Exogenous Spermidine Improves Seed Germination of White Clover under Water Stress via Involvement in Starch Metabolism, Antioxidant Defenses and Relevant Gene Expression
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Exogenous Spermidine on Seed Germination Characteristics
| PEG (%) | Germination Percentage (%) | Germination Vigor (%) | Germination Index | Mean Germination Time (d) | ||||
|---|---|---|---|---|---|---|---|---|
| Water | Spd | Water | Spd | Water | Spd | Water | Spd | |
| 0 | 95.5 ± 2.5 a | 97.5 ± 1.0 a | 95.5 ± 2.5 a | 97.5 ± 5.1 a | 45.38 ± 1.65 a | 47.88 ± 0.48 a * | 1.11 ± 0.05 d | 1.04 ± 0.03 d * |
| 10 | 92.0 ± 1.6 a | 96.5 ± 1.0 a * | 87.3 ± 8.3 a | 94.7 ± 2.3 a | 39.62 ± 4.15 b | 43.56 ± 1.80 b | 1.41 ± 0.32 c | 1.27 ± 0.12 c |
| 15 | 78.7 ± 3.1 b | 86.7 ± 3.6 b * | 74.7 ± 3.1 b | 83.3 ± 5.0 b * | 31.93 ± 2.20 c | 38.16 ± 1.97 c * | 1.54 ± 0.11 b c | 1.33 ± 0.04 c * |
| 18 | 54.7 ± 8.1 c | 59.5 ± 4.4 c | 53.3 ± 4.1 c | 58.7 ± 4.2 c | 16.53 ± 0.55 d | 21.98 ± 1.21 d * | 1.72 ± 0.18 b a | 1.70 ± 0.11 b |
| 20 | 23.5 ± 6.6 d | 26.0 ± 7.1 d | 23.0 ± 7.0 d | 25.0 ± 8.2 d | 6.36 ± 1.87 e | 6.86 ± 1.90 e | 1.89 ± 0.10 a | 1.90 ± 0.12 a |
| PEG (%) | Seedling Fresh Weight (mg·10 Seedling−1) | Seedling Dry Weight (mg·10 Seedling−1) | Root Length (cm) | Seed Vigour Index | ||||
|---|---|---|---|---|---|---|---|---|
| Water | Spd | Water | Spd | Water | Spd | Water | Spd | |
| 0 | 86.7 ± 4.8 a | 86.1 ± 4.4 a | 5.5 ± 0.4 b | 5.6 ± 0.07 c | 2.97 ± 0.15 b | 3.46 ± 0.15 a * | 4.02 ± 0.48 a | 4.01 ± 0.17 a |
| 10 | 55.3 ± 6.3 b | 65.1 ± 2.4 b * | 5.7 ± 0.6 b a | 8.0 ± 0.10 a * | 3.20 ± 0.03 a | 3.45 ± 0.21 a * | 2.26 ± 0.45 b | 2.84 ± 0.10 b |
| 15 | 47.4 ± 1.1 c | 50.7 ± 3.6 c | 6.0 ± 0.06 b a | 7.2 ± 0.03 b a * | 2.69 ± 0.10 c | 2.88 ± 0.12 b | 1.52 ± 0.14 c | 1.91 ± 0.09 c * |
| 18 | 37.4 ± 2.9 d | 40.5 ± 1.4 d | 5.9 ± 0.09 b a | 6.5 ± 0.10 b | 2.23 ± 0.22 d | 2.37 ± 0.08 c | 0.70 ± 0.18 d | 0.88 ± 0.01 d |
| 20 | 24.2 ± 4.5 e | 24.4 ± 1.2 e | 6.2 ± 0.02 a | 6.7 ± 0.03 b | 1.47 ± 0.05 e | 1.60 ± 0.14 d | 0.16 ± 0.07 e | 0.23 ± 0.02 e |
2.2. Effect of Exogenous Spermidine on Carbohydrate Levels
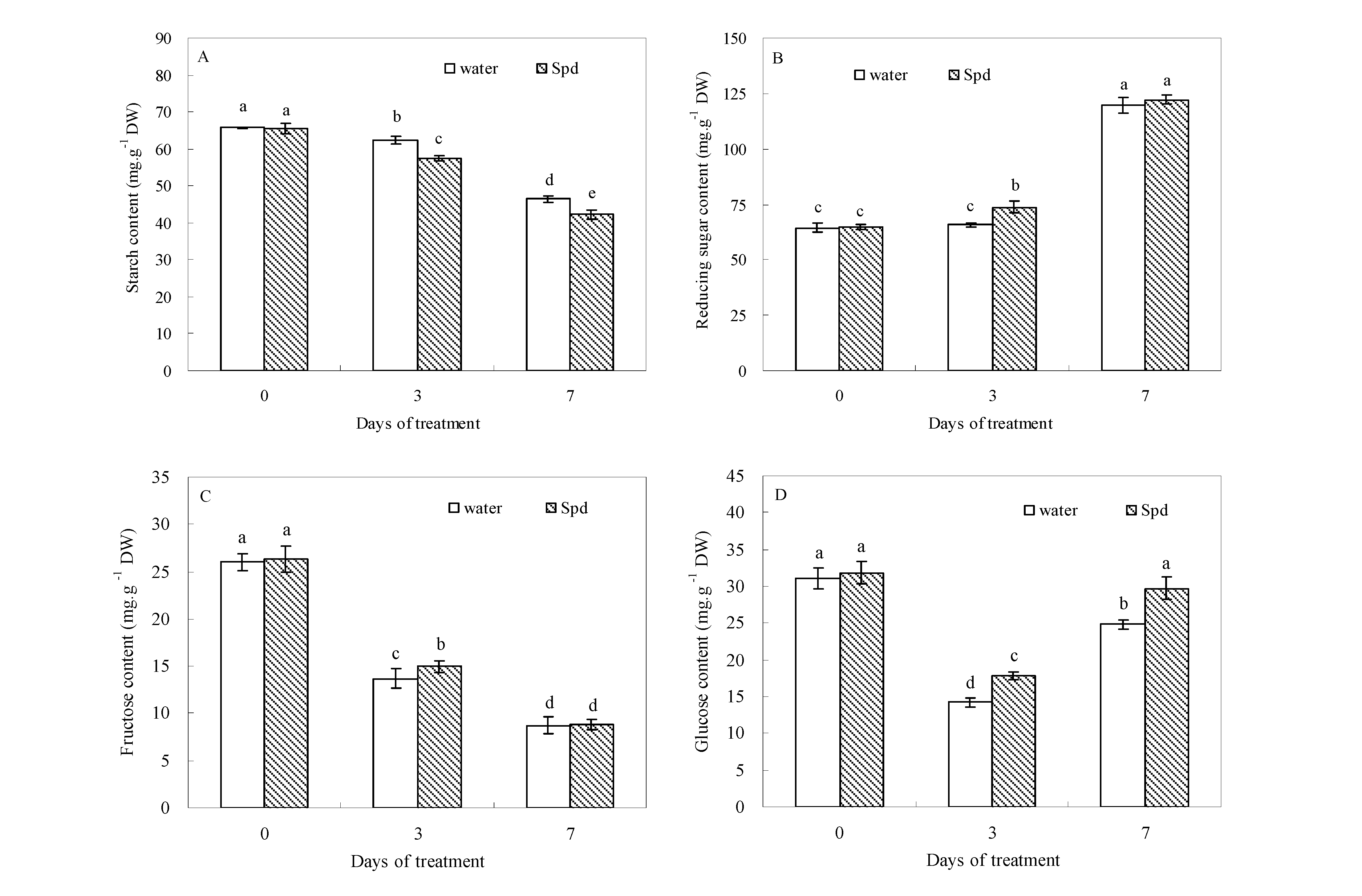
2.3. Effect of Exogenous Spermidine on Amylase Activities and Gene Relative Expression
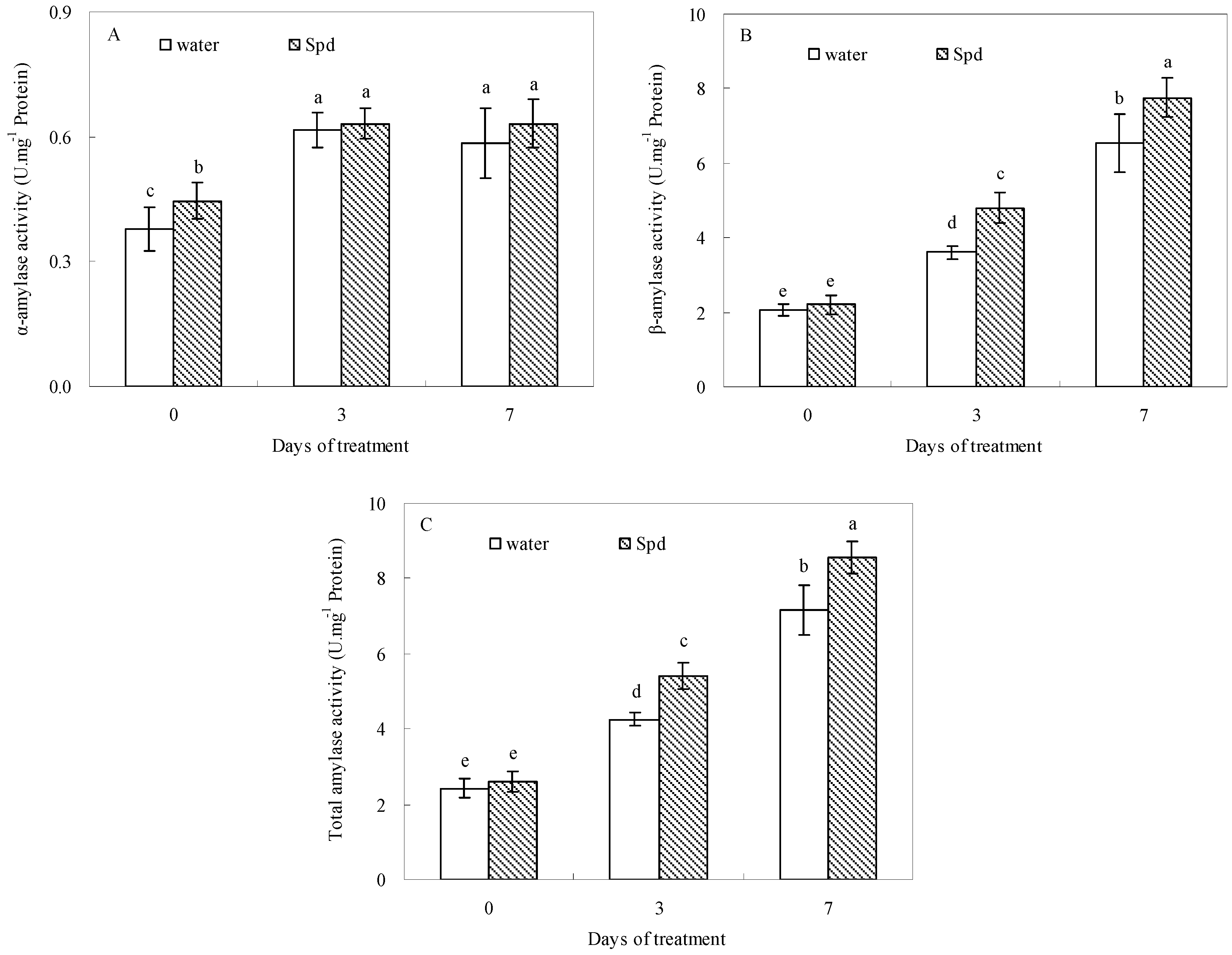
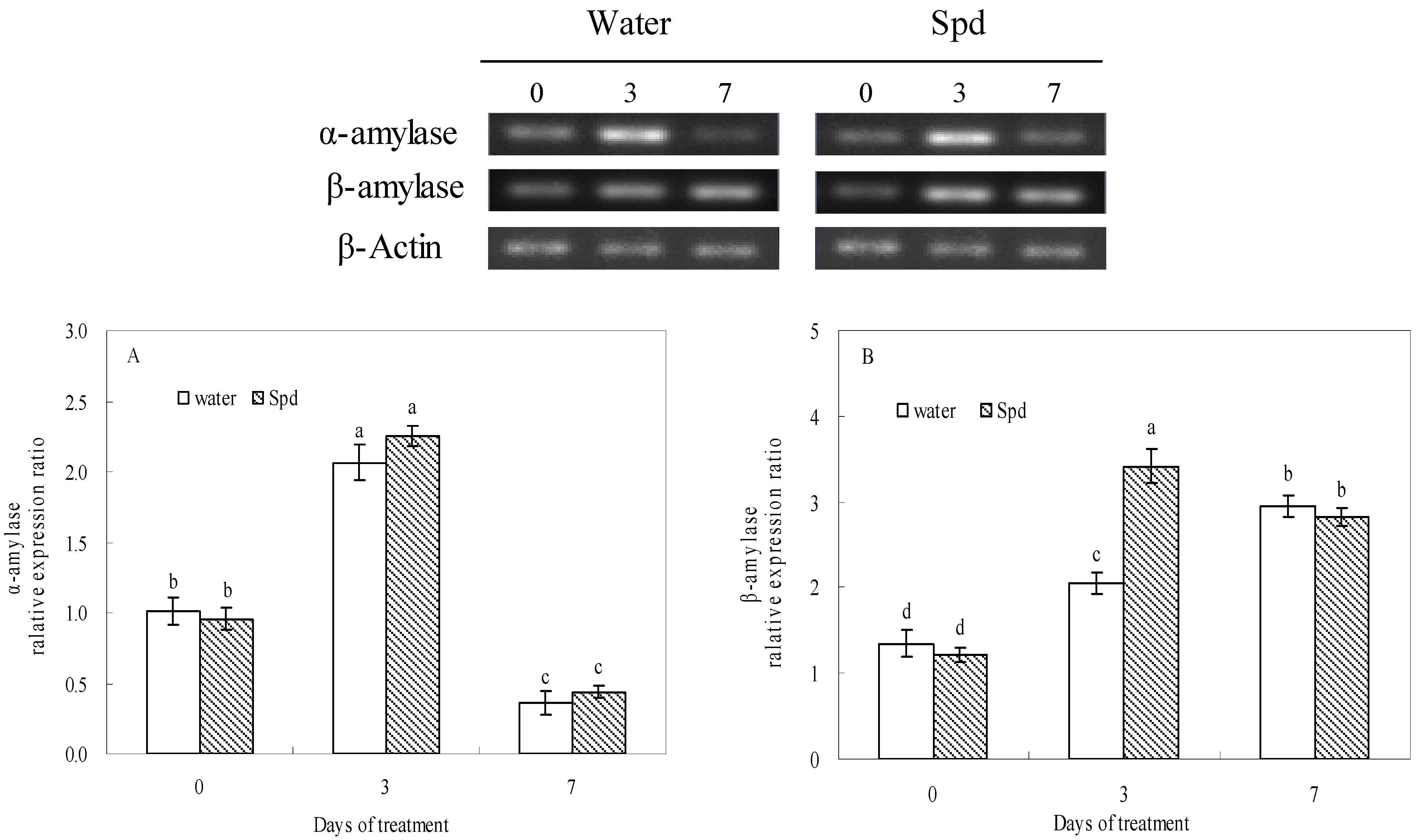
2.4. Effect of Exogenous Spermidine on Reactive Oxygen Species Production and Membrane Damage
2.5. Effect of Exogenous Spermidine on Antioxidant Enzyme Activities and Gene Relative Expression
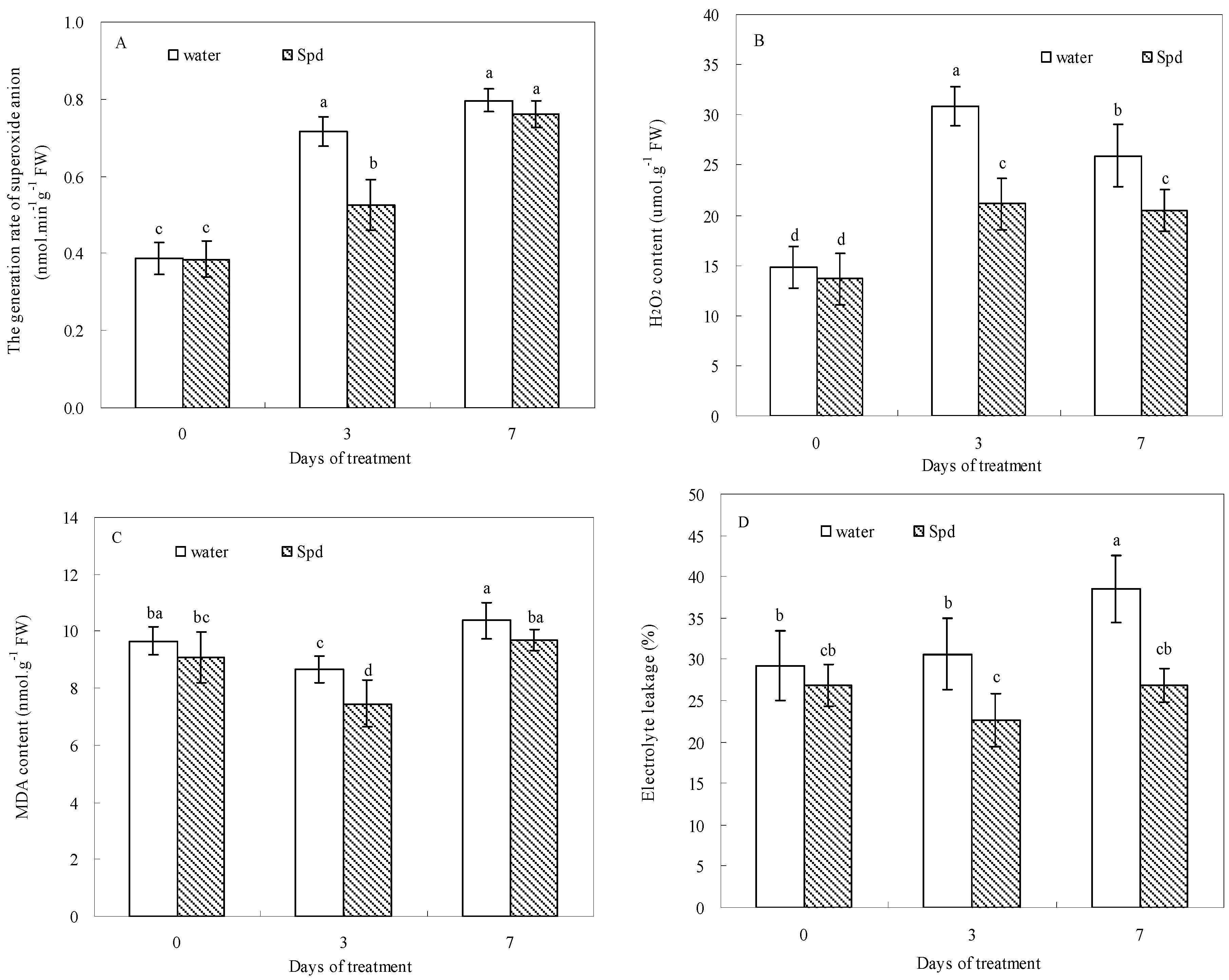
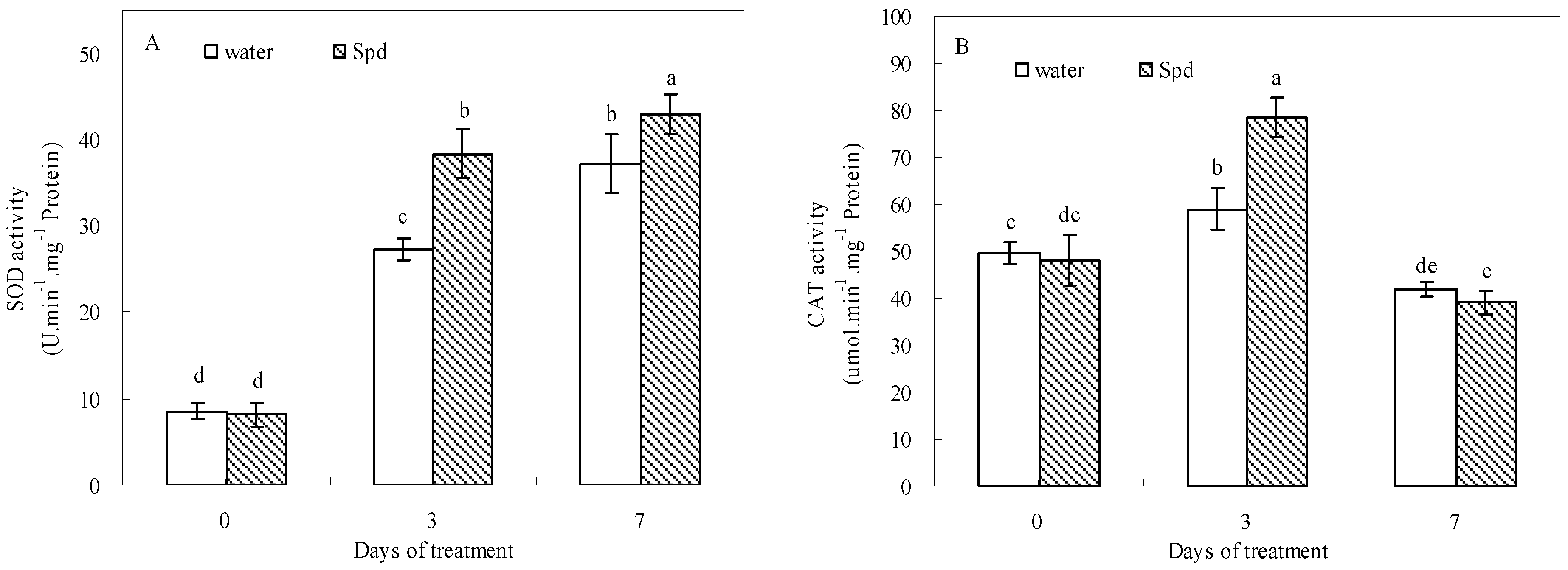
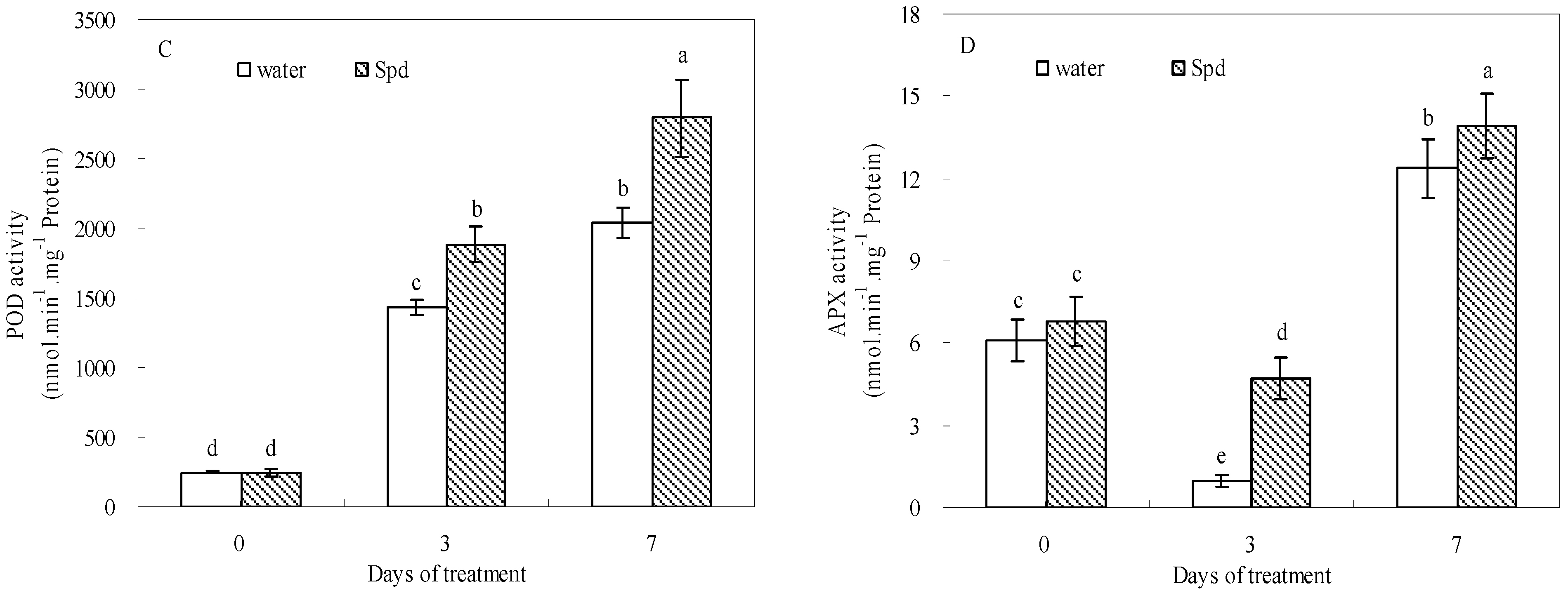
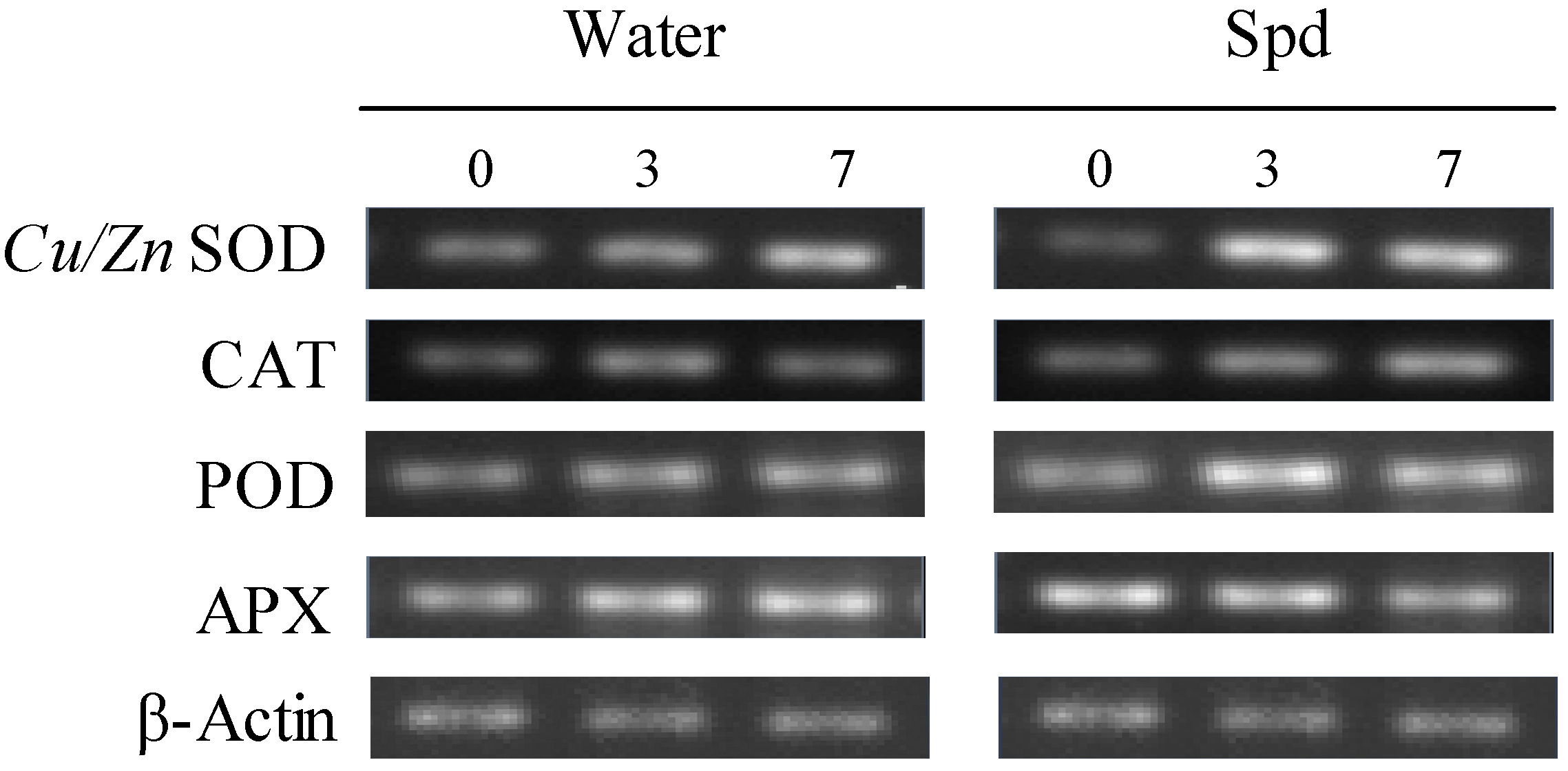

| Enzyme Activity | Gene Expression | |||||
|---|---|---|---|---|---|---|
| 0 day | 3 days | 7 days | 0 day | 3 days | 7 days | |
| α-amylase | + | ns | ns | ns | ns | ns |
| β-amylase | ns | + | + | ns | + | ns |
| Cu/ZnSOD | ns | + | + | ns | + | + |
| CAT | ns | + | ns | ns | ns | + |
| POD | ns | + | + | ns | + | + |
| APX | ns | + | + | + | ns | — |
| Treatment | Content (umol.g−1 FW) | |||
|---|---|---|---|---|
| AsA | DAsA | AsA + DAsA | AsA/DAsA | |
| Water | 18.77 ± 0.66 b | 3.45 ± 0.46 a | 20.97 ± 1.31 b | 5.21 ± 1.14 b |
| Spd | 21.42 ± 1.46 a | 1.52 ± 0.20 b | 23.59 ± 1.04 a | 14.59 ± 1.33 a |
2.6. Effect of Exogenous Spermidine on Ascorbic Acid and Glutathione Content
| Treatment | Content (umol.g−1 FW) | |||
|---|---|---|---|---|
| GSH | GSSG | GSH+GSSG | GSH/GSSG | |
| water | 1.37 ± 0.423 b | 2.01 ± 0.21 a | 3.29 ± 0.27 b | 0.75 ± 0.21 b |
| Spd | 2.29 ± 0.21 a | 1.86 ± 0.11 a | 4.01 ± 0.30 a | 1.07 ± 0.19 a |
2.7. Effect of Exogenous Spermidine on Root Viability
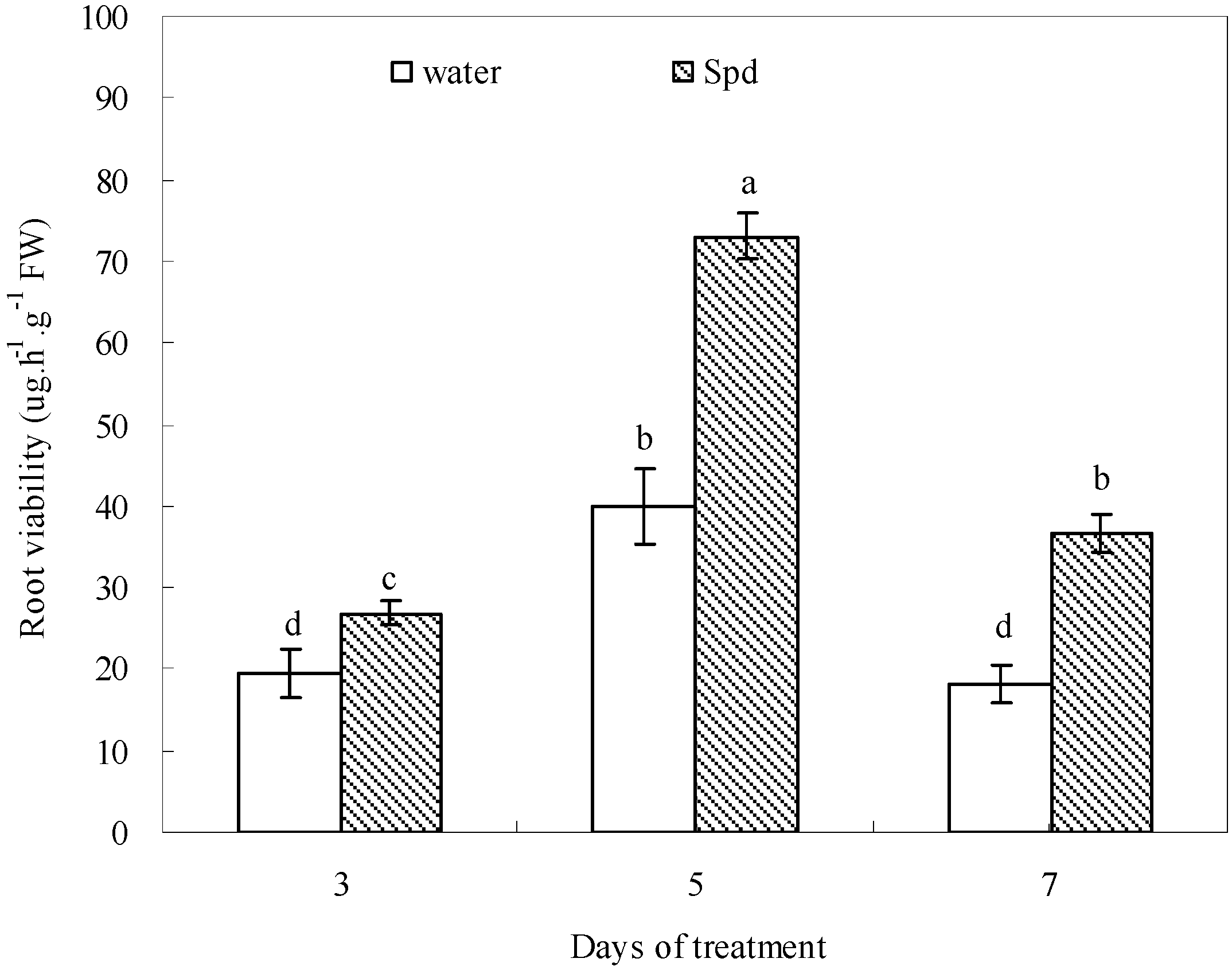
2.8. Discussion
3. Experimental Section
3.1. Plant Materials and Treatments
3.2. Determination of Seed Germination Characteristics
3.3. Carbohydrate and Amylase Activity
3.4. Reactive Oxygen Species and Electrolyte Leakage
3.5. Antioxidant Enzyme Activity, Ascorbic Acid, Glutathione and Malondialdehyde Content
3.6. Root Viability
3.7. Gene Expression Analysis
| Target Gene | Accession No. | Forward Primer (5'–3') | Reverse Primer (5'–3') |
|---|---|---|---|
| α-amylase | FY463398.1 | ATGGATGCGACCAAACCTTACT | GCAGCACTAATCCAGTCAACGA |
| β-amylase | AF049098.1 | TCAGCAGGTATTGAGTGGAGGTT | TTGACACCTTGTGGTCTTGCAT |
| Cu/ZnSOD | FY461274 | TCACCTTCCACTTTCAAACCTCTC | TGTTGGACCTTCGTCTTCTTGAGT |
| CAT | FY464988 | GTCTTCTTTGTTCACGATGGGATG | GAAAGTGGGAGAAGAAGTCAAGGAT |
| POD | AJ011939 | TCTAGGGCAACGGTTAATTCATTC | GGTACGGATTTTCCCATTTCTTG |
| APX | FY460674 | GCAGCATCAGTTGGCAAGACC | GGCAAACCTGAGACTAAATACACGA |
| β-Actin | JF968419 | TTACAATGAATTGCGTGTTG | AGAGGACAGCCTGAATGG |
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Foolad, M.; Subbiah, P.; Kramer, C.; Hargrave, H.; Lin, G.Y. Genetic relationships among cold, salt and drought tolerance during seed germination in an interspecific cross of tomato. Euphytica 2003, 130, 199–206. [Google Scholar] [CrossRef]
- Farooq, M.; Aziz, T.; Rehman, H.U.; Rehman, A.U.; Cheema, S.A. Evaluating surface drying and re-drying for wheat seed priming with polyamines: Effects on emergence, early seedling growth and starch metabolism. Acta Physiol. Plant. 2011, 33, 1707–1713. [Google Scholar] [CrossRef]
- Liptay, A.; Schopfer, P. Effect of water stress, seed coat restraint, and abscisic acid upon different germination capabilities of two tomato lines at low temperature. Plant Physiol. 1983, 73, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Siegen, N.; Lewak, S. Growth regulators differentially affect photosensitivity induced by low temperature and osmotic stresses in germinating white clover seeds. Plant Growth Regul. 1992, 11, 133–137. [Google Scholar] [CrossRef]
- Bradford, K.J. Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. Hortscience 1986, 21, 1105–1112. [Google Scholar]
- Naeem, M.A.; Muhammad, S. Effect of seed priming on growth of barley (Hordeum vulgare) by using brackish water in salt affected soils. Pak. J. Bot. 2006, 38, 613–622. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Khalid, M.; Tabassum, R.; Mehmood, T. Nutrient homeostasis, reserves metabolism and seedling vigor as affected by seed priming in coarse rice. Can. J. Bot. 2006, 84, 1196–1202. [Google Scholar] [CrossRef]
- Nathawat, N.S.; Nair, J.S.; Kumawat, S.M.; Yadava, N.S.; Singh, G.; Ramaswamy, N.K.; Sahu, M.P.; D’Souza, S.F. Effect of seed soaking with thiols on the antioxidant enzymes and photosystem activities in wheat subjected to water stress. Biol. Plant. 2007, 51, 93–97. [Google Scholar] [CrossRef]
- Shang, J.; Zhao, K. Organic substance metabolism during seed germination of Pinus bungeana. J. Northeast For. Univ. 1993, 4, 66–69. [Google Scholar]
- Sun, Z.T.; Henson, C.A. A quantitative assessment of the importance of barley seed α-amylase, β-amylase, debranching enzyme, and α-glucosidase in starch degradation. Arch. Biochem. Biophys. 1991, 284, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Machovic, M.; Janecek, S. Starch-binding domains in the post-genome era. Cell. Mol. Life Sci. 2006, 63, 2710–2724. [Google Scholar] [CrossRef] [PubMed]
- Catusse, J.; Job, C.; Jon, D. Proteomics reveals a potential role of the perisperm in starch remobilization during sugabeet seed germination. Bus. Media Dordr. 2012, 2, 27–41. [Google Scholar]
- Zheng, C.F.; Jiang, D.; Liu, F.; Dai, T.B.; Liu, W.H.; Jing, Q.; Cao, W.X. Exogenous nitric oxide improves seeds germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ. Exp. Bot. 2009, 67, 222–227. [Google Scholar] [CrossRef]
- Hendry, G.A.F. Oxygen free radical process and seed longevity. Seed Sci. Res. 1993, 3, 141–153. [Google Scholar] [CrossRef]
- Tambussi, E.A.; Bartoli, C.G.; Beltrano, J.; Guiamet, J.J.; Arans, J.L. Oxidative damage to thylakoid proteins in water stressed leaves of wheat (Triticum aestivum). Physiol. Plant. 2000, 108, 398–404. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.Q.; Kong, B.B.; Lin, T.B. Exogenous trehalose differentially modulate antioxidant defense system in wheat callus during water deficit and subsequent recovery. Plant Growth Regul. 2013, 70, 275–285. [Google Scholar] [CrossRef]
- Martin-Tanguy, J. Metabolism and function of polyamines in plants: Recent development (new approaches). Plant Growth Regul. 2001, 34, 135–148. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Basu, S.; Sengupta, D.N. Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J. Plant Physiol. 2011, 168, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, R.K.; Sawhney, V.K. Polyamine research in plants—A changing perspective. Physiol. Plant. 2002, 116, 281–292. [Google Scholar] [CrossRef]
- Tang, W.; Newton, R.J. Polyamines reduce salt-induced oxidative damage by increasing the activities of antioxidant enzymes and decreasing lipid peroxidation in Virginia pine. Plant Growth Regul. 2005, 46, 31–43. [Google Scholar] [CrossRef]
- Alcazar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Serafini-Fracassini, D. Biochemistry and Physiology of Polyamines in Plants. In Cell Cycle-Dependent Changes in Plant Polyamine Metabolism; Slocum, R.D., Flores, H.E., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 159–171. [Google Scholar]
- Xu, S.C.; Hu, J.; Li, Y.P.; Ma, W.G.; Zheng, Y.Y.; Zhu, S.J. Chilling tolerance in Nicotiana tabacum induced by seed proming with putrescine. Plant Growth Regul. 2011, 63, 279–290. [Google Scholar] [CrossRef]
- Zhao, M.G.; Liu, R.J.; Chen, L.; Tian, Q.Y.; Zhang, W.H. Glucose-induced inhibition of seed germination in Lotus japonicus is alleviated by nitric oxide and spermine. J. Plant Physiol. 2009, 166, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Basra, S.M.A.; Hussain, M.; Rehman, H.; Saleem, B.A. Incorporation of polyamines in the priming media enhances the germination and early seedling growth in hybrid sunflower (Helianthus annus L.). Int. J. Agric. Biol. 2007, 9, 868–872. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Rehman, H.; Hussain, M. Seed priming with polyamines improves the germination and early seedling growth in fine rice. J. New Seeds 2008, 9, 145–155. [Google Scholar] [CrossRef]
- Yiu, J.C.; Liu, C.W.; Fang, D.Y.T.; Lai, Y.S. Waterlogging tolerance of welsh onion (Allium fistulosum L.) enhanced by exogenous spermidine and spermine. Plant Physiol. Biochem. 2009, 47, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Kasukabe, Y.; He, L.; Nada, K.; Misawa, S.; Iharu, I.; Tachibana, S. Overexpression of spermidine synthase enhances tolerance to multiple environmental stress and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.R.; Lynch, T.J. Abscisic acid inhibition of radicle emergence but not seedling growth is suppressed by sugars. Plant Physiol. 2000, 122, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Beligni, M.V.; Lamattina, L. Nitric oxide stimulates seed germination and de-etiolation and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 2000, 210, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.J.; Serrano, M.; Pretel, M.T.; Amoros, A.; Botella, M.A. Polyamines and ethylene changes during germination of different plant species under salinity. Plant Sci. 2004, 167, 781–788. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Lee, D.J. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol. Plant. 2009, 31, 937–945. [Google Scholar] [CrossRef]
- Sinska, I.; Lewandowska, U. Polyamines and ethylene in the removal of embryonal dormancy in apple seeds. Physiol. Plant. 1991, 81, 59–64. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, A.; Feng, Z. Amelioration of chilling stress by triadimefon in cucumber seedlings. Plant Growth Regul. 2003, 39, 277–283. [Google Scholar] [CrossRef]
- Li, Z.; Shi, P.; Peng, Y. Improved drought tolerance through drought preconditioning associated with changes in antioxidant enzyme activities, gene expression and osmoregulatory solutes accumulation in white clover (Trifolium repens L.). Plant Omics 2013, 6, 481–489. [Google Scholar]
- Xu, Y.C.; Wang, J.; Shan, L.; Dong, X.; Li, M.M. Effect of exogenous polyamines on glycolate oxidase activity and active oxygen species accumulation in wheat seedlings under osmotic stress. Isr. J. Plant. Sci. 2001, 49, 173–178. [Google Scholar] [CrossRef]
- Kubis, J. The effect of exogenous spermidine on superoxide dismutase activity, H2O2 and superoxide radical level in barley leaves under water deficit conditions. Acta Physiol. Plant. 2005, 27, 289–295. [Google Scholar] [CrossRef]
- He, L.X.; Ban, Y.; Inoue, H.; Matsuda, N.; Liu, J.H.; Moriguchi, T. Enhancement of spermidine content and antioxidant capacity in transgentic pear shoots overexpressing apple spermidine synthase in response to salinity and hyperosmosis. Phytochemistry 2008, 69, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.P.; Ban, Y.; Inoue, H.; Matsuda, N.; Moriguchi, T. Aluminum tolerance in a spermidine synthase-overexpressiong transgenic European pear is correlated with the enhanced level of spermidine via alleviating oxidative status. Environ. Exp. Bot. 2009, 66, 471–478. [Google Scholar] [CrossRef]
- Wen, X.P.; Ban, Y.; Inoue, H.; Matsuda, N.; Kita, M.; Moriguchi, T. Antisense inhibition of a spermidine synthase gene highlights the role of polyamines for stress alleviation in pear shoots subjected to salinity and cadmium. Environ. Exp. Bot. 2011, 72, 157–166. [Google Scholar] [CrossRef]
- Li, C.Z.; Jiao, J.; Wang, G.X. The important roles of reactive oxygen species in the relationship between ethylene and polyamines in leaves of spring wheat seedlings under root osmotic stress. Plant Sci. 2004, 166, 303–315. [Google Scholar] [CrossRef]
- Asada, K. Ascorbate peroxidase—A hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant. 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Ma, X. Different response on drought tolerance and post-drought recovery between the small-leafed and the large-leafed white clover (Trifolium repens L.) associated with antioxidantive enzyme protection and lignin metabolism. Acta Physiol. Plant. 2013, 35, 214–222. [Google Scholar]
- Xu, L.X.; Han, L.B.; Huang, B.R. Antioxidant enzyme activities and gene expression patterns in leaves of Kentucky bluegrass in response to drought and post-drought recovery. J. Am. Soc. Hortic. Sci. 2011, 136, 247–255. [Google Scholar]
- Huang, H.; Villanueva, V.R. Amino acids, polyamines and proteins during seed germination of two species of Dipterocarpaceae. Trees Struct. Funct. 1992, 7, 189–193. [Google Scholar]
- Cvikrova, M.; Binarova, P.; Cenklova, V.; Edera, J.; Macháčková, I. Reinitiation of cell division and polyamine and aromatic monoamine levels in alfalfa explants during the induction of somatic embryogenesis. Physiol. Plant. 1999, 105, 330–337. [Google Scholar] [CrossRef]
- Liu, H.P.; Yu, B.J.; Zhang, W.H.; Liu, Y.L. Effect of osmotic stress on the activity of H+-ATPase and the levels of covalently and non-covalently conjugated polyamines in plasma membrane preparation from wheat seedling roots. Plant Sci. 2005, 168, 1599–1607. [Google Scholar] [CrossRef]
- Stark, F.; Pfannstiel, J.; Klaiber, I.; Raabe, T. Protein kinase CK2 links polyamine metabolism to MAPK signalling in Drosophila. Cell. Signal. 2011, 23, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, A.F.; Besford, R.T.; Capell, T.; Borell, A.; Testillano, P.S.; Risueno, M.C. Mechanism of polyamine action during senescence responses induced by osmotic stress. J. Exp. Bot. 1994, 45, 1789–1800. [Google Scholar] [CrossRef]
- Sudha, G.; Ravishankar, G.A. Involvement and interaction of various signaling compounds on the plant metabolic events during defense responses, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell Tissue Org. 2002, 71, 181–212. [Google Scholar] [CrossRef]
- Childs, A.C.; Mehta, D.J.; Germer, E.W. Polyamine-dependent gene expression. Cell. Mol. Life Sci. 2003, 60, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Du, H.M.; Zhou, P.; Huang, B.R. Antioxidant enzymatic activities and gene expression associated with heat tolerance in a cool-season perennial grass species. Environ. Exp. Bot. 2013, 87, 159–166. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, J.; Zhang, Y.; Xie, X.J.; Knapp, A. Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aust. J. Agric. Res. 2007, 58, 811–815. [Google Scholar] [CrossRef]
- Fu, J.; Dernoeden, P.H. Carbohydrate level, photosynthesis, and respiration in creeping bentgrass as influenced by spring and summer coring. J. Am. Soc. Hortic. Sci. 2009, 134, 41–47. [Google Scholar]
- Ting, S. Rapid calorimetric methods for simultaneous determination of total reducing sugars and fructose in citrus juices. J. Agric. Food Chem. 1956, 4, 263–266. [Google Scholar] [CrossRef]
- Smith, D. Removing and Analyzing Total Nonstructural Carbohydrates from Plant Tissue; Research Division, College of Agricultural and Life Sciences, University of Wisconsin: Madison, WI, USA, 1969; pp. 1–11. [Google Scholar]
- Tarrago, J.F.; Nicolas, G. Starch degradation in the cotyledons of germinating lentils. Plant Physiol. 1976, 58, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Kishorekumar, A.; Abdul, J.C.; Manivannan, P.; Sankar, B.; Sridharan, R.; Panneerselvam, R. Comparative effects of different triazole compounds on growth, photosynthetic pigments and carbohydrate metabolism of Solenostemon rotundifolius. Colloids Surf. B 2007, 60, 207–212. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidase. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Millhollon, E.P.; Lucas, M.C. Antioxidant response to NaCl stress in salt-tolerant to and salt-sensitive cultivars of cotton. Crop Sci. 1994, 34, 706–714. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Dhindsa, P.P.; Thorpe, T.A. Leaf senescence: Correlated with increased leaves of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Knievel, D.P. Procedure for estimating ratio of living to dead root dry matter in the root core sample. Crop Sci. 1973, 13, 124–126. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Peng, Y.; Zhang, X.-Q.; Ma, X.; Huang, L.-K.; Yan, Y.-H. Exogenous Spermidine Improves Seed Germination of White Clover under Water Stress via Involvement in Starch Metabolism, Antioxidant Defenses and Relevant Gene Expression. Molecules 2014, 19, 18003-18024. https://doi.org/10.3390/molecules191118003
Li Z, Peng Y, Zhang X-Q, Ma X, Huang L-K, Yan Y-H. Exogenous Spermidine Improves Seed Germination of White Clover under Water Stress via Involvement in Starch Metabolism, Antioxidant Defenses and Relevant Gene Expression. Molecules. 2014; 19(11):18003-18024. https://doi.org/10.3390/molecules191118003
Chicago/Turabian StyleLi, Zhou, Yan Peng, Xin-Quan Zhang, Xiao Ma, Lin-Kai Huang, and Yan-Hong Yan. 2014. "Exogenous Spermidine Improves Seed Germination of White Clover under Water Stress via Involvement in Starch Metabolism, Antioxidant Defenses and Relevant Gene Expression" Molecules 19, no. 11: 18003-18024. https://doi.org/10.3390/molecules191118003
APA StyleLi, Z., Peng, Y., Zhang, X.-Q., Ma, X., Huang, L.-K., & Yan, Y.-H. (2014). Exogenous Spermidine Improves Seed Germination of White Clover under Water Stress via Involvement in Starch Metabolism, Antioxidant Defenses and Relevant Gene Expression. Molecules, 19(11), 18003-18024. https://doi.org/10.3390/molecules191118003







