Sulfur Amino Acids in Diet-induced Fatty Liver: A New Perspective Based on Recent Findings
Abstract
:1. Sulfur Amino Acids in Diet-Induced Fatty Liver: Early Findings
1.1. Diets Inducing Fatty Liver
1.2. Lipotropic Factors, Methionine and Choline
| Diet | Casein (% of Diet) | Liver % Fat |
|---|---|---|
| Fat 20% Cholesterol 2% Casein varied | 5 | 32.1 |
| 10 | 26.4 | |
| 15 | 17.3 | |
| 20 | 14.8 |
| Diet | Methionine Added | Liver % Fat |
|---|---|---|
| Egg albumin 5% | 0 | 23.6 |
| Fat 40% | 0.25% | 14.0 |
| Egg albumin 5% | 0 | 33.8 |
| Fat 30% | 0.25% | 19.1 |
| Cholesterol 2% |
| Diet | Choline | Diet A no Cystine | Diet B + Cystine |
|---|---|---|---|
| % of Diet | % Fat in Liver | ||
| Diet A: Casein 8% Gelatin 12% | 0 | 15.1 | 34.5 |
| 0.01 | 12.3 | ||
| 0.02 | 9.8 | 34.5 | |
| 0.04 | 6.0 | 27.6 | |
| Diet B: Protein 12% Fat 15% Cystine 0.2% | 0.06 | 6.0 | 13.7 |
| 0.08 | 8.9 | ||
| 0.16 | 6.0 | 6.2 | |
| 0.32 | 5.2 | ||
| Diet | Casein (Met) | Glyceride % of Liver | Cholesterol Ester % of Liver | ||
|---|---|---|---|---|---|
| % of Diet | Choline 0.75% | Choline 0.75% | |||
| Fat 20% Cholesterol 2% Choline ±0.75% | 5 (0.12) | 0.98 | 3.42 | ||
| 10 (0.25) | 3.40 | 4.66 | |||
| 15 (0.36) | 2.26 | 4.57 | |||
| 30 (0.75) | 2.71 | 6.45 | |||
1.3. Cystine
| Diet | Glyceride % of Liver | Cholesterol % of Liver | Cholesterol Ester % of Liver | |
|---|---|---|---|---|
| Casein 5% | Control | 13.2 | 0.29 | 3.20 |
| Beef fat 20% | Cystine 0.2% | 32.7 | 0.26 | 3.35 |
| Cholesterol 2% |
| Diet | Cystine Varied (met 0.62%) | Methionine Varied (cystine 0.62%) | ||
|---|---|---|---|---|
| Cys % | % Fat in Liver | Met % | % Fat in Liver | |
| Casein 5% Agar 2% Lard 40% Choline 0 | 0.117 | 11.6 | 0.155 | 35.1 |
| 0.217 | 11.2 | 0.310 | 32.8 | |
| 0.417 | 14.0 | 0.465 | 19.0 | |
| 0.617 | 12.0 | 0.775 | 9.9 | |
| 0.817 | 15.7 | |||
| 1.017 | 19.7 | |||
1.4. The “Antilipotropic” Effect of Methionine
| Diet | Exp. | Methionine (% of Diet) | Liver (% Fat) |
|---|---|---|---|
| Casein 9% Corn oil 5% Choline 0.15% | 1 | 0 | 3.7 |
| 0.3 | 11.0 | ||
| 2 | 0 | 4.2 | |
| 0.3 | 9.7 | ||
| 3 | 0 | 4.8 | |
| 0.1 | 7.8 | ||
| 0.2 | 8.3 | ||
| 0.6 | 7.5 |
2. Hyperhomocysteinemia (HHE)
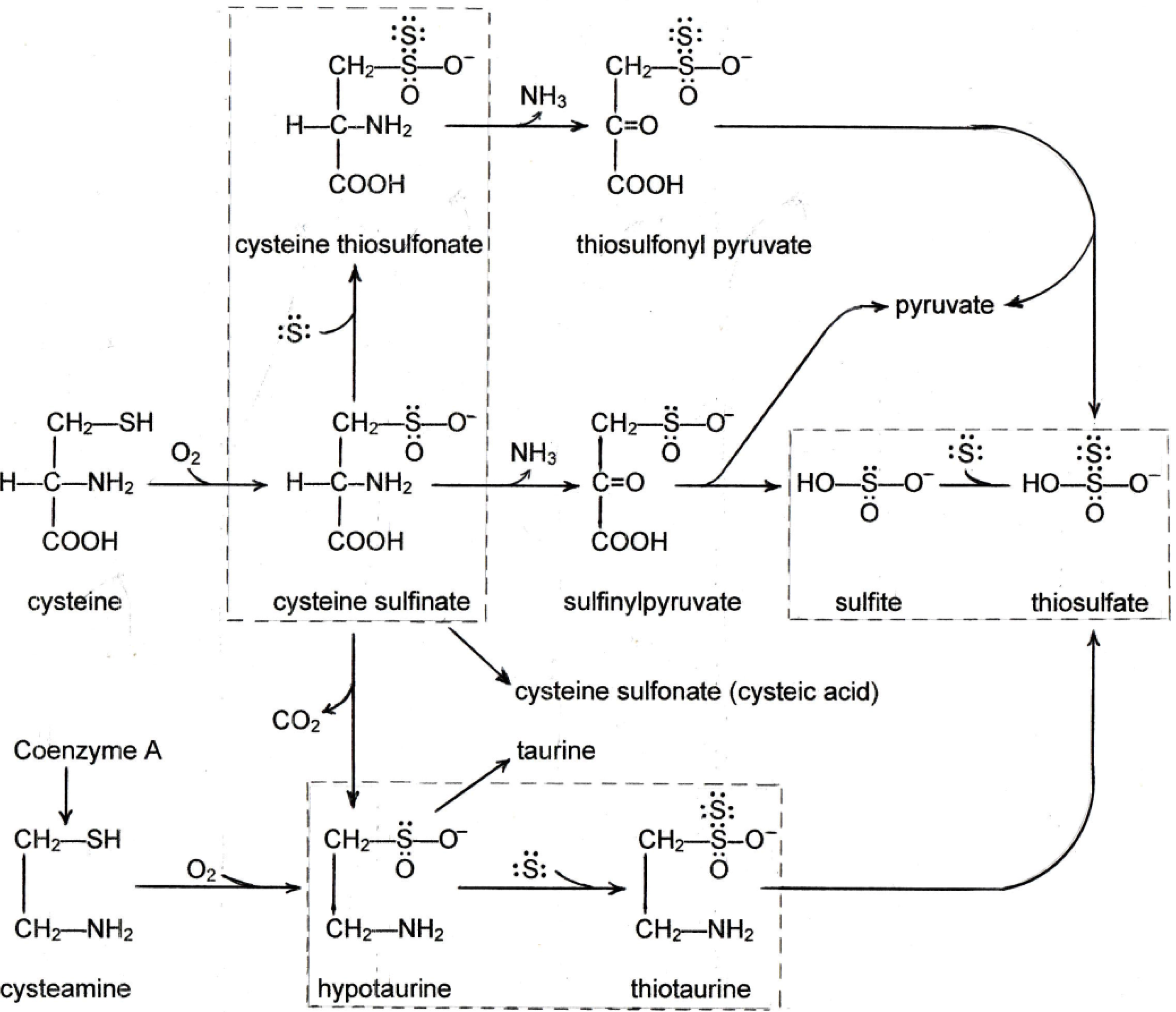
3. Sulfane Sulfur
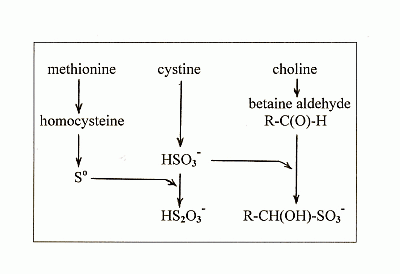
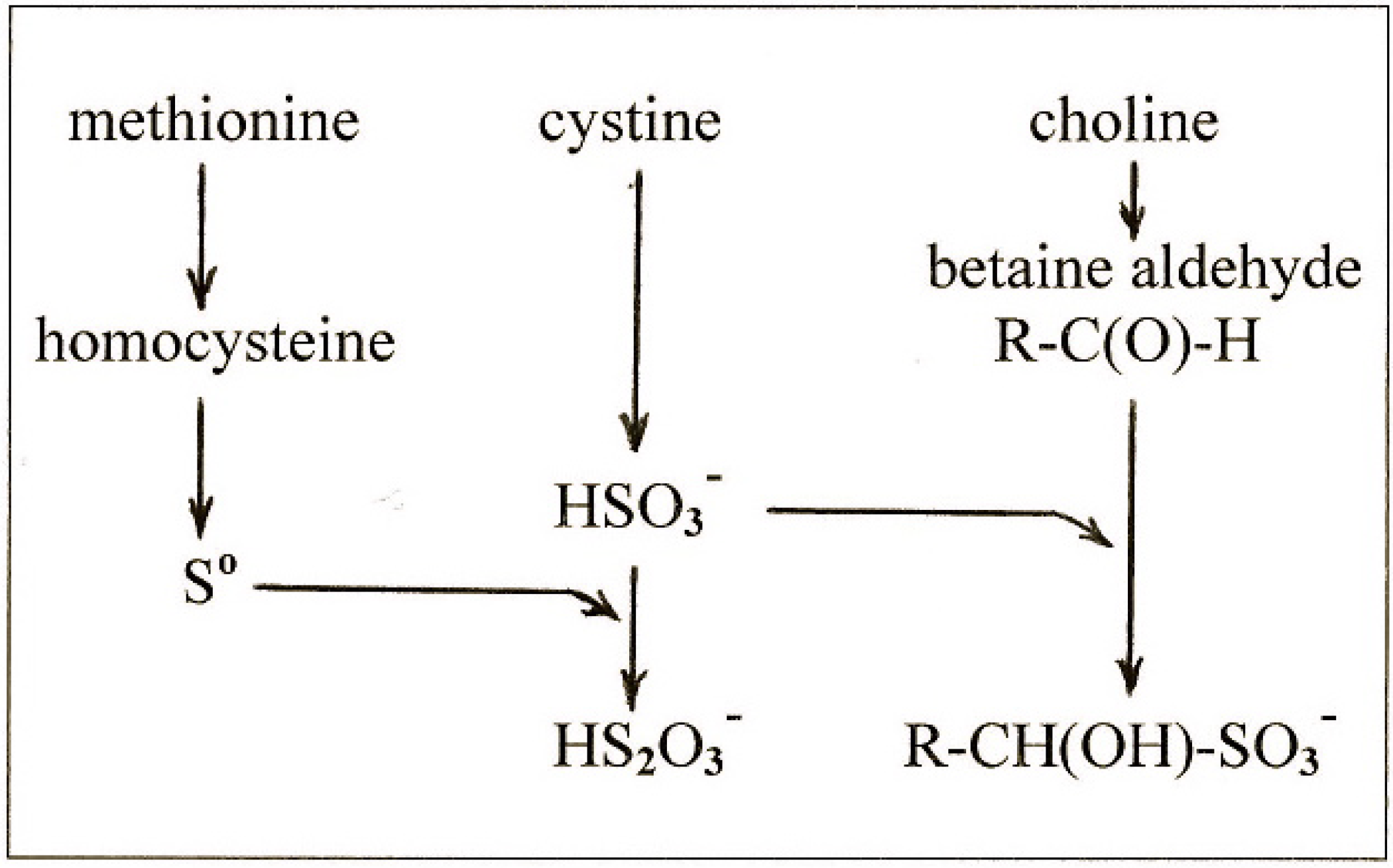
4. New Findings on the Effects of Sulfur on Fatty Liver
4.1. New Findings
4.2. The Lipotropic Effect of Methionine Explained
4.3. The Hepatosteatogenic Effect of Cystine Explained
4.4. The Relationship between Cystine and Choline
4.5. The Antilipotropic Effect, the Double-Edged Sword
4.6. Health Implications
5. Conclusions
- -
- methionine gives rise to So which is the ultimate lipotropic agent;
- -
- excess cystine gives rise to excess sulfite which depletes So to suboptimal levels causing fatty liver;
- -
- and choline gives rise to betaine aldehyde which removes the sulfite and blocks the depletion of So.
| Condition Causing Fatty Liver | Fatty Liver Prevented by: | Ref. |
|---|---|---|
| Deficient methionine → low So | Choline → decreased SO3→ increased So | [9] |
| Deficient choline → high SO3→ low So | Methionine → increased So production | [7] |
| Excess methionine → high So | Cystine → increased SO3→ decreased So | [16,19] |
| High cystine → high SO3→ low So | Choline → decreased SO3→ increased So | [9,10] |
| Same | Methionine → increased So | [13] |
| High cystine, low methionine, low choline → high SO3→ low So | Increased homocysteine → increased So | [38] |
| Same | NaHS containing So | [39] |
Conflicts of Interest
References
- Savard, C.; Tartaglione, E.V.; Kuver, R.; Haigh, G.C.; Farrel, G.C.; Subramanian, S.; Chait, A.; Yeh, M.W.; Quinn, L.S.; Ioannou, G.N. Synergistic Interaction of Dietary Cholesterol and Dietary Fat in Inducing Experimental Steatohepatitis. Hepatology 2013, 57, 81–92. [Google Scholar] [CrossRef]
- Ferguson, T.M.; Rigdon, R.H.; Couch, J.R. A Pathologic Study of Vitamin B12-Deficient Chick Embryos. Arch. Pathol. 1955, 60, 393–399. [Google Scholar]
- Johnson, E.M. A Histologic Study of Postnatal Vitamin B12 Deficiency in the Rat. Am. J. Path. 1964, 44, 73–79. [Google Scholar]
- Kennedy, D.G.; Young, P.B.; Blanchflower, W.J.; Scott, J.M.; Weir, D.G.; Molloy, A.M.; Kennedy, S. Cobalt-Vitamin B12 Deficiency Causes Lipid Accumulation, Lipid Peroxidation and Decreased Alpha-tocopherol Concentrations in the Liver of Sheep. Int. J. Vitam. Nutr. Res. 1994, 64, 270–276. [Google Scholar]
- Farrell, G.C.; George, J.; Hall, P.M.; McCullough, A.J. Fatty Liver Disease: NASH and Related Disorders; Blackwell Publishing: Malden, MA, USA, 2005. [Google Scholar]
- Beeston, A.W.; Channon, H.J.; Wilkinson, H. The Influence of the Caseinogen Content of Diets on the Nature of the “Cholesterol” Fatty Liver. Biochem. J. 1935, 29, 2659–1663. [Google Scholar]
- Channon, H.J.; Manifold, M.C.; Platt, A.P. The Action of Cystine and Methionine on Liver Fat Deposition. Biochem. J. 1938, 32, 969–975. [Google Scholar]
- Kulinaki, A.; Vance, D.E.; Vance, J.E. A Choline-deficient Diet in Mice Inhibits Neither the CDP-choline Pathway for Phosphatidylcholine Synthesis in Hepatocytes nor Apolipoproteins B Secretion. J. Biol. Chem. 2004, 279, 23916–23924. [Google Scholar] [CrossRef]
- Best, C.H.; Lucas, C.C.; Rideout, J.H.; Patteron, J.M. Dose-Response Curves in the Estimation of Potency of Lipotropic Agents. J. Biol. Chem. 1950, 186, 317–329. [Google Scholar]
- Young, R.J.; Lucas, C.C.; Patterson, J.M.; Best, C.H. Lipotropic Dose-Response Studies in Rats: Comparison of Choline, Betaine, and Methionine. Can. J. Biochem. 1956, 34, 713–720. [Google Scholar] [CrossRef]
- Du Vigneaud, V.; Chandler, J.P.; Moyer, A.W.; Keppel, D.M. The Effect of Choline on the Ability of Homocystine to Replace Methionine in the Diet. J. Biol. Chem. 1939, 131, 57–76. [Google Scholar]
- Singal, S.A.; Eckstein, H.C. Supplementary Proteins and Amino Acids and Dietary Production of Fatty Livers in Mice. Proc. Soc. Exp. Biol. Med. 1939, 41, 512–513. [Google Scholar] [CrossRef]
- Treadwell, C.R.; Groothuis, M.; Eckstein, H.C. The Influence of Supplementary Casein, Cystine, and Methionine on Liver Lipid Content. J. Biol. Chem. 1942, 142, 653–658. [Google Scholar]
- Earle, D.P.; Kendall, A.F. Liver Damage and Urinary Excretion of Sulfate in Rats Fed l-Cystine, dl-Methionine, and Cysteic acid. J. Exp. Med. 1942, 75, 191–195. [Google Scholar] [CrossRef]
- Victor, J.; Pappenheimer, A.M. The Influence of Choline, Cystine, and of a-Tocopherol Upon the Occurrence of Ceroid Pigment in Dietary Cirrhosis of Rats. J. Exp. Med. 1945, 82, 375–383. [Google Scholar] [CrossRef]
- Harper, A.E.; Benton, D.A.; Winje, M.E.; Elvehjem, C.A. “Antiliptropic” Effect of Methionine in Rats Fed Threonine Deficient Diets Containing Choline. J. Biol. Chem. 1954, 209, 159–163. [Google Scholar]
- Williams, J.N.; Hurlebaus, A.J. Response of Liver to Prolonged Protein Depletion: Production of Fatty Livers by Certain Amino Acids Fed in a Protein-free Ration. J. Nutr. 1965, 85, 73–84. [Google Scholar]
- Leclerc, J. Fatty Liver Induced by Methionine Supplementation of Low Protein Diet: Effect of Inositol Supply. J. Nutr. 1991, 121, 1139. [Google Scholar]
- Ables, G.P.; Perrone, C.E.; Orentreich, D.; Orentreich, N. Methionine-restricted C57BL/6J Mice are Resistant to Diet-induced Obesity and Insulin Resistance but Have Low Bone Density. PLoS One 2012, 7, e51357. [Google Scholar]
- Gibson, J.B.; Carson, N.A.J.; Neill, D.W. Pathological Findings in Homocystinuria. J. Clin. Pathol. 1964, 17, 427–433. [Google Scholar] [CrossRef]
- Watanabe, M.; Osada, J.; Aratani, Y.; Kluckman, K.; Reddick, R.; Malinow, M.R.; Maeda, N. Mice Deficient in Cystathionine ß-synthase: Animal Models for Mild and Severe Homocyst(e)inemia. Proc. Natl. Acad. Sci. USA 1995, 92, 1585–1589. [Google Scholar] [CrossRef]
- Frenkel, E.P.; Kitchens, R.; Johnston, J.M. The Effect of Vitamin B12 Deprivation on the Enzymes of Fatty Acid Synthesis. J. Biol. Chem. 1973, 248, 7540–7546. [Google Scholar]
- Karmin, O.; Lynn, E.G.; Chung, Y.H.; Siow, Y.; Man, R.K.; Choy, P.C. Homocysteine Stimulates the Production and Secretion of Cholesterol in Hepatic Cells. Biochim. Biophys. Acta 1998, 1393, 317–324. [Google Scholar] [CrossRef]
- Toohey, J.I. Sulphane Sulphur in Biological Systems: A Possible Regulatory Role. Biochem. J. 1989, 264, 625–632. [Google Scholar]
- Schmokel, M.S.; Cenedese, S.; Overgaard, J.; Jorgensen, M.R.; Chen, Y.S.; Gatti, C.; Stalke, D.; Iversen, B.B. Testing the Concept of Hypervalency: Charge Density Analysis of K2SO4. Inorg. Chem. 2012, 51, 8607–8616. [Google Scholar] [CrossRef]
- Miaskiewicz, K.; Steudel, R. Sulfur Compounds. Part 140. Structures and Relative Stabilities of Seven Isomeric Forms of H2S2O2. J. Chem. Soc. Dalton Trans. 1991, 2395–2398. [Google Scholar] [CrossRef]
- Steudel, R.; Drozdova, Y.; Miaskiewwicz, K.; Hertwig, R.H.; Koch, W. How Unstable are Thiosulfoxides? An ab initio MO Study of Various Disulfanes RSSR (R=H, Me, Pr, All), Their Branched Isomers R2SS, and the Related Transition States. J. Am. Chem. Soc. 1997, 119, 1990–1996. [Google Scholar] [CrossRef]
- Toohey, J.I. Persulfide Sulfur is a Growth Factor for Cells Defective in Sulfur Metabolism. Biochem. Cell Biol. 1986, 64, 758–765. [Google Scholar] [CrossRef]
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S.; Sarahroodi, S. Therapeutic Uses and Pharmacological Properties of Garlic, Shallot, and Their Biologically Active Compounds. Iran J. Basic Med. Sci. 2013, 16, 1031–1048. [Google Scholar]
- Yang, G. Hydrogen Sulfide in Cell Survival: A Double-edged Sword. Exp. Rev. Clin. Pharm. 2011, 4, 33–42. [Google Scholar]
- Toohey, J.I. Sulfur Signaling: Is the Agent Sulfide or Sulfane? Anal. Biochem. 2011, 413, 1–7. [Google Scholar] [CrossRef]
- Cavallini, D.; de Marco, C.; Mondovi, B. Cleavage of Cystine by a Pyridoxal Model. Arch. Biochem. Biophys. 1960, 87, 281–288. [Google Scholar] [CrossRef]
- Costa, M.T.; Wolf, A.M.; Giarnieri, D. Cleavage of Cystine by Cystathionase. Enzymologia 1972, 43, 271–279. [Google Scholar]
- Gupta, V.J.; Wilcken, D.E. The Detection of Cysteine-homocysteine Mixed Disulfide in Plasma of Normal Fasting Man. J. Clin. Invest. 1978, 8, 205–207. [Google Scholar] [CrossRef]
- Frimpter, G.W. The Disulfide of L-Cysteine and L-Homocysteine in Urine of Patients with Cystinuria. J. Biol. Chem. 1961, 236, PC51–64. [Google Scholar]
- Schneider, J.A.; Bradley, K.H.; Seegmiller, J.H. Identification and Measurement of Cysteine-homocysteine Mixed Disulfide in Plasma. J. Lab. Clin. Med. 1968, 71, 122–125. [Google Scholar]
- Molloy, M.H.; Rassin, D.K.; Gaull, G.E. Plasma Cyst(e)ine in Homocysteinemia. Am. J. Clin. Nutr. 1981, 34, 2619–2621. [Google Scholar]
- Maclean, K.N.; Greiner, L.S.; Evans, J.R.; Sood, S.K.; Lhotek, S.; Markham, N.E.; Stabler, S.P.; Allen, R.H.; Austin, R.C.; Balasubramaniam, V.; et al. Cystathionine Protects Against Endoplasmic Reticulum Stress-induced Lipid Accumulation, Tissue Injury, and Apoptotic Cell Death. J. Biol. Chem. 2012, 287, 31994–32005. [Google Scholar] [CrossRef]
- Luo, Z.L.; Tang, L.; Wang, T.; Dai, R.W.; Ren, J.D.; Cheng, L.; Xiang, K.; Tian, F.Z. Effects of Treatment with Hydrogen Sulfide on Methionine-choline Deficient Diet-induced Non-alcoholic Steatohepatitis in Rats. J. Gastroenterol. Hepatol. 2013, 29, 215–222. [Google Scholar]
- Greiner, R.; Palinkas, Z.; Basell, K.; Becher, D.; Antelmann, H.; Dick, T.P. Polysulfides Link H2S to Protein Thiol Oxidation. Antiox. Redox. Signal. 2013, 19, 1749–1765. [Google Scholar] [CrossRef]
- De Marco, C.; Coletta, M.; Cavllini, D. Spontaneous Transulfuration of Sulfinates by Organic Persulfides. Arch. Biochem. Biophys. 1961, 93, 178–180. [Google Scholar] [CrossRef]
- Fellman, J.H.; Avedovech, N.A. Cysteine Thiosulfonate in Cystine Metabolism. Arch. Biochem. Biophys. 1982, 218, 303–308. [Google Scholar] [CrossRef]
- Cavallini, D.; de Marco, C.; Mondovi, B.; Mori, B.G. The Cleavage of Cystine by Cystathionase and the Transulfuration of Hypotaurine. Enzymologia 1960, 22, 161–173. [Google Scholar]
- Cavalllini, D.; de Marco, C.; Mondovi, B. Chromatographic Evidence on the Occurrence of Thiotaurine in the Urine of Rats Fed with Cystine. J. Biol. Chem. 1959, 234, 854–857. [Google Scholar]
- Roy, A.B.; Trudinger, P.A. The Biochemistry of Inorganic Compounds of Sulfur; Cambridge University Press: Cambridge, UK, 1970; pp. 20–34. [Google Scholar]
- Tan, W.H.; Eichler, F.S.; Hoda, S.; Lee, M.S.; Baris, H.; Hanley, C.A.; Grant, P.E.; Krishnamoorthy, K.S.; Shih, V.E. Isolated Sulfite Oxidase Deficiency; A Case Report with a Novel Metabolism and Review of the Literature. Pediatrics 2005, 116, 757–766. [Google Scholar] [CrossRef]
- De Marco, C.; Rinaldi, A. Oxidative Deamination of Sulfinyl, Sulfonyl and Thiosulfonyl Amino Acids by D-Aspartate Oxidase. Eur. J. Biochem. 1972, 24, 470–476. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Dominy, J.E.; Lee, J.; Coloso, R.M. Mammalian Cysteine Metabolism; New Insights into Regulation of Cysteine Metabolism. J. Nutr. 2006, 136, 1652S–1659S. [Google Scholar]
- Rothschild, H.A.; Barron, H.A. The Oxidation of Betaine Aldehyde by Betaine Aldehyde Dehydrogenase. J. Biol. Chem. 1954, 209, 511–523. [Google Scholar]
- Bai, J.Y.; Meng, Z.Q. Sulfur Dioxide-induced Liver Pathology. Chin. J. Pathol. 2004, 33, 155–157. [Google Scholar]
- Click, R.E. Obesity, Longevity, Quality of Life: Alteration by Dietary 2-Mercaptoethanol. Virulence 2010, 1, 509–515. [Google Scholar] [CrossRef]
- Click, R.E. Dietary Supplemented 2-Mercaptoethanol Prevents Spontaneous and Delays Virally-induced Murine Mammary Tumorigenesis. Cancer Biol. Ther. 2013, 14, 521–526. [Google Scholar] [CrossRef]
- Heidrick, M.L.; Hendrick, L.; Cook, D.E. Effect of Dietary 2-Mercaptoethanol on the Life Span, Immune System, Tumor Incidence and Lipid Peroxidation Damage in Spleen Lymphocytes of Ageing BC3F1 Mice. Mech. Ageing Devel. 1984, 31, 341–356. [Google Scholar] [CrossRef]
- Harman, D. Prolongation of the Normal Lifespan and Inhibition of Spontaneous Cancer by Antioxidants. J. Gerontol. 1961, 16, 24–54. [Google Scholar]
- Toohey, J.I. Sulfur Metabolism in AIDS. Cystamine as an Anti-HIV Agent. AIDS Res. Hum. Retrov. 2009, 25, 1057–1060. [Google Scholar] [CrossRef]
- Fujisawa, R.; Rubin, B.; Suzuki, A.; Patel, P.S.; Gahl, W.A.; Joshi, B.H.; Puri, R.K. Cysteamine Suppresses Invasion, Metastasis and Prolongs Survival by Inhibiting Matrix Metalloproteinases in a Mouse Model of Human Pancreatic Cancer. PLoS One 2012, 7, e34437. [Google Scholar] [CrossRef]
- Gibrat, C.; Cicchetti, F. Potential of Cystamine and Cysteamine in the Treatment of Neurodegenerative Diseases. Prog. Neuropsychopharmacol. Biol. Pyschiatry 2011, 35, 380–389. [Google Scholar] [CrossRef]
- Capuozzo, E.; Pecci, L.; Baseggo, C.A.; Fontana, M. Thiotaurine Prevents Apoptosis of Human Neutrophils: A Putative Role in Inflammation. Adv. Exp. Med. Biol. 2013, 775, 227–236. [Google Scholar] [CrossRef]
- Budhram, R.; Pandya, K.G.; Lau-Cam, C.A. Protection by Taurine and Thiotaurine Against Biochemical and Cellular Alterations Induced by Diabetes in a Rat Model. Adv. Exp. Med. Biol. 2013, 775, 321–343. [Google Scholar]
- Zeisel, S.H.; da Costa, K.A. Choline: An Essential Nutrient for Public Health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Toohey, J.I. Sulfur Amino Acids in Diet-induced Fatty Liver: A New Perspective Based on Recent Findings. Molecules 2014, 19, 8334-8349. https://doi.org/10.3390/molecules19068334
Toohey JI. Sulfur Amino Acids in Diet-induced Fatty Liver: A New Perspective Based on Recent Findings. Molecules. 2014; 19(6):8334-8349. https://doi.org/10.3390/molecules19068334
Chicago/Turabian StyleToohey, John I. 2014. "Sulfur Amino Acids in Diet-induced Fatty Liver: A New Perspective Based on Recent Findings" Molecules 19, no. 6: 8334-8349. https://doi.org/10.3390/molecules19068334
APA StyleToohey, J. I. (2014). Sulfur Amino Acids in Diet-induced Fatty Liver: A New Perspective Based on Recent Findings. Molecules, 19(6), 8334-8349. https://doi.org/10.3390/molecules19068334