Flavonoids from Gynostemma pentaphyllum Exhibit Differential Induction of Cell Cycle Arrest in H460 and A549 Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC-MS Analysis of Flavonoids from G. pentaphyllum
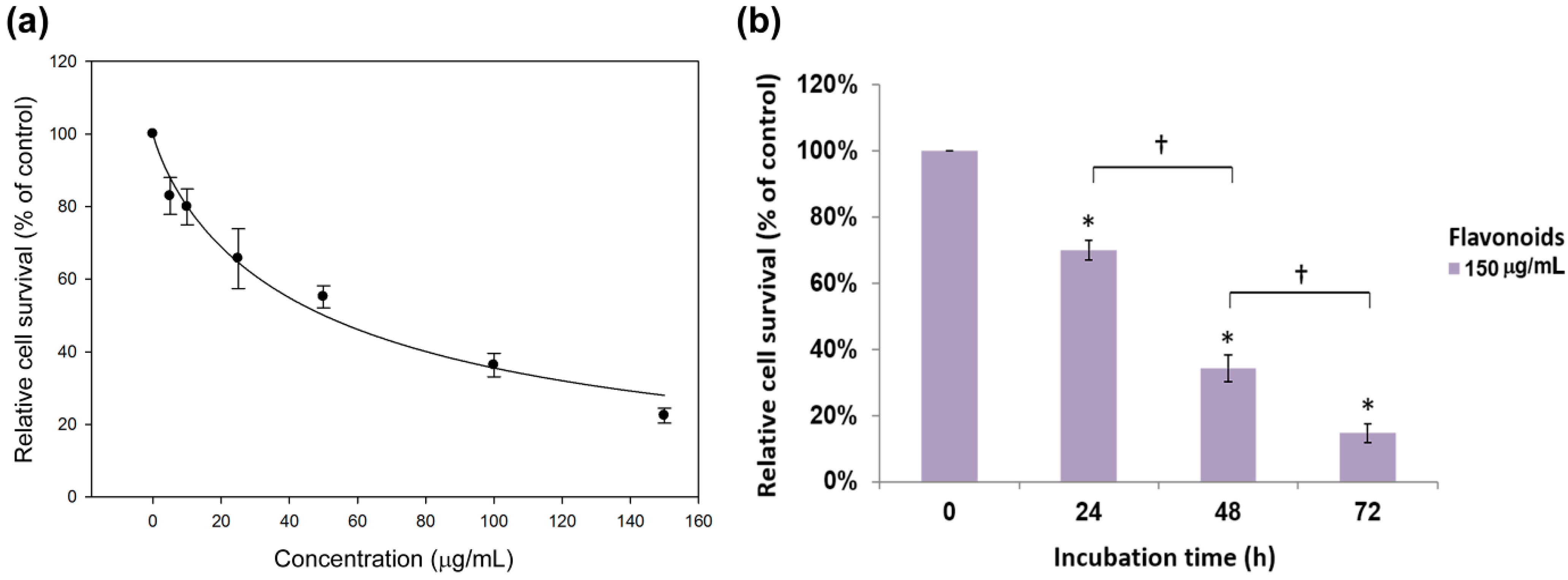
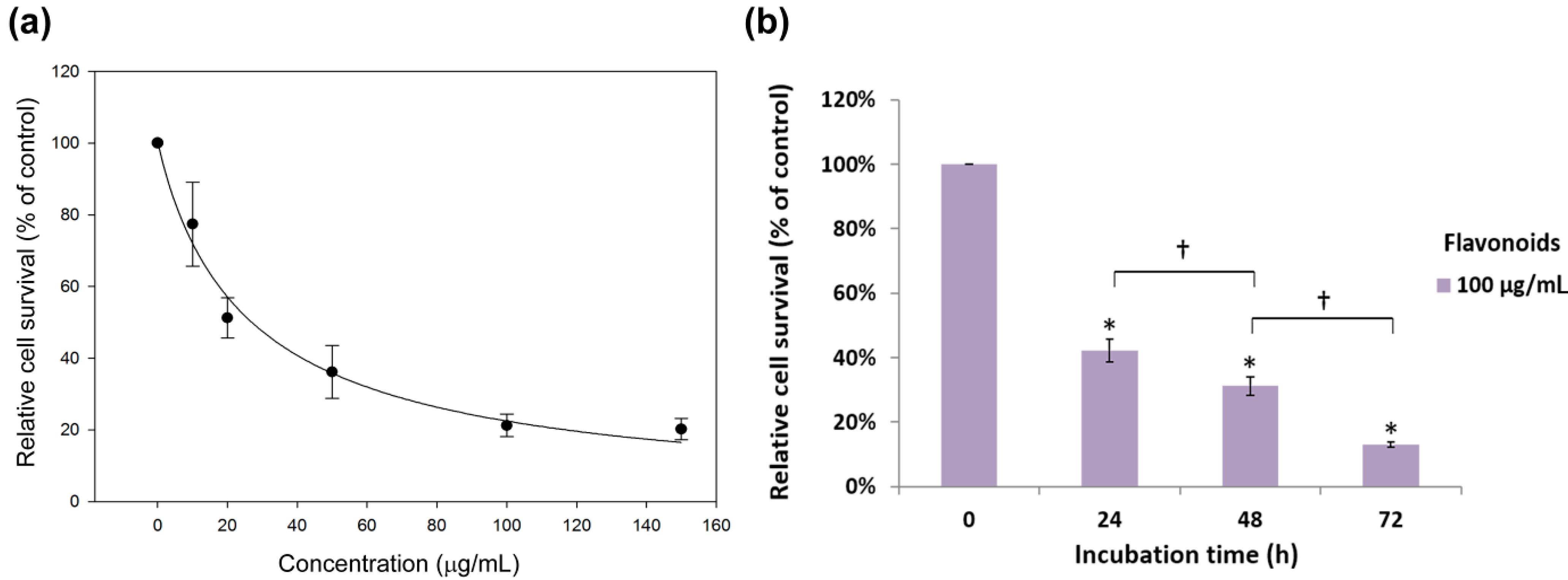
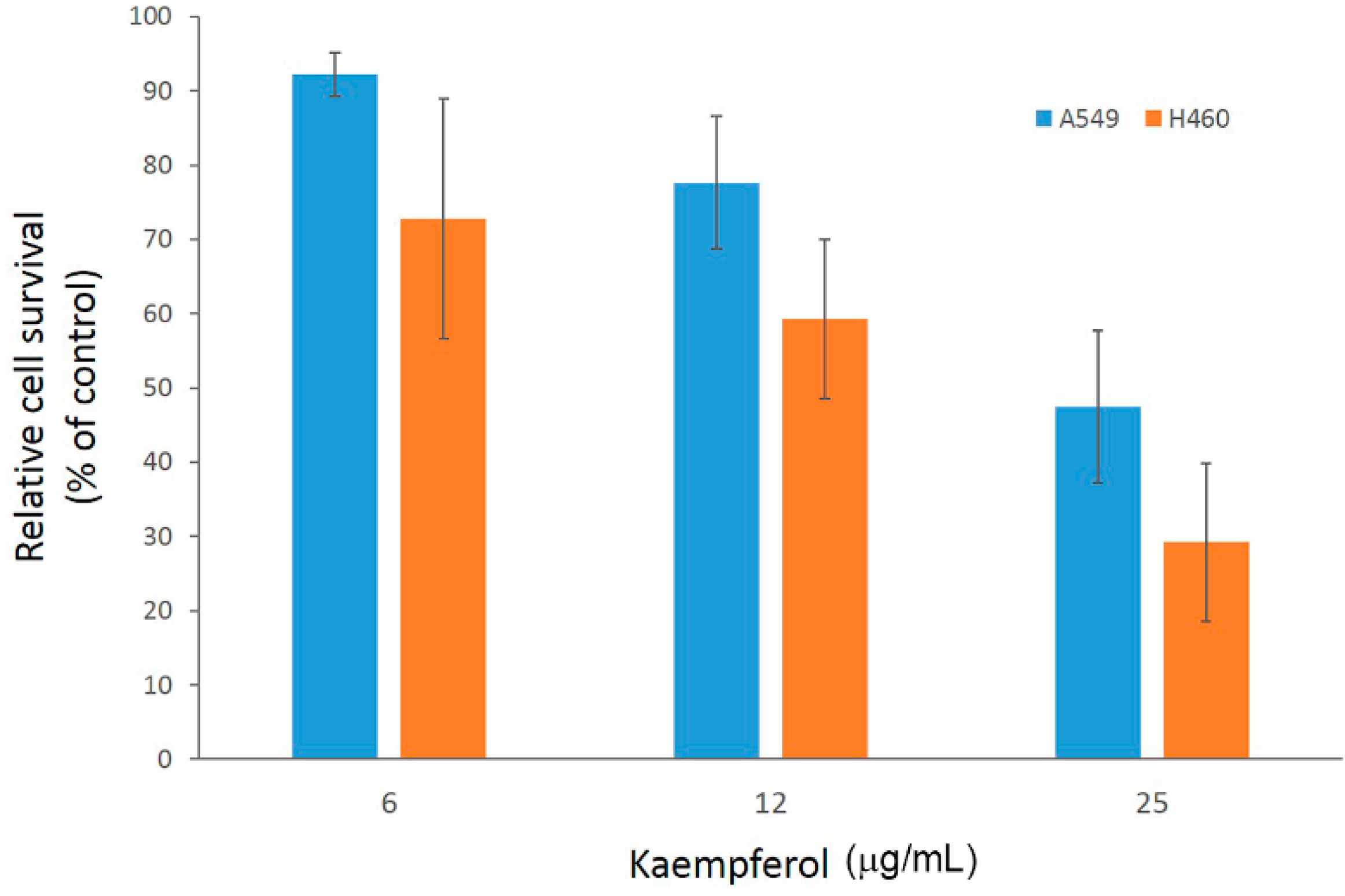
2.2. Antiproliferation Effects of Flavonoids on H460 and A549
2.3. Cell Cycle Analysis


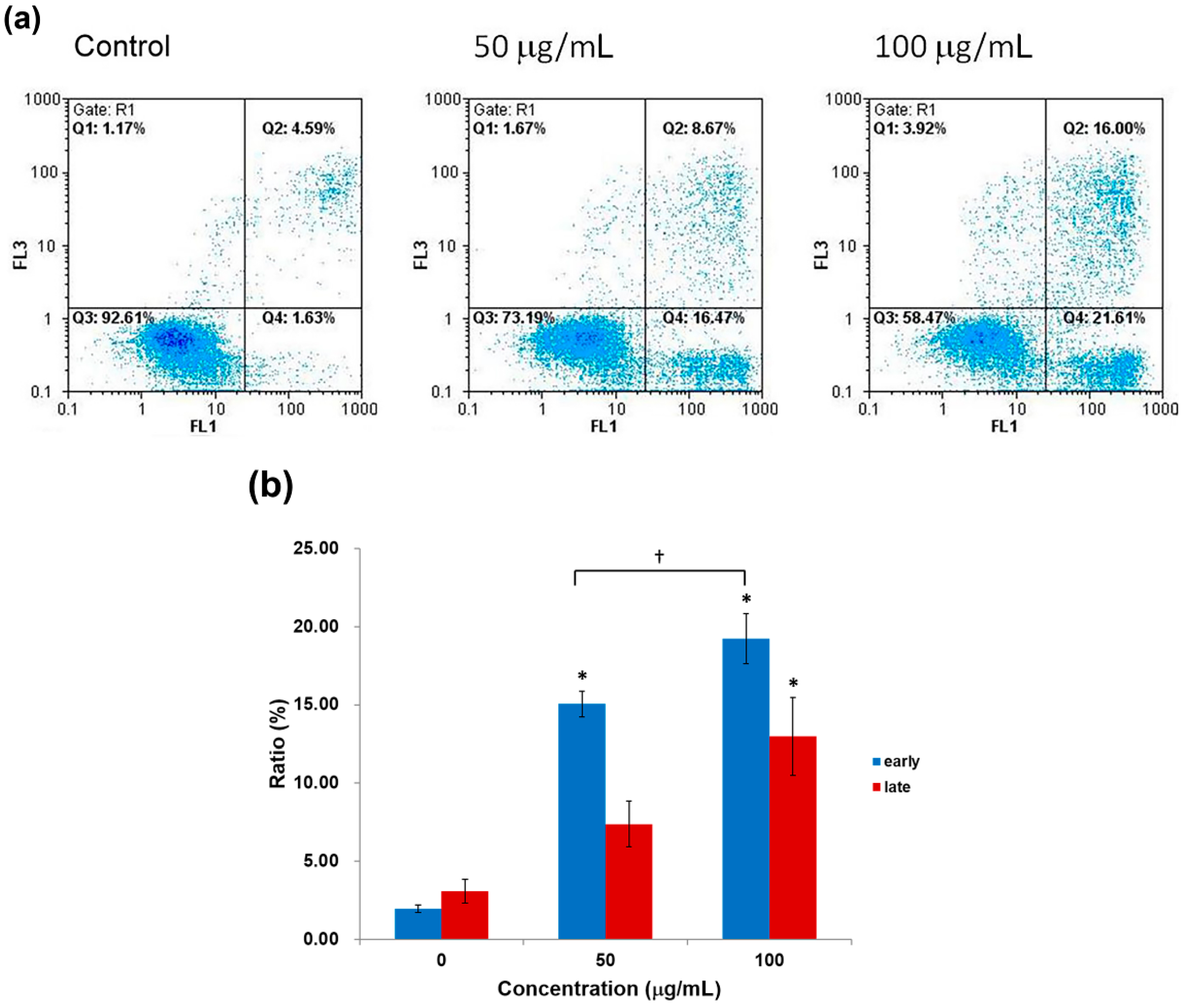
2.4. Apoptosis Analysis by Annexin-V/PI Staining Assay

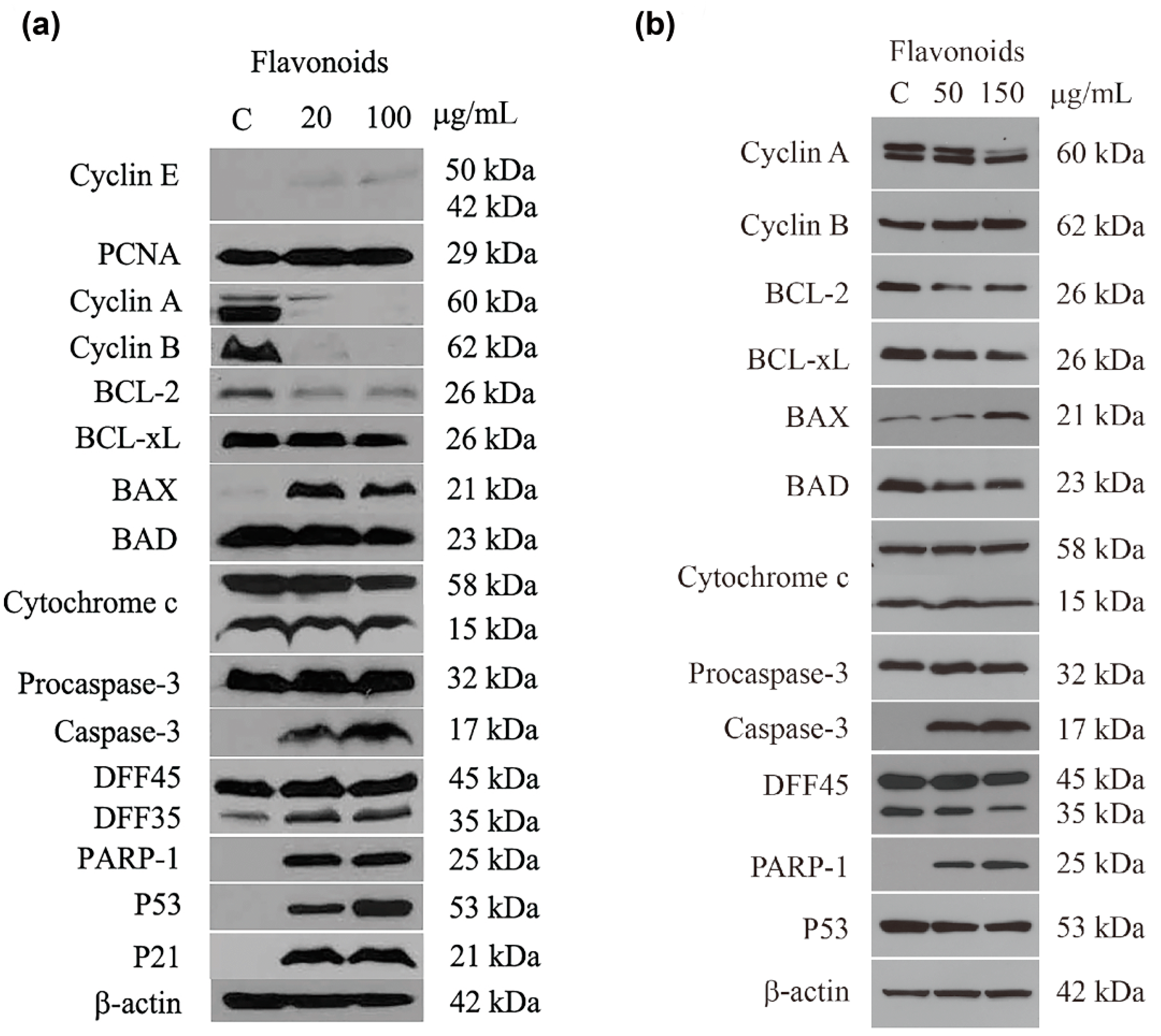
2.5. Expression of Proteins Associated with Cell Cycle Control and Apoptosis
3. Experimental Section
3.1. Materials
3.2. Extraction and Preparation of Flavonoids from G. pentaphyllum
3.3. MTT Assay
3.4. Cell Cycle Analysis
3.5. Annexin V and PI Staining Assay
3.6. Western Blotting
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar]
- Wall, M.E.; Wani, M.C. Camptothecin and taxol: Discovery to clinic—Thirteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 1995, 55, 753–760. [Google Scholar]
- Schild, L.; Chen, B.H.; Makarov, P.; Kattengell, K.; Heinitz, K.; Keilhoff, G. Selective induction of apoptosis in glioma tumour cells by a Gynostemma pentaphyllum extract. Phytomedicine 2010, 17, 589–597. [Google Scholar]
- Tsai, Y.C.; Lin, C.L.; Chen, B.H. Preparative chromatography of flavonoids and saponins in Gynostemma pentaphyllum and their antiproliferation effect on hepatoma cell. Phytomedicine 2010, 18, 2–10. [Google Scholar]
- Tsai, Y.C.; Wu, W.B.; Chen, B.H. Preparation of carotenoids and chlorophylls from Gynostemma pentaphyllum (Thunb.) Makino and their antiproliferation effect on hepatoma cell. J. Med. Food 2010, 13, 1431–1442. [Google Scholar]
- Yuan, G.; Wei, J.; Zhou, J.; Guo, X.; Yang, M. Apoptosis of human hepatoma cells induced by Gynostemma pentaphyllum Makino. Chin. Ger. J. Clin. Oncol. 2006, 5, 173–177. [Google Scholar]
- Lu, K.W.; Chen, J.C.; Lai, T.Y.; Yang, J.S.; Weng, S.W.; Ma, Y.S.; Lin, H.Y.; Wu, R.S.; Wu, K.C.; Wood, W.G.; et al. Gypenosides suppress growth of human oral cancer SAS cells in vitro and in a murine xenograft model: The role of apoptosis mediated by caspase-dependent and caspase-independent pathways. Integr. Cancer Ther. 2012, 11, 129–140. [Google Scholar]
- Cheng, T.C.; Lu, J.F.; Wang, J.S.; Lin, L.J.; Kuo, H.I.; Chen, B.H. Antiproliferation effect and apoptosis mechanism of prostate cancer cell PC-3 by flavonoids and saponins prepared from Gynostemma pentaphyllum. J. Agric. Food Chem. 2011, 59, 11319–11329. [Google Scholar]
- Piao, X.L.; Wu, Q.; Yang, J.; Park, S.Y.; Chen, D.J.; Liu, H.M. Dammarane-type saponins from heat-processed Gynostemma pentaphyllum show fortified activity against A549 cells. Arch. Pharm. Res. 2013, 36, 874–879. [Google Scholar]
- Liou, C.J.; Huang, W.C.; Kuo, M.L.; Yang, R.C.; Shen, J.J. Long-term oral administration of Gynostemma pentaphyllum extract attenuates airway inflammation and Th2 cell activities in ovalbumin-sensitized mice. Food Chem. Toxicol. 2010, 48, 2592–2598. [Google Scholar]
- Huang, W.C.; Kuo, M.L.; Li, M.L.; Yang, R.C.; Liou, C.J.; Shen, J.J. Gynostemma pentaphyllum decreases allergic reactions in a murine asthmatic model. Am. J. Chin. Med. 2008, 36, 579–592. [Google Scholar]
- Yang, F.; Shi, H.; Zhang, X.; Yang, H.; Zhou, Q.; Yu, L.L. Two new saponins from tetraploid jiaogulan (Gynostemma pentaphyllum), and their anti-inflammatory and alpha-glucosidase inhibitory activities. Food Chem. 2013, 141, 3606–3613. [Google Scholar]
- Xie, Z.; Liu, W.; Huang, H.; Slavin, M.; Zhao, Y.; Whent, M.; Blackford, J.; Lutterodt, H.; Zhou, H.; Chen, P.; et al. Chemical composition of five commercial Gynostemma pentaphyllum samples and their radical scavenging, antiproliferative, and anti-inflammatory properties. J. Agric. Food Chem. 2010, 58, 11243–11249. [Google Scholar]
- Li, L.; Jiao, L.; Lau, B.H. Protective effect of gypenosides against oxidative stress in phagocytes, vascular endothelial cells and liver microsomes. Cancer Biother. 1993, 8, 263–272. [Google Scholar]
- Megalli, S.; Davies, N.M.; Roufogalis, B.D. Anti-hyperlipidemic and hypoglycemic effects of Gynostemma pentaphyllum in the Zucker fatty rat. J. Pharm. Pharm. Sci. 2006, 9, 281–291. [Google Scholar]
- Huyen, V.T.; Phan, D.V.; Thang, P.; Hoa, N.K.; Ostenson, C.G. Antidiabetic effect of Gynostemma pentaphyllum tea in randomly assigned type 2 diabetic patients. Horm. Metab. Res. 2010, 42, 353–357. [Google Scholar]
- Norberg, A.; Hoa, N.K.; Liepinsh, E.; van Phan, D.; Thuan, N.D.; Jornvall, H.; Sillard, R.; Ostenson, C.G. A novel insulin-releasing substance, phanoside, from the plant Gynostemma pentaphyllum. J. Biol. Chem. 2004, 279, 41361–41367. [Google Scholar]
- Circosta, C.; De Pasquale, R.; Occhiuto, F. Cardiovascular effects of the aqueous extract of Gynostemma pentaphyllum Makino. Phytomedicine 2005, 12, 638–643. [Google Scholar]
- Megalli, S.; Aktan, F.; Davies, N.M.; Roufogalis, B.D. Phytopreventative anti-hyperlipidemic effects of Gynostemma pentaphyllum in rats. J. Pharm. Pharm. Sci. 2005, 8, 507–515. [Google Scholar]
- Tanner, M.A.; Bu, X.; Steimle, J.A.; Myers, P.R. The direct release of nitric oxide by gypenosides derived from the herb Gynostemma pentaphyllum. Nitric Oxide 1999, 3, 359–365. [Google Scholar]
- Shi, L.; Song, D.P.; Pan, M.J.; Liu, D.B. Research advances on the saponins of Gynostemma pentaphyllum. Drug Eval. Res. 2011, 34, 456–464. [Google Scholar]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar]
- Huang, W.W.; Tsai, S.C.; Peng, S.F.; Lin, M.W.; Chiang, J.H.; Chiu, Y.J.; Fushiya, S.; Tseng, M.T.; Yang, J.S. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int. J. Oncol. 2013, 42, 2069–2077. [Google Scholar]
- Yan, H.; Wang, X.; Wang, Y.; Wang, P.; Xiao, Y. Antiproliferation and anti-migration induced by gypenosides in human colon cancer SW620 and esophageal cancer Eca-109 cells. Hum. Exp. Toxicol. 2014, 33, 522–533. [Google Scholar]
- Chen, J.C.; Lu, K.W.; Lee, J.H.; Yeh, C.C.; Chung, J.G. Gypenosides induced apoptosis in human colon cancer cells through the mitochondria-dependent pathways and activation of caspase-3. Anticancer Res. 2006, 26, 4313–4326. [Google Scholar]
- Lin, J.J.; Hsu, H.Y.; Yang, J.S.; Lu, K.W.; Wu, R.S.; Wu, K.C.; Lai, T.Y.; Chen, P.Y.; Ma, C.Y.; Wood, W.G.; et al. Molecular evidence of anti-leukemia activity of gypenosides on human myeloid leukemia HL-60 cells in vitro and in vivo using a HL-60 cells murine xenograft model. Phytomedicine 2011, 18, 1075–1085. [Google Scholar]
- Lu, K.W.; Chen, J.C.; Lai, T.Y.; Yang, J.S.; Weng, S.W.; Ma, Y.S.; Tang, N.Y.; Lu, P.J.; Weng, J.R.; Chung, J.G. Gypenosides causes DNA damage and inhibits expression of DNA repair genes of human oral cancer SAS cells. In Vivo 2010, 24, 287–291. [Google Scholar]
- Chen, J.C.; Lu, K.W.; Tsai, M.L.; Hsu, S.C.; Kuo, C.L.; Yang, J.S.; Hsia, T.C.; Yu, C.S.; Chou, S.T.; Kao, M.C.; et al. Gypenosides induced G0/G1 arrest via CHk2 and apoptosis through endoplasmic reticulum stress and mitochondria-dependent pathways in human tongue cancer SCC-4 cells. Oral Oncol. 2009, 45, 273–283. [Google Scholar]
- Lu, H.F.; Chen, Y.S.; Yang, J.S.; Chen, J.C.; Lu, K.W.; Chiu, T.H.; Liu, K.C.; Yeh, C.C.; Chen, G.W.; Lin, H.J.; et al. Gypenosides induced G0/G1 arrest via inhibition of cyclin E and induction of apoptosis via activation of caspases-3 and -9 in human lung cancer A-549 cells. In Vivo 2008, 22, 215–221. [Google Scholar]
- Park, K.I.; Park, H.S.; Nagappan, A.; Hong, G.E.; Lee do, H.; Kang, S.R.; Kim, J.A.; Zhang, J.; Kim, E.H.; Lee, W.S.; et al. Induction of the cell cycle arrest and apoptosis by flavonoids isolated from Korean Citrus aurantium L. in non-small-cell lung cancer cells. Food Chem. 2012, 135, 2728–2735. [Google Scholar]
- Youn, H.; Jeong, J.C.; Jeong, Y.S.; Kim, E.J.; Um, S.J. Quercetin potentiates apoptosis by inhibiting nuclear factor-kappaB signaling in H460 lung cancer cells. Biol. Pharm. Bull. 2013, 36, 944–951. [Google Scholar]
- Howlader, N.N.A.; Krapcho, M.; Garshell, J.; Neyman, N.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Cho, H.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2010; National Cancer Institute: Bethesda, MD, USA, 2008. Based on November 2012 SEER Data Submission, Posted to the SEER Website. Available online: http://seer.cancer.gov/csr/1975_2010/ (accessed on 19 September 2013).
- Levine, A.J. p53, the cellular gatekeeper for growth and division. Cell 1997, 88, 323–331. [Google Scholar]
- Ii, T.; Satomi, Y.; Katoh, D.; Shimada, J.; Baba, M.; Okuyama, T.; Nishino, H.; Kitamura, N. Induction of cell cycle arrest and p21(CIP1/WAF1) expression in human lung cancer cells by isoliquiritigenin. Cancer Lett. 2004, 207, 27–35. [Google Scholar]
- Choi, E.J.; Ahn, W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract. 2008, 2, 322–325. [Google Scholar]
- Kuo, P.C.; Liu, H.F.; Chao, J.I. Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J. Biol. Chem. 2004, 279, 55875–55885. [Google Scholar]
- Nguyen, T.T.; Tran, E.; Ong, C.K.; Lee, S.K.; Do, P.T.; Huynh, T.T.; Nguyen, T.H.; Lee, J.J.; Tan, Y.; Ong, C.S.; et al. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J. Cell. Physiol. 2003, 197, 110–121. [Google Scholar]
- Nguyen, T.T.; Tran, E.; Nguyen, T.H.; Do, P.T.; Huynh, T.H.; Huynh, H. The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis 2004, 25, 647–659. [Google Scholar]
- Sample Availability: Samples of the flavonoids extracted from G. pentaphyllum are available from the authors J.F.L. and B.H.C.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsui, K.-C.; Chiang, T.-H.; Wang, J.-S.; Lin, L.-J.; Chao, W.-C.; Chen, B.-H.; Lu, J.-F. Flavonoids from Gynostemma pentaphyllum Exhibit Differential Induction of Cell Cycle Arrest in H460 and A549 Cancer Cells. Molecules 2014, 19, 17663-17681. https://doi.org/10.3390/molecules191117663
Tsui K-C, Chiang T-H, Wang J-S, Lin L-J, Chao W-C, Chen B-H, Lu J-F. Flavonoids from Gynostemma pentaphyllum Exhibit Differential Induction of Cell Cycle Arrest in H460 and A549 Cancer Cells. Molecules. 2014; 19(11):17663-17681. https://doi.org/10.3390/molecules191117663
Chicago/Turabian StyleTsui, Ko-Chung, Tzu-Hsuan Chiang, Jinn-Shyan Wang, Li-Ju Lin, Wei-Chih Chao, Bing-Huei Chen, and Jyh-Feng Lu. 2014. "Flavonoids from Gynostemma pentaphyllum Exhibit Differential Induction of Cell Cycle Arrest in H460 and A549 Cancer Cells" Molecules 19, no. 11: 17663-17681. https://doi.org/10.3390/molecules191117663
APA StyleTsui, K.-C., Chiang, T.-H., Wang, J.-S., Lin, L.-J., Chao, W.-C., Chen, B.-H., & Lu, J.-F. (2014). Flavonoids from Gynostemma pentaphyllum Exhibit Differential Induction of Cell Cycle Arrest in H460 and A549 Cancer Cells. Molecules, 19(11), 17663-17681. https://doi.org/10.3390/molecules191117663






