Dysidinoid A, an Unusual Meroterpenoid with Anti-MRSA Activity from the South China Sea Sponge Dysidea sp.
Abstract
:1. Introduction

2. Results and Discussion
 +35.4 (c 0.50, MeOH). Its IR spectrum showed absorption bands assignable to amide (3276 cm−1) and carbonyl (1775 and 1714) functionalities. The positive ESIMS of 1 exhibited quasimolecular ion peaks at m/z 302.2 [M+H]+ and 324.2 [M+Na]+, respectively. The molecular formula of C19H27NO2 with seven degrees of unsaturation, was deduced from HRESIMS at m/z 324.1941 [M+Na]+ (calcd. for C19H27NO2, 324.1939), which was supported by the 1H- and 13C-NMR data (Table 1). The 1H-NMR spectrum of 1 showed resonances attributable to two olefinic protons at δH 5.16 (H-3) and 6.26 (H-18), three tertiary methyl groups at δH 1.55 (H3-11), 1.00 (H3-12), and 0.88 (H3-14), a secondary methyl group at δH 0.95 (H3-13). In addition, the spectrum showed resonances due to an exchangable amine proton at δH 7.33 (20-NH), as well as partially overlapping signals with complex coupling patterns between δH 1.08 and 2.61 that could be attributed to several aliphatic methylene and methine units. The 13C-NMR and DEPT spectra of 1 showed 19 carbon resonances, corresponding to two carbonyl groups (δC 171.7 and 170.4), two olefinic quaternary carbons (δC 143.9 and 147.9), two aliphatic quaternary carbons (δC 38.3 and 42.4), two olefinic methine carbons (δC 120.5 and 130.4), two aliphatic methine carbons (δC 37.4 and 47.0), five aliphatic methylene carbons (δC 19.0, 26.3, 36.2, 27.4, and 32.5), and four methyl carbons (δC 17.7, 19.8, 16.3, and 18.0). The above spectroscopic signatures suggested the presence of a 9,4-friedodrime sesquiterpene moiety and accounted for four degrees of unsaturation, indicating three rings in the structure of 1.
+35.4 (c 0.50, MeOH). Its IR spectrum showed absorption bands assignable to amide (3276 cm−1) and carbonyl (1775 and 1714) functionalities. The positive ESIMS of 1 exhibited quasimolecular ion peaks at m/z 302.2 [M+H]+ and 324.2 [M+Na]+, respectively. The molecular formula of C19H27NO2 with seven degrees of unsaturation, was deduced from HRESIMS at m/z 324.1941 [M+Na]+ (calcd. for C19H27NO2, 324.1939), which was supported by the 1H- and 13C-NMR data (Table 1). The 1H-NMR spectrum of 1 showed resonances attributable to two olefinic protons at δH 5.16 (H-3) and 6.26 (H-18), three tertiary methyl groups at δH 1.55 (H3-11), 1.00 (H3-12), and 0.88 (H3-14), a secondary methyl group at δH 0.95 (H3-13). In addition, the spectrum showed resonances due to an exchangable amine proton at δH 7.33 (20-NH), as well as partially overlapping signals with complex coupling patterns between δH 1.08 and 2.61 that could be attributed to several aliphatic methylene and methine units. The 13C-NMR and DEPT spectra of 1 showed 19 carbon resonances, corresponding to two carbonyl groups (δC 171.7 and 170.4), two olefinic quaternary carbons (δC 143.9 and 147.9), two aliphatic quaternary carbons (δC 38.3 and 42.4), two olefinic methine carbons (δC 120.5 and 130.4), two aliphatic methine carbons (δC 37.4 and 47.0), five aliphatic methylene carbons (δC 19.0, 26.3, 36.2, 27.4, and 32.5), and four methyl carbons (δC 17.7, 19.8, 16.3, and 18.0). The above spectroscopic signatures suggested the presence of a 9,4-friedodrime sesquiterpene moiety and accounted for four degrees of unsaturation, indicating three rings in the structure of 1.| Position | δC | δH (J in Hz) | HMBC (H→C) | NOESY |
|---|---|---|---|---|
| 1α | 19.0, CH2 | 1.83, m | C-2, 3, 5, 9, 10 | H-1β, 2β, 10 |
| 1β | 1.53, m | C-2, 5, 10 | H3-12, 14, H-1α, 2β | |
| 2α | 26.3, CH2 | 1.93, m | H-1α, 1β, 10 | |
| 2β | 2.07, m | C-3, 4, 10 | H-1α, 1β, 2α | |
| 3 | 120.5, CH | 5.16, br s | C-5, 11 | H3-11, H-2α, 2β |
| 4 | 143.9, C | |||
| 5 | 38.3, C | |||
| 6α | 36.2, CH2 | 1.08, m | C-8 | H-6β, 7a, 8, 10 |
| 6β | 1.68, dt (12.8, 3.4) | C-7, 8, 10, 12 | H3-11, 12, H-6α, 7b | |
| 7a | 27.4, CH2 | 1.41, m | C-5, 6, 9, 13 | H-6, 8 |
| 7b | 1.40, dd (6.9, 3.5) | H-6, 8, H3-12, 13, 14 | ||
| 8 | 37.4, CH | 1.28, m | C-7, 9, 13 | H-7b, 10, H3-13 |
| 9 | 42.4, C | |||
| 10 | 47.0, CH | 1.12, dd (12.4, 1.6) | C-2, 4, 5, 9, 12, 14, 15 | H-1α, 2α, 8, 15α, 15β |
| 11 | 17.7, CH3 | 1.55, br s | C-3, 4, 5 | H3-12, H-3 |
| 12 | 19.8, CH3 | 1.00, s | C-4, 5, 6, 10 | H3-11, 14, H-6β, 7β |
| 13 | 16.3, CH3 | 0.95, d (6.7) | C-7, 8, 9 | H3-14, H-7β, 8 |
| 14 | 18.0, CH3 | 0.88, s | C-8, 9, 10, 15 | H3-12, 13, H-1β, 7β |
| 15α | 32.5, CH2 | 2.61, d (14.1) | C-8, 9, 10, 14, 16, 17, 18 | H3-14, H-1α, 10 |
| 15β | 2.43, dd (14.1, 1.2) | C-8, 9, 10, 14, 16, 17, 18 | H3-13 | |
| 16 | 147.9, C | |||
| 17 | 171.7, C | |||
| 18 | 130.4, CH | 6.26, d (1.0) | C-15, 16, 19 | H3-13, H-10, 15α, 15β |
| 19 | 170.4, C | |||
| 20-NH | 7.33, br s |
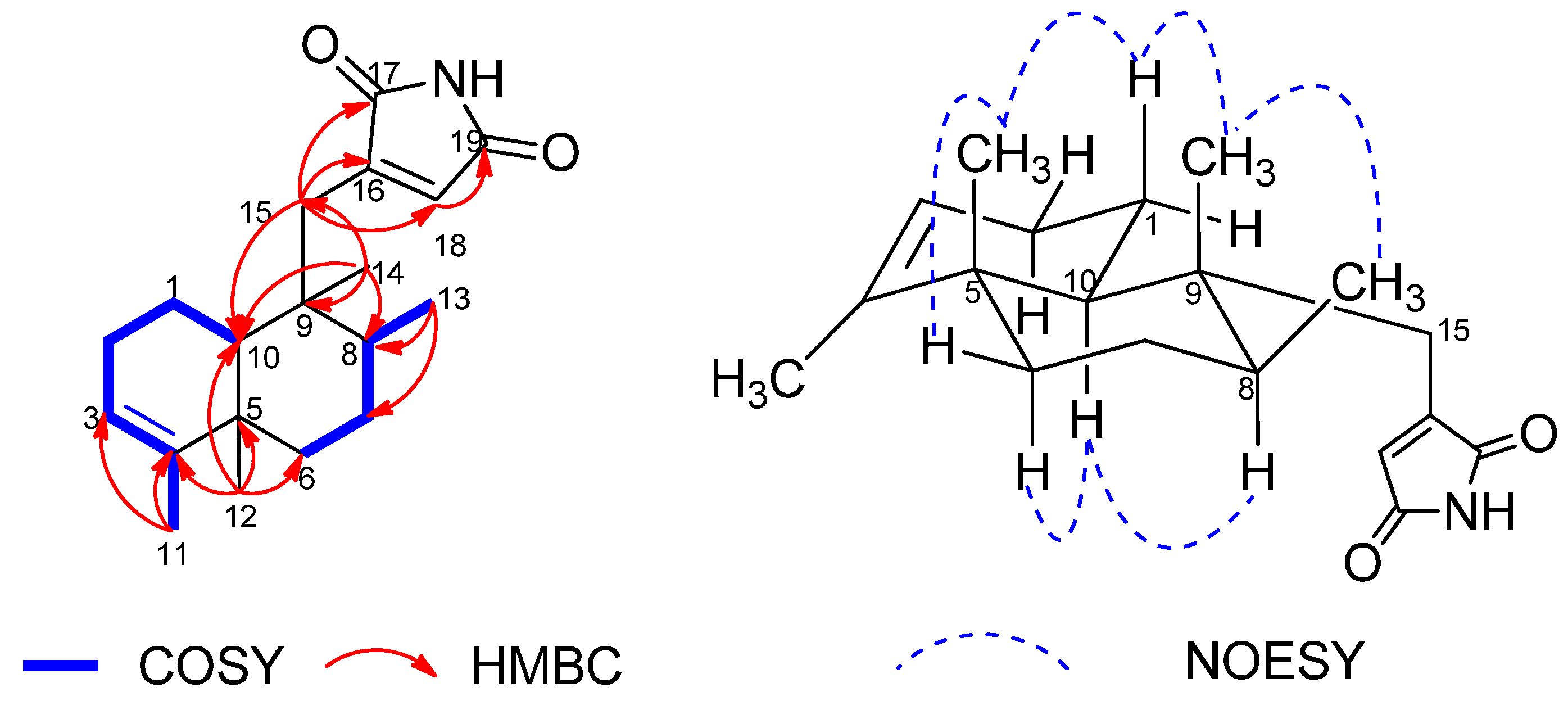
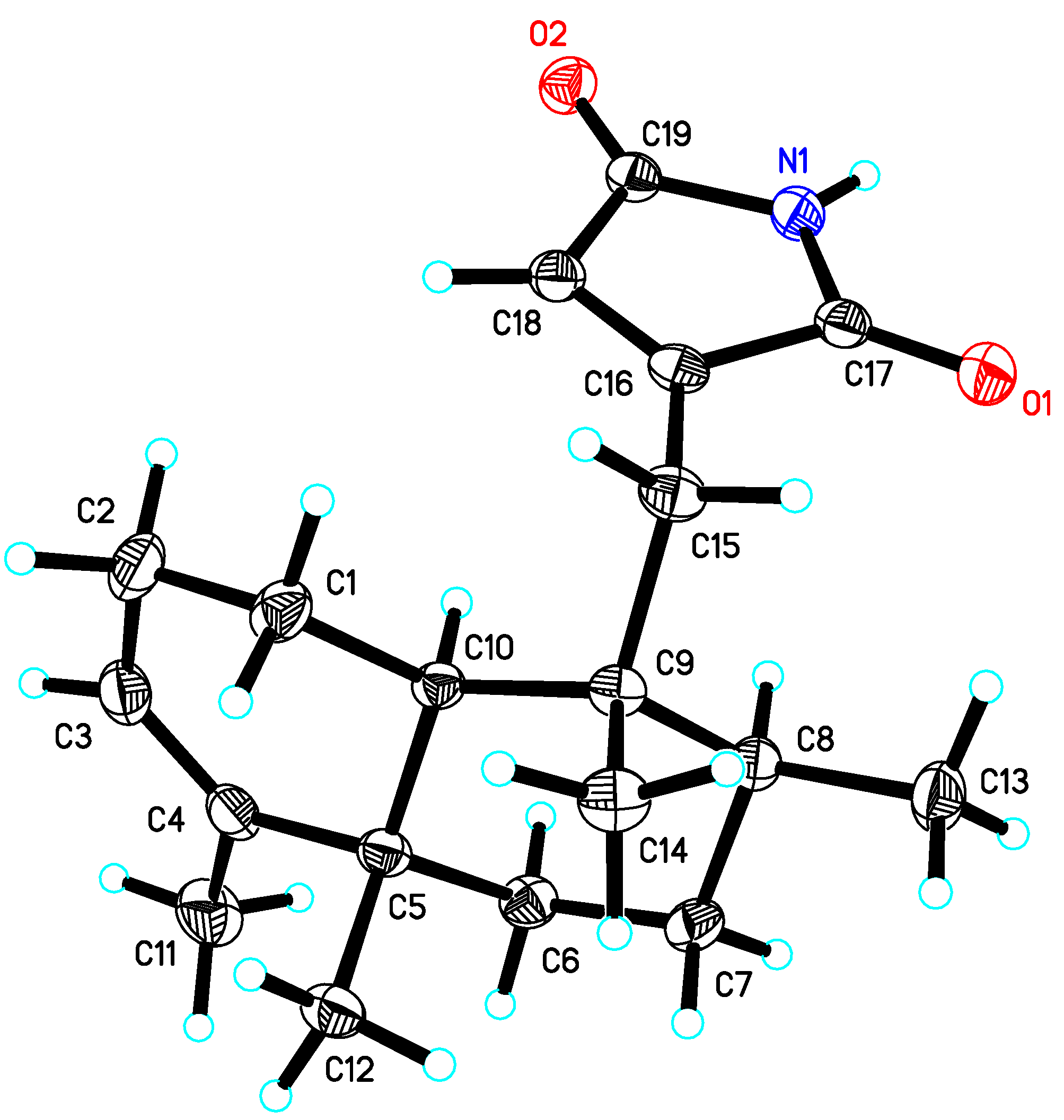
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction, Isolation and Characterization
 +35.4 (c 0.5, MeOH); UV (MeOH) λmax (log ε) 209 (4.05), 235 (398) nm; 1H and 13C-NMR, see Table 1; IR (KBr) υmax 3276, 2961, 2928, 2857, 1775, 1714, 1621, 1453, 1344, 1124, 1075, 871, 626 cm−1; positive ESIMS m/z 302.2 [M+H]+, 324.2 [M+Na]+; positive HRESIMS m/z 324.1941 [M+Na]+ (calcd for C19H27NO2, 324.1939).
+35.4 (c 0.5, MeOH); UV (MeOH) λmax (log ε) 209 (4.05), 235 (398) nm; 1H and 13C-NMR, see Table 1; IR (KBr) υmax 3276, 2961, 2928, 2857, 1775, 1714, 1621, 1453, 1344, 1124, 1075, 871, 626 cm−1; positive ESIMS m/z 302.2 [M+H]+, 324.2 [M+Na]+; positive HRESIMS m/z 324.1941 [M+Na]+ (calcd for C19H27NO2, 324.1939).3.4. X-ray Crystallographic Analysis of Dysidinoid A (1)
3.5. Antimicrobial Assays
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Figueroa, M.; Jarmusch, A.K.; Raja, H.A.; El-Elimat, T.; Kavanaugh, J.S.; Horswill, A.R.; Cooks, R.G.; Cech, N.B.; Oberlies, N.H. Polyhydroxyanthraquinones as quorum sensing inhibitors from the guttates of penicillium restrictum and their analysis by desorption electrospray ionization mass spectrometry. J. Nat. Prod. 2014, 77, 1351–1358. [Google Scholar]
- Li, T.; Song, Y.; Zhu, Y.; Du, X.; Li, M. Current status of Staphylococcus aureus infection in a central teaching hospital in Shanghai, China. BMC Microbiol. 2003, 13. [Google Scholar] [CrossRef]
- Payne, D.J. Desperately seeking new antibiotics. Science 2008, 321, 1644–1645. [Google Scholar]
- Plaza, A.; Keffer, J.L.; Bifulco, G.; Lloyd, J.R.; Bewley, C.A. Chrysophaentins A–H, antibacterial bisdiarylbutene macrocycles that inhibit the bacterial cell division protein FtsZ. J. Am. Chem. Soc. 2010, 132, 9069–9077. [Google Scholar]
- Li, M.; Du, X.; Villaruz, A.; Diep, B.; Wang, D.; Song, Y.; Tian, Y.; Hu, J.; Yu, F.; Lu, Y.; et al. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat. Med. 2012, 18, 816–819. [Google Scholar]
- Jiao, W.H.; Xu, T.T.; Yu, H.B.; Chen, G.D.; Huang, X.J.; Yang, F.; Li, Y.S.; Han, B.N.; Liu, X.Y.; Lin, H.W. Dysideanones A–C, unusual sesquiterpene quinones from the South China Sea sponge Dysidea avara. J. Nat. Prod. 2014, 77, 346–350. [Google Scholar]
- Jiao, W.H.; Xu, T.T.; Yu, H.B.; Mu, F.R.; Li, J.; Li, Y.S.; Yang, F.; Han, B.N.; Lin, H.W. Dysidaminones A–M, cytotoxic and NF-κB inhibitory sesquiterpene aminoquinones from the South China Sea sponge Dysidea fragilis. RSC Adv. 2014, 4, 9236–9246. [Google Scholar]
- Marcos, I.S.; Conde, A.; Moro, R.F.; Basabe, P.; Diez, D.; Urones, J.G. Quinone/hydroquinone sesquiterpenes. Mini-Rev. Org. Chem. 2010, 7, 230–254. [Google Scholar]
- Ueda, K.; Kadekaru, T.; Siwu, E.R.; Kita, M.; Uemura, D. Haterumadysins A–D, sesquiterpenes from the Okinawan Marine sponge Dysidea c hlorea. J. Nat. Prod. 2006, 69, 1077–1079. [Google Scholar]
- Lu, Q.; Faulkner, D.J. Three dolabellanes and a macrolide from the sponge Dysidea sp. from Palau. J. Nat. Prod. 1998, 61, 1096–1100. [Google Scholar]
- De Almeida Leone, P.; Redburn, J.; Hooper, J.N. A.; Quinn, R.J. Polyoxygenated Dysidea sterols that inhibit the binding of [I125] IL-8 to the human recombinant IL-8 receptor type A. J. Nat. Prod. 2000, 63, 694–697. [Google Scholar]
- Bai, R.; Paull, K.; Herald, C.; Malspeis, L.; Pettit, G.; Hamel, E. Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data. J. Biol. Chem. 1991, 266, 15882–15889. [Google Scholar]
- Fu, X.; Ferreira, M.L.G.; Schmitz, F.J.; Kelly-Borges, M. New diketopiperazines from the sponge Dysidea chlorea. J. Nat. Prod. 1998, 61, 1226–1231. [Google Scholar]
- Kazlauskas, R.; Murphy, P.; Warren, R.; Wells, R.; Blount, J. New quinones from a dictyoceratid sponge. Aust. J. Chem. 1978, 31, 2685–2697. [Google Scholar]
- Ciavatta, M.L.; Lopez Gresa, M.P.; Gavagnin, M.; Romero, V.; Melck, D.; Manzo, E.; Guo, Y.W.; van Soest, R.; Cimino, G. Studies on puupehenone-metabolites of a Dysidea sp.: Structure and biological activity. Tetrahedron 2007, 63, 1380–1384. [Google Scholar]
- Urban, S.; Capon, R.J. 5-epi-Isospongiaquinone, a new sesquiterpene/quinone antibiotic from an Australian Marine sponge, Spongia hispida. J. Nat. Prod. 1992, 55, 1638–1642. [Google Scholar]
- Pérez-García, E.; Zubía, E.; Ortega, M.J.; Carballo, J.L. Merosesquiterpenes from two sponges of the genus Dysidea. J. Nat. Prod. 2005, 68, 653–658. [Google Scholar]
- Jiao, W.H.; Huang, X.J.; Yang, J.S.; Yang, F.; Piao, S.J.; Gao, H.; Li, J.; Ye, W.C.; Yao, X.S.; Chen, W.S.; et al. Dysidavarones A–D, new sesquiterpene quinones from the marine sponge Dysidea avara. Org. Lett. 2012, 14, 202–205. [Google Scholar]
- McNamara, C.E.; Larsen, L.; Perry, N.B.; Harper, J.L.; Berridge, M.V.; Chia, E.W.; Kelly, M.; Webb, V.L. Anti-inflammatory sesquiterpene-quinones from the New Zealand sponge Dysidea cf. cristagalli. J. Nat. Prod. 2005, 68, 1431–1433. [Google Scholar]
- Utkina, N.K.; Denisenko, V.A.; Krasokhin, V.B. Sesquiterpenoid aminoquinones from the marine sponge Dysidea sp. J. Nat. Prod. 2010, 73, 788–791. [Google Scholar]
- Sample Availability: Sample of the compound 1is available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, W.-H.; Li, J.; Liu, Q.; Xu, T.-T.; Shi, G.-H.; Yu, H.-B.; Yang, F.; Han, B.-N.; Li, M.; Lin, H.-W. Dysidinoid A, an Unusual Meroterpenoid with Anti-MRSA Activity from the South China Sea Sponge Dysidea sp. Molecules 2014, 19, 18025-18032. https://doi.org/10.3390/molecules191118025
Jiao W-H, Li J, Liu Q, Xu T-T, Shi G-H, Yu H-B, Yang F, Han B-N, Li M, Lin H-W. Dysidinoid A, an Unusual Meroterpenoid with Anti-MRSA Activity from the South China Sea Sponge Dysidea sp. Molecules. 2014; 19(11):18025-18032. https://doi.org/10.3390/molecules191118025
Chicago/Turabian StyleJiao, Wei-Hua, Jing Li, Qian Liu, Ting-Ting Xu, Guo-Hua Shi, Hao-Bing Yu, Fan Yang, Bing-Nan Han, Min Li, and Hou-Wen Lin. 2014. "Dysidinoid A, an Unusual Meroterpenoid with Anti-MRSA Activity from the South China Sea Sponge Dysidea sp." Molecules 19, no. 11: 18025-18032. https://doi.org/10.3390/molecules191118025
APA StyleJiao, W.-H., Li, J., Liu, Q., Xu, T.-T., Shi, G.-H., Yu, H.-B., Yang, F., Han, B.-N., Li, M., & Lin, H.-W. (2014). Dysidinoid A, an Unusual Meroterpenoid with Anti-MRSA Activity from the South China Sea Sponge Dysidea sp. Molecules, 19(11), 18025-18032. https://doi.org/10.3390/molecules191118025






