Design and Synthesis of Arylthiophene-2-Carbaldehydes via Suzuki-Miyaura Reactions and Their Biological Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
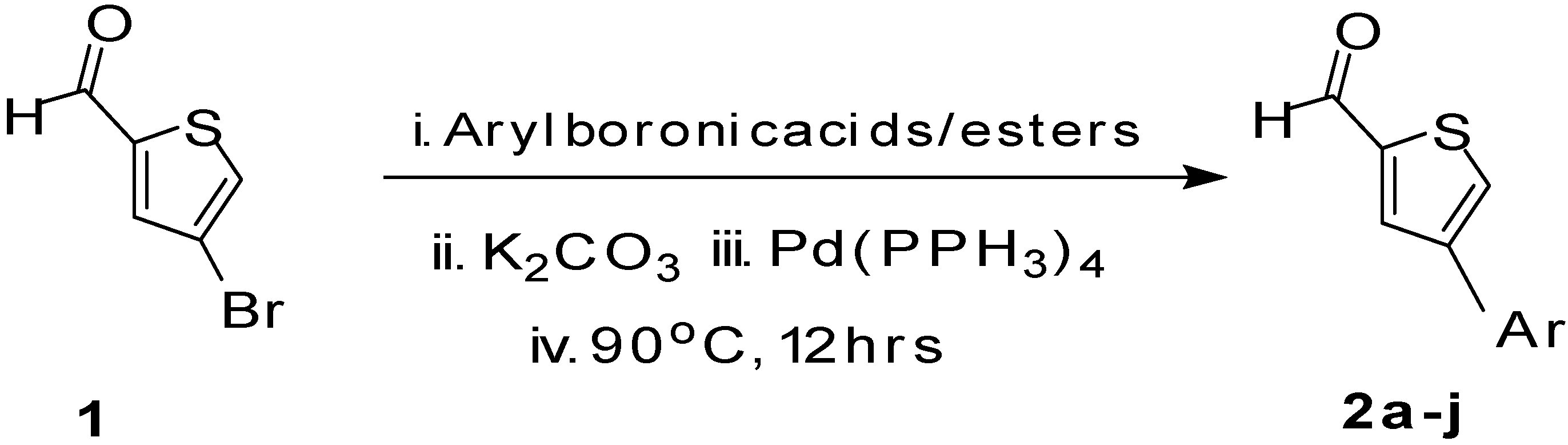
| Entry | ArylBoronic Acids/Esters | Products | Solvent/H2O (4:1) | Yields% |
|---|---|---|---|---|
| 1 |  |  | Toluene | 67 |
| 2 |  |  | 1,4-Dioxane | 64 |
| 3 |  |  | 1,4-Dioxane | 70 |
| 4 |  |  | 1,4-Dioxane | 66 |
| 5 |  |  | 1,4-Dioxane | 62 |
| 6 |  |  | Toluene | 64 |
| 7 |  |  | 1,4-Dioxane | 57 |
| 8 |  | 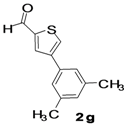 | 1,4-Dioxane | 71 |
| 9 |  | 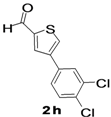 | 1,4-Dioxane | 37 |
| 10 |  |  | 1,4-Dioxane | 40 |
| 11 |  | 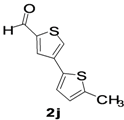 | 1,4-Dioxane | 69 |
2.2. Biological Evaluation
2.2.1. Antibacterial Activity
| Compound | Gram Positive Bacteria | Gram Negative Bacteria | ||||
|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | P. aeruginosa | E.coli | S. dysenteriae | S. typhi | |
| 2b | 36.6 ± 0.4 | 23 ± 0.09 | 42 ± 0.02 | 18 ± 0.13 | 33 ± 0.05 | 33 ± 0.20 |
| 2c | 34 ± 0.01 | 36.2 ± 0.05 | 45 ± 0.019 | 14 ± 0.14 | 35 ± 0.01 | 34 ± 0.05 |
| 2d | 33 ± 0.01 | 28.3 ± 0.75 | 46 ± 0.02 | 32.5 ± 0.12 | 35 ± 0.15 | 30 ± 0.15 |
| 2e | 28 ± 0.2 | 22.4 ± 0.13 | 44 ± 0.02 | 32 ± 0.05 | 41 ± 0.05 | 22.2 ± 0.14 |
| 2f | 34 ± 0.05 | 24.4 ± 0.03 | 34 ± 0.04 | 9 ± 0.03 | 42 ± 0.7 | 36 ± 0.01 |
| 2g | 27 ± 0.08 | 36 ± 0.08 | 25 ± 0.06 | 22.5 ± 0.11 | 32 ± 0.01 | 32.2 ± 0.11 |
| 2h | 39.4 ± 0.05 | 28 ± 0.03 | 40 ± 0.041 | 32 ± 0.03 | 29 ± 0.19 | 30 ± 0.13 |
| 2i | 27.8 ± 0.12 | 36.5 ± 0.04 | 42 ± 0.04 | 16 ± 0.043 | 43 ± 0.105 | 33 ± 0.25 |
| 2j | 35.2 ± 0.01 | 27.10 ± 0.01 | 36 ± 0.16 | 26 ± 0.03 | 35 ± 0.2 | 29 ± 0.1 |
| Streptomycin | 15 ± 0.034 | 34 ± 0.015 | 41.5 ± 0.002 | 29.3 ± 0.002 | 48 ± 0.05 | 33 ± 0.001 |
| Compound | Gram Positive Bacteria | Gram Negative Bacteria | ||||
|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | P. aeruginosa | E.coli | S. dysenteriae | S. typhi | |
| 2a | 63 ± 0.18 | 61 ± 0.14 | 67.6 ± 0.001 | 47 ± 0.15 | 63 ± 0.18 | 61 ± 0.13 |
| 2b | 62.4 ± 0.1 | 59 ± 0.1 | 67 ± 0.01 | 38 0.18 | 62.4 ± 0.1 | 63 ± 0.25 |
| 2c | 64 ± 0.11 | 60 ± 0.01 | 60 ± 0.009 | 24 ± 0.17 | 64 ± 0.11 | 64 ± 0.5 |
| 2d | 63 ± 0.31 | 58 ± 0.35 | 67 ± 0.01 | 40.5 ± 0.19 | 63 ± 0.31 | 64 ± 0.05 |
| 2e | 58 ± 0.01 | 54 ± 0.17 | 60 ± 0.03 | 62 ± 0.01 | 58 ± 0.01 | 53 ± 0.12 |
| 2f | 61.4 ± 0.56 | 54 ± 0.23 | 64 ± 0.04 | 8 ± 0.01 | 61.4 ± 0.56 | 63 ± 0.11 |
| 2g | 59 ± 0.44 | 56 ± 0.24 | 56 ± 0.04 | 44.5 ± 0.19 | 59 ± 0.44 | 61 ± 0.16 |
| 2h | 59.4 ± 0.45 | 58 ± 0.22 | 67 ± 0.021 | 60 ± 0.24 | 59.4 ± 0.45 | 57 ± 0.01 |
| 2i | 58 ± 0.16 | 56 ± 0.5 | 66 ± 0.01 | 26 ± 0.023 | 58 ± 0.16 | 59.5 ± 0.25 |
| 2j | 65 ± 0.09 | 60 ± 0.05 | 69 ± 0.16 | 66 ± 0.43 | 65 ± 0.09 | 63 ± 0.1 |
| Streptomycin | 55 ± 0.03 | 53 ± 0.001 | 63 ± 0.002 | 63 ± 0.002 | 55 ± 0.034 | 53 ± 0.001 |
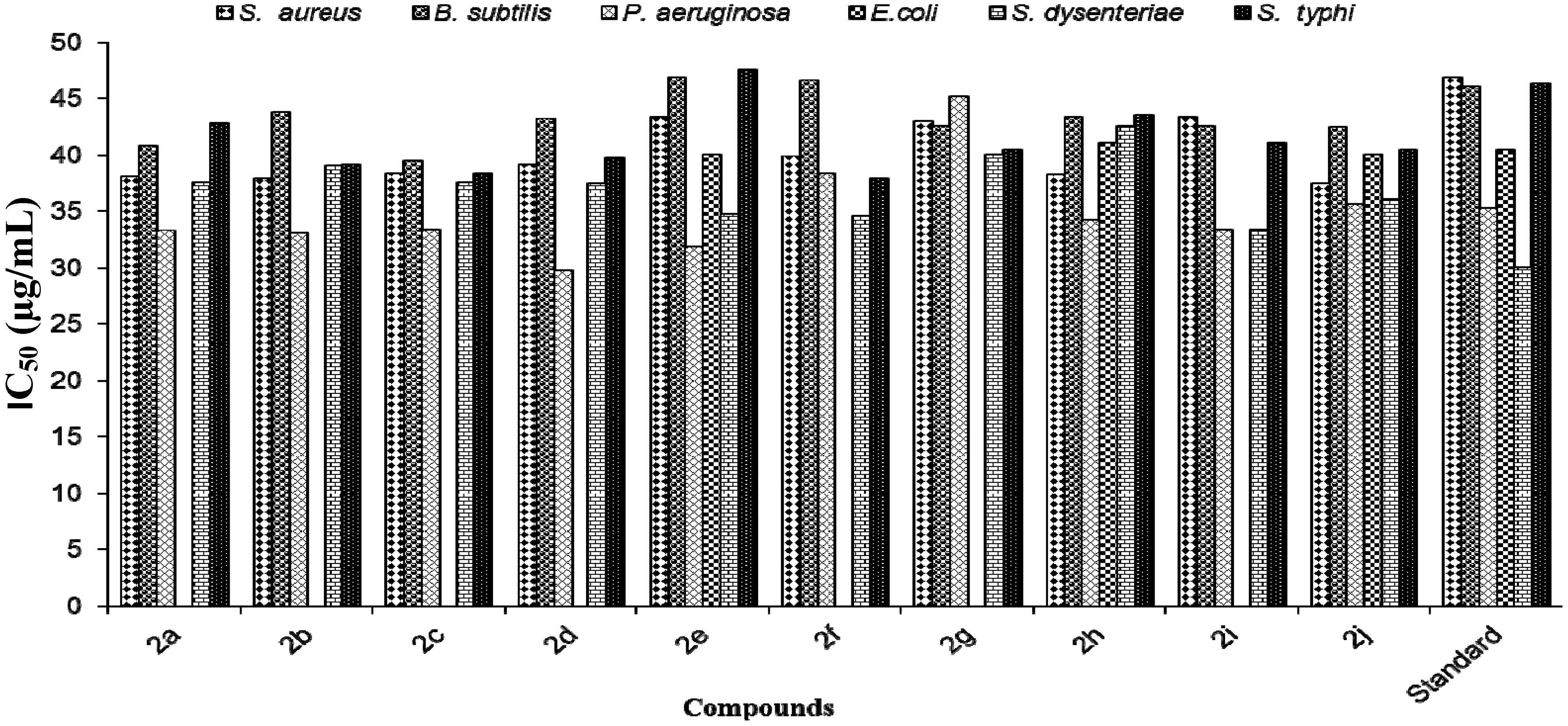
2.2.2. Antiurease Activity
| Entry | %age Activity at 25 μg | %age Activity at 50 μg | IC50 (µg/mL) |
|---|---|---|---|
| 2a | 45 ± 0.0162 | 86.9 ± 0.012 | 27.9 |
| 2b | 35 ± 0.014 | 87.0 ± 0.084 | 32.2 |
| 2c | 44 ± 0.0042 | 87.8 ± 0.006 | 28.4 |
| 2d | 37 ± 0.0042 | 88.7 ± 0.005 | 31.2 |
| 2e | 44.5 ± 0.057 | 76.5 ± 0.05 | 29.2 |
| 2f | 42.3 ± 0.0007 | 80.2 ± 0.0017 | 30.0 |
| 2g | 42.5 ± 0.0070 | 87.2 ± 0.5 | 29.1 |
| 2h | 44 ± 0.084 | 86.4 ± 0.44 | 28.5 |
| 2i | 46.2 ± 0.0042 | 90.6 ± 0.05 | 27.1 |
| 2j | 2 ± 0.219 | 9.7 ± 0.2 | Zero |
| Thiourea | 47 ± 0.007 | 77 ± 0.015 | 27.5 |
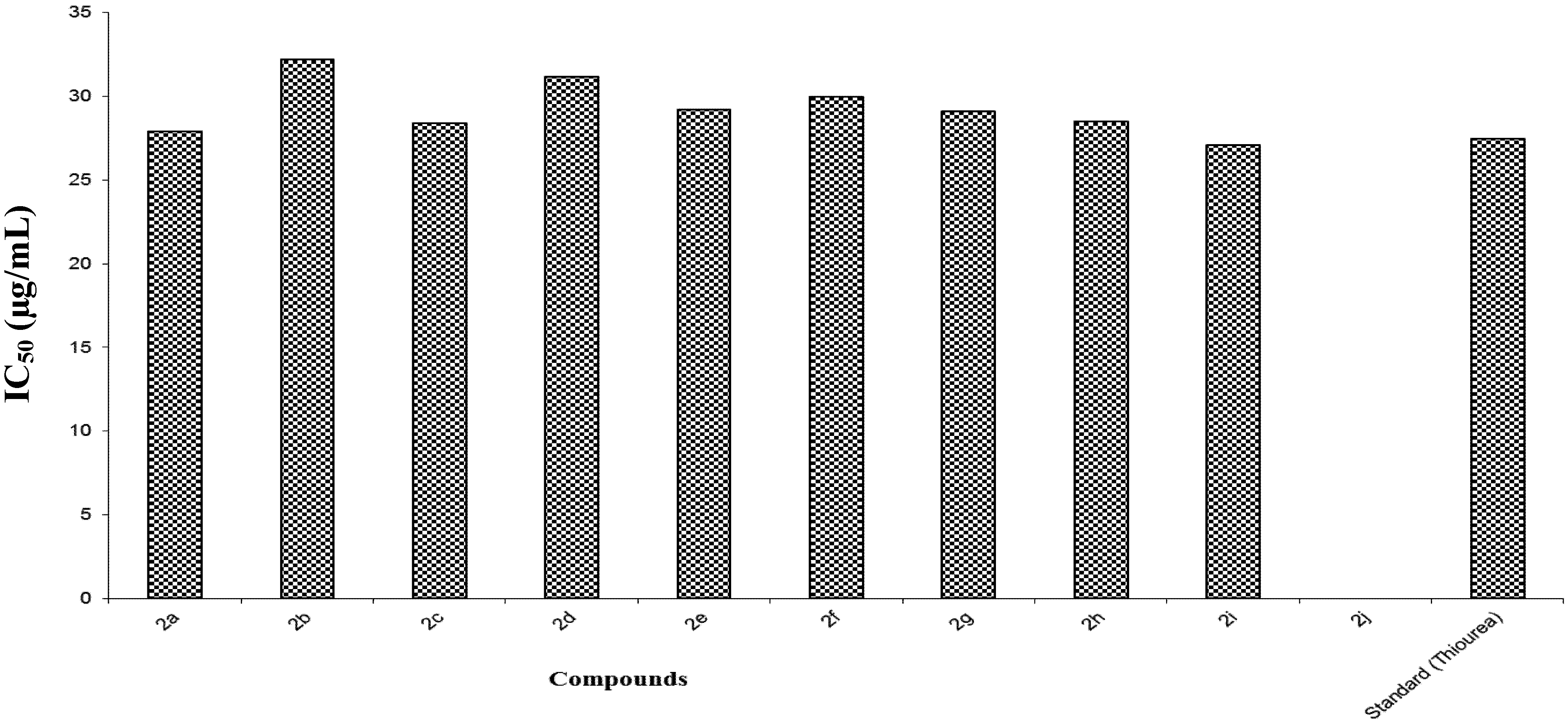
2.2.3. Nitric Oxide (NO) Scavenging Assay
| Compound | % Activity at 25 µg | % Activity at 50 µg | % Activity at 100 µg | IC50 µg/mL |
|---|---|---|---|---|
| 2a | 12 ± 0.037 | 33 ± 0.0035 | 48 ± 0.049 | 92.5 |
| 2b | 10 ± 0.02 | 45 ± 0.001 | 60 ± 0.021 | 66.6 |
| 2c | 18 ± 0.007 | 40 ± 0.038 | 55 ± 0.040 | 83.3 |
| 2d | 12.0 ± 0.025 | 58 ± 0.010 | 73 ± 0.0028 | 45.6 |
| 2e | −15 ± 0.04 | −12 ± 0.037 | 3 ± 0.0014 | NA |
| 2f | 5 ± 0.005 | 10 ± 0.028 | 25 ± 0.005 | NA |
| 2g | 13 ± 0.002 | 28 ± 0.007 | 43 ± 0.002 | NA |
| 2h | 13 ± 0.002 | 20 ± 0.025 | 35 ± 0.077 | NA |
| 2i | 12 ± 0.05 | 57 ± 0.0021 | 72 ± 0.009 | 46.1 |
| 2j | 15 ± 0.021 | 50 ± 0.024 | 65 ± 0.013 | 49.3 |
| Ascorbic Acid | 40 ± 0.038 | 61 ± 0.004 | 77 ± 0.001 | 56.33 |
2.2.4. Haemolytic Activity
| Entry | Average ± SD | Entry | Average ± SD |
|---|---|---|---|
| 2a | 3.947 ± 0.078 | 2f | 6.083 ± 0.143 |
| 2b | 5.003 ± 0.078 | 2g | 4.525 ± 0.075 |
| 2c | 3.934 ± 0.095 | 2h | 3.487 ± 0.054 |
| 2d | 3.306 ± 0.058 | 2i | 9.779 ± 0.095 |
| 2e | 3.356 ± 0.075 | 2j | 4.135 ± 0.095 |
| Standard | 99.824 ± 0.536 |
3. Experimental
3.1. General
3.2. General Procedure of Synthesis of 4-Arylthiophene-2-Carbaldehyde
3.3. Characterization Data
3.4. General Antibacterial Activity Assay Procedure
3.5. Antiurease Activity Assay Procedure
3.6. Haemolytic Activity Assay Procedure
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compound. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Miyaura, N.; Yanagi, N.; Suzuki, A. Palladium-catalyzed cross-coupling reaction of phenylboronic acids with haloareenes in the presence of base. Syn. Commun. 1981, 11, 513–519. [Google Scholar] [CrossRef]
- Jeong, S.J.; Higuchi, R.; Miyamoto, T.; Ono, M.; Kuwano, M.; Mawatari, S.F. Bryoanthrathiophene, a new antiangiogenic constituent from the bryozoan Watersipora subtorquata. J. Nat. Prod. 2002, 65, 1344–1345. [Google Scholar] [CrossRef]
- Kelly, T.R.; Fu, Y.; Sieglen, J.T.; de Silva, H. Synthesis of an orange anthrathiophene pigment isolated from a Japanese bryozoan. Org. Lett. 2000, 2, 2351–2352. [Google Scholar] [CrossRef]
- Ankita, C.; Jha, K.; Sachin, K. Biological diversity of thiophene. J. Adv. Sci. 2012, 3, 3–10. [Google Scholar]
- Koumura, N.; Wang, Z.S.; Mori, S.; Miyashita, M.; Suzuki, E.; Hara, J.K. Alkyl-functionalized organic dyes for efficient molecular photovoltaics. J. Am. Chem. Soc. 2006, 128, 14256–14257. [Google Scholar] [CrossRef]
- Garnier, F. Functionalized conducting polymer. Angew. Chem. Int. Ed. Engl. 1989, 28, 513–517. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.; Jenekhe, S.A. Thiophene-linked polyquinoxaline: A new electron transport conjugated polymer for light emitting diodes. Macromolecules 1999, 32, 3824–3826. [Google Scholar] [CrossRef]
- Thayumanavan, S.; Mendez, J.; Marder, S.R. Synthesis of functionalized organic second-order nonlinear optical chromophores for electro-optic applications. J. Org. Chem. 1999, 64, 4289–4297. [Google Scholar] [CrossRef]
- Russel, K.R.; Press, B.J.; Rampulla, A.R. Thiophene system 9, Thienopyrimidinedione derivatives as potential antihypertensive agent. J. Med. Chem. 1988, 31, 1786–1789. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, S.; Sing, I.; Saxena, K.K.; Kumar, A. Synthesis, characterization and biological activity of various substituted benzothiazol derivatives. Dig. J. Nanomater. Bios. 2010, 5, 67–76. [Google Scholar]
- Badar, S.M.I. Synthesis and anti-inflammatory activity of novel 2,5-disubstituted thiophene derivatives. Turk. J. Chem. 2001, 35, 131–143. [Google Scholar]
- Mabkhot, Y.N.; Barakat, A.; Al-Majid, A.M.; Alsharani, S.; Yousaf, S.; Choudhary, M.T. Synthesis, Reactions and biologicalactivity of some new bis-heterocyclic ring compounds containing sulphur atom. Chem. Cent. J. 2013, 7, 112–121. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Satyendra, D.; Apurba, T.; Patel, M.; Monika, K.; Girish, K.; Mohan, S.; Saravanan, J. Synthesis and antimicrobial screening of some novel substituted thiophene. Hygeia J. Drug Med. 2012, 4, 112–118. [Google Scholar]
- Al-Adiwish, W.A.; Yaacob, W.A.; Adan, D.; Nazlina, I. Synthesis and antibacterial activity of thiophenes. Int. J. Adv. Sci. Eng. Inf. Technol. 2012, 2, 27–30. [Google Scholar]
- Thomas, A.B.; Nanda, R.K.; Kothapalli, L.P.; Deshpande, A.D. Synthesis and antibacterial activity of N-[2-(aryl/substituted aryl)-4-oxo-1,3-thiazolidin-3-yl]pridine-4-carboxamide. J. Kor. Chem. Soc. 2011, 55, 960–968. [Google Scholar] [CrossRef]
- Sigmundova, I.; Zahradnik, P.; Magdolen, P.; Bujdakova, H. Synthesis and study of new antibacterial benzothiazoles substituted on heterocyclic ring. ARKIVOC 2008, 8, 183–192. [Google Scholar]
- Dang, T.T.; Rasool, N.; Langer, P. Synthesis of tetraarylthiophenes by regioselective Suzuki cross coupling reaction of tetrabromothiophene. Tetrahedron Lett. 2007, 48, 845–847. [Google Scholar] [CrossRef]
- Handy, S.T.; Mayi, D. Regioselective double Suzuki couplings of 4,5-dibromothiophene-2-carbaldehyde. Tetrahedron Lett. 2007, 48, 8108–8110. [Google Scholar] [CrossRef]
- Kaspady, M.; Narayanaswamy, K.V.; Raju, M.; Rao, K.G. Synthesis, antibacterial activity of 2,4-disubstituted oxazoles and thiazoles as bioesters. Lett. Drug Des. Discov. 2009, 6, 21–28. [Google Scholar] [CrossRef]
- Atta-ur-Rehman; Choudhary, M.T.; Thomsen, W. Manual of Bioassay Techniques for Natural Product Chemists; Harwood Academic Publishers: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Srivastava, S.; Das, B. Synthesis and evaluation of some novel thiophene as potential antibacterial and mycolytic agents. Pharm. Chem. 2011, 3, 103–111. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M. Role of free radical and metal ions in human disease: An overview. Meth. Enzymol. 1990, 186, 1–85. [Google Scholar]
- Vamanu, V. Study of antioxidant and antimicrobial activities of Pleurotusodstreatus PS1101109 Mycellum. Pakistan J. Bot. 2013, 45, 311–317. [Google Scholar]
- Marcocci, L.; Packer, L.; Droy-Lefai, M.T.; Sekaki, A.; Albert, M. Antioxidant action of Ginko biloba extracts EGb 761. Meth. Enzymol. 1994, 234, 462–475. [Google Scholar]
- Fadda, A.A.; Berghot, M.A.; Amer, F.A.; Badway, D.S.; Bayoumy, N.M. Synthesis and antioxidant and antitumor activity of novel pyridine, chromine, thiophene and thiazoles derivatives. Arch. Pharm. 2012, 435, 378–385. [Google Scholar]
- Garrat, D. The Quantitative Analysis of Drugs, 3rd ed.; Chapman and Hall Ltd.: Tokyo, Japan, 1964; pp. 456–458. [Google Scholar]
- Mavrova, A.T.; Wesselinova, D.; Tsenov, Y.A.; Denkova, P. Synthesis, cytotoxicity and effects of some 1,2,3-triazol and 1,3,4-thiadiazole derivatives on immunocompetent cells. Eur. J. Med. Chem. 2009, 44, 63–69. [Google Scholar] [CrossRef]
- Powell, W.A.; Catranis, C.M.; Maynard, C.A. Design of self-processing antimicrobial peptide for plant protection. Lett. Appl. Microbiol. 2000, 31, 163–165. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, J.D. In vitro hemolysis of human erythrocytes by plant extraction with antiplasmodial activity. J. Ethnopharmacol. 2001, 74, 239–243. [Google Scholar] [CrossRef]
- Nasrullah, M.; Khan, M.A.; Humphrey, M.G.; Nasim, F.H.; Abidi, M.G.; Khan, M.N.; Farooq, U.; Munawar, M.A. Diaryl pyrazole-4-carbaldehyde benzoylhydrazones metal complexes: Synthesis and their antibacterial and antioxidant screening. Asian J. Chem. 2013, 25, 419–423. [Google Scholar]
- Riaz, M.; Rasool, N.; Bukhari, I.H.; Shahid, M.; Zubair, M.; Rizwan, K.; Rashid, U. In vitro antimicrobial, antioxidant, cytotoxicity and GC-MS analysis of Mazus goodenifolius. Molecules 2012, 17, 14275–14287. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ali, S.; Rasool, N.; Ullah, A.; Nasim, F.-u.-H.; Yaqoob, A.; Zubair, M.; Rashid, U.; Riaz, M. Design and Synthesis of Arylthiophene-2-Carbaldehydes via Suzuki-Miyaura Reactions and Their Biological Evaluation. Molecules 2013, 18, 14711-14725. https://doi.org/10.3390/molecules181214711
Ali S, Rasool N, Ullah A, Nasim F-u-H, Yaqoob A, Zubair M, Rashid U, Riaz M. Design and Synthesis of Arylthiophene-2-Carbaldehydes via Suzuki-Miyaura Reactions and Their Biological Evaluation. Molecules. 2013; 18(12):14711-14725. https://doi.org/10.3390/molecules181214711
Chicago/Turabian StyleAli, Shaukat, Nasir Rasool, Aman Ullah, Faiz-ul-Hassan Nasim, Asma Yaqoob, Muhammad Zubair, Umer Rashid, and Muhammad Riaz. 2013. "Design and Synthesis of Arylthiophene-2-Carbaldehydes via Suzuki-Miyaura Reactions and Their Biological Evaluation" Molecules 18, no. 12: 14711-14725. https://doi.org/10.3390/molecules181214711
APA StyleAli, S., Rasool, N., Ullah, A., Nasim, F.-u.-H., Yaqoob, A., Zubair, M., Rashid, U., & Riaz, M. (2013). Design and Synthesis of Arylthiophene-2-Carbaldehydes via Suzuki-Miyaura Reactions and Their Biological Evaluation. Molecules, 18(12), 14711-14725. https://doi.org/10.3390/molecules181214711







