Contribution of Bacillus Isolates to the Flavor Profiles of Vanilla Beans Assessed through Aroma Analysis and Chemometrics
Abstract
:1. Introduction
2. Results and Discussion
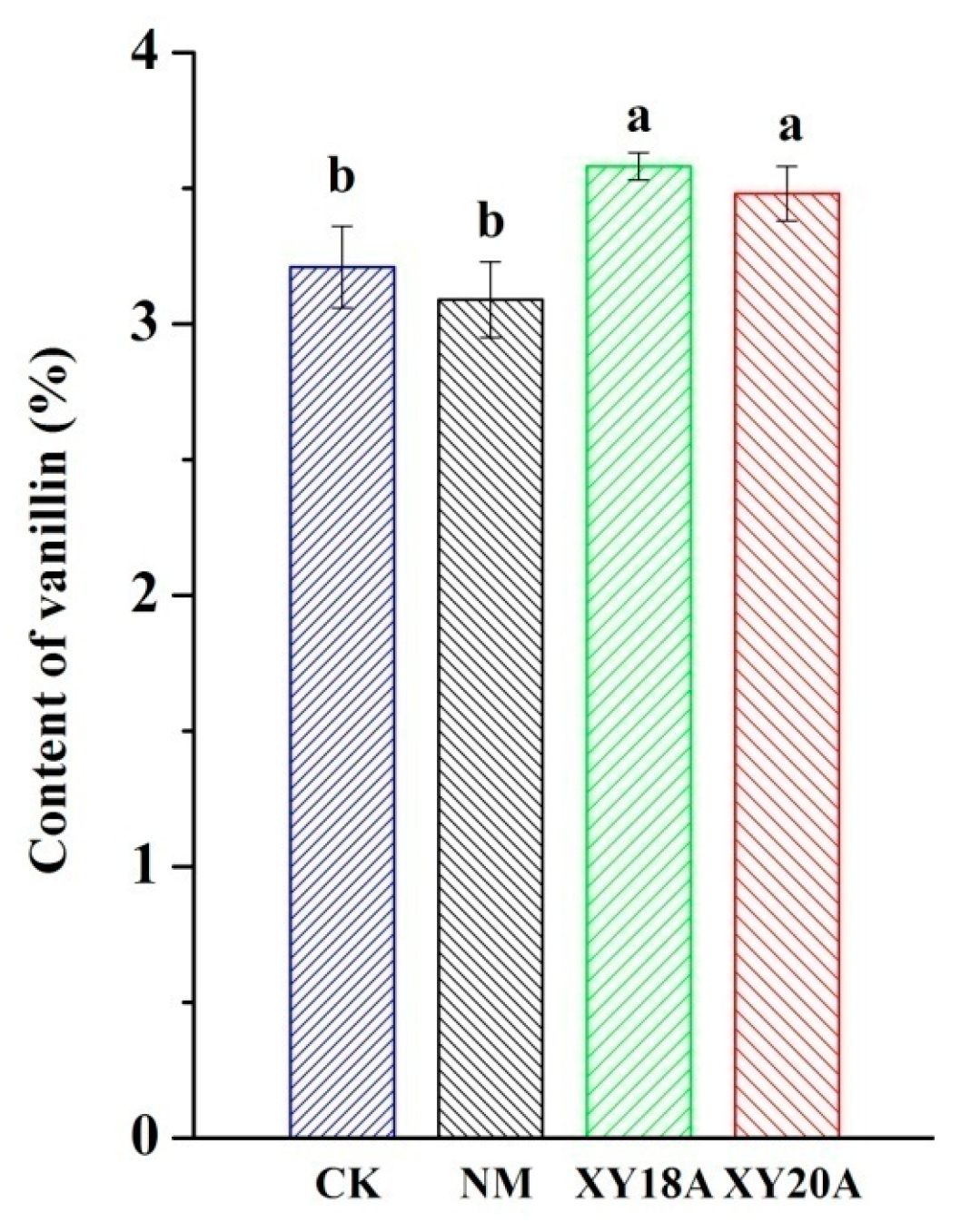
2.1. Effect of Bacillus Strains on Vanillin Formation
2.2. Volatiles Identified in the Headspace of VFs
2.2.1. Volatiles Formed from Endogenous Vanilla Metabolism
| Compounds | RIDB-WAX | RIliterature | CK | NM | XY18A | XY20A |
|---|---|---|---|---|---|---|
| Acids | ||||||
| Acetic acid | 1432 | 1446 | * | * | * | * |
| Propionic acid | 1524 | 1530 | * | * | * | * |
| Butanoic acid | 1616 | 1621 | * | * | * | * |
| Hexanoic acid | 1837 | 1840 | * | – | * | * |
| Homovanillic acid | 2312 | – | – | * | * | * |
| Benzoic acid | 2493 | 2415 | – | – | * | – |
| Alcohols | ||||||
| 1-Octanol | 1548 | 1555 | * | * | * | * |
| 2,3-Butanediol | 1566 | 1566 | * | * | * | * |
| Benzyl alcohol | 1851 | 1869 | * | * | * | * |
| Phenylethyl alcohol | 1883 | 1906 | * | * | * | * |
| p-Anisyl alcohol | 2248 | 2191 | * | * | * | * |
| Vanillyl alcohol | 2769 | – | * | * | * | * |
| Aldehydes | ||||||
| Hexanal | 1048 | 1080 | * | * | * | * |
| Octanal | 1273 | 1289 | * | * | * | * |
| Heptenal | 1304 | 1328 | * | * | * | * |
| Nonanal | 1377 | 1392 | * | * | * | * |
| Benzaldehyde | 1493 | 1519 | * | * | * | * |
| 4-Methoxybenzaldehyde | 1982 | 2018 | * | * | * | * |
| Piperonal | 2184 | – | * | * | * | * |
| Vanillin | 2530 | 2578 | * | * | * | * |
| Isovanillin | 2570 | – | * | * | * | * |
| p-Formylphenol | 2914 | – | * | * | * | * |
| Bases | ||||||
| 2-Acetyl-1H-pyrrole | 1940 | 1991 | * | – | * | * |
| 2-Formyl-1H-pyrrole | 1988 | 1965 | * | * | * | * |
| Esters | ||||||
| 2-Phenylethyl acetate | 1786 | 1822 | * | – | * | * |
| Methyl 3-phenylacrylate | 2040 | 2035 | * | * | * | * |
| 4-Formyl-2-methoxyphenyl acetate | 2467 | – | * | * | * | * |
| Ethers | ||||||
| 2-(2-ethoxyethoxy)ethanol | 1603 | 1577 | * | * | * | * |
| Butyl carbitol | 1776 | 1793 | * | * | * | * |
| Furans | ||||||
| Furfural | 1439 | 1452 | * | * | * | * |
| 2-Acetylfuran | 1479 | 1494 | * | * | * | * |
| 5-Methylfurfural | 1544 | 1568 | * | * | * | * |
| Hydrocarbons | ||||||
| 3-Carene | 1125 | 1146 | * | * | * | * |
| Limonene | 1177 | 1198 | * | * | * | * |
| Tetradecane | 1392 | 1400 | * | * | * | * |
| Ketones | ||||||
| Acetoin | 1263 | 1283 | * | * | * | * |
| 6-Methyl-5-hepten-2-one | 1320 | 1342 | * | * | * | * |
| 6,10,14-Trimethyl-2-pentadecanone | 2110 | 2115 | * | * | * | * |
| Apocynin | 2600 | – | – | – | * | – |
| Lactone | ||||||
| Butyrolactone | 1587 | 1643 | * | * | * | * |
| Phenols | ||||||
| cis-Anethole | 1794 | 1845 | * | * | * | * |
| Guaiacol | 1831 | 1857 | * | * | * | * |
| Creosol | 1926 | 1927 | * | * | * | * |
| Phenol | 1979 | 2003 | * | * | * | – |
| 4-Methylphenol | 2056 | 2074 | * | * | * | * |
| 2-Methoxy-4-vinylphenol | 2164 | 2192 | * | * | * | * |
| 4-(Ethoxymethyl)phenol | 2514 | – | – | * | * | * |
2.2.2. Volatiles Formed from Exogenous Bacillus Metabolism
2.2.3. Volatiles Formed from Interaction of Bacillus and Vanilla Metabolism
| No. | Compounds | Quantities(%) * | |||
|---|---|---|---|---|---|
| CK | NM | XY18A | XY20A | ||
| A1 | Acetic acid | 0.080 ± 0.0081 a | 0.080 ± 0.0121 a | 0.087 ± 0.0076 a | 0.086 ± 0.0078 a |
| A2 | Butanoic acid | 0.026 ± 0.0154 a | 0.024 ± 0.0185 a | 0.033 ± 0.0042 ab | 0.060 ± 0.0226 b |
| A3 | 2,3-Butanediol | 0.021 ± 0.0019 a | 0.023 ± 0.0009 a | 0.023 ± 0.0007 a | 0.024 ± 0.0026 a |
| A4 | Vanillyl alcohol | 0.004 ± 0.0024 a | 0.002 ± 0.0005 a | 0.003 ± 0.0002 a | 0.003 ± 0.0033 a |
| A5 | Heptenal | 0.010 ± 0.0098 a | 0.011 ± 0.0110 a | 0.009 ± 0.0018 a | 0.011 ± 0.0018 a |
| A6 | Acetoin | 0.033 ± 0.0058 b | 0.026 ± 0.0038 ab | 0.030 ± 0.0011 b | 0.021 ± 0.0051 a |
| A7 | Guaiacol | 0.028 ± 0.0013 b | 0.022 ± 0.0035 a | 0.023 ± 0.0013 a | 0.025 ± 0.0028 ab |
| A8 | Creosol | 0.004 ± 0.0012 a | 0.004 ± 0.0007 a | 0.004 ± 0.0001 a | 0.004 ± 0.0004 a |
| A9 | 2-Methoxy-4-vinylphenol | 0.003 ± 0.0017 a | 0.003 ± 0.0011 a | 0.003 ± 0.0003 a | 0.005 ± 0.0016 a |
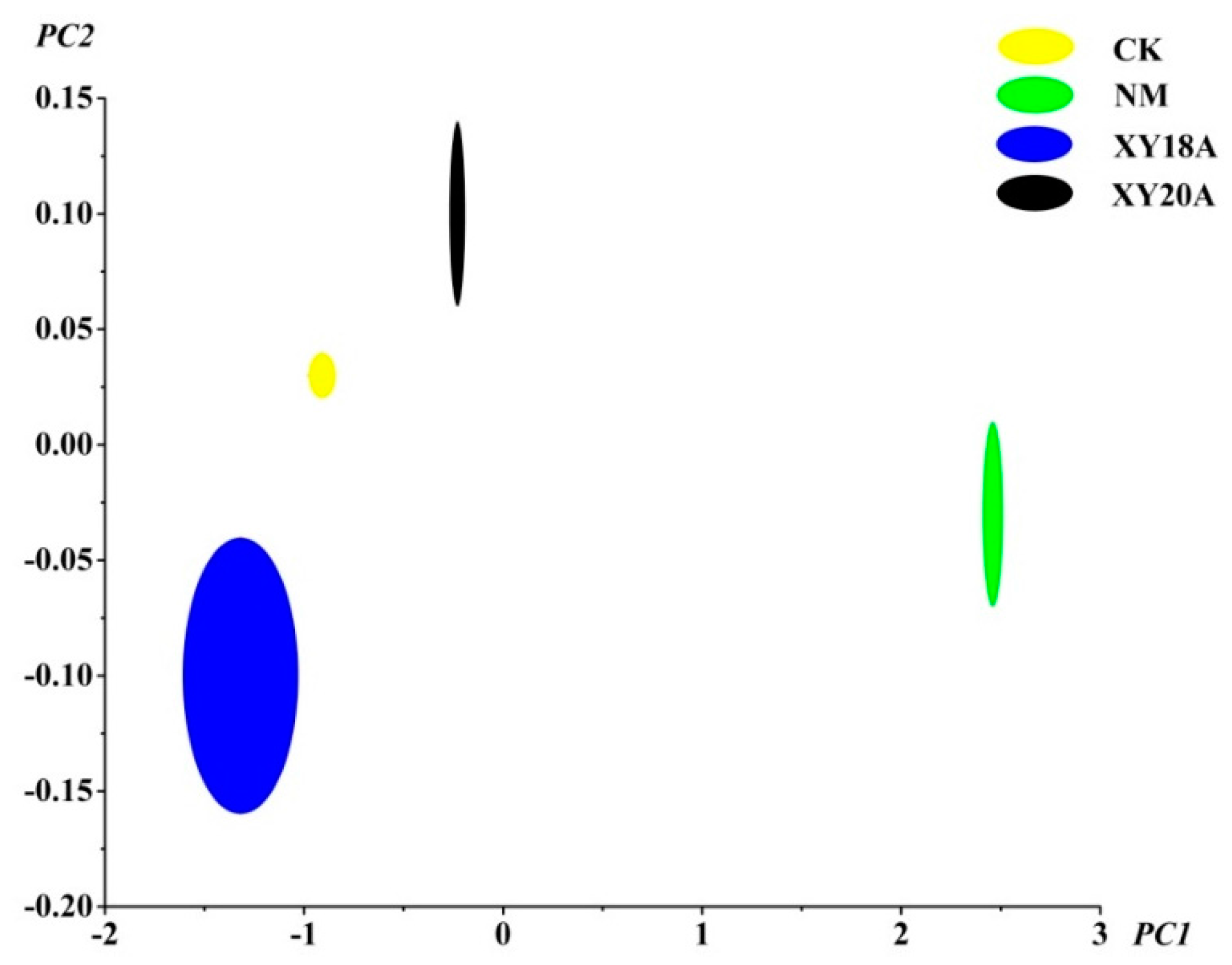
2.3. E-nose Analysis of VFs
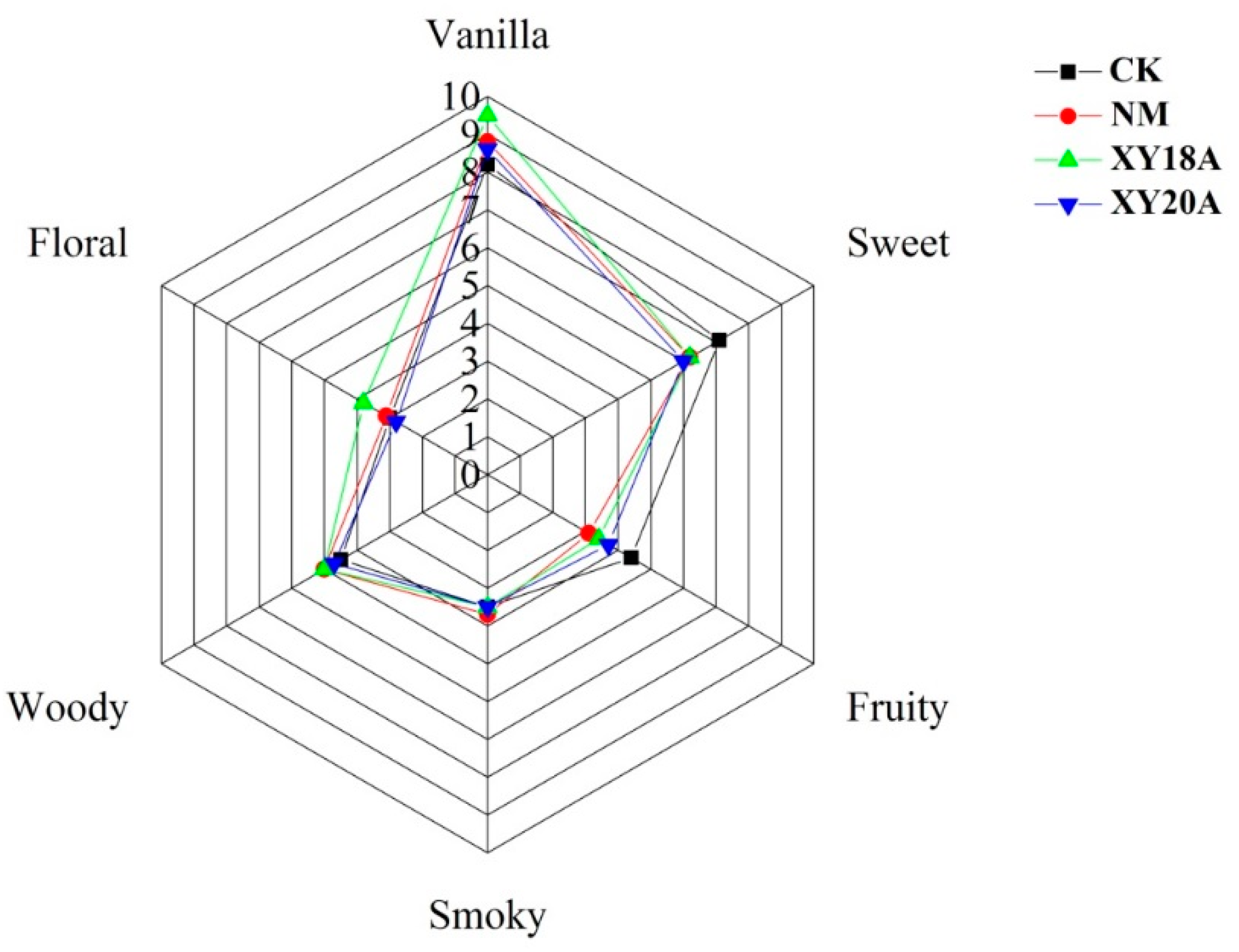
2.4. Sensory Characteristics of VFs
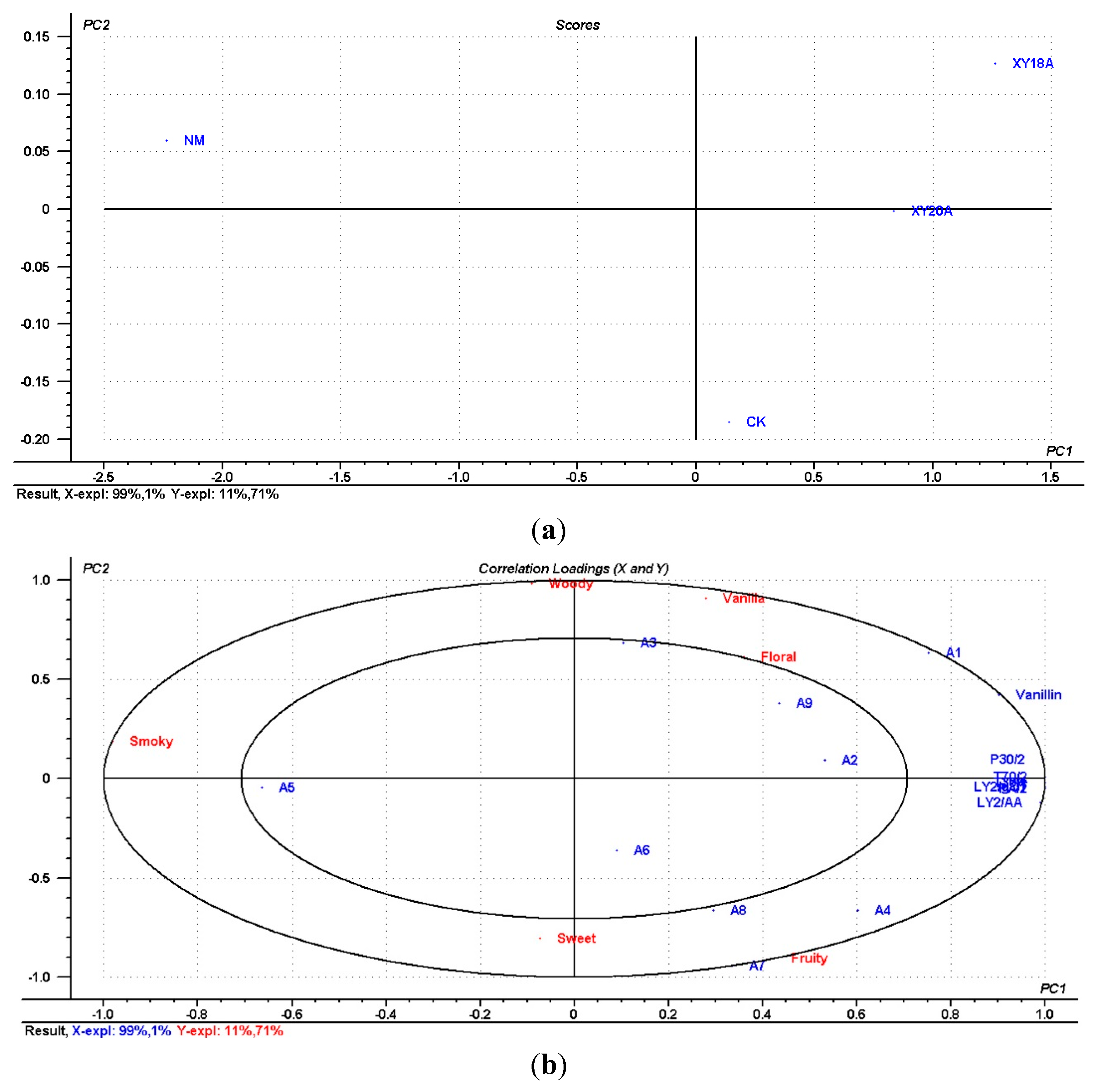
2.5. Comparison of the Characteristic Compounds, E-nose Response, and Sensory Attributes of VFs
3. Experimental Section
3.1. Samples and Chemicals
3.2. Sample Preparation and Analysis
3.2.1. Determination of Vanillin
3.2.2. GC-MS Analysis
3.2.3. E-nose Analysis
3.2.4. Sensory Evaluation
3.2.5. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| VF | Vanilla flavor |
| CK | Control (hot air processing) |
| NM | Non-microorganism-assisted curing sample |
| XY18A | XY18-assisted curing sample |
| XY20A | XY20-assisted curing sample |
| GC-MS | Gas chromatography-mass spectrometry |
| SPME | Solid-phase microextraction |
| CAR/PDMS | Carboxen/polydimethylsiloxane |
| E-nose | Electronic nose |
| HPLC | High-performance liquid chromatography |
| PLSR | Partial least squares regression |
| PCA | Principal component analysis |
| RI | Retention index |
References
- Lubinsky, P.; Bory, S.; Hernandez, J.H.; Kim, S.C.; Gomez-Pompa, A. Origins and dispersal of cultivated vanilla (Vanilla planifolia Jacks. [Orchidaceae]). Econ. Bot. 2008, 62, 127–138. [Google Scholar] [CrossRef]
- Pérez-Silva, A.; Gunata, Z.; Lepoutre, J.P.; Odoux, E. New insight on the genesis and fate of odor-active compounds in vanilla beans (Vanilla planifolia G. Jackson) during traditional curing. Food Res. Int. 2011, 44, 2930–2937. [Google Scholar] [CrossRef]
- Dignum, M.J.W.; Heijden, R.V.D.; Kerler, J.; Winkel, C.; Verpoorte, R. Identification of glucosides in green beans of Vanilla planifolia Andrews and kinetics of vanilla β-glucosidase. Food Chem. 2004, 85, 199–205. [Google Scholar] [CrossRef]
- Odoux, E.; Chauwin, A.; Brillouet, J.M. Purification and characterization of vanilla bean (Vanilla planifolia Andrews) β-d-glucosidase. J. Agr. Food Chem. 2003, 51, 3168–3173. [Google Scholar] [CrossRef] [PubMed]
- Dignum, M.J.W.; Kerler, J.; Verpoorte, R. Vanilla production: Technological, chemical, and biosynthetic aspects. Food Res. Int. 2001, 17, 199–219. [Google Scholar] [CrossRef]
- Mariezcurrena, M.D.; Zavaleta, H.A.; Waliszewski, K.N.; Sánchez, V. The effect of killing conditions on the structural changes in vanilla (Vanilla planifolia, Andrews) pods during the curing process. Int. J. food Sci. Tech. 2008, 43, 1452–1457. [Google Scholar] [CrossRef]
- Sreedhar, R.V.; Roohie, K.; Maya, P.; Venkatachalam, L.; Bhagyalakshmi, N. Biotic elicitors enhance flavor compounds during accelerated curing of vanilla beans. Food Chem. 2009, 112, 461–468. [Google Scholar] [CrossRef]
- Ranadive, A.S. Vanilla-cultivation, curing, chemistry, technology and commercial products. In Developments in Food Science Spices, Herbs and Edible Fungi; Charalambrous, D.G., Ed.; Elsevier Publishers: Amsterdam, The Netherland, 1994; pp. 517–577. [Google Scholar]
- Westcott, R.J.; Cheetham, P.S.J.; Arraclough, A.J.B. Use of organized viable vanilla plant aerial roots of the production of natural vanillin. Phytochemistry 1994, 35, 135–138. [Google Scholar] [CrossRef]
- Odoux, E.; Escoute, J.; Verdeil, J.L.; Brillouet, J.M. Localization of β-d-glucosidase activity and glucovanillin in vanilla bean (Vanilla planifolia Andrews). Ann. Bot-Lond. 2003, 92, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.L.; Xu, F.; Tan, L.H.; Wu, H.S.; Chu, Z.; Wang, Q.H. Optimization of enzymatic process for vanillin extraction using response surface methodology. Molecules 2012, 17, 8753–8761. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Sarkar, B.C.; Sharma, H.K.; Singh, C. Optimization of enzymatic hydrolysis pretreatment conditions for enhanced juice recovery from guava fruit using response surface methodology. Food Bioprocess Tech. 2009, 2, 96–100. [Google Scholar] [CrossRef]
- Madhava Naidu, M.; Kumar, P.V.S.; Shyamala, B.N.; Sulochanamma, G.; Prakash, M.; Thakur, M.S. Enzyme-assisted process for production of superior quality vanilla extracts from green vanilla pods using tea leaf enzymes. Food Bioprocess Tech. 2012, 5, 527–532. [Google Scholar] [CrossRef]
- Chen, Y.G.; Gu, F.L.; Li, J.H.; He, S.Z.; Xu, F.; Fang, Y. Involvement of colonizing Bacillusisolates in glucovanillin hydrolysis during the curing of Vanilla planifolia Andrews. Appl. Environ. Microbiol. 2015, 81, 4947–4954. [Google Scholar] [CrossRef] [PubMed]
- Niebler, J.; Buettner, A. Identification of odorants in frankincense (Boswellia sacra Flueck.) by aroma extract dilution analysis and two-dimensional gas chromatography-mass spectrometry/olfactometry. Phytochemistry 2015, 109, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Lyroni, E.; Tzia, C. Sensory profiling and hedonic judgement of probiotic ice cream as a function of hydrocolloids, yogurt and milk fat content. LWT-Food Sci. Technol. 2010, 43, 1351–1358. [Google Scholar] [CrossRef]
- Odoux, E. Changes invanillin and glucovanillin concentrations during the various stages of the process traditionally used for curing Vanilla fragrans beans in Réunion. Fruits 2000, 55, 119–125. [Google Scholar]
- Frenkel, C.; Ranadive, A.S.; Vázquez, J.T.; Havkin-Frenkel, D. Curing of vanilla. In Handbook of Vanilla Science and Technology; Havkin-Frenkel, D., Belanger, F.C., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 79–106. [Google Scholar]
- Perera, C.O.; Owen, E. Effect of tissue disruption by different methods followed by incubation with hydrolyzing enzymes on the production of vanillin from Tongan vanilla beans. Food Bioprocess Tech. 2010, 3, 49–54. [Google Scholar] [CrossRef]
- Toth, S.; Lee, K.J.; Havkin-Frenkel, D.; Belanger, F.C.; Hartman, T.G. Volatile compounds in vanilla. In Handbook of Vanilla Science and Technology; Havkin-Frenkel, D., Belanger, F.C., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 183–219. [Google Scholar]
- Zhang, S.Y.; Christoph, M. Comparative analysis of volatiles in traditionally cured Bourbon and Ugandan vanilla Bean (Vanilla planifolia) extracts. J. Agr. Food Chem. 2012, 60, 10433–10444. [Google Scholar] [CrossRef] [PubMed]
- Sostaric, T.; Boyce, M.C.; Spickett, E.E. Analysis of the volatile components in vanilla extracts and flavorings by solid-phase microextraction and gas chromatography. J. Agr. Food Chem. 2000, 48, 5802–5807. [Google Scholar] [CrossRef]
- Volatile Compounds in Food 16.1. Available online: http://www.vcf-online.nl/VcfHome.cfm (accessed on 21 September 2015).
- Search ChemSpider. Available online: http://www.chemspider.com/ (accessed on 21 September 2015).
- Gilberta, J.A.; van der Lelie, D.; Zarraonaindia, I. Microbial terroir for wine grapes. Proc. Natl. Acad. Sci. USA 2004, 111, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.J.; Nam, Y.D.; Roh, S.W.; Bae, J.W. Unexpected convergence of fungal and bacterial communities during fermentation of traditional Korean alcoholic beverages inoculated with various natural starters. Food Microbiol. 2012, 30, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Hartman, T.G. Composition of vanilla beans from different geographical regions. In Proceedings of First International Congress on the Future of Vanilla and Vanillin in Vanilla, Princeton, NJ, USA, 11–12 November 2003.
- Pérez-Silva, A.; Odoux, E.; Brat, P.; Ribeyre, F.; Rodriguez-Jimenes, G.; Robles-Olvera, V.; García-Alvarado, M.A.; Günata, Z. GC-MS and GC-olfactometry analysis of aroma compounds in a representative organic aroma extract from cured vanilla (Vanilla planifolia G. Jackson) beans. Food Chem. 2006, 99, 728–735. [Google Scholar] [CrossRef]
- Zigová, J.S.; Šturdík, E.; Vandák, D.; Schlosser, S. Butyric acid production by Clostridium butyricum with integrated extraction and pertraction. Process Biochem. 1999, 34, 835–843. [Google Scholar] [CrossRef]
- Zigová, J.S.; Šturdík, E. Advances in biotechnological production of butyric acid. J. Ind. Microbiol. Biot. 2000, 24, 153–160. [Google Scholar] [CrossRef]
- Zhang, C.H.; Yang, H.; Yang, F.X.; Ma, Y.J. Current progress on butyric acid production by fermentation. Current Microbiol. 2009, 59, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.O.; Bignon, J.; Papoport, G.; Debarbouille, M. Regulation of the acetoin catabolic pathway is controlled by sigma L in Bacillus subtilis. J. Bacteriol. 2011, 183, 2497–2504. [Google Scholar]
- Zhang, Y.J.; Mo, L.M.; Chen, F.; Lu, M.Q.; Dong, W.J.; Wang, Q.H.; Xu, F.; Gu, F.L. Optimized production of vanillin from green vanilla pods by enzyme-assisted extraction combined with pre-freezing and thawing. Molecules 2014, 19, 2181–2198. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Z.; Gu, F.L.; Xu, F.; Wang, Q.H. Comparison of four kinds of extraction techniques and kinetics of microwave-assisted extraction of vanillin from Vanilla planifolia Andrews. Food Chem. 2014, 149, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Katsumata, T.; Egi, M. Taste properties of Maillard-reaction products prepared from 1000 to 5000 Da peptide. Food Chem. 2006, 99, 600–604. [Google Scholar] [CrossRef]
- Schlichtherle-Cerny, H.; Amadò, R. Analysis of taste-active compounds in an enzymatic hydrolysate of deamidated wheat gluten. J. Agr. Food Chem. 2006, 50, 1515–1522. [Google Scholar] [CrossRef]
- Gonzalez, N.J.; Adhikari, K.; Sancho-Madriz, M.F. Sensory characteristics of peach-flavored yogurt drinks containing prebiotics and synbiotics. LWT-Food Sci. Technol. 2011, 44, 158–163. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.L. Quantitative descriptive analysis, developments, application and the future. Food Technol. 1998, 52, 48–52. [Google Scholar]
- Hariom; Shyamala, B.N.; Prakash, M.; Bhat, S.S. Vanilla flavour evaluation by sensory and electronic nose techniques. J. Sens. Stud. 2006, 21, 228–239. [Google Scholar] [CrossRef]
- Song, S.Q.; Zhang, X.M.; Xiao, Z.B.; Niu, Y.W.; Hayat, K.; Eric, K. Contribution of oxidized tallow to aroma characteristics of beeflike process flavour assessed by gas chromatography-mass spectrometry and partial least squares regression. J. Chromatogr. A 2012, 1254, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, F.; Chen, Y.; Fang, Y.; Wu, G.; Tan, L. Contribution of Bacillus Isolates to the Flavor Profiles of Vanilla Beans Assessed through Aroma Analysis and Chemometrics. Molecules 2015, 20, 18422-18436. https://doi.org/10.3390/molecules201018422
Gu F, Chen Y, Fang Y, Wu G, Tan L. Contribution of Bacillus Isolates to the Flavor Profiles of Vanilla Beans Assessed through Aroma Analysis and Chemometrics. Molecules. 2015; 20(10):18422-18436. https://doi.org/10.3390/molecules201018422
Chicago/Turabian StyleGu, Fenglin, Yonggan Chen, Yiming Fang, Guiping Wu, and Lehe Tan. 2015. "Contribution of Bacillus Isolates to the Flavor Profiles of Vanilla Beans Assessed through Aroma Analysis and Chemometrics" Molecules 20, no. 10: 18422-18436. https://doi.org/10.3390/molecules201018422
APA StyleGu, F., Chen, Y., Fang, Y., Wu, G., & Tan, L. (2015). Contribution of Bacillus Isolates to the Flavor Profiles of Vanilla Beans Assessed through Aroma Analysis and Chemometrics. Molecules, 20(10), 18422-18436. https://doi.org/10.3390/molecules201018422





