The Use of Headspace Solid-Phase Microextraction (HS-SPME) to Assess the Quality and Stability of Fruit Products: An Example Using Red Mombin Pulp (Spondias purpurea L.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Volatile Composition of Red Mombin Pulp
| Peak | Compound | RI | Integral pulp | Powder | ||||
|---|---|---|---|---|---|---|---|---|
| After SD | PP | LP | ||||||
| (day 0) | (day 60) | (day 120) | (day 60) | (day 120) | ||||
| 1 | hexane * | >700 | 10.7 ± 1.3 | 1.2 ± 0.1 | 1.6 ± 0.3 | 1.1 ± 0.3 | 1.6 ± 0.5 | 1.3 ± 0.4 |
| 2 | ethyl acetate a | >700 | 107.5 ± 8.4 | nd | nd | nd | nd | nd |
| 3 | 2,3-pentanedione * | >700 | 41.6 ± 4.3 | nd | nd | nd | nd | nd |
| 4 | 3,3-dimethyl pentane * | >700 | 11.17 ± 0.47 | nd | nd | nd | nd | nd |
| 5 | ciclohexane * | >700 | 6.44 ± 0.12 | nd | nd | nd | nd | nd |
| 6 | 2-methyl-butanol * | >700 | 4.01 ± 0.31 | nd | nd | nd | nd | nd |
| 7 | 3,4-dimethyl-pentanol * | >700 | 4.31 ± 0.35 | nd | nd | nd | nd | nd |
| 8 | 1-penten-3-ol * | >700 | 27.9 ± 6.6 | nd | nd | nd | nd | nd |
| 9 | 2-penten-1-ol * | 793 | 20.1 ± 1.7 | 2.0 ± 0.7 | 1.7 ± 0.3 | 1.9 ± 0.5 | 1.2 ± 0.1 | 1.5 ± 0.3 |
| 10 | 2,3-butanediol * | 794 | 18.2 ± 0.5 | 0.9 ± 0.3 | 1.0 ± 0.3 | 0.7 ± 0.1 | 0.6 ± 0.0 | 0.7 ± 0.1 |
| 11 | 5-hexen-2-one * | 795 | 1.6 ± 0.1 | 0.9 ± 0.2 | 0.7 ± 0.0 | 0.9 ± 0.4 | 0.8 ± 0.1 | 0.7 ± 0.2 |
| 12 | isopentyl acetate b | 795 | 4.2 ± 0.1 | nd | nd | nd | nd | nd |
| 13 | 4-pentenal * | 796 | 2.4 ± 0.1 | nd | nd | nd | nd | nd |
| 14 | butyl ethanoate * | 797 | 1.0 ± 0.0 | nd | nd | nd | nd | nd |
| 15 | Hexanal a | 801 | 194.2 ± 13.6 | 57.9 ± 10.3 | 45.4 ± 1.7 | 41.6 ± 4.9 | 34.1 ± 2.3 | 33.7 ± 3.5 |
| 16 | trans-2-hexenal a | 848 | 4.5 ± 0.3 | 2.8 ± 0.8 | 3.1 ± 1.1 | 1.9 ± 0.6 | 1.7 ± 0.2 | 1.4 ± 0.2 |
| 17 | ethyl 2-methylbutanoate * | 852 | 4.7 ± 0.5 | 2.7 ± 0.5 | 2.0 ± 0.0 | 2.1 ± 0.8 | 1.8 ± 0.1 | 1.8 ± 0.1 |
| 18 | ethyl 3-methylbutanoate | 857 | nd | 2.2 ± 0.7 | 2.3 ± 0.7 | 2.3 ± 0.4 | 2.2 ± 0.2 | 1.3 ± 1.0 |
| 19 | 3-hexen-1-ol a,b | 858 | 165.2 ± 16.3 | 97.5 ± 7.3 | 88.0 ± 6.1 | 66.8 ± 9.4 | 89.3 ± 2.7 | 90.1 ± 8.1 |
| 20 | NI | 867 | 21.5 ± 2.3 | 6.0 ± 2.0 | 7.1 ± 2.6 | 6.4 ± 1.2 | 2.9 ± 0.0 | 2.7 ± 0.6 |
| 21 | 2,6-dimethyl-1-heptene * | 870 | 36.9 ± 0.9 | 14.1 ± 2.8 | 15.5 ± 0.2 | 19.2 ± 3.9 | 14.4 ± 2.0 | 13.9 ± 1.1 |
| 22 | 2-hexen-1-ol a | 873 | 114.7 ± 10.8 | 66.2 ± 3.8 | 75.6 ± 7.0 | 66.9 ± 6.1 | 71.3 ± 5.0 | 72.7 ± 4.4 |
| Peak | Compound | RI | Integral pulp | Powder | ||||
| After SD | After SD | After SD | ||||||
| (day 0) | (day 0) | (day 0) | (day 0) | (day 0) | ||||
| 23 | β-pinene b | 945 | nd | 5.8 ± 0.5 | 11.5 ± 4.21 | 13.5 ± 5.3 | 9.3 ± 0.1 | 9.1 ± 0.3 |
| 24 | β-myrcene b | 991 | 1.9 ± 0.2 | nd | nd | nd | nd | nd |
| 25 | Limonene a,b | 1030 | 14.0 ± 0.2 | 27.3 ± 5.4 | 31.0 ± 3.0 | 27.0 ± 5.1 | 23.5 ± 1.7 | 24.1 ± 3.3 |
| 26 | Copaene b | 1373 | 15.0 ± 1.6 | nd | nd | nd | nd | nd |
| 27 | β-caryophyllene a | 1415 | 45.2 ± 7.3 | 1.7 ± 0.3 | 1.6 ± 0.4 | 1.4 ± 0.4 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| 28 | non identified terpene | 1446 | 2.8 ± 0.5 | 0.5 ± 0.0 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.0 | 0.4 ± 0.2 |
| 29 | α-caryophyllene a | 1452 | 14.6 ± 1.5 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.0 |
| 30 | NI terpene | 1470 | 1.6 ± 0.2 | 1.2 ± 0.4 | 0.7 ± 0.0 | 0.7 ± 0.2 | 0.6 ± 0.0 | 0.6 ± 0.1 |
| 31 | NI terpene | 1472 | 4.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| 32 | α-muurolene * | 1494 | 4.8 ± 0.5 | tr | tr | 0.4 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| 33 | pentadecane * | 1500 | 2.5 ± 0.1 | 2.5 ± 3.7 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| 34 | NI terpene | 1514 | 8.2 ± 0.4 | 1.5 ± 2.2 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| 35 | NI | 1615 | 81.9 ± 3.0 | 3.3 ± 2.1 | 4.5 ± 0.9 | 5.1 ± 1.4 | 4.8 ± 0.1 | 5.3 ± 0.9 |
| 36 | α-bisabolol * | 1683 | 19.2 ± 2.1 | 0.9 ± 0.2 | 0.7 ± 0.0 | 0.6 ± 0.2 | 0.9 ± 0.1 | 1.3 ± 0.7 |
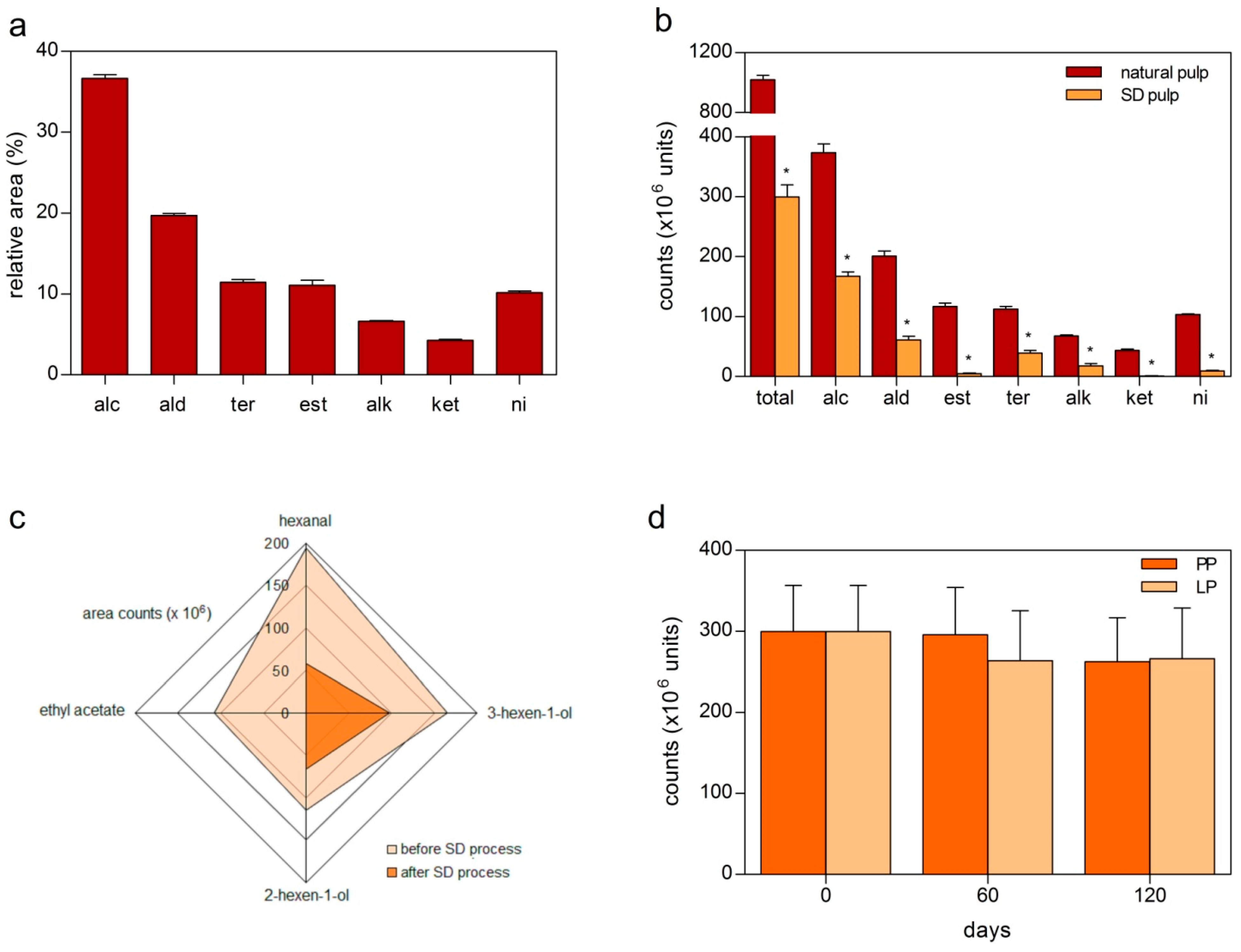
2.2. Effects of Spray-Drying (SD) on the Volatiles Composition of Red Mombin Pulp
2.3. Volatile Compound Stability of the Red Mombin Pulp Stored in Different Packages
3. Experimental Section
3.1. Materials
3.2. Headspace Extraction Procedure
3.3. Gas Chromatography-Flame Ionization Detector (GC-FID) Analyses
3.4. Gas Chromatography-Mass Spectrometry (GC-MS) Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Genovese, M.I.; Pinto, M.S.; Gonçalves, A.E.S.; Lajolo, F.M. Bioactive compounds and antioxidant capacity of exotic fruits and commercial frozen pulps from Brazil. Food Sci. Technol. Int. 2008, 14, 207–214. [Google Scholar] [CrossRef]
- Narain, N.; Galvão, M.S.; Santana, K.L.; Moreira, J.J.S. Volatile compounds in tomato-based dried products. Dry. Technol. 2010, 28, 232–239. [Google Scholar] [CrossRef]
- Augusto, P.E.D.; Cristianini, M.; Ibarz, A. Effect of temperature on dynamic and steady-state shear rheological properties of siriguela (Spondias purpurea L.) pulp. J. Food Eng. 2012, 108, 283–289. [Google Scholar] [CrossRef]
- Nardini, G.S.; Merib, J.O.; Dias, A.N.; Dutra, J.N.B.; Silveira, C.D.S.; Budziak, D.; Matrendal, E.; Carasek, E. Determination of volatile profile of citrus fruit by HS-SPME/GC-MS with oxidized NiTi fibers using two temperatures in the same extraction procedure. Microchem. J. 2013, 109, 128–133. [Google Scholar] [CrossRef]
- Gokbulut, I.; Karabulut, I. SPME–GC–MS detection of volatile compounds in apricot varieties. Food Chem. 2012, 132, 1098–1102. [Google Scholar] [CrossRef]
- Torres, J.D.; Chiralt, A.; Escriche, I. Development of volatile fraction of fresh cut osmotically treated mango during cold storage. Food Chem. 2012, 130, 921–927. [Google Scholar] [CrossRef]
- Sunthonvit, N.; Srzednicki, G.; Craske, J. Effects of drying treatments on the composition of volatile compounds in dried nectarines. Dry. Technol. 2007, 25, 877–881. [Google Scholar] [CrossRef]
- Thuwapanichayanan, R.; Prachayawarakorn, S.; Kunwisawa, J.; Soponronnarit, S. Determination of effective moisture diffusivity and assessment of quality attributes of banana slices during drying. LWT Food Sci. Technol. 2011, 44, 1502–1510. [Google Scholar] [CrossRef]
- Zotarelli, M.F.; Porciuncula, B.D.A.; Laurindo, J.B. A convective multi-flash drying process for producing dehydrated crispy fruits. J. Food Eng. 2012, 108, 523–531. [Google Scholar] [CrossRef]
- Demarchi, S.M.; Ruiz, N.A.Q.; Concellón, A.; Giner, S.A. Effect of temperature on hot-air drying rate and on retention of antioxidant capacity in apple leathers. Food Bioprod. Process. 2013, 91, 310–318. [Google Scholar] [CrossRef]
- Borrmann, D.; Pierucci, A.P.T.R.; Leite, S.G.F.; Leão, M.H.M.R. Microencapsulation of passion fruit (Passiflora) juice with n-octenylsuccinate-derivatised starch using spray-drying. Food Bioprod. Process. 2013, 91, 23–27. [Google Scholar] [CrossRef]
- Cheong, K.W.; Tan, C.P.; Mirhosseini, H.; Hamid, N.S.A.; Osman, A.; Basri, M. Equilibrium headspace analysis of volatile flavor compounds extracted from soursop (Annona muricata) using solid-phase microextraction. Food Res. Int. 2010, 43, 1267–1276. [Google Scholar] [CrossRef]
- Dong, L.; Piao, Y.; Zhang, X.; Zhao, C.; Hou, Y.; Shi, Z. Analysis of volatile compounds from a malting process using headspace solid-phase micro-extraction and GC-MS. Food Res. Int. 2013, 51, 783–789. [Google Scholar] [CrossRef]
- Ceva-Antunes, P.M.N.; Bizzo, H.R.; Silva, A.S.; Carvalho, C.P.S.; Antunes, O.A.C. Analysis of volatile composition of red mombin (Spondias purpurea L.) by solid phase microextraction (SPME). LWT Food Sci. Technol. 2006, 39, 436–442. [Google Scholar] [CrossRef]
- Augusto, F.; Valente, A.L.P.; Tada, E.S.; Rivellino, S.R. Screening of Brazilian fruits aromas using solid-phase microextraction-gas chromatography-mass spectrometry. J. Chromatogr. A 2000, 873, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.; Wright, C.J.; McBurney, T.; Taylor, A.J.; Linforth, R.S.T. Influence of harvest date and light integral on the development of strawberry flavour compounds. J. Exp. Bot. 2002, 53, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Yu-Tao, W.; Shao-Wen, H.; Rong-Le, L.; Ji-Yun, J. Effects of nitrogen application on flavor compounds of cherry tomato fruits. J. Plant Nutr. Soil Sci. 2007, 170, 461–468. [Google Scholar] [CrossRef]
- Song, J.; Fan, L.; Forney, C.F.; Jordan, M.A. Using volatile emission and chlorophyll fluorescence as indicators of heat injury in apples. J. Am. Soc. Hortic. Sci. 2001, 126, 771–777. [Google Scholar]
- Hewett, E.W. An overview of preharvest factors influencing postharvest quality of horticultural products. Int. J. Postharvest Technol. Innov. 2006, 1, 4–15. [Google Scholar] [CrossRef]
- Ceva-Antunes, P.M.N.; Bizzo, H.R.; Alves, S.M.; Antunes, O.A.C. Analysis of volatile compounds of tapereba (Spondias mombin L.) and caja (Spondias mombin L.) by simultaneous distillation and extraction (SDE) and solid phase microextraction (SPME). J. Agric. Food Chem. 2003, 51, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.R.B.; Shibamoto, T. Volatile composition of some brazilian fruits: Umbu-caja (Spondias citherea), camu-camu (Myrciaria dubia), Araça-boi (Eugenia stipitata), and cupuaçu (Theobroma grandiflorum). J. Agric. Food Chem. 2000, 48, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Secouard, S.; Maliac, C.; Grisel, M.; Decroix, B. Release of limonene from polysaccharide matrices: Viscosity and synergy effect. Food Chem. 2003, 82, 227–234. [Google Scholar] [CrossRef]
- Gonzalez-Palomares, S.; Estarrón-Espinoza, M.; Gómez-Leyva, J.F.; Andrade-González, I. Effect of the temperature on the spray drying of roselle extracts (Hibiscus sabdariffa L.). Plant Foods Hum. Nutr. 2009, 64, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Tze, N.G.; Han, C.P.; Yusof, Y.A.; Ling, C.N.; Talib, R.A.; Taip, F.S.; Aziz, M.G. Physiochemical and nutritional properties of spray-dried pitaya fruit powder as natural colorant. Food Sci. Biotechnol. 2012, 21, 675–682. [Google Scholar] [CrossRef]
- NIST, National Institute of Standards and Technology. Database Standard Reference Number 69. Available online: http://webbook.nist.gov/chemistry/ (accessed on 16 October 2013).
- Sample Availability: Samples of red mombin pulp powder are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todisco, K.M.; Castro-Alves, V.C.; Garruti, D.D.S.; Costa, J.M.C.d.; Clemente, E. The Use of Headspace Solid-Phase Microextraction (HS-SPME) to Assess the Quality and Stability of Fruit Products: An Example Using Red Mombin Pulp (Spondias purpurea L.). Molecules 2014, 19, 16851-16860. https://doi.org/10.3390/molecules191016851
Todisco KM, Castro-Alves VC, Garruti DDS, Costa JMCd, Clemente E. The Use of Headspace Solid-Phase Microextraction (HS-SPME) to Assess the Quality and Stability of Fruit Products: An Example Using Red Mombin Pulp (Spondias purpurea L.). Molecules. 2014; 19(10):16851-16860. https://doi.org/10.3390/molecules191016851
Chicago/Turabian StyleTodisco, Katieli Martins, Victor Costa Castro-Alves, Deborah Dos Santos Garruti, José Maria Correia da Costa, and Edmar Clemente. 2014. "The Use of Headspace Solid-Phase Microextraction (HS-SPME) to Assess the Quality and Stability of Fruit Products: An Example Using Red Mombin Pulp (Spondias purpurea L.)" Molecules 19, no. 10: 16851-16860. https://doi.org/10.3390/molecules191016851
APA StyleTodisco, K. M., Castro-Alves, V. C., Garruti, D. D. S., Costa, J. M. C. d., & Clemente, E. (2014). The Use of Headspace Solid-Phase Microextraction (HS-SPME) to Assess the Quality and Stability of Fruit Products: An Example Using Red Mombin Pulp (Spondias purpurea L.). Molecules, 19(10), 16851-16860. https://doi.org/10.3390/molecules191016851





