Elderberries: A Source of Ribosome-Inactivating Proteins with Lectin Activity
Abstract
:1. Introduction: Uses of Sambucus in Ethno-Pharmacology
2. Special Chemical Compounds Found in Sambucus
3. Ribosome-Inactivating Proteins (RIPs)
3.1. Type 1 (Single Chain) Ribosome-Inactivating Proteins
3.1.1. Ebulitins α, β and γ
3.1.2. Nigritins f1 and f2
3.2. Type 2 Ribosome-Inactivating Proteins from S. ebulus L.
3.2.1. Ebulin l
| Tissue | Protein | Mr (SDS-PAGE) (kDa) | Sugar Spec. | Yield a | IC50 (nM) | |||
|---|---|---|---|---|---|---|---|---|
| Whole | A Chain | B Chain | ||||||
| A | leaves | ebulin l | 56.0 | 26.0 | 30.0 | Gal | 3.2 | 0.15 |
| fruits | ebulin f | 56.0 | 26.0 | 30.0 | Gal | 1.6 | 0.03 | |
| blossoms | ebulin blo | 60.0 b | 34.0 | 30.0 | Gal | 5.9 | - | |
| rhizome | ebulin r1 | 56.0 | 26.0 | 30.0 | Gal | 1.34 | 0.34 | |
| rhizome | ebulin r2 | 56.0 | 26.0 | 30.0 | Gal | 0.56 | 1.14 | |
| rhizome | SEA I | 140.0 | 30.0 | 35.0 | Neu5Ac-Gal/GalNAc | 6 | 1 | |
| B | bark | nigrin b | 58.0 | 26.0 | 32.0 | Gal/GalNAc | 254 | 0.1 |
| bark | basic nigrin b | 64.0 | 32.0 | 32.0 | no determined | 90 | 0.02 | |
| bark | SNA-I | 240.0 | 33.0 | 35.0 | Neu5Ac-Gal/GalNAc | 100 | 1.65 | |
| bark | SNA-I’ | 120.0 | 32.0 | 35.0 | Neu5Ac-Gal/GalNAc | 0.2 | 1.25 | |
| bark | SNRLP1 | 68.0 | 34.0 | 34.0 | GalNAc oligomers | 100 c | 7 | |
| bark | SNRLP2 | 62.0 | 30.0 | 32.0 | GalNAc oligomers | - | 7 | |
| bark | SNA-V | 120.0 | - | - | Gal/GalNAc | - | - | |
| fruits | nigrin f | 58.0 | 26.0 | 31.6 | Gal | 1.3 | 0.03 | |
| seeds | nigrin s | 57.3 | 26.0 | 31.0 | Gal | - | - | |
| C | bark | sieboldin b | 60.0 | 27.0 | 33.0 | Neu5Ac-Gal/GalNAc | 2.6 | 0.9 |
| bark | SSA | 115.4 | 28.7 | 29.0 | Neu5Ac-Gal/GalNAc | - | >100 | |
3.2.2. Ebulins r1 and r2
3.2.3. Ebulin f and Polyebulin f
3.2.4. Ebulin Blo
3.2.5. SEA
3.3. Type 2 Ribosome-Inactivating Proteins from Sambucus nigra L.
3.3.1. Nigrin b (SNA-V)
3.3.2. Basic Nigrin b (bNgb)
3.3.3. Nigrin s
3.3.4. Nigrin f
3.3.5. Nigrins l1 and l2
3.3.6. SNA-I
3.3.7. SNA-I’
3.3.8. SNLRP1 and SNLRP2
3.4. Type 2 Ribosome-Inactivating Proteins from S. sieboldiana L.
3.4.1. Sieboldin b
3.4.2. SSA
4. Hololectins of Sambucus
5. Potential Biological Roles of Sambucus Lectins
6. Uses of Ribosome-Inactivating Proteins of Sambucus in Targeted Therapy
7. Toxicity and Histological Analysis of Sambucus Type 2 RIPs Administered at High Concentrations to Mice
7.1. Nigrin b
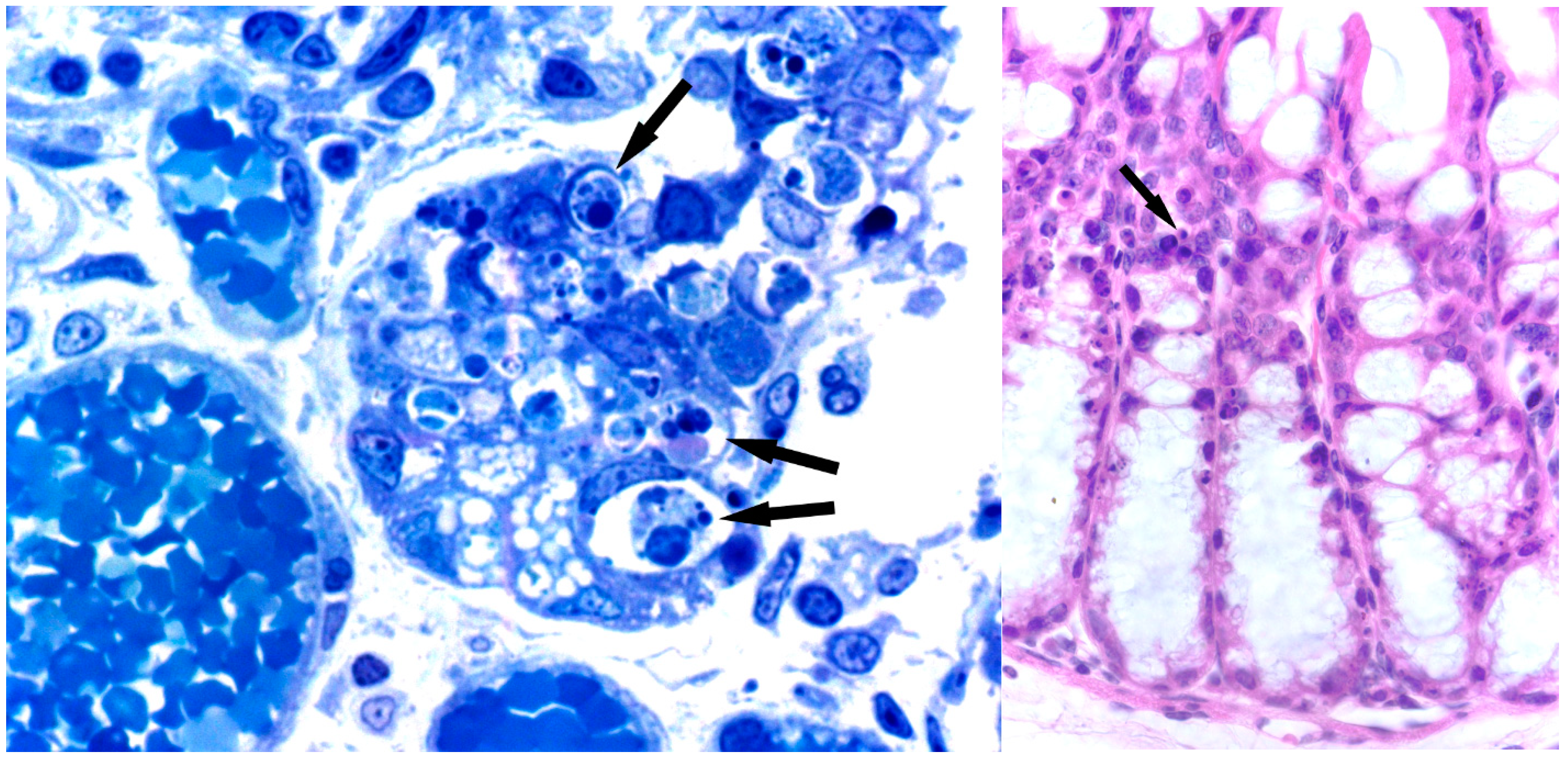
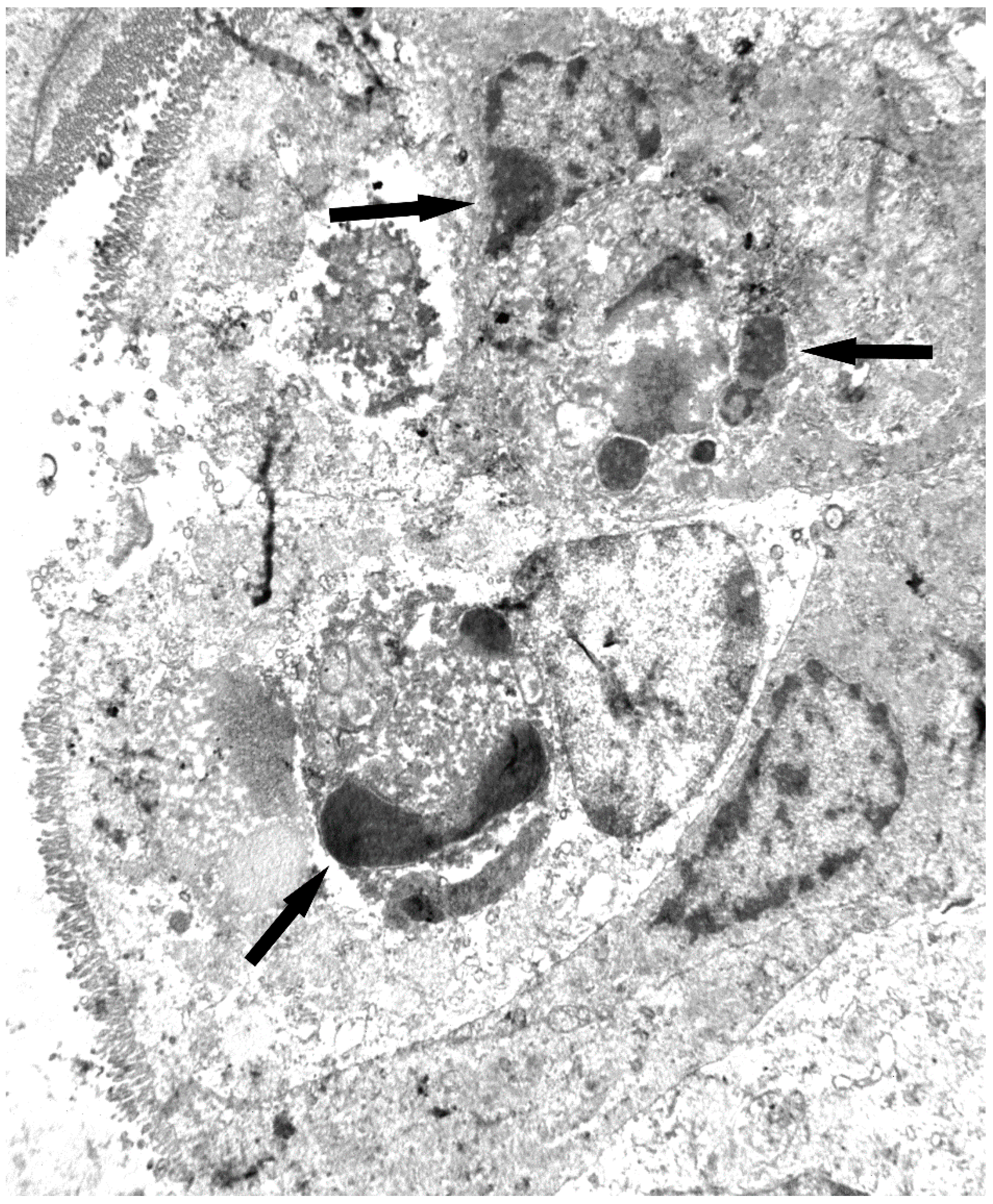
7.2. Ebulin f
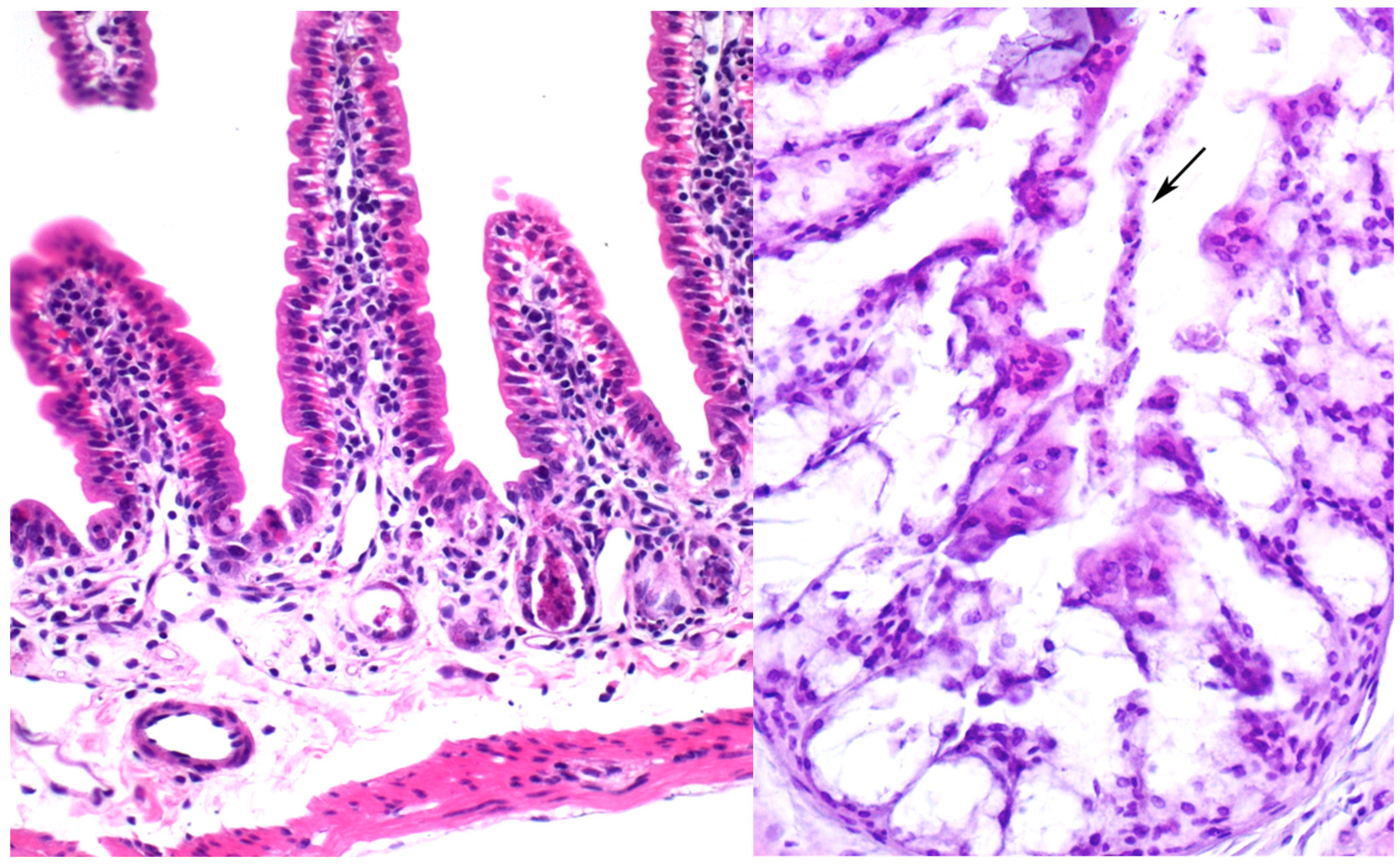
7.3. Ebulin Blo
8. Entomotoxicity of Sambucus Proteins
9. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mariotti-Lippi, L.; Bellini, C.; Mori, S. Palaeovegetational reconstruction based on pollen and seeds/fruits from a Bronze Age archaeological site in Tuscany (Italy). Plant Biosyst. 2010, 144, 902–908. [Google Scholar] [CrossRef]
- Martin, L.; Jacomet, S.; Thiebault, S. Plant economy during the neolithic in a mountain context: The case of “Le Chenet des Pierres” in the French Alps (Bozel-Savoie, France). Veg. Hist. Archaeobotany 2008, 17, s113–s122. [Google Scholar] [CrossRef]
- Ellis, P. The Fabrication of “Celtic” Astrology. Available online: http://cura.hree.fr/xv/13ellis2.html (accessed on 22 October 2014).
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Christensen, K.B.; Petersen, R.K.; Kristiansen, K.; Christensen, L.P. Identification of bioactive compounds from flowers of black elder (Sambucus nigra L.) that activate the human peroxisome proliferator-activated receptor (PPAR) gamma. Phytother. Res. 2010, 24, s129–s132. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Christensen, K.B.; Olsen, L.C.; Christensen, L.P.; Grevsen, K.; Færgeman, N.J.; Kristiansen, K.; Young, J.F.; Oksbjerg, N. Bioactive components from flowers of Sambucus nigra L. increase glucose uptake in primary porcine myotube cultures and reduce fat accumulation in Caenorhabditis elegans. J. Agric. Food Chem. 2013, 61, 11033–11040. [Google Scholar] [CrossRef] [PubMed]
- Yesilada, E.; Gürbüz, I.; Toker, G. Anti-ulcerogenic activity and isolation of the active principles from Sambucus ebulus L. leaves. J. Ethnopharmacol. 2014, 153, 478–483. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.H.; Shi, X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Cooke, D.; Brown, K.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and metabolism of the putative cancer chemopreventive agent cyanidin-3-glucoside in mice. Cancer Chemother. Pharmacol. 2009, 64, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Tomassini, L.; Foddai, S.; Ventrone, A.; Nicoletti, M. Two new non-glycosidic iridoids from Sambucus ebulus. Nat. Prod. Res. 2013, 27, 2012–2015. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, S.; Zeller, I.; Pölzelbauer, P.; Frotschnig, S.; Laufer, G.; Messner, B.; Pieri, V.; Stuppner, H.; Bernhard, D. Identification and pharmacological characterization of the anti-inflammatory principal of the leaves of dwarf elder (Sambucus ebulus L.). J. Ethnopharmacol. 2011, 133, 704–709. [Google Scholar] [CrossRef]
- Girbes, T.; Ferreras, J.M.; Arias, F.J.; Stirpe, F. Description, distribution, activity and phylogenetic relationship of ribosome-inactivating proteins in plants, fungi and bacteria. Mini Rev. Med. Chem. 2004, 4, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F. Ribosome-inactivating proteins. Toxicon 2004, 44, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Wong, J.H.; Wang, H. Recent progress in research on ribosome inactivating proteins. Curr. Protein Pept. Sci. 2010, 11, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, R.; Arias, F.J.; Rojo, M.A.; Escarmis, C.; Ferreras, J.M.; Girbes, T. Molecular action of the type 1 ribosome-inactivating protein saporin 5 on Vicia sativa ribosomes. FEBS Lett. 1993, 325, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Arias, F.J.; Rojo, M.A.; Iglesias, R.; Ferreras, J.M.; Girbes, T. Vicia sativa L. run-off and purified ribosomes: Polyphenylalanine synthesis and molecular action of ribosome-inactivating proteins. J. Exp. Bot. 1993, 44, 1297–1304. [Google Scholar] [CrossRef]
- Girbés, T.; Barbieri, L.; Ferreras, M.; Arias, F.J.; Rojo, M.A.; Iglesias, R.; Alegre, C.; Escarmis, C.; Stirpe, F. Effects of ribosome-inactivating proteins on Escherichia coli and Agrobacterium tumefaciens translation systems. J. Bacteriol. 1993, 175, 6721–6724. [Google Scholar]
- Iglesias, R.; Citores, L.; Ferreras, J.M.; Pérez, Y.; Jiménez, P.; Gayoso, M.J.; Olsnes, S.; Tamburino, R.; Di Maro, A.; Parente, A.; et al. Sialic acid-binding dwarf elder four-chain lectin displays nucleic acid N-glycosidase activity. Biochimie 2010, 92, 71–80. [Google Scholar] [CrossRef]
- Barbieri, L.; Ciani, M.; Girbes, T.; Liu, W.Y.; Van Damme, E.J.; Peumans, W.J.; Stirpe, F. Enzymatic activity of toxic and non-toxic type 2 ribosome-inactivating proteins. FEBS Lett. 2004, 563, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Battelli, M.G.; Stirpe, F. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta 1993, 1154, 237–282. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Bovi, P.D.; Ciliento, R.; Gaudio, L.; Di Maro, A.; Aceto, S.; Lorito, M.; Rao, R. Inducible expression of a Phytolacca heterotepala ribosome-inactivating protein leads to enhanced resistance against major fungal pathogens in tobacco. Phytopathology 2005, 95, 206–215. [Google Scholar] [CrossRef]
- Girbes, T.; de Torre, C.; Iglesias, R.; Ferreras, J.M.; Mendez, E. RIP for viruses. Nature 1996, 379, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Nsimba-Lubaki, M.; Peumans, W.J.; Allen, A.K. Isolation and characterization of glycoprotein lectins from the bark of three species of elder, Sambucus ebulus, S. nigra and S. racemosa. Planta 1986, 168, 113–118. [Google Scholar]
- Citores, L.; Rojo, M.A.; Jimenez, P.; Ferreras, J.M.; Iglesias, R.; Aránguez, I.; Girbes, T. Transient occurrence of an ebulin-related d-galactose-lectin in shoots of Sambucus ebulus L. Phytochemistry 2008, 69, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.; Cabrero, P.; Basterrechea, J.E.; Tejero, J.; Cordoba-Diaz, D.; Girbes, T. Isolation and molecular characterization of two lectins from dwarf elder (Sambucus ebulus L.) blossoms related to the Sam n1 allergen. Toxins (Basel) 2013, 5, 1767–1779. [Google Scholar] [CrossRef]
- De Benito, F.M.; Citores, L.; Iglesias, R.; Ferreras, J.M.; Soriano, F.; Arias, J.; Mendez, E.; Girbes, T. Ebulitins: A new family of type 1 ribosome-inactivating proteins (rRNA N-glycosidases) from leaves of Sambucus ebulus L. that coexist with the type 2 ribosome-inactivating protein ebulin 1. FEBS Lett. 1995, 360, 299–302. [Google Scholar] [CrossRef] [PubMed]
- De Benito, F.M.; Iglesias, R.; Ferreras, J.M.; Citores, L.; Camafeita, E.; Mendez, E.; Girbes, T. Constitutive and inducible type 1 ribosome-inactivating proteins (RIPs) in elderberry (Sambucus nigra L.). FEBS Lett. 1998, 428, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Girbes, T.; Citores, L.; Iglesias, R.; Ferreras, J.M.; Muñoz, R.; Rojo, M.A.; Arias, F.J.; Garcia, J.R.; Mendez, E.; Calonge, M. Ebulin 1, a nontoxic novel type 2 ribosome-inactivating protein from Sambucus ebulus L. leaves. J. Biol. Chem. 1993, 268, 18195–18199. [Google Scholar]
- Svinth, M.; Steinghardt, J.; Hernandez, R.; Duh, J.K.; Kelly, C.; Day, P.; Lord, M.; Girbés, T.; Robertus, J.D. Differences in cytotoxicity of native and engineered RIPs can be used to assess their ability to reach the cytoplasm. Biochem. Biophys. Res. Commun. 1998, 249, 637–642. [Google Scholar] [CrossRef]
- Pascal, J.M.; Day, P.J.; Monzingo, A.F.; Ernst, S.R.; Robertus, J.D.; Iglesias, R.; Perez, Y.; Ferreras, J.M.; Citores, L.; Girbes, T. 2.8-A crystal structure of a nontoxic type-II ribosome-inactivating protein, ebulin l. Proteins 2001, 43, 319–326. [Google Scholar] [CrossRef]
- Citores, L.; Muñoz, R.; de Benito, F.M.; Iglesias, R.; Ferreras, J.M.; Girbes, T. Differential sensitivity of HeLa cells to the type 2 ribosome-inactivating proteins ebulin l, nigrin b and nigrin f as compared with ricin. Cell. Mol. Biol. 1996, 42, 473–476. [Google Scholar]
- Rojo, M.A.; Citores, L.; Arias, F.J.; Ferreras, J.M.; Jiménez, P.; Girbés, T. Molecular cloning of a cDNA coding for the d-galactose-binding dimeric lectin of dwarf elder (Sambucus ebulus L.) leaves. Int. J. Biochem. Cell Biol. 2003, 35, 1061–1065. [Google Scholar] [CrossRef]
- Citores, L.; de Benito, F.M.; Iglesias, R.; Ferreras, J.M.; Argüeso, P.; Jiménez, P.; Testera, A.; Camafeita, E.; Méndez, E.; Girbés, T. Characterization of a new non-toxic two-chain ribosome-inactivating protein and a structurally-related lectin from rhizomes of dwarf elder (Sambucus ebulus L.). Cell. Mol. Biol. 1997, 43, 485–499. [Google Scholar]
- Citores, L.; de Benito, F.M.; Iglesias, R.; Miguel, F.J.; Argueso, P.; Jimenez, P.; Mendez, E.; Girbes, T. Presence of polymerized and free forms of the non-toxic type 2 ribosome -inactivating protein ebulin and a structurally related new homodimeric lectin in fruits of Sambucus ebulus L. Planta 1998, 204, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.; Tejero, J.; Cabrero, P.; Cordoba-Diaz, D.; Girbes, T. Differential sensitivity of d-galactose-binding lectins from fruits of dwarf elder (Sambucus ebulus L.) to a simulated gastric fluid. Food Chem. 2013, 136, 794–802. [Google Scholar] [CrossRef]
- Jiménez, P.; Gayoso, M.J.; Tejero, J.; Cabrero, P.; Cordoba-Diaz, D.; Basterrechea, J.E.; Girbés, T. Toxicity in mice of lectin ebulin f present in dwarf elderberry (Sambucus ebulus L.). Toxicon 2013, 61, 26–29. [Google Scholar] [CrossRef]
- Förster-Waldl, E.; Marchetti, M.; Schöll, I.; Focke, M.; Radauer, C.; Kinaciyan, T.; Nentwich, I.; Jäger, S.; Schmid, E.R.; Boltz-Nitulescu, G.; et al. Type I allergy to elderberry (Sambucus nigra) is elicited by a 33.2 kDa allergen with significant homology to ribosomal inactivating proteins. Clin. Exp. Allergy 2003, 33, 1703–1710. [Google Scholar]
- Girbes, T.; Citores, L.; Ferreras, J.M.; Rojo, M.A.; Iglesias, R.; Munoz, R.; Arias, F.J.; Calonge, M.; Garcia, J.R.; Mendez, E. Isolation and partial characterization of nigrin b, a non-toxic novel type 2 ribosome-inactivating protein from the bark of Sambucus nigra L. Plant Mol. Biol. 1993, 22, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J. History of plant lectin research. Methods Mol. Biol. 2014, 1200, 3–13. [Google Scholar] [PubMed]
- Shang, C.; Van Damme, E.J. Comparative analysis of carbohydrate binding properties of Sambucus nigra lectins and ribosome-inactivating proteins. Glycoconj. J. 2014, 31, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.; Barre, A.; Rougé, P.; Van Leuven, F.; Peumans, W.J. Characterization and molecular cloning of Sambucus nigra agglutinin V (nigrin b), a GalNAc-specific type-2 ribosome-inactivating protein from the bark of elderberry (Sambucus nigra). Eur. J. Biochem. 1996, 237, 505–513. [Google Scholar] [CrossRef] [PubMed]
- De Benito, F.M.; Citores, L.; Iglesias, R.; Ferreras, J.M.; Camafeita, E.; Mendez, E.; Girbes, T. Isolation and partial characterization of a novel and uncommon two-chain 64-kDa ribosome-inactivating protein from the bark of elder (Sambucus nigra L.). FEBS Lett. 1997, 413, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gayoso, M.J.; Muñoz, R.; Arias, Y.; Villar, R.; Rojo, M.A.; Jiménez, P.; Ferreras, J.M.; Aranguez, I.; Girbés, T. Specific dose-dependent damage of Lieberkühn crypts promoted by large doses of type 2 ribosome-inactivating protein nigrin b intravenous injection to mice. Toxicol. Appl. Pharmacol. 2005, 207, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Musiani, S.; Buonamici, L.; Santi, S.; Riccio, M.; Maraldi, N.M.; Girbés, T.; Stirpe, F. Interaction of volkensin with HeLa cells: Binding, uptake, intracellular localization, degradation and exocytosis. Cell. Mol. Life Sci. 2004, 61, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Citores, L.; Iglesias, R.; Muñoz, R.; Ferreras, J.M.; Jimenez, P.; Girbes, T. Elderberry (Sambucus nigra L.) seed proteins inhibit protein synthesis and display strong immunoreactivity with rabbit polyclonal antibodies raised against the type 2 ribosome-inactivating protein nigrin b. J. Exp. Bot. 1994, 45, 513–516. [Google Scholar] [CrossRef]
- Citores, L.; de Benito, F.M.; Iglesias, R.; Ferreras, J.M.; Jimenez, P.; Argueso, P.; Farias, G.; Mendez, E.; Girbes, T. Isolation and characterization of a new non-toxic two-chain ribosome-inactivating protein from fruits of elder (Sambucus nigra L). J. Exp. Bot. 1996, 47, 1577–1585. [Google Scholar] [CrossRef]
- Shibuya, N.; Goldstein, I.J.; Broekaert, W.F.; Nsimba-Lubaki, M.; Peeters, B.; Peumans, W.J. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2–6)Gal/GalNAc sequence. J. Biol. Chem. 1987, 262, 1596–1601. [Google Scholar] [PubMed]
- Van Damme, E.J.; Barre, A.; Rougé, P.; Van Leuven, F.; Peumans, W.J. The NeuAc(alpha-2,6)-Gal/GalNAc-binding lectin from elderberry (Sambucus nigra) bark, a type-2 ribosome-inactivating protein with an unusual specificity and structure. Eur. J. Biochem. 1996, 235, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Mechanisms of cancer-associated glycosylation changes. Front. Biosci. (Landmark Ed.) 2012, 17, 670–699. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Sialosignaling: Sialyltransferases as engines of self-fueling loops in cancer progression. Biochim. Biophys. Acta 2014, 1840, 2752–2764. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, J.; Feijoo-Carnero, C.; Merino-Trigo, A.; Páez de la Cadena, M.; Rodríguez-Berrocal, F.J.; de Carlos, A.; Butrón, M.; Martínez-Zorzano, V.S. Immunohistochemical analysis of sialic acid and fucose composition in human colorectal adenocarcinoma. Tumour Biol. 2000, 21, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.; Gutiérrez, E.; Rodríguez, J.A.; Gomes, C.; David, L. Construction and validation of a Sambucus nigra biosensor for cancer-associated STn antigen. Biosens. Bioelectron. 2014, 57, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.; Roy, S.; Barre, A.; Citores, L.; Mostafapous, K.; Rougé, P.; Van Leuven, F.; Girbés, T.; Goldstein, I.J.; Peumans, W.J. Elderberry (Sambucus nigra) bark contains two structurally different Neu5Ac(alpha2,6)Gal/GalNAc-binding type 2 ribosome-inactivating proteins. Eur. J. Biochem. 1997, 245, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.; Barre, A.; Rougé, P.; Van Leuven, F.; Peumans, W.J. Isolation and molecular cloning of a novel type 2 ribosome-inactivating protein with an inactive B chain from elderberry (Sambucus nigra) bark. J. Biol. Chem. 1997, 272, 8353–8360. [Google Scholar] [CrossRef] [PubMed]
- Rojo, M.A.; Yato, M.; Ishii-Minami, N.; Minami, E.; Kaku, H.; Citores, L.; Girbes, T.; Shibuya, N. Isolation, cDNA cloning, biological properties, and carbohydrate binding specificity of sieboldin-b, a type II ribosome-inactivating protein from the bark of Japanese elderberry (Sambucus sieboldiana). Arch. Biochem. Biophys. 1997, 340, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kaku, H.; Tanaka, Y.; Tazaki, K.; Minami, E.; Mizuno, H.; Shibuya, N. Sialylated oligosaccharide-specific plant lectin from Japanese elderberry (Sambucus sieboldiana) bark tissue has a homologous structure to type II ribosome-inactivating proteins, ricin and abrin. cDNA cloning and molecular modelling study. J. Biol. Chem. 1996, 271, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Kaku, H.; Peumans, W.J.; Goldstein, I.J. Isolation and characterization of a second lectin (SNA-II) present in elderberry (Sambucus nigra L.) bark. Arch. Biochem. Biophys. 1990, 277, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.J.; Kellens, J.T.C.; Allen, A.K.; Van Damme, E.J. Isolation and characterization of a seed lectin from elderberry (Sambucus nigra) and its relationship to the bark lectins. Carbohydr. Res. 1991, 213, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Mach, L.; Waltraud, S.; Ammann, M.; Poetsch, J.; Bertsch, W.; Marz, L.; Glossl, J. Purification and partial characterization of a novel lectin from elder (Sambucus nigra L.) fruit. Biochem. J. 1991, 278, 667–671. [Google Scholar] [PubMed]
- Rojo, M.A.; Kaku, H.; Ishii-Minami, N.; Minami, E.; Yato, M.; Hisajima, S.; Yamaguchi, T.; Shibuya, N. Characterization and cDNA cloning of monomeric lectins that correspond to the B-chain of a type 2 ribosome-inactivating protein from the bark of Japanese elderberry (Sambucus sieboldiana). J. Biochem. 2004, 135, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Desmyter, S.; Ciani, M.; Proost, P.; Peumans, W.J.; Van Damme, E.J. Analysis of the in planta antiviral activity of elderberry ribosome-inactivating proteins. Eur. J. Biochem. 2004, 271, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Shahidi-Noghabi, S.; Van Damme, E.J.; Mahdian, K.; Smagghe, G. Entomotoxic action of Sambucus nigra agglutinin I in Acyrthosiphon pisum aphids and Spodoptera exigua caterpillars through caspase-3-like-dependent apoptosis. Arch. Insect Biochem. Physiol. 2010, 75, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Shahidi-Noghabi, S.; van Damme, E.J.; Smagghe, G. Carbohydrate-binding activity of the type-2 ribosome-inactivating protein SNA-I from elderberry (Sambucus nigra) is a determining factor for its insecticidal activity. Phytochemistry 2008, 69, 2972–2978. [Google Scholar] [CrossRef] [PubMed]
- Pastan, I.; Hassan, R.; FitzGerald, D.J.; Kreitman, R.J. Immunotoxin treatment of cancer. Annu. Rev. Med. 2007, 58, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Suh, D.Y.; Frankel, A.E. Toxin conjugate therapy of cancer. Semin. Oncol. 2005, 32, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Polito, L. Immunotoxins and other conjugates: Preclinical studies. Mini Rev. Med. Chem. 2004, 4, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M. Immunotoxins and toxin constructs in the treatment of leukemia and lymphoma. Adv. Pharmacol. 2004, 51, 209–228. [Google Scholar] [PubMed]
- Fracasso, G.; Bellisola, G.; Castelletti, D.; Tridente, G.; Colombatti, M. Immunotoxins and other conjugates: Preparation and general characteristics. Mini Rev. Med. Chem. 2004, 4, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J. Immunotoxins for targeted cancer therapy. AAPS J. 2006, 8, E532–E551. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Pastan, I. Immunotoxins in the treatment of hematologic malignancies. Curr. Drug Targets 2006, 7, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.M.; Roberts, L.M.; Robertus, J.D. Ricin: Structure, mode of action, and some current applications. FASEB J. 1994, 8, 201–208. [Google Scholar] [PubMed]
- Lambert, J.M.; Goldmacher, V.S.; Collinson, A.R.; Nadler, L.M.; Blattler, W.A. An immunotoxin prepared with blocked ricin: A natural plant toxin adapted for therapeutic use. Cancer Res. 1991, 51, 6236–6242. [Google Scholar] [PubMed]
- Ferreras, J.M.; Citores, L.; Iglesias, R.; Jiménez, P.; Girbés, T. Use of ribosome-inactivating proteins from Sambucus for the construction of immunotoxins and conjugates for cancer therapy. Toxins 2011, 3, 420–441. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Arias, Y.; Ferreras, J.M.; Jimenez, P.; Rojo, M.A.; Girbes, T. Sensitivity of cancer cell lines to the novel non-toxic type 2 ribosome-inactivating protein nigrin b. Cancer Lett. 2001, 167, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Citores, L.; Ferreras, J.M.; Munoz, R.; Benitez, J.; Jimenez, P.; Girbes, T. Targeting cancer cells with transferrin conjugates containing the non-toxic type 2 ribosome-inactivating proteins nigrin b or ebulin l. Cancer Lett. 2002, 184, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bodey, B.; Bodey, B., Jr.; Siegel, S.E.; Kaiser, H.E. Immunocytochemical detection of endoglin is indicative of angiogenesis in malignant melanoma. Anticancer Res. 1998, 18, 2701–2710. [Google Scholar]
- Fonsatti, E.; Altomonte, M.; Nicotra, M.R.; Natali, P.G.; Maio, M. Endoglin (CD105): A powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene 2003, 22, 6557–6563. [Google Scholar] [CrossRef] [PubMed]
- Zijlmans, H.J.; Fleuren, G.J.; Hazelbag, S.; Sier, C.F.; Dreef, E.J.; Kenter, G.G.; Gorter, A. Expression of endoglin (CD105) in cervical cancer. Br. J. Cancer 2009, 100, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, P.; Hawinkels, L.J.; de Jonge-Muller, E.S.; Biemond, I.; Lamers, C.B.; Verspaget, H.W. Angiogenic markers endoglin and vascular endothelial growth factor in gastroentero-pancreatic neuroendocrine tumors. World J. Gastroenterol. 2011, 17, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Fonsatti, E.; Jekunen, A.P.; Kairemo, K.J.; Coral, S.; Snellman, M.; Nicotra, M.R.; Natali, P.G.; Altomonte, M.; Maio, M. Endoglin is a suitable target for efficient imaging of solid tumors: In vivo evidence in a canine mammary carcinoma model. Clin. Cancer Res. 2000, 6, 2037–2043. [Google Scholar] [PubMed]
- Bredow, S.; Lewin, M.; Hofmann, B.; Marecos, E.; Weissleder, R. Imaging of tumor neo-vasculature by targeting the TGF-beta binding receptor endoglin. Eur. J. Cancer 2000, 36, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Arias, Y.; Ferreras, J.M.; Rojo, M.A.; Gayoso, M.J.; Nocito, M.; Benitez, J.; Jimenez, P.; Bernabeu, C.; Girbes, T. Targeting a marker of the tumor neovasculature using a novel anti-human CD105-immunotoxin containing the non-toxic type 2 ribosome-inactivating protein nigrin b. Cancer Lett. 2007, 256, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Benitez, J.; Ferreras, J.M.; Muñoz, R.; Arias, Y.; Iglesias, R.; Cordoba-Diaz, M.; del Villar, R.; Girbes, T. Cytotoxicity of an ebulin l-anti-human CD105 immunotoxin on mouse fibroblasts (L929) and rat myoblasts (L6E9) cells expressing human CD105. Med. Chem. 2005, 1, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; Arias, Y.; Ferreras, J.M.; Jiménez, P.; Rojo, M.A.; Bernabéu, C.; Cordoba-Diaz, D.; Girbés, T. Transient injury-dependent up-regulation of CD105 and its specific targeting with an anti-vascular anti-mouse endoglin-nigrin b immunotoxin. Med. Chem. 2012, 8, 996–1002. [Google Scholar] [PubMed]
- Muñoz, R.; Arias, Y.; Ferreras, J.M.; Jiménez, P.; Langa, C.; Rojo, M.A.; Gayoso, M.J.; Cordoba-Diaz, D.; Bernabéu, C.; Girbés, T. In vitro and in vivo effects of an anti-mouse endoglin (CD105)-immunotoxin on the early stages of mouse B16MEL4A5 melanoma tumors. Cancer Immunol. Immunother. 2013, 62, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Roodink, I.; Leenders, W.P. Targeted therapies of cancer: Angiogenesis inhibition seems not enough. Cancer Lett. 2010, 299, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G.D. Understanding ricin from a defensive viewpoint. Toxins (Basel) 2011, 3, 1373–1392. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Dickers, K.J.; Rice, P.; Griffiths, G.D.; Vale, J.A. Ricin poisoning. Toxicol. Rev. 2003, 22, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.J.; Jolliffe, N.A.; Marsden, C.J.; Pateman, C.S.; Smith, D.C.; Spooner, R.A.; Watson, P.D.; Roberts, L.M. Ricin. Mechanisms of cytotoxicity. Toxicol. Rev. 2003, 22, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, P.; Gayoso, M.J.; Garrosa, M.; Cordoba-Diaz, D.; Cabrero, P.; Tejero, J.; Aracil, M.; Girbés, T. Paneth cells are also target of the ribotoxic lectin nigrin b. Histol. Histopathol. 2014, 29, 1057–1063. [Google Scholar] [PubMed]
- Jiménez, P.; Cordoba-Diaz, D.; Cabrero, P.; Aracil, M.; Gayoso, M.J.; Garrosa, M.; Cordoba-Diaz, M.; Girbés, T. Plasma accumulation of vitamin B6 from an oral dose in a new gut injury-regeneration reversible mouse model. Food Nutr. Sci. 2013, 4, 908–917. [Google Scholar] [CrossRef]
- Jiménez, P.; Cabrero, P.; Tejero, J.; Gayoso, M.J.; Garrosa, M.; Cordoba-Diaz, D.; Cordoba-Diaz, M.; Girbés, T. Concentrated extract of green tea polyphenols enhances the toxicity of the elderberry lectin nigrin b to mice. Food Nutr. Sci. 2014, 5, 466–471. [Google Scholar] [CrossRef]
- Battelli, M.G.; Citores, L.; Buonamici, L.; Ferreras, J.M.; de Benito, F.M.; Stirpe, F.; Girbes, T. Toxicity and cytotoxicity of nigrin b, a two-chain ribosome-inactivating protein from Sambucus nigra: Comparison with ricin. Arch. Toxicol. 1997, 71, 360–364. [Google Scholar] [CrossRef] [PubMed]
- He, H.X.; McMahon, S.; Henderson, T.D.; Griffey, S.M.; Cheng, L.W. Ricin toxicokinetics and its sensitive detection in mouse sera or feces using immuno-PCR. PLoS One 2010, e12858. [Google Scholar] [CrossRef]
- Kiselova, Y.; Ivanova, D.; Chervenkov, T.; Gerova, D.; Galunska, B.; Yankova, T. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother. Res. 2006, 20, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.; Cabrero, P.; Basterrechea, J.E.; Tejero, J.; Cordoba-Diaz, D.; Cordoba-Diaz, M.; Girbes, T. Effects of short-term heating on total polyphenols, anthocyanins, antioxidant activity and lectins of different parts of dwarf elder (Sambucus ebulus L.). Plant Foods Hum. Nutr. 2014, 69, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Shahidi-Noghabi, S.; Van Damme, E.J.; Iga, M.; Smagghe, G. Exposure of insect midgut cells to Sambucus nigra L. agglutinins I and II causes cell death via caspase-dependent apoptosis. J. Insect Physiol. 2010, 56, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Shahidi-Noghabi, S.; Van Damme, E.J.; De Vos, W.H.; Smagghe, G. Internalization of Sambucus nigra agglutinins I and II in insect midgut CF-203 cells. Arch. Insect Biochem. Physiol. 2011, 76, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Walski, T.; van Damme, E.J.; Smagghe, G. Penetration through the peritrophic matrix is a key to lectin toxicity against Tribolium castaneum. J. Insect Physiol. 2014, 70, 94–101. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejero, J.; Jiménez, P.; Quinto, E.J.; Cordoba-Diaz, D.; Garrosa, M.; Cordoba-Diaz, M.; Gayoso, M.J.; Girbés, T. Elderberries: A Source of Ribosome-Inactivating Proteins with Lectin Activity. Molecules 2015, 20, 2364-2387. https://doi.org/10.3390/molecules20022364
Tejero J, Jiménez P, Quinto EJ, Cordoba-Diaz D, Garrosa M, Cordoba-Diaz M, Gayoso MJ, Girbés T. Elderberries: A Source of Ribosome-Inactivating Proteins with Lectin Activity. Molecules. 2015; 20(2):2364-2387. https://doi.org/10.3390/molecules20022364
Chicago/Turabian StyleTejero, Jesús, Pilar Jiménez, Emiliano J. Quinto, Damián Cordoba-Diaz, Manuel Garrosa, Manuel Cordoba-Diaz, Manuel J. Gayoso, and Tomás Girbés. 2015. "Elderberries: A Source of Ribosome-Inactivating Proteins with Lectin Activity" Molecules 20, no. 2: 2364-2387. https://doi.org/10.3390/molecules20022364
APA StyleTejero, J., Jiménez, P., Quinto, E. J., Cordoba-Diaz, D., Garrosa, M., Cordoba-Diaz, M., Gayoso, M. J., & Girbés, T. (2015). Elderberries: A Source of Ribosome-Inactivating Proteins with Lectin Activity. Molecules, 20(2), 2364-2387. https://doi.org/10.3390/molecules20022364







