The Interactions of Oxygen with Small Gold Clusters on Nitrogen-Doped Graphene
Abstract
:1. Introduction
2. Methodology
3. Results and Discussion
3.1. The Structural and Electronic Properties of Gold Clusters
| Aun cluster | Spin multiplicity | Average bond length (Å) | Binding energy per atom (eV) | HOMO–LUMO energy gap (eV) |
|---|---|---|---|---|
| Au2 | 1 | 2.549 (2.53, 2.47) a,b | 1.12 | 2.01 (1.96) d |
| Au3 | 2 | 2.695 (2.60) c | 1.13 | 1.83 (2.70) e |
| Au4 | 1 | 2.710 (2.68) a | 1.48 | 0.97 (0.927) a |
| Au5 | 2 | 2.678 (2.63) c | 1.62 | 0.96 (1.142) a |
| Au6 | 1 | 2.712 (2.68) a | 1.85 | 2.10 (2.05) d |
| Au7 | 2 | 2.722 (2.70) c | 1.81 | 1.00 (1.077) a |
| Au8 | 1 | 2.695 (2.67) a | 1.93 | 1.46 (1.420) a |
| Au9 | 2 | 2.739 (2.72) c | 1.91 | 0.71 (0.97) c |
| Au10 | 1 | 2.742 (2.71) a | 1.99 | 1.31 (1.172) a |
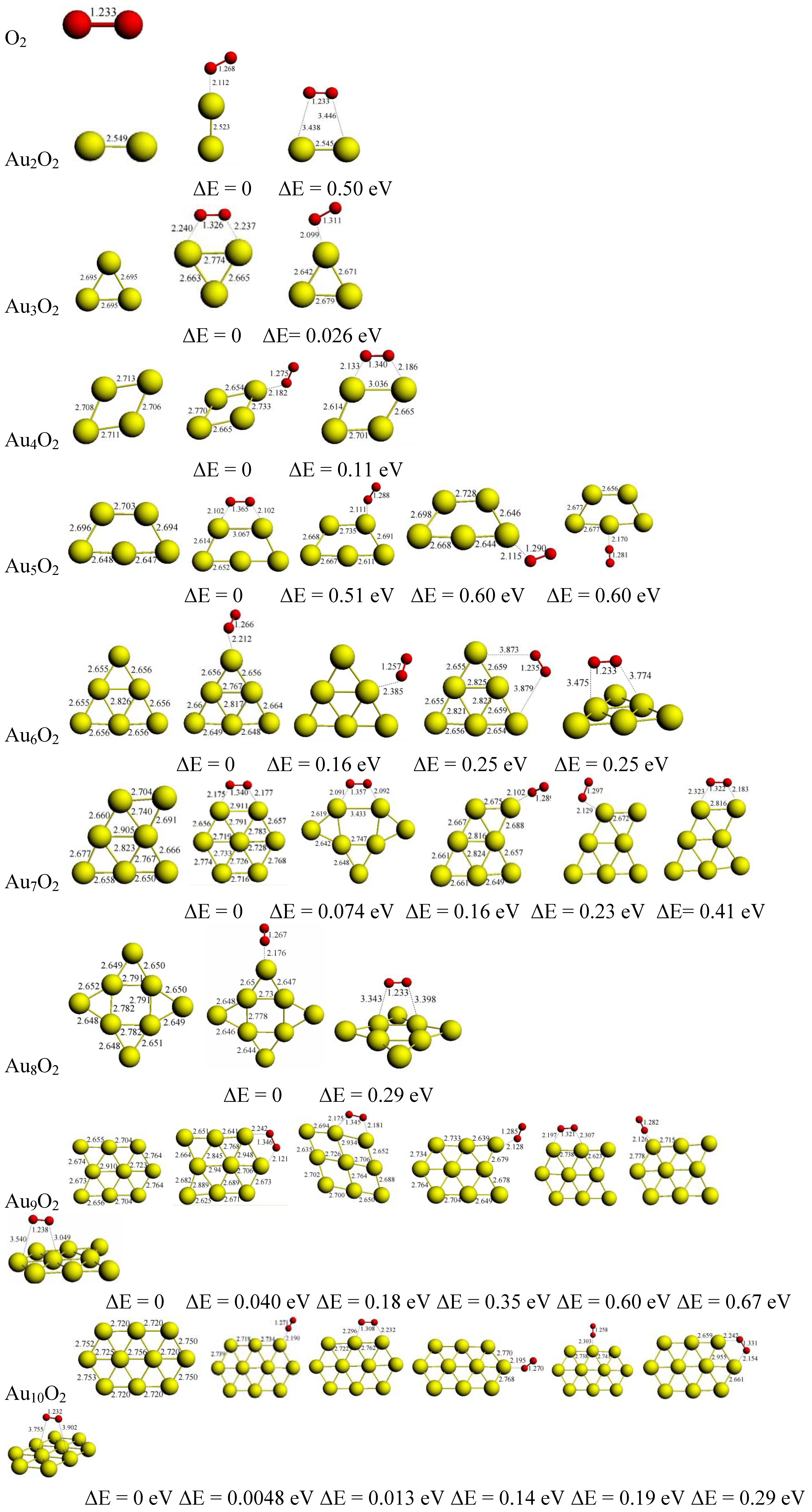
3.2. The Geometries, Energetics, and the Electronic Properties of AunO2 Complexes
3.2.1. Structural Evolution
3.2.2. O2 Adsorption Energies
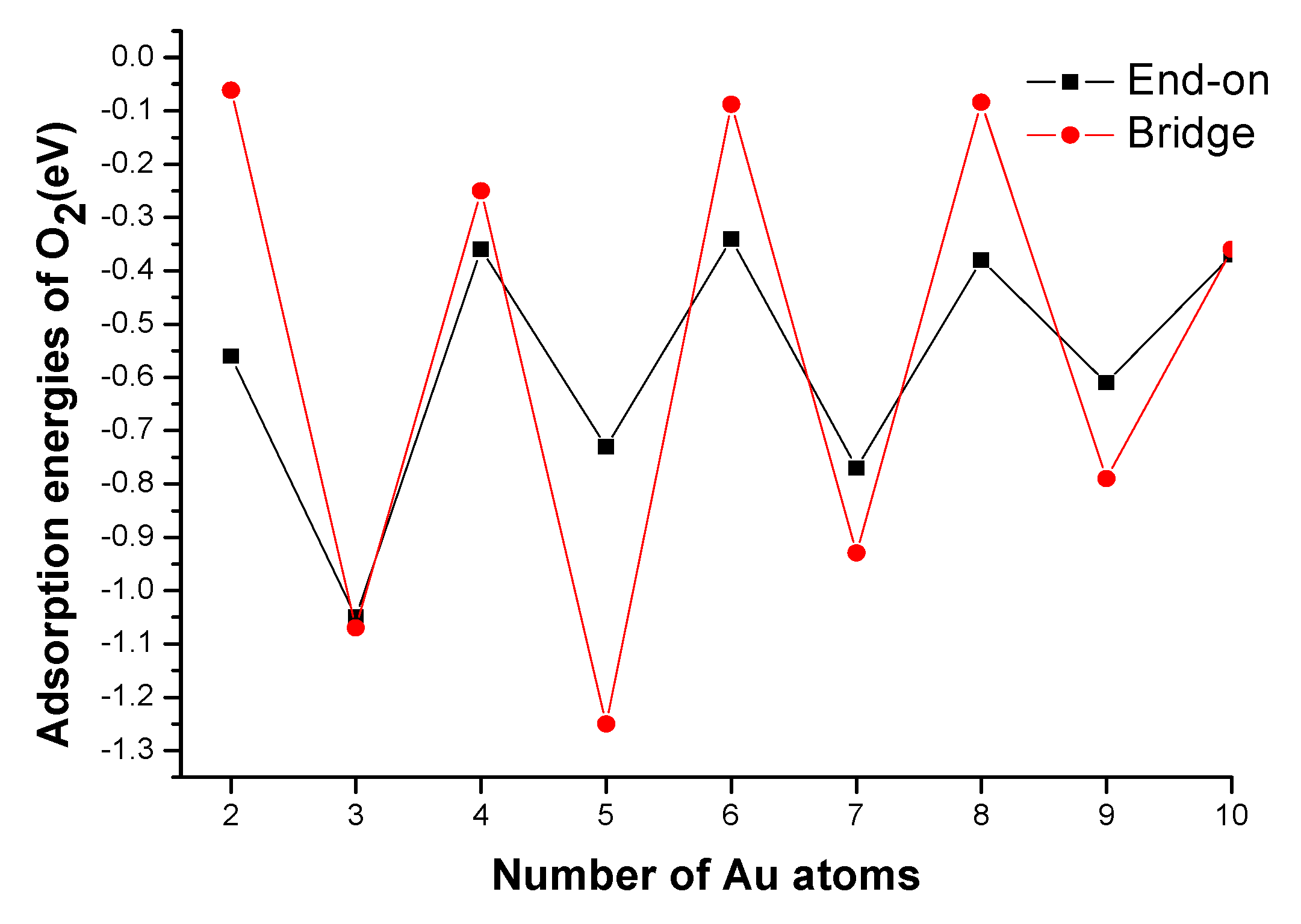
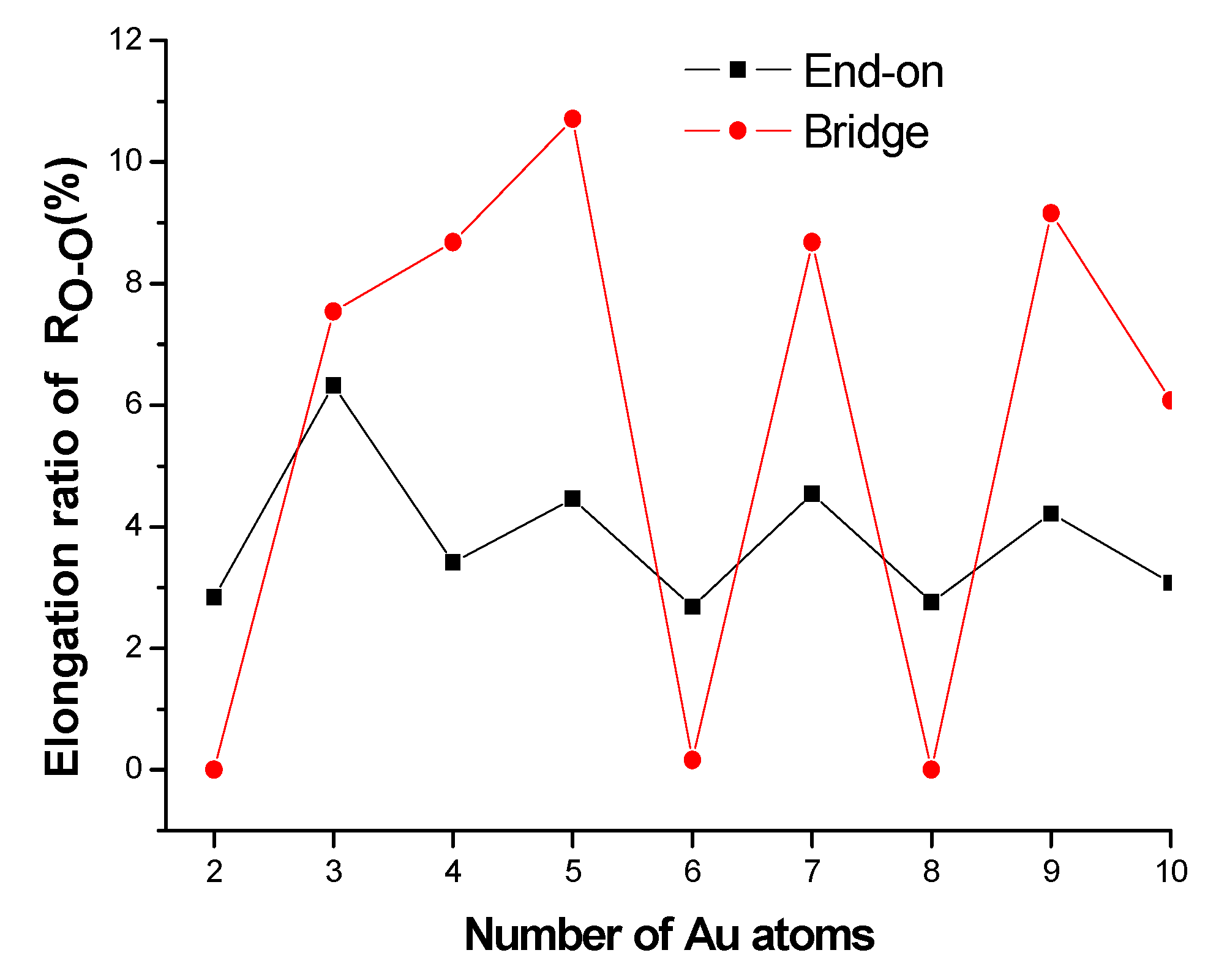
3.2.3. Activation of O2 Molecules
3.3. The Geometries, Energetics, and the Electronic Properties of AunO2/N-Graphene
3.3.1. Pure Aun Clusters on N-Graphene
| Aun cluster | Spin multiplicity | E3 (eV) | MDC-q charge |
|---|---|---|---|
| Au2, ┴ | 2 | –0.58 | –0.103 |
| Au2, ‖ | 2 | –0.30 | 0.058 |
| Au3, ┴ | 1 | –0.51 | –0.107 |
| Au3, ‖ | 1 | –0.30 | 0.030 |
| Au4, ┴ | 2 | –0.81 | –0.089 |
| Au4, ‖ | 2 | –0.57 | 0.080 |
| Au5, ┴ | 1 | –0.67 | 0.002 |
| Au5, ‖ | 1 | –0.79 | –0.019 |
| Au6, ┴ | 2 | –0.64 | 0.098 |
| Au6, ‖ | 2 | –0.74 | –0.164 |
| Au7, ┴ | 1 | –0.42 | 0.023 |
| Au7, ‖ | 1 | –1.15 | –0.171 |
3.3.2. O2 on N-Graphene Supported Aun Clusters
| Aun cluster | E1 (eV) | E2 (eV) | ΔQ (O2) | ΔQ (O2, with support) | ||||
|---|---|---|---|---|---|---|---|---|
| End-on | Bridge | End-on | Bridge | End-on | Bridge | End-on | Bridge | |
| Au2 | –0.56 | ––– | –0.83 | ––– | 0.041 | ––– | –0.096 | ––– |
| Au3 | –1.05 | –1.07 | –1.26 | –1.69 | –0.161 | –0.191 | –0.261 | –0.272 |
| Au4 | –0.36 | –0.25 | –0.42 | –0.30 | –0.077 | –0.073 | –0.157 | –0.091 |
| Au5 | –0.73 | –1.25 | –0.77 | –1.32 | –0.084 | –0.203 | –0.259 | –0.223 |
| Au6 | –0.34 | ––– | –0.52 | ––– | –0.126 | ––– | –0.134 | ––– |
| Au7 | –0.77 | –0.93 | –0.86 | –0.99 | –0.169 | –0.231 | –0.198 | –0.248 |
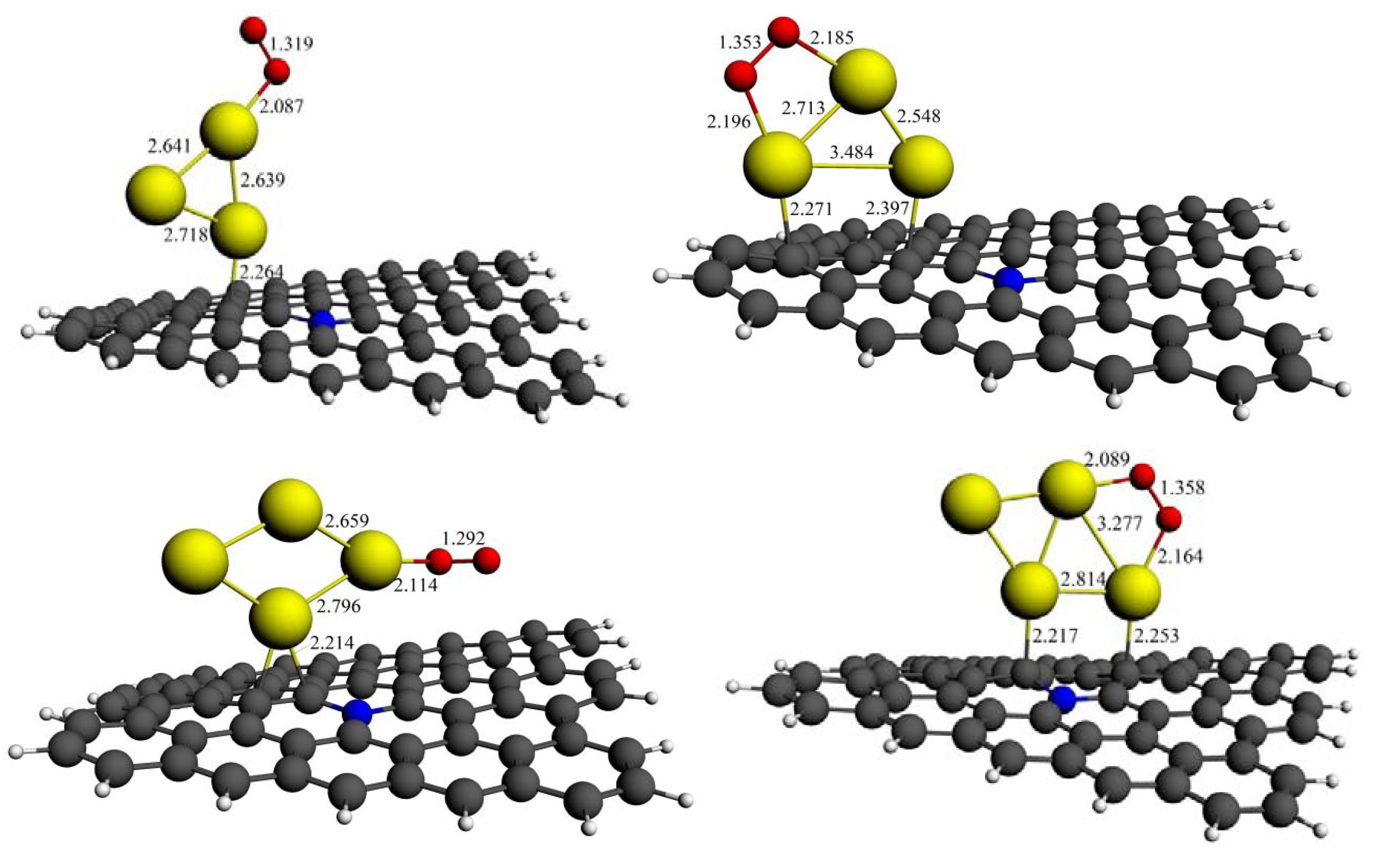
| Aun cluster | E1 (O) | E2 (O, with support) | E1 (OH) | E2 (OH, with support) |
|---|---|---|---|---|
| Au4 | –3.43 | –3.96 | –2.75 | –2.97 |
| Au7, ┴ | –4.21 | –3.99 | –3.80 | –3.50 |
| Au7, ‖ | –4.34 | –3.65 |
3.3.3. Improved Structural Stability of Aun Clusters
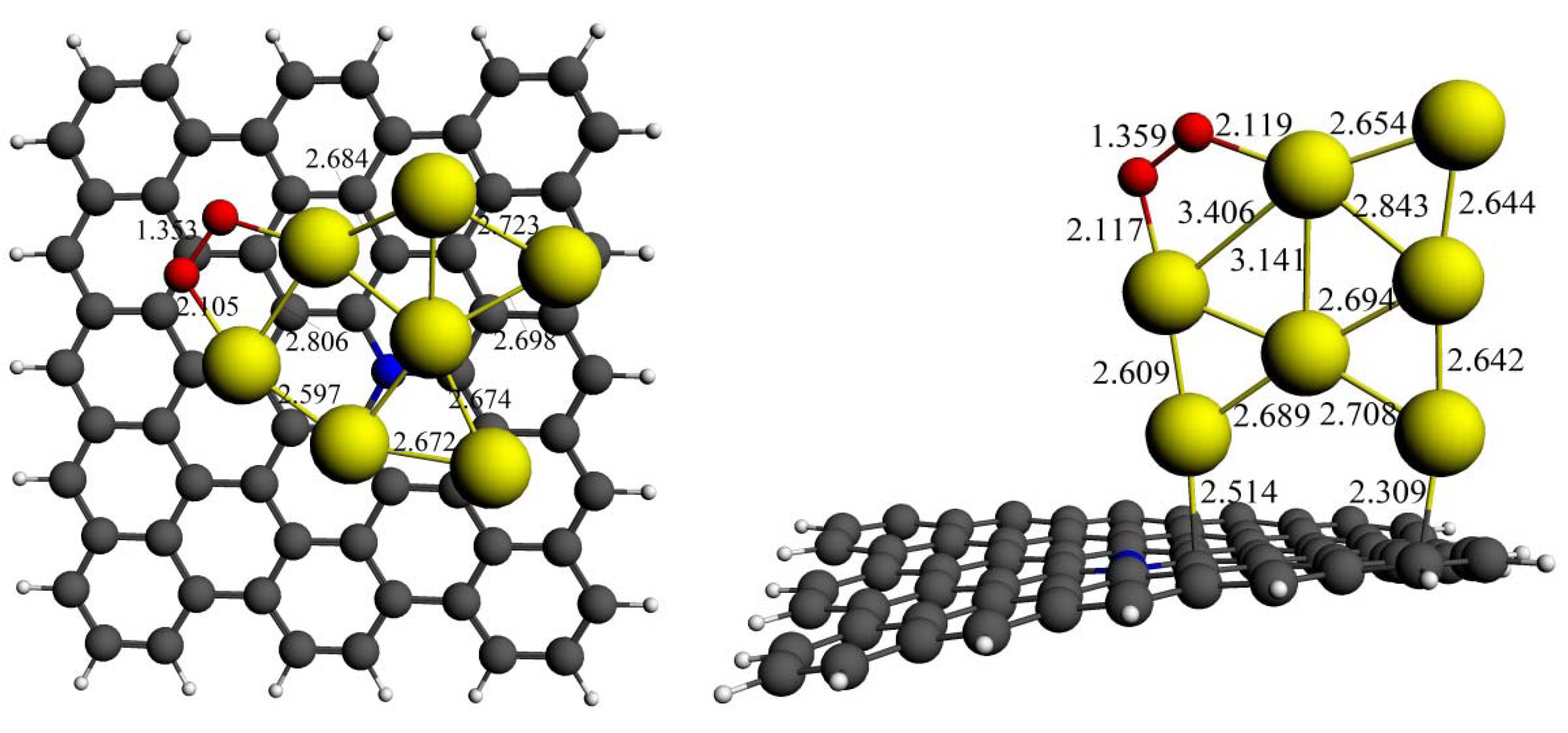
4. Conclusions
Acknowledgments
References
- Wallace, W.T.; Whetten, R.L. Coadsorption of CO and O2 on selected gold clusters: Evidence for efficient room-temperature CO2 generation. J. Am. Chem. Soc. 2002, 124, 7499–7505. [Google Scholar] [CrossRef]
- Hagen, J.; Socaciu, L.D.; Elijazyfer, M.; Heiz, U.; Bernhardt, T.M.; Woste, L. Coadsorption of CO and O2 on small free gold cluster anions at cryogenic temperatures: Model complexes for catalytic CO oxidation. Phys. Chem. Chem. Phys. 2002, 4, 1707–1709. [Google Scholar] [CrossRef]
- Hakkinen, H.; Landman, U. Gas-phase catalytic oxidation of CO by Au2–. J. Am. Chem. Soc. 2001, 123, 9704–9705. [Google Scholar] [CrossRef]
- Lopez, N.; Norskov, J.K. Catalytic CO oxidation by a gold nanoparticle: A density functional study. J. Am. Chem. Soc. 2002, 124, 11262–11263. [Google Scholar] [CrossRef]
- Molina, L.M.; Hammer, B. Active role of oxide support during CO oxidation at Au/MgO. Phys. Rev. Lett. 2003, 90, 206102. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A. FTIR study of CO oxidation on Au/TiO2 at 90 K and room temperature. An insight into the nature of the reaction centers. J. Phys. Chem. B 2000, 104, 5414–5416. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Goodman, D.W. Oxidation catalysis by supported gold nano-clusters. Top. Catal. 2002, 21, 25–34. [Google Scholar] [CrossRef]
- Assadollahzadeh, B.; Schwerdtfeger, P. A systematic search for minimum structures of small gold clusters Aun (n = 2–20) and their electronic properties. J. Chem. Phys. 2009, 131, 064306. [Google Scholar] [CrossRef]
- Kang, G.J.; Chen, Z.X.; Li, Z.; He, X. A theoretical study of the effects of the charge state and size of gold clusters on the adsorption and dissociation of H2. J. Chem. Phys. 2009, 130, 034701. [Google Scholar] [CrossRef]
- Li, G.P.; Hamilton, I.P. Complexes of small neutral gold clusters and hydrogen sulphide: A theoretical study. Chem. Phys. Lett. 2006, 420, 474–479. [Google Scholar] [CrossRef]
- Yoon, B.; Hakkinen, H.; Landman, U. Interaction of O2 with gold clusters: Molecular and dissociative adsorption. J. Phys. Chem. A 2003, 107, 4066–4071. [Google Scholar] [CrossRef]
- Mills, G.; Gordon, M.S.; Metiu, H. The adsorption of molecular oxygen on neutral and negative Aun clusters (n = 2–5). Chem. Phys. Lett. 2002, 359, 493–499. [Google Scholar] [CrossRef]
- Ding, X.; Li, Z.; Yang, J.; Hou, J.G.; Zhu, Q. Adsorption energies of molecular oxygen on Au clusters. J. Chem. Phys. 2004, 120, 9594–9600. [Google Scholar] [CrossRef]
- Fernandez, E.M.; Ordejon, P.; Balbas, L.C. Theoretical study of O2 and CO adsorption on Aun clusters (n = 5–10). Chem. Phys. Lett. 2005, 408, 252–257. [Google Scholar] [CrossRef]
- Lyalin, A.; Taketsugu, T. Cooperative adsorptionof O2 and C2H4 on small gold clusters. J. Phys. Chem. C 2009, 113, 12930–12934. [Google Scholar] [CrossRef]
- Coquet, R.; Howard, K.L.; Willock, D.J. Theory and simulation in heterogeneous gold catalysis. Chem. Soc. Rev. 2008, 37, 2046–2076. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef]
- Novoselov, K.S.; McCann, E.; Morozov, S.V.; Fal’ko, V.I.; Katsnelson, M.I.; Zeitler, U.; Jiang, D.; Schedin, F.; Geim, A.K. Unconventional quantum Hall effect and Berry's phase of 2π in bilayer graphene. Nat. Phys. 2006, 2, 177–180. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Yoo, E.; Okata, T.; Akita, T.; Kohyama, M.; Nakamura, J.; Honma, I. Enhanced electrocatalytic activity of Pt subnanoclusters on graphene nanosheet surface. Nano Lett. 2009, 9, 2255–2259. [Google Scholar] [CrossRef]
- Scheuermann, G.M.; Rumi, L.; Steurer, P.; Bannwarth, W.; Mulhaupt, R. Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki−Miyaura coupling reaction. J. Am. Chem. Soc. 2009, 131, 8262–8270. [Google Scholar] [CrossRef]
- Pulido, A.; Boronat, M.; Corma, A. Theoretical investigation of gold clusters supported on graphene sheets. New J. Chem. 2011, 35, 2153–2161. [Google Scholar] [CrossRef]
- Chen, G.; Li, S.J.; Su, Y.; Wang, V.; Mizuseki, H.; Kawazoe, Y. Improved stability and catalytic properties of Au16 cluster supported on graphane. J. Phys. Chem. C 2011, 115, 20168–20174. [Google Scholar] [CrossRef]
- te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J.Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Fonseca Guerra, C.; Snijders, J.G.; te Velde, G.; Baerends, E.J. Towards an order-N DFT method. Theor. Chem. Acc. 1998, 99, 391–403. [Google Scholar]
- ADF2009.01, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. SCM Home Page. Available online: http://www.scm.com (accessed on 5 March 2013).
- Swart, M.; van Duijnen, P.T.; Snijders, J.G. A charge analysis derived from an atomic multipole expansion. J.Comput. Chem. 2001, 22, 79–88. [Google Scholar] [CrossRef]
- Häkkinen, H.; Moseler, M.; Landman, U. Bonding in Cu, Ag, and Au clusters: Relativistic effects, trends, and surprises. Phys. Rev. Lett. 2002, 89, 033401. [Google Scholar] [CrossRef]
- Häkkinen, H.; Yoon, B.; Landman, U.; Li, X.; Zhai, H.J.; Wang, L.S. On the electronic and atomic structures of small AuN− (N = 4−14) clusters: A photoelectron spectroscopy and density-functional study. J. Phys. Chem. A 2003, 107, 6168–6175. [Google Scholar] [CrossRef]
- Deka, A.; Deka, R.C. Structural and electronic properties of stable Aun (n = 2–13) clusters: A density functional study. J. Mol. Struc.-Theochem. 2008, 870, 83–93. [Google Scholar] [CrossRef]
- Hakkinen, H.; Landman, U. Gold clusters (AuN, 2<=N<=10) and their anions. Phys. Rev. B 2000, 62, R2287–R2290. [Google Scholar] [CrossRef]
- Lee, H.M.; Ge, M.; Sahu, B.R.; Tarakeswar, P.; Kim, K.S. Geometrical and electronic structures of gold, silver, and gold-silver binary clusters: Origins of ductility of gold and gold-silver alloy formation. J. Phys. Chem. B 2003, 107, 9994–10005. [Google Scholar] [CrossRef]
- Fernandez, E.M.; Soler, J.M.; Garzon, I.L.; Balbas, L.C. Trends in the structure and bonding of noble metal clusters. Phys. Rev. B 2004, 70, 165403. [Google Scholar] [CrossRef]
- Kuang, X.J.; Wang, X.Q.; Liu, G.B. All-electron scalar relativistic calculation of water molecule adsorption onto small gold clusters. J. Mol. Model. 2011, 17, 2005–2016. [Google Scholar] [CrossRef]
- Bishea, G.A.; Morse, M.D. Spectroscopic studies of jetcooled AgAu and Au2. J. Chem. Phys. 1991, 95, 5646–5659. [Google Scholar] [CrossRef]
- Shafai, G.S.; Shetty, S.; Krishnamurty, S.; Shah, V.; Kanhere, D.G. Density functional investigation of the interaction of acetone with small gold clusters. J. Chem. Phys. 2007, 126, 014704. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Zhao, J. Density-functional study of Aun (n = 2–20) clusters: Lowest-energy structures and electronic properties. Phys. Rev. B 2002, 66, 035418. [Google Scholar] [CrossRef]
- De, H.S.; Krishnamurty, S.; Mishra, D.; Pal, S. Finite temperature behavior of gas phase neutral Aun (3≤n≤10) clusters: A first principles investigation. J. Phys. Chem. C 2011, 115, 17278–17285. [Google Scholar] [CrossRef]
- Zhou, J.C.; Li, W.J.; zhu, J.B. Particle swarm optimization computer simulation of Ni clusters. Trans. Nonferrous. Met. Soc. China 2008, 18, 410–415. [Google Scholar] [CrossRef]
- Ding, X.L.; Li, Z.Y.; Yang, J.L.; Hou, J.G.; Zhu, Q.S. Theoretical study of nitric oxide adsorption on Au clusters. J. Chem. Phys. 2004, 121, 2558–2562. [Google Scholar]
- Wu, X.; Senapati, L.; Nayak, S.K.; Selloni, A.; Hajaligol, M. A density functional study of carbon monoxide adsorption on small cationic, neutral, and anionic gold clusters. J. Chem. Phys. 2002, 117, 4010–4015. [Google Scholar] [CrossRef]
- Ghebriel, H.W.; Kshirsagar, A. Adsorption of molecular hydrogen and hydrogen sulfide on Au clusters. J. Chem. Phys. 2007, 126, 244705. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Z. Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells. J. Phys. Chem. C 2011, 115, 11170–11176. [Google Scholar] [CrossRef]
- Yu, L.; Pan, X.; Cao, X.; Hu, P.; Bao, X. Oxygen reduction reaction mechanism on nitrogen-doped graphene: A density functional theory study. J. Catal. 2011, 282, 183–190. [Google Scholar]
- Xu, Y.; Ruban, A.V.; Mavrikakis, M. Adsorption and dissociation of O2 on Pt-Co and Pt-Fe alloys. J. Am. Chem. Soc. 2004, 126, 4717–4725. [Google Scholar] [CrossRef]
- Chen, X.; Li, F.; Wang, X.; Sun, S.; Xia, D. Density functional theory study of the oxygen reduction reaction on a cobalt−polypyrrole composite catalyst. J. Phys. Chem. C 2012, 116, 12553–12558. [Google Scholar] [CrossRef]
- Chen, X.; Sun, S.; Wang, X.; Li, F.; Xia, D. DFT study of polyaniline and metal composites as nonprecious metal catalysts for oxygen reduction in fuel cells. J. Phys. Chem. C 2012, 116, 22737–22742. [Google Scholar] [CrossRef]
- Uribe, F.A.; Zawodzinski, T.A., Jr. A study of polymer electrolyte fuel cell performance at high voltages. Dependence on cathode catalyst layer composition and on voltage conditioning. Electrochim. Acta 2002, 47, 3799–3806. [Google Scholar] [CrossRef]
- Lamas, E.J.; Balbuena, P.B. Adsorbate effects on structure and shape of supported nanoclusters: A molecular dynamics study. J. Phys. Chem. B 2003, 107, 11682–11689. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, X.; Sun, S.; Li, F.; Wang, X.; Xia, D. The Interactions of Oxygen with Small Gold Clusters on Nitrogen-Doped Graphene. Molecules 2013, 18, 3279-3291. https://doi.org/10.3390/molecules18033279
Chen X, Sun S, Li F, Wang X, Xia D. The Interactions of Oxygen with Small Gold Clusters on Nitrogen-Doped Graphene. Molecules. 2013; 18(3):3279-3291. https://doi.org/10.3390/molecules18033279
Chicago/Turabian StyleChen, Xin, Shaorui Sun, Fan Li, Xiayan Wang, and Dingguo Xia. 2013. "The Interactions of Oxygen with Small Gold Clusters on Nitrogen-Doped Graphene" Molecules 18, no. 3: 3279-3291. https://doi.org/10.3390/molecules18033279
APA StyleChen, X., Sun, S., Li, F., Wang, X., & Xia, D. (2013). The Interactions of Oxygen with Small Gold Clusters on Nitrogen-Doped Graphene. Molecules, 18(3), 3279-3291. https://doi.org/10.3390/molecules18033279




