Polyphenolic Content, Antioxidant and Antimicrobial Activities of Lycium barbarum L. and Lycium chinense Mill. Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC Analysis of Polyphenols
| Polyphenolic Compound | m/z | RT ± SD (min) | L. barbarum | L. chinense |
|---|---|---|---|---|
| Gentisic acid | 179 | 3.52 ± 0.04 | <0.02 | NF |
| Caffeic acid | 179 | 5.60 ± 0.04 | <0.02 | NF |
| Chlorogenic acid | 353 | 5.62 ± 0.05 | 5899.29 ± 4.46 | 12045.96 ± 9.25 |
| p-Coumaric acid | 163 | 9.48 ± 0.08 | 30.29 ± 0.23 | 54.97 ± 0.43 |
| Ferulic acid | 193 | 12.8 ± 0.10 | <0.02 | 112.25 ± 0.87 |
| Isoquercitrin | 463 | 19.60 ± 0.10 | 25.08 ± 0.72 | 20.46 ± 0.21 |
| Rutin | 609 | 20.20 ± 0.15 | 5646.66 ± 3.32 | 16205.28 ± 8.09 |
| Quercitrin | 447 | 23.64 ± 0.13 | 13.00 ± 0.12 | 5.52 ± 0.07 |
| Quercetin | 301 | 26.80 ± 0.15 | 5.59 ± 0.06 | 4.49 ± 0.05 |
| Kaempferol | 285 | 32.48 ± 0.17 | NF | 2.83 ± 0.03 |
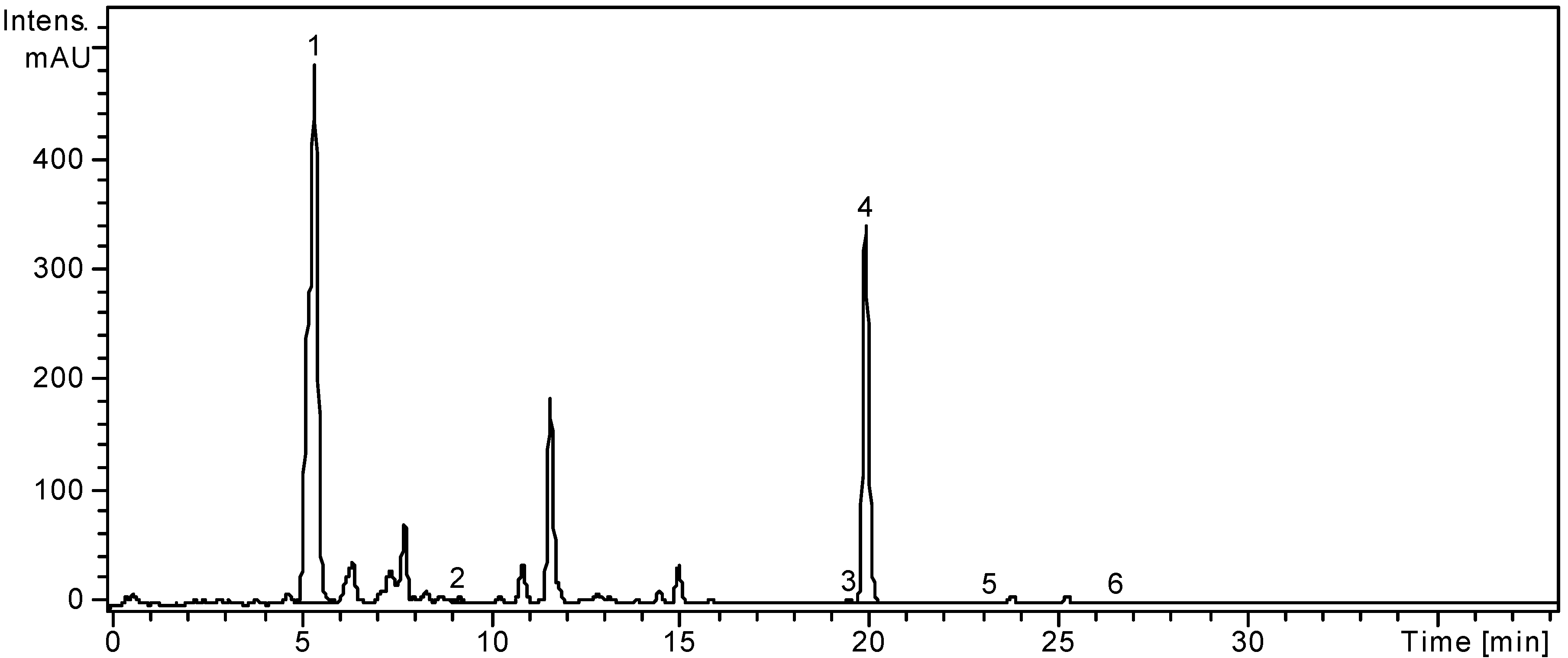
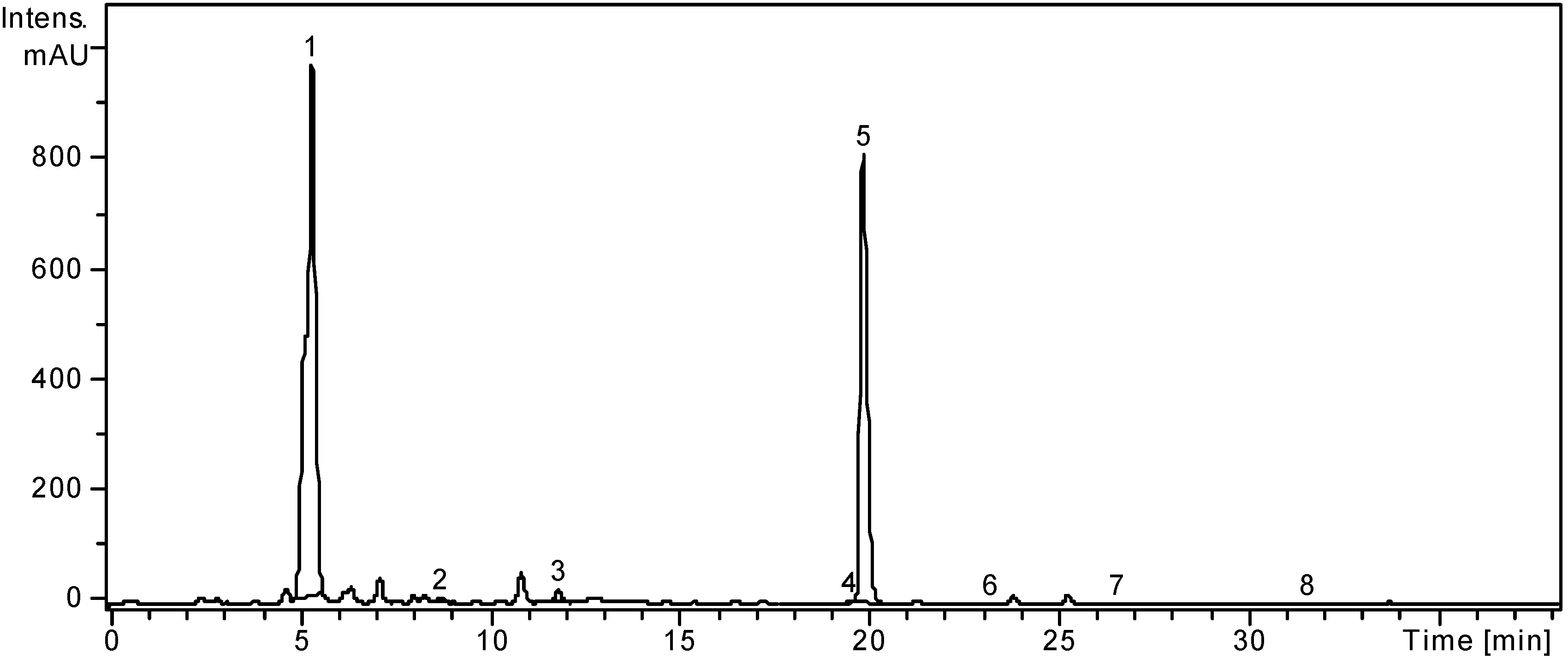
2.2. Determination of Phenolic Compounds Content
| Samples | TPC (mg GAE/g Plant Material) | Flavonoids (mg RE/g Plant Material) | Caffeic Acid Derivatives (mg CAE/g Plant Material) |
|---|---|---|---|
| L. barbarum | 61.59 ± 1.68 | 43.73 ± 1.43 | 16.95 ± 0.57 |
| L. chinense | 80.64 ± 2.02 | 61.65 ± 0.95 | 18.80 ± 0.61 |
2.3. Antioxidant Activity
| Samples | DPPH (µg QE/mg Plant Material) | TEAC (µg TE/mg Plant Material) | HAPX (%) |
|---|---|---|---|
| L. barbarum | 29.30 ± 4.34 | 35.72 ± 6.29 | 29.69 ± 2.21 |
| L. chinense | 36.80 ± 0.65 | 55.95 ± 0.88 | 40.86 ± 2.21 |
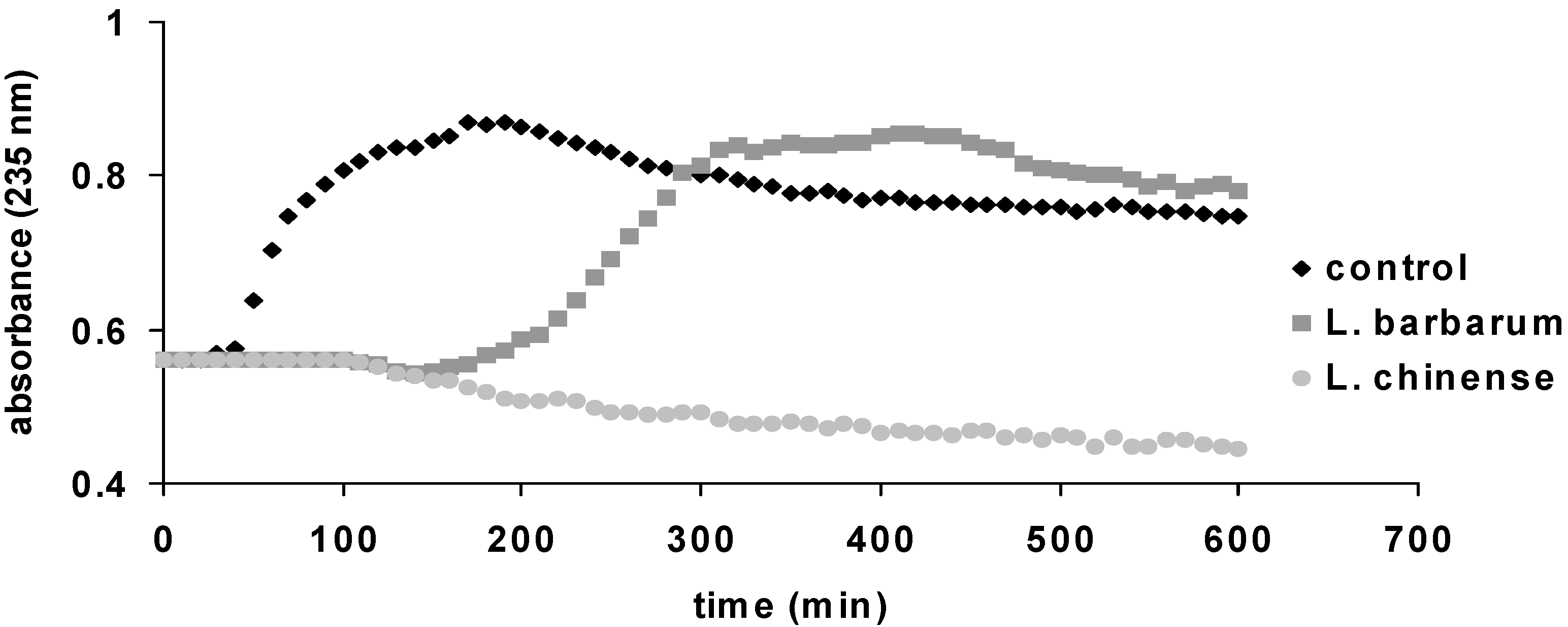
2.4. Antimicrobial Activity
| Bacterial Strains | Standard Antibiotic | Inhibition Zone (mm) | |
|---|---|---|---|
| Gentamicin | L. barbarum | L. chinense | |
| Staphylococcus aureus | 9.1 ± 0.9 | 13.1 ± 0.9 | 12.1 ± 0.9 |
| Bacillus subtilis | 17.2 ± 0.8 | 17.2 ± 0.6 | 24.2 ± 0.6 |
| Listeria monocytogenes | 12.3 ± 0.8 | 13.1 ± 0.3 | 21.6 ± 0.8 |
| Escherichia coli | 12.3 ± 0.9 | 12.3 ± 0.8 | 14.5 ± 0.4 |
| Salmonella typhimurium | 15.1 ± 0.8 | 12.4 ± 0.7 | 19.6 ± 0.3 |
| Bacterial Strains | MIC (µg/mL) | |
|---|---|---|
| L. barbarum | L. chinense | |
| Staphylococcus aureus | >100 | >100 |
| Bacillus subtilis | 100 | 75 |
| Listeria monocytogenes | >100 | >100 |
| Escherichia coli | 100 | 75 |
| Salmonella typhimurium | 75 | 50 |
3. Experimental Section
3.1. Plant Materials and Extraction Procedure
3.2. Chemicals and Instrumentation
3.3. HPLC-MS Analysis
3.3.1. Apparatus and Chromatographic Conditions for the Analysis of Polyphenols
| Peak No. | Phenolic Compound | m/z | RT ± SD | Peak No. | Phenolic Compound | m/z | RT ± SD |
|---|---|---|---|---|---|---|---|
| 1. | Caftaric acid | 311 | 3.54 ± 0.05 | 11. | Rutin | 609 | 20.76 ± 0.15 |
| 2. | Gentisic acid | 153 | 3.69 ± 0.04 | 12. | Myricetin | 317 | 21.13 ± 0.12 |
| 3. | Caffeic acid | 179 | 6.52 ± 0.04 | 13. | Fisetin | 285 | 22.91 ± 0.15 |
| 4. | Chlorogenic acid | 353 | 6.43 ± 0.05 | 14. | Quercitrin | 447 | 23.64 ± 0.13 |
| 5. | p-Coumaric acid | 163 | 9.48 ± 0.08 | 15. | Quercetin | 301 | 27.55 ± 0.15 |
| 6. | Ferulic acid | 193 | 12.8 ± 0.10 | 16. | Patuletin | 331 | 29.41 ± 0.12 |
| 7. | Sinapic acid | 223 | 15.00 ± 0.10 | 17. | Luteolin | 285 | 29.64 ± 0.19 |
| 8. | Cichoric acid | 473 | 15.96 ± 0.13 | 18. | Kaempferol | 285 | 32.48 ± 0.17 |
| 9. | Hyperoside | 463 | 19.32 ± 0.12 | 19. | Apigenin | 279 | 39.45 ± 0.15 |
| 10. | Isoquercitrin | 463 | 20.29 ± 0.10 |
3.3.2. Identification and Quantification of Polyphenols
3.4. Determination of Total Polyphenols, Flavonoids Content and Caffeic Acid Derivatives
3.5. In Vitro Antioxidant Activity Assays
3.5.1. DPPH Bleaching Assay
3.5.2. TEAC Assay (Trolox Equivalent Antioxidant Capacity)
3.5.3. Hemoglobin/Ascorbate Peroxidase Activity Inhibition (HAPX) Assay
3.5.4. Inhibition of Lipid Peroxidation Catalyzed by Cytochrome c
3.6. Determination of Antimicrobial Activity
3.6.1. Microorganisms and Culture Growth
3.6.2. Antimicrobial Activity Assay
3.6.3. Minimum Inhibitory Concentration
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Dahech, I.; Farah, W.; Trigui, M.; Hssouna, A.B.; Belghith, H.; Belghith, K.S.; Abdallah, F.B. Antioxidant and antimicrobial activities of Lycium shawii fruits extract. Int. J. Biol. Macromol. 2013, 60, 328–333. [Google Scholar] [CrossRef]
- Vlase, L.; Pârvu, M.; Pârvu, E.A.; Toiu, A. Chemical Constituents of Three Allium Species from Romania. Molecules 2013, 18, 114–127. [Google Scholar]
- Oliviera, C.B.S.; Meurer, Y.S.R.; Oliviera, M.G.; Medeiros, W.M.T.Q.; Silva, F.O.N.; Brito, A.C.F.; de Pontes, D.L.; Andrade-Neto, V.F. Comparative Study on the Antioxidant and Anti-Toxoplasma Activities of Vanilin and Its Resorcinarene Derivative. Molecules 2014, 19, 5898–5912. [Google Scholar]
- Hasnat, M.A.; Pervin, M.; Lim, B.O. acetylcholinesterase inhibition and in vitro and in vivo antioxidant activities of Ganoderma lucidum grown on germinated brown rice. Molecules 2013, 18, 6663–6678. [Google Scholar]
- Pratt, D.E. Phenolic Compounds in Food and Their Effects on Health II; American Chemical Society: Washington, DC, USA, 1992; pp. 352–391. [Google Scholar]
- Li, X.M.; Li, X.L.; Zhou, A.G. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur. Polym. J. 2007, 43, 488–497. [Google Scholar] [CrossRef]
- Serafini, M.; Bellocco, R.; Wolk, A.; Ekstrom, A.M. Total antioxidant potential of fruit and vegetables and risk of gastric cancer. Gastroenterology 2002, 123, 985–991. [Google Scholar] [CrossRef]
- Di Matteo, V.; Cacchio, M.; di Giulio, C.; Esposito, E. Role of serotonin 2C receptors in the control of brain dopaminergic function. Pharmacol. Biochem. Behav. 2002, 71, 727–734. [Google Scholar] [CrossRef]
- Pop, A.; Berce, C.; Bolfă, P.; Nagy, A.; Cătoi, C.; Dumitrescu, I.B.; Silaghi-Dumitrescu, L.; Loghin, F. Evaluation of the possible endocrine disruptive effect of butylated hydroxitoluene and propyl gallate in immature female rats. Farmacia 2013, 61, 202–211. [Google Scholar]
- Popa, D.S.; Bolfă, P.; Kiss, B.; Vlase, L.; Păltinean, R.; Pop, A.; Cătoi, C.; Crișan, G.; Loghin, F. Influence of Genista tinctoria L. or Methylparaben on Subcronic Toxicity of Bisphenol A in rats. Biomed. Environ. Sci. 2014, 27, 85–96. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar]
- Inbaraj, B.S.; Lu, H.; Kao, T.H.; Chen, B.H. Simultaneos determination of phenolic acids and flavonoids in Lycium barbarum Linnaeus by HPLC-DAD-ESI-MS. J. Pharm. Biomed. Anal. 2010, 51, 549–556. [Google Scholar]
- Li, X.-M. Protective effect of Lycium barbarum polysaccharides on streptozocin-induced oxidative stress in rats. Int. J. Biol. Macromol. 2007, 40, 461–465. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Liu, D.; Huang, A.-G. The efficiency of flavonoids in polar extracts of Lycium chinense Mill fruits as free radical scavenger. Food Chem. 2004, 87, 283–288. [Google Scholar]
- Fukuda, T.; Yokoyama, J.; Ohashi, H. Phylogeny and biogeography of the genus Lycium (Solanaceae): Inferences from chloroplast DNA sequences. Mol. Phylogenet. Evol. 2001, 19, 246–258. [Google Scholar]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar]
- Ciocârlan, V. Illustrated Flora of Romania. Pteridophyta et Spermatophyta; Ceres Publishing House: Bucharest, Romania, 2009; p. 709. [Google Scholar]
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 2009, 29, 19–25. [Google Scholar] [CrossRef]
- Jin, M.; Huang, Q.; Zhao, K.; Shang, P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013, 54, 16–23. [Google Scholar] [CrossRef]
- Dong, J.Z.; Lu, D.Y.; Wang, Y. Analysis of Flavonoids from Leaves of Cultivated Lycium barbarum L. Plant Foods Hum. Nutr. 2009, 64, 199–204. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Lu, H.; Hung, C.F.; Wu, W.B.; Lin, C.L.; Chen, B.H. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC–DAD–APCI–MS. J. Pharm. Biomed. Anal. 2008, 47, 812–818. [Google Scholar]
- Cheng, D.; Kong, H. The effect of Lycium Barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules 2011, 16, 2542–2550. [Google Scholar] [CrossRef]
- Cui, B.K.; Liu, Su.; Lin, X.J.; Wang, J.; Li, S.H.; Wang, Q.B.; Li, S.P. Effects of Lycium Barbarum aqueous and ethanol extracts on high-fat-diet induced oxidative stress in rat liver tissue. Molecules 2011, 16, 9116–9128. [Google Scholar] [CrossRef]
- Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar]
- Wu, S.; Wang, Y.; Gong, G.; Li, F.; Ren, H.; Liu, Y. Adsorbtion and desorption properties of macroporous resins for flavonoids from the extract of Chinese wolfberry (Lycium barbarum L.). Food Bioprod. Process. 2014. [Google Scholar] [CrossRef]
- Li, X.M.; Ma, Y.L.; Liu, X.J. Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. J. Ethnopharmacol. 2007, 111, 504–511. [Google Scholar]
- Yu, M.S.; Leung, S.K.Y.; Lai, S.W.; Che, C.M.; Zee, S.Y.; So, K.F.; Yuen, W.H.; Chang, R.C.C. Neuroprotective effects of anti-aging oriental medicine Lycium barbarum against b-amyloid peptide neurotoxicity. Exp. Gerontol. 2005, 40, 716–727. [Google Scholar] [CrossRef]
- Chan, H.C.; Chang, R.C.C.; Ip, A.K.C.; Chiu, K.; Yuen, W.H.; Zee, S.Y.; So, K.F. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp. Neurol. 2007, 203, 269–273. [Google Scholar] [CrossRef]
- Niu, A.J.; Wu, J.M.; Yu, D.H.; Wang, R. Protective effect of Lycium barbarum polysaccharides on oxidative stress damage in skeletal muscle of exhaustive exercise rats. Int. J. Biol. Macromol. 2008, 42, 447–449. [Google Scholar]
- Zhu, J.; Liu, W.; Yu, J.; Zou, S.; Wang, J.; Yao, W.; Gao, X. Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Corbohydr. Polym. 2013, 98, 8–16. [Google Scholar]
- Wu, H.T.; He, X.J.; Hong, Y.K.; Ma, T.; Xu, Y.P.; Li, H.H. Chemical characterization of Lycium barbarum polysaccharides and its inhibition against liver oxidative injury of high-fat-mice. Int. J. Biol. Macromol. 2010, 46, 540–543. [Google Scholar] [CrossRef]
- Ming, M.; Guanhua, L.; Zhanhai, Y.; Guang, C.; Xuan, Z. Effect of the Lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chem. 2009, 113, 872–877. [Google Scholar]
- Zhang, M.; Chen, H.; Huang, J.; Li, Z.; Zhu, C.; Zhang, S. Effect of lycium barbarum polysaccharide on human hepatoma QGY7703 cells: Inhibition of proliferation and induction of apoptosis. Life Sci. 2005, 76, 2115–2124. [Google Scholar]
- Liu, H.; Fan, Y.; Wang, W.; Liu, N.; Zhang, H.; Zhu, Z.; Liu, A. Polysaccharides from Lycium barbarum leaves: Isolation, characterization and splenocyte proliferation activity. Int. J. Biol. Macromol. 2012, 51, 417–422. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, X.; Wang, F.; Zhang, Q.; Zhang, Z. Characterization of Lycium barbarum polysaccharide and its effect on human hepatoma cells. Int. J. Biol. Macromol. 2013, 61, 270–275. [Google Scholar]
- Zhang, X.; Li, Y.; Cheng, J.; Liu, G.; Qi, C.; Zhou, W.; Zhang, Y. Immune activities comparison of polysaccharide and polysaccharide-protein complex from Lycium barbarum L. Int. J. Biol. Macromol. 2014, 65, 441–445. [Google Scholar]
- Wang, J.; Hu, Y.; Wang, D.; Zhang, F.; Zhao, X.; Abula, S.; Fan, Y.; Guo, L. Lycium barbarum polysaccharide inhibits the infectivity of Newcastle disease virus to chicken embryo fibroblast. Int. J. Biol. Macromol. 2010, 46, 212–216. [Google Scholar] [CrossRef]
- Shen, L.; Du, G. Lycium barbarum polysaccharide stimulates proliferation of MCF-7 cells by the ERK pathway. Life Sci. 2012, 91, 353–357. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhou, W.X.; Zhang, Y.X.; Qi, C.H.; Yan, H.; Wang, Z.F.; Wang, B. Macrophages, rather than T and B cells are principal immunostimulatory target cells of Lycium barbarum L. polysaccharide LBPF4-OL. J. Ethnopharmacol. 2011, 136, 465–472. [Google Scholar] [CrossRef]
- Wang, N.T.; Lin, H.I.; Yeh, D.Y.; Chou, T.Y.; Chen, C.F.; Leu, F.C.; Wang, D.; Hu, R.T. Effects of the antioxidants Lycium barbarum and ascorbic acid on Reperfusion Liver injury in rats. Transplant. Proc. 2009, 41, 4110–4113. [Google Scholar] [CrossRef]
- Lin, C.L.; Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Antioxidative activity of polysaccharide fractions isolated from Lycium barbarum Linnaeus. Int. J. Biol. Macromol. 2009, 45, 146–151. [Google Scholar] [CrossRef]
- Liang, B.; Jin, M.; Liu, H. Water-soluble polysaccharides from dried Lycium barbarum fruits: Isolation, structural features and antioxidant activity. Carbohydr. Polym. 2011, 83, 1947–1951. [Google Scholar] [CrossRef]
- Jiang, L.F. Preparation and antioxidant activity of Lycium barbarum oligosaccharides. Carbohydr. Polym. 2014, 99, 646–648. [Google Scholar]
- Yeh, Y.C.; Hahm, T.S.; Sabliov, C.M.; Lo, Y.M. Effects of Chinese wolfberry (Lycium chinense P. Mill.) leaf hydrolysates on the growth of Pediococcus acidilactici. Bioresour. Technol. 2008, 99, 1383–1393. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, K.A.; Piao, M.J.; Kim, K.C.; Kim, A.D.; Chae, S.; Park, J.S.; Youn, U.J.; Hyun, J.W. Cytoprotective effect of the fruits of Lycium chinense Miller against oxidative stress-induced hepatotoxicity. J. Ethnopharmacol. 2010, 130, 299–306. [Google Scholar] [CrossRef]
- Chung, I.M.; Ali, M.; Praveen, N.; Yu, B.R.; Kim, S.H.; Ahmad, A. New polyglucopyranosyl and polyarabinopyranosyl of fatty acid derivatives from the fruits of Lycium chinense and its antioxidant activity. Food Chem. 2014, 151, 435–443. [Google Scholar] [CrossRef]
- Chung, I.M.; Ali, M.; Nagella, P.; Ahmad, A. New glycosidic constituents from fruits of Lycium chinense and their antioxidant activities. Arabian J. Chem. 2013. [Google Scholar] [CrossRef]
- Xie, L.W.; Atanasov, A.; Guo, D.A.; Malainer, C.; Zhang, J.X.; Zehl, M.; Guan, S.H.; Heiss, E.H.; Urban, E.; Dirsch, V.M.; et al. Activity-guided isolation of NF-κB inhibitors and PPARγ agonists from the root bark of Lycium chinense Miller. J. Ethnopharmacol. 2014, 152, 470–477. [Google Scholar] [CrossRef]
- Duan, H.; Chen, Y.; Chen, G. Far infrared-assisted extraction followed by capillary electrophoresis for the determination of bioactive constituents in the leaves of Lycium barbarum Linn. J. Chromatogr. A 2010, 1217, 4511–4516. [Google Scholar] [CrossRef]
- Terauchi, M.; Kanamori, H.; Nobuso, M.; Yahara, S.; Nohara, T. Detection and determination of antioxidative components in Lycium chinense. Nat. Med. 1997, 51, 387–391. [Google Scholar]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, D.; Csillag, I.; Sevastre, B.; Mot, A.C.; Silaghi-Dumitrescu, R.; Tilea, I. Evaluation of antioxidant and antimicrobial activities and phenolic profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef]
- Benedec, D.; Vlase, L.; Oniga, I.; Mot, A.C.; Damian, G.; Hanganu, D.; Duma, M.; Silaghi-Dumitrescu, R. Polyphenolic Composition, Antioxidant and Antimicrobial Activities for Two Romanian Subspecies of Achillea distans Waldst. et Kit. ex Willd. Molecules 2013, 18, 8725–8739. [Google Scholar] [CrossRef]
- Mot, A.C.; Bischin, C.; Damian, G.; Silaghi-Dumitrescu, R. Antioxidant activity evaluation involving hemoglobin-related free radical reactivity. In Advanced Protocols in Oxidative Stress III. Methods in Molecular Biology; Springer: New York, NY, USA, 2013; in press. [Google Scholar]
- Bischin, C.; Tusan, C.; Bartok, A.; Septelean, R.; Damian, G.; Silaghi-Dumitrescu, R. Evalution of the biochemical effects of silyl-phosphaalkenes on oxidative and nitrosative stress pathways involving metallocenters. Phosphorus Sulfur Silicon Relat. Elem. 2014. [Google Scholar] [CrossRef]
- Bischin, C.; Deac, F.; Silaghi-Dumitrescu, R.; Worrall, J.A.; Rajagopal, B.S.; Damian, G.; Cooper, C.E. Ascorbate peroxidase activity of cytochrome c. Free Radic. Res. 2011, 45, 439–444. [Google Scholar]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar]
- Marinova, E.M.; Toneva, A.; Yanishlieva, N. Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem. 2009, 114, 1498–1502. [Google Scholar] [CrossRef]
- Varadarajan, P.; Rathinaswamy, G.; Asirvatahm, D. Antimicrobial properties and phytochemical constituents of Rheo discolor Hance. Ethnobot.Leaflets 2008, 12, 841–845. [Google Scholar]
- Salvat, A.; Antonacci, L.; Fortunato, R.H.; Suarez, E.Y.; Godo, H.M. Antimicrobial activity in methanolic extracts of several plant species from Northern Argentina. Phytomedicine 2004, 11, 230–234. [Google Scholar] [CrossRef]
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in Their Reaction with Ligands; North-Holland Publishing Company: Amsterdam, The Netherlands, 1971; pp. 98–134. [Google Scholar]
- Mot, A.C.; Damian, G.; Sarbu, C.; Silaghi-Dumitrescu, R. Redox reactivity in propolis: Direct detection of free radicals in basic medium and interaction with hemoglobin. Redox Rep. 2009, 14, 267–274. [Google Scholar]
- Romanian Pharmacopoeia Commission National Medicines Agency. Romanian Pharmacopoeia, Xth ed.; Medical Publishing House: Bucharest, Romania, 1993; p. 335. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar]
- Sample Availability: Samples are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.-M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic Content, Antioxidant and Antimicrobial Activities of Lycium barbarum L. and Lycium chinense Mill. Leaves. Molecules 2014, 19, 10056-10073. https://doi.org/10.3390/molecules190710056
Mocan A, Vlase L, Vodnar DC, Bischin C, Hanganu D, Gheldiu A-M, Oprean R, Silaghi-Dumitrescu R, Crișan G. Polyphenolic Content, Antioxidant and Antimicrobial Activities of Lycium barbarum L. and Lycium chinense Mill. Leaves. Molecules. 2014; 19(7):10056-10073. https://doi.org/10.3390/molecules190710056
Chicago/Turabian StyleMocan, Andrei, Laurian Vlase, Dan Cristian Vodnar, Cristina Bischin, Daniela Hanganu, Ana-Maria Gheldiu, Radu Oprean, Radu Silaghi-Dumitrescu, and Gianina Crișan. 2014. "Polyphenolic Content, Antioxidant and Antimicrobial Activities of Lycium barbarum L. and Lycium chinense Mill. Leaves" Molecules 19, no. 7: 10056-10073. https://doi.org/10.3390/molecules190710056
APA StyleMocan, A., Vlase, L., Vodnar, D. C., Bischin, C., Hanganu, D., Gheldiu, A.-M., Oprean, R., Silaghi-Dumitrescu, R., & Crișan, G. (2014). Polyphenolic Content, Antioxidant and Antimicrobial Activities of Lycium barbarum L. and Lycium chinense Mill. Leaves. Molecules, 19(7), 10056-10073. https://doi.org/10.3390/molecules190710056










