaria, Psychotria, Hedyotis, Ophiorrhiza and Morinda. The occurrence and distribution of iridoids, alkaloids and anthraquinones point out their chemotaxonomic correlation among tribes and subfamilies. From an evolutionary point of view, Rubioideae is the most ancient subfamily, followed by Ixoroideae and finally Cinchonoideae. The chemical biosynthetic pathway, which is not so specific in Rubioideae, can explain this and large amounts of both iridoids and indole alkaloids are produced. In Ixoroideae, the most active biosysthetic pathway is the one that produces iridoids; while in Cinchonoideae, it produces indole alkaloids together with other alkaloids. The chemical biosynthetic pathway now supports this botanical conclusion.
Keywords:
Rubiaceae; Rubioideae; Cinchonoideae; Ixoroideae; iridoids; alkaloid; anthraquinones; triterpenes 1. Introduction
The Rubiaceae family is characterized by the production of bioactive metabolites with great pharmacological potential. These metabolites can be used as chemotaxonomic markers even for genera and subfamilies [1,2]. Usually, taxa are classified according to different botanical characteristics; classical taxonomic systems only consider the plant morphological characters, while modern systems correlate their various combinations, including the chemical composition. Studies correlating classical plant taxonomy to chemical data can be found as far back as 1699 [3].
Phytochemical compounds can be a useful tool for characterizing, describing and classifying plant species. The distribution of secondary metabolites in Rubiaceae follows patterns that may help characterize the botanical group (subfamily, tribe or genera). These patterns relative to chemotaxonomy are often used to establish the botanical origin [4].
In recent years, Rubiaceae species have been thoroughly studied from a phytochemical viewpoint. However, very few studies have used this knowledge as a tool in taxonomic studies. When conducting bioprospecting studies of a plant, all botanical and chemotaxonomic information is of great importance, since it increases the likelihood of finding bioactive compounds, which enables the discovery of new Nature-originated drugs [5]. Therefore, the present study aims to conduct a literature survey on phytochemical studies addressing species of Rubiaceae published from 1990 to 2014, and describe their secondary metabolites occurrence and distribution in the subfamilies, tribes and main genera of this family.
2. Taxonomic Classification of Rubiaceae
The Rubiaceae family has a cosmopolitan distribution, mostly concentrated in the tropics. Being one of the largest in the Magnoliopsida class, it ranks fourth in diversity of species among Angiosperms [4]. It includes approximately 637 genera and 13,000 species [5,6]. In Brazil, nearly 120 genera and 1400 species occur, representing one of the most important economic, ornamental and medicinal plant families in the Brazilian flora [7].
The Rubiaceae family taxonomic classification is complex and there are still some gaps which have to be filled. According to the classification of Robbrecht [8], the Rubiaceae family is divided into four subfamilies: Rubioideae, Cinchonoideae, Antirheoideae and Ixoroideae. However, more recent studies suggest this family to be divided into three subfamilies: Rubioideae, Cinchonoideae and Ixoroideae, as some authors do not recognize Antirheoideae as a subfamily, since molecular studies have shown it to be polyphyletic with no standardized occurrence of a chemical marker [9,10,11,12,13,14,15,16]. Due to the abundance of species, the subfamilies were divided into 43 tribes (an intermediate clade between genus and subfamily) [16], which are listed in Figure 1.
Due to the lack of studies that can complement the extant information on geographical distribution, morpho-anatomical characteristics and molecular data, there are still genera and species not allocated into any tribe [16]. The evaluation of the chemical profile of these species may indicate a more complete phylogenetic distribution, since the secondary metabolites are the results of adaptation and evolution of a specific taxon to environment [17]. Thus, the profile of secondary metabolites distribution can bring new information for the taxonomic classification of this family.
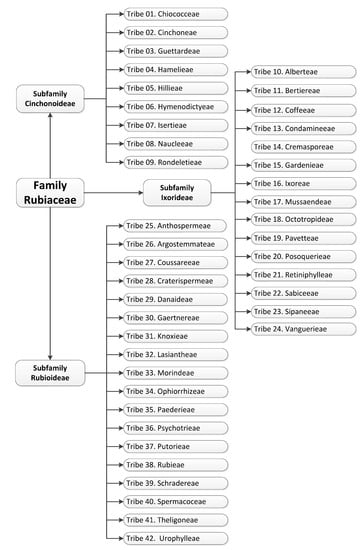
Figure 1.
Subfamilies and tribes belonging to the Rubiaceae family [16].
3. Chemical and Biological Aspects of Rubiaceae
The Rubiaceae family presents a large diversity of substances such as iridoids, indole alkaloids, anthraquinones, terpenoids (diterpenes and triterpenes), flavonoids and other phenolic derivatives, with emphasis on production of bioactive alkaloids [2]. Alkaloids are secondary metabolites that can generate various drugs with important pharmacological effects and used to find out physiological responses and biochemical mechanisms of action [18].
The number of described products, the structural diversity and pharmacological activities reported for various species of Rubiaceae demonstrate this family to be a promising source of new bioactive substances, which may give rise to new products as active molecules or even drug prototypes. Many of these plants have widespread use in folk medicine and some showed anti-inflammatory, analgesic, antibacterial, mutagenic, antiviral, antioxidant, effect on vascular diseases as well as activity on the central nervous system [19].
In the Ixoroideae subfamily, the genus Coffea is one of the most economically important, mainly the species Coffea arabica, popularly known as coffee, which has caffeine as one of its principal chemical components. This substance acts as stimulant of the central nervous system, as well as vasoconstrictor, bronchodilator and diuretic, besides being one of the components of migraine drugs [18]. Genipa, the Brazilian jenipapo (Genipa americana) with antiangiogenic, anti-inflammatory and antioxidant activity [20,21,22] is another important genus from which genipin was isolated, a colorless iridoid, used by indigenous people to tattoo their skin, since it produces a black coloration when it reacts with skin proteins. Its fruits are used to make wines, liqueurs, jams, soft drinks, etc. [23].
In the Cinchonoideae subfamily, Cinchona species are the source of quinine, isolated in 1820 by Pelletier and Caventou [24], and which for about 200 years was the only active substance against malaria, and can be considered as responsible for the development of synthetic antimalarials [1,25]. More than 50 new substances were isolated from alkaloid-rich Uncaria species [19], as Uncaria tomentosa, known as “unha de gato”, is one of most used plants in Brazilian folk medicine. Studies have shown that alkaloids isolated from this plant have immunostimulant and antitumor activity [26,27]. Other groups of substances such as triterpenes and procyanidins presented anti-inflammatory activity [28,29].
Psychotria, belonging to the Rubioideae subfamily, are plants that produce substances with activity on the central nervous system, such as Psychotria viridis, popularly known as “ayahuasca” which means “soul wine”. P. viridis is used in religious ceremonies in association with Banisteriopsis caapi, a species from the Malpighiaceae family [30,31]. Their hallucinogenic effect is due to the synergy that occurs between the alkaloid N,N-dimethyltryptamine (DMT), present in the leaves of P. viridis, and β-carboline indole alkaloids (harmine, harmaline and tetrahydroharmine) present in the bark of B. caapi [32]. Cephaelis is another important genus, especially C. ipecacuanha, a plant traditionally used by the Brazilian population, an important source of emetine, an alkaloid with emetic, antihelminthic and expectorant effects [33,34]. In Brazil, species of Palicourea are considered responsible for about half of all cattle deaths brought about by natural poisoning [35]. Some selected isolated compounds from Rubiaceae species are shown in Table 1 and Figure 2.
4. Chemotaxonomic Considerations
Chemotaxonomic studies use chemical characteristics, particularly secondary metabolites from a group of organisms to determine their taxonomic classification [36]. This correlation between phytochemical compounds and morphological data becomes an important tool to determine plant classification, phylogeny and evolution [37,38,39].
The plant evolution process, from a morphological point of view, occured by the successive appearance of small weeds, larger herbs, shrubs and, finally, trees achieving the climax with primitive angiosperms. Then, the evolutionary polarity became inverted, woody plant being gradually replaced by herbaceous plants [40,41]. As explained by Gottlieb: “The most conspicuous evolutionary trend in the gross morphology of land plants concerns the successive appearance of small weeds, larger herbs, shrubs and, finally, trees. This trend had attained or even had passed its climax with the primitive angiosperms and within this division the evolutionary polarity became inverted, woody plants being gradually replaced by herbaceous plants.

Table 1.
Some metabolites isolated from Rubiaceae.
| Genera | Class | Substance | Structure * |
|---|---|---|---|
| Cephaelis | Alkaloid | Emetine | I |
| Lactone | Chelidonic acid | II | |
| Alkaloid | Cephalin | III | |
| Alkaloid | Psycotrin | IV | |
| Cinchona | Alkaloid | Quinine | V |
| Triterpene | Cincholic acid | VI | |
| Triterpene | Quinovic acid | VII | |
| Alkaloid | Quinidine | VIII | |
| Alkaloid | Cinchonine | IX | |
| Alkaloid | Cinchonidine | X | |
| Coffea | Methyl xantine | Caffeine | XI |
| Diterpene | Cafestol | XII | |
| Anthraquinone | Galiosin | XIII | |
| Anthraquinone | Copareolatin | XIV | |
| Anthraquinone | Munjistin | XV | |
| Corynanthe | Alkaloid | Yohimbine | XVI |
| Galium | Iridoide | Macedonine | XVII |
| Genipa | Monoterpene | Genipin | XVIII |
| Hedyotis | Anthraquinone | Alizarin | XIX |
| Landerbergia | Alkaloid | Quinidine | VIII |
| Alkaloid | Cinchonine | IX | |
| Alkaloid | Cinchonidine | X | |
| Morinda | Anthraquinone | Alizarin | XIX |
| Mussaenda | Triterpene | Arjunolic acid | XX |
| Oldenlandia | Anthraquinone | Alizarin | XIX |
| Psychotria | Alkaloid | Psycotrin | IV |
| Alkaloid | Cephalin | III | |
| Relbunium | Anthraquinone | Purpurin | XXI |
| Remijia | Alkaloid | Quinidine | VIII |
| Alkaloid | Cinchonine | IX | |
| Alkaloid | Cinchonidine | X | |
| Rubia | Anthraquinone | Purpurin | XXI |
| Anthraquinone | Alizarin | XIX |
* shown in Figure 2.
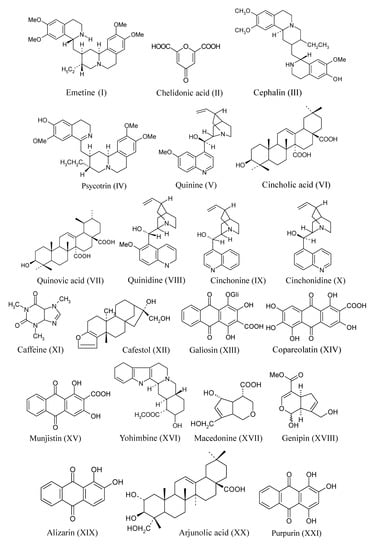
Figure 2.
Different classes of compounds isolated from Rubiaceae.
These successional phenomena are paralleled by micromolecular compositions. The ubiquitous flavonoids excepted, polyketides and terpenoids dominate the chemical compositions of bryophytes and pteridophytes. Shikimate-derived aromatics became numerically significant only in gymnosperms and attain predominance over other biosynthetic classes in primitive angiosperms. Concomitantly, here secondary metabolism reflects the trend from woody to herbaceous forms by inactivation of cinnamoyl/cinnamyl-derivatives through two phenomena: (i) extension of the shikimate pathway by reduction of cinnamyl alcohols to allylphenols and propenylphenols and (ii) gradual curtailment of the final steps of the shikimate pathway. The former alternative is most frequent in the primitive magnolialean block, where oxidative oligomerization of the precursors leads to neolignans. The first consequence of the latter alternative, the accumulation of phenylalanine and tyrosine, again very frequent in the magnolialean block, occurs also in the rosiflorean block. Oxidative elaboration of these amino acids leads to benzylisoquinolines. Further shortening of the shikimate pathway is restricted to the rosiflorean block. It leads to the accumulation of chorismic acid, the precursor of anthranilate- and of tryptophane-derived alkaloids, and of shikimic acid, the precursor of gallic acid- and ellagic acid-derived tannins. With gallic acid, the possibilities of diversifying the production of micromolecules through gradual curtailment of the shikimate pathway seem to be exhausted. In the most highly advanced, mostly sympetalous, angiosperms, shikimate-derived secondary metabolites play a relatively minor role. In these lineages, the full potential of acetate utilization leads to polyacetylenes, while mevalonate utilization leads to steroidal alkaloids, iridoids, alkaloids, sesquiterpene lactones, etc. In comparison with the polyketides and terpenoids of less advanced plant groups mentioned above, these compounds all show a high state of oxidation.” [40].
Regarding the distribution of the major secondary metabolites in Rubiaceae, indole alkaloids are indicated as the main chemical markers of this family [42,43,44,45,46]. Iridoids, anthraquinones, triterpene glycosides, flavonoids, lignoids, terpenes and phenols derivatives, were also reported [47]. Indole alkaloids occur just in families belonging to the Gentianales order (Loganiaceae, Rubiaceae, Apocynaceae and Naucleaceae), where one observes monoterpene indole alkaloids mainly [48]. The occurrence of indole alkaloids out of Gentianales order is quite rare and when found they are usually simple indole alkaloids.
A good correlation between the biosynthetic pathways and morphological aspects of the Ixoroideae, Cinchonoideae and Rubioideae subfamilies is obtained by evaluating chemical data, combined with the parameters cited by Robbrecht [8]. Each one of these subfamilies presents a different and typical profile of indole alkaloids, iridoids and anthraquinones which are considered as Rubiaceae chemotaxonomic markers [49]. Other studies based on chemotaxonomic data obtained by gas chromatography coupled to mass spectrometry show that the iridoid glycosides are present in several different species belonging to the Rubiaceae subfamilies [50,51,52]. Monoterpene indole alkaloids, especially which are derivatives of tryptamine and monoterpene (iridoid) secologanin are another predominant class in Rubiaceae. Quinoline alkaloids, which are products from the monoterpene indole and isoquinoline alkaloids rearrangement, yielding emetine-type alkaloids, are also characteristic of Rubiaceae, however, strychnine class alkaloids are not present in this family. Other alkaloid types are quite heterogeneous leading to a hard chemotaxonomic correlation [53].
Several studies have reported the use of chemical data to assist plant taxonomy [53]. Interest in this area increased due to the appearance of fast and accurate analytical techniques. However, there are still limitations on the application of chemical data in systematics. Even with a growing number of phytochemical studies, there are still many plants that remain without any chemical study.
5. Data Obtained Through the Bibliographic Survey
The present study sought to survey phytochemical studies of all species of Rubiaceae published in ScienceDirect and CAS SciFinder websites between 1990 and 2014. The data compiled in this review show the distribution of the studied species classified by their respective tribes and subfamilies as well as the isolated compounds and their chemical classes (Table 2).
Based on the obtained data, the main occurrence of iridoids, anthraquinones, triterpenes, indole alkaloids and alkaloids belonging to different chemical subclasses, was observed. The chemical profile, as expressed by the occurrence of major categories of secondary metabolites (alkaloids, anthraquinones and iridoids) showed to be quite different for each subfamily. Furthermore, the study of specific classes may contribute to chemotaxonomic correlations, since there are compounds with restricted distribution [54]. These same classes of substances served as a distribution pattern to create and modify plant classification systems as proposed by Dahlgren [54].
In Ixoroideae subfamily, the iridoids are found as chemotaxonomic markers, in Cinchonoideae the indole alkaloids predominate over other substances and in Rubioideae the anthraquinones are the major class of secondary metabolites (Figure 3). These global findings corroborate those found in the Brazilian Rubiaceae chemotaxonomic study by Bolzani [15].
Other studies also describe indole alkaloids as the class of substances of major occurrence in Cinchonoideae, especially in Guettardeae tribe [50,55]. Studies by Wijinsma and Verpoorte [56] and Bolzani et al. [15] describe the occurrence of standardized chemical markers: iridoids in Ixoroideae; indole alkaloids in Cinchonoideae and anthraquinones in Rubioideae. These data corroborate the one presented in this review.
Therefore, it was observed triterpenes widely distributed in all subfamilies, therefore a chemotaxonomic correlation cannot be established. The occurrence of a common pattern in secondary metabolism may suggest, strongly, taxons having a common ancestor. Thus, if there are morphological similarities, they can either be due to a common ancestry or convergent evolution [54]. Furthermore, the seco-iridoids are iridoids precursors and also participate in the biosynthesis of monoterpene indole alkaloids, so they may be involved in two distinct chemotaxonomic subdivisions [57,58]. Thus, different species may exhibit different chemical substance classes, but having the same precursor, which may indicates a phylogenetic relationship [59,60,61,62,63,64].

Table 2.
Compounds isolated from Rubiaceae species, organized by subfamily and tribe.
| Subfamily | Tribe | Species | Compound (s) | References |
|---|---|---|---|---|
| Cinchonoideae | CHI | Chiococca alba | Triterpene glycosides: chiococcasaponins I–V | [65] |
| Cetoalcohols: 4-hydroxy-heptadecan-7-one; 5-hydroxy-octadecan-11-one Phenylcoumarines: 5,7,4′-trimethoxy-4-phenylcoumarine Lignans: exostemin; matairesinol; d-mannitol | [66] | |||
| Seco-iridoids: albosides I–III | [67] | |||
| Nor-seco-pimarane: merilactone | [68] | |||
| Triterpene: 3-β-hydroxyolean-12,15-dien-28-oic acid | [69] | |||
| Triterpene glycosides: O-α-d-apiofuranosyl (1→3)-[α-d-apiofuranosyl (1→4)]-α-l-rhamnopyranosyl (1→2)-α-l-arabinopyranosyl 3-O-β-d-glucopyranosyl-3-β-hydroxyolean-12,15-dien-28-oate; 28-O-α-d-apiofuranosyl (1→3)-α-l-rhamnopyranosyl (1→2)-α-l-arabinopyranosyl 3-O-β-d-glucopyranosyl-3-β-hydroxyolean-12,15-dien-28-oate | [70] | |||
| Ent-kaurane diterpenes: 1-hydroxy-18-nor-kaur-4,16-dien-3-one; 15-hydroxy-kaur-16-en-3-one; kaur-16-en-19-ol; kaurenoic acid; merilactone; ribenone | [71] | |||
| Ent-kaurane: ent-17-hydroxy-16α-kauran-3-one | [72] | |||
| Chiococca braquiata | Flavonoids: 4′-methoxykaempferol-7-(acetyloxy)-3,5-O-α-l-rhamnoside; apigenin; 7-O-methoxyquercetrin; quercetrin Triterpenes: α-amirin; β-amirin; ursolic acid; oleanolic acid | [73] | ||
| Coutarea hexandra | Coumarins: 5-O-β-d-glucopyranosyl-4-(4-hydroxyphenyl)-7-methoxy-2H-chromen-2-one; 5-O-β-d-galactopyranosyl-4-(4-hydroxyphenyl)-7-methoxy-2H-chromen-2-one Cucurbitacins: 23,24-dihydrocucurbitacin F; 23,24-dihydro-25-acetylcucurbitacin F; 2-O-β-d-glucopyranosyl-23,24-dihydrocucurbitacin F | [74] | ||
| Exostema acuminatum | Nor-diterpenes: ent-16,17-diidroxicauran-19-nor-4-en-3-one; ent-16,17-dihydroxy-kauran-19-nor-4-en-3-one Phenylcoumarins: 5,7,4′-trimethoxy-4-phenylcoumarin; 7,4′-dimethoxy-5-hydroxy-4-phenylcoumarin; 5,7,4′-trimethoxy-3′-hydroxy-4- phenylcoumarin; 5,7,4′-trimethoxy-8-hydroxy-4-phenylcoumarin (exostemin I); 5,7,4′-trimethoxy-8,3′-dihydroxy-4′-phenylcoumarin; | [75] | ||
| 7,4′-dimethoxy-5,3′-hydroxy-4′-phenylcoumarin | [75] | |||
| Exostema caribaeum | Phenylcoumarin: 5-O-β-d-galactopyranosyl-7-methoxy-3′, 4′-dihydroxy-4-phenylcoumarin | [76] | ||
| Hintonia latiflora | Phenylcoumarin: 5-O-(6′′acetyl-β-d-glucopyranosyl)-7,3′,4′-trihydroxy-4-phenylcoumarin Phenylstyrene: 6-O-β-d-glucopyranosyl-2,3′,4β-trihydroxy-4-methoxy-β-phenylstyrene | [77] | ||
| Hintonia standleyana | Phenylcoumarin: 3-O-β-d-glucopyranosyl-23,24-dihydrocucurbitacin F; 5-O-[β-d-apiofuranosyl-(1→6)-β-d-glucopyranosyl]-7-methoxy-3′,4′-dihydroxy-4-phenyl-coumarin; desoxycordifolinic acid | [78] | ||
| CIN | Cinchona ledgeriana | Quinolinic alkaloids: quinine; quinidine; cinchonidine and cinchonine | [79,80] | |
| Cinchona robusta | Anthraquinones: robustaquinones A–H; 1,3,8-trihydroxy-2-methoxyanthraquinone; copareolatin 6-methyl ether | [81] | ||
| Ladenbergia oblongifolia | Alkaloids: epicinchonicinol; cinchonidicinol; mixture of dihydrocinchonicinol and dihydrocinchonidicinol | [82] | ||
| Remijia peruviana | Quinolinic alkaloids: quinine; cuprein; cinchonine; acetylcupreine; N-ethylquinine | [83] | ||
| Alkaloids: remijinine; epiremijinine; 5-acetylapocinchonamine; N-acetyldeoxy-cinchonicinol; N-acetylcinchonicinol | [84] | |||
| Sickingia tinctoria | Indole alkaloids: sickingin; 5-carboxystrictosidine; ophiorines A–B; lyalosidic acid | [85] | ||
| Sickingia williamsii | Indole alkaloids: sickingin; 5α-carboxystrictosidine; ophiorines A–B; lyalosidic acid | [85] | ||
| GUE | Antirhea acutata | Triterpene-methyl ester: nor-seco-cycloartane | [86] | |
| Antirhea lucida | Indole alkaloids: N,N-methyl-3′-indolylmethyl-5-methoxytryptamine; N,N-dimethyltryptamine; 6-methoxy-2-methyl-1,2,3,4-tetrahydro-13-carboline | [87] | ||
| Antirhea portoricensis | Indole alkaloids: 20-epiantirhine; isoantirhine; antirhine; yohimbol; epi-yohimbol; 19(S)-hydroxydihydrocorinanteol | [88] | ||
| Chomelia obtusa | Triterpenes: 3-O-β-d-quinovopyranosyl-28-O-β-d-glycopyranosyl quinovic acid; 3-O-β-d-quinovopyranosyl-28-O-β-d-glycopyranosyl cincholic acid; ursolic acid; oleanolic acid Flavonoids: (3-O-β-d-glycopyranosyl quercetin; 3-O-[α-l-rhamnopyranosyl-(1→6)-β-d-galactopyranoside] quercetin; 3,5-O-dicaffeoyl quinic acid; 4,5-O-dicaffeoyl quinic acid | [89] | ||
| Guettarda grazielae | Triterpenes: α-amyrin acetate; cycloartenone; 3β,19α,23-trihydroxyurs-12-ene; 3-β-O-β-d-glucopyranosylquinovic acid; 3β,6β,19α,23-tetrahydroxyurs-12-en-28-oic; acid ursolic acid | [90] | ||
| Iridoid: guettardodiol Seco-iridoid: sarracenin; 7α-morroniside; 7β-morroniside | [91] | |||
| Guettarda noumeana | Quinolinic alkaloids: cupreine; dihydrocupreine; N-methyldihydroquinicinol; N-methylquinicinol | [92] | ||
| Guettarda pohliana | Triterpenes: ursolic acid; oleanolic acid; pomolic acid; rotundic acid; 3β,6β,19α,23-tetra-hydroxyurs-12-en-28-oic acid; clethric acid Monoterpene: 5-O-caffeoylquinic acid; loliolide Seco-iridoid: secoxiloganin | [93] | ||
| Triterpenes glycosides: 28-O-β-d-glycopyranosyl-3-O-β-d-quinovopyranosyl quinovic acid; 28-O-β-d-glycopyranosyl-3-O-β-d-glycopyranosyl quinovic acid; 3-O-β-d-glycopyranosyl quinovic acid; 28-O-β-d-glycopyranosyl-3-O-β-d-glycopyranosyl cincholic acid; quinovic acid; daucosterol Phenolic compound: 4,5-O-dicaffeoylquinic acid | [94] | |||
| Guettarda speciosa | Phenolic compounds: 1-O-α-d-glucuronide 3-O-benzoyl ester; guettardionoside Indole alkaloid: cadambine Iridoid glycoside: sweroside; morroniside Steroids: ecdysone; icariside D1 Triterpene: quinovic glycoside C | [95] | ||
| Machaonia brasiliensis | Steroids: 3β-O-β-glucopyranosyl stigmasterol; 3β-O-β-glucopyranosyl sitosterol Seco-iridoid: secologanoside Flavonoid: 7-O-β-glucopyranosyl quercetagetin Clorogenic acids: 4,5-O-dicaffeoylquinic acid; 5-O-caffeoylquinic acid. | [96] | ||
| Neolamarckia cadamba | Indole alkaloids: neolamarckines A–B | [97] | ||
| Neolaugeria resinosa | Oxindole alkaloids: neolaugerine; isoneolaugerine; 15-hydroxyneolaugerine | [98] | ||
| Timonius timon | Triterpenes: 3β,6β,23-trihydroxy-olean-12-en-28-oic acid; 3β,6β,19α,23-tetrahydroxy-olean-12-en-28α-oic acid | [99] | ||
| HAM/HIL | Chione venosa var. venosa | Acetophenone derivatives: ortho-hydroxy-acetophenone-azine; acetophenone-2-O-β-d-glucopyranoside; acetophenone-2-O-[β-d-apiofuranosyl-(1→6′)-O-β-d-glucopyranosyl] Iridoid glycosides: 4α-morroniside; sweroside; diderroside Triterpene: daucosterol | [100] | |
| HAM | Deppea blumenaviensis | β-carboline alkaloids: deppeaninol | [101] | |
| Hamelia magniflora | Indole alkaloids: magniflorine; ajmalicine | [102] | ||
| Hamelia patens | Indole alkaloids: (−)-hamelin; tetrahydroalstonin; aricine; pteropodine; isopteropodine; uncarine F; speciophylline; palmirine; mitraphylline; rumberine | [103] | ||
| HYM | Hymenodictyon excelsum | Triterpenes: 3β-hydroxy-11-oxours-12-en-28-oic acid; 3β-hydroxy-27-p-(Z)-coumaroyloxyolean-12-en-28-oic acid; 3-oxo-11α,12α-epoxyurs-13β,28-olide; 3β-hydroxy-11α,12α-epoxyurs-13β,28-olide; 3β-hydroxyurs-11-en-13(28)-lactone; oleanolic acid; uncarinic acid E (3β-hydroxy-27-(E)-p-coumaroyloxyolean-12-en-28-oic acid; ursolic acid; ursonic acid; 3β-(formyloxy)-urs-12-en-28-oic acid | [104] | |
| Hymenodictyon floribundum | Glycosides: scopolin; himexelsin or xeroboside; scopoletin | [105] | ||
| Iridoids: floribundane A–B | [106] | |||
| ISE | Isertia haenkeana | Indole alkaloids: dihydroquinamine; epidihydroquinamine; apodihydrocinchonamine; 3-carbomethoxy-5-(l′-hydroxyethyl) pyridine | [107] | |
| Isertia pittieri | Triterpene glycosides: pyrocincholic acid 3β-O-α-d-quinovopyranosyl-28-[β-d-glucopyranosyl(1→6)-β-d-glucopyranosyl] ester; pyrocincholic acid 3β-O-β-d-quinovopyranosyl(1→6)-α-d-glucopyranosyl-28-[-β-d-glucopyranosyl(1→2)-β-d-glucopyranosyl] ester; quinovic acid 3α-O-R-l-rhamnopyranosyl(28→1)-β-d-glucopyranosyl ester; quinovic acid 3β-O-β-d-glucopyranosyl(1→4)-R-l-rhamnopyranosyl-(28→1)-β-d-glucopyranosyl ester | [108] | ||
| NAU | Adina cordifolia | Coumarins: umbelliferone; skimmin; 7-methoxycoumarin and 7-hydroxy-8-acetyl coumarin | [109] | |
| Adina racemosa | Flavonoid glycosides: quercetin 3-O-R-l-rhamnopyranosyl(16)-(3-O-trans-p-coumaroyl)-α-d-galactopyranoside; quercetin 3-O-R-l-rhamnopyranosyl(1→6)-[(4-O-trans-p-coumaroyl)-R-l-rhamnopyranosyl(1→2)]-(4-O-trans-p-coumaroyl)-α-d-galactopyranoside; kaempferol 3-O-R-l-rhamnopyranosyl(1→6)-[(4-O-trans-p-coumaroyl)-R-l-rhamno-pyranosyl(1→2)]-(4-O-trans-p-coumaroyl)-β-d-galactopyranoside; quercetin 3-O-R-l-rhamnopyranosyl(1→6)-[(4-O-trans-p-coumaroyl)-R-l-rhamnopyranosyl(1→2)]-(3-O-trans-p-coumaroyl)-β-d-galactopyranoside; quercetin 3-O-R-l-rhamnopyranosyl(1→6)-[(4-O-trans-caffeoyl)-R-l-hamnopyranosyl-(1→2)]-(3-O-trans-p-coumaroyl)-β-d-galactopyranoside | [110] | ||
| Secoiridoid glucosides: adinosides A–E; grandifloroside 11-methyl ester | [111] | |||
| Adina rubella | Triterpenes glycosides: quinovic acid 3-O-β-d-glucopyranosyl (l→4)-β-d-fucopyranoside; quinovic acid 3-O-β-d-glucopyranosyl (1→4)-β-d-fucopyranoside (28→1)-β-d-glucopyranosyl ester; quinovic acid 3-O-β-d-glucopyranosyl (1→4)-α-l-rhamnopyranosyl-(28→1)-β-d-glucopyranosyl ester; quinovic acid 3-O-β-d-glucopyranosyl (1→2)-β-d-glucopyranosyl-(28→1)-β-d-glucopyranosyl ester | [112] | ||
| 27-Nor-triterpene glycosides: rubellosides C–D | [113] | |||
| Adina polycephala | Iridoids: genipin-1-O-α-l-rhamnopyranosyl (1→6)-α-d-glucopyranoside | [114] | ||
| Cephalanthus glabratus | Oxindole alkaloids: tetrahydroalstonine; mitraphylline; uncarine E | [115] | ||
| Cephalanthus occidentalis | Triterpenes glycosides: 3-O-α-glucopyranosylcincholic acid; cincholic acid 28-O-α-glucopyranosyl ester; 3-O-β-glucopyranosyl-(1→4)-β-fucopyranosylcincholic acid; 3-O-β-glucopyranosyl-(1→4)-β-fucopyranosylcincholic acid 28-O-β-glucopyranosyl ester; 3-O-β-glucopyranosylcincholic acid 28-O-α-arabinopyranosyl-(1→2)-β-glucopyranosyl ester; 3-O-β-glucopyranosylquinovic acid 28-O-α-arabinopyranosyl-(1→2)-β-glucopyranosyl ester | [116] | ||
| Corynanthe pachyceras | Indole alkaloids: corynanthine; α-yohimbine; dihydrocorynanthine; corynantheine; corynantheidine | [117] | ||
| Mitragyna diversifolia | Monoterpe indole alkaloids: mitradiversifoline; specionoxeine-N(4)-oxide; 7-hydroxyisopaynantheine; 3-dehydropaynantheine; 3-isopaynantheine-N(4)-oxide | [118] | ||
| Mitragyna inermis | 27-Nor-glycosides triterpene: inermisides I–II Triterpenes: quinovic acid; 3-O-[β-d-glucopyranosyl-(1→4)-α-l-rhamnopyranosyl]; β-d-glucopyranosyl-[3-O-(β-d-glucopyranosyl)]-quinovic acid; 3-O-(β-d-6-deoxy-glucopyranosyl) quinovic acid | [119] | ||
| Indole alkaloids: naucleactonin D; nauclefilline; angustoline; angustine; naucleficine; nauclefidine Triterpenes: barbinervic acid; quinovic acid; 3-O-α-l-rhamnopyranoside acid; betulinic acid; oleanolic acid; ursolic acid; strictosamide | [120] | |||
| Oxindole alkaloids: mitraphylline; isomitraphylline; speciophylline; pteropodine | [121] | |||
| Mitragyna parvifolia | Oxindole alkaloids: 16,17-dihydro-17β-hydroxyisomitraphylline; 16,17-dihydro-17β-hydroxymitraphylline; 2-isomitraphylline; mitraphylline | [122] | ||
| Mitragyna rotundifolia | Triterpene glycosides: quinovic acid 3-O-β-d-6-deoxy-glucopyranoside 28-O-β-d-glucopyranosyl ester; quinovic acid 27-O-α-l-rhamnopyranosyl ester; 3-O-α-l-rhamnopyranoside; quinovic acid 27-O-β-d-glucopyranosyl ester; quinovic acid 3-O-6-deoxy- glucopyranoside; quinovic acid 27-O-β-d-glucopyranosyl ester; cincholic acid 3-O-β-d-6-deoxy-glucopyranoside; cincholic acid 28-O-β-d-glucopyranosyl ester | [123] | ||
| Mitragyna speciosa | Indole alkaloids: mitragynine; speciogynine; speciociliatine; 7-hydroxy-mitragynine; paynantheine | [124] | ||
| Nauclea cadamba | Gluco-indole alkaloids: 3β-dihydroisocadambine; cadambine; 3α-dihydrocadambine; 16-carbomethoxynaufoline; nauclechine; 5,11,12,5α-tetrahydroindolo[3,2-g]-pyridino-[4,3-b]indolizine | [125] | ||
| Nauclea diderrichii | Triterpene glycosides: quinovic acid 3-O-α-l-rhamnopyranosyl (28→1)-β-d-gluco-pyranosyl ester; quinovic acid 3-O-β-d-glucopyranosyl (1→2)-d-glucopyranoside; quinovic acid 3-O-β-l-fucopyranosyl (28→1)-β-d-glucopyranosyl ester | [126] | ||
| Indole alkaloids: 3α-5α-tetrahydrodeoxycordifoline; cadambine acid | [127] | |||
| Nauclea latifolia | Indole alkaloids: latifoliamides A–E; angustoline | [128] | ||
| Nauclea officinalis | Indole alkaloids: naucleficines A–E; naucleidinal; angustoline | [129] | ||
| Indole alkaloids: naucline; angustine; angustidine; nauclefine; naucletine | [130] | |||
| Triterpenes: 3β,19α,23,24-tetrahydroxyurs-12-en-28-oic acid; 2β,3β,19α,24-tetrahydroxyurs-12-en-28-oic acid; 3-oxo-urs-12-ene-27; 28-dioic acid; quinovic acid 3-β-rhamnopyranoside | [131] | |||
| Nauclea orientalis | Tetrahydro-β-carboline monoterpene alkaloid glucosides: naucleaorine; epimethoxynaucleaorine; strictosidine lactam Triterpenes: oleanolic acid; 3,4,5-trimethoxyphenol; 3-hydroxyurs-12-en-28-oic acid methyl ester; 3α,23-dihydroxyurs-12-en-28-oic acid; 3α,19α,23-trihydroxyurs-12-en-28-oic acid methyl ester | [132] | ||
| Indole alkaloids: nauclealines A–B; naucleosides A–B; strictosamide; vincosamide; pumiloside | [133] | |||
| Indole alkaloids: naucleaorals A–B | [134] | |||
| Nauclea pobeguinii | Indole alkaloids: naucleidinal; magniflorine; naucleofficine D; diastereoisomers of 3,14-dihydroangustoline; strictosidine; desoxycordifoline; 3α,5α-tetrahydrodeoxycordifoline lactam Phenolic compound: kelampayoside A | [135] | ||
| Indole alkaloid: nauclequinine; nauclefoline; nauclefidine | [136] | |||
| Neonauclea purpurea | Quinolinic alkaloid: 2,6-dimethoxy-1,4-benzoquinone | [137] | ||
| Indole alkaloids: cadambine; α-dihydrocadambine | ||||
| Neonauclea sessilifolia | Triterpene glycosides: 3-O-β-d-glucopyranosyl quinovic acid; 3-O-β-d-glucopyranosyl-(1→2)-β-d-quinovopyranosyl quinovic acid; 3-O-β-d-quinovopyranosyl pyrocincholic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl ester; 3-O-α-l-rhamnopyranosyl-(1→4)-β-d quinovopyranosyl pyrocincholic acid 28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl ester | [138] | ||
| Triterpene: ursolic acid | [139] | |||
| Chromone-secoiridoid glycosides: sessilifoside; 7′′-O-β-d glucopyranosylsessilifoside Indole alkaloid glycosides: neonaucleosides A–C Glycosides: 5-hydroxy-2-methylchromone-7-O-β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside; sweroside; loganin; grandifloroside; quinovic acid 3β-O-β-d-quinovopyranoside-28-O-β-d-glucopyranoside | [140] | |||
| Ochreinauclea maingayii | Indole alkaloids: neonaucline; cadamine; naucledine | [141] | ||
| Pausinystalia johimbe | Monoterpene indole alkaloid: yohimbine | [142] | ||
| Uncaria attenuata | Oxindole alkaloids: corynoxine; corynoxine B; isocorynoxeine; epi-allo-corynantheine; dihydrocorynantheine pseudoindoxyl Indole alkaloids: 19-epi-3-iso-ajmalicine Triterpene: ursolic acid | [19] | ||
| Uncaria borneensis | Alkaloids: isorhynchophylline; rhynchophylline; isocorynoxeine; corynoxeine; Indole alkaloids: allo-yohimbine; pseudo-yohimbine; 3-epi-β-yohimbine | [143] | ||
| Uncaria callophylla | Indole alkaloids: dihydro-corynantheine; gambirine; isogambirine; gambireine; rotundifoline; callophylline; callophyllines A–B; yohimbine; pseudoyohimbine; β-yohimbine; α-yohimbine | [144] | ||
| Indole alkaloids: callophyllines A–B; 3-epi-β-yohimbine; gambirine | [144] | |||
| Uncaria cordata var. cordata and Uncaria cordata var. ferruginea | Indole alkaloids: dihydrocorynantheine | [143] | ||
| Uncaria elliptica | Pentacyclic oxindole alkaloids: formosanine; isomitraphylline; mitraphylline Indole alkaloids: ajmalicine | [145] | ||
| Triterpenes: 3β,6β,19α-trihydroxy-23-oxo-urs-12-en-28-oic acid; 3β,6β,19α,23-trihydroxy-23-oxo-urs-en-28-oic acid; 3,6-dioxo-19α-hydroxy-urs-12-ene-28-oic acid; 3β,6β-diacetoxi-19-hydroxy-urs-12-ene-28-oic acid; quinovic acid 3β-O-β-d-quinopyranosyl-(28→1)-β-d-glucopyranosyl ester | [145] | |||
| Uncaria gambir | Proanthocyanidins: gambiriins A1–A2 ; gambiriins B1–B2; (+)-catechin; (+)-epicatechin; procyanidin B1; procyanidin B3; gambiriin | [146] | ||
| Uncaria glabrata | Monoterpene indole alkaloids: 14α-hydroxyrauniticine; rauniticine; uncarine C–E; glabratine; deoxycordifoline | [147] | ||
| Uncaria guianensis | Indole alkaloid: 3-isoajmalicine Oxindole alkaloids: isomitraphylline; mitraphylline; isomitraphylinic acid | [38] | ||
| Indole alkaloid: ajmalicine Oxindole alkaloids: formosanine or uncarine B; isomitraphylline; mitraphylline | [148] | |||
| Triterpenes: quinovic acid 3β-O-β-d-quinovopyranoside; quinovic acid 3β-O-β-d-fucopyranosyl-(27→1)-β-d-quinovopyranosyl ester; quinovic acid 3β-O-[β-d-glucopyranosyl-(1→3)-β-d-fucopyranosyl]-(27→1)-β-d-glucopyranosyl ester; quinovic acid 38-O-β-d-fucopyranoside | [149] | |||
| Uncaria hirsuta | Bis(monoterpenoid) indole alkaloid glucosides: hirsutaside D; bahienoside A–B; neonaucleoside B | [150] | ||
| Phenolic compound: chlorogenic acid Alkaloid: uncarine B Flavonoids: quercitrin; rutin; hiperin; neohesperidin | [151] | |||
| Uncaria lanosa var. glabrata and Uncaria lanosa var. ferrea | Pentacyclic oxindole alkaloids: isopteropodine; pteropodine | [143] | ||
| Uncaria longiflora var. longiflora | Alkaloids: isorhynchophylline; rhynchophylline; iso-corynoxeine; corynoxeine | [143] | ||
| Uncaria longiflora var. pteropoda | Pentacyclic oxindole alkaloids: pteropodine; isopteropodine | [143] | ||
| Pentacyclic oxindole alkaloids: pteropodine; isopteropodine | [152] | |||
| Uncaria macrophylla | Oxindole alkaloids: rhynchophylline; isorhynchophylline; corynoxine; corynoxine B | [153] | ||
| Uncaria rhynchophylla | Indole alkaloids: tetrahydroalstonine; tetrahydroalstonine-N-oxide; akuamigine; (4R)-akuamigina-N-oxide; (4S)-akuamigine-N-oxide; corynantheine; dihydrocorynantheine; dihydrocorynantheine-N-oxide; hirsuteine; geissoschizine methyl ether; hirsutine N-oxide; akuamigine pseudoindoxyl; rauniticine pseudoindoxyl; 3-isorauninticine pseudoindoxyl; dihydrocorynantheine pseudoindoxyl; vallesiachotamine; vincoside lactam; strictosamide; rhynchophyne; 2′-O-β-d-glucopyranosyl-11-hydroxyvincoside lactam; angustine; angustoline; angustidine | [154] | ||
| Sesquiterpene indole alkaloids: (5S)-5-carboxystrictosidine; 3,4-dehydro-(5S)-5-carboxystrictosidine Indole alkaloids: cadambine; 3α-dihydrocadambine; 3β-isodihydrocadambine Pentacyclic oxindole alkaloids: isorhynchophylline; rhynchophylline; corynoxeine; isocorynoxeine; corynoxeine; rhynchophylline N-oxide; isorhynchophylline N-oxide; macrophylline A; 18-19-dehydrocorynoxinic acid; 22-O-demethyl-22-O-β-d-glucopyranosyl isocorynoxeine | [154] | |||
| Oxindole alkaloids: rhynchophylline; corynoxeine; corynanteine; hirsutine | [155] | |||
| Oxindole alkaloids: isocorynoxeine; isorhynchophylline; orynoxeine; rhynchophylline Indole alkaloids: corynanteine; dihydrocorynanteine | [156] | |||
| Pentacyclic oxindole alkaloids: 22-O-demethyl-22-O-β-glucopyranosyl isorhynchophylline; 22-O-demethyl-22-O-β-glucopyranosyl rhynchophylline; 22-O-demethyl-22-O-β-glucopyranosyl isocorynoxeine; isorhynchophylline acid; 9-hydroxy isocorynoxeine; 18,19-dehydrocorynoxinic acid; 18,19 dehydrocorynoxinic acid B; rhynchophyllic acid; 9-hydroxycorynoxeine; isocorynoxeine N-oxide; rhynchophylline acid N-oxide; corynoxeine N-oxide; isocorynoxeine; rhynchophylline; isorhynchophylline N-oxide; isorhynchophylline; corynoxeine Indole alkaloid: vincoside lactam Phenolic compounds: chlorogenic acid; neochlorogenic; cryptochlorogenic; quinic acid; cis-5-caffeoylquinic acid; procyanidin b1; procyanidin b2; catechin; epi-catechin; rutin | [157] | |||
| Uncaria salaccensis | Oxindole alkaloids: 3-oxo-7-hydroxy-3,7-secorhynchophylline | [158] | ||
| Uncaria sinensis | Alkaloids: isohynchophyllic acid; pteropodic acid; 3α-dihydrocadambine; 3β-isodihydrocadambine | [159] | ||
| Proanthocyanidin: procyanidin B-1 | [160] | |||
| Uncaria tomentosa | Pentacyclic alkaloids: isomitraphylline; mitraphylline; uncarine F; speciophylline; isopterophylline; pterophylline; isocorynoxeine Tetratacyclic alkaloids: corynoxeine; isorincophylline; rincophylline | [161] | ||
| Alkaloids: cinchonain Ia; cinchonain Ib | [162] | |||
| Oxindole alkaloids: uncarines C–E; mitraphylline; isomitraphylline Iridoid glycosides: 7-deoxyloganic acid | [163] | |||
| Triterpenes glycosides: 3-oxo-6β-19α-dihydroxyurs-12-en-28-oic acid; 3β,6β,19α,23-tetrahydroxyurs-12-en-28-oic acid; 3β-methoxy-16α-hydroxyurs-12,19(29)-dien-27,28-dioic acid; 3β-hydroxyurs-12-en-27,28-dioic acid | [164] | |||
| Oxindole alkaloids: pteropodine; isopteropodine; speciophylline; uncarine F; mitraphylline; isomitraphylline; rincophylline; isorincophylline | [165] | |||
| Oxindole alkaloids: mitraphylline | [166,167] | |||
| Indole alkaloid: 3-isoajmalicine | [168] | |||
| Alkaloids: cinchonain Ia; cinchonain Ib | [162] | |||
| Iridoids: tomentosides A–B Phenolic compound: (−)-epi-cathequin | [169] | |||
| Triterpenes: oleanolic acid; 3β,6β,19α-trihydroxyurs-12-en-28-oic acid | [170] | |||
| Triterpenes: 3β,6β,19α-trihydroxyurs-12-en-23-al-28-oic acid; 3β,19α-dihydroxy-6-oxo-urs-12-en-23-al-28-oic acid; 3β,19α-dihydroxy-6-oxo-urs-12-en-23-ol-28-oic acid | [171] | |||
| Triterpene: 23-nor-24-esomethylene-3β,6β-19α-trihydroxyurs-12-en-28 oic acid; 3β,6β,19α-trihydroxyurs-12-en-28-oic acid; 3-oxo-6β,19α-dihydroxyurs-12-en-28 oic acid; oleanic acid | [169] | |||
| Uncaria villosa | Indole alkaloids: villocarines A–D | [172] | ||
| Ixorideae | ALB | Alberta magna | Iridoids: (+)-5-acetaldehyde-l-formyl-2-methylcyclopentan; 5-acetaldehyde-1-formyl-2- methylcyclopent-1-ene; 1,4α,5,6,7α-hexahydro-1-hydroxy-7-methylcyclopenta-pyran-4-carboxaldeyde; 4,4α,5,7α-tetrahydro-1-hydroxy-4-(hydroxymethylene)-7-methylcyclopentane-pyran-3-(1H)-one; 5-deoxystansioside; 6,10-bisdeoxyaucubin; boschnaloside | [173] |
| COF | Coffea sp | Alkaloid: caffeine | [174] | |
| Coffea bengalensis | Alkaloid: caffeine Diterpene: 16-epicafestol | [175] | ||
| Nematostylis anthophylla | Triterpene glycosides: randianin; 2′′-O-acetylrandianin; 6′′-O-acetylrandianin | [176] | ||
| Tricalysia dubia | Diterpenes: tricalysiol A–B; tricalysiolide B; tricalysioside G tricalysioside L | [177] | ||
| Ent-kaurane glycosides: tricalysiosides A–G | [178] | |||
| Tricalysia okelensis | Ent-kaurane glycosides: ent-kauran-3α,16α,17-triol-19-al 3-O-[5-O-vanilloyl-β-d-apiopyranosyl(1→6)]-β-d-glucopyranoside; ent-kauran-3α,16α,17-triol-19-al; 3-O-[5-O-E-sinapoyl-β-d-apiopyranosyl(1→6)]-β-d-glucopyranoside | [179] | ||
| CON | Calycophyllum spruceanum | Seco-iridoids: 7-methoxydiderroside,6′-O-acetyldiderroside; 8-O-tigloyldiderroside; loganetin; loganin; secoxyloganin; kingiside; diderroside | [180] | |
| Chimarrhis turbinata | Indole monoterpene alkaloids: strictosidine; strictosidine acid; 5α-arboxystrictosidine; isovallesiachotamine; vallesiachotamine; turbinatine; 3,4-dehydro-strictosidine; turbinatine β-Carboline alkaloids: cordifoline; deoxycordifoline; harman-3-carboxylic acid | [181] | ||
| Crossopteryx febrifuga | Triterpene glycosides: 3β-(α-l-rhamnopyranosyloxi)-28-O-(β-d-glucopyranosyl)urs-12,20(30)-diene-27,28-dioic acid | [182] | ||
| Emmenopterys henryi | Triterpenes: 3β,19α,23-trihydroxyurs-12-en-24-al-28-oic acid; 3β,19α,24-trihydroxy-23-norurs-12-en-28-oic acid; 3β,12β-dihydroxy-5α-pregnane-14,16-dien-20-one; and 12β-hydroxy-5α-pregnane-14,16-dien-3,20-dione; 3β,19α,23,24-tetrahydroxyurs-12-en-28-oic acid; pomolic acid; 3β,6β,19α,23-tetrahydroxyurs-12-en-28-oic acid; 3β,6β,23-trihydroxyolean-12-en-28-oic acid; 3β,6β,19α,23-tetrahydroxyolean-12-en-28-oic acid; 3β,23,24-trihydroxyolean-12-en-28-oic acid; 3β,12β-dihydroxy-5α-pregnane-16-en-20-one; 12β-dihydroxy-5α-pregnane-16-en-3,20-dione | [183] | ||
| Pogonopus speciosus | Alkaloids: 1′,2′,3′,4′-tetradehydrotubulosine; tubulosine; psychotrine | [184] | ||
| Pogonopus tubulosus | Alkaloid: tubulosine | [185] | ||
| Alkaloids: tubulosine; psychotrine; cephaeline | [186] | |||
| Simira glaziovii | Alkaloids: aribin; ophiorine B; lyaloside Monoterpenes: methyl 3,4-dimethoxycinamate | [187] | ||
| Simira eliezeriana | Diterpenes: simirane A [(5R,6R,8R,9R,10S,11S,13S)-6 β,11β -dihydroxy-2,4(18),15-erythroxylatrien-1-one]; simirane B [(5S,8R,9R,10S,11S,13S)-11-hydroxy-2,4(18),15-erythroxylatrien-1-one] | [188] | ||
| GAR | Alibertia edulis | Iridoids: 6β-hydroxy-7-epigardoside methyl ester | [189] | |
| Alibertia macrophylla | Diterpene: ent-kaurane-2β,3α,16α-triol Triterpenes: lupenone; germanicone; α-amirenone; β-amirenone; lupeol; oleanolic acid; ursolic acid Glucosidic iridoids: 6α-hydroxygeniposide; 6β-hydroxygeniposide; gardenoside; shanziside methylester Phenolic acids: protocatechuic; vanilic; caffeic | [190] | ||
| Alibertia myrciifolia | Coumarin: scopoletin | [64] | ||
| Flavonoid: corymbosin | [191] | |||
| Iridoid: 10-O-vanilloylgeniposidic acid | [192] | |||
| Triterpenes: pomolic acid methyl ester; ursolic acid methyl ester; oleanolic acid methyl ester | [193] | |||
| Alibertia sessilis | Phenolic compounds: 3,4,5-trimethoxyphenyl-1-O-β-d-(5-O-syringoyl)-apiofuranosyl-(1→6)-β-d-glucopyranoside Iridoids: geniposidic acid; geniposide; 6α-hydroxygeniposide; 6β-hydroxygeniposide Lignans glycosides: (+)-lyoniresinol-3α-O-β-d-glucopyranoside; (−)-lyoniresinol-3α-O-β-d-glucopyranoside | [64] | ||
| Flavonoids: quercetin-3-O-β-d-(2′′-O-trans-p-coumaroyl)-rutinoside; kaempherol-3-O-β-d-(2′′-O-trans-p-coumaroyl)-rutinoside Triterpenes: oleanolic acid; ursolic acid; epi-betulinic acid Iridoids: gardenoside; deacetylasperuloside; 10-dehydrogardenoside; β-gardiol; α-gardiol | [46] | |||
| Burchellia bubalina | Iridoids: β-gardiol; α-gardiol; garjasmine | [60] | ||
| Canthium gilfillanii | Iridoid: geniposidic acid | [61] | ||
| Catunaregam nilotica | Triterpene glycosides: 28-O-β-d-glucopyranosyl-3-O(O-α-l-rhamnopyranosyl-(1→3)-O-β-d-glucopyranosyl]-(1→3)]-β-d-glucopyranosyl) oleanolate; 3-O-[2′,3′-di-O-(β-d-glucopyranosyl)-β-d-glucopyranosyl] oleanolic acid; 3-O-(O-α-l-rhamnopyranosyl-(1→3)-O-[O-β-d-glucopyranosyl-(1→3)]-β-d-glucopyranosyl) oleanolic acid; 3-O-[O-β-d-glucopyranosyl-(1→3)-β-d-glucopyranosyl] oleanolic acid | [194] | ||
| Catunaregam spinosa | Triterpene glycosides: catunarosides A–D; swartziatrioside; aralia-saponin V–IV | [195] | ||
| Coptosapelta flavescens | Anthraquinones: 1,4-dimethoxy-2-methylanthraquinone; 2-amino-3-methoxycarbonyl-1,4-naphtoquinone | [196] | ||
| Duroia hirsuta | Iridoid: plumericin | [197] | ||
| Iridoid lactone: duroin Flavonol: ether flavonol-3-O-methyl | [198] | |||
| Duroia macrophylla | Triterpenes: oleanolic acid; ursolic acid | [199] | ||
| Gardenia collinsae | Triterpenes: 20R,24R-epoxy-3-oxodammarane-25ξ, 26-diol; C-24-epimer; 20R,24R-ocotilone | [200] | ||
| Gardenia gummifera | Cycloartane triterpenes: dikamaliartanes A–F Flavonoid: 3′,5,5′-trihydroxy-4′,6,7,8-tetramethoxyflavone | [201] | ||
| Gardenia jasminoides | Coumarines: ferrulic acid; skimmin; uracil; 5,8-di-(3-methyl-2,3-dihydroxy-butyloxypsoralen); 3-O-α-d-glucopyranosyl-(1→4)-β-d-glucopyranosyloxypeucedanin | [202] | ||
| Iridoids: genipin 1-O-β-d-d-isomaltoside; 1,10-di-O-β-d-glucopyranoside; genipin 1-O-β-d-gentiobioside; geniposide; scandoside methyl ester; deacetylasperulosidic acid methyl ester; 6-O-methyldeacetylasperulosidic acid methyl ester; gardenoside | [59] | |||
| Iridoids: 8-epi-apodantheroside; 7β,8β-epoxy-8α-dihydrogeniposide | [203] | |||
| Iridoids: 6′-O-[(E)-sinapoyl] gardoside; 4′′-O-[(E)-p-coumaroyl]-gentiobiosylgenipin; 6′-O-[(E)-caffeoyl]-deacetylasperulosidic acid methyl ester | [204] | |||
| Iridoid: 6-O-sinapoylgeniposide | [205] | |||
| Monoterpenes: gardenone; gardendiol | [206] | |||
| Carotenoids: crocetin; crocetin mono (β-d-glucosyl) ester; crocetin di-(β-d-glucosyl) ester; crocetin mono-(β-gentiobiosyl) ester; crocetin (β-d-glucosyl)-(β-gentiobiosyl) ester; crocin [crocetin-di-(β-gentiobiosyl)ester]; crocetin (β-gentiobiosyl)-(β-neapolitanosyl) ester; crocetin-di-(β-neapolitanosyl) ester | [207] | |||
| Monoterpenes: jasminosides J–K; 6′-O-trans-sinapoyljasminoside B; 6′-O-trans-sinapoyljasminoside L; jasminosides M–P; jasminoside C; jasminol E; sacranoside B | [208] | |||
| Flavonoid: luteolin-7-O-β-d-glucopyranoside Triterpenes: ursolic acid; oleanolic acid; methyl 3,4-di-O-caffeoylquinate; methyl 5-O-caffeoyl-3-O-sinapoylquinate; methyl 3,5-di-O-caffeoyl-4-O-(3-hydroxy-3-methyl)glutaroylquinate; methyl 5-O-caffeoyl-4-O-sinapoylquinate Glycosides: 2-methyl-l-erythritol-4-O-(6-O-trans-sinapoyl)-β-d-glucopyranoside; 2-methyl-l-erythritol-1-O-(6-O-trans-sinapoyl)-β-d-glucopyranoside | [209] | |||
| Iridoids: 6′-O-trans-p-coumaroyl geniposidic acid; 11-(6-O-trans-sinapoyl glucopyranosyl)-gardendiol; 10-(6-O-trans-sinapoyl glucopyranosyl)gardendiol; 6′′-O-trans-sinapoylgenipin gentiobioside; 6′′-O-trans-cinnamoylgenipin gentiobioside; 10-O-succinoylgeniposide; 6′-O-acetylgeniposide; 6′′-O-trans-p-coumaroylgenipin gentiobioside | [210] | |||
| Iridoids: gardaloside | [211] | |||
| Iridoids: garjasmine; dunnisin; α-gardiol; β-gardiol; diffusoside A diffusoside B; genameside C; deacetylasperulosidic acid | [212] | |||
| Gardenia jasminoides var. radicans | Iridoid glycoside: 6′′-O-trans-feruloylgenipin gentiobioside; 2′-O-trans-p-coumaroylgardoside; 2′-O-trans-feruloylgardoside | [213] | ||
| Gardenia lucida | Cycloartane triterpenes: dikamaliartanes A–F Flavonoid: 3′,5,5′-trihydroxy-4′,6,7,8-tetramethoxyflavone | [201] | ||
| Gardenia saxatilis | Triterpenes: lupenone; lupeol; betulinic acid; messagenic acid A; messagenic acid B; oleanolic acid; ursolic acid; acid (27-O-feruloyloxybetulinic acid; 27-O-p-(Z)- and 27-O-p-(E)-coumarate esters of betulinic acid and a mixture of uncarinic acid E (27-O-p-(E)-coumaroyloxyoleanolic acid) and 27-O-p-(E)-coumaroyloxyursolic acid | [214] | ||
| Gardenia sootepensis | Sesquiterpene: sootepdienone | [215] | ||
| Gardenia thailandica | Flavonoids: 5,7-dihydroxy-7,2′,3′,4′,5′,6′-hexamethoxyflavone; 5,7-dihydroxy-2′,3′,4′,5′,6′-pentamethoxyflavone; 5-hydroxy-7,2′,3′,4′,5′-pentamethoxyflavone; 5,7-dihydroxy-2′,3′,4′,5′-tetramethoxyflavone Triterpenes: thailandiol; gardenolic acid; quadrangularic E acid; 3β-hydroxy-5α-cycloart-24(31)-en-28-oic acid | [216] | ||
| Gardenia fructus | Iridoids: genipin 1-O-β-gentiobioside; 10-O-acetylgeniposide; 6α-hydroxygeniposide; 6β-hydroxygeniposide; gardenoside; picrocrocinic acid; 6′-O-sinapoyljasminoside; 10-O-(4′′-O-methylsuccinoyl) geniposide; jasminosides Q–R; 6-O-p-coumaroylgeniposide; 6′-O-acetylgeniposide; 6′-O-sinapoylgeniposide | [217] | ||
| Iridoids: geniposidic acid; genipin 1-β-gentiobioside; geniposide; genipin Flavonoids: rutin; crocin-1; crocin-2 Phenolic compound: chlorogenic acid | [218] | |||
| Iridoid glycosides: gardenoside; genipin 1-O-β-d-isomaltoside; genipin 1,10-di-O-β-d-glucopyranoside; genipin 1-O-β-d-gentiobioside; geniposide; scandoside methyl ester; deacetylasperulosidic acid methyl ester | [59] | |||
| Genipa americana | Iridoids: genipaol; genipin; tarenoside; geniposidic acid; geniposide; genamesides A–D; genipin-gentiobioside; gardenoside; gardendiol; shanzhiside | [219] | ||
| Monoterpenes: genipacetal; genipic acid; genipinic acid | ||||
| Genipa spruceana | Cycloartane triterpene: genipatriol | [220] | ||
| Lamprothamnus zanguebaricus | Phenolic acids: 1-(3-hydroxy-4-methoxy-5-methylphenyl)-ethanone; 1-(3-hydroxy-4-methoxyphenyl)-ethanone | [221] | ||
| Oxyanthus pallidus | Cycloartane glycosides: pallidiosides A–C Triterpenes: oleanolic acid; 3-O-β-d-glucopyranosyl-β-sitosterol | [222] | ||
| Oxyanthus pyriformis | Cyanogenic glycosides: prunasin; amygdalin | [223] | ||
| Oxyanthus speciosus | Phenolic compounds: 2-(2-hydroxy)-ethanol-β-d-glucopyranoside | [61] | ||
| Cyanogenic glycosides: holocalin | [223] | |||
| Pavetta owariensis | Proanthocyanidins: pavetannin A1; pavetannin A2; cinnamtannin B1; pavetanninB1; pavetannin B3; pavetannin B5; pavetannin B6 | [224] | ||
| Psydrax livida | Phenolic compounds: psydroside Monoterpene: psydrin | [61] | ||
| Randia dumetorum | Iridoid: 11-methylixoside | [225] | ||
| Triterpenes: α-l-arabinosyl(1→3)-β-galactopyranosyl(1→3)-3-β-hydroxyolean-12-en-28-methyloate | [226] | |||
| Randia Formosa | Triterpenes glycosides: randiasaponins I–VII; ilexoside XXVII; ilexoside XXXVII | [227] | ||
| Randia siamensis | Triterpenes: ursolic acid; pseudoginsenoside-RP 1; pseudoginsenoside-RT 1 | [228] | ||
| Randia spinosa | Iridoid glycosides: randinoside; galioside; deacetylasperulosidic acid methyl ester; scandoside methyl ester; geniposide; gardenoside | [229] | ||
| Rothmannia macrophylla | Iridoids: macrophylloside | [230] | ||
| Rothmannia urcelliformis | Iridoid: genipin Iridoid alcaloidal: gardenamide A; 4-oxonicotinamide-1-(1′-β-d-ribofuranoside) | [231] | ||
| Schumanniophyton problematicum | Alkaloids: rohitukine; rohitukine N-oxide; flavopiridol | [232] | ||
| Scyphiphora hydrophyllacea | Iridoid: scyphiphorin A1–A2; scyphiphorin B1–B2 | [233,234] | ||
| Tocoyena brasiliensis | Triterpene glycosides: 3-O-β-d-quinovopyranosyl quinovic acid; 3-O-β-d-glucopyranosyl quinovic acid; 28-O-β-glucopyranosyl ester derivative of quinovic acid Flavonoid: ramnazin-3-O-rutinoside | [235] | ||
| Tocoyena bullata | Iridoid glycoside: gardenoside | [236] | ||
| Tocoyena formosa | Iridoids: α-gardiol; β-gardiol; gardenoside | [237] | ||
| IXO | Enterospermum madagascariensis | Sesquiterpenes: 2-hydroxy-10-epi-zonarene; 2,15-dihydroxycalamenene; guaia-4,6-dien-3-one | [238] | |
| Enterospermum pruinosum | Triterpenes glycosides: longispinogenin; 3,16-di-O-β-d-glucopyranoside; triacetyllongispinogenin; diglucoside | [239] | ||
| Ixora coccinea | Triterpene: ursolic acid | [240] | ||
| Proanthocyanidins: ixoratannin A-2; epicatechin; procyanidin A2; cinnamtannin B-1 Flavonoids: kaempferol-7-O-α-l-rhamnoside; kaempferol-3-O-α-l-rhamnoside; quercetin-3-O-α-l-rhamnopyranoside; kaempferol-3,7-O-α-l-dirhamnnoside | [241] | |||
| Triterpenes: lupeol; ixorene; 17β-dammara-12,20-diene-3β-ol | [242,243] | |||
| Fenolic compounds: 3-O-caffeoylquinic acid; 5-O-caffeoylquinic acid; catechin; epicatechin; rutin; quercetin; kaempferol; quercetin 3-O-glucoside; quercetin 3-O-galactoside; kaempferol 7-O-glucoside | [244] | |||
| MUS | Heinsia crinata | Triterpene glycosides: heinsiagenin A-3β-O-(β-glucopyranosyl-(1→2)-β-d-glucopyranosyl-(1→6)-[α-l-rhamnpyranosyl-(1→2)]-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside); heinsiagenin A-3β-O-(α-l-rhamnopynosyl-(1→2)-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside) | [245] | |
| Mussaenda dona aurora | Iridoid glycoside: shanshiside D | [246] | ||
| Mussaenda erythrophylla | Flavonoid: 5-hydroxy-7,4′-dimethoxyflavones; Phenolic compounds: 3-iso-cumaryloxycyclopropane-1-oic acid; 4-hydroxy-3-methoxy cinnamic acid | [247] | ||
| Mussaenda incana | Iridolactona: shanzhilactone Iridoid glycosides: barlerin; mussaenoside Triterpene: lupeol | [248] | ||
| Mussaenda macrophylla | Iridoid: 6-epi-barlerin | [249] | ||
| Mussaenda roxburghii | Iridoid: shanzhiol | [250] | ||
| Mussaenda pubescens | Monoterpenes: mussaenins A–C | [251] | ||
| Triterpene glycosides: mussaendosides R-S; 6 α-hydroxygeniposide; 3β-O-β-d-glucopyranosyl quinovic acid 28-O-β-d-glucopyranosyl ester | [252] | |||
| OCT | Villaria odorata | Alkenoyloxy alkenol: villarinol | [253] | |
| Iridoids: morindolide; hydrophylin A; hydrophylin B Sesquiterpene: vomifoliol | [254] | |||
| PAV | Pavetta owariensis | Proanthocyanidins: proanthocyanidin A-2; proanthocyanidin A-4; pavetannin A Flavonoids: (+)-catechin; (−)-epicatechin; (+)-epicatechin | [224] | |
| Tarenna attenuata | Iridoids: tarenninosides A–G | [255] | ||
| Tarenna gracilipes | Cycloartane glycosides: tareciliosides H–M | [256] | ||
| Cycloartane glycosides: tareciliosides A–G | [257] | |||
| Tarenna madagascariensis | Iridoids: tarennin; gardenoside; geniposidic acid Phenolic compounds: p-cumaric acid; cafeic acid; chlorogenic acid Flavonoids: kaempferol 3-O-β-d-glucopyranoside-7-O-α-l-rhamnopyranoside; kaempferol 3-O-α-l-rhamnopyranoside-7-O-α-l-rhamnopyranoside; quercetin 3-O-α-l-rhamnopyranoside-7-O-α-l-rhamnopyranoside; kaempferol 3-O-α-l-(3′′-O-acetyl)-rhamnopyranoside-7-O-α-l-rhamnopyranoside; kaempferol 3-O-α-l-(4′′-O-acetyl) rhamnopyranoside-7-O-α-l-rhamnopyranoside | [258] | ||
| POS | Molopanthera paniculata | Iridoid glycosides: barlerin; shanzhiside methyl ester | [259] | |
| SAB | Sabicea brasiliensis | Phenolic compounds: 5-O-caffeoylquinic acid; 3,5-O-dicaffeoylquinic acid; 4,5-O-dicaffeoylquinic acid Coumarine: scopoletin Triterpene: ursolic acid | [260] | |
| Sabicea grisea var. grisea | Steroid: octacosanol | [261] | ||
| Coumarine: scopoletin Phenolic compounds: ethyl caffeate; salicylic acid Steroid: 3-O-β-d-glucopyranosylsitosterol Triterpene: vanillic acid | [262] | |||
| VAN | Canthium berberidifolium | Iridoid glycosides: 6-O-β-d-apiofuranosyl-mussaenosidic acid Phenolic diglycosides: canthosides A–D | [263] | |
| Canthium multiflorum | Iridoid: 6-oxo-genipin; macrophylloside; garjasmine; gardenine; gardenamide; deacetylasperulosidic acid; 6α-hydroxygeniposide; galioside; aitchisonide B Triterpenes: vanillic acid 4-O-β-d-(6-O-benzoylglucopyranoside); oleanolic acid; quinovic acid | [264] | ||
| Canthium schimperianum | Cyanogenic glycoside esterified with an iridoid glycoside: 2R-[(2-methoxybenzoyl-genoposidyl)-5-O-β-d-apiofuranosyl-(1→6)-β-glucopyranosyl-oxy]-2-phenyl acetonitrile; oxyanthin | [265] | ||
| Fadogia agrestis | Monoterpene glycosides: (2E,6Z)-2,6-dimethyl-8-[(O-α-l-rhamnopyranosyl-(1→3)-α-l-rhamnopyranosyl)-oxy]-octadien-1-yl-α-l-rhamnopyranoside; (2E,6Z)-2,6-dimethyl-8-[(O-α-l-rhamnopyranosyl-(1→3)-α-l-rhamnopyranosyl)-oxy]-octadien-1-yl-O-β-d-glucopyranosyl-(1→2)-α-l-rhamnopyranoside; (2E,6Z)-2,6-dimethyl-8-[(O-β-d-glucopyranosyl-(12)-α-l-rhamnopyranosyl)-oxy]-octadien-1-yl-O-β-d-glucopyranosyl-(1→2)-α-l-rhamnopyranoside; (2E,6Z)-2,6-dimethyl-8-[(O-α-l-rhamnopyranosyl-(1→3)-(2-O-((2E,6Z)-8-hydroxy-2,6-dimethyloctadienoyl)-α-l-rhamnopyranosyl)-(1→3)-α-l-rhamnopyranosyl) oxy]-octadien-1-yl α-l-rhamnopyranoside; (2E,6Z)-2,6-dimethyl-8-[(O-α-l-rhamnopyranosyl-(1→3)-(2-O-((2E,6Z)-8-hydroxy-2,6-dimethyloctadienoyl)-α-l-rhamnopyranosyl)-(1→3)-4-O-acetyl-α-l-rhamnopyranosyl) oxy]-octadien-1-yl α-l-rhamnopyranoside; (2E,6Z)-2,6-dimethyl-8-[(O-α-l-rhamnopyranosyl-(1→3)-(2-O-((2E,6Z)-8-hydroxy-2,6-dimethyloctadienoyl)-α-l-rhamnopyranosyl)-(1→3)-α-l-rhamnopyranosyl)-oxy]-octadien-1-yl-O-β-d-glucopyranosyl-(1→2)-α-l-rhamnopyranoside | [266] | ||
| Fadogia ancylantha | Triterpene glycosides: 3-O-β-d-glucopyranosyl-3-β-hydroxyolean-12-en-28-oic acid 28-O-[R-l-rhamnopyranosyl-(1→2)-β-d-glucopyranosyl] ester; 3-O-β-d-glucopyranosyl-3-β-hydroxyolean-12-en-28-oic acid 28-O-[-d-apiofuranosyl-(1→2)-β-d-glucopyranosyl] ester | [267] | ||
| Fadogia homblei | Coumarine: scopoletin Flavones: luteolin; quercetin-3-O-β-d-galactoside Triterpenes: lupeol; betulinic acid; 3β-dodecanoyllup-20(29)-en-28-al; lup-20(29)-en-3β-ylhexadecanoate; oleanolic acid; ursolic acid Lignan: 4,4′-dihydroxy-3,3′-dimethoxy-7,9′; 7′,9-diepoxylignan-((−)-pinoresinol) | [268] | ||
| Vangueria spinosa | Proanthocyanidin: (−)-epicatechin-3-O-β-glucopyranoside | [269] | ||
| * | Augusta longifolia | Triterpenes: ursolic acid; acyl lupeol Coumarin: scopoletin Flavonoids: naringenin; kaempferol; quercetin; myricitrin; rutin | [270] | |
| Myrioneuron nutans | Alkaloid: myrobotinol | [271] | ||
| Wendlandia formosana | Iridoid glycosides: 10-O-caffeoyl scandoside methyl ester; 6-methoxy scandoside methyl ester; scandoside methyl ester; methyl deacetyl asperulosidate; 10-O-caffeoyl daphylloside Triterpene: ursolic acid | [272] | ||
| Wendlandia tinctoria | Iridoid glycosides: 5-dehydro-8-epi-adoxosidic acid; 5-dehydro-8-epi-mussaenoside; 10-O-dihydroferuloyldeacetyldaphylloside; wendoside; 8-epi-mussaenoside | [273,274] | ||
| Iridoids: 5-dehydro-8-epi-adoxosidic acid; wendoside | [273] | |||
| Rubioideae | ARG | Argostemma yappii | Pyrrolidinoindole alkaloid: (+)-isochimonanthine | [275] |
| COU | Anthocephalus chinensis | Seco-iridoid glycoside: 3′-O-caffeoylsweroside; loganine; 8-epikingiside; loganic acid; sweroside Phenolic apiglycosides: kelampayosides A–B Indole alkaloids: cadambine; strictosidine lactam; 5α-carboxystrictosidine; desoxycordifoline | [276] | |
| Coussarea brevicaulis | Triterpenes: 3-epi-spathodic acid; coussaric acid; barbinervic acid; scutellaric acid | [277] | ||
| Coussarea hydrangeifolia | Phenylpropanoid glycosides: 1′-O-benzyl-α-l-rhamnopyranosyl-(1′′→6′)-β-d-glucopyranoside; α-l-xylopyranosyl-(4′′→2′)-(3-O-β-d-glucopyranosyl)-10-O-(E)-caffeoyl-β-d-glucopyranoside; 1,6-di-O-caffeoyl-β-d-glucopyranoside; 1-O-(E)-caffeoyl-β-d-glucopyranoside 1-O-(E)-feruloyl-β-d-glucopyranoside | [278] | ||
| Coussarea paniculata | Triterpenes: lupeol; lupeyl acetate; botulin; betulinic acid; 3-epi-betulinic acid; 3-epi-betulinaldehyde; oleanolic acid; ursolic acid; lup-20(29)-en-3β,25-diol; lup-20(29)-en-11R-ol-25,3β-lactone; 3-deoxybetulonic acid | [279] | ||
| Coussarea platyphylla | Triterpenes: betulonic acid; betulinic acid Iridoid: monotropein Diterpene: trans-phytol | [280] | ||
| Cruckshanksia pumila | Iridoids: asperuloside; 7-α-methoxysweroside; swertiamarine | [246,281] | ||
| Heterophyllaea pustulata | Anthraquinones: soranjidiol; soranjidiol-1-methyl ether; rubiadin; rubiadin-1-methyl ether; damnacanthal; damnacanthol | [282] | ||
| Anthraquinones: soranjidiol; rubiadin; rubiadin-1-methyl ether | [283] | |||
| KNO | Knoxia corymbosa | Chromone glycosides: corymbosins K1–K4; noreugenin; undulatoside A | [284] | |
| Knoxia valerianoides | Anthraquinones: 2-hydroxymethylknoxiavaledin; 2-ethoxymethylknoxiavaledin; 2-formylknoxiavaledin | [285] | ||
| Anthraquinones: lucidin; lucidin-ω-methyl ether; rubiadin; damnacanthol; 1,3,6-trihydroxy-2-methoxymethylanthraquinone; 3,6-dihydroxy-2-hydroxymethyl-9,10-anthraquinone; 1,3,6-trihydroxy-2-hydroxymethyl-9,10-anthraquinone 3-O-β-primeveroside; vanillic acid | [286] | |||
| Pentas bussei | Pentacyclic cyclol-type naphthohydroquinone: eriobrucinol; methyl 5,10-dihydroxy-7-methoxy-1,1,3α-trimethyl-1a,2,3,3a,10c,10d-hexahydro-1H-4-oxacyclobuta[cd]-indeno[5,6-a]naphthalene-9-carboxylate | [287] | ||
| Benzochromene: methyl-5,10-dihydroxy-7-methoxy-3-methyl-3-[4-methyl-3-pentenyl]-3H-benzo[f]chromene-9-carboxylate | [288] | |||
| Pentas lanceolata | Anthraquinones: 5,6-dihydroxydamnacanthol; nordamnacanthal ; lucidin-ω-methyl ether; damnacanthol | [289] | ||
| Iridoid: tudoside; 13(R)-epi-gaertneroside; 13(R)-epi-epoxygaertneroside; (E)-uenfoside; (Z)-uenfoside | [290] | |||
| Pentas longiflora | Quinones: pentalongin; mollugin | [291] | ||
| Quinones: pentalongin; mollugin; trans-3,4-dihydroxy-3,4-dihydromollugin; methyl-2,3-epoxy-3-prenyl-1,4-naphthoquinone-2-carboxylate; tectoquinone; 3-hydroxymollugin | [289] | |||
| Pentas micrantha | Anthraquinones: tectoquinone; lucidin-ω-methyl ether; damnacanthol; rubiadin-1-methyl ether; rubiadin; damnacanthal; 5,6-dihydroxydamnacanthol; munjistin methyl ester | [292] | ||
| Pentas schimperi | Anthraquinones: schimperiquinones A–B; cleomiscosin A; 2-hydroxymethylanthraquinone Triterpene: oleanolic acid | [293] | ||
| Triterpenes: oleanolic acid; ursolic acid | [294] | |||
| LAS | Lasianthus fordii | Iridoid glycosides: asperuloside; deacetylasperuloside; methyl deacetyl-asperuloside; megastigmane glucoside; lasianthionoside A–C | [295] | |
| Lasianthus gardneri | Triterpenes: lupenone; lupeol; ursolic acid; canaric acid; 3,4-seco-lupane | [296] | ||
| Lasianthus wallichii | Iridoids: iridolactone; iridoid dimer of asperuloside; asperulosidic acid | [297] | ||
| Ronabea emetic | Iridoid glycosides: asperuloside; 6-hydroxygeniposide; deacetylasperulosidic acid; asperulosidic acid | [298] | ||
| MOR | Coelospermum billardieri | Iridoids: coelobillardin | [299] | |
| Morinda citrifolia | Anthraquinone glycosides: digiferruginol-1-methylether-11-O-β-gentiobioside; digiferruginol-11-O-β-primeveroside; damnacanthol-11-O-β-primeveroside; 1-methoxy-2-primeverosyloxymethyl-anthraquinone-3-olate; 1-hydroxy-2-primeverosyloxymethyl-anthraquinone-3-olate; 1-hydroxy-5,6-dimethoxy-2-methyl-7-primeverosyloxyanthraquinone | [300] | ||
| Anthraquinones: alizarin or 1,2-dihydroxyanthraquinone | [301] | |||
| Anthraquinones: 5,15-dimethylmorindol; alizarin 1-methyl ether; anthragallol 1,3-dimethyl ether; anthragallol 2-dimethyl ether; 6-hydroxy-anthragallol-1,3-dimethyl ether; demorindone-5-dimethylether Iridoids: morindacin; asuperlosidic acid; deacetylasperulosidic acid | [302] | |||
| Fatty acid glucosides: 1,6-di-O-octanoyl-β-d-glicopiranose; 6-O-(-β-d-glucopyranosyl)-1-O-decanoyl-β-d-glicopyranose | [303] | |||
| Iridoid glycosides: 6R-hydroxyadoxoside; 6β,7β-epoxy-8-epi-splendoside; americanin A; narcissoside; asperuloside; asperulosidic acid; borreriagenin; citrifolinin B epimer a; citrifolinin B epimer b; cytidine; deacetylasperuloside; dehydromethoxygaertneroside; epi-dihydrocornin; methylR-d-fructofuranoside; methyl-β-d-fructofuranoside; nicotifloroside Fatty acid glycoside : β-sitosterol 3-O-β-d-glucopyranoside | [304] | |||
| Iridoid glycosides: 9-epi-6α-methoxy geniposidic acid | [305] | |||
| Iridoids: morindacin | [302] | |||
| Triterpenes: 1-O-(3′-methylbut-3′-enyl)-β-d-glucopyranose; 1-n-butyl-4-(5′-formyl-2′-furanyl)methylsuccinate; 4-epi-borreriagenin Iridoid glycosides: asperulosidic acid; deacetylasperulosidic acid; 1-n-butyl-4-methyl-2-hydroxysuccinate; 1-n-butyl-4-methyl-3-hydroxysuccinate | [306] | |||
| Iridoid glycoside: citrifoside | [307] | |||
| Morinda coreia | Iridoid glycosides: yopaaosides A–C; 10-O-acetylmonotropein; 6-O-acetylscandoside Phenolic glycosides: 3,4,5-trimethoxyphenyl 1-O-β-apiofuranosyl (1′→6′′)-β-glucopyranoside | [308] | ||
| Morinda elliptica | Anthraquinones: 2-formyl-1-hydroxyanthraquinone; 1-hydroxy-2-methylanthraquinone; nordamnacanthal; damnacanthal; lucidin-ω-methyl ether; rubiadin; soranjidiol; morindone; rubiadin-l-methyl ether; alizarin-l-methyl ether; morindone-5-methyl ether | [309,310,311] | ||
| Morinda longissima | Coumarine: scopoletin | [312] | ||
| Morinda lucida | Anthraquinones: oruwal; oruwalol; damnacanthal; nor-damnacanthal; soranjidiol; alizarin-l-methyl ether; rubiadin; rubiadin-l-methyl ether; 2-methylanthraquinone; anthraquinone-2-aldehyde; l-hydroxy-2-methylanthraquinone; l-methoxy-2-methyl-anthraquinone; hexacosanoic acid | [313] | ||
| Morinda morindoides | Flavonoids: quercetin; quercetin 7,4'-dimethylether; luteolin 7-glucoside; apigenin 7-glucoside; quercetin 3-rhamnoside; kaempferol 3-rhamnoside; quercetin 3-rutinoside; kaempferol 3-rutinoside; chrysoeriol 7-neohesperidoside | [314] | ||
| Flavonoids: quercetin; quercetin-3-O-rutinoside; kaempferol-7-O-rhamnosylsophoroside; chrysoeriol-7-O-neohesperidoside; quercetin-7,4′-dimethylether; quercetin-3-O-rhamnoside; kaempferol-3-O-rhamnoside; kaempferol-3-O-rutinoside; apigenin-7-O-glucoside; luteolin-7-O-glucoside; kaempferol; apigenin; luteolin Iridoids: epoxygaertneroside; methoxygaertneroside; gaertneroside; gaertneric acid | [315] | |||
| Iridoid: 6′-O-acetyl-3′′-methoxygaertneroside | [316] | |||
| Morinda officinalis | Monoterpene: monotropein | [317] | ||
| Anthraquinones: 1,3,8-trihydroxy-2-methoxy anthraquinone; 2-hydroxy-1-methoxy-anthraquinone; rubiadin | [318] | |||
| Morinda pandurifolia | Anthraquinones: soranjidiol; lucidin-ω-methyl ether; damnacanthal; 1-methoxy-2-methyl anthraquinone; 3-hydroxy-1-methoxy-2-methoxymethyl anthraquinone; anthragallol; nordamnacanthal; flavopurpurin; damnacanthal; lucidin; soranjidiol Iridoid glycoside: asperulosidic acid | [319] | ||
| Morinda royoc | Anthraquinones: nordamnacanthal; damnacanthal; lucidin; soranjidiol; rubiadin 1-methylether | [320] | ||
| Morinda umbellata | nor-Iridoids: umbellatolides A–B | [321] | ||
| OPH | Lerchea bracteata | Alkaloids: dihydrocorynantheol; dihydrositsirikine; β-hunterburnin methoclhoride; α-hunterburnine methoclhoride; dihydrocorynantheol; melinonine B; methobromide; yombine methobromide; 4-methylanthirine; diploceline; malindine; iso-malindine; dihydro-3-epi-corynantheol methoclhoride (lercheine) | [322] | |
| Myrioneuron faberi | Alkaloid: myriberine A | [323] | ||
| Ophiorrhiza blumeana | Indole alkaloids: bracteatine; ophiorrhizine; ophiorrhizine-12-carboxylate; cinchonamine | [324] | ||
| Ophiorrhiza bracteata | Indole alkaloids: bracteatine | [325] | ||
| Ophiorrhiza communis | Indole alkaloids: harman; strictosidinic acid | [326] | ||
| Ophiorrhiza hayatana | Anthraquinones: ophiohayatones A–C | [327] | ||
| Ophiorrhiza kunstleri | Indole alkaloids: ophiorrhines A–B | [328] | ||
| Ophiorrhiza liukiuensis | Monoterpene glycosides: demethylsecologanol; 3-O-glucosylsenburiside II Indole alkaloids: camptothecin; 9-methoxycamptothecin; pumiloside; (3R)-deoxypumiloside; 10-methoxycamptothecin; estrictosamide; lyalosidic acid; ophiorrhines A–B; harman Iridoids: loganic acid; loganin; swertiaside A Triterpene: ursolic acid; epi-vogeloside Monoterpene: sweroside Flavonoid: hyperin Coumarin: scopoletin | [329] | ||
| β-Carbolinic alkaloids: lyalosidic acid; lyaloside; 10-hydroxylyalosidic acid; ophiorrhines A–B; ophiorrhines methyl ester A–B | [330] | |||
| Ophiorrhiza japonica | β-Carbolinic alkaloids: lyaloside; lyalosidic acid; 10-hydroxylyalosidic acid; ophiorrhines A–B; ophiorrhines methyl ester A–B | |||
| Ophiorrhiza pumila | Pentacyclic alkaloid: camptothecin | [331] | ||
| Anthraquinones:1-hydroxy-2-methylanthraquinone; 3-hydroxy-2-methylanthraquinone; 3-hydroxyanthraquinone-2-carbaldehyde; 1-hydroxy-2-hydroxymethylanthraquinone; 3-hydroxy-2-hydroxymethylanthraquinone; 1,3-dihydroxy-2-methylanthraquinone | [332] | |||
| Alkaloids: camptothecin; 9-methoxycamptothecin; pumiloside; (3R)-deoxypumiloside | [329] | |||
| Alkaloids: camptothecin; (3S)-pumiloside; (3S)-deoxypumiloside; (3R)-deoxy-pumiloside; strictosamide | [333] | |||
| Alkaloids: camptothecin; pumiloside; (3S)-deoxypumiloside; (3R)-deoxypumiloside; strictosamide 9-methoxycamptothecin | [330] | |||
| Ophiorrhiza rosacea | Indole alkaloids: ophiorrhines A and B | [328] | ||
| Ophiorrhiza rugosa var decumbens | Anthraquinones: 1-hydroxy-2-hydroxymethyl-3-methoxyanthraquinone; 2-n-butoxy-methyl-1,3-dihydroxyanthraquinone | [334] | ||
| Ophiorrhiza trichocarpon | Indole alkaloids: ophiorrhisides A–F; 3,4,5,6-tetradehydrodolichantoside; lyaloside; dolichantoside; 5-oxostrictosidine | [335] | ||
| Ophiorrhiza tomentosa | Indole alkaloids: harman; strictosidinic acid | [326] | ||
| PAE | Paederia foetidae | Phenolic acid: ethyl p-methoxy-trans-cinnamate | [336] | |
| Paederia scandens | Iridoid glycosides: paederoside; paederoside B; asperuloside; paederosidic acid; methylpaederosidate; saprosmoside E | [337] | ||
| Iridoid glycosides: paederoside; asperuloside; paederosidic acid; asperulosidic acid; paederosidic acid methyl ester; geniposide | [338] | |||
| Iridoid glycosides: paederosidic acid; paederoside; asperulosidic acid; asperuloside; geniposidic acid; deacetylasperulosidic acid; decatilasperuloside methyl ester | [339] | |||
| Iridoid: 6β-O-β-d glucosylparderosic acid | [340] | |||
| Iridoid glycosides: asperuloside; paederoside; scanderoside | [341,342] | |||
| Iridoid glycosides: 6′-O-E-feruloyl monotropein; 10-O-E-feruloyl monotropein | [343] | |||
| Iridoid glycoside: paederoside B | [344] | |||
| PRI | Rennellia elliptica | Anthraquinone: 1,2-dimethoxy-6-methyl-9,10-anthraquinone; 1-hydroxy-2-methoxy-6-methyl-9,10-anthraquinone; nordamnacanthal; 2-formyl-3-hydroxy-9,10-anthraquinone; damnacanthal; lucidin-ω-methyl ether; 3-hydroxy-2-methyl-9,10-anthraquinone; rubiadin; 3-hydroxy-2-methoxy-6-methyl-9,10-anthraquinone; rubiadin-1-methyl ether; 3-hydroxy-2-hydroxymethyl-9,10-anthraquinone | [345] | |
| PSY | Camptotheca acuminata | Alkaloids: camptothecin; 10-hydroxycamptothecin | [346] | |
| Carapichea affinis | Alkaloids: cephaeline; emetine; ipecoside; 6-O-methylipecoside; 6-O-methyl-trans-cephaeloside; borucoside | [347] | ||
| Cephaelis acuminata | Alkaloids: 2-O-β-d-glucopyranosyldemethylalangiside; demethylalangiside; 6′′-O-β-d-glucopyranosylipecoside; 6′′-O-α-d-glucopyranosylipecoside; ipecoside; (4R)-4-hydroxy-6,7-di-O-methyl ipecoside; (4S)-4-hydroxy-6,7-di-O-methylipecoside; 6,7-di-O-methylipecoside tetraacetate | [348] | ||
| Alkaloids: emetine; cephaeline; neocephaeline 7-O-demethylcephaeline; 10-O-demethylcephaeline; 2′-n-(1′′-deoxy-1′′-β-d-buctopyranosyl) cephaeline; 2′′-n-(1′′-deoxy-1′′-β-d-fructopyranosyl) pyranosyl | [349] | |||
| Cephaelis acuminata | Alkaloids: neocephaeline; 7′-O-demethylcephaeline; 10-O-demethylcephaeline; 2′-n-(10-deoxy-10-β-d-fructopyranosyl) cephaeline; 2′-n-(10-deoxy-10′′-β-d-fructopyranosyl) neocephaeline; emetine; cephaeline; psychotrine; protoemetine; 9-demethylprotoemetinol; isocephaeline | [349] | ||
| Cephaelis dichroa | Indole alkaloids: vallesiachotamine lactone; vallesiachotamine; strictosamide; strictosidine; angustine | [350] | ||
| Cephaelis ipecacuanha | Tetrahydroisoquinoline-monoterpene glucosides: 3-O-demethyl-2-O-methylalangiside; alangiside or ipecoside; 6-O-methylipecoside; 7-O-methylipecoside; 3-O-demethyl-2-O-methylalangiside; 2-O-methylalangiside | [351] | ||
| Alkaloids: emetine; cephaeline; psychotrine; emetamine; O-methylpsycotrine | [352] | |||
| Chassalia curviflora var. ophioxyloides | Indole alkaloids: alstrostine A; rudgeifoline | [353] | ||
| Margaritopsis cymuligera | Pyrrolidinoindoline alkaloids: hodgkinsine; quadrigemine C | [354] | ||
| Palicourea acuminata | Indole alkaloid: strictosidinic acid; methylester strictosidine; palicoside; bahienoside B; 5α-carboxystrictosidine; desoxycordifoline; lagamboside; vallesiachotamine | [355] | ||
| Palicourea adusta | Monoterpenoid glucoindole alkaloids: lyaloside; tetra-(O-acetyl)-lyaloside; (E)-O-(6′)-cinnamoyl-4′′-hydroxy-3′′-methoxylyaloside; (E)-tetra-(O-acetyl)-O-(6′)-cinnamoyl-4′-hydroxy-3′-methoxylyaloside; (E)-tetra-(O-acetyl)-O-(6′)-cinnamoyl-4′′-hydroxy-3′′,5′′-dimethoxylyaloside | [356] | ||
| Palicourea crocea | Monoterpenoid indole alkaloids: 3,4-dihydro-1-(1-β-d-glucopyranosyloxy-1,4α,5,7-tetrahydro-4-methoxycarbonylcyclopenta[c]pyran-7-yl)-β-carboline-N2-oxide; croceaine A; psychollatine | [357] | ||
| Palicourea coriacea | Glucoindole alkaloids: 3-epi-strictosidinic acid; strictosidinic acid; strictosidinic ketone Alkaloid: calycanthine Triterpene: ursolic acid | [358] | ||
| Palicourea crocea | Monoterpene Indole Alkaloids: croceaines A–B | [359] | ||
| Palicourea rigida | Indole alkaloid: vallesiachotamine | [360] | ||
| Prismatomeris connata | Anthraquinone glycosides: 1-O-methylrubiadin 3-O-β-primeveroside; damnacanthol 3-O-β-primeveroside; rubiadin 3-O-β-primerveroside; lucidin 3-O-β-primeverosideo; 1,3-dihydroxy-2-(methoxymethyl) anthraquinone 3-O-β-primerveroside; digiferruginol ω-gentiobiose | [361] | ||
| Phenolic compound glycoside: prismaconnatoside | [362] | |||
| Prismatomeris malayana | Anthraquinone: 1,3-dihydroxy-5,6-dimethoxy-2-methoxymethyl-9,10-anthraquinone; 2-hydroxymethyl-1-methoxy-9,10-anthraquinone; tectoquinone; 1-hydroxy-2-methyl-9,10-anthraquinone; rubiadin; rubiadin-1-methyl ether; 1,3-dihydroxy-5,6-dimethoxy-2-methyl-9,10-anthraquinone; nordamnacanthal; damnacanthal | [363] | ||
| Prismatomeris tetrandra | Iridoids: prismatomerin | [364,365] | ||
| Psychotria bahiensis | Bis(monoterpenoid) indole alkaloid glucosides: bahienoside A; bahienoside B; 5R-carboxystrictosidine; angustine; strictosamide; (E)- and (Z)-vallesiachotamine | [366] | ||
| Psychotria barbiflora | β-Carbolinic alkaloids: harman; strictosidinic acid | [367] | ||
| Psychotria brachyceras | Monoterpene indole alkaloids: brachycerine | [368] | ||
| Psychotria camponutans | Pyranonaphthoquinones: pentalongin; psychorubrin; 1-hydroxy-3,4-dihydro-1H-benz[g]isochromene-5,10-dione | [369] | ||
| Psychotria colorata | Alkaloids: (−)-calycanthine; isocalycanthine; (+)-chimonanthine; hodgkinsine; quadrigemine C; (8-8a),(8′-8′a)-tetradehydroisocalycanthine 3a(R),3′a(R) | [370] | ||
| Psychotria calocarpa | Alkaloids: psychotriasine | [371] | ||
| Psychotria correae | Indole alkaloids: isodolichantoside; correantoside; 10-hydroxycorreantoside; correantines A–C e 20-epi-correantine B C13-Norisoprenoids: megastigm-5-ene-3,9-diol; S(+)-dehydrovomifoliol Carotenoids: lutein | [372] | ||
| Psychotria glomerulata | Quinoline alkaloids: glomerulatines A−C; calycanthine; iso-calycanthine | [373] | ||
| Psychotria ipecacuanha | Alkaloids: emetine; cephaeline | [374] | ||
| Psychotria leiocarpa | Indole alkaloids: umbellatine; brachicerine; lyaloside; strictosamide; myrianthosines A–B; n,β-D-glucopyranosyl vincosamide quadrigemine A Iridoid glucosides: asperuloside; deacetylasperuloside; loganin | [375] | ||
| Psychotria myriantha | Indole alkaloids: strictosidinic acid | [376] | ||
| Indole alkaloids: strictosidinic acid | [377] | |||
| Psychotria nuda | Alkaloid: strictosamide | [378] | ||
| Psychotria lyciiflora | Alkaloids: meso-chimonanthine; hodgkinsine; N-demethyl-meso- chimonanthine; quadrigemine C; isopsycotridine B; psychotridine; quadrigemine I; oleoidine; caledonine | [379] | ||
| Psychotria oleoides | ||||
| Psychotria prunifolia | Alkaloids: strictosamide; 10-hydroxyiso-deppeaninol; N-oxide-10-hydroxy-antirhine | [380] | ||
| Indole-β-carboline alkaloids: 10-hydroxyisodeppeaninol; N-oxide-10-hydroxy-antirhine; 14-oxoprunifoleine; strictosamide | [381] | |||
| Indole-β-carboline alkaloids: 14-oxoprunifoleine; strictosamide; 10-hydroxyantirhine N-oxide; 10-hydroxyisodeppeaninol | [382] | |||
| Psychotria suterella | Indole alkaloids: lyaloside; naucletine; strictosamide | [383] | ||
| Psychotria umbellata | Indole alkaloids: psycollatine | [384] | ||
| Psychotria vellosiana | Triterpenes: squalene; lupeolids Coumarin: scopoletin | [385] | ||
| Psychotria viridis | Alkaloid: dimethyltryptamine | [386] | ||
| Rudgea jasminoides | Anthraquinone: 1,4-naphthohydroquinone | [387] | ||
| PUT | Plocama pendula | Naphthohydroquinones: mollugin 6-methyl ether; plocanaphthin Lignans: syringaresinol; pinoresinol; lariciresinol Coumarin: scopoletin | [388] | |
| Anthraquinones: balonone; balonone; methyl ether; plocamanones A–C; knoxiadin; 5,6-dimethyl ether; plocamanone D; chionone; isozyganein dimethyl ether; lucidin 1,3-dimethyl ether; lucidin; 1-hydroxy-2-methyl-9,10-anthraquinone; tectoquinone; rubiadin 3-methyl ether; rubiadin 1-methyl ether; rubiadin dimethyl ether; rubiadin; lucidin 3-methyl ether; munjistin ethyl ester; ibericin; damnacanthol ω-ethyl ether; alizarin dimethyl ether; alizarin 1-methyl ether; anthragallol 1,2-dimethyl ether; 3-hydroxy-2-(hydroxymethyl)-9,10-anthraquinone | [389] | |||
| Triterpenes: 3-epi-pomolic acid 3α-acetate; baloic acid; meth; 19α-hydroxyoleanonic acid; 3β-hydroxyolean-11,13(18)-dien-28-oic acid; 3α-acetoxy-19α-hydroxyursa-12-en-28-oic acid; baloic acid;19α-hydroxyoleanonic acid | [390] | |||
| Putoria calabrica | Flavonoids: calabricosides A–B Iridoid: asperuloside; paederosidic acid; paederoside Lignan glycosides: liriodendrin; dihydrodehydrodiconiferyl alcohol-4-O-β-d-glucopyranoside; 7S,8R,8′R-(–)-lariciresinol-4,4′-bis-O-β-d-glucopyranoside. | [391] | ||
| SPE | Borreria verticillata | Indole alkaloids: spermacoceine; borrerine; borreverine; isoborreverine | [392] | |
| Indole alkaloids: verticillatines A–B Iridoids: scandoside methyl ester; 6′-O-(2-glyceryl) scandoside methyl ester; asperuloside acid | [393] | |||
| Dunnia sinensis | Iridoid: dunnisinine Iridoid glycoside: dunnisinoside | [394] | ||
| Galianthe brasiliensis | Iridoid glycosides: asperuloside; deacetylasperuloside; mixture of Z- and E-6-O-p-coumaroylscandoside methyl ester | [395] | ||
| Galianthe ramosa | Phenolic compound: epicatechin Triterpene: ursolic acid β-carboline indole alkaloid: 1-(hydroxymethyl)-3-(2-hydroxypropan-2-yl)-2-(5-methoxy-9H-β-carbolin-1-yl) cyclopentanol | [396] | ||
| β-carboline alkaloid: 1-(hydroxymethyl)-3-(2-hydroxypropan-2-yl)-2-(5-methoxy-9H-β-carbolin-1-yl) cyclopentanol; 9-methoxyindole alkaloid | [396] | |||
| Galianthe thalictroides | β-carboline indole alkaloid: 1-methyl-3-(2-hydroxypropan-2-yl)-2-(5-methoxy-9H-β-carbolin-1-yl)-cyclopentanol; 1-(hydroxymethyl)-3-(2-hydroxypropan-2-yl)-2-(5-methoxy-9H-β-carbolin-1-yl)-cyclopentanol Anthraquinones: 1-methylalizarin; morindaparvin-A Coumarin: scopoletin | [397] | ||
| Hedyotis auricularia | β-Carboline alkaloid: auricularine | [398] | ||
| Hedyotis capitellata | β-Carboline alkaloids: capitelline; cyclocapitelline; isocyclocapitelline; hedyocapitelline; hedyocapitine | |||
| Hedyotis chrysotricha | β-Carboline alkaloid: chrysotricine | |||
| Hedyotis capitellata | Anthraquinones: capitellataquinone A–D; rubiadin; anthragallol; 2-methyl ether; alizarin-1-methyl eter; digiferruginol; lucidin-3-O-β-glucoside | [399] | ||
| β-Carboline alkaloids: capitelline; (−)-isocyclocapitelline; (+)-cyclocapitelline; isochrysotricine; chrysotricine | [400] | |||
| β -Carboline alkaloids: capitelline; (+)-isocyclocapitelline; (+)-cyclocapitelline; isochrysotricine; chrysotricine | [401] | |||
| Hedyotis chrysotricha | β-Carboline alkaloid: chrysotricine | [402] | ||
| Hedyotis corymbosa | Iridoid glucosides: asperuloside; scandoside methyl ester | [403] | ||
| Iridoids: hedycoryside A–C | [404] | |||
| Hedyotis crassifolia | Triterpenes: ursolic acid; 3β-hydroxyurs-11-ene-23(13)-lactone; 3α,13β-dihydroxyurs-11-ene-28-oic acid; oleanolic acid; 3-β-d-glucopyranosyl-β-sitosterol and 3β,6β-dihydroxyolean-12-ene-28-oic acid | [405] | ||
| Hedyotis diffusa | Iridoid glycosides: dunnisinoside; E-6-O-p-methoxycinnamoyl scandoside methyl ester; Z-6-O-p-methoxycinnamoyl scandoside methyl ester; E-6-O-p-feruloyl scandoside methyl ester; E-6-O-p-coumaroyl scandoside methyl ester; Z-6-O-p-coumaroyl scandoside methyl ester | [406] | ||
| Iridoid glucosides: diffusosides A–B | [407] | |||
| Anthraquinones: 2-methyl-3-methoxyanthraquinone; 2-methyl-3-hydroxyanthraquinone; 2-methyl-3-hydroxy-4-methoxyanthraquinone; 2,3-dimethoxy-6-methylanthraquinone | [398] | |||
| Flavonoids: quercetin; quercetin 3-O-glucopyranoside; quercetin 3-O-sambubioside; quercetin 3-O-sophoroside; quercetin 3-O-rutinoside | ||||
| Hedyotis dichotoma | Anthraquinones:1,4-dihydroxy-2,3-dimethoxyanthraquinone; 1,4-dihydroxy-2-hydroxy-methylanthraquinone; 2,3-dimethoxy-9-hydroxy-1,4-anthraquinone; 2-hydroxymethyl-10-hydroxy-1,4-anthraquinone Flavonoids: isovitexin | [398] | ||
| Hedyotis intricata | Triterpene: lupeol; oleanolic acid Iridoid: asperuloside | [408] | ||
| Hedyotis hedyotidea | Iridoids: deacetylasperulosidic acid ethyl ester; hedyotoside; asperulosidic acid; asperuloside; deacetylasperuloside | [409] | ||
| Hedyotis herbacea | Flavonoids: kaempferol 3-O-rutinoside; rutin; kaempferol 3-O-glucoside; kaempferol 3-O-arabinopyranoside; kaempferol-3-O-arabino pyranoside; quercetin 3-O-galactoside | [398,410] [410] | ||
| Hedyotis nudicaulis | Triterpene glycosides: nudicaucins A–C; guaiacin D | [411] | ||
| Hedyotis pinifolia | Anthraquinones:1,6-dihydroxy-7-methoxy-2-methylanthraquinone; 1,6-dihydroxy-2-methylanthraquinone; 3,6-dihydroxy-2-methylanthraquinon; 1,3,6-trihydroxy-2-methylanthraquinone | [412] | ||
| Hedyotis tenelliflora | Iridoids: teneoside B | [413] | ||
| Hedyotis verticillata | Flavonoids: kaempferitrin | [398] | ||
| Hedyotis vestita | Stereoid: phytol Flavonoids: rutine; isohrametin 3-O-rutinoside; vomifoliol 9-O-β-d-glucopyranoside; auricularin Iridoid: 6α-methoxygenyposide; Phenolic compound: sodium (1S,4aR,5R,7aR)-7-hydroxymethyl-5-methoxy-1-β-d-glucopyranosyloxy-1,4α,5,7α-tetrahydrocyclopenta[c]pyran-4-carboxylate | [414] | ||
| Mitracarpus frigidus | Pyranonaphthoquinone: psychorubrin | [415] | ||
| Mitracarpus scaber | Pentalongin hydroquinone diglycoside: harounoside | [416] | ||
| Phenolic compounds: pentadecanoic; (Z)-octadec-9-enoic; tetradecanoic; (Z,Z)-octadeca-9,12-dienoic; (Z)-hexadec-9-enoic; octadecanoic; dodecanoic acid | [417] | |||
| Mitracarpus villosus | Triterpenes: methyl ursalate; ursolic acid | [418] | ||
| Oldenlandia corymbosa | Iridoid glycosides: geniposidic acid; scandoside; feretoside; 10-O-benzoylscandoside methyl ester; odenlandoside III; asperulosidic acid; deacetylasperulosidic acid | [419] | ||
| Oldenlandia difusa | Triterpenes: ursolic acid | [420] | ||
| Triterpenes: 2,6-dihydroxy-1-methoxy-3-methylanthraquinone; 2-hydroxy-1-methoxy-3-methylanthraquinone; 2-hydroxy-3-methylanthraquinone; quercetin-3-O-[2-O-(6-O-E-sinapoyl)-β-d-glucopyranosyl]-β-glucopyranoside; quercetina-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-glucopyranoside; kaempferol-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-galactopyranoside; quercetin-3-O-(2-O-β-d-glucopyranosyl)-β-d-glucopyranoside; rutin; quercertin | [421] | |||
| Oldenlandia umbellata | Anthraquinones: 1,2,3-trimethoxyanthraquinone; 1,3-dimethoxy-2-hydroxy-anthraquinone; 1,2-dimethoxyanthraquinone; 1-methoxy-2-hydroxyanthraquinone; 1,2-dihydroxyanthraquinone | [422] | ||
| Richardia grandiflora | Phenolic compounds: o-hydroxybenzoic acid; m-methoxy-p-hydroxybenzoic acid | [423] | ||
| Saprosma fragrans | Anthraquinones: 4-dihydroxy-1-methoxyanthraquinone-2-corboxaldehyde; damnacanthal | [424] | ||
| Saprosma hainanense | Alkaloids: saprosmine A; saprosmine B; marcanine A; quinolone; cleistopholine; 4-methoxycarbonyl-5; 10-benzogquinolinequinone; liriodenine | [425] | ||
| Saprosma scortechinii | Iridoid: 6-O-epi-acetylscandoside | [426] | ||
| Iridoids: 10-O-benzoyl deacetylasperulosidic acid; 3,4-dihydro-3α-methoxy-paederoside; saprosmosides A–H | [426] | |||
| Bis-iridoid glucosides: saprosmosides A–F Iridoid glucosides: 3,4-dihydro-3-methoxypaederoside; 10-O-benzoyldeacetylasperulosidic acid; deacetylasperuloside; asperuloside; paederoside; deacetylasperulosidic acid; scandoside; asperulosidic acid; 10-acetylscandoside; paederosidic acid; 6-epi-paederosidic acid; methylpaederosidate; monotropein | [427] | |||
| Saprosma ternatum | Alkaloid: vittadinoside Coumarins: scopoletin Iridoid glycosides: epiasperuloside; epipaederosidic acid; epipaederosi Triterpenes: betulinic acid; betulinaldehyde | [428] | ||
| Spermacoce verticillata | Triterpenes: morolic acid; oleanolic acid; ursolic acid; 3,5-dioxofriedelane Flavonoids: 3-O-α-l-rhamnopyranosyl quercetin; quercetin Anthraquinones: 2-hydroxy-3-methylanthraquinone | [429] | ||
| RUB | Asperula maximowiczii | Iridoids: asperuloides A–C | [430] | |
| Crucianella graeca | Coumarins: daphnin; daphnetin; daphnetin glucoside Iridoids: deacetylasperulosidic acid; scandoside; asperuloside; asperulosidic acid; methyl ester of deacetylasperulosidic acid; dafiloside; geniposidic acid; 10-hydroxyloganin; deacetylasperuloside | [431] | ||
| Crucianella maritima | Iridoid: deacetylasperulosidic acid 6'-glucoside sodium salt; Anthraquinones: 1-hydroxy-2-carbomethoxyanthraquinone; 6-methylanthragallol-2-methyl ether; 6-methylanthragallol-2,3-dimethyl ether; 6-methoxy-2-methylquinizarin; 1-hydroxy-2-methyl-6-methoxyanthraquinone | [432] | ||
| Iridoids: asperuloside; asperulosidic acid; deacetylasperulosidic acid | [433] | |||
| Cruciata glabra | Coumarins: daphnin; daphnetin; daphnetin glucoside Iridoids: scandoside | [431] | ||
| Cruciata laevipes | Coumarins: daphnin; daphnetin glucoside Iridoids: scandoside; asperuloside; asperulosidic acid; methyl ester of deacetylasperulosidic acid; daphylloside | |||
| Cruciata pedemontana | Coumarins: daphnin; daphnetin glucoside Iridoids: scandoside; asperuloside; asperulosidic acid; methyl ester of deacetylasperulosidic acid; daphylloside | |||
| Cruciata taurica | Monoterpenoid glycosides: cruciaside A (2,5-O-β-d-diglucopyranosyl-3-hydroxy-p-cymene); cruciaside B (5-O-β-d-glucopyranosyl-2,3-dihydroxy-p-cymene) | [434] | ||
| Coumarin glucosides: daphnin; daphnetin glucoside; 7-O-(6′-acetoxy-β-d-glucopyranosyl)-8-hydroxycoumarin; 7-O-[6′-O-(3′′,4′′-dihydroxycinnamoyl)-β-d-glucopyranosyl]-8-hydroxycoumarin | [435] | |||
| Crucianella graeca | Iridoids: deacetylasperulosidic acid; scandoside; asperuloside; asperulosidic acid; geniposidic acid; 10-hydroxyloganin; deacetylasperuloside; iridoid V3 | [431] | ||
| Galium album | Iridoid glycosides: secogalioside; asperuloside; deacetyl asperulosidic acid; scandoside; monotropein; asperulosidic acid; geniposidic acid; 10-hydroxyloganin; 10-hydroxymorroniside (isomers 7α e7β); daphylloside | [436] | ||
| Galium aparine | Anthraquinone aldehyde: nordamnacanthal | [437] | ||
| Galium lovcense | Iridoid glycosides: secogalioside; asperuloside; deacetyl asperulosidic acid; scandoside; monotropein; asperulosidic acid; geniposidic acid; 10-hydroxyloganin; 10-hydroxymorroniside (isomers 7α e7β); daphylloside; 7-β-hydroxy-11-methyl forsythide; 7-O-acetyl-10-acetoxyloganin | [436] | ||
| Galium rivale | Iridoid glycosides: monotropein; scandoside; eacetylasperulosidic acid; geniposidic acid; asperulosidic acid Triterpene glycosides: rivalosides A–E e momordin II | [438] | ||
| Galium macedonicum | Iridoid: macedonine | [439] | ||
| Galium sinaicum | Anthraquinones: 6,7-dimethoxyxanthopurpurin; 6-hydroxy-7-methoxyrubiadin; 5-hydroxy-6-hydroxymethyl anthragallol 1,3-dimethyl ether; 7-carboxyanthragallol 1,3-dimethyl ether; anthragallol l-methyl ether 3-O-β-d-glucopyranoside; anthragallol l-methyl ether 3-O-rutinoside; anthragallol 3-O-rutinoside; alizarin 1-methyl ether 2-O-primeveroside | [440] | ||
| Galium spurium | Flavonoids: asperulosidic acid ester ; asperuloside; caffeic acid; kaempferol-3-O-l-rhamnopyranoside; quercetin-3-O-[α-l-rhamnopyranosyl(1→6)-β-d-glucopyranoside]; isorhamnetin-3-O-glucopyranoside; quercetin-3-O-α-l-rhamnopyranoside; kaempferol-3-O-[α-l-rhamnopyranosyl(1→6)-β-d-glucopyranoside]; quercetin | [441] | ||
| Galium verum | Anthraquinones: 1,3-dihydroxy-2 methoxy methyl; 1,3-dimethoxy-2-hydroxy; 1,3-dihydroxy-2-acetoxy; 1-hydroxy-2-hydroxy-methyl; 1,3-dihydroxy-2-methyl; 1-methoxy-2-hydroxy; 1,3-dihydroxy-2-hydroxy-methyl-6-methoxy; 1,6-dihydroxy-2-methyl anthraquinones | [442] | ||
| Galium verum var. asiaticum | Iridoid glycoside: 10-p-dihydrocoumaroyl-6-α-hydroxygeniposide; 10-p-dihydrocoumaroyl deacetylasperuloside; asperulosidic acid methyl ester; asperuloside; asperulosidic acid; deacetylasperuloside; scandoside | [443] | ||
| Rubia akane | Anthraquinones: 1,3-dihydroxyanthraquinone-2-al; lucidin-3-O-primeveroside | [437] | ||
| Rubia cordifolia | Naphtoquinones: dihydromollugin; 2-carbomethoxy-3-(3'-hydroxy)-isopentyl-1,4-naphthohydroquinone 1,4-O-di-β-glucoside; 2-carbomethoxy-3-(3'-hydroxy) isopentyl-1,4-naphthohydroquinona 4-O-β-glucoside Anthraquinones: xanthopurpurin; 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone 3-O-β-glucoside; 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone; 2-methyl-1-hydroxy-9,10-anthraquinone; 3-O-α-rhamnosyl(1→2)-β-glucoside; 3-O-(6'-O-acetyl)-α-rhamnosyl (1→2)-β-glucoside; 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone 3-O-(4′,6′-O-diacetyl)-α-rhamnosyl (1→2)-β-glucoside; 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone 3-O-(3′,6′-O-diacetyl)-α-rhamnosyl (1→2)-β-glucoside | [444] | ||
| Iridoids glycoside: 6-methoxygeniposidic acid; 6-methoxygeniposidic acid methyl ester Triterpene: oleanolic aldehyde acetate Fenolic compound: furomollugin | [445] | |||
| Rubia peregrina | Anthocyanins: cyanidin 3-O-glucoside; delphinidin 3-O-glucoside; cyanidin 3-O-arabinoside | [446] | ||
| Rubia schumanniana | Anthraquinones glycosides: 1,3,6-trihydroxy-2-methyl anthraquinone; (2-methyl-1,3,6-trihydroxy-9,10-anthraquinone-3-O-α-L-rhamnopyranosyl (1→2)-β-d-glucopyranoside); 1-hydroxy-2-hydroxy-methylene-9,10-anthraquinone-11-O-β-d-glucopyranosyl (1→6)-β-d-glucopyranoside; digiferruginol glycoside | [447] | ||
| Triterpenes: 3β-hydroxy-urs-30-p-Z-hydroxycinnamoyl-12-en-28-oic-acid; 3β-hydroxy-olean-30-p-E-hydroxycinnamoyl-12-en-28-oic-acid; 3β,6α-dihydroxy-urs-14-en-12-one | [448] | |||
| Cyclopeptides: rubischumanins A–C; C-6β-oxy-RA IV; RA-IV; O-seco-RA-V | [448] | |||
| Rubia yunnanensis | Triterpene: rubiarbonol K | [449] | ||
| Rubia tinctorum | Anthraquinones: alizarin; lucidin; mollugin; xanthopurpurin; rubiadin | [450] | ||
| Anthraquinones: 1-hydroxy-2-hydroxymethylanthraquinone 3-glucoside 2-hydroxymethyl-anthraquinone 3-glucoside; 3,8-dihydroxymethylanthraquinone 3-glucoside Anthraquinone glycosides: alizarin; lucidian-ω-ethyl ether; lucidin primeveroside Iridoid: asperuloside | [451] | |||
| Anthraquinones: pseudopurpurin; lucidin; alizarin; purpurin; alizarin-2-methylether; lucidin-ω-ethylether; nordamnacanthal; munjistin ethyl ester; lucidin primeveroside; ruberithric acid | [452,453] | |||
| Rubia yunnanensis | Cyclic hexapeptides: rubiyunnanins A–B | [454] | ||
| Triterpenes: rubiarbonones D–F; rubiarbosides F–G; rubiarbonone A; rubiarbonol A–B; rubiarbonone B; rubiarbonol A; rubiarbonol B; rubiarbonol F; rubiarbonol G; rubiarboside A | [455] | |||
| ** | * | Luculia pinciana | Triterpene: luculiaoic acid A | [456] |
| Triterpenes: vogeloside; epi-vogeloside; loganoside; loganin; cincholic acid 28-O-β-d-glucopyranosyl ester; cincholic acid-3-O-β-d-glucopyranoside, 28-O-β-d-glucopyranosyl ester; cincholic acid-3-O-β-d-glucopyranoside | [457] |
ALB: Alberteae; ARG: Argostemmateae; CHI: Chiococceae; CIN: Cinchoneae; COF: Coffeeae; CON: Condamineeae; COU: Coussareeae; GAR: Gardenieae; GUE: Guettardeae; HAM: Hamelieae; HIL: Hillieae; HYM: Hymenodictyeae; ISE: Isertieae; IXO: Ixoreae; KNO: Knoxieae; LAS: Lasiantheae; MOR: Morindeae; MUS: Mussaendeae; NAU: Naucleeae; OCT: Octotropideae; OPH: Ophiorrhizeae; PAE: Paederieae; PAV: Pavetteae; POS: Posoquerieae; PRI: Prismatomerideae; PSY: Psychotrieae; PUT: Putorieae; RUB: Rubieae; SAB: Sabiceeae; SPE: Spermacoceae; VAN: Vanguerieae. * Genera not allocated to any tribe. ** Genera unclassified to subfamily.
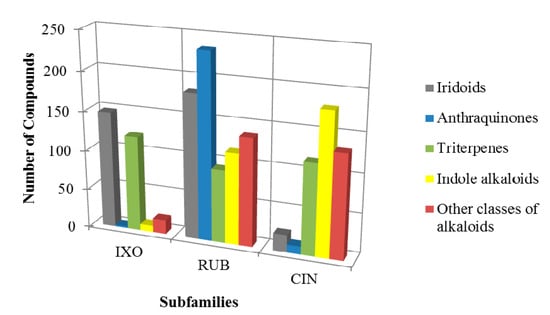
Figure 3.
Chemical diversity and major secondary metabolites distribution among Rubiaceae subfamilies observed in this revision. IXO: Ixoroideae, CIN: Cinchonoideae, RUB: Rubioideae.
This survey found Rubioideae subfamily has the highest chemical diversity in Rubiaceae subfamily. Among the described tribes, the most chemically studied are: Naucleeae (44), Gardenieae (39), Psycotrieae (34), Spermacoceae (35), Rubieae (25) and Ophiorrhizeae (14); other tribes have around five to six studied species. In general, the species with the largest number of phytochemical studies recorded from 1990 to 2014 belong to the genera Uncaria, Psychotria, Hedyotis, Ophiorrhiza and Morinda. Plants from the Psycotrieae tribe were shown to be the major producers of alkaloids, since all phytochemical studies with genera belonging to this tribe (Camptotheca, Carapichea, Cephaelis, Chassalia, Margaritopsis, Palicourea and Psychotria) resulted in the isolation of alkaloids. In the Gardenieae tribe, the presence of iridoids was observed, not only in this survey, but also in other studies [59,60,61,62,64]. Studies showed Rubia, Galium and Morinda genera (subfamily Rubioideae) as important sources of anthraquinones, such as aglycone and rarely glycosides [56].
However, studies establishing a chemotaxonomic classification of plants are quite complex, since there are different types of secondary metabolites that can be distinct in correlated species. These differences in the production of secondary metabolites can be attributed to a number of factors such as genetic mutation, blocking of a biosynthetic pathway and changes in the metabolism due to infection. Soil and climatic variations such as altitude, soil type, macronutrients, micronutrients and water availability, plant age, ultraviolet radiation, rainfall, seasonality and circadian rhythm, also have great influence on the production of metabolites. Besides the fact that the chemical composition can be variable in accordance with the plant organ, it is necessary to study the plant as a whole, to be able to infer a degree of similarity [59,60,61,62,63,64].
Considering the chemical profile of the Rubiaceae family and the metabolic pathways used to produce it, Rubioideae is the most ancient subfamily from an evolutive point of view [16], then it was subdivided into Ixoroideae and finally into Cinchonoideae. The chemical biosynthetic pathway now supports this botanical conclusion. In Rubioideae, anthraquinones are the main metabolites and the pathways are not so specific, being iridoids and indole alkaloids produced also in a large amount. In Ixoroideae, the most active biosysthetic pathway is the one that produces iridoids; while in Cinchonoideae, it is the one that produces indole alkaloids together with other alkaloids.
6. Conclusions
This review has encompassed phytochemical studies of Rubiaceae species for the past 24 years. These substances have been isolated mainly from Uncaria, Psychotria, Hedyotis, Ophiorrhiza and Morinda genera. From the Rubioideae subfamily, 139 species were studied; 80 from the Ixoroideae, and 74 from the Cinchonoideae. Some correlations between iridoids, triterpenes, alkaloids and anthraquinones occurrence and distribution between tribes and subfamilies could be observed, providing chemotaxonomic clues. From an evolutionary point of view, the Rubioideae is the most ancient subfamily [16], then it was subdivided into the Ixoroideae and finally into the Cinchonoideae.
Acknowledgments
The authors are thankful to the Brazilian Agencies CNPq, CAPES and FAPEAM for the financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barreiro, E.J. Produtos naturais bioativos de origem vegetal e o desenvolvimento de fármacos. Quím. Nova 1990, 13, 29–39. [Google Scholar]
- Farias, F.M. Psychotria myriantha müll arg. (rubiaceae): Caracterização dos alcalóides e avaliação das atividades antiquimiotáxica e sobre o sistema nervoso central. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil, 2006. [Google Scholar]
- Fairbrothers, D.E. Chemosystematics with emphasis on systematic serology. In Modern Methods in Plant Taxonomy; Heywood, V.H., Ed.; International Association for Plant Taxonomy: Stockholm, Sweden, 1968; Volume 18, pp. 141–174. [Google Scholar]
- Mabberley, D.J. The Plant-book: A Portable Dictionary of the Vascular Plants Utilizing Kubitzki's The Families and Genera of Vascular Plants (1990-), Cronquist’s An Integrated System of Classification of Flowering Plants (1981), and Current Botanical Literature, Arranged Largely on the Principles of Editions 1–6 (1896/97–1931) of Willis’s A Dictionary of the Flowering Plants and Ferns, 2nd ed.; Cambridge university press: Cambridge, UK, 1997. [Google Scholar]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Tech. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Mongrand, S.; Badoc, A.; Patouille, B.; Lacomblez, C.; Chavent, M.; Bessoule, J.J. Chemotaxonomy of the Rubiaceae family based on leaf fatty acid composition. Phytochemistry 2005, 66, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.C.; Lorenzi, H. Botânica sistemática: Guia ilustrado para identificação de Fanerógamas nativas e exóticas no Brasil, baseado em APG II; Instituto Plantarum: Nova Odessa, Brazil, 2008. [Google Scholar]
- Robbrecht, E. Tropical woody Rubiaceae. Oper. Bot. Belg. 1988, 1, 599–602. [Google Scholar]
- Verdcourt, B. Remarks on the classification of the Rubiaceae. Bull. Jard. Bot. l'Etat Brux./Bull. Rijksplant. Bruss. 1958, 28, 209–290. [Google Scholar] [CrossRef]
- Bremekamp, C.E.B. Remarks on the position, the delimitation and the subdivision of the Rubiaceae. Acta Bot. Neerl. 1966, 15, 1–33. [Google Scholar] [CrossRef]
- Andersson, L. Circumscription of the tribe Isertieae (Rubiaceae). In Proceedings of the Second International Rubiaceae Conference, Meise, Belgium, 13–15 September, 1995; Volume 7, pp. 139–164.
- Bremer, B.; Andreasen, K.; Olsson, D. Subfamilial and tribal relationships in the Rubiaceae based on rbcL sequence data. Ann. Mo. Bot. Gard. 1995, 82, 383–397. [Google Scholar] [CrossRef]
- Andersson, L.; Rova, J.H.; Guarin, F.A. Relationships, circumscription, and biogeography of Arcytophyllum (Rubiaceae) based on evidence from cpDNA. Brittonia 2002, 54, 40–49. [Google Scholar] [CrossRef]
- Rova, J.H.; Delprete, P.G.; Andersson, L.; Albert, V.A. A trnL-F cpDNA sequence study of the Condamineeae-Rondeletieae-Sipaneeae complex with implications on the phylogeny of the Rubiaceae. Am. J. Bot. 2002, 89, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Bolzani, V.D.S.; Young, M.C.M.; Furlan, M.; Cavalheiro, A.J.; Araújo, A.R.; Silva, D.H.S.; Loped, M.N. Secondary metabolites from Brazilian Rubiaceae plant species: Chemotaxonomical and biological significance. Rec. Res. Dev. Phytochem. 2001, 5, 19–31. [Google Scholar]
- Bremer, B. A review of molecular phylogenetic studies of rubiaceae 1. Ann. Mo. Bot. Gard. 2009, 96, 4–26. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Simões, C.M.O.; Schenkel, E.P.; Gosmann, G.; Mello, J.C.P.; Mentz, L.A.; Petrovick, P.R. Farmacognosia: Da planta ao medicamento, 6th ed; UFSC University Press: Florianópolis, Brazil, 2004; p. 1104. [Google Scholar]
- Heitzman, M.E.; Neto, C.C.; Winiarz, E.; Vaisberg, A.J.; Hammond, G.B. Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae). Phytochemistry 2005, 66, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Almog, J.; Cohen, Y.; Azoury, M.; Hahn, T.R. Genipin—A novel fingerprint reagent with colorimetric and fluorogenic activity. J. Forensic Sci. 2004, 49, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Song, Y.S.; Kim, H.J.; Lee, Y.H.; Hong, S.M.; Kim, S.J.; Kim, B.C.; Jin, C.; Lim, C.J.; Park, E.H. Antiinflammatory effects of genipin, an active principle of gardenia. Eur. J. Pharmacol. 2004, 495, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Kim, H.G.; Lee, S.A.; Lim, S.; Park, E.H.; Kim, S.J.; Lim, C.J. Genipin-induced apoptosis in hepatoma cells is mediated by reactive oxygen species/c-Jun NH 2-terminal kinase-dependent activation of mitochondrial pathway. Biochem. pharmacol. 2005, 70, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.C. O Brasil dos viajantes e dos exploradores e a química de produtos naturais brasileira. Quim. Nova 1995, 18, 608–615. [Google Scholar]
- Pelletier, P.J.; Caventou, J.B. Recherches chimiques sur les quinquinas. In Annales de Chimie et de Physique, 1st ed.; Gay-Lussac, J.L., Arago, F. Eds., Eds.; Chez Crochard: Paris, France, 1820; Volume 3. [Google Scholar]
- Viegas, C.; Bolzani, V.S.; Barreiro, E.J. Os produtos naturais e a química medicinal moderna. Quim. Nova 2006, 29, 326–337. [Google Scholar] [CrossRef]
- Lemaire, I.; Assinewe, V.; Cano, P.; Awang, D.V.; Arnason, J.T. Stimulation of interleukin-1 and-6 production in alveolar macrophages by the neotropical liana, Uncaria tomentosa (una de gato). J. Ethnopharmacol. 1999, 64, 109–115. [Google Scholar] [CrossRef]
- Gonçalves, C.; Dinis, T.; Batista, M.T. Antioxidant properties of proanthocyanidins of Uncaria tomentosa bark decoction: A mechanism for anti-inflammatory activity. Phytochemistry 2005, 66, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Callaway, J.C.; Raymon, L.P.; Hearn, W.L.; McKenna, D.J.; Grob, C.S.; Brito, G.S.; Mash, D.C. Quantitation of N,N-dimethyltryptamine and harmala alkaloids in human plasma after oral dosing with ayahuasca. J. Anal. Toxicol. 1996, 20, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Grob, C.S.; Mckenna, D.J.; Callaway, J.C.; Brito, G.S.; Neves, E.S.; Oberlaender, G.; Saide, O.L.; Labigalini, E.; Tacla, C.; Miranda, C.T. Human psychopharmacology of hoasca, a plant hallucinogen used in ritual context in Brazil. J. Nerv. Ment. Dis. 1996, 184, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Deulofeu, V. Chemical compounds isolated from Banisteriopsis and related species. In Ethnopharmacological Search for Psychoactive Drugs; Efron, D., Ed.; U.S. Govt. Printing Office: Washington, WA, USA, 1967; Volume 18, pp. 393–402. [Google Scholar]
- Freedland, C.S.; Mansbach, R.S. Behavioral profile of constituents in ayahuasca, an Amazonian psychoactive plant mixture. Drug Alcohol. Depend. 1999, 54, 183–194. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109 (Suppl S1), 69. [Google Scholar] [CrossRef] [PubMed]
- De-Moraes-Moreau, R.L.; Haraguchi, M.; Morita, H.; Palermo-Neto, J. Chemical and biological demonstration of the presence of monofluoroacetate in the leaves of Palicourea marcgravii St. Hil. Braz. J. Med. Biol. Res. 1995, 28, 685–692. [Google Scholar] [PubMed]
- Di Stasi, L.C.; Hiruma-Lima, C.A. Plantas medicinais na Amazônia e na Mata Atlântica, 2nd ed.; UNESP University Press: São Paulo, Brazil, 2008; Volume 1, p. 604. [Google Scholar]
- Domínguez, X.A. Métodos de investigación fitoquímica; Limusa: Mexico City, Mexico, 1973; Volume 1, p. 281. [Google Scholar]
- Bremer, B. Combined and separate analyses of morphological and molecular data in the plant family Rubiaceae. Cladistics 1996, 12, 21–40. [Google Scholar] [CrossRef]
- Otto, A.; Wilde, V. Sesqui-, di-, and triterpenoids as chemosystematic markers in extant conifers—A review. Bot. Rev. 2001, 67, 141–238. [Google Scholar] [CrossRef]
- Carbonezi, C.A.; Hamerski, L.; Flausino, O.A., Jr.; Furlan, M.; Bolzani, V.D.S.; Young, M.C.M. Determinação por RMN das configurações relativas e conformações de alcalóides oxindólicos isolados de Uncaria guianensis. Quim. Nova 2004, 27, 878–881. [Google Scholar] [CrossRef]
- Dahlgren, R. A revised system of classification of the angiosperms. Bot. J. Linn. Soc. 1980, 80, 91–124. [Google Scholar] [CrossRef]
- Gottlieb, O.R. The role of oxygen in phytochemical evolution towards diversity. Phytochemistry 1989, 28, 2545–2558. [Google Scholar] [CrossRef]
- Gottlieb, O.R. Phytochemicals: Differentiation and function. Phytochemistry 1990, 29, 1715–1724. [Google Scholar] [CrossRef]
- Young, M.C.M.; Araújo, A.R.; da Silva, C.A.; Lopes, M.N.; Trevisan, L.M.; Bolzani, V.D.S. Triterpenes and saponins from Rudgea viburnioides. J. Nat. Prod. 1998, 61, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Young, M.C.M.; Braga, M.R.; Dietrich, S.M.; Gottlieb, H.E.; Trevisan, L.M.; Bolzani, V.D.S. Fungitoxic non-glycosidic iridoids from Alibertia macrophylla. Phytochemistry 1992, 31, 3433–3435. [Google Scholar] [CrossRef]
- Bolzani, V.D.S.; Trevisan, L.M.; Young, M.C.M. Caffeic acid esters and triterpenes of Alibertia macrophylla. Phytochemistry 1991, 30, 2089–2091. [Google Scholar] [CrossRef]
- Koike, K.; Cordell, G.A.; Farnsworth, N.R.; Freer, A.A.; Gilmore, C.J.; Sim, G.A. New cytotoxic diterpenes from Rondeletia panamensis (Rubiaceae). Tetrahedron 1980, 36, 1167–1172. [Google Scholar] [CrossRef]
- Olea, R.S.G.; Roque, N.F.; Bolzani, V.D.S. Acylated flavonol glycosides and terpenoids from the leaves of Alibertia sessilis. J. Braz. Chem. Soc. 1997, 8, 257–259. [Google Scholar] [CrossRef]
- Schripsema, J.; Dagnina, D.; Grosman, G. Alcalóides indólicos. In Farmacognosia da planta ao medicamento; Simões, C.M.O., Ed.; Editora da UFSC. 2004: Florianópolis, Brazil, 2004; Volume 5, pp. 819–846. [Google Scholar]
- Young, M.; Braga, M.; Dietrich, S.; Bolzani, V.; Trevisan, L.; Gottlieb, O. Chemosystematic Markers of Rubiaceae, Proceedings of the Second International Rubiaceae Conference, Meise, Belgium, 13–15 September, 1995; pp. 205–212.
- Inouye, H.; Takeda, Y.; Nishimura, H.; Kanomi, A.; Okuda, T.; Puff, C. Chemotaxonomic studies of rubiaceous plants containing iridoid glycosides. Phytochemistry 1988, 27, 2591–2598. [Google Scholar] [CrossRef]
- Valant-Vetschera, K.M.; Wollenweber, E. Exudate flavonoid aglycones in the alpine species of Achillea sect. Ptarmica: Chemosystematics of A. moschata and related species (Compositae–Anthemideae). Biochem. Syst. Ecol. 2001, 29, 149–159. [Google Scholar] [CrossRef]
- Zidorn, C.; Stuppner, H. Chemosystematics of taxa from the Leontodon section Oporinia. Biochem. Syst. Ecol. 2001, 29, 827–837. [Google Scholar] [CrossRef]
- Rycroft, D.S. Chemosystematics and the liverwort genus Plagiochila. J. Hattori Bot. Lab. 2003, 93, 331–342. [Google Scholar]
- Gottlieb, O.R. Micromolecular Evolution, Systematics and Ecology: An Essay into a Novel Botanical Discipline, 1st ed.; Springer Science & Business Media: Berlin, Germany, 1982; Volume 19, p. 94. [Google Scholar]
- Dahlgren, G. The last Dahlgrenogram. System of classification of the dicotyledons. In Plant Taxonomy Phytogeography and Related Subjects: The Davis and Hedge Festschrift; Tan, K., Mill, R.R., Elias, T.S., Davis, P.H., Hedge, I.C., Davis, P.H., Hedge, I.C., Eds.; University Press: Edinburgh, UK, 1989; pp. 249–260. [Google Scholar]
- Santos, A.R.D.; Barros, M.P.D.; Santin, S.M.D.O.; Sarragiotto, M.H.; Souza, M.C.D.; Eberlin, M.N.; Meurer, E.C. Polar constituents of the leaves of Machaonia brasiliensis (Rubiaceae). Quim. Nova 2004, 27, 525–527. [Google Scholar] [CrossRef]
- Wijnsma, R.; Verpoorte, R. Anthraquinones in the Rubiaceae. In Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer-Verlag Wien: Viena, Austria, 1986; pp. 79–149. [Google Scholar]
- Nagakura, N.; Ruffer, M.; Zenk, M.H. The biosynthesis of monoterpenoid indole alkaloids from strictosidine. J. Chem. Soc. Perkin. 1979. [Google Scholar] [CrossRef]
- Poser, G.V.; Mentz, L.; Simões, C.; Schenkel, E.; Gosmann, G.; Mello, J.D.; Mentz, L.; Petrovick, P. Diversidade biológica e sistemas de classificação. In Farmacognosia: da planta ao medicamento; Simões, C.M.O., Ed.; University Press: Florianópolis, Brazil, 2004; Volume 5, p. 82. [Google Scholar]
- Chen, Q.C.; Zhang, W.Y.; Youn, U.J.; Kim, H.J.; Lee, I.S.; Jung, H.J.; Na, M.K.; Min, B.S.; Bae, K.H. Iridoid glycosides from Gardeniae Fructus for treatment of ankle sprain. Phytochemistry 2009, 70, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Drewes, S.E.; Horn, M.M.; Munro, O.Q.; Ramesar, N.; Ochse, M.; Bringmann, G.; Peters, K.; Peters, E.M. Stereostructure, conformation and reactivity of P-and a-gardiol from Burchellia bubalina. Phytochemistry 1999, 50, 387–394. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Rockenbach, J.; Wray, V. Phenylpropanoid glycosides, a furanone glucoside and geniposidic acid from members of the rubiaceae. Phytochemistry 1995, 39, 375–378. [Google Scholar] [CrossRef]
- Bailleul, F.; Delaveau, P.; Koch, M. Apodantheroside, an iridoid glucoside from Feretia apodanthera. Phytochemistry 1980, 19, 2763–2764. [Google Scholar] [CrossRef]
- Bringmann, G.; Ochse, M.; Wolf, K.; Kraus, J.; Peters, K.; Peters, E.M.; Herderich, M.; Aké Assi, L.; Tayman, F.S.K. 4-Oxonicotinamide-1-(1′-β-ribofuranoside) from Rothmannia longiflora Salisb. (Rubiaceae). Phytochemistry 1999, 51, 271–276. [Google Scholar]
- Luciano, J.H.S.; Lima, M.A.S.; Souza, E.B.; Silveira, E.R. Chemical constituents of Alibertia myrciifolia Spruce ex K. Schum. Biochem. Syst. Ecol. 2004, 32, 1227–1229. [Google Scholar] [CrossRef]
- Borges, R.M.; Valença, S.S.; Lopes, A.A.; Barbi, N.S.; Silva, A.J.R. Saponins from the roots of Chiococca alba and their in vitro anti-inflammatory activity. Phytochem. Lett. 2013, 6, 96–100. [Google Scholar] [CrossRef]
- Abd El-Hafiz, M.A.; Weniger, B.; Quirion, J.C.; Anton, R. Ketoalcohols, lignans and coumarins from Chiococca alba. Phytochemistry 1991, 30, 2029–2031. [Google Scholar] [CrossRef]
- Carbonezi, C.A.; Martins, D.; Young, M.C.M.; Lopes, M.N.; Furlan, M.; Bolzani, V.S. Iridoid and seco-iridoid glucosides from Chiococca alba (Rubiaceae). Phytochemistry 1999, 51, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Argáez, R.B.; Medina, L.B.; Pat, F.M.; Rodrigues, L.M.P. Merilactone, an Unusual C19 Metabolite From the Root Extract of Chiocacca alba. J. Nat. Prod. 2001, 64, 228–231. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Cunha, E.V.L. A triterpenoid from the root-bark of Chiococca alba. Phytochemistry 1992, 31, 2546–2547. [Google Scholar] [CrossRef]
- Borges, R.M.; Tinoco, L.W.; Souza Filho, J.D.D.; Barbi, N.D.S.; Silva, A.J.R.D. Two new oleanane saponins from Chiococca alba (L.) Hitch. J. Braz. Chem. Soc. 2009, 20, 1738–1741. [Google Scholar] [CrossRef]
- Dzib-Reyes, E.V.; García-Sosa, K.; Simá-Polanco, P.; Peña-Rodríguez, L.M. Diterpenoids from the root extract of Chiococca alba. Rev. Latinoam. Quím. 2012, 40, 123–129. [Google Scholar]
- Borges-Argáez, R.; Medina-Baizabál, L.; May-Pat, F.; Peña-Rodríguez, L.M. A new ent-kaurane from the root extract of Chiococca alba. Can. J. Chem. 1997, 75, 801–804. [Google Scholar] [CrossRef]
- Lopes, M.N.; Oliveira, A.C.D.; Young, M.C.M.; Bolzani, V.D.S. Flavonoids from Chiococca braquiata (Rubiaceae). J. Braz. Chem. Soc. 2004, 15, 468–471. [Google Scholar] [CrossRef]
- Olmedo, D.; Rodríguez, N.; Vásquez, Y.; Solís, P.; López-Pérez, J.; Feliciano, A.S.; Gupta, M. A new coumarin from the fruits of Coutarea hexandra. Nat. Prod. Res. 2007, 21, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Chai, H.B.; Shin, Y.G.; García, R.; Mejía, M.; Gao, Q.; Fairchild, C.R.; Lane, K.E.; Menendez, A.T.; Farnsworth, N.R. Cytotoxic Constituents of the Roots of Exostema acuminatum. Tetrahedron 2000, 56, 6401–6405. [Google Scholar] [CrossRef]
- Calera, M.R.; Mata, R.; Anaya, A.L.; Lotina-Hennsen, B. 5-O-β-d-Galactopyranosyl-7-methoxy-3′,4′-dihydroxy-4-phenylcoumarin, an inhibitor of photophosphorylation in spinach chloroplasts. Photosynth. Res. 1995, 45, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Mata, R.; Camacho, M.D.R.; Mendoza, S.; Cruz, M.D.C. A phenylstyrene from Hintonia latiflora. Phytochemistry 1992, 31, 3199–3201. [Google Scholar] [CrossRef]
- Déciga-Campos, M.; Guerrero-Analco, J.A.; Quijano, L.; Mata, R. Antinociceptive activity of 3-O-β-d-glucopyranosyl-23,24-dihydrocucurbitacin F from Hintonia standleyana (Rubiaceae). Pharmacol. Biochem. Behav. 2006, 83, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Maehara, S.; Simanjuntak, P.; Kitamura, C.; Ohashi, K.; Shibuya, H. Bioproduction of Cinchona Alkaloids by the Endophytic Fungus Diaporthe sp. Associated with Cinchona ledgeriana. Chem. Pharm. Bull. 2012, 60, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Maehara, S.; Simanjuntak, P.; Maetani, Y.; Kitamura, C.; Ohashi, K.; Shibuya, H. Ability of endophytic filamentous fungi associated with Cinchona ledgeriana to produce Cinchona alkaloids. J. Nat. Med. 2013, 67, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Schripsema, J.; Ramos-Valdivia, A.; Verpoorte, R. Robustaquinones, novel anthraquinones from an elicited Cinchona robusta suspension culture. Phytochemistry 1999, 51, 55–60. [Google Scholar] [CrossRef]
- Okunade, A.L.; Lewis, W.H.; Elvin-Lewis, M.P.; Casper, S.J.; Goldberg, D.E. Cinchonicine-derived alkaloids from the bark of the Peruvian Ladenbergia oblongifolia. Fitoterapia 2001, 72, 717–719. [Google Scholar] [CrossRef]
- Ruiz-Mesia, L.; Ruiz-Mesía, W.; Reina, M.; Martínez-Diaz, R.; de Inés, C.; Guadaño, A.; González-Coloma, A. Bioactive cinchona alkaloids from Remijia peruviana. J. Agric. Food Chem. 2005, 53, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.G.; Sazatornil, J.G.; Rodríguez, M.L.; Mesía, L.R.; Arana, G.V. Five New Alkaloids from the Leaves of Remijia peruviana. J. Nat. Prod. 2004, 67, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.; Garofalo, L.; Tommasi, N.; Ugaz, O.L.; Pizza, C. Glucoindole alkaloids from bark of two Sickingia species. Phytochemistry 1994, 37, 1471–1475. [Google Scholar] [CrossRef]
- Lee, D.; Cuendet, M.; Axelrod, F.; Chavez, P.I.; Fong, H.H.S.; Pezzuto, J.M.; Douglas Kinghorn, A. Novel 29-nor-3,4-seco-cycloartane triterpene methyl esters from the aerial parts of Antirhea acutata. Tetrahedron 2001, 57, 7107–7112. [Google Scholar] [CrossRef]
- Weniger, B.; Rafik, W.; Bastida, J.; Quirion, J.C.; Anton, R. Indole alkaloids from Antirhea lucida. Planta Med. 1995, 61, 569–569. [Google Scholar] [CrossRef] [PubMed]
- Weniger, B.; Anton, R.; Varea, T.; Quirion, J.C.; Bastida, J.; Garcia, R. Indole alkaloids from Antirhea portoricensis. J. Nat. Prod. 1994, 57, 287–290. [Google Scholar] [CrossRef]
- Barros, M.P.D.; Santin, S.M.D.O.; Costa, W.F.D.; Vidotti, G.J.; Sarragiotto, M.H.; Souza, M.C.D.; Bersani-Amado, C.A. Chemical constituents and anti-inflammatory and antioxidant activities evaluation of the leaves extracts of Chomelia obtusa Cham. & Schltdl.(Rubiaceae). Quim. Nova 2008, 31, 1987–1989. [Google Scholar]
- Lima, G.S.; Moura, F.S.; Lemos, R.P.L.; Conserva, L.M. Triterpenes from Guettarda grazielae M: RV Barbosa (Rubiaceae). Rev. Bras. Farmacogn. 2009, 19, 284–289. [Google Scholar] [CrossRef]
- Moura, F.S.; Lima, G.S.; Meneghetti, M.R.; Lyra Lemos, R.P.; Conserva, L.M. A new iridoid from Guettarda grazielae MRV Barbosa (Rubiaceae). Nat. Prod. Res. 2011, 25, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Montagnac, A.; Litaudon, M.; País, M. Quinine-and quinicine-derived alkaloids from Guettarda noumeana. Phytochemistry 1997, 46, 973–975. [Google Scholar] [CrossRef]
- Testa, G.; Oliveira, P.R.N.; Silva, C.C.; Schuquel, I.T.A.; Oliveira Santin, S.M.; Kato, L.; Oliveira, C.M.A.; Arruda, L.L.M.; Bersani-Amado, C.A. Constituintes químicos das folhas e avaliação da atividade anti-inflamatória de extratos das raízes e folhas de Guettarda pohliana Müll. Arg.(Rubiaceae). Quim. Nova 2012, 35, 527–529. [Google Scholar] [CrossRef]
- De Oliveira, P.R.N.; Testa, G.; de Sena, S.B.; da Costa, W.F.; Helena, M.; de Souza, M.C. Saponinas triterpênicas das raízes de Guettarda pohliana Müll. Arg.(Rubiaceae). Quim. Nova 2008, 31, 755–758. [Google Scholar] [CrossRef]
- Cai, W.H.; Matsunami, K.; Otsuka, H.; Shinzato, T.; Takeda, Y. A glycerol α-d-glucuronide and a megastigmane glycoside from the leaves of Guettarda speciosa L. J. Nat. Med. 2011, 65, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.R.; de Barros, M.P.; de OSantin, S.; Sarragiotto, M.H.; de Souza, M.C.; Eberlin, M.N.; Meurer, E.C. Constituintes polares das folhas de Machaonia brasiliensis (Rubiaceae). Quim. Nova 2004, 27, 525–527. [Google Scholar] [CrossRef]
- Qureshi, A.K.; Mukhtar, M.R.; Hirasawa, Y.; Hosoya, T.; Nugroho, A.E.; Morita, H.; Shirota, O.; Mohamad, K.; Hadi, A.H.A.; Litaudon, M. Neolamarckines A and B, new indole alkaloids from Neolamarckia cadamba. Chem. Pharm. Bull. 2011, 59, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Weniger, B.; Jiang, Y.; Anton, R.; Bastida, J.; Varea, T.; Quirion, J.C. Oxindole alkaloids from Neolaugeria resinosa. Phytochemistry 1993, 32, 1587–1590. [Google Scholar] [CrossRef]
- Khan, I.A.; Sticher, O.; Rali, T. New triterpenes from the leaves of Timonius timon. J. Nat. Prod. 1993, 56, 2163–2165. [Google Scholar] [CrossRef]
- Lendl, A.; Werner, I.; Glasl, S.; Kletter, C.; Mucaji, P.; Presser, A.; Reznicek, G.; Jurenitsch, J.; Taylor, D.W. Phenolic and terpenoid compounds from Chione venosa (sw.) urban var. venosa (Bois Bandé). Phytochemistry 2005, 66, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Kan-Fan, C.; Zuanazzi, J.A.; Quirion, J.C.; Husson, H.P.; Henriques, A. Deppeaninol, A New β-Carboline Alkaloid from Deppea blumenaviensis (Rubiaceae). Nat. Prod. Lett. 1995, 7, 317–321. [Google Scholar] [CrossRef]
- Rumbero, A.; Vásquez, P. Structure and stereochemistry of magniflorine, a new indole alkaloid from Hamelia magniflora Wernha. Tetrahedron Lett. 1991, 32, 5153–5154. [Google Scholar] [CrossRef]
- Paniagua-Vega, D.; Cerda-Garcia-Rojas, C.M.; Ponce-Noyola, T.; Ramos-Valdivia, A.C. A new monoterpenoid oxindole alkaloid from Hamelia patens micropropagated plantlets. Nat. Prod. Commun. 2012, 7, 1441–1444. [Google Scholar] [PubMed]
- Nareeboon, P.; Komkhunthot, W.; Lekcharoen, D.; Wetprasit, N.; Piriyapolsart, C.; Sutthivaiyakit, S. Acetylenic fatty acids, triglyceride and triterpenes from the leaves of Hymenodictyon excelsum. Chem. Pharm. Bull. 2009, 57, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Mitaine-Offer, A.C.; Tapondjou, L.; Djoukeng, J.; Bouda, H.; Lacaille-Dubois, M.A. Glycoside derivatives of scopoletin and β-sitosterol from Hymenodictyon floribundum. Biochem. Syst. Ecol. 2003, 31, 227–228. [Google Scholar] [CrossRef]
- Borges, C.M.; Diakanawma, C.; de Mendonça, D.I. Iridoids from Hymenodictyon floribundum. J. Braz. Chem. Soc. 2010, 21, 1121–1125. [Google Scholar] [CrossRef]
- Bruix, M.; Rumbero, A.; Vázquez, P. Apodihydrocinchonamine, an indole alkaloid from Isertia haenkeana. Phytochemistry 1993, 33, 1257–1261. [Google Scholar] [CrossRef]
- Um, B.-H.; Weniger, B.; Lobstein, A.; Pouplin, T.; Polat, M.; Aragón, R.; Anton, R. Triterpenoid Saponins from Isertia pittieri. J. Nat. Prod. 2001, 64, 1588–1589. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, P.F.; Bhat, A.R.; Azam, A. Antiamoebic coumarins from the root bark of Adina cordifolia and their new thiosemicarbazone derivatives. Eur. J. Med. Chem. 2009, 44, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Tanahashi, T.; Nagakura, N.; Takenaka, Y.; Chen, C.C.; Pelletier, J. Flavonoid glycosides from Adina racemosa and their inhibitory activities on eukaryotic protein synthesis. J. Nat. Prod. 2004, 67, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Fujii, K.; Tomatsu, S.; Takao, C.; Tanahashi, T.; Nagakura, N.; Chen, C.C. Six Secoiridoid Glucosides from Adina racemosa. J. Nat. Prod. 2003, 66, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.J.; He, Z.S. Triterpenoid glycosides from Adina rubella. Phytochemistry 1997, 44, 1139–1143. [Google Scholar] [PubMed]
- He, Z.; Fang, S.Y.; Wang, P.; Gao, J.H. 27-nor-triterpenoid glycosides from Adina rubella. Phytochemistry 1996, 42, 1391–1393. [Google Scholar]
- Zhang, Y.; Gan, M.; Lin, S.; Liu, M.; Song, W.; Zi, J.; Wang, S.; Li, S.; Yang, Y.; Shi, J. Glycosides from the bark of Adina polycephala. J. Nat. Prod. 2008, 71, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Jorge, T.C.M.; Ozima, A.P.; Düsman, L.T.; Souza, M.C.; Pereira, G.F.; Vidotti, G.J.; Sarragiotto, M.H. Alkaloids from Cephalanthus glabratus (Rubiaceae). Biochem. Syst. Ecol. 2006, 34, 436–437. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Zhang, S. Six new triterpenoid saponins from the root and stem bark of Cephalanthus occidentalis. Planta Med. 2005, 71, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Staerk, D.; Lemmich, E.; Christensen, J.; Kharazmi, A.; Olsen, C.E.; Jaroszewski, J.W. Leishmanicidal, antiplasmodial and cytotoxic activity of indole alkaloids from Corynanthe pachyceras. Planta Med. 2000, 66, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.F.; Wang, J.S.; Wang, X.B.; Luo, J.; Wang, H.Y.; Kong, L.Y. Monoterpene indole alkaloids from the stem bark of Mitragyna diversifolia and their acetylcholine esterase inhibitory effects. Phytochemistry 2013, 96, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.H.; Yu, B.Y.; Yang, X.W. 27-Nor-triterpenoid glycosides from Mitragyna inermis. Phytochemistry 2002, 61, 379–382. [Google Scholar] [CrossRef]
- Donfack, E.V.; Lenta, B.N.; Kongue, M.D.T.; Fongang, Y.F.; Ngouela, S.; Tsamo, E.; Dittrich, B.; Laatsch, H. Naucleactonin D, an Indole Alkaloid and other Chemical Constituents from Roots and Fruits of Mitragyna inermis. Z. Naturforsch. B 2012, 67, 1159–1165. [Google Scholar] [CrossRef]
- Toure, H.; Babadjamian, A.; Balansard, G.; Faure, R.; Houghton, P. Complete 1H and 13C NMR chemical shift assignments for some pentacyclic oxindole alkaloids. Spectroscopy Lett. 1992, 25, 293–300. [Google Scholar] [CrossRef]
- Pandey, R.; Singh, S.C.; Gupta, M.M. Heteroyohimbinoid type oxindole alkaloids from Mitragyna parvifolia. Phytochemistry 2006, 67, 2164–2169. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Hao, X. Triterpenoid saponins from Mitragyna rotundifolia. Biochem. Syst. Ecol. 2006, 34, 585–587. [Google Scholar] [CrossRef]
- Takayama, H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem. Pharm. Bull. 2004, 52, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Takayama, H.; Tsutsumi, S.I.; Kitajima, M.; Santiarworn, D.; Liawruangrath, B.; Aimi, N. Gluco-indole Alkaloids from Nauclea cadamba in Thailand and Transformation of 3a-Dihydrocadambine into the Indolopyridine Alkaloid, 16-Carbomethoxynaufoline. Medica 1983, 49, 188–190. [Google Scholar]
- Lamidi, M.; Ollivier, E.; Faure, R.; Debrauwer, L.; Nze-Ekekang, L.; Balansard, G. Quinovic acid glycosides from Nauclea diderrichii. Planta Med. 1995, 61, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Lamidi, M.; Ollivier, E.; Mahiou, V.; Faure, R.; Debrauwer, L.; Nze Ekekang, L.; Balansard, G. Gluco-indole alkaloids from the bark of Nauclea diderrichii. 1H and 13C NMR assignments of 3–5 tetrahydrodeoxycordifoline lactam and cadambine acid. Magn. Reson. Chem. 2005, 43, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Agomuoh, A.A.; Ata, A.; Udenigwe, C.C.; Aluko, R.E.; Irenus, I. Novel Indole Alkaloids from Nauclea latifolia and Their Renin-Inhibitory Activities. Chem. Biodivers. 2013, 10, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lou, H.; Dai, S.; Xu, H.; Zhao, F.; Liu, K. Indole alkoloids from Nauclea officinalis with weak antimalarial activity. Phytochemistry 2008, 69, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Y.; Mukhtar, M.R.; Hadi, A.H.A.; Awang, K.; Mustafa, M.R.; Zaima, K.; Morita, H.; Litaudon, M. Naucline, a new indole alkaloid from the bark of Nauclea officinalis. Molecules 2012, 17, 4028–4036. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.Y.; Dai, S.J.; Zhao, F.; Liu, J.F.; Fang, W.S.; Liu, K. New ursane-type triterpene with NO production suppressing activity from Nauclea officinalis. J. Asian Nat. Prod. Res. 2012, 14, 97–104. [Google Scholar] [CrossRef] [PubMed]
- He, Z.D.; Ma, C.Y.; Zhang, H.J.; Tan, G.T.; Tamez, P.; Sydara, K.; Bouamanivong, S.; Southavong, B.; Soejarto, D.D.; Pezzuto, J.M. Antimalarial constituents from Nauclea orientalis (L.) L. Chem. Biodivers. 2005, 2, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; ElSohly, H.N.; Jacob, M.R.; Pasco, D.S.; Walker, L.A.; Clark, A.M. New Indole Alkaloids from the Bark of Nauclea o rientalis. J. Nat. Prod. 2001, 64, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Sichaem, J.; Surapinit, S.; Siripong, P.; Khumkratok, S.; Jong-aramruang, J.; Tip-pyang, S. Two new cytotoxic isomeric indole alkaloids from the roots of Nauclea orientalis. Fitoterapia 2010, 81, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Foubert, K.; Dhooghe, L.; Lemière, F.; Cimanga, K.; Mesia, K.; Apers, S.; Pieters, L. Chromatographic profiling and identification of two new iridoid-indole alkaloids by UPLC–MS and HPLC-SPE-NMR analysis of an antimalarial extract from Nauclea pobeguinii. Phytochem. Lett. 2012, 5, 316–319. [Google Scholar] [CrossRef]
- Anam, E.M. Novel Nauclequiniine from the Root Extract of Nauclea pobequinii (Pob. & Pellegr.) Petit (Rubiaceae). J. Chem. B Organ. Chem. Med. Chem. 1997, 36, 54–56. [Google Scholar]
- Karaket, N.; Supaibulwatana, K.; Ounsuk, S.; Bultel-Ponce, V.; Pham, V.C.; Bodo, B. Chemical and bioactivity evaluation of the bark of Neonauclea purpurea. Nat. Prod. Commun. 2012, 7, 169–170. [Google Scholar] [PubMed]
- Itoh, A.; Tanahashi, T.; Nagakura, N.; Nishi, T. Two triterpenoid saponins from Neonauclea sessilifolia. Chem. Pharm. Bull. 2003, 51, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.Y.; Li, G.H.; Hao, X.J. Two New Triterpenes from Neonauclea sessilifolia. Acta Bot. Sin. 2003, 45, 1003–1007. [Google Scholar]
- Itoh, A.; Tanahashi, T.; Nagakura, N.; Nishi, T. Two chromone-secoiridoid glycosides and three indole alkaloid glycosides from Neonauclea sessilifolia. Phytochemistry 2003, 62, 359–369. [Google Scholar] [CrossRef]
- Mukhtar, M.R.; Osman, N.; Awang, K.; Hazni, H.; Qureshi, A.K.; Hadi, A.H.A.; Zaima, K.; Morita, H.; Litaudon, M. Neonaucline, a new indole alkaloid from the leaves of Ochreinauclea maingayii (Hook. f.) Ridsd.(Rubiaceae). Molecules 2011, 17, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Raman, V.; Avula, B.; Galal, A.M.; Wang, Y.H.; Khan, I.A. Microscopic and UPLC–UV–MS analyses of authentic and commercial yohimbe (Pausinystalia johimbe) bark samples. J. Nat. Med. 2013, 67, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.S.; Lee, K.H.; Goh, S.H. Alkaloid distribution in Malaysian Uncaria. Phytochemistry 1992, 31, 2031–2034. [Google Scholar] [CrossRef]
- Kam, T.S.; Lee, K.H.; Goh, S.H. Dimeric indole alkaloids from Uncaria callophylla. Phytochemistry 1991, 30, 3441–3444. [Google Scholar] [CrossRef]
- Diyabalanage, T.K.K.; Kumarihamy, B.M.M.; Wannigama, G.P.; Jayasinghe, L.; Merlini, L.; Scaglioni, L. Alkaloids of Uncaria elliptica. Phytochemistry 1997, 45, 1731–1732. [Google Scholar] [CrossRef]
- Taniguchi, S.; Kuroda, K.; Doi, K.I.; Tanabe, M.; Shibata, T.; Yoshida, T.; Hatano, T. Revised structures of gambiriins A1, A2, B1, and B2, chalcane-flavan dimers from gambir (Uncaria gambir extract). Chem. Pharm. Bull. 2007, 55, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Arbain, D.; Ibrahim, S.; Sargent, M.V.; Skelton, B.W.; White, A.H. The alkaloids of Uncaria cf. glabrata. Aust. J. Chem. 1998, 51, 961–964. [Google Scholar] [CrossRef]
- Laus, G.; Keplinger, K. Alkaloids of peruvian Uncaria guianensis (Rubiaceae). Phyton 2003, 43, 1–8. [Google Scholar]
- Yépez, A.M.P.; de Ugaz, O.L.; Alvarez, C.M.P.; de Feo, V.; Aquino, R.; de Simone, F.; Pizza, C. Quinovic acid glycosides from Uncaria guianensis. Phytochemistry 1991, 30, 1635–1637. [Google Scholar] [CrossRef]
- Xin, W.B.; Chou, G.X.; Wang, Z.T. Bis (monoterpenoid) indole alkaloid glucosides from Uncaria hirsuta. Phytochem. Lett. 2011, 4, 380–382. [Google Scholar] [CrossRef]
- Wu, T.S.; Chan, Y.Y. Constituents of leaves of Uncaria hirsuta Haviland. J. Chin. Chem. Soc. 1994, 41, 209–212. [Google Scholar] [CrossRef]
- Salim, F.; Ahmad, R. Alkaloids from Malaysian Uncaria longiflora var. pteropoda. Biochem. Syst. Ecol. 2011, 39, 151–152. [Google Scholar] [CrossRef]
- Sakakibara, I.; Takahashi, H.; Terabayashi, S.; Yuzurihara, M.; Kubo, M.; Ishige, A.; Higuchi, M.; Komatsu, Y.; Okada, M.; Maruno, M. Effect of oxindole alkaloids from the hooks of Uncaria macrophylla on thiopental-induced hypnosis. Phytomedicine 1998, 5, 83–86. [Google Scholar] [CrossRef]
- Ndagijimana, A.; Wang, X.; Pan, G.; Zhang, F.; Feng, H.; Olaleye, O. A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies. Fitoterapia 2013, 86, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.C.; Lin, R.D.; Chen, C.T.; Lee, M.H. Monoamine oxidase B (MAO-B) inhibition by active principles from Uncaria rhynchophylla. J. Ethnopharmacol. 2005, 100, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Laus, G.; Teppner, H. The alkaloids of an Uncaria rhynchophylla (Rubiaceae-Coptosapelteae). Phyton 1996, 36, 185–196. [Google Scholar]
- Xie, S.; Shi, Y.; Wang, Y.; Wu, C.; Liu, W.; Feng, F.; Xie, N. Systematic identification and quantification of tetracyclic monoterpenoid oxindole alkaloids in Uncaria rhynchophylla and their fragmentations in Q-TOF-MS spectra. J. Pharm. Biomed. Anal. 2013, 81, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ponglux, D.; Wongseripipatana, S.; Aimi, N.; Nishimura, M.; Ishikawa, M.; Sada, H.; Haginiwa, J.; Sakai, S.I. Structure and synthesis of two new types of oxindole alkaloids found from Uncaria salaccensis. Chem. Pharm. Bull. 1990, 38, 573–575. [Google Scholar] [CrossRef]
- Liu, H.; Feng, X.; Lu, Y.; Zheng, Q. Isorhynchophyllic acid, a new alkaloid from Uncaria sinensis. Chin. Chem. Lett. 1992, 3, 425–426. [Google Scholar]
- Sekiya, N.; Shimada, Y.; Shibahara, N.; Takagi, S.; Yokoyama, K.; Kasahara, Y.; Sakakibara, I.; Terasawa, K. Inhibitory effects of Choto-san (Diao-teng-san), and hooks and stems of Uncaria sinensis on free radical-induced lysis of rat red blood cells. Phytomedicine 2002, 9, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Montoro, P.; Carbone, V.; de Dioz Zuniga Quiroz, J.; De Simone, F.; Pizza, C. Identification and quantification of components in extracts of Uncaria tomentosa by HPLC-ES/MS. Phytochem. Anal. 2004, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wirth, C.; Wagner, H. Pharmacologically active procyanidines from the bark of Uncaria tomentosa. Phytomedicine 1997, 4, 265–266. [Google Scholar] [CrossRef]
- Muhammad, I.; Dunbar, D.C.; Khan, R.A.; Ganzera, M.; Khan, I.A. Investigation of Una De Gato I. 7-Deoxyloganic acid and 15N NMR spectroscopic studies on pentacyclic oxindole alkaloids from Uncaria tomentosa. Phytochemistry 2001, 57, 781–785. [Google Scholar] [CrossRef]
- Aquino, R.; Tommasi, N.; Simone, F.; Pizza, C. Triterpenes and quinovic acid glycosides from Uncaria tomentosa. Phytochemistry 1997, 45, 1035–1040. [Google Scholar] [CrossRef]
- Laus, G.; Keplinger, D. Separation of stereoisomeric oxindole alkaloids from Uncaria tomentosa by high performance liquid chromatography. J. Chromatogr. A 1994, 662, 243–249. [Google Scholar] [CrossRef]
- García Prado, E.; Gimenez, G.; de la Puerta Vázquez, R.; Espartero Sánchez, J.L.; Sáenz Rodríguez, M.T. Antiproliferative effects of mitraphylline, a pentacyclic oxindole alkaloid of Uncaria tomentosa on human glioma and neuroblastoma cell lines. Phytomedicine 2007, 14, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Duran, R.; González-Aspajo, G.; Ruiz-Martel, C.; Bourdy, G.; Doroteo-Ortega, V.H.; Alban-Castillo, J.; Robert, G.; Auberger, P.; Deharo, E. Anti-inflammatory activity of Mitraphylline isolated from Uncaria tomentosa bark. J. Ethnopharmacol. 2012, 143, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Wurm, M.; Kacani, L.; Laus, G.; Keplinger, K.; Dierich, M.P. Pentacyclic oxindole alkaloids from Uncaria tomentosa induce human endothelial cells to release a lymphocyte-proliferation-regulating factor. Planta Med. 1998, 64, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Hashimoto, K.I.; Yokoya, M.; Takayama, H.; Sandoval, M.; Aimi, N. Two new nor-triterpene glycosides from peruvian “Uña de Gato”(Uncaria tomentosa). J. Nat. Prod. 2003, 66, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.; de Feo, V.; de Simone, F.; Pizza, C.; Cirino, G. Plant metabolites. New compounds and anti-inflammatory activity of Uncaria tomentosa. J. Nat. Prod. 1991, 54, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Hashimoto, K.I.; Yokoya, M.; Takayama, H.; Aimi, N. Two New 19-Hydroxyursolic Acid-type Triterpenes from Peruvian “Uña de Gato” (Uncaria tomentosa). Tetrahedron 2000, 56, 547–552. [Google Scholar] [CrossRef]
- Matsuo, H.; Okamoto, R.; Zaima, K.; Hirasawa, Y.; Ismail, I.S.; Lajis, N.H.; Morita, H. New Vasorelaxant Indole Alkaloids, Villocarines A–D from Uncaria villosa. Bioorg. Med. Chem. 2011, 19, 4075–4079. [Google Scholar] [CrossRef] [PubMed]
- Drewes, S.E.; Horn, M.M.; Connolly, J.D.; Bredenkamp, B. Enolic iridolactone and other iridoids from Alberta magna. Phytochemistry 1998, 47, 991–996. [Google Scholar] [CrossRef]
- Ashihara, H.; Sano, H.; Crozier, A. Caffeine and related purine alkaloids: Biosynthesis, catabolism, function and genetic engineering. Phytochemistry 2008, 69, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Begum, B.; Hasan, C.M.; Rashid, M.A. Caffeine from the Mature Leaves of Coffea bengalensis. Biochem. Syst. Ecol. 2003, 31, 1219–1220. [Google Scholar] [CrossRef]
- Dai, Y.; Harinantenaina, L.; Brodie, P.J.; Birkinshaw, C.; Randrianaivo, R.; Applequist, W.; Ratsimbason, M.; Rasamison, V.E.; Shen, Y.; TenDyke, K. Two antiproliferative triterpene saponins from Nematostylis anthophylla from the highlands of Central Madagascar. Chem. Biodivers. 2013, 10, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Hitotsuyanagi, Y.; Sugeta, N.; Sakakura, K.; Fujita, K.; Fukaya, H.; Aoyagi, Y.; Hasuda, T.; Kinoshita, T.; He, D.H. Tricalysiolides AF, new rearranged ent-kaurane diterpenes from Tricalysia dubia. Tetrahedron 2006, 62, 1512–1519. [Google Scholar] [CrossRef]
- He, D.H.; Otsuka, H.; Hirata, E.; Shinzato, T.; Bando, M.; Takeda, Y. Tricalysiosides AG: Rearranged ent-kauranoid glycosides from the leaves of Tricalysia dubia. J. Nat. Prod. 2002, 65, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Jacob, M.R.; Agarwal, A.K.; Clark, A.M.; Liang, Z.S.; Li, X.C. Ent-Kaurane Glycosides from Tricalysia okelensis. Chem. Pharm. Bull. 2010, 58, 261. [Google Scholar] [CrossRef] [PubMed]
- Zuleta, L.M.C.; Cavalheiro, A.J.; Silva, D.H.S.; Furlan, M.; Young, M.C.M.; Albuquerque, S.; Castro-Gamboa, I.; Bolzani, V.S. Seco-Iridoids from Calycophyllum spruceanum (Rubiaceae). Phytochemistry 2003, 64, 549–553. [Google Scholar] [CrossRef]
- Cardoso, C.L.; Silva, D.H.S.; Young, M.C.M.; Castro-Gamboa, I.; Bolzani, V.D.S. Indole monoterpene alkaloids from Chimarrhis turbinata DC Prodr.: A contribution to the chemotaxonomic studies of the Rubiaceae family. Rev. Bras. Farmacogn. 2008, 18, 26–29. [Google Scholar] [CrossRef]
- Ngalamulume, T.; Kilonda, A.; Toppet, S.; Compernolle, F.; Hoornaert, G. An ursadienedioic acid glycoside from Crossopteryx febrifuga. Phytochemistry 1991, 30, 3069–3072. [Google Scholar]
- Wu, X.D.; He, J.; Li, X.Y.; Dong, L.B.; Gong, X.; Gao, X.; Song, L.D.; Li, Y.; Peng, L.Y.; Zhao, Q.S. Triterpenoids and Steroids with Cytotoxic Activity. Planta Med. 2013, 79, 1356–1361. [Google Scholar] [PubMed]
- Ito, A.; Lee, Y.H.; Chai, H.B.; Gupta, M.P.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. 1′,2′,3′,4′-tetradehydrotubulosine, a cytotoxic alkaloid from Pogonopus speciosus. J. Nat. Prod. 1999, 62, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Anderson, J.; McKenzie, A.; Byrn, S.; McLaughlin, J.; Hudson, M. Tubulosine: An antitumor constituent of Pogonopus speciosus. J. Nat. Prod. 1990, 53, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Sauvain, M.; Moretti, C.; Bravo, J.A.; Callapa, J.; Munoz, V.; Ruiz, E.; Richard, B.; le Men-Olivier, L. Antimalarial activity of alkaloids from Pogonopus tubulosus. Phytother. Res. 1996, 10, 198–201. [Google Scholar] [CrossRef]
- Bastos, A.B.F.D.O.; Carvalho, M.G.; Velandia, J.R.; Braz-Filho, R. Chemical constituents from Simira glaziovii (K. schum) steyerm. and ¹H and 13C NMR assignments of ophiorine and its derivatives. Quim. Nova 2002, 25, 241–245. [Google Scholar] [CrossRef]
- De Araújo, M.F.; Curcino Vieira, I.J.; Braz-Filho, R.; de Carvalho, M.G. Simiranes A and B: Erythroxylanes diterpenes and other compounds from Simira eliezeriana (Rubiaceae). Nat. Prod. Res. 2011, 25, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.C.; Giannini, M.J.S.M.; Carbone, V.; Piacente, S.; Pizza, C.; Bolzani, V.S.; Lopes, M.N. New antifungal terpenoid glycosides from Alibertia edulis (Rubiaceae). Helv. Chim. Acta 2008, 91, 1355–1362. [Google Scholar] [CrossRef]
- Da Silva, V.C.; de Oliveira Faria, A.; da Silva Bolzani, V.; Nasser Lopes, M. A New ent-Kaurane Diterpene from Stems of Alibertia macrophylla K. Schum.(Rubiaceae). Helv. Chim. Acta 2007, 90, 1781–1785. [Google Scholar] [CrossRef]
- Zani, C.; Chaves, P.; Queiroz, R.; de Oliveira, A.; Cardoso, J.; Anjos, A.; Grandi, T. Brine shrimp lethality assay as a prescreening system for anti-Trypanosoma cruzi activity. Phytomedicine 1995, 2, 47–50. [Google Scholar] [CrossRef]
- Luciano, J.H.S.; Lima, M.A.S.; Souza, E.B.; Silveira, E.R.; Vasconcelos, I.M.; Fernandes, G.S.; Souza, E.B. Antifungal iridoids, triterpenes and phenol compounds from Alibertia myrciifolia Sprunge Ex. Schum. Quim. Nova 2010, 33, 292–294. [Google Scholar] [CrossRef]
- Ahmad, V.U. Handbook of Natural Products Data: Pentacyclic Triterpenoids; Elsevier Science: New York, NY, USA, 1994; Volume 2, p. 1556. [Google Scholar]
- Lemmich, E.; Cornett, C.; Furu, P.; Jorstian, C.L.; Knudsen, A.D.; Olsen, C.E.; Salih, A.; Thilborg, S.T. Molluscicidal saponins from Catunaregam nilotica. Phytochemistry 1995, 39, 63–68. [Google Scholar] [CrossRef]
- Gao, G.; Lu, Z.; Tao, S.; Zhang, S.; Wang, F. Triterpenoid saponins with antifeedant activities from stem bark of Catunaregam spinosa (Rubiaceae) against Plutella xylostella (Plutellidae). Carbohydr. Res. 2011, 346, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Kongyen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Sawangjaroen, N.; Songsing, P.; Madardam, H. Anthraquinone and naphthoquinone derivatives from the roots of Coptosapelta flavescens. Nat. Prod. Commun. 2014, 9, 219–220. [Google Scholar] [PubMed]
- Page, J.E.; Madrinan, S.; Towers, G.H.N. Identification of a plant growth inhibiting iridoid lactone from Duroia hirsuta, the allelopathic tree of the “Devil’s Garden”. Cell. Mol. Life Sci. 1994, 50, 840–842. [Google Scholar] [CrossRef]
- Aquino, R.; de Tommasi, N.; Tapia, M.; Lauro, M.R.; Rastrelli, L. New 3-methyoxyflavones, an iridoid lactone and a flavonol from Duroia hirsuta. J. Nat. Prod. 1999, 62, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Carrion, L.L.; Ramos, D.F.; Salome, K.S.; da Silva, P.E.A.; Barison, A.; Nunez, C.V. Triterpenes and the Antimycobacterial Activity of Duroia macrophylla Huber (Rubiaceae). Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Nuanyai, T.; Sappapan, R.; Vilaivan, T.; Pudhom, K. Dammarane triterpenes from the apical buds of Gardenia collinsae. Phytochem. Lett. 2011, 4, 183–186. [Google Scholar] [CrossRef]
- Kunert, O.; Sreekanth, G.; Babu, G.S.; Rao, B.V.R.A.; Radhakishan, M.; Kumar, B.R.; Saf, R.; Rao, A.V.N.A.; Schühly, W. Cycloartane triterpenes from dikamali, the gum resin of Gardenia gummifera and Gardenia lucida. Chem. Biodivers. 2009, 6, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.I.; Oh, J.S.; Kim, J.S.; Chen, P.C.; Zee, O.P. Phytochemical Compounds from the Underground Parts of Gardenia jasminoides var. radicans Makino. Korean J. Pharmacogn. 2002, 33, 1–4. [Google Scholar]
- Machida, K.; Takehara, E.; Kobayashi, H.; Kikuchi, M. Studies on the constituents of Gardenia species. III. New iridoid glycosides from the leaves of Gardenia jasminoides cv. fortuneana Hara. Chem. Pharm. Bull. 2003, 51, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.M.; Chou, G.X.; Wang, Z.T. Iridoid Glycosides from Gardenia jasminoides Ellis. Helv. Chim. Acta 2008, 91, 646–653. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Bi, Z.M.; Li, P.; Tang, D.; Cai, H.X. A new iridoid glycoside from Gardenia jasminoides. Chin. Chem. Lett. 2007, 18, 1221–1223. [Google Scholar] [CrossRef]
- Zhao, W.M.; Xu, J.P.; Qin, G.W.; Xu, R.S. Two monoterpenes from fruits of Gardenia jasminoides. Phytochemistry 1994, 37, 1079–1081. [Google Scholar]
- Pfister, S.; Meyer, P.; Steck, A.; Pfander, H. Isolation and structure elucidation of carotenoid-glycosyl esters in Gardenia fruits (Gardenia jasminoides Ellis) and saffron (Crocus sativus Linne). J. Agric. Food Chem. 1996, 44, 2612–2615. [Google Scholar] [CrossRef]
- Yang, L.; Peng, K.; Zhao, S.; Chen, L.; Qiu, F. Monoterpenoids from the fruit of Gardenia jasminoides Ellis (Rubiaceae). Biochem. Syst. Ecol. 2013, 50, 435–437. [Google Scholar] [CrossRef]
- Yang, L.; Peng, K.; Zhao, S.; Zhao, F.; Chen, L.; Qiu, F. 2-Methyl-l-erythritol glycosides from Gardenia jasminoides. Fitoterapia 2013, 89, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xie, Z.L.; Gao, H.; Ma, W.W.; Dai, Y.; Wang, Y.; Zhong, Y.; Yao, X.S. Bioactive iridoid glucosides from the fruit of Gardenia jasminoides. J. Nat. Prod. 2009, 72, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.L.; Wang, H.Y.; Shi, L.S.; Lai, J.H.; Lin, H.C. Immunosuppressive Iridoids from the Fruits of Gardenia jasminoides. J. Nat. Prod. 2005, 68, 1683–1685. [Google Scholar] [CrossRef] [PubMed]
- Song, J.L.; Wang, R.; Shi, Y.P.; Qi, H.Y. Iridoids from the flowers of Gardenia jasminoides Ellis and their chemotaxonomic significance. Biochem. Syst. Ecol. 2014, 56, 267–270. [Google Scholar] [CrossRef]
- Qin, F.M.; Meng, L.J.; Zou, H.L.; Zhou, G.X. Three new iridoid glycosides from the fruit of Gardenia jasminoides var. radicans. Chem. Pharm. Bull. 2013, 61, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Suksamrarn, A.; Tanachatchairatana, T.; Kanokmedhakul, S. Antiplasmodial triterpenes from twigs of Gardenia saxatilis. J. Ethnopharmacol. 2003, 88, 275–277. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Naovanit, S.; Taylor, W.C.; Bubb, W.A.; Dampawan, P. A sesquiterpene from Gardenia sootepensis. Phytochemistry 1998, 48, 197–200. [Google Scholar] [CrossRef]
- Tuchinda, P.; Saiai, A.; Pohmakotr, M.; Yoosook, C.; Kasisit, J.; Napaswat, C.; Santisuk, T.; Reutrakul, V. Anti-HIV-1 cycloartanes from leaves and twigs of Gardenia thailandica. Planta Med. 2004, 70, 366–369. [Google Scholar] [PubMed]
- Akihisa, T.; Watanabe, K.; Yamamoto, A.; Zhang, J.; Matsumoto, M.; Fukatsu, M. Melanogenesis inhibitory activity of monoterpene glycosides from Gardeniae fructus. Chem. Biodivers. 2012, 9, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, Y.; Yin, F.; Mao, C.; Li, L.; Cai, B.; Lu, T. Quality control and producing areas differentiation of Gardeniae Fructus for eight bioactive constituents by HPLC–DAD–ESI/MS. Phytomedicine 2014, 21, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Ueno, M.; Masuoka, C.; Ikeda, T.; Nohara, T. Iridoid glucosides from the fruit of Genipa americana. ChemInform 2006, 37, 1342–1344. [Google Scholar] [CrossRef]
- Hossain, C.F.; Jacob, M.R.; Clark, A.M.; Walker, L.A.; Nagle, D.G. Genipatriol, a new cycloartane triterpene from Genipa spruceana. J. Nat. Prod. 2003, 66, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Rutaihwa, D.S.D.; Mhehe, G.L. 1-(3-Hydroxy-4-methoxy-5-methylphenyl) ethanone, a new compound from the stem bark of Lamprothamnus zanguebaricus. Fitoterapia 2003, 74, 741–742. [Google Scholar] [CrossRef]
- Tigoufack, I.B.N.; Ngnokam, D.; Tapondjou, L.A.; Harakat, D.; Voutquenne, L. Cycloartane glycosides from leaves of Oxyanthus pallidus. Phytochemistry 2010, 71, 2182–2186. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, J.; Nahrstedt, A.; Wray, V. Cyanogenic glycosides from PS Psydrax and Oxyanthus species [a/t]. Phytochemistry 1992, 31, 567–570. [Google Scholar] [CrossRef]
- Balde, A.; Pieters, L.; Gergely, A.; Kolodziej, H.; Claeys, M.; Vlietinck, A. A-type proanthocyanidins from stem-bark of Pavetta owariensis. Phytochemistry 1991, 30, 337–342. [Google Scholar] [CrossRef]
- Jangwan, J.S.; Aquino, R.P.; Mencherini, T.; Singh, R. Isolation and in vitro cytotoxic activity of 11-methylixoside isolated from bark of Randia dumetorum Lamk. Herb. Polon. 2013, 59, 44–52. [Google Scholar] [CrossRef]
- Jangwan, J.S.; Singh, R. In vitro cytotoxic activity of triterpene isolated from bark of Randia Dumetorum Lamk. J. Curr. Chem. Pharm. Sci. 2014, 4, 1–9. [Google Scholar]
- Sahpaz, S.; Gupta, M.P.; Hostettmann, K. Triterpene saponins from Randia formosa. Phytochemistry 2000, 54, 77–84. [Google Scholar] [CrossRef]
- Jansakul, C.; Intarit, K.; Itharat, A.; Phadungcharoen, T.; Ruangrungsi, N.; Merica, A.; Lange, G.L. Biological activity of crude extract and saponin pseudoginsenoside-RT1 derived from the fruit of Randia siamensis. Pharm. Biol. 1999, 37, 42–45. [Google Scholar] [CrossRef]
- Hamerski, L.; Furlan, M.; Siqueira Silva, D.H.; Cavalheiro, A.J.; Eberlin, M.N.; Tomazela, D.M.; da Silva Bolzani, V. Iridoid glucosides from Randia spinosa (Rubiaceae). Phytochemistry 2003, 63, 397–400. [Google Scholar] [CrossRef]
- Ling, S.K.; Tanaka, T.; Kouno, I. Iridoids from Rothmannia macrophylla. J. Nat. Prod. 2001, 64, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Hamm, A.; Kraus, J.; Ochse, M.; Noureldeen, A.; Jumbam, D.N. Gardenamide A from Rothmannia urcelliformis (Rubiaceae)—Isolation, Absolute Stereostructure, and Biomimetic Synthesis from Genipine. European J. Org. Chem. 2001, 2001, 1983–1987. [Google Scholar] [CrossRef]
- Kumara, P.M.; Soujanya, K.N.; Ravikanth, G.; Vasudeva, R.; Ganeshaiah, K.N.; Shaanker, R.U. Rohitukine, a chromone alkaloid and a precursor of flavopiridol, is produced by endophytic fungi isolated from Dysoxylum binectariferum Hook. f and Amoora rohituka (Roxb). Wight & Arn. Phytomedicine 2014, 21, 541–546. [Google Scholar] [PubMed]
- Zeng, Y.B.; Mei, W.L.; Zhao, Y.X.; Zhuang, L.; Hong, K.; Dai, H.F. Two new epimeric pairs of iridoid from mangrove plant Scyphiphora hydrophyllacea. Chin. Chem. Lett. 2007, 18, 1509–1511. [Google Scholar] [CrossRef]
- Tao, S.H.; Wu, J.; Qi, S.H.; Zhang, S.; Li, M.Y.; Li, Q.X. Scyphiphorins A and B, two new iridoid glycosides from the stem bark of a Chinese mangrove Scyphiphora hydrophyllacea. Helv. Chim. Acta 2007, 90, 1718–1722. [Google Scholar] [CrossRef]
- Hamerski, L.; Carbonezi, C.A.; Cavalheiro, A.J.; Bolzani, V.S.; Young, M.C.M. Triterpenoid saponins from Tocoyena brasiliensis Mart.(Rubiaceae). Quim. Nova 2005, 28, 601–604. [Google Scholar] [CrossRef]
- Von Poser, G.L.; Seibt, L.T. Gardenoside from Tocoyena bullata. Biochem. Syst. Ecol. 1998, 26, 669–670. [Google Scholar] [CrossRef]
- Bolzani, V.S.; Izumisawa, C.M.; Young, M.C.M.; Trevisan, L.; Kingston, D.G.I.; Gunatilaka, A.L. Iridoids from Tocoyena formosa. Phytochemistry 1997, 46, 305–308. [Google Scholar] [CrossRef]
- Raharivelomanana, P.; Bianchini, J.P.; Ramanoelina, A.R.P.; Rasoharahona, J.R.E.; Chatel, F.; Faure, R. Structures of Cadinane- and Guaiane-type Sesquiterpenoids from Enterospermum madagascariensis (Baill.) Homolle. Magn. Reson. Chem. 2005, 43, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Rasoanaivo, P.; Multari, G.; Federici, E.; Galeffi, C. Triterpenoid diglucoside of Enterospermum pruinosum. Phytochemistry 1995, 39, 251–253. [Google Scholar] [CrossRef]
- Latha, P.G.; Nayar, M.N.S.; Sing, O.V.; George, K.R.; Panikkar, K.R.; Pushpangadan, P. Isolation of antigenotoxic ursolic acid from Ixora coccinea flowers. Actual. Biol. 2001, 23, 21–24. [Google Scholar]
- Idowu, T.O.; Ogundaini, A.O.; Salau, A.O.; Obuotor, E.M.; Bezabih, M.; Abegaz, B.M. Doubly linked, A-type proanthocyanidin trimer and other constituents of Ixora coccinea leaves and their antioxidant and antibacterial properties. Phytochemistry 2010, 71, 2092–2098. [Google Scholar] [CrossRef] [PubMed]
- Versiani, M.A.; Ikram, A.; Khalid, S.; Faizi, S.; Tahiri, I.A. Ixoroid: A new triterpenoid from the flowers of Ixora coccinea. Nat. Prod. Commun. 2012, 7, 831–834. [Google Scholar] [PubMed]
- Ikram, A.; Versiani, M.A.; Shamshad, S.; Ahmed, S.K.; Ali, S.T.; Faizi, S. Ixorene, a New Dammarane Triterpene from the Leaves of Ixora coccinea Linn. Rec. Nat. Prod. 2013, 7, 302–306. [Google Scholar]
- Jaiswal, R.; Karar, M.G.E.; Gadir, H.A.; Kuhnert, N. Identification and Characterisation of Phenolics from Ixora coccinea L.(Rubiaceae) by Liquid Chromatography Multi-stage Mass Spectrometry. Phytochem. Anal. 2014, 25, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Wynants, C.; Toppet, S.; Kilonda, A.; Hoornaert, G. Two triterpenoid saponins from Heinsia crinata. Phytochemistry 1994, 36, 1489–1492. [Google Scholar]
- Vidyalakshmi, K.; Rajamanickam, G. An iridoid with anticancer activity from the sepals of Mussaenda dona aurora. Indian J. Chem. B 2009, 48, 1019–1022. [Google Scholar]
- Eswaraiah, M.C.; Elumalai, A. Isolation of phytoconstituents from the stems of Mussaenda erythrophylla. Pharm. Sin. 2011, 2, 132–142. [Google Scholar]
- Dinda, B.; Debnath, S.; Majumder, S.; Arima, S.; Sato, N.; Harigaya, Y. Chemical constituents of Mussaenda incana. Indian J. Chem. 2005, 44, 2362–2366. [Google Scholar] [CrossRef]
- Dinda, B.; Majumder, S.; Arima, S.; Sato, N.; Harigaya, Y. Iridoid glucoside and sterol galactoside from Mussaenda macrophylla. J. Nat. Med. 2008, 62, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.U.; Ghosh, R.; Chowdhury, S.; Dinda, B. New iridoid from aerial parts of Mussaenda roxburghii. Nat. Prod. Commun. 2012, 7, 1–2. [Google Scholar]
- Zhao, W.; Yang, G.; Xu, R.; Qin, G. Three monoterpenes from Mussaenda pubescens. Phytochemistry 1996, 41, 1553–1555. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, J.; Qin, G.; Xu, R. Saponins from Mussaenda pubescens. Phytochemistry 1995, 39, 191–193. [Google Scholar] [CrossRef]
- Macabeo, A.; Avila, J.A.; Alejandro, G.; Franzblau, S.G.; Kouam, S.F.; Hussain, H.; Krohn, K. Villarinol, a new alkenoyloxyalkenol derivative from the endemic Philippine Rubiaceae species Villaria odorata. Nat. Prod. Commun. 2012, 7, 779–780. [Google Scholar] [PubMed]
- Tan, M.A.; Villacorta, R.A.U.; Alejandro, G.J.D.; Takayama, H. Iridoids and a Norsesquiterpenoid from the Leaves of Villaria odorata. Nat. Prod. Commun. 2014, 9, 1229–1230. [Google Scholar] [PubMed]
- Yang, X.W.; Ma, Y.L.; He, H.P.; Wang, Y.H.; Di, Y.T.; Zhou, H.; Li, L.; Hao, X.J. Iridoid Constituents of Tarenna attenuata. J. Nat. Prod. 2006, 69, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Matsunami, K.; Otsuka, H.; Shinzato, T.; Takeda, Y. Tareciliosides HM: Further cycloartane glycosides from leaves of Tarenna gracilipes. Chem. Pharm. Bull. 2011, 59, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Matsunami, K.; Otsuka, H.; Shinzato, T.; Takeda, Y. Tareciliosides AG: Cycloartane glycosides from leaves of Tarenna gracilipes (HAY.) OHWI. Chem. Pharm. Bull. 2008, 56, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Djoudi, R.; Bertrand, C.; Fiasson, K.; Fiasson, J.L.; Comte, G.; Fenet, B.; Antoine Rabesa, Z. Polyphenolics and iridoid glycosides from Tarenna madagascariensis. Biochem. Syst. Ecol. 2007, 35, 314–316. [Google Scholar] [CrossRef]
- Kato, L.; Oliveira, C.; Melo, M.P.; Freitas, C.S.; Schuquel, I.T.A.; Delprete, P.G. Glucosidic iridoids from Molopanthera paniculata Turcz.(Rubiaceae, Posoquerieae). Phytochem. Lett. 2012, 5, 155–157. [Google Scholar] [CrossRef]
- Batista, J.C.; Santin, S.M.D.O.; Schuquel, I.T.A.; Arruda, L.L.M.D.; Bersani-Amado, C.A.; Oliveira, C.M.A.D.; Kato, L.; Ferreira, H.D.; Silva, C.C.D. Chemical constituents and evaluation of antioxidant and anti-inflammatory activities of roots of Sabicea brasiliensis wernh (Rubiaceae). Quim. Nova 2014, 37, 638–642. [Google Scholar] [CrossRef]
- Oliveira, A.M.D.; Conserva, L.M.; de Souza Ferro, J.N.; Brito, F.D.A.; Lemos, R.P.L.; Barreto, E. Antinociceptive and anti-inflammatory effects of octacosanol from the leaves of Sabicea grisea var. grisea in mice. Int. J Mol. Sci. 2012, 13, 1598–1611. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.; Lima, R.; Ferro, J.; Lemos, R.; Conserva, L.; Barreto, E. Chemical Constituents from the Stems and Preliminary Antinociceptive Activity of Sabicea grisea var. grisea. Chem. Nat. Compd. 2014, 49, 1119–1120. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Iridoid and phenolic diglycosides from Canthium berberidifolium. Phytochemistry 2002, 61, 461–464. [Google Scholar] [CrossRef]
- Kouam, S.F.; Ngouonpe, A.W.; Bullach, A.; Lamshöft, M.; Kuigoua, G.M.; Spiteller, M. Monoterpenes with antibacterial activities from a Cameroonian medicinal plant Canthium Multiflorum (Rubiaceae). Fitoterapia 2013, 91, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, B.; Wray, V.; Proksch, P. A cyanogenic glycoside from Canthium schimperianum. Phytochemistry 1996, 42, 633–636. [Google Scholar] [CrossRef]
- Anero, R.; Díaz-Lanza, A.; Ollivier, E.; Baghdikian, B.; Balansard, G.; Bernabé, M. Monoterpene glycosides isolated from Fadogia agrestis. Phytochemistry 2008, 69, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Mencherini, T.; Picerno, P.; del Gaudio, P.; Festa, M.; Capasso, A.; Aquino, R. Saponins and polyphenols from Fadogia ancylantha (Makoni tea). J. Nat. Prod. 2010, 73, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.M.A.; Coombes, P.H.; Crouch, N.R.; Mulholland, D.A. Chemical Constituents from Fadogia homblei De Wild (Rubiaceae). Int. Lett. Chem. Phys. Astron. 2013, 9, 116–124. [Google Scholar] [CrossRef]
- Chatterjee, S.K.; Bhattacharjee, I.; Chandra, G. Isolation and identification of bioactive antibacterial components in leaf extracts of Vangueria spinosa (Rubiaceae). Asian Pac. J. Trop. Med. 2011, 4, 35–40. [Google Scholar] [CrossRef]
- Choze, R.; Delprete, P.G.; Lião, L.M. Chemotaxonomic significance of flavonoids, coumarins and triterpenes of Augusta longifolia (Spreng.) Rehder, Rubiaceae-Ixoroideae, with new insights about its systematic position within the family. Rev. Bras. Farmacogn. 2010, 20, 295–299. [Google Scholar] [CrossRef]
- Pham, V.C.; Jossang, A.; Sevenet, T.; Nguyen, V.H.; Bodo, B. Absolute Configuration of Myrobotinol, New Fused-Hexacyclic Alkaloid Skeleton from Myrioneuron nutans. J. Org. Chem. 2007, 72, 9826–9829. [Google Scholar] [CrossRef] [PubMed]
- Lakshmana Raju, B.; Lin, S.J.; Hou, W.C.; Lai, Z.Y.; Liu, P.C.; Hsu, F.L. Antioxidant iridoid glucosides from Wendlandia formosana. Nat. Prod. Res. 2004, 18, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Debnath, S.; Arima, S.; Sato, N.; Harigaya, Y. Iridoid glucosides from Wendlandia tinctoria roots. Chem. Pharm. Bull. 2006, 54, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Debnath, S.; Banik, R.; Sato, N.; Harigaya, Y. Iridoid glucosides from Wendlandia tinctoria roots. Nat. Prod. Commun. 2011, 6, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Sargent, M.V.; Wahyuni, F.S. (+)-Isochimonanthine, a Pyrrolidinoindole Alkaloid from Argostemma yappii King. Aust. J. Chem. 2000, 53, 159–160. [Google Scholar]
- Kitagawa, I.; Wei, H.; Nagao, S.; Mahmud, T.; Hori, K.; Kobayashi, M.; Uji, T.; Shibuya, H. Indonesian Medicinal Plants. XIV. Characterization of 3′-O-Caffeoylsweroside, a new secoiridoid glucoside, and kelampayosides A and B. two new phenolic apioglucosides, from the bark of Anthocephalus chinensis (Rubiaceae). Chem. Pharm. Bull. 1996, 44, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Su, B.N.; Kang, Y.H.; Pinos, R.E.; Santarsiero, B.D.; Mesecar, A.D.; Soejarto, D.D.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Isolation and absolute stereochemistry of coussaric acid, a new bioactive triterpenoid from the stems of Coussarea brevicaulis. Phytochemistry 2003, 64, 293–302. [Google Scholar] [CrossRef]
- Hamerski, L.; Bomm, M.D.; Silva, D.H.S.; Young, M.C.M.; Furlan, M.; Eberlin, M.N.; Castro-Gamboa, I.; Cavalheiro, A.J.; Bolzani, S.V. Phenylpropanoid glucosides from leaves of Coussarea hydrangeifolia (Rubiaceae). Phytochemistry 2005, 66, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Prakash Chaturvedula, V.; Schilling, J.K.; Johnson, R.K.; Kingston, D.G. New cytotoxic lupane triterpenoids from the twigs of Coussarea paniculata. J. Nat. Prod. 2003, 66, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Araujo, F.C.V.D.; Marques, F.G.; Silva, C.C.D.; Santin, S.M.D.O.; Nakamura, C.V.; Zamuner, M.L.M.; Souza, M.C.D. Terpenes isolated of Coussarea platyphylla Müll. Arg. (Rubiaceae). Quim. Nova 2009, 32, 1760–1763. [Google Scholar] [CrossRef]
- Piovano, M.; Chamy, M.C.; Garbarino, J.A.; Nicoletti, M. Iridoids from Cruckshanksia pumila (Rubiaceae). Biochem. Syst. Ecol. 2003, 31, 1201–1203. [Google Scholar] [CrossRef]
- Núñez-Montoya, S.C.; Comini, L.R.; Sarmiento, M.; Becerra, C.; Albesa, I.; Argüello, G.A.; Cabrera, J.L. Natural anthraquinones probed as Type I and Type II photosensitizers: singlet oxygen and superoxide anion production. J. Photochem. Photobiol. B 2005, 78, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Vázquez, M.F.; Comini, L.R.; Martini, R.E.; Núñez-Montoya, S.C.; Bottini, S.; Cabrera, J.L. Comparisons between conventional, ultrasound-assisted and microwave-assisted methods for extraction of anthraquinones from Heterophyllaea pustulata Hook f. (Rubiaceae). Ultrason. Sonochem. 2014, 21, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Huang, R.; Zhang, H.B.; Li, L. Chromone glycosides from Knoxia corymbosa. J. Asian Nat. Prod. Res. 2006, 8, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Jiang, S.H.; Zhu, D.Y.; Lin, L.Z.; A Cordell, G. Anthraquinones from Knoxia valerianoides. Phytochemistry 1994, 36, 765–768. [Google Scholar] [CrossRef]
- Yoo, N.H.; Jang, D.S.; Lee, Y.M.; Jeong, I.H.; Cho, J.H.; Kim, J.H.; Kim, J.S. Anthraquinones from the roots of Knoxia valerianoides inhibit the formation of advanced glycation end products and rat lens aldose reductase in vitro. Arch. Pharm. Res. 2010, 33, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Bukuru, J.; Nguyen Van, T.; Van Puyvelde, L.; He, W.; De Kimpe, N. New pentacyclic cyclol-type naphthohydroquinone from the roots of Pentas bussei. Tetrahedron 2003, 59, 5905–5908. [Google Scholar] [CrossRef]
- Bukuru, J.F.; Van, T.N.; van Puyvelde, L.; Mathenge, S.G.; Mudida, F.P.; De Kimpe, N. A Benzochromene from the Roots of Pentas bussei. J. Nat. Prod. 2002, 65, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Endale, M.; Patrick, A.J.; Akala-Hoseah, M.; Rono-Nelson, K.; Eyase-Fredrick, L.; Solomon, D.; Albert, N.; Njogu, M.M.; Per, S.; Mate, E. Antiplasmodial Quinonesfrom Pentas longiflora and Pentas lanceolata. Planta Med. 2012, 78, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Schripsema, J.; Caprini, G.P.; van der Heijden, R.; Bino, R.; de Vos, R.; Dagnino, D. Iridoids from Pentas lanceolata. J. Nat. Prod. 2007, 70, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Hari, L.; de Buyck, L.F.; de Pootert, H.L. Naphthoquinoid pigments from Pentas longiflora. Phytochemistry 1991, 30, 1726–1727. [Google Scholar] [CrossRef]
- Endale, M.; Ekberg, A.; Alao, J.P.; Akala, H.M.; Ndakala, A.; Sunnerhagen, P.; Erdélyi, M.; Yenesew, A. Anthraquinones of the Roots of Pentas micrantha. Molecules 2012, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Donfack, A.R.N.; Tala, M.F.; Wabo, H.K.; Jerz, G.; Zeng, G.Z.; Winterhalter, P.; Tan, N.H.; Tane, P. Two new anthraquinone dimers from the stem bark of Pentas schimperi (Rubiaceae). Phytochem. Lett. 2014, 8, 55–58. [Google Scholar] [CrossRef]
- Cai, Y.F.; Huang, Q.S. Determination of oleanolic acid and ursolic acid in Damnacanthus indicus from different places by RP-hPLC]. Zhong Yao Cai 2012, 35, 694–696. [Google Scholar] [PubMed]
- Takeda, Y.; Shimizu, H.; Masuda, T.; Hirata, E.; Shinzato, T.; Bando, M.; Otsuka, H. Lasianthionosides AC, megastigmane glucosides from leaves of Lasianthus fordii. Phytochemistry 2004, 65, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Dallavalle, S.; Jayasinghe, L.; Kumarihamy, B.M.M.; Merlini, L.; Musso, L.; Scaglioni, L. A new 3, 4-seco-lupane derivative from Lasianthus gardneri. J. Nat. Prod. 2004, 67, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Shimidzu, H.; Mizuno, K.; Inouchi, S.; Masuda, T.; Hirata, E.; Shinzato, T.; Aramoto, M.; Otsuka, H. An iridoid glucoside dimer and a non-glycosidic iridoid from the leaves of Lasianthus wallichii. Chem. Pharm. Bull. 2002, 50, 1395–1397. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Fasshuber, H.; Schinnerl, J.; Robien, W.; Brecker, L.; Valant-Vetschera, K. Iridoids as chemical markers of false ipecac (Ronabea emetica), a previously confused medicinal plant. J. Ethnopharmacol. 2011, 138, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Magiatis, P.; Skaltsounis, A.L.; Tillequin, F.; Seguin, E.; Cosson, J.P. Coelobillardin, an iridoid glucoside dimer from Coelospermum billardieri. Phytochemistry 2002, 60, 415–418. [Google Scholar] [CrossRef]
- Kamiya, K.; Hamabe, W.; Tokuyama, S.; Satake, T. New anthraquinone glycosides from the roots of Morinda citrifolia. Fitoterapia 2009, 80, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Hemwimon, S.; Pavasant, P.; Shotipruk, A. Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia. Sep. Purif. Technol. 2007, 54, 44–50. [Google Scholar] [CrossRef]
- Kamiya, K.; Tanaka, Y.; Endang, H.; Umar, M.; Satake, T. New anthraquinone and iridoid from the fruits of Morinda citrifolia. Chem. Pharm. Bull. 2005, 53, 1597–1599. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Kwon, M.K.; Kim, J.N.; Kim, C.K.; Lee, Y.J.; Shin, H.J.; Lee, J.; Lee, H.S. Identification of novel fatty acid glucosides from the tropical fruit Morinda citrifolia L. Phytochem. Lett. 2010, 3, 238–241. [Google Scholar] [CrossRef]
- Sang, S.; Wang, M.; He, K.; Liu, G.; Dong, Z.; Badmaev, V.; Zheng, Q.Y.; Ghai, G.; Rosen, R.T.; Ho, C.T. Chemical components in noni fruits and leaves (Morinda citrifolia L.). ACS Symp. Ser. 2002, 803, 134–150. [Google Scholar]
- Akihisa, T.; Seino, K.I.; Kaneko, E.; Watanabe, K.; Tochizawa, S.; Fukatsu, M.; Banno, N.; Metori, K.; Kimura, Y. Melanogenesis inhibitory activities of iridoid-, hemiterpene-, and fatty acid-glycosides from the fruits of Morinda citrifolia (Noni). J. Oleo. Sci. 2010, 59, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Samoylenko, V.; Zhao, J.; Dunbar, D.C.; Khan, I.A.; Rushing, J.W.; Muhammad, I. New constituents from noni (Morinda citrifolia) fruit juice. J. Agric. Food Chem. 2006, 54, 6398–6402. [Google Scholar] [CrossRef] [PubMed]
- Takashima, J.; Ikeda, Y.; Komiyama, K.; Hayashi, M.; Kishida, A.; Ohsaki, A. New constituents from the leaves of Morinda citrifolia. Chem. Pharm. Bull. 2007, 55, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Iridoid and phenolic glycosides from Morinda coreia. Phytochemistry 2002, 59, 551–556. [Google Scholar] [CrossRef]
- Abdullah, M.A.; Ali, A.M.; Marziah, M.; Lajis, N.H.; Ariff, A.B. Establishment of cell suspension cultures of Morinda elliptica for the production of anthraquinones. Plant Cell Tissue Organ Cult. 1998, 54, 173–182. [Google Scholar] [CrossRef]
- Ismail, N.H.; Ali, A.M.; Aimi, N.; Kitajima, M.; Takayama, H.; Lajis, N.H. Anthraquinones from Morinda elliptica. Phytochemistry 1997, 45, 1723–1725. [Google Scholar] [CrossRef]
- Chiang, L.; Abdullah, M.A. Enhanced anthraquinones production from adsorbent-treated Morinda elliptica cell suspension cultures in production medium strategy. Process Biochem. 2007, 42, 757–763. [Google Scholar] [CrossRef]
- Pham, M.H.; Nguyen, D.T.; Do, T.D. Isolation and Identification of Scopoletin From Roots of Nho Dong (Morinda longissima Y.Z. Ruan, Rubiaceae). Tap. Chi. Duoc. Hoc. 2005, 45, 12–13. [Google Scholar]
- Rath, G.; Ndonzao, M.; Hostettmann, K. Antifungal anthraquinones from Morinda lucida. Pharm. Biol. 1995, 33, 107–114. [Google Scholar] [CrossRef]
- Cimanga, K.; De Bruyne, T.; Lasure, A.; Li, Q.; Pieters, L.; Claeys, M.; Berghe, D.V.; Kambu, K.; Tona, L.; Vlietinck, A. Flavonoid O-glycosides from the leaves of Morinda morindoides. Phytochemistry 1995, 38, 1301–1303. [Google Scholar] [CrossRef]
- Cimanga, R.K.; Kambu, K.; Tona, L.; Hermans, N.; Apers, S.; Totté, J.; Pieters, L.; Vlietinck, A.J. Cytotoxicity and in vitro susceptibility of Entamoeba histolytica to Morinda morindoides leaf extracts and its isolated constituents. J. Ethnopharmacol. 2006, 107, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Kubata, B.K.; Itagaki, S.; Horii, T.; Taba, M.K.; Murakami, N. New anti-malarial phenylpropanoid conjugated iridoids from Morinda morindoides. Bioorg. Med. Chem. Lett. 2010, 20, 1520–1523. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Yun, K.J.; Chung, K.S.; Seo, K.H.; Park, H.J.; Cho, Y.M.; Baek, N.I.; Jang, D.; Lee, K.T. Monotropein isolated from the roots of Morinda officinalis ameliorates proinflammatory mediators in RAW 264.7 macrophages and dextran sulfate sodium (DSS)-induced colitis via NF-κB inactivation. Food Chem. Toxicol. 2013, 53, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Qin, L.; Liu, L.; Wu, Y.; Han, T.; Xue, L.; Zhang, Q. Anthraquinone compounds from Morinda officinalis inhibit osteoclastic bone resorption in vitro. Chem. Biol. Interact. 2011, 194, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ruksilp, T.; Sichaem, J.; Khumkratok, S.; Siripong, P.; Tip-pyang, S. Anthraquinones and an iridoid glycoside from the roots of Morinda pandurifolia. Biochem. Syst. Ecol. 2011, 39, 888–892. [Google Scholar] [CrossRef]
- Borroto, J.; Coll, J.; Rivas, M.; Blanco, M.; Concepción, O.; Tandrón, Y.A.; Hernández, M.; Trujillo, R. Anthraquinones from in vitro root culture of Morinda royoc L. Plant Cell Tissue Organ. Cult. 2008, 94, 181–187. [Google Scholar] [CrossRef]
- Ban, N.K.; Giang, V.H.; Linh, T.M.; Lien, L.Q.; Ngoc, N.T.; Thao, D.T.; Nam, N.H.; Cuong, N.X.; van Kiem, P.; van Minh, C. Two new 11-noriridoids from the aerial parts of Morinda umbellata. Phytochem. Lett. 2013, 6, 267–269. [Google Scholar] [CrossRef]
- Arbain, D.; Lajis, N.H.; Putra, D.P.; Sargent, M.V.; Skelton, B.W.; White, A.H. A New Quaternary Corynanthe Alkaloid from Lerchea bracteata. ChemInform 1993, 24. [Google Scholar] [CrossRef]
- Huang, S.D.; Zhang, Y.; Cao, M.M.; Di, Y.T.; Tang, G.H.; Peng, Z.G.; Jiang, J.D.; He, H.P.; Hao, X.J. Myriberine A, a new alkaloid with an unprecedented heteropentacyclic skeleton from Myrioneuron faberi. Org. Lett. 2013, 15, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Arbain, D.; Dachriyanus, F.; Sargent, M.V.; Skelton, B.W.; White, A.H. Unusual indole alkaloids from Ophiorrhiza blumeana Korth. J. Chem. Soc. Perkin Trans. 1 1998, 2537–2540. [Google Scholar] [CrossRef]
- Arbain, D.; Byrne, L.T.; Sargent, M.V. Isomalindine-16-carboxylate, a zwitterionic alkaloid from Ophiorrhiza cf. communis. Aust. J. Chem. 1997, 50, 1109–1110. [Google Scholar] [CrossRef]
- Hamzah, A.S.; Arbain, D.; V Sargent, M.; Lajis, M.N. The Alkaloids of Ophiorrhiza communis and O. tomentosa. Pertanika J. Sci. Technol. 1994, 2, 33–38. [Google Scholar]
- Chan, H.H.; Li, C.Y.; Damu, A.G.; Wu, T.S. Anthraquinones from Ophiorrhiza hayatana OHWI. Chem. Pharm. Bull. 2005, 53, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Arbain, D.; Putra, D.P.; Sargent, M.V.; Susila, R.; Wahyuni, F.S. Indole alkaloids from two species of Ophiorrhiza. Aust. J. Chem. 2000, 53, 221–224. [Google Scholar]
- Kitajima, M.; Fujii, N.; Yoshino, F.; Sudo, H.; Saito, K.; Aimi, N.; Takayama, H. Camptothecins and two new monoterpene glucosides from Ophiorrhiza liukiuensis. Chem. Pharm. Bull. 2005, 53, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M. Chemical studies on monoterpenoid indole alkaloids from medicinal plant resources Gelsemium and Ophiorrhiza. J. Nat. Med. 2007, 61, 14–23. [Google Scholar] [CrossRef]
- Saito, K.; Sudo, H.; Yamazaki, M.; Koseki-Nakamura, M.; Kitajima, M.; Takayama, H.; Aimi, N. Feasible production of camptothecin by hairy root culture of Ophiorrhiza pumila. Plant Cell Rep. 2001, 20, 267–271. [Google Scholar]
- Kitajima, M.; Fischer, U.; Nakamura, M.; Ohsawa, M.; Ueno, M.; Takayama, H.; Unger, M.; Stöckigt, J.; Aimi, N. Anthraquinones from Ophiorrhiza pumila tissue and cell cultures. Phytochemistry 1998, 48, 107–111. [Google Scholar] [CrossRef]
- Yamazaki, M.; Mochida, K.; Asano, T.; Nakabayashi, R.; Chiba, M.; Udomson, N.; Yamazaki, Y.; Goodenowe, D.B.; Sankawa, U.; Yoshida, T. Coupling deep transcriptome analysis with untargeted metabolic profiling in Ophiorrhiza pumila to further the understanding of the biosynthesis of the anti-cancer alkaloid camptothecin and anthraquinones. Plant and Cell Physiol. 2013, 54, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, V.V.; Vijayan, F.P.; Padikkala, J. Antitumor activities of an anthraquinone fraction isolated from in vitro cultures of Ophiorrhiza rugosa var decumbens. Integr. Cancer Ther. 2011, 11, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Ohara, S.; Kogure, N.; Santiarworn, D.; Takayama, H. β-Carboline-type indole alkaloid glycosides from Ophiorrhiza trichocarpon. Tetrahedron 2013, 69, 9451–9456. [Google Scholar] [CrossRef]
- Uddin, N.; Hossain, M.K.; Haque, M.R.; Hasan, C.M. Chemical Investigation of Paederia foetidae (Rubiaceae). Asian J. Chem. 2013, 25, 1163–1164. [Google Scholar]
- Suzuki, S.; Endo, Y. Studies on the Constituents of the Fruits of Paederia scandens. Structure of A New Iridoid, Paederia lactone. J. Tohoku Pharm. Univ. 2004, 51, 17–21. [Google Scholar]
- Quang, D.N.; Hashimoto, T.; Tanaka, M.; Dung, N.X.; Asakawa, Y. Iridoid glucosides from roots of Vietnamese Paederia scandens. Phytochemistry 2002, 60, 505–514. [Google Scholar] [CrossRef]
- Wu, Z.J.; Wang, J.H.; Fang, D.M.; Zhang, G.L. Analysis of iridoid glucosides from Paederia scandens using HPLC–ESI-MS/MS. J. Chromatogr.B 2013, 923, 54–64. [Google Scholar] [CrossRef] [PubMed]
- He, D.H.; Chen, J.S.; Wang, X.L.; Ding, K.Y. A new iridoid glycoside from Paederia scandens. Chin. Chem. Lett. 2010, 21, 437–439. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, L.; Chen, Z.; Hu, C. Analgesic effect of iridoid glycosides from Paederia scandens (LOUR.) MERRILL (Rubiaceae) on spared nerve injury rat model of neuropathic pain. Pharmacol. Biochem. Behav. 2012, 102, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhu, W.; Pang, M.; Jeffry, J.; Zhou, L. Protective effect of iridoid glycosides from Paederia scandens (LOUR.) MERRILL (Rubiaceae) on uric acid nephropathy rats induced by yeast and potassium oxonate. Food Chem. Toxicol. 2014, 64, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.L.; Chin, Y.-W.; Kim, J.; Park, J.H. Two new acylated iridoid glucosides from the aerial parts of Paederia scandens. Chem. Pharm. Bull. 2004, 52, 1356–1357. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Peng, S.; Liu, X.; Bai, B.; Ding, L. Sulfur-containing iridoid glucosides from Paederia scandens. Fitoterapia 2006, 77, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Osman, C.P.; Ismail, N.H.; Ahmad, R.; Ahmat, N.; Awang, K.; Jaafar, F.M. Anthraquinones with antiplasmodial activity from the roots of Rennellia elliptica Korth.(Rubiaceae). Molecules 2010, 15, 7218–7226. [Google Scholar] [CrossRef] [PubMed]
- Lorence, A.; Medina-Bolivar, F.; Nessler, C.L. Camptothecin and 10-hydroxycamptothecin from Camptotheca acuminata hairy roots. Plant Cell Rep. 2004, 22, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, M.; Fasshuber, H.; Robien, W.; Brecker, L.; Greger, H. Dopamine-iridoid alkaloids in Carapichea affinis (Psychotria borucana) confirm close relationship to the vomiting root Ipecac. Biochem. Syst. Ecol. 2011, 39, 232–235. [Google Scholar] [CrossRef]
- Itoh, A.; Baba, Y.; Tanahashi, T.; Nagakura, N. Tetrahydroisoquinoline-monoterpene glycosides from Cephaelis acuminata. Phytochemistry 2002, 59, 91–97. [Google Scholar] [CrossRef]
- Itoh, A.; Ikuta, Y.; Baba, Y.; Tanahashi, T.; Nagakura, N. Ipecac alkaloids from Cephaelis acuminata. Phytochemistry 1999, 52, 1169–1176. [Google Scholar] [CrossRef]
- Solis, P.N.; Wright, C.W.; Gupta, M.P.; Philipson, J.D. Alkaloids from Cephaelis dichroa. Phytochemistry 1993, 33, 1117–1119. [Google Scholar] [CrossRef]
- Itoh, A.; Tanahashi, T.; Nagakura, N.; Nayeshiro, H. Tetrahydroisoquinoline-monoterpene glucosides from Alangium lamarckii and Cephaelis ipecacuanha. Phytochemistry 1994, 36, 383–387. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Shimomura, K. Emetic alkaloid formation in root culture of Cephaelis ipecacuanha. Phytochemistry 1991, 30, 505–507. [Google Scholar] [CrossRef]
- Schinnerl, J.; Orlowska, E.A.; Lorbeer, E.; Berger, A.; Brecker, L. Alstrostines in Rubiaceae: Alstrostine A from Chassalia curviflora var. ophioxyloides and a novel derivative, rudgeifoline from Rudgea cornifolia. Phytochem. Lett. 2012, 5, 586–590. [Google Scholar]
- Brand, G.; Henriques, A.T.; Passos, C.S.; Baldoqui, D.C.; Oliveira Santin, S.M.; Ferreira da Costa, W.; Sarragiotto, M.H. Pyrrolidinoindoline alkaloids from Margaritopsis cymuligera (Muell. Arg.) CM Taylor (Rubiaceae). Biochem. Syst. Ecol. 2012, 45, 155–157. [Google Scholar] [CrossRef]
- Berger, A.; Fasshuber, H.; Schinnerl, J.; Brecker, L.; Greger, H. Various types of tryptamine-iridoid alkaloids from Palicourea acuminata (Psychotria acuminata, Rubiaceae). Phytochem. Lett. 2012, 5, 558–562. [Google Scholar] [CrossRef]
- Valverde, J.; Tamayo, G.; Hesse, M. β-Carboline monoterpenoid glucosides from Palicourea adusta. Phytochemistry 1999, 52, 1485–1489. [Google Scholar] [CrossRef]
- Narine, L.L.; Maxwell, A.R. Monoterpenoid indole alkaloids from Palicourea crocea. Phytochem. Lett. 2009, 2, 34–36. [Google Scholar] [CrossRef]
- Nascimento, C.A.; Gomes, M.S.; Liao, L.M.; de Oliveira, C.; Kato, L.; da Silva, C.C.; Tanaka, C. Alkaloids from Palicourea coriacea (Cham.) K. Schum. Z. Naturforsch. B 2006, 61, 1443–1446. [Google Scholar] [CrossRef]
- Düsman, L.T.; Marinho Jorge, T.C.; Souza, M.C.D.; Eberlin, M.N.; Meurer, E.C.; Bocca, C.C.; Basso, E.A.; Sarragiotto, M.H. Monoterpene Indole Alkaloids from Palicourea crocea. J. Nat. Prod. 2004, 67, 1886–1888. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.R.O.; Oliveira, P.L.; Oliveira, C.M.A.; Kato, L.; Guillo, L.A. In vitro antiproliferative effects of the indole alkaloid vallesia chotamine on human melanoma cells. Arch. Pharm. Res. 2012, 35, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Feng, S.X.; Qiu, S.X.S.; Chen, T. Anthraquinone Glycosides from the Roots of Prismatomeris connata. Chin. J. Nat. Med. 2011, 9, 42–45. [Google Scholar] [CrossRef]
- Feng, S.X.; Bai, J.; Qiu, S.; Li, Y.; Chen, T. Iridoid and phenolic glycosides from the roots of Prismatomeris connata. Nat. Prod. Commun. 2012, 7, 561–562. [Google Scholar] [PubMed]
- Tuntiwachwuttikul, P.; Butsuri, Y.; Sukkoet, P.; Prawat, U.; Taylor, W.C. Anthraquinones from the roots of Prismatomeris malayana. Nat. Prod. Res. 2008, 22, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.; Gehle, D.; Dey, S.K.; Nahar, N.; Mosihuzzaman, M.; Sultana, N.; Sohrab, M.H.; Stephens, P.J.; Pan, J.J.; Sasse, F. Prismatomerin, a new iridoid from Prismatomeris tetrandra. Structure elucidation, determination of absolute configuration, and cytotoxicity. J. Nat. Prod. 2007, 70, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Pan, J.J.; Krohn, K. Determination of the absolute configurations of pharmacological natural products via density functional theory calculations of vibrational circular dichroism: the new cytotoxic iridoid prismatomerin. J. Org. Chem. 2007, 72, 7641–7649. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Maxwell, A.; Reynolds, W. Novel bis (monoterpenoid) indole alkaloids from Psychotria bahiensis. J. Nat. Prod. 2003, 66, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.; Lemos, R.P.L.; Conserva, L.M. β-Carboline alkaloids from Psychotria barbiflora DC. (Rubiaceae). Biochem. Syst. Ecol. 2013, 50, 339–341. [Google Scholar] [CrossRef]
- Nascimento, N.C.; Menguer, P.K.; Henriques, A.T.; Fett-Neto, A.G. Accumulation of brachycerine, an antioxidant glucosidic indole alkaloid, is induced by abscisic acid, heavy metal, and osmotic stress in leaves of Psychotria brachyceras. Plant Physiol. Biochem. 2013, 73, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Claessens, S.; de Kimpe, N. First straightforward synthesis of 1-hydroxy-3,4-dihydro-1H-benz [g] isochromene-5,10-dione and structure revision of a bioactive benz [g] isochromene-5, 10-dione from Psychotria camponutans. Tetrahedron 2008, 64, 412–418. [Google Scholar] [CrossRef]
- Verotta, L.; Pilati, T.; Tatò, M.; Elisabetsky, E.; Amador, T.A.; Nunes, D.S. Pyrrolidinoindoline Alkaloids from Psychotria colorata. J. Nat. Prod. 1998, 61, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; He, H.P.; Wang, Y.H.; Hao, X.J. A new dimeric alkaloid from the leaf of Psychotria calocarpa. Helv. Chim. Acta 2010, 93, 1650–1652. [Google Scholar] [CrossRef]
- Achenbach, H.; Lottes, M.; Waibel, R.; Karikas, G.A.; Correa, M.D.; Gupta, M.P. Alkaloids and other compounds from Psychotria correae. Phytochemistry 1995, 38, 1537–1545. [Google Scholar] [CrossRef]
- Solís, P.N.; Ravelo, A.G.; Antonio Palenzuela, J.; Gupta, M.P.; González, A.; David Phillipson, J. Quinoline alkaloids from Psychotria glomerulata. Phytochemistry 1997, 44, 963–969. [Google Scholar] [CrossRef]
- Garcia, R.M.A.; Oliveira, L.O.; Moreira, M.A.; Barros, W.S. Variation in emetine and cephaeline contents in roots of wild Ipecac (Psychotria ipecacuanha). Biochem. Syst. Ecol. 2005, 33, 233–243. [Google Scholar] [CrossRef]
- Lopes, S.; Von Poser, G.L.; Kerber, V.A.; Farias, F.M.; Konrath, E.L.; Moreno, P.; Sobral, M.E.; Zuanazzi, J.A.S.; Henriques, A.T. Taxonomic significance of alkaloids and iridoid glucosides in the tribe Psychotrieae (Rubiaceae). Biochem. Syst. Ecol. 2004, 32, 1187–1195. [Google Scholar] [CrossRef]
- Farias, F.M.; Passos, C.S.; Arbo, M.D.; Zuanazzi, J.A.S.; Steffen, V.M.; Henriques, A.T. Monoamine levels in rat striatum after acute intraperitoneal injection of strictosidinic acid isolated from Psychotria myriantha Mull. Arg. (Rubiaceae). Phytomedicine 2010, 17, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Farias, F.M.; Passos, C.S.; Arbo, M.D.; Barros, D.M.; Gottfried, C.; Steffen, V.M.; Henriques, A.T. Strictosidinic acid, isolated from Psychotria myriantha Mull. Arg. (Rubiaceae), decreases serotonin levels in rat hippocampus. Fitoterapia 2012, 83, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Farias, F.M.; Konrath, E.L.; Zuanazzi, J.A.S.; Henriques, A.T. Strictosamide from Psychotria nuda (Cham. et Schltdl) Wawra (Rubiaceae). Biochem. Syst. Ecol. 2008, 36, 919–920. [Google Scholar] [CrossRef]
- Jannic, V.; Guéritte, F.; Laprévote, O.; Serani, L.; Martin, M.T.; Sévenet, T.; Potier, P. Pyrrolidinoindoline Alkaloids from Psychotria oleoides and Psychotria lyciiflora. J. Nat. Prod. 1999, 62, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Faria, E.O.; Kato, L.; de Oliveira, C.M.; Carvalho, B.G.; Silva, C.C.; Sales, L.S.; Schuquel, I.T.; Silveira-Lacerda, E.P.; Delprete, P.G. Quaternary β-carboline alkaloids from Psychotria prunifolia (Kunth) Steyerm. Phytochem. Lett. 2010, 3, 113–116. [Google Scholar] [CrossRef]
- De Oliveira Figueiredo, P.; Perdomo, R.T.; Garcez, F.R.; Matos, M.D.F.C.; de Carvalho, J.E.; Garcez, W.S. Further constituents of Galianthe thalictroides (Rubiaceae) and inhibition of DNA topoisomerases I and IIα by its cytotoxic β-carboline alkaloids. Bioorg. Med. Chem. Lett. 2014, 24, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Lucilia, K.; Oliveira, C.; Faria, E.O.; Ribeiro, L.C.; Carvalho, B.G.; Silva, C.C.D.; Schuquel, I.T.; Santin, S.M.; Nakamura, C.V.; Britta, E.A. Antiprotozoal alkaloids from Psychotria prunifolia (Kunth) steyerm. J. Braz. Chem. Soc. 2012, 23, 355–360. [Google Scholar] [CrossRef]
- Van De Santos, L.; Fett Neto, A.G.; Kerber, V.A.; Elisabetsky, E.; Quirion, J.C.; Henriques, A.T. Indole monoterpene alkaloids from leaves of Psychotria suterella Mull. Arg. (Rubiaceae). Biochem. Syst. Ecol. 2001, 29, 1185–1187. [Google Scholar] [CrossRef]
- Fragoso, V.; Nascimento, N.C.; Moura, D.J.; Richter, M.F.; Saffi, J.; Fett-Neto, A.G. Antioxidant and antimutagenic properties of the monoterpene indole alkaloid psychollatine and the crude foliar extract of Psychotria umbellata Vell. Toxicol. in Vitro 2008, 22, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Moreno, B.P.; Fiorucci, L.L.R.; do Carmo, M.R.B.; Sarragiotto, M.H.; Baldoqui, D.C. Terpenoids and a coumarin from aerial parts of Psychotria vellosiana Benth. (Rubiaceae). Biochem. Syst. Ecol. 2014, 56, 80–82. [Google Scholar] [CrossRef]
- Blackledge, R.D.; Taylor, C.M. Psychotria Viridis—A Botanical Source of Dimethyltryptamine (DMT). Microgram J. 2003, 1, 18–22. [Google Scholar]
- Oliveira, M.D.C.; Negri, G.; Salatino, A.; Braga, M.R. Detection of anthraquinones and identification of 1,4-naphthohydroquinone in cell suspension cultures of Rudgea jasminoides (Cham.) Müll. Arg. (Rubiaceae). Braz. J. Bot. 2007, 30, 167–172. [Google Scholar] [CrossRef]
- Fraga, B.M.; Díaz, C.E.; Quintana, N. Naphthohydroquinones and lignans from the roots of Plocama pendula, a canary island paleoendemism. Biochem. Syst. Ecol. 2010, 38, 784–788. [Google Scholar] [CrossRef]
- Fraga, B.M.; Quintana, N.; Díaz, C.E. Anthraquinones from natural and transformed roots of Plocama pendula. Chem. Biodivers. 2009, 6, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Fraga, B.M.; Díaz, C.E.; Quintana, N. Triterpenes from Natural and Transformed Roots of Plocama pendula. J. Nat. Prod. 2006, 69, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Calis, I.; Heilmann, J.; Tasdemir, D.; Linden, A.; Ireland, C.M.; Sticher, O. Flavonoid, Iridoid, and Lignan Glycosides from Putoria calabrica. J. Nat. Prod. 2001, 64, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Baldé, A.; Pieters, L.; Gergely, A.; Wray, V.; Claeys, M.; Vlietinck, A. Spermacoceine, a bis-indole alkaloid from Borreria verticillata. Phytochemistry 1991, 30, 997–1000. [Google Scholar] [CrossRef]
- Moreira, V.F.; Oliveira, R.R.; Mathias, L.; Braz-Filho, R.; Curcino Vieira, I.J. New chemical constituents from Borreria verticillata (Rubiaceae). Helv. Chim. Acta 2010, 93, 1751–1757. [Google Scholar] [CrossRef]
- Wei, X.; Xie, H.; Ge, X.; Zhang, F. Iridoids from Dunnia sinensis. Phytochemistry 2000, 53, 837–840. [Google Scholar] [CrossRef]
- Moura, V.M.; Santos, A.R.; Nurnberg, V.; de Souza, M.C.; Santin, S.M.O. Iridoid glycosides from Galianthe brasiliensis. Biochem. Syst. Ecol. 2005, 33, 451–453. [Google Scholar] [CrossRef]
- De Freitas, C.S.; Kato, L.; de Oliveira, C.; Queiroz, L., Jr.; Santana, M.J.; Schuquel, I.T.; Delprete, P.G.; da Silva, R.A.; Quintino, G.O.; da Silva, N.B. β-Carboline Alkaloids from Galianthe ramosa Inhibit Malate Synthase from Paracoccidioides spp. Planta Med. 2014, 80, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.O.; Garcez, F.R.; Maria de Fátima, C.; Perdomo, R.T.; Queiroz, L.M.; Pott, A.; Garcez, A.J.; Garcez, W.S. A New Cytotoxic β-Carboline Alkaloid from Galianthe thalictroides. Planta Med. 2011, 77, 1852–1854. [Google Scholar] [CrossRef] [PubMed]
- Lajis, N.H.; Ahmad, R. Phytochemical studies and pharmacological activities of plants in genus Hedyotis oldenlandia. Stud. Nat. Prod. Chem. 2006, 33, 1057–1090. [Google Scholar]
- Ahmad, R.; Shaari, K.; Lajis, N.H.; Hamzah, A.S.; Ismail, N.H.; Kitajima, M. Anthraquinones from Hedyotis capitellata. Phytochemistry 2005, 66, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.M.; van Sung, T.; Porzel, A.; Schmidt, J.; Merzweiler, K.; Adam, G. β-Carboline alkaloids from Hedyotis capitellata. Phytochemistry 1999, 52, 1725–1729. [Google Scholar] [CrossRef]
- Phuong, N.M.; van Sung, T.; Schmidt, J.; Porzel, A.; Adam, G. Capitelline-A New Indole Alkaloid from Hedyotis capitellata. Nat. Prod. Lett. 1998, 11, 93–100. [Google Scholar] [CrossRef]
- Peng, J.N.; Feng, X.Z.; Zheng, Q.T.; Liang, X.T. A β-carboline alkaloid from Hedyotis chrysotricha. Phytochemistry 1997, 46, 1119–1121. [Google Scholar] [CrossRef]
- Sudarsono, A. Distribution of Asperuloside, Scandoside Methyl Ester in Plant Organs of Hedyotis corymbosa (L.) Lamk (Oldenlandia Corymbosa Linn) of Rubiaceae Family. Maj. Farm. Indones. 2004, 15, 62–67. [Google Scholar]
- Jiang, W.; Kuang, L.S.; Hou, A.J.; Qian, M.; Li, J.Z. Iridoid glycosides from Hedyotis corymbosa. Helv. Chim. Acta 2007, 90, 1296–1301. [Google Scholar] [CrossRef]
- Huu, B.C.; Phi Phung, N.K. Contribution to the study on chemical constituents of Hedyotis crassifolia L., (Rubiaceae). Vietnam J. Chem. 2014, 45, 363. [Google Scholar]
- Xu, G.H.; Kim, Y.H.; Chi, S.W.; Choo, S.J.; Ryoo, I.J.; Ahn, J.S.; Yoo, I.D. Evaluation of human neutrophil elastase inhibitory effect of iridoid glycosides from Hedyotis diffusa. Bioorg. Med. Chem. Lett. 2010, 20, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Fan, C.; Ye, W.; Luo, J. Two new iridoid glucosides from Hedyotis diffusa. Fitoterapia 2010, 81, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, X.; Sanchez, H.; Palacios Estrada, T. Estudio Quimico de Hedyotis intricata. Rubiaceae. Rev. Latinoam. Quím. 1992, 22, 46–46. [Google Scholar]
- Peng, J.N.; Feng, X.Z.; Liang, X.T. Iridoids from Hedyotis hedyotidea. Phytochemistry 1998, 47, 1657–1659. [Google Scholar] [CrossRef]
- Hamzah, A.S.; Aimi, N.; Lajis, N.H.J. Constituents of Hedyotis herbacea (Rubiaceae). Biochem. Syst. Ecol. 1996, 24, 273. [Google Scholar] [CrossRef]
- Konishi, M.; Hano, Y.; Takayama, M.; Nomura, T.; Hamzah, A.S.; Jasmani, H. Triterpenoid saponins from Hedyotis nudicaulis. Phytochemistry 1998, 48, 525–528. [Google Scholar] [CrossRef]
- Duy, L.H.; Phi Phung, N.K. Anthraquinones from Hedyotis pinifolia. Vietnam J. Chem. 2014, 47. [Google Scholar] [CrossRef]
- Zhao, J.F.; Yuan, Q.M.; Yang, X.D.; Zhang, H.B.; Li, L. Two new iridoid glycosides from Hedyotis tenelliflora Blume. Helv. Chim. Acta 2005, 88, 2532–2536. [Google Scholar] [CrossRef]
- Hang, N.H.; Khoi, N.D.T.; Truong, T.L.; Linh, N.P.; Tuyen, P.N.K.; Phung, N.K.P.; Nga, V.T. Further study on the chemical constituents of Hedyotis vestita (Rubiaceae). Vietnam J. Chem. 2014, 51, 648–652. [Google Scholar]
- Fabri, R.L.; Grazul, R.M.; Carvalho, L.O.; Coimbra, E.S.; Cardoso, G.M.M.; Souza-Fagundes, E.M.; Silva, A.D.; Scio, E. Antitumor, antibiotic and antileishmanial properties of the Pyranonaphthoquinone Psychorubrin from Mitracarpus frigidus. An. Acad. Bras. Cienc. 2012, 84, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Harouna, H.; Faure, R.; Elias, R.; Debrauwer, L.; Saadou, M.; Balansard, G.; Boudon, G. Harounoside a pentalongin hydroquinone diglycoside from Mitracarpus scaber. Phytochemistry 1995, 39, 1483–1484. [Google Scholar] [CrossRef]
- Ekpendu, T.O.E.; Adesomoju, A.A.; Ekundayo, O.; Okogun, J.I.; Laakso, I. Constituents of the volatile oil of Mitracarpus scaber Zucc. Flavour Frag. J. 1993, 8, 269–271. [Google Scholar] [CrossRef]
- Ekpendu, T.O.E.; Adesomoju, A.A.; Okogun, J.I. Chemical Studies of Mitracarpus villosus (Sw.) Dc—A Medicinal Rubiaceous Weed. J. Chem. Soc. Niger. 2001, 26, 69–71. [Google Scholar]
- Otsuka, H.; Yoshimura, K.; Yamasaki, K.; Cantoria, M.C. Isolation of 10-O-acyl iridoid glucosides from a Philippine medicinal plant, Oldenlandia corymbosa L.(Rubiaceae). Chem. Pharm. Bull. 1991, 39, 2049–2052. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, B.Z.; Ryu, S.Y. Antitumour effects of ursolic acid isolated from Oldenlandia diffusa. Phytother. Res. 1998, 12, 553–556. [Google Scholar] [CrossRef]
- Lu, H.C.; He, J. A study on chemical constituents of Oldenlandia diffusa (Willd) Roxb. Nat. Prod. Res. Dev. 1996, 8, 34–37. [Google Scholar]
- Siva, R.; Mayes, S.; Behera, S.K.; Rajasekaran, C. Anthraquinones dye production using root cultures of Oldenlandia umbellata L. Ind. Crops Prod. 2012, 37, 415–419. [Google Scholar] [CrossRef]
- Tomaz, A.C.D.A.; Nogueira, R.B.S.; Pinto, D.S.; Agra, M.D.F.; Souza, M.D.F.V.D.; Da-Cunha, E.V.L. Chemical constiuents from Richardia grandiflora (Cham. & Schltdl.) Steud. (Rubiaceae). Rev. Bras. Farmacogn. 2008, 18, 47–52. [Google Scholar]
- Singh, D.; Verma, N.; Raghuwanshi, S.; Shukla, P.; Kulshreshtha, D. Antifungal anthraquinones from Saprosma fragrans. Bioorg. Med. Chem. Lett. 2006, 16, 4512–4514. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, G.Y.; Han, C.R.; Yuan, Y.; Yang, B.; Zhang, Y.; Wang, J.; Zhong, X.Q.; Huang, X. Two novel alkaloids from the stem of Saprosma hainanense and their cytotoxic activities in vitro. Chem. Pharm. Bull. 2011, 59, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.K.; Komorita, A.; Tanaka, T.; Fujioka, T.; Mihashi, K.; Kouno, I. Iridoids and anthraquinones from the Malaysian medicinal plant, Saprosma scortechinii (Rubiaceae). Chem. Pharm. Bull. 2002, 50, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.K.; Komorita, A.; Tanaka, T.; Fujioka, T.; Mihashi, K.; Kouno, I. Sulfur-Containing Bis-iridoid Glucosides and Iridoid Glucosides from Saprosma s cortechinii. J. Nat. Prod. 2002, 65, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Cao, X.; Liu, X.Y.; Long, C.; Liu, J.H.; Xu, Q.Z.; Jiao, B.H. Iridoid glycosides from Saprosma ternatum. Planta Med. 2010, 76, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.R.J.C.; Lemos, R.P.L.; Conserva, L.M. Chemical constituents from Spermacoce verticillata (Rubiaceae). Biochem. Syst. Ecol. 2012, 44, 208–211. [Google Scholar] [CrossRef]
- Park, A.; Kim, H.J.; Lee, J.S.; Woo, E.R.; Park, H.; Lee, Y.S. New Iridoids from Asperula m aximowiczii. J. Nat. Prod. 2002, 65, 1363–1366. [Google Scholar] [CrossRef] [PubMed]
- Mitova, M.I.; Anchev, M.E.; Panev, S.G.; Handjieva, N.V.; Popov, S.S. Coumarins and Iridoids from Crucianella graeca, Cruciata glabra, Cruciata laevipes and Cruciata pedemontana (Rubiaceae). Z. Naturforsch. B 1996, 51, 631–634. [Google Scholar]
- El Lakany, A.M.; Kader, M.S.A.; Sabri, N.N. Anthraquinones with antibacterial activities from Crucianella maritima L. growing in Egypt. Nat. Prod. Sci. 2004, 10, 63–68. [Google Scholar]
- Venditti, A.; Altieri, A.; Bianco, A. Monoterpenoids glycosides content from two Mediterranean populations of Crucianella maritima L. Nat. Prod. Res. 2014, 28, 586–588. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Mitova, M.; Handjieva, N.; Ersoz, T.; Calis, I. Aromatic monoterpenoid glycosides from Cruciata taurica. Nat. Prod. Res. 2003, 17, 109–113. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Mitova, M.; Handjieva, N.; Çalış, I.H. Coumarin glucosides from Cruciata taurica. Phytochemistry 2002, 59, 447–450. [Google Scholar] [CrossRef]
- Handjieva, N.; Mitova, M.; Ancev, M.; Popov, S. Iridoid glucosides from Galium album and G. lovcense. Phytochemistry 1996, 43, 625–628. [Google Scholar] [CrossRef]
- Morimoto, M.; Tanimoto, K.; Sakatani, A.; Komai, K. Antifeedant activity of an anthraquinone aldehyde in Galium aparine L. against Spodoptera litura F. Phytochemistry 2002, 60, 163–166. [Google Scholar] [CrossRef]
- Rosa, S.; Iodice, C.; Mitova, M.; Handjieva, N.; Popov, S.; Anchev, M. Triterpene saponins and iridoid glucosides from Galium rivale. Phytochemistry 2000, 54, 751–756. [Google Scholar] [CrossRef]
- Mitova, M.; Handjieva, N.; Spassov, S.; Popov, S. Macedonine, a non-glycosidic iridoid from Galium macedonicum. Phytochemistry 1996, 42, 1227–1229. [Google Scholar] [CrossRef]
- El-Gamal, A.A.; Takeya, K.; Itokawa, H.; Halim, A.F.; Amer, M.M.; Saad, H.E.A.; Awad, S.A. Anthraquinones from the polar fractions of Galium sinaicum. Phytochemistry 1996, 42, 1149–1155. [Google Scholar] [CrossRef]
- Yang, S.W. Antioxidative constituents of the aerial parts of Galium spurium. Biomol. Ther. 2011, 19, 336–341. [Google Scholar] [CrossRef]
- Banthorpe, D.V.; White, J.J. Novel anthraquinones from undifferentiated cell cultures of Galium verum. Phytochemistry 1995, 38, 107–111. [Google Scholar] [CrossRef]
- Lee, T.G.; Kim, D.K. Articles: Iridoid Compounds from the Whole Plant of Galium verum var. asiaticum. Nat. Prod. Sci. 2013, 19, 227–230. [Google Scholar] [CrossRef]
- Miyazawa, M.; Kawata, J. Identification of the key aroma compounds in dried roots of Rubia cordifolia. J. Oleo Sci. 2006, 55, 37–39. [Google Scholar] [CrossRef]
- Wu, L.J.; Wang, S.X.; Hua, H.M.; Li, X.; Zhu, T.R.; Miyase, T.; Ueno, A. 6-Methoxygeniposidic acid, an iridoid glycoside from Rubia cordifolia. Phytochemistry 1991, 30, 1710–1711. [Google Scholar] [CrossRef]
- Longo, L.; Scardino, A.; Vasapollo, G. Identification and quantification of anthocyanins in the berries of Pistacia lentiscus L., Phillyrea latifolia L. and Rubia peregrina L. Innov. Food Sci. Emerg. 2007, 8, 360–364. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Bai, Y.; Duddeck, H.; Hiegemann, M. Digiferriginol glycoside from Rubia schumanniana. Phytochemistry 1991, 30, 947–949. [Google Scholar]
- Kuang, B.; Han, J.; Zeng, G.Z.; Chen, X.Q.; He, W.J.; Tan, N.H. Three new triterpenoids from Rubia schumanniana. Nat. Prod. Bioprosp. 2012, 2, 166–169. [Google Scholar] [CrossRef]
- Zou, C.; Hao, X.J.; Chen, C.; Zhou, J. A new antitumor glycocyclohexapeptide and arborane type new triterpenoids Rubia yunnanensis. Acta Bot. Yunn. 1992, 14, 114. [Google Scholar]
- Marec, F.; Kollarova, I.; Jegorov, A. Mutagenicity of natural anthraquinones from Rubia tinctorum in the Drosophila wing spot test. Planta Med. 2001, 67, 127–131. [Google Scholar] [CrossRef] [PubMed]
- El-Emary, N.A.; Backheet, E.Y. Three hydroxymethylanthraquinone glycosides from Rubia tinctorum. Phytochemistry 1998, 49, 277–279. [Google Scholar] [CrossRef]
- Perassolo, M.; Quevedo, C.; Busto, V.; Ianone, F.; Giulietti, A.M.; Talou, J.R. Enhance of anthraquinone production by effect of proline and aminoindan-2-phosphonic acid in Rubia tinctorum suspension cultures. Enzyme Microb. Technol. 2007, 41, 181–185. [Google Scholar] [CrossRef]
- Orbán, N.; Boldizsár, I.; Szucs, Z.; Dános, B. Influence of different elicitors on the synthesis of anthraquinone derivatives in Rubia tinctorum L. cell suspension cultures. Dyes Pigments 2008, 77, 249–257. [Google Scholar] [CrossRef]
- Fan, J.T.; Chen, Y.S.; Xu, W.Y.; Du, L.; Zeng, G.Z.; Zhang, Y.M.; Su, J.; Li, Y.; Tan, N.H. Rubiyunnanins A and B, two novel cyclic hexapeptides from Rubia yunnanensis. Tetrahedron Lett. 2010, 51, 6810–6813. [Google Scholar] [CrossRef]
- Liou, M.-J.; Wu, T.S. Triterpenoids from Rubia yunnanensis. J. Nat. Prod. 2002, 65, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.Y.; Du, Z.Z.; Yang, X.S.; Hao, X.J. Note: A new triterpene from Luculia pinciana Hook. J. Asian Nat. Prod. Res. 2005, 7, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Hao, X. Terpenoid glycosides from stem of Luculia pinceana. J. Chin. Mater. Med. 2007, 32, 2606–2609. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).