Selenium in the Environment, Metabolism and Involvement in Body Functions
1. General Overview
1.1. Physicochemical Properties of Selenium and Its Compounds
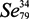 ) is a metalloid of the same family as oxygen and sulfur (S). The name is derived from Selene—goddess of the moon, by reference to the fact that it is always linked to tellurium, metalloid initially appointed by reference to the Earth [1]. Six isotopes coexist in Nature. Their mass numbers are very close to 74, 76, 77, 78, 80 and 82 [2]. It resembles S in terms of atomic size, bond energies, ionization potentials and main oxidation states [3].
) is a metalloid of the same family as oxygen and sulfur (S). The name is derived from Selene—goddess of the moon, by reference to the fact that it is always linked to tellurium, metalloid initially appointed by reference to the Earth [1]. Six isotopes coexist in Nature. Their mass numbers are very close to 74, 76, 77, 78, 80 and 82 [2]. It resembles S in terms of atomic size, bond energies, ionization potentials and main oxidation states [3].1.2. The Physical and Chemical Forms of Selenium
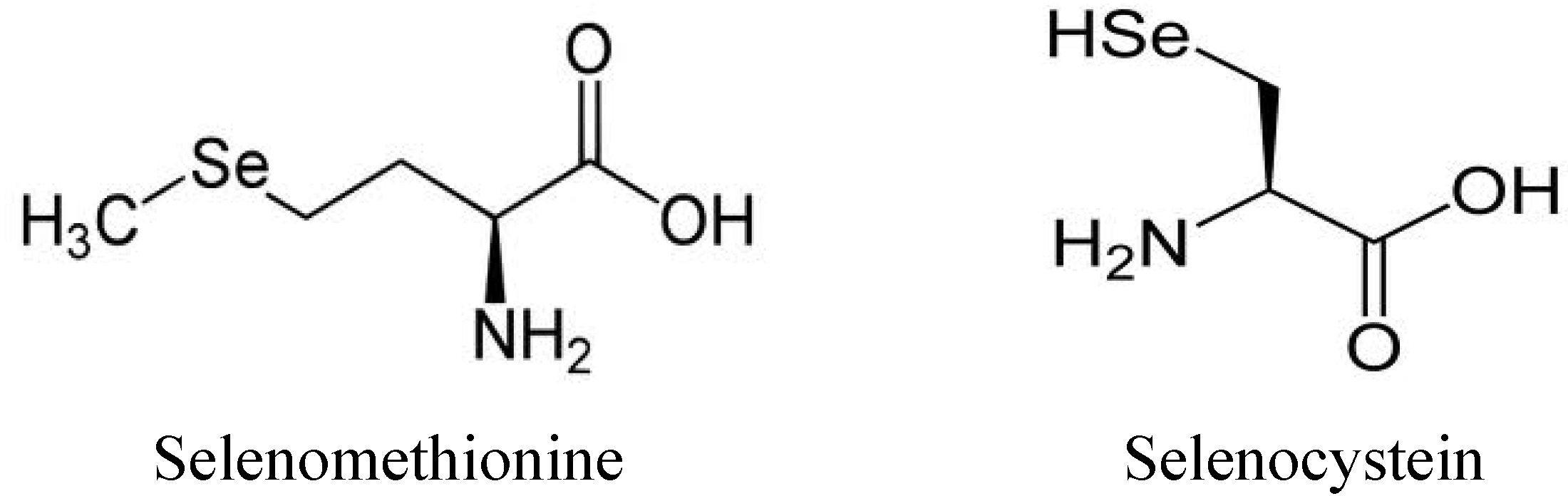
1.3. Use and Production
2. Sources of Selenium in the Environment and its Location
2.1. In Soils
2.2. Plant Sources
| Foods | Average content (mg/kg DM) |
|---|---|
| Trial conducted in France [22] | |
| Meadow grass | 0.24 |
| Alfalfa | 0.23 |
| Peas fodder | 0.32 |
| Maize silage | 0.16 |
| Fresh grass silage | 0.19 |
| Meadow hay | 0.14 |
| Alfalfa hay | 0.37 |
| Straw | 0.16 |
| Barley | 0.09 |
| Oat | 0.14 |
| Wheat | 0.11 |
| Soya bean meal | 0.40 |
| Peanut seed meal | 0.32 |
| Rapeseed meal | 0.15 |
| Urea | 0.10 |
| Dried sugar beet pulp | 0.16 |
| Green wheat | 0.30 |
| Trial conducted in Southern Belgium [23] | |
| Lolium perenne Elgon | 0.055 |
| Lolium perenne Ritz | 0.269 |
| Trifolium pratense | 0.090 |
| Rumex acetosa | 0.463 |
| Plantago major | 0.631 |
| Plantago lanceolata | 0.288 |
| Sanguisorba officinalis | 0.605 |
| Knautia arvensis | 0.123 |
| Trial conducted in Switzerland [24] | |
| Grass | 0.026 |
| Grass silage | 0.054 |
| Hay | 0.034 |
| Silage but | 0.018 |
| fodder beet | 0.026 |
| Compound feedstuffs for dairy cows | 0.02–0.79 |
2.3. Selenium in Water
2.4. Sources of Selenium in the Air
2.5. Food and Feed Sources of Selenium
| Feeds | Se content |
|---|---|
| Tinggi [3] | (mg·kg−1 FM) |
| Cereal, cereal products | 0.01–0.31 |
| Bread | 0.06–0.15 |
| Rice (white) | 0.05–0.08 |
| Pasta/spaghetti | 0.01–0.10 |
| Meat and meat products | 0.06–0.34 |
| Chicken | 0.081–0.142 |
| Pork | 0.032–0.198 |
| Beef | 0.042–0.142 |
| Lamb | 0.033–0.260 |
| Milk and dairy products | <0.001–0.11 |
| Fairweather-Tait et al. [34] | (mg·kg−1 DM) |
| Onions | <0.5 |
| Lentils | 0.24–0.36 |
| Potatoes | 0.12 |
| Crustaceans | 0.36–1.33 |
| Cod | 1.5 |
| Tuna | 5.6 |
3. Role of Selenium in the Body
3.1. Selenoproteins
3.1.1. Glutathione Peroxidase (GPx)
3.1.2. Deiodinases
3.1.3. Selenoprotein-P (SelP)
3.1.4. Thioredoxin Reductase
3.1.5. Other Selenoproteins
| Groupe/nom | Abbreviation | Location | Main Functions |
|---|---|---|---|
| Selenoprotein-W | SelW | Prostate, brain, colon, heart and skeletal muscle | Antioxidant in human lung cancer cells, protect the developing myoblast Calcium-binding [34,50] |
| Selenoprotein-N | SelN | Most tissues, transmembrane glycoprotein associated with endoplasmic reticulum | Proper muscle development. Cell proliferation, redox signalling, calcium homeostasis [51] |
| Selenoprotein-S | SelS | Plasma membranes, endoplasmic reticulum | Elimination of misfolded proteins from the ER reticulum, regulation of inflammation [52] |
| Selenoprotein-K | SelK | Spleen, immune cells and endoplasmic reticulum | Possible antioxidant and development activity [53] |
| Selenoprotein-H | SelH | Spleen, brain, nucleus | Gene regulation of the glutathione synthesis, transcription factor, increasing of cell viability [51,54] |
| Selenoprotein-R | SelR | Liver, kidney | Antioxidant, methionine metabolism and proteins repair. Reduction of sulfoxymethyl group [51] |
| Selenoprotein-M | SelM | Endoplasmic reticulum, neuronal cells | Protein folding, antioxidant activity [48,51] |
| 15kDselenoprotein | Sel15 | Endoplasmic reticulum | Plays a role in protein folding Protects against cancer? [34,35] |
| Mitochondrial capsular selenoprotein | MCSeP | Sperm mitochondrial capsule | GPX4 storage [34,35] |
| Selenophosphate synthetase-2 | SPS-2 | Kidney, liver, testis | Synthesis of selenophosphate for selenoprotein synthesis, Secys biosynthesis [55,56] |
3.2. Roles of Selenium in the Immune Response
3.3. Cancer and Cardiovascular Diseases
3.4. Role of Selenium in Reproduction
4. Metabolism of Selenium
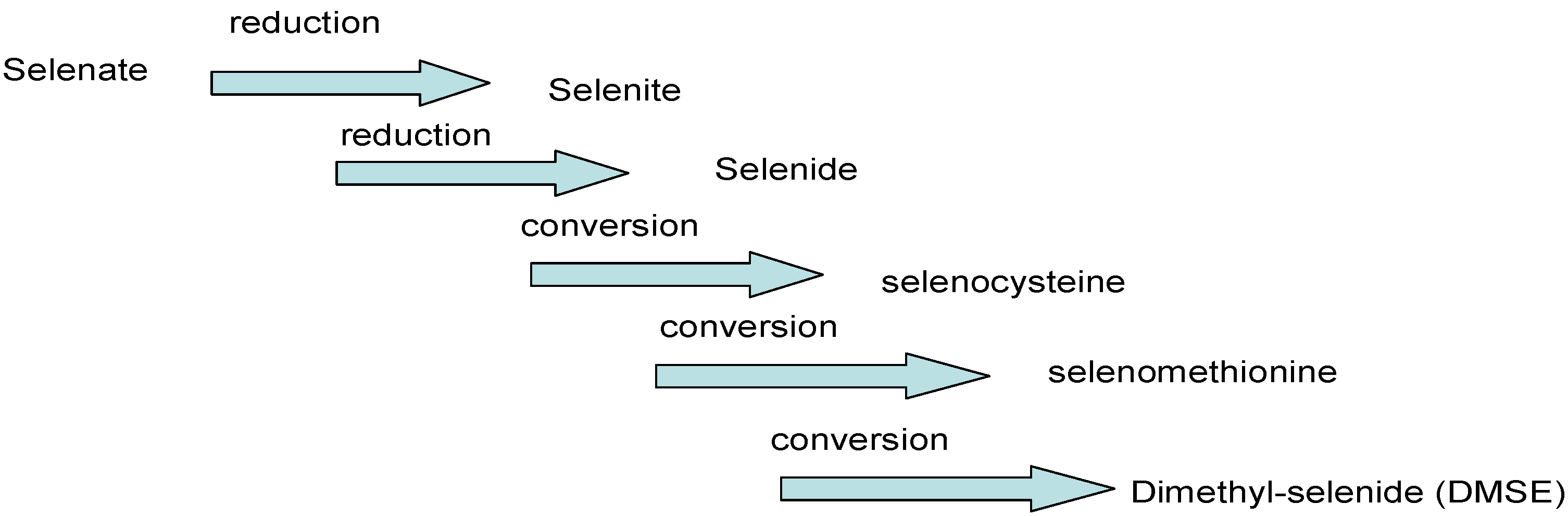
4.1. Transformation, Absorption and Transport
4.2. Excretion
4.3. Homeostasis
5. Nutritional Requirements and Effects of Deficiencies or Excesses in Selenium
5.1. In Animals
| Disorder | Description and consequences | Predilection site | Species affected |
|---|---|---|---|
| Exudative diathesis | Increased capillary permeability: oedema, swelling and bruising | Thorax, neck, wings | Poultry mainly, pig |
| Hepatosis | Necrosis | Liver | Pig |
| Mulberry heart disease | Microangiopathy | Heart mainly, brain | Pig |
5.2. In Humans
6. Methods to Assess Selenium Content in Feed and Selenic Status
7. Conclusions
References
- Reilly, C. Selenium in Food and Health; Springer Science Media: New York, NY, USA, 2006. [Google Scholar]
- Patai, S.; Rappoport, Z. The Chimestry of Organis Selenium and Tellurium Compounds; Willey: New York, NY, USA, 1986; Volume 1. [Google Scholar]
- Tinggi, U. Essentiality and toxicity of selenium and its status in australia: A review. Toxicol. Lett. 2003, 137, 103–110. [Google Scholar] [CrossRef]
- Simonoff, M.; Simonoff, G. Le sélénium et la vie; Masson: Paris, France, 1991; p. 242. [Google Scholar]
- Burk, R.F. Selenium in Biology and Human Health; Springer-Verlag New York Inc.: New York, NY, USA, 1994; p. 221. [Google Scholar]
- Bonnard, N.; Brondeau, M.T.; Jargot, D.; Pillière, F.; Schneider, O.; Serre, P. Fiche toxicologique. Sélénium et composés. Available online: http://www.inrs.fr/default/dms/inrs/FicheToxicologique/TI-FT.../ft150.pdf (accessed on 12 October 2011).
- Bisson, M.; Gay, G.; Guillard, D.; Ghillebaert, F.; Tack, K. Le sélénium et ses composés. Available online: http://www.ineris.fr/substances/fr/substance/getDocument/3012 (accessed on 28 January 2012).
- Graham, T.W. Trace element deficiencies in cattle. Vet. Clin. North. Am. Food Anim. Pract. 1991, 7, 153–215. [Google Scholar]
- Maroc, L. Exposition professionnelle au sélénium et ses effets sur l’homme. Ph.D. Thesis, Université Paris 11 Chatenay, Paris, France, 1990. [Google Scholar]
- George, M.W. Selenium and tellurium. Available online: http://minerals.usgs.gov/minerals/pubs/commodity/selenium/selenmyb04.pdf (accessed on 15 March 2012).
- Schamberger, R.J. Selenium. In Biochemistry of the Essential Ultratrace Elements; Frieden, E., Ed.; Plenum Press: New York, NY, USA, 1984; pp. 201–237. [Google Scholar]
- Martens, D.A.; Suarez, D.L. Selenium speciation of soil/sediment determined with sequential extractions and hydride generation atomic absorption spectrophotometry. Environ. Sci. Technol. 1996, 31, 133–139. [Google Scholar] [CrossRef]
- Barceloux, D.G. Selenium. J. Toxicol. Clin. Toxicol. 1999, 37, 145–172. [Google Scholar] [CrossRef]
- Lebreton, P.; Salat, O.; Nicol, J.M. Un point sur le sélénium. Bull. Tech. GTV 1998, 35–47. [Google Scholar]
- Underwood, E.J.; Suttle, N.F. The Mineral Nutrition of Livestock, 3 ed.; CABI Publishing: Cambridge, UK, 2004; p. 614. [Google Scholar]
- Stadlober, M.; Sager, M.; Irgolic, K.J. Effects of selenate supplemented fertilisation on the selenium level of cereals—Identification and quantification of selenium compounds by HPLC-ICP-MS. Food Chem. 2001, 73, 357–366. [Google Scholar] [CrossRef]
- Coughtrey, P.J.; Jackson, D.; Thorne, M.C. Selenium. In Radionuclide Distribution and Transport in Terrestrial and Aquatic Ecosystems; A. Balkema: Rotterdam, The Netherlands, 1983; Volume 3, p. 372. [Google Scholar]
- Neve, J.; Favier, A. Selenium in Medecine and Biology. In Proceedings of the Second International Congress on trace elements in Medecine and Biology, Avoriaz, France, March 1988; New York Walter de Gruyer: Avoriaz, France, 1988. [Google Scholar]
- William, G.; Rambour, S.; Evrard, C.M. Physiologie des plantes; De boeck: Bruxelles, Belgique, 2003; p. 514. [Google Scholar]
- Minson, D.J. Forage in Ruminant Nutrition; Academic Press: New York, NY, USA, 1990; pp. 369–381. [Google Scholar]
- Fournier, E. Bioaccumulation du sélénium et effets biologiques induits chez le bivalve filtreur corbicula fluminea. Prise en compte de l'activité ventilatoire, de la spéciation du sélénium et de la voie de contamination. Ph.D. Thesis, Universite de Bordeaux 1, Bordeaux, France, 2005. [Google Scholar]
- Richy, B. Le sélénium en élevage. Ph.D. Thesis, Université de Lyon, Lyon, France, 1978. [Google Scholar]
- Hambuckers, A.; Dotreppe, O.; Istasse, L. Problem of applying sodium selenate to increase selenium concentration in grassland plants in southern belgium. Commun. Soil Sci. Plant Anal. 2010, 41, 1283–1292. [Google Scholar] [CrossRef]
- Kessler, J. Carence en sélénium chez les ruminants: Mesures prophylactiques. Revue suisse d'agriculture 1993, 25, 21–26. [Google Scholar]
- Terry, N.; Zayed, A.M. Selenium Volatilization by Plants. In Selenium in the Environment; Frankenberger, W., Jr., Benson, S., Eds.; Dekker: New York, NY, USA, 1994; pp. 343–367. [Google Scholar]
- Ellis, D.R.; Salt, D.E. Plants, selenium and human health. Curr. Opin. Plant Biol. 2003, 6, 273–279. [Google Scholar] [CrossRef]
- Vernoux, J.F.; Barbier, J.; Chery, L. Les anomalies en sélénium dans les captages d'ile-de-france (essonne,seine-et-marne), rapport brgm r401104, 46p. Available online: http://www.brgm.fr/Rapport?code=RR-40114-FR (accessed on 23 December 2012).
- IRSN Fiche radionucléide. Sélénium 79 et environnement. Direction de l’environnement et de l’intervention. Available online: http://www.irsn.fr/EN/Research/publications-documentation/radionuclides-sheets/Documents/Selenium_Se79_v2.pdf (accessed on 23 February 2012).
- Wen, H.; Carignan, J. Reviews on atmospheric selenium: Emissions, speciation and fate. Atmospheric Environ. 2007, 41, 7151–7165. [Google Scholar] [CrossRef]
- Sannac, S. Développement d’un protocole métrologique pour l’analyse de spéciation du sélénium et du mercure dans des matrices environnementales et agroalimentaires par hplc-id-icp-ms. Ph.D. Thesis, Université de Pau et des pays de l’adour, Pau, France, 2009. [Google Scholar]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar]
- Whanger, P.D. Selenium and its relationship to cancer: An update. Br. J. Nutr. 2004, 91, 11–28. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef]
- Suttle, N.F. Mineral Nutrition of Livestock, 4th ed.; MPG Books Group: London, UK, 2010; p. 565. [Google Scholar]
- Cabaraux, J.F.; Dotreppe, O.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Les oligo-éléments dans l'alimentation des ruminants: État des lieux, formes et efficacité des apports avec une attention particulière pour le sélénium, 2007. CRA-W-Fourrages Actualités 12ème journée. 2007, 28–36. [Google Scholar]
- Mostert, V. Selenoprotein P: Properties, functions, and regulation. Arch. Biochem. Biophys. 2000, 376, 433–438. [Google Scholar] [CrossRef]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2012, in press. [Google Scholar]
- Brigelius-Flohe, R.; Aumann, K.D.; Blocker, H.; Gross, G.; Kiess, M.; Kloppel, K.D.; Maiorino, M.; Roveri, A.; Schuckelt, R.; Usani, F.; et al. Phospholipid-hydroperoxide glutathione peroxidase. Genomic DNA, cDNA, and deduced amino acid sequence. J. Biol. Chem. 1994, 269, 7342–7348. [Google Scholar]
- Ducros, V.; Favier, A. Selenium metabolism. EMC Endocrinol. Nutr. 2004, 1, 19–28. [Google Scholar] [CrossRef]
- Meschy, F. Nutrition minérale des ruminants; Editions Quae: Versaille, France, 2010; p. 208. [Google Scholar]
- Flohé, L. The Selenoprotein Glutathione Peroxidise. In Glutathione: Chemical, Biochemical and Medical Aspects; Part A; Dolphin, D., Poulson, R., Avramovic, O., Eds.; John Wiley & Sons Inc: New York, NY, USA, 1989; pp. 643–731. [Google Scholar]
- Bareither, M.L.; Verhage, H.G. Control of the secretory cell cycle in cat oviduct by estradiol and progesterone. Am. J. Anat. 1981, 162, 107–118. [Google Scholar] [CrossRef]
- Chu, F.F.; Doroshow, J.H.; Esworthy, R.S. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J. Biol. Chem. 1993, 268, 2571–2576. [Google Scholar]
- Schwaab, V.; Faure, J.; Dufaure, J.P.; Drevet, J.R. Gpx3: The plasma-type glutathione peroxidase is expressed under androgenic control in the mouse epididymis and vas deferens. Mol. Reprod. Dev. 1998, 51, 362–372. [Google Scholar] [CrossRef]
- Maiorino, M.; Scapin, M.; Ursini, F.; Biasolo, M.; Bosello, V.; Flohe, L. Distinct promoters determine alternative transcription of gpx-4 into phospholipid-hydroperoxide glutathione peroxidase variants. J. Biol. Chem. 2003, 278, 34286–34290. [Google Scholar]
- Papp, L.V.; Holmgren, A.; Khanna, K.K. Selenium and selenoproteins in health and disease. Antioxid. Redox Signal. 2010, 12, 793–795. [Google Scholar] [CrossRef]
- Eberle, B.; Haas, H.J. Purification of selenoprotein Ph from human plasma. J. Trace Elem. Electrolytes Health Dis. 1993, 7, 217–221. [Google Scholar]
- Yao, H.D.; Wu, Q.; Zhang, Z.W.; Li, S.; Wang, X.L.; Lei, X.G.; Xu, S.W. Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim. Biophys. Acta 2013, 1830, 3112–3120. [Google Scholar] [CrossRef]
- Arbogast, S.; Ferreiro, A. Selenoproteins and protection against oxidative stress: Selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxid. Redox Signal. 2010, 12, 893–904. [Google Scholar] [CrossRef]
- Cox, A.J.; Lehtinen, A.B.; Xu, J.; Langefeld, C.D.; Freedman, B.I.; Carr, J.J.; Bowden, D.W. Polymorphisms in the selenoprotein s gene and subclinical cardiovascular disease in the diabetes heart study. Acta Diabetol. 2012. [Google Scholar] [CrossRef]
- Liu, J.; Srinivasan, P.; Pham, D.N.; Rozovsky, S. Expression and purification of the membrane enzyme selenoprotein k. Protein Expr. Purif. 2012, 86, 27–34. [Google Scholar]
- Mehta, S.L.; Mendelev, N.; Kumari, S.; Andy Li, P. Overexpression of human selenoprotein h in neuronal cells enhances mitochondrial biogenesis and function through activation of protein kinase a, protein kinase b, and cyclic adenosine monophosphate response element-binding protein pathway. Int. J. Biochem. Cell Biol. 2013, 45, 604–611. [Google Scholar] [CrossRef]
- Davis, C.D.; Tsuji, P.A.; Milner, J.A. Selenoproteins and cancer prevention. Annu. Rev. Nutr. 2012, 32, 73–95. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Finch, J.M.; Turner, R.J. Effects of selenium and vitamin e on the immune responses of domestic animals. Res. Vet. Sci. 1996, 60, 97–106. [Google Scholar] [CrossRef]
- Sordillo, L.M. Selenium-dependent regulation of oxidative stress and immunity in periparturient dairy cattle. Vet. Med. Int. 2013, 2013, e154045. [Google Scholar]
- Ren, F.; Chen, X.; Hesketh, J.; Gan, F.; Huang, K. Selenium promotes T-cell response to TCR-stimulation and ConA, but not PHA in primary porcine splenocytes. PLoS One 2012, 7, e35375. [Google Scholar]
- Hefnawy, A.E.G; Tórtora-Pérez, J.L. The importance of selenium and the effects of its deficiency in animal health. Small Rumin. Res. 2010, 89, 185–192. [Google Scholar] [CrossRef]
- Cortes-Jofre, M.; Rueda, J.R.; Corsini-Munoz, G.; Fonseca-Cortes, C.; Caraballoso, M.; Bonfill Cosp, X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst. Rev. (Online) 2013, 10, CD002141. [Google Scholar]
- Koyama, H.; Mutakin; Abdulah, R.; Yamazaki, C.; Kameo, S. Selenium supplementation trials for cancer prevention and the subsequent risk of type 2 diabetes mellitus. Nihon Eiseigaku Zasshi. Jpn. J. Hyg. 2013, 68, 1–10. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Tanguy, S.; Grauzam, S.; de Leiris, J.; Boucher, F. Impact of dietary selenium intake on cardiac health: Experimental approaches and human studies. Mol. Nutr. Food Res. 2012, 56, 1106–1121. [Google Scholar] [CrossRef]
- Joseph, J. Selenium and cardiometabolic health: Inconclusive yet intriguing evidence. Am. J. Med. Sci. 2012. [Google Scholar] [CrossRef]
- Derbeneva, S.A.; Bogdanov, A.R.; Pogozheva, A.V.; Gladyshev, O.A.; Vasilevskaia, L.S.; Zorin, S.N.; Mazo, V.K. Effect of diet enriched with selenium on the psycho-emotional and adaptive capacity of patients with cardiovascular diseases and obesity. Vopr. Pitan. 2012, 81, 35–41. [Google Scholar]
- Aréchiga, C.F.; Vázquez-Flores, S.; Ortiz, O.; Hernández-Cerón, J.; Porras, A.; McDowell, L.R.; Hansen, P.J. Effect of injection of β-carotene or vitamin e and selenium on fertility of lactating dairy cows. Theriogenology 1998, 50, 65–76. [Google Scholar] [CrossRef]
- Harrison, J.H.; Russell Conrad, H. Effect of dietary calcium on selenium absorption by the nonlactating dairy cow1,2,3. J. Dairy Sci. 1984, 67, 1860–1864. [Google Scholar] [CrossRef]
- Spears, J.W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef]
- Gutierrez, C.; Corbera, J.A.; Morales, I.; Morales, M.; Navarro, R. Uterine prolapse in 2 dromedary camels. Can. Vet. J. 2001, 42, 803–804. [Google Scholar]
- Mistry, H.D.; Pipkin, F.B.; Redman, C.W.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Maiorino, M.; Flohe, L.; Roveri, A.; Steinert, P.; Wissing, J.B.; Ursini, F. Selenium and reproduction. BioFactors 1999, 10, 251–256. [Google Scholar] [CrossRef]
- Thomson, C.D.; Robinson, M.F. Urinary and fecal excretions and absorption of a large supplement of selenium: Superiority of selenate over selenite. Am. J. Clin. Nutr. 1986, 44, 659–663. [Google Scholar]
- Vendeland, S.C.; Deagen, J.T.; Butler, J.A.; Whanger, P.D. Uptake of selenite, selenomethionine and selenate by brush border membrane vesicles isolated from rat small intestine. Biometals 1994, 7, 305–312. [Google Scholar]
- Van Ryssen, J.B.J.; Van Malsen, P.S.M.; Hartmann, F. Contribution of dietary sulphur to the interaction between selenium and copper in sheep. J. Agric. Sci. 1998, 130, 107–114. [Google Scholar] [CrossRef]
- Neathery, M.W.; Miller, W.J.; Gentry, R.P.; Crowe, C.T.; Alfaro, E.; Fielding, A.S.; Pugh, D.G.; Blackmon, D.M. Influence of high dietary lead on selenium metabolism in dairy calves. J. Dairy Sci. 1987, 70, 645–652. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Miranda, M.; Benedito, J.L.; Blanco-Penedo, I.; Lopez-Alonso, M. Effect of type of muscle and Cu supplementation on trace element concentrations in cattle meat. Food Chem. Toxicol. 2011, 49, 1443–1449. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogra, Y.; Ishiwata, K.; Takayama, H.; Aimi, N.; Suzuki, K.T. Selenosugars are key and urinary metabolites for selenium excretion within the required to low-toxic range. Proc. Natl. Acad. Sci. USA 2002, 99, 15932–15936. [Google Scholar]
- Dubois, F.; Belleville, F. Selenium: Physiologic role and value in human pathology. (in French). Pathol. Biol. (Paris) 1988, 36, 1017–1025. [Google Scholar]
- Seboussi, R. Métabolisme du sélénium chez le dromadaire; SupAgro: Montpellier France, 2008. [Google Scholar]
- Schrauzer, G.N. Selenomethionine: A review of its nutritional significance, metabolism and toxicity. J. Nutr. 2000, 130, 1653–1656. [Google Scholar]
- CSS Recommandations nutritionnelles pour la belgique. Publication du conseil supérieur de la santé. N° 8309. Available online: http://www.health.belgium.be/internet2Prd/groups/public/@public/@shc/documents/ie2divers/12352470_fr.pdf (accessed on 30 April 2012).
- Planté, P. Quelques oligo-éléments. Available online: http://world-medical-clinic.com/france/articles/plante/oe.htm (accessed on 28 April 2004).
- Oster, O.; Prellwitz, W. The daily dietary selenium intake of west german adults. Biol. Trace Elem. Res. 1989, 20, 1–14. [Google Scholar] [CrossRef]
- Radostits, O.M.; Gay, C.C.; Blood, D.C.; Hinchcliff, K.W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses, 9th ed.; W.B Saunders Ltd: Philadelphia, PA, USA, 2000; pp. 1515–1533. [Google Scholar]
- Guyot, H.; Rollln, F. The diagnosis of selenium and iodine deficiencies in cattle. Le diagnostic des carences en sélénium et iode chez les bovins 2007, 151, 166–191. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mehdi, Y.; Hornick, J.-L.; Istasse, L.; Dufrasne, I. Selenium in the Environment, Metabolism and Involvement in Body Functions. Molecules 2013, 18, 3292-3311. https://doi.org/10.3390/molecules18033292
Mehdi Y, Hornick J-L, Istasse L, Dufrasne I. Selenium in the Environment, Metabolism and Involvement in Body Functions. Molecules. 2013; 18(3):3292-3311. https://doi.org/10.3390/molecules18033292
Chicago/Turabian StyleMehdi, Youcef, Jean-Luc Hornick, Louis Istasse, and Isabelle Dufrasne. 2013. "Selenium in the Environment, Metabolism and Involvement in Body Functions" Molecules 18, no. 3: 3292-3311. https://doi.org/10.3390/molecules18033292
APA StyleMehdi, Y., Hornick, J.-L., Istasse, L., & Dufrasne, I. (2013). Selenium in the Environment, Metabolism and Involvement in Body Functions. Molecules, 18(3), 3292-3311. https://doi.org/10.3390/molecules18033292




