1-[2-(2-Methoxyphenylamino)ethylamino]-3-(naphthalene-1- yloxy)propan-2-ol May Be a Promising Anticancer Drug
Abstract
:1. Introduction
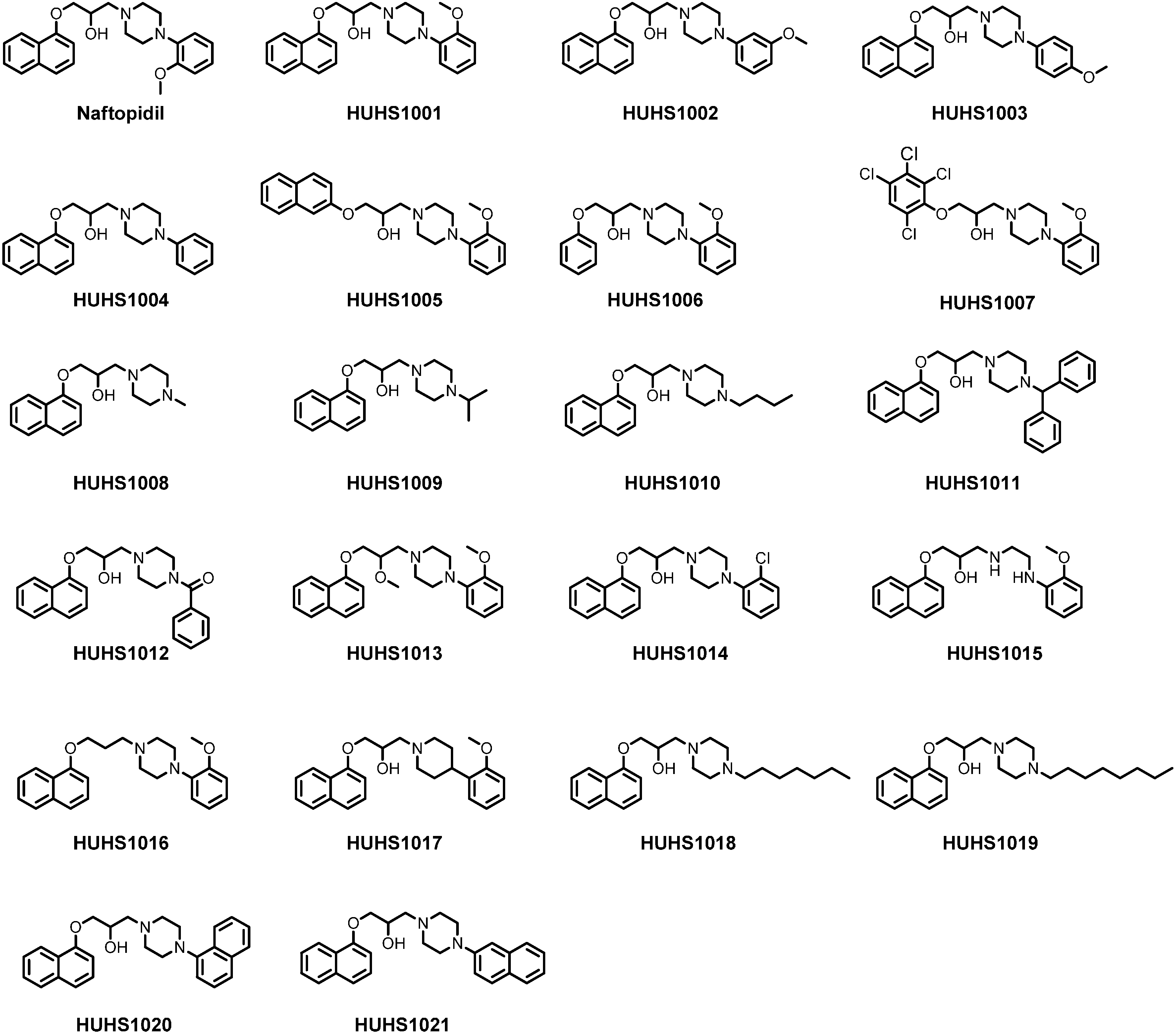
2. Synthesis of HUHS1015
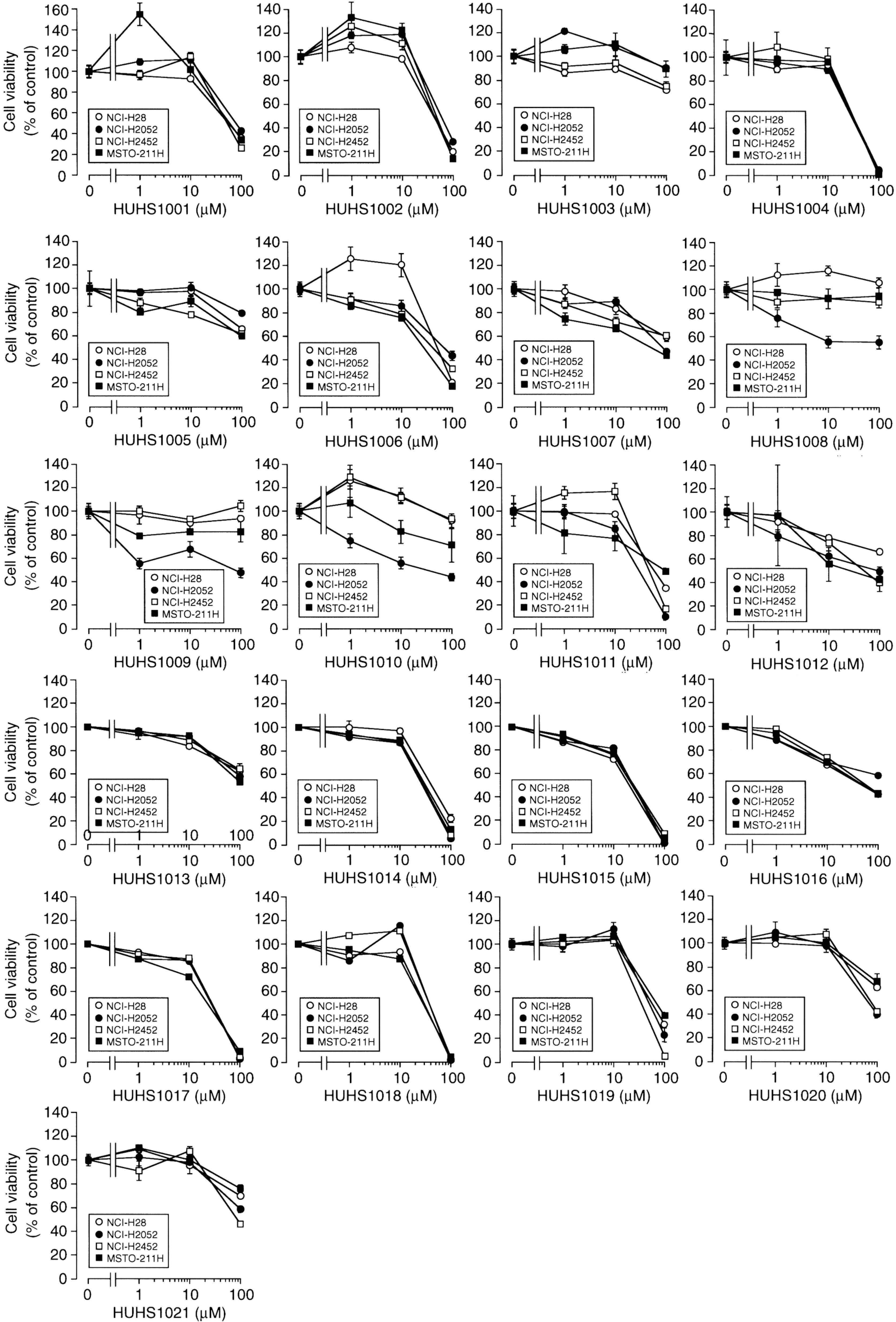
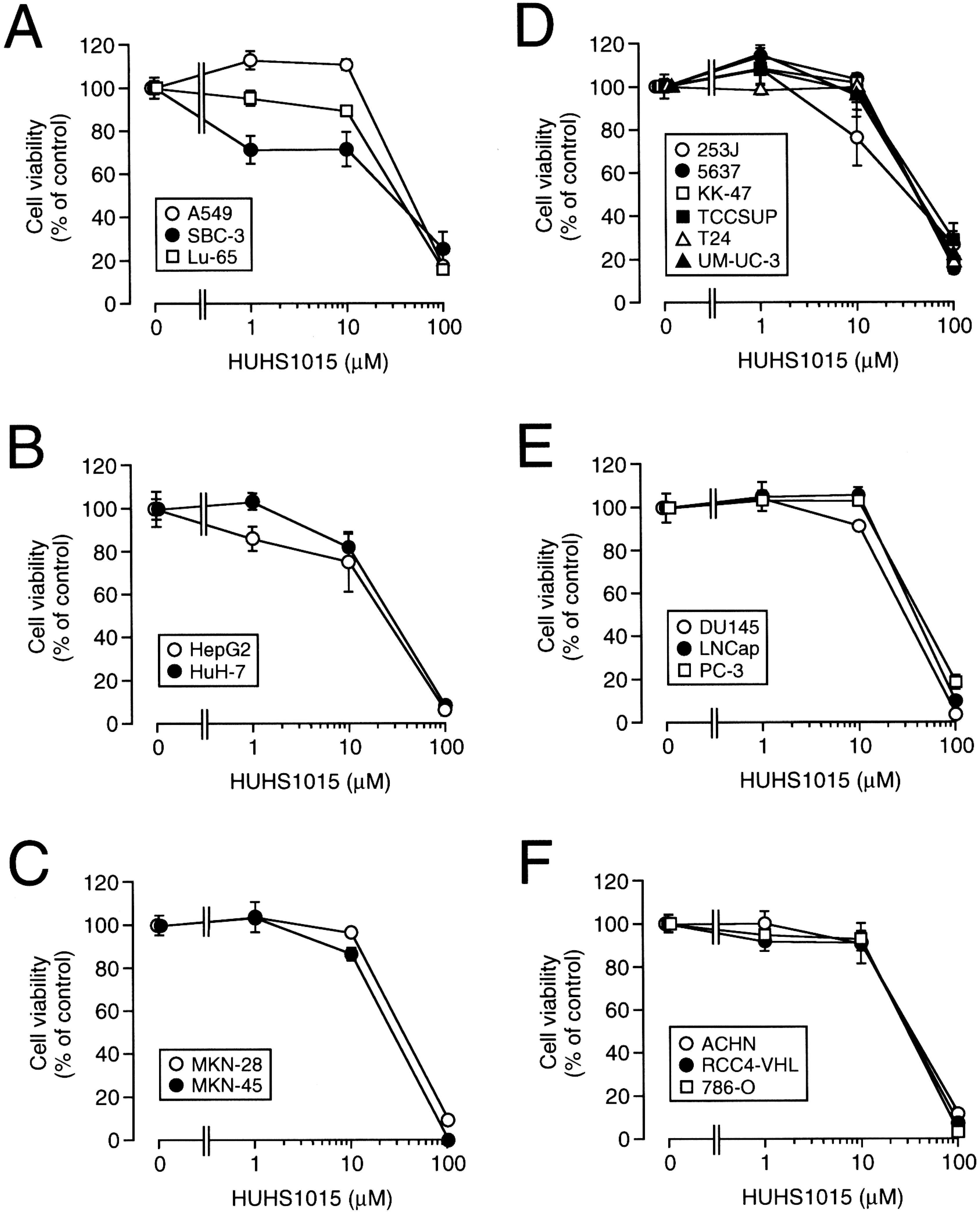
3. The Naftopidil Analogue HUHS1015 Induces Cell Death for a Wide Variety of Human Cancer Cell Lines
4. HUHS1015 Induces Caspase-Dependent and -Independent Apoptosis of Cancer Cells
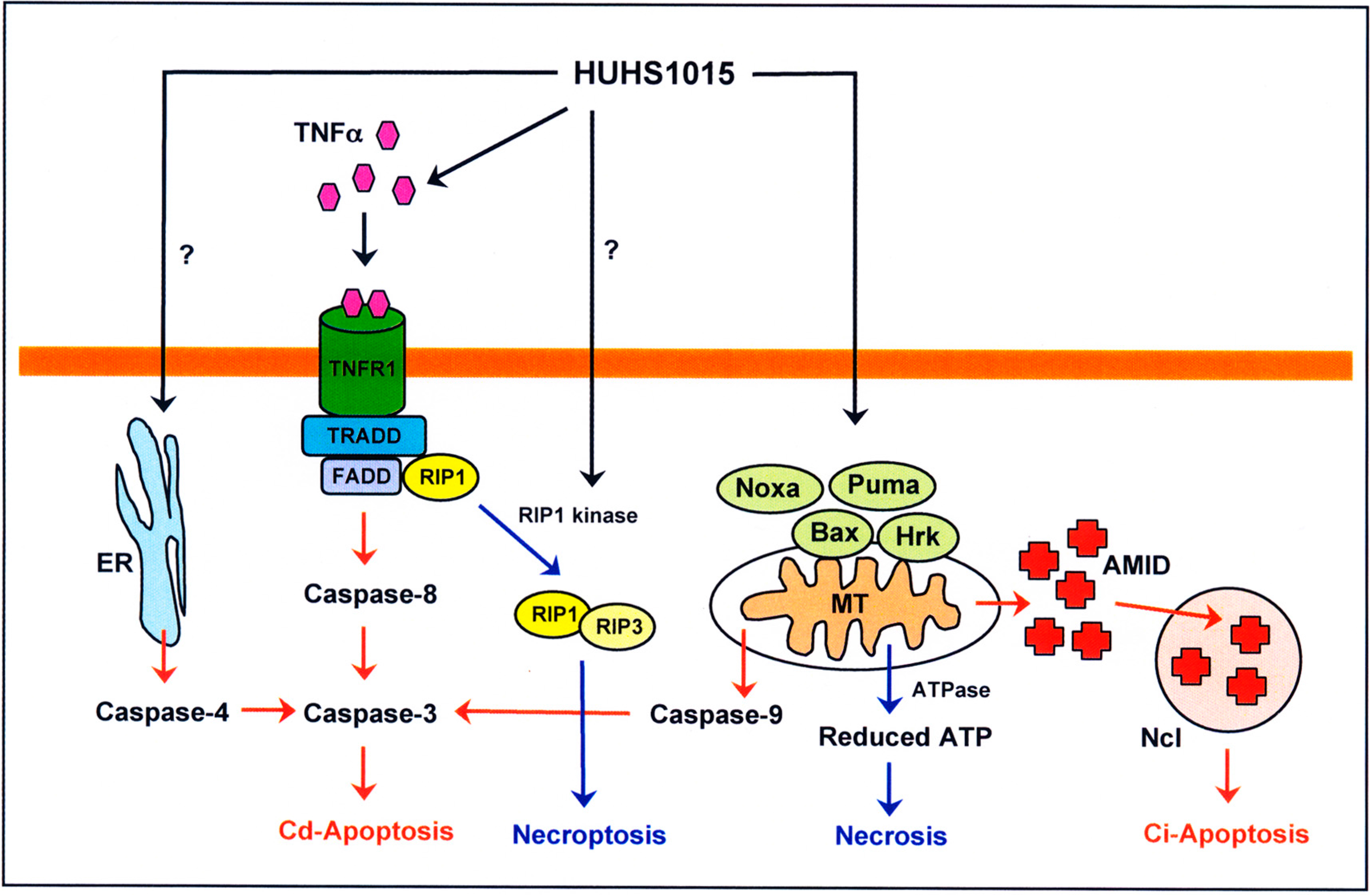
5. HUHS1015 Activates Caspase-8 by Upregulating Tumor Necrosis Factor α (TNFα)
6. HUHS1015 Induces Caspase-Independent Apoptosis by Accumulating AMID in the Nucleus
7. HUHS1015 Induces Necrosis (Necroptosis) of Cancer Cells
8. HUHS1015 Suppresses Tumor Growth in Xenograft Model Mice
9. Conclusions
Author Contributions
Abbreviation
| HUHS1015 | 1-[2-(2-methoxyphenylamino)ethylamino]- 3-(naphthalene-1-yloxy)propan-2-ol |
| MPM | malignant pleural mesothelioma |
| PKC | protein kinase C |
| TUNEL | terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling |
| AIF | apoptosis-inducing factor |
| AMID@ | AIF-like mitochondrion-associated inducer of death |
| ER | endoplasmic reticulum |
| TNFα | tumor necrosis factor α |
| FADD | Fas-associated death domain |
| TNFR1 | TNF receptor 1 |
| TRADD | TNFR1-associated death domain protein |
| PI | propidium iodide |
| Nec-1 | necrostatin-1 |
| RIP1 | receptor interacting protein 1 |
| tBid | truncated Bid |
Conflicts of Interest
References
- Takei, R.; Ikegaki, I.; Shibata, K.; Tsujimoto, G.; Asano, T. Naftopidil, a novel α1-adrenoceptor antagonist, displays selective inhibition of canine prostatic pressure and high affinity binding to cloned human a1-adrenoceptors. Jpn. J. Pharmacol. 1999, 79, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Ishii, K.; Kanda, H.; Iwamoto, Y.; Nishikawa, K.; Soga, N.; Kise, H.; Arima, K.; Sugimura, Y. Naftopidil, a selective α1-adrenoceptor antagonist, suppresses human prostate tumor growth by altering interactions between tumor cells and stroma. Cancer Prev. Res. (Phila) 2011, 4, 87–96. [Google Scholar] [CrossRef]
- Kanda, H.; Ishii, K.; Ogura, Y.; Imamura, T.; Kanai, M.; Arima, K.; Sugimura, Y. Naftopidil, a selective α-1 adrenoceptor antagonist, inhibits growth of human prostate cancer cells by G1 cell cycle arrest. Int. J. Cancer 2008, 122, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, A.; Nagaya, H.; Kanno, T.; Nishizaki, T. Anti-tumor action of α1-adrenoceptor blockers on human bladder, prostate, and renal cancer cells. Pharmacology 2012, 90, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Masachika, E.; Kanno, T.; Nakano, T.; Gotoh, A.; Nishizaki, T. Naftopidil induces apoptosis in malignant mesothelioma cell lines independently of α1-adrenoceptor blocking. Anticancer Res. 2013, 33, 887–894. [Google Scholar] [PubMed]
- Brede, M.; Philipp, M.; Knaus, A.; Muthig, V.; Hein, L. α2-adrenergic receptor subtypes—Novel functions uncovered in gene-targeted mouse models. Biol. Cell 2004, 96, 343–348. [Google Scholar] [PubMed]
- Kaumann, A.J.; Molenaar, P. Modulation of human cardiac function through 4 β-adrenoceptor populations. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997, 355, 667–681. [Google Scholar] [CrossRef]
- Zhong, H.; Minneman, K.P. α1-adrenoceptor subtypes. Eur. J. Pharmacol. 1999, 375, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Tanaka, A.; Shimizu, T.; Nakano, T.; Nishizaki, T. 1-[2-(2-Methoxyphenylamino)ethylamino]-3-(naphthalene-1-yloxy)propan-2-ol as a potential anticancer drug. Pharmacology 2013, 91, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Kaku, Y.; Nagaya, H.; Tsuchiya, A.; Kanno, T.; Gotoh, A.; Tanaka, A.; Shimizu, T.; Tabata, C.; Nakano, T.; Nishizaki, T. Newly synthesized anticancer drug HUHS1015 is effective on malignant pleural mesothelioma. Cancer Sci. 2014, 105, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Li, J.Y.; Xu, W. A review of the role of Puma, Noxa and Bim in the tumorigenesis, therapy and drug resistance of chronic lymphocytic leukemia. Cancer Gene Ther. 2013, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tomek, M.; Akiyama, T.; Dass, C.R. Role of Bcl-2 in tumour cell survival and implications for pharmacotherapy. J. Pharm. Pharmacol. 2012, 64, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, J.; Katayama, T.; Eguchi, Y.; Kudo, T.; Taniguchi, M.; Koyama, Y.; Manabe, T.; Yamagishi, S.; Bando, Y.; Imaizumi, K.; et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J. Cell Biol. 2004, 165, 347–356. [Google Scholar] [CrossRef]
- Kim, S.J.; Zhang, Z.; Hitomi, E.; Lee, Y.C.; Mukherjee, A.B. Endoplasmic reticulum stress-induced caspase-4 activation mediates apoptosis and neurodegeneration in INCL. Hum. Mol. Genet. 2006, 15, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Binet, F.; Chiasson, S.; Girard, D. Evidence that endoplasmic reticulum (ER) stress and caspase-4 activation occur in human neutrophils. Biochem. Biophys. Res. Commun. 2010, 391, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, C.; Fulda, S.; Srinivasan, A.; Friesen, C.; Li, F.; Tomaselli, K.J.; Debatin, K.M.; Krammer, P.H.; Peter, M.E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998, 17, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Kaku, Y.; Tsuchiya, A.; Kanno, T.; Nishizaki, T. HUHS1015 induces necroptosis and caspase-independent apoptosis of MKN28 human gastric cancer cells in association with AMID accumulation in the nucleus. Anticancer Agents Med. Chem. 2014. [Google Scholar] [CrossRef]
- Cande, C.; Cecconi, F.; Dessen, P.; Kroemer, G. Apoptosis-inducing factor (AIF): Key to the conserved caspase-independent pathways of cell death? J. Cell Sci. 2002, 115, 4727–4734. [Google Scholar] [CrossRef] [PubMed]
- Daugas, E.; Susin, S.A.; Zamzami, N.; Ferri, K.F.; Irinopoulou, T.; Larochette, N.; Prévost, M.C.; Leber, B.; Andrews, D.; Penninger, J.; et al. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000, 14, 729–739. [Google Scholar]
- Bilyy, R.; Kit, Y.; Hellman, U.; Stoika, R. AMID: New insights on its intracellular localization and expression at apoptosis. Apoptosis 2008, 13, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, L.; Liang, Q.; Wang, J.; Mo, W.; Zhou, B. Yeast AMID homologue Ndi1p displays respiration-restricted apoptotic activity and is involved in chronological aging. Mol. Biol. Cell 2006, 17, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.R.; Gong, M.; Wodke, L.; Lamb, J.H.; Jones, D.J.; Farmer, P.B.; Scrutton, N.S.; Munro, A.W. The human apoptosis-inducing protein AMID is an oxidoreductase with a modified flavin cofactor and DNA binding activity. J. Biol. Chem. 2005, 280, 30735–30740. [Google Scholar] [CrossRef] [PubMed]
- Ohiro, Y.; Garkavtsev, I.; Kobayashi, S.; Sreekumar, K.R.; Nantz, R.; Higashikubo, B.T.; Duffy, S.L.; Higashikubo, R.; Usheva, A.; Gius, D.; et al. A novel p53-inducible apoptogenic gene, PRG3, encodes a homologue of the apoptosis-inducing factor (AIF). FEBS Lett. 2002, 524, 163–171. [Google Scholar] [CrossRef]
- Varecha, M.; Amrichova, J.; Zimmermann, M.; Ulman, V.; Lukasova, E.; Kozubek, M. Bioinformatic and image analyses of the cellular localization of the apoptotic proteins endonuclease G, AIF, and AMID during apoptosis in human cells. Apoptosis 2007, 12, 1155–1171. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xu, L.G.; Li, X.; Zhai, Z.; Shu, H.B. AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J. Biol. Chem. 2002, 277, 25617–25623. [Google Scholar] [CrossRef] [PubMed]
- Vanags, D.M.; Pörn-Ares, M.I.; Coppola, S.; Burgess, D.H.; Orrenius, S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J. Biol. Chem. 1996, 271, 31075–31085. [Google Scholar] [CrossRef] [PubMed]
- Pietra, G.; Mortarini, R.; Parmiani, G.; Anichini, A. Phases of apoptosis of melanoma cells, but not of normal melanocytes, differently affect maturation of myeloid dendritic cells. Cancer Res. 2001, 61, 8218–8226. [Google Scholar] [PubMed]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Christofferson, D.E.; Yuan, J. Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 2010, 22, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, H.; Xu, C.J.; Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Kvansakul, M.; Hinds, M.G. Structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis. 2013, 4, e909. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.X.; Thompson, C.B. Necrotic death as a cell fate. Genes Dev. 2006, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishizaki, T.; Kanno, T.; Tsuchiya, A.; Kaku, Y.; Shimizu, T.; Tanaka, A. 1-[2-(2-Methoxyphenylamino)ethylamino]-3-(naphthalene-1- yloxy)propan-2-ol May Be a Promising Anticancer Drug. Molecules 2014, 19, 21462-21472. https://doi.org/10.3390/molecules191221462
Nishizaki T, Kanno T, Tsuchiya A, Kaku Y, Shimizu T, Tanaka A. 1-[2-(2-Methoxyphenylamino)ethylamino]-3-(naphthalene-1- yloxy)propan-2-ol May Be a Promising Anticancer Drug. Molecules. 2014; 19(12):21462-21472. https://doi.org/10.3390/molecules191221462
Chicago/Turabian StyleNishizaki, Tomoyuki, Takeshi Kanno, Ayako Tsuchiya, Yoshiko Kaku, Tadashi Shimizu, and Akito Tanaka. 2014. "1-[2-(2-Methoxyphenylamino)ethylamino]-3-(naphthalene-1- yloxy)propan-2-ol May Be a Promising Anticancer Drug" Molecules 19, no. 12: 21462-21472. https://doi.org/10.3390/molecules191221462
APA StyleNishizaki, T., Kanno, T., Tsuchiya, A., Kaku, Y., Shimizu, T., & Tanaka, A. (2014). 1-[2-(2-Methoxyphenylamino)ethylamino]-3-(naphthalene-1- yloxy)propan-2-ol May Be a Promising Anticancer Drug. Molecules, 19(12), 21462-21472. https://doi.org/10.3390/molecules191221462



