Ionic Liquid-Based Vacuum Microwave-Assisted Extraction Followed by Macroporous Resin Enrichment for the Separation of the Three Glycosides Salicin, Hyperin and Rutin from Populus Bark
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of Ionic Liquids
2.1.1. Anion Effect
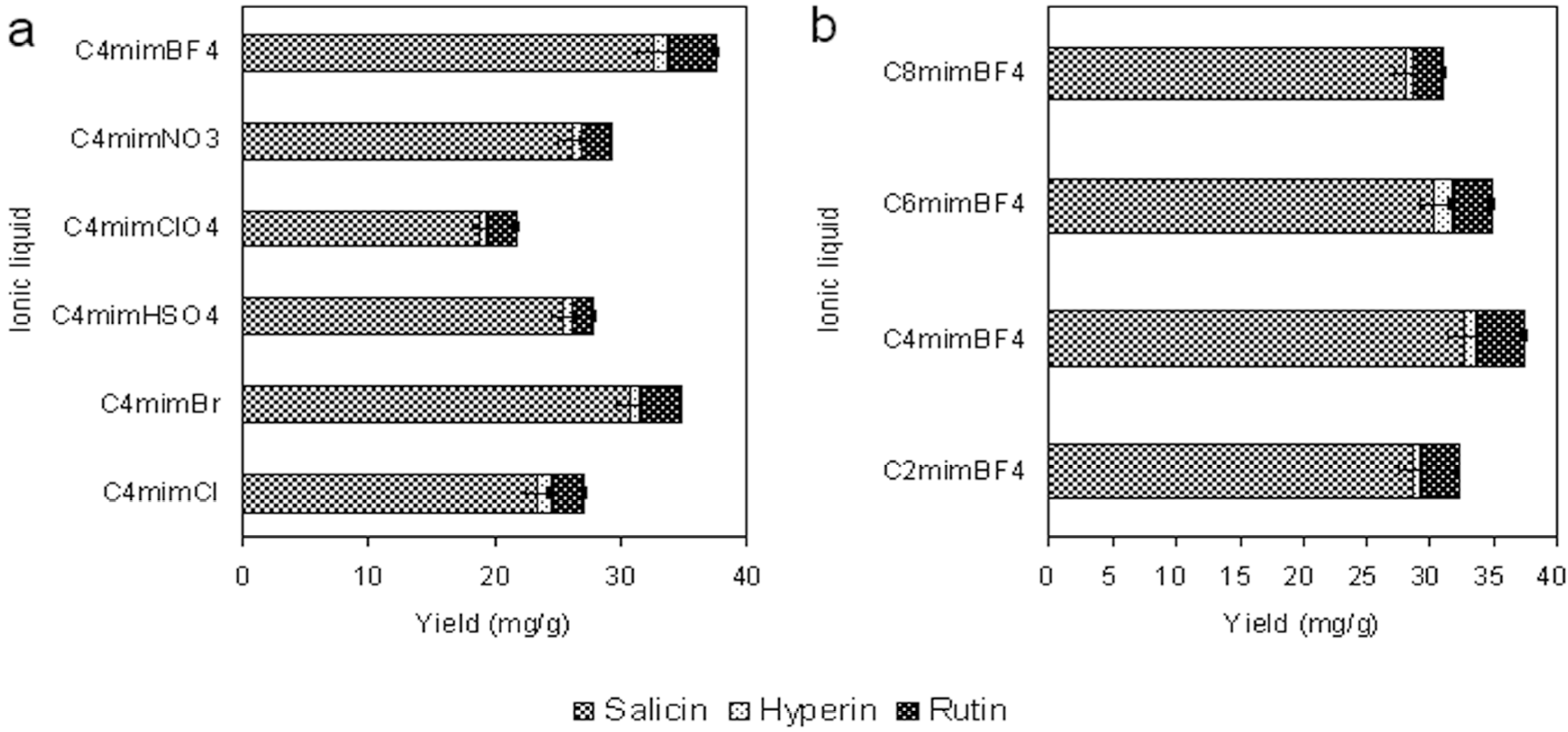
2.1.2. Effect of the Alkyl Chain Length of the Ionic Liquid Cation
2.2. Optimization of Salicin, Hyperin and Rutin Extraction Using a Factorial Design
2.2.1. Effect of Concentration
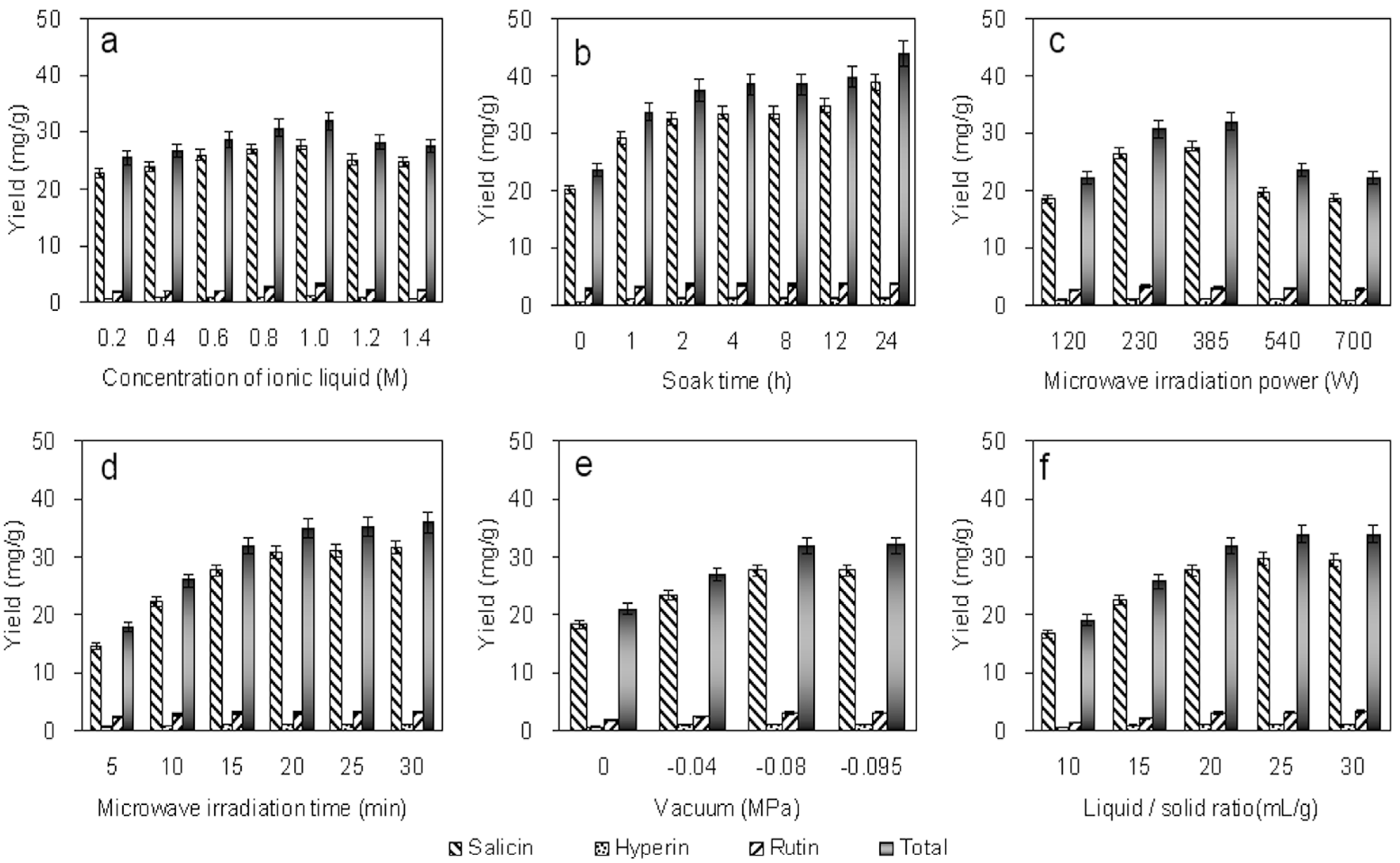
2.2.2. Effect of Bark Soaking Time
2.2.3. Effect of Microwave Irradiation Power
2.2.4. Effect of Microwave Irradiation Time
2.2.5. Effect of the Vacuum
2.2.6. Effect of the Liquid/Solid Ratio
2.3. Optimization of Salicin, Hyperin and Rutin Extraction Using Response Surface Methodology (RSM)
| Run | BBD Experiments | ANOVA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A (min) | B(W) | C (mL/g) | Y (mg/g) | Source | Sum of Squares | Degree of Freedom | Mean Square | F-value | p-value | |||||
| 1 | 20 | 385 | 25 | 41.22 | Modelb | 81.45 | 9 | 9.05 | 36.10 | < 0.0001 c | ||||
| 2 | 10 | 385 | 25 | 35.83 | A | 12.98 | 1 | 12.98 | 51.63 | 0.0002 c | ||||
| 3 | 10 | 230 | 20 | 31.43 | B | 4.21 | 1 | 4.21 | 16.73 | 0.0046 c | ||||
| 4 | 15 | 385 | 20 | 37.12 | C | 17.02 | 1 | 17.02 | 67.71 | <0.0001 c | ||||
| 5 | 15 | 385 | 20 | 36.21 | AB | 0.13 | 1 | 0.13 | 0.50 | 0.5018 | ||||
| 6 | 15 | 230 | 15 | 32.24 | AC | 5.81 | 1 | 5.81 | 23.10 | 0.0020 c | ||||
| 7 | 15 | 230 | 25 | 34.34 | BC | 0.16 | 1 | 0.16 | 0.62 | 0.4567 | ||||
| 8 | 15 | 540 | 25 | 35.70 | A2 | 0.01 | 1 | 0.01 | 0.02 | 0.8855 | ||||
| 9 | 10 | 540 | 20 | 33.72 | B2 | 40.96 | 1 | 40.96 | 162.93 | < 0.0001 c | ||||
| 10 | 10 | 385 | 15 | 34.90 | C2 | 0.05 | 1 | 0.05 | 0.18 | 0.6846 | ||||
| 11 | 15 | 540 | 15 | 32.81 | Residual | 1.76 | 7 | 0.25 | ||||||
| 12 | 15 | 385 | 20 | 37.10 | Lack of fit | 1.20 | 3 | 0.40 | 2.87 | 0.1674 | ||||
| 13 | 15 | 385 | 20 | 36.67 | Pure error | 0.56 | 4 | 0.14 | ||||||
| 14 | 20 | 385 | 15 | 35.47 | Corrected total | 83.21 | 16 | |||||||
| 15 | 20 | 540 | 20 | 35.48 | Credibility analysis of the regression equations | |||||||||
| 16 | 15 | 385 | 20 | 36.84 | Index mark | Standard deviation | Mean | Coefficient of variation % | Press | R2 | Adjust R2 | Predicted R2 | Adequacy precision | |
| 17 | 20 | 230 | 20 | 33.90 | Y | 0.50 | 35.35 | 1.42 | 20.10 | 0.9789 | 0.9517 | 0.7585 | 24.278 | |
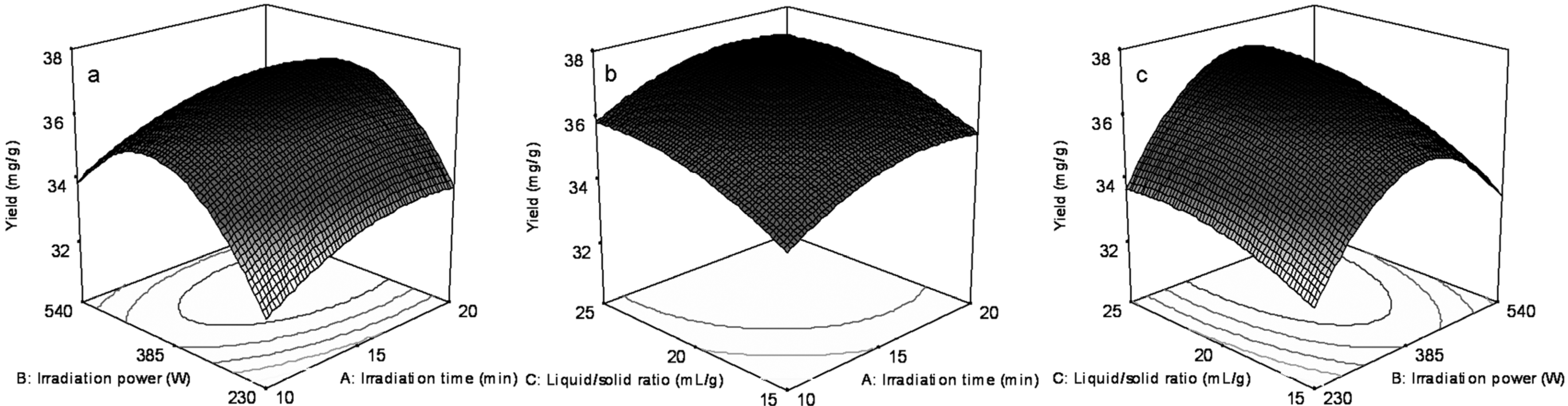
2.4. Verification Test under Optimum Condition
2.5. Method Validation
2.5.1. Stability Studies of Standard Stock Solutions of Salicin, Hyperin and Rutin in Methanol
| Indicators | Number | Compounds | ||
|---|---|---|---|---|
| Salicin | Hyperin | Rutin | ||
| Standard solution | 1 | 14.62 | 2.54 | 2.66 |
| 2 | 7.31 | 1.27 | 1.33 | |
| 3 | 1.462 | 0.254 | 0.266 | |
| Recovered concentration after 4 h (mg/mL) | 1 | 14.60 | 2.55 | 2.65 |
| 2 | 7.32 | 1.26 | 1.32 | |
| 3 | 1.462 | 0.253 | 0.267 | |
| RSD% (n = 5) | 1 | 1.11 | 0.97 | 0.96 |
| 2 | 0.98 | 0.98 | 0.98 | |
| 3 | 0.98 | 0.93 | 1.01 | |
| Average recovery of intra-day (%) | 1 | 99.86 | 100.39 | 99.62 |
| 2 | 100.14 | 99.21 | 99.25 | |
| 3 | 100.00 | 99.61 | 100.38 | |
| Recovered concentration after 5d (mg/mL) | 1 | 14.61 | 2.54 | 2.66 |
| 2 | 7.31 | 1.26 | 1.33 | |
| 3 | 1.463 | 0.254 | 0.265 | |
| RSD% (n = 5) | 1 | 1.05 | 0.99 | 0.97 |
| 2 | 0.97 | 0.97 | 0.98 | |
| 3 | 1.00 | 0.97 | 0.99 | |
| Average recovery of inter-day (%) | 1 | 99.93 | 100.00 | 100.00 |
| 2 | 100.00 | 99.21 | 100.00 | |
| 3 | 100.07 | 100.00 | 99.62 | |
2.5.2. Stability Studies of Salicin, Hyperin and Rutin Standards under the ILVMAE Conditions
| Compounds | Initial Concentration (mg/mL) | Intra-day | Inter-day | ||||
|---|---|---|---|---|---|---|---|
| Recovered Concentration (mg/mL) | RSD% | Average Recovery (%) | Recovered Concentration (mg/mL) | RSD% | Average Recovery (%) | ||
| Salicin | 1.46 | 1.43 | 0.98 | 97.9 | 1.42 | 0.99 | 97.1 |
| Hyperin | 0.25 | 0.26 | 1.02 | 102.0 | 0.24 | 0.95 | 96.8 |
| Rutin | 0.27 | 0.26 | 0.97 | 97.1 | 0.26 | 0.97 | 94.8 |
2.5.3. Recovery
| Sample | Spiked Mean Mass (mg) | Mean Mass in Sample (before Addition) (mg) | Detected Mean Mass (after Addition) (mg) | Recovery (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salicin | Hyperin | Rutin | Salicin | Hyperin | Rutin | Salicin | Hyperin | Rutin | Salicin | Hyperin | Rutin | |
| 1 | 0.5 | 0.05 | 0.1 | 1.12 | 0.13 | 0.22 | 1.56 | 0.18 | 0.31 | 96.3 | 100.0 | 96.9 |
| 2 | 1.0 | 0.1 | 0.2 | 1.12 | 0.13 | 0.22 | 2.17 | 0.24 | 0.42 | 102.4 | 104.3 | 100.0 |
| 3 | 1.5 | 0.15 | 0.3 | 1.12 | 0.13 | 0.22 | 2.73 | 0.27 | 0.53 | 104.2 | 96.4 | 101.9 |
| Average | 101.0 | 100.2 | 99.6 | |||||||||
2.5.4. Repeatability
| Repeat Number | Yield (mg/g) | ||
|---|---|---|---|
| Salicin | Hyperin | Rutin | |
| 1 (1st day) | 36.551 | 1.388 | 2.359 |
| 2 (1st day) | 33.922 | 1.296 | 2.326 |
| 3 (2nd day) | 35.663 | 1.341 | 2.342 |
| 4 (3rd day) | 34.548 | 1.363 | 2.337 |
| 5 (5th day) | 36.916 | 1.212 | 2.586 |
| Average | 35.52 | 1.32 | 2.39 |
| RSD (%) | 3.6 | 5.2 | 4.6 |
2.6. Comparison of ILVMAE with Other Methods
| Number | Method | Extraction Time (min) | Extraction Yield ± SD (mg/g) | |||
|---|---|---|---|---|---|---|
| Salicin | Hyperin | Rutin | Total | |||
| 1 | ILVMAE | 15 | 35.53 ± 1.40 | 1.32 ± 0.04 | 2.40 ± 0.15 | 39.25 ± 1.59 |
| 2 | ILMAE | 15 | 28.43 ± 1.14 | 0.96 ± 0.04 | 3.12 ± 0.14 | 32.51 ± 1.32 |
| 3 | ILHRE | 120 | 24.49 ± 1.03 | 0.92 ± 0.03 | 2.86 ± 0.12 | 28.27 ± 1.18 |
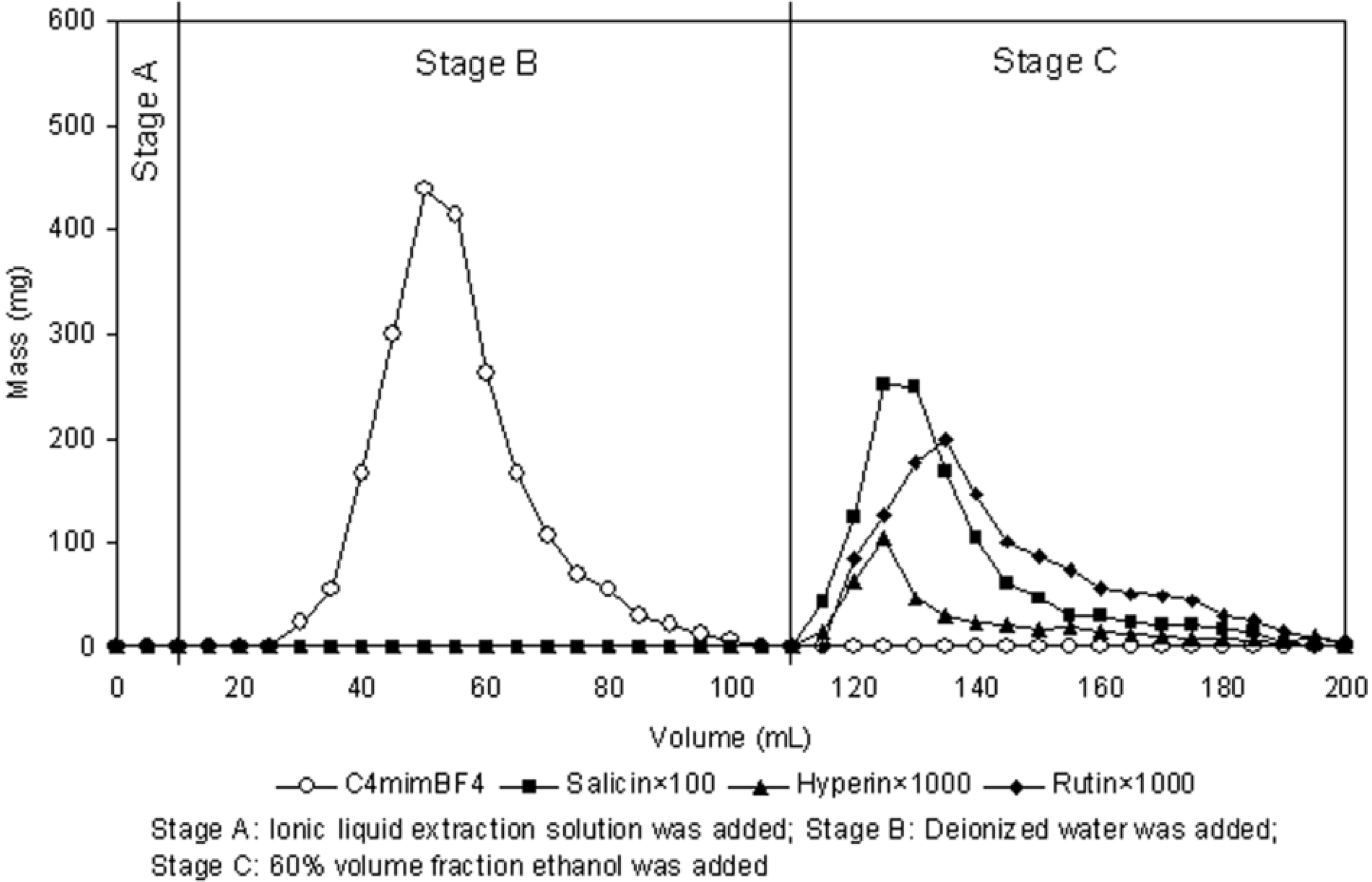
2.7. Separation of Target Analytes from Ionic Liquid Extraction Solution
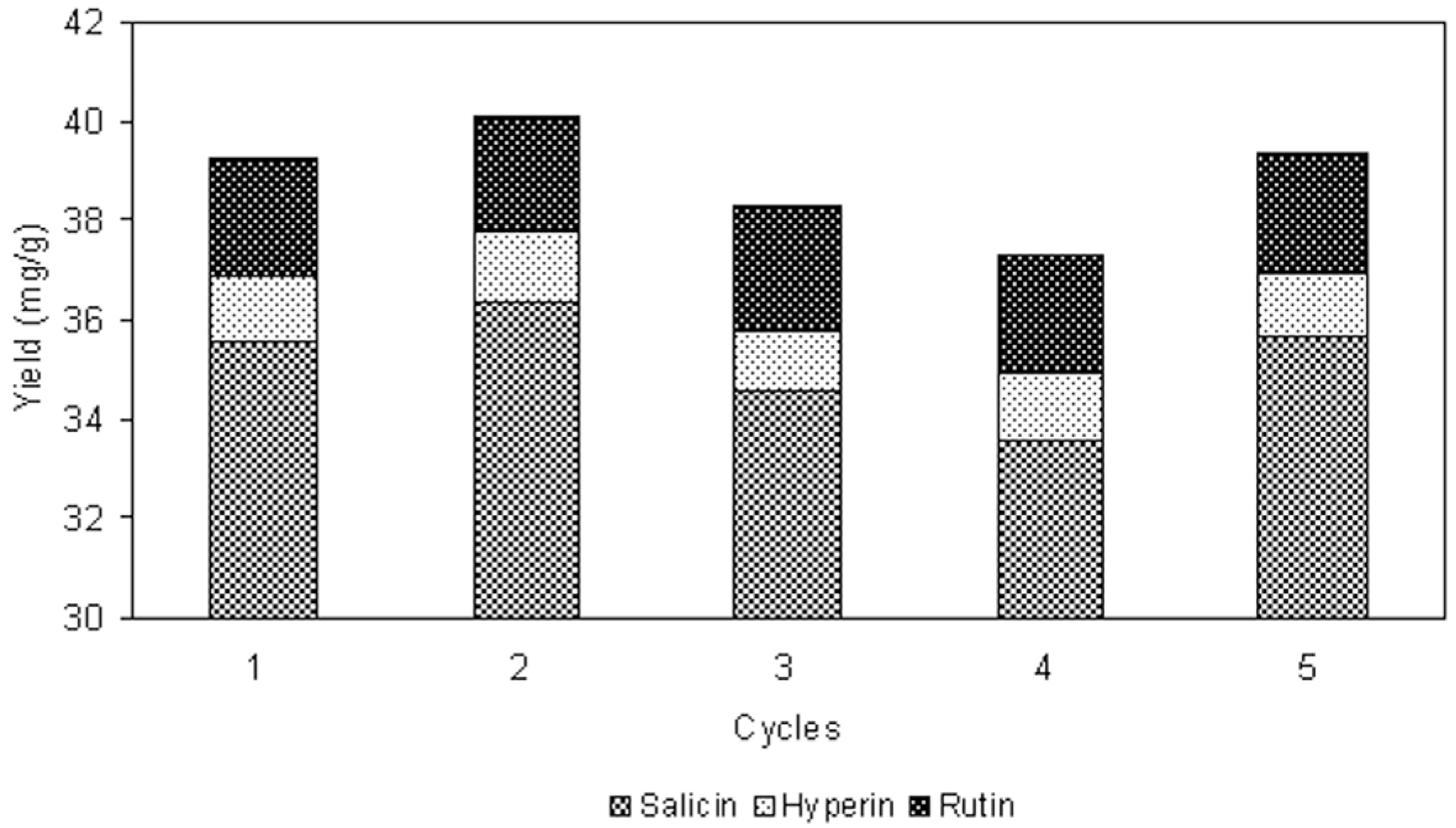
2.8. Recovery and Recycling of Ionic Liquid
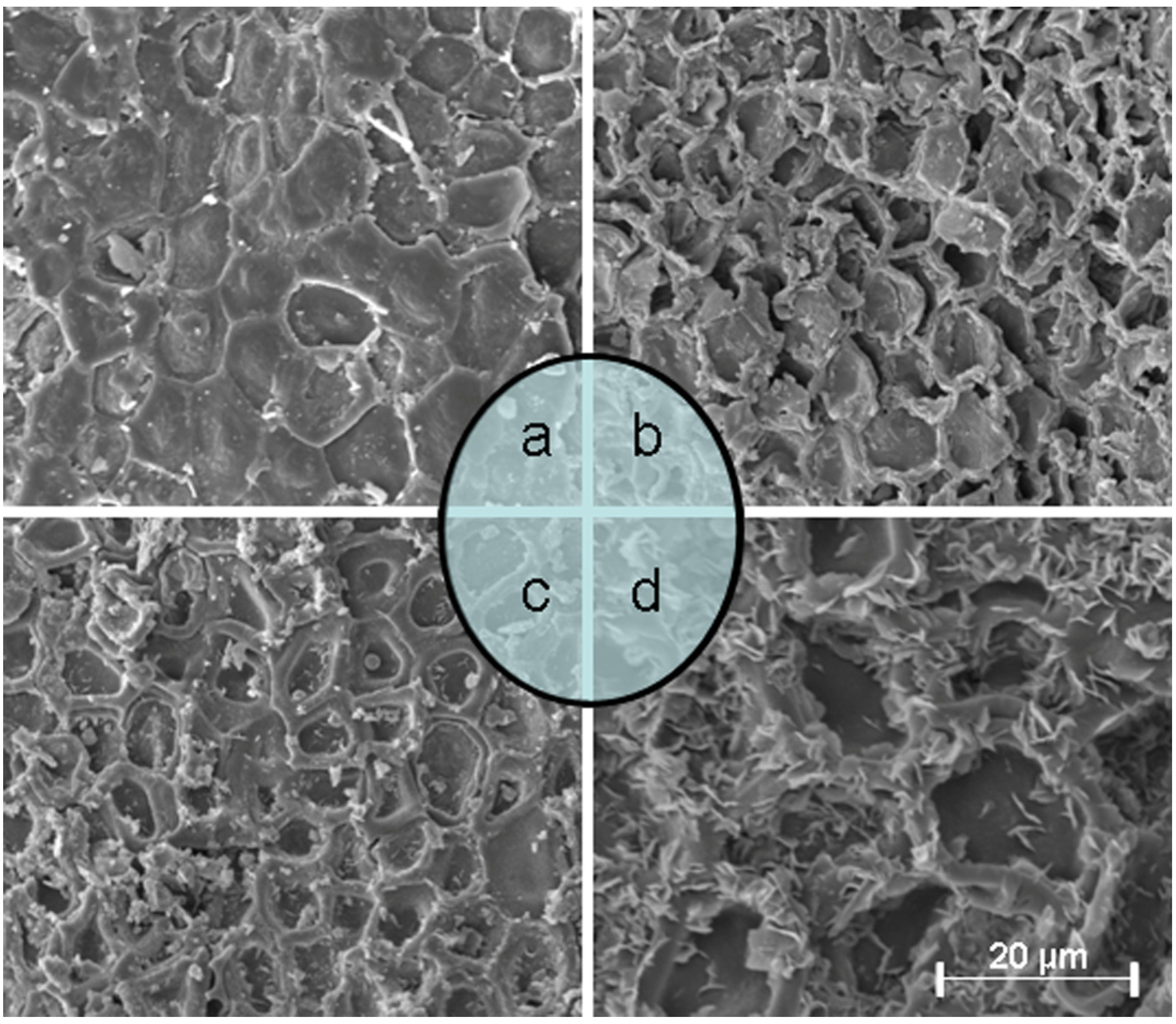
2.9. Structural Changes after Extraction
3. Experimental Section
3.1. Materials and Chemicals
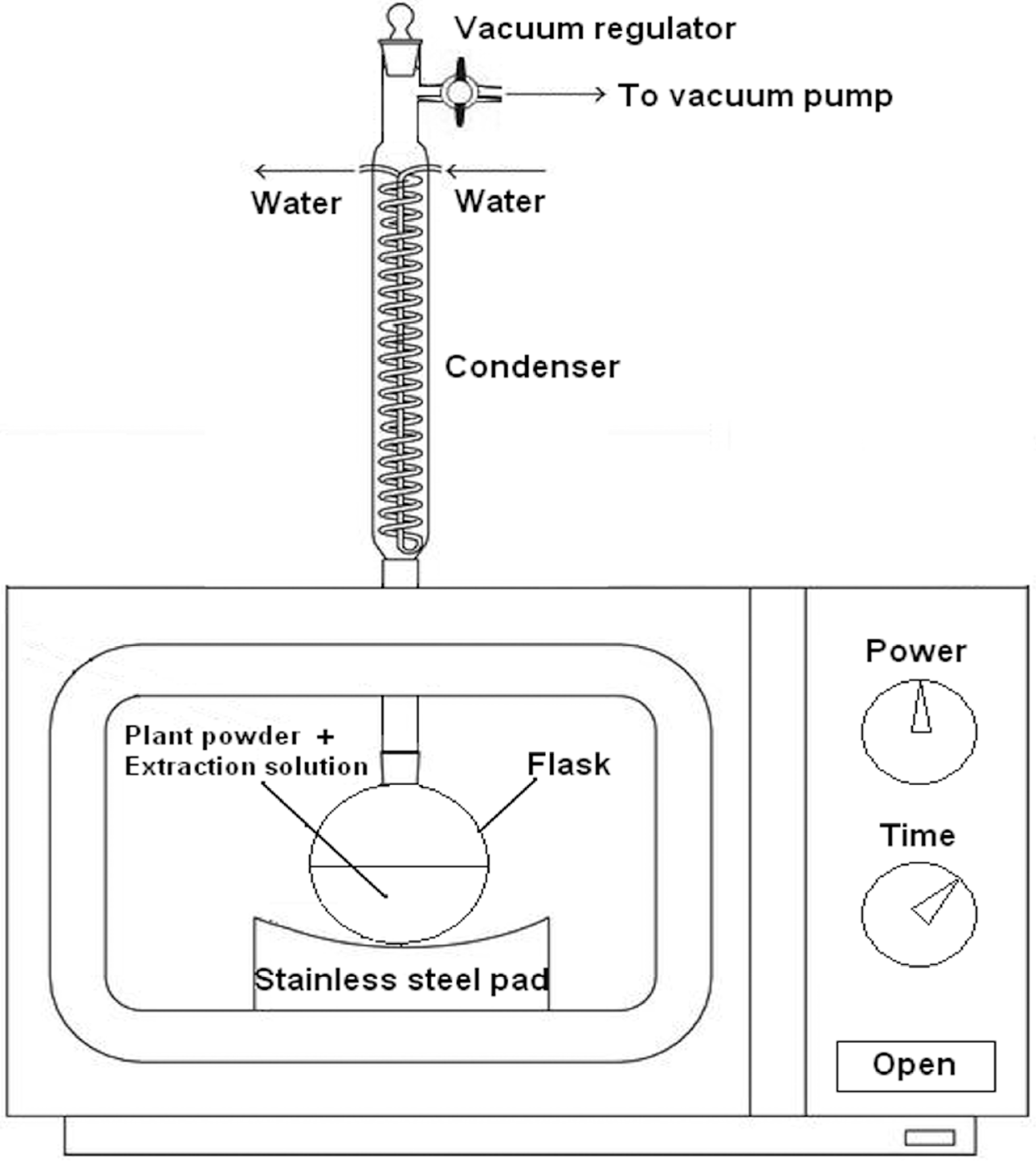
3.2. Apparatus
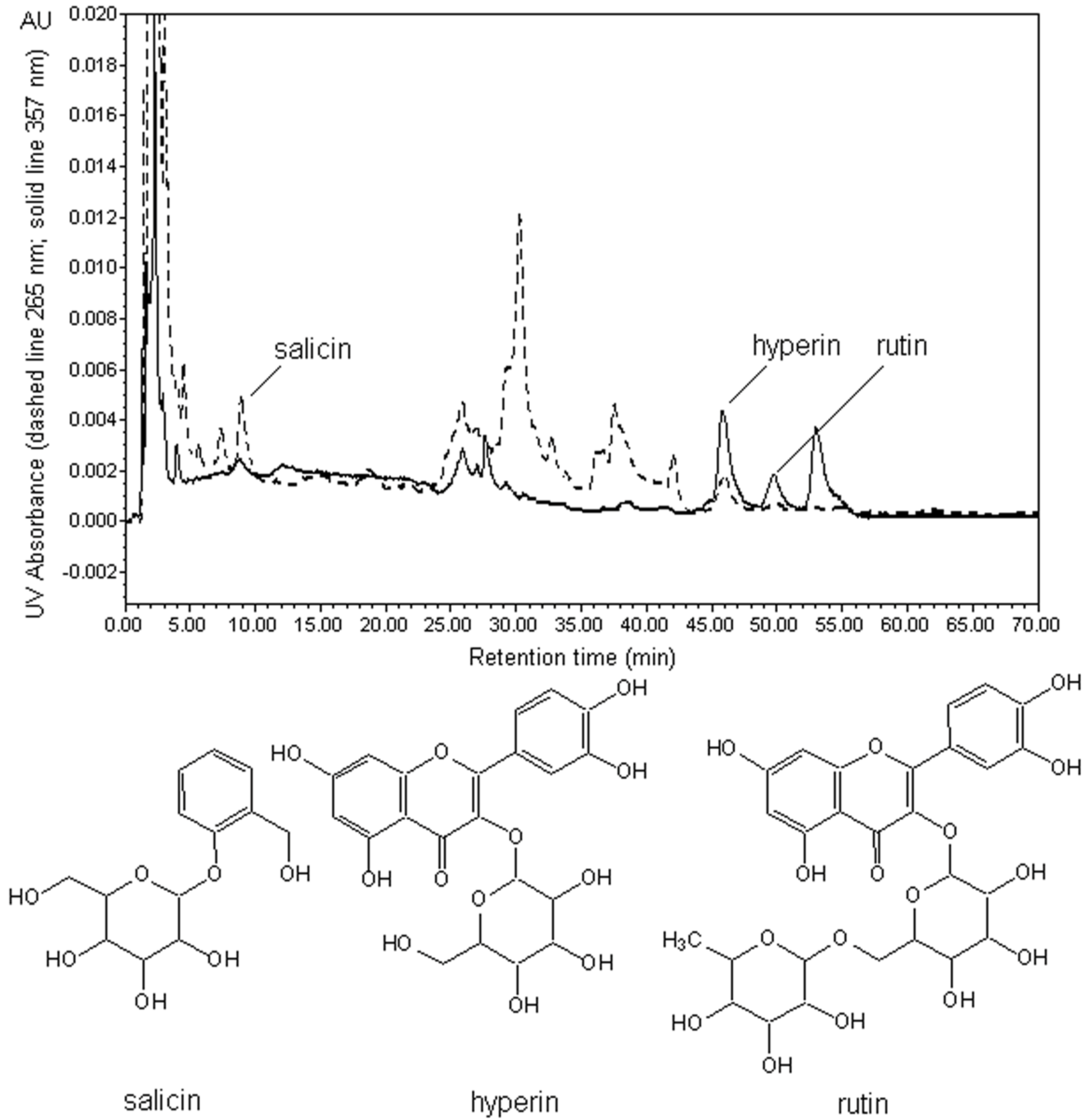
3.3. HPLC Analysis and Quantification
3.3.1. Preparation of Standard Solutions of Salicin, Hyperin and Rutin
3.3.2. Stability Test of Standard Mixtures
3.3.3. HPLC analytical Conditions
3.4. Ionic Liquid Vacuum Microwave-Assisted Extraction (ILVMAE)

3.5. Optimization of ILVMAE by RSM
3.6. Stability and Repeatability of ILVMAE
3.7. Comparison of ILVMAE with Reference Extraction Methods
3.8. Separation of Salicin, Hyperin and Rutin from Ionic Liquid Extraction Solution
3.9. SEM
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, X.; Wang, Q.; Xu, G.J.; Xu, L. Advances in the research of constituents and pharmacology of Populus L. Nat. Prod. Res. Develop. 1999, 11, 65–74. [Google Scholar]
- Zhang, C.; Zheng, H.; Liu, G.; Hu, F. Development and validation of HPLC method for determination of salicin in poplar buds: Application for screening of counterfeit propolis. Food Chem. 2011, 127, 345–350. [Google Scholar]
- Li, M.M.; Liu, A.T.; Zou, C.J.; Xu, W.D.; Shimizu, H.; Wang, K.Y. An overview of the “Three-North” Shelterbelt project in China. For. Stud. China 2012, 14, 70–79. [Google Scholar]
- Clausen, T.P.; Reichardt, P.B.; Bryant, J.P.; Werner, R.A.; Post, K.; Frisby, K. Chemical model for short-term induction in quaking aspen (Populus tremuloides) foliage against herbivores. J. Chem. Ecol. 1989, 15, 2335–2346. [Google Scholar]
- Nan, Y.; Zhou, L. Comparative study: Salicin and rutin contents in propolis, and poplar leaves and buds. World Sci. Tech-Moder. Tradit. Chin. Med. Mater. Med. 2008, 10, 59–61. [Google Scholar]
- Albrecht, M.; Nahrstedt, A.; Wray, V. Isolation, structure elucidation, and HPLC analysis of flavonol glycosides from leaves of Populus tremuloides and P. Tremula. Planta Med. 1989, 55, 611. [Google Scholar]
- Du, Q.; Jerz, G.; He, Y.; Li, L.; Xu, Y.; Zhang, Q.; Zheng, Q.; Winterhalter, P.; Ito, Y. Semi-industrial isolation of salicin and amygdalin from plant extracts using slow rotary counter-current chromatography. J. Chromatogr. A. 2005, 1074, 43–46. [Google Scholar]
- Zaugg, S.E.; Cefalo, D.; Walker, E.B. Capillary electrophoretic analysis of salicin in Salix spp. J. Chromatogr. A. 1997, 781, 487–490. [Google Scholar]
- Kenstavièienë, P.; Nenortienë, P.; Kiliuvienë, G.; Ževžikovas, A.; Lukošius, A.; Kazlauskiene, D. Application of high-performance liquid chromatography for research of salicin in bark of different varieties of Salix. Medicina (Kaunas) 2009, 45, 644–651. [Google Scholar]
- Akao, T.; Yoshino, T.; Kobashi, K.; Hattori, M. Evaluation of salicin as an antipyretic prodrug that does not cause gastric injury. Planta Med. 2002, 68, 714–718. [Google Scholar]
- Afanas'eva, I.B.; Ostrakhovitch, E.A.; Mikhal'chik, E.V.; Ibragimova, G.A.; Korkina, L.G. Enhancement of antioxidant and anti-inflammatory activities of bioflavonoid rutin by complexation with transition metals. Biochem. Pharmcol. 2001, 61, 677–684. [Google Scholar]
- Erlund, I.; Alfthan, G.; Mäenpää, J.; Aro, A. Tea and coronary heart disease: The flavonoid quercetin is more bioavailable from rutin in women than in men. Arch. Intern. Med. 2001, 161, 1919–1920. [Google Scholar]
- Chen, W.M.; Jin, M.; Wu, W. Experimental study on inhibitory effect of rutin against platelet activation induced by platelet activating factor in rabbits. Chin. J. Integr. Med. 2012, 22, 283–285. [Google Scholar]
- Shukla, P.; Gopalkrishna, B.; Shukla, P. Isolation of rutin from Phyllanthus amarus. Int. J. Pharm. Sci. Res. 2012, 3, 1198–1201. [Google Scholar]
- Lee, S.; Jung, S.H.; Lee, Y.S.; Yamada, M.; Kim, B.K.; Ohuchi, K.; Shin, K.H. Antiinflammatory activity of hyperin from Acanthopanax chiisanensis roots. Arch. Pharm. Res. 2004, 27, 628–632. [Google Scholar]
- Kim, S.J.; Um, J.Y.; Lee, J.Y. Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-êB activation in mouse peritoneal macrophages. Am. J. Chin. Med. 2011, 39, 171–181. [Google Scholar]
- Piao, M.J.; Kang, K.A.; Zhang, R.; Ko, D.O.; Wang, Z.H.; You, H.J.; Kim, H.S.; Kim, J.S.; Kang, S.S.; Hyun, J.W. Hyperoside prevents oxidative damage induced by hydrogen peroxide in lung fibroblast cells via an antioxidant effect. Biochim. Biophys. Acta 2008, 1780, 1448–1457. [Google Scholar]
- Xing, H.Y.; Liu, Y.; Chen, J.H.; Sun, F.J.; Shi, H.Q.; Xia, P.Y. Hyperoside attenuates hydrogen peroxide-induced L02 cell damage via MAPK-dependent Keap1-Nrf2-ARE signaling pathway. Biochem. Biophys. Res. Commun. 2011, 410, 759–765. [Google Scholar]
- Wu, L.L.; Yang, X.B.; Huang, Z.M.; Liu, H.Z.; Wu, G.X. In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) Medik. Acta Pharm. Sin. 2007, 28, 404–409. [Google Scholar]
- Haas, J.S.; Stolz, E.D.; Betti, A.H.; Stein, A.C.; Schripsema, J.; Poser, G.L.; Rates, S.M. The anti-immobility effect of hyperoside on the forced swimming test in rats is mediated by the D2-like receptors activation. Planta Med. 2011, 77, 334–339. [Google Scholar]
- Zheng, M.; Liu, C.; Pan, F.; Shi, D.; Zhang, Y. Antidepressant-like effect of hyperoside isolated from Apocynum venetum leaves: Possible cellular mechanisms. Phytomedicine 2012, 19, 145–149. [Google Scholar]
- Zhang, X.N.; Li, J.M.; Yang, Q.; Feng, B.; Liu, S.B.; Xu, Z.H.; Guo, Y.Y.; Zhao, M.G. Anti-apoptotic effects of hyperoside via inhibition of NR2B-containing NMDA receptors. Pharmacol. Reports 2010, 62, 949–955. [Google Scholar]
- Choi, J.H.; Kim, D.W.; Yun, N.; Choi, J.S.; Islam, M.N.; Kim, Y.S.; Lee, S.M. Protective effects of hyperoside against carbon tetrachloride-induced liver damage in mice. J. Nat. Prod. 2011, 74, 1055–1060. [Google Scholar]
- Li, Z.L.; Liu, J.C.; Hu, J.; Li, X.Q.; Wang, S.W.; Yi, D.H.; Zhao, M.G. Protective effects of hyperoside against human umbilical vein endothelial cell damage induced by hydrogen peroxide. J. Ethnopharmacol. 2012, 139, 388–394. [Google Scholar]
- Liu, T.; Sui, X.; Zhang, R.; Yang, L.; Zu, Y.; Zhang, L.; Zhang, Y.; Zhang, Z. Application of ionicliquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from Rosmarinus officinalis. J. Chromatogr. A 2011, 1218, 8480–8489. [Google Scholar]
- Chen, F.L.; Hou, K.X.; Li, S.Y.; Zu, Y.G.; Yang, L. Extraction and Chromatographic Determination of Shikimic Acid in Chinese Conifer Needles with 1-Benzyl-3-methylimidazolium Bromide Ionic Liquid Aqueous Solutions. J. Anal. Methods Chem. 2014, 2014, 1–12. [Google Scholar]
- Yang, L.; Wang, H.; Zu, Y.; Zhao, C.; Zhang, L.; Chen, X.; Zhang, Z. Ultrasound-assisted extraction of the three terpenoid indole alkaloids vindoline, catharanthine and vinblastine using ionic liquid solution from Catharanthus roseus. Chem. Eng. J. 2011, 172, 705–712. [Google Scholar]
- Ma, C.; Wang, S.; Yang, L.; Zu, Y. Ionic liquid-based ultrasonic-assisted extraction of camptothecin and 10-hydroxycamptothecin from samara of Camptotheca acuminate. Chem. Eng. Pro. 2012, 57–58, 59–64. [Google Scholar]
- Lertlapwasin, R.; Bhawawet, N.; Imyim, A.; Fuangswasdi, S. Ionic liquid extraction of heavy metal ions by 2-aminothiophenol in 1-butyl-3-methylimidazolium hexafluorophosphate and their association constants. Sep. Purif. Technol. 2010, 72, 70–76. [Google Scholar]
- Li, X.Q.; Guo, R.L.; Zhang, X.P.; Li, X.Y. Extraction of glabridin using imidazolium-based ionic liquids. Sep. Purif. Technol. 2012, 88, 146–150. [Google Scholar]
- Yang, L.; Li, L.; Liu, T.; Zu, Y.; Yang, F.; Zhao, C.; Zhang, L.; Chen, X.; Zhang, Z. Development of sample preparation method for isoliquiritigenin, liquiritin, and glycyrrhizic acid analysis in licorice by ionic liquids- ultrasound based extraction and high-performance liquid chromatography detection. Food Chem. 2013, 138, 173–179. [Google Scholar]
- Matsumoto, M.; Mochiduki, K.; Fukunishi, K.; Kondo, K. Extraction of organic acids using imidazolium-based ionic liquids and their toxicity to Lactobacillus rhamnosus. Sep. Purif. Technol. 2004, 40, 97–101. [Google Scholar]
- Yang, L.; Ge, H.; Wang, W.; Zu, Y.; Yang, F.; Zhao, C.; Zhang, L.; Zhang, Y. Development of sample preparation method for eleutheroside B and E analysis in Acanthopanax senticosus by ionic liquids- ultrasound based extraction and high-performance liquid chromatography detection. Food Chem. 2013, 141, 2426–2433. [Google Scholar]
- Ma, C.; Liu, T.; Yang, L.; Zu, Y.; Wang, S.; Zhang, R. Study on ionic liquid based ultrasonic-assisted extraction of biphenyl cyclooctene lignans from the fruit of Schisandra chinensis Baill. Anal. Chim. Acta 2011, 689, 110–116. [Google Scholar]
- Liu, Y.; Yang, L.; Zu, Y.; Zhao, C.; Zhang, L.; Zhang, Y.; Zhang, Z.; Wang, W. Development of an ionic liquid-based microwave-assisted method for simultaneous extraction and distillation for determination of proanthocyanidins and essential oil in Cortex cinnamomi. Food Chem. 2012, 135, 2514–2521. [Google Scholar]
- Wang, J.X.; Xiao, X.H.; Li, G.K. Study of vacuum microwave-assisted extraction of polyphenolic compounds and pigment from Chinese herbs. J. Chromatogr. A 2008, 1198–1199, 45–53. [Google Scholar]
- Xiao, X.H.; Wang, J.H.; Wang, G.; Wang, J.Y.; Li, G.K. Evaluation of vacuum microwave-assisted extraction technique for the extraction of antioxidants from plant samples. J. Chromatogr. A 2009, 1216, 8867–8873. [Google Scholar]
- Hu, Y.; Li, Y.; Zhang, Y.; Li, G.; Chen, Y. Development of sample preparation method for auxin analysis in plants by vacuum microwave-assisted extraction combined with molecularly imprinted clean-up procedure. Anal. Bioanal. Chem. 2011, 399, 3367–3374. [Google Scholar]
- Lou, Z.; Er, C.; Li, J.; Wang, H.; Zhu, S.; Sun, J.T. Removal of caffeine from green tea by microwave-enhanced vacuum ice water extraction. Anal. Chim. Acta 2012, 716, 49–53. [Google Scholar]
- Xiao, X.; Song, W.; Wang, J.; Li, G. Microwave-assisted extraction performed in low temperature and in vacuo for the extraction of labile compounds in food samples. Anal. Chim. Acta 2012, 71, 285–293. [Google Scholar]
- Design-Expert software; Version 7.0; Stat-Ease Inc.: Minneapolis, MN, USA, 2005.
- Yang, F.; Yang, L.; Wang, W.; Liu, Y.; Zhao, C.; Zu, Y. Enrichment and purification of syringin, eleutheroside E and isofraxidin from Acanthopanax senticosus by macroporous resin. Inter. J. Mol. Sci. 2012, 13, 8970–8986. [Google Scholar]
- Ma, C.; Liu, T.; Yang, L.; Zu, Y.; Chen, X.; Zhang, L.; Zhang, Y.; Zhao, C. Ionic liquid based microwave simultaneous extraction of essential oil and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J. Chromatogr. A 2011, 1218, 8573–8580. [Google Scholar]
- Wu, K.; Zhang, Q.; Liu, Q.; Tang, F.; Long, Y.; Yao, S. Ionic liquid surfactant-mediated ultrasonic-assisted extraction coupled to HPLC: Application to analysis of tanshinones in Salvia miltiorrhiza bunge. J. Sep. Sci. 2009, 32, 4220–4226. [Google Scholar]
- Ma, C.; Liu, T.; Yang, L.; Zu, Y.; Yang, F.; Zhao, C.; Zhang, L.; Zhang, Z. Preparation of high purity biphenyl cyclooctene lignans from Schisandra extract by ion exchange resin catalytic transformation combined with macroporous resin separation. J. Chromatogr. B 2011, 879, 3444–3451. [Google Scholar]
- Yang, F.; Ma, C.; Yang, L.; Zhao, C.; Zhang, Y.; Zu, Y. Enrichment and purification of deoxyschizandrinand ã-schizandrin from the extract of Schisandra chinensis fruit by macroporous resins. Molecules 2012, 17, 3510–3523. [Google Scholar]
- Wu, J.; Yang, Z. Separation and purification of hyperin from Folium Crataegi by macroporous adsorption resin. Strait Pharmaceutical J. 2007, 19, 53–55. [Google Scholar]
- Duan, H.; Zhai, K.; Gao, G.; Cao, W. Macroporous resin adsorption for purification of salicin from Alangium chinense (Lour.) Harms. Food Sci 2012, 33, 99–102. [Google Scholar]
- Liu, J.; Wu, X.; Shang, X.; Liu, X.; Chen, X. Study on the effect of separation and purification of rutin from tartary buckwheat with different macroporous resin. Food Res. Dev. 2008, 29, 22–24. [Google Scholar]
- Chen, F.; Li, T.; Li, S.; Hou, K.; Liu, Z.; Li, L.; Cui, G.; Zu, Y.; Yang, L. Preparation and characterization of Tripterygium wilfordii multi-glycoside nanoparticle using supercritical anti-solvent process. Int. J. Mol. Sci. 2014, 15, 2695–2711. [Google Scholar]
- Sample Availability: Samples Not Available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Mo, K.; Liu, Z.; Yang, F.; Hou, K.; Li, S.; Zu, Y.; Yang, L. Ionic Liquid-Based Vacuum Microwave-Assisted Extraction Followed by Macroporous Resin Enrichment for the Separation of the Three Glycosides Salicin, Hyperin and Rutin from Populus Bark. Molecules 2014, 19, 9689-9711. https://doi.org/10.3390/molecules19079689
Chen F, Mo K, Liu Z, Yang F, Hou K, Li S, Zu Y, Yang L. Ionic Liquid-Based Vacuum Microwave-Assisted Extraction Followed by Macroporous Resin Enrichment for the Separation of the Three Glycosides Salicin, Hyperin and Rutin from Populus Bark. Molecules. 2014; 19(7):9689-9711. https://doi.org/10.3390/molecules19079689
Chicago/Turabian StyleChen, Fengli, Kailin Mo, Zhaizhi Liu, Fengjian Yang, Kexin Hou, Shuangyang Li, Yuangang Zu, and Lei Yang. 2014. "Ionic Liquid-Based Vacuum Microwave-Assisted Extraction Followed by Macroporous Resin Enrichment for the Separation of the Three Glycosides Salicin, Hyperin and Rutin from Populus Bark" Molecules 19, no. 7: 9689-9711. https://doi.org/10.3390/molecules19079689
APA StyleChen, F., Mo, K., Liu, Z., Yang, F., Hou, K., Li, S., Zu, Y., & Yang, L. (2014). Ionic Liquid-Based Vacuum Microwave-Assisted Extraction Followed by Macroporous Resin Enrichment for the Separation of the Three Glycosides Salicin, Hyperin and Rutin from Populus Bark. Molecules, 19(7), 9689-9711. https://doi.org/10.3390/molecules19079689




