Baicalein Prevents 6-Hydroxydopamine-Induced Mitochondrial Dysfunction in SH-SY5Y Cells via Inhibition of Mitochondrial Oxidation and Up-Regulation of DJ-1 Protein Expression
Abstract
:1. Introduction
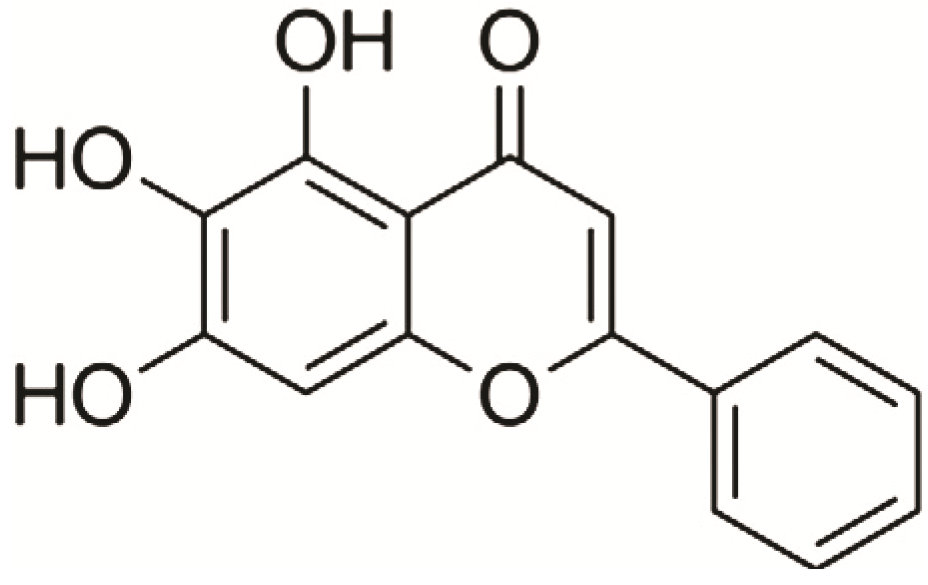
2. Results and Discussion
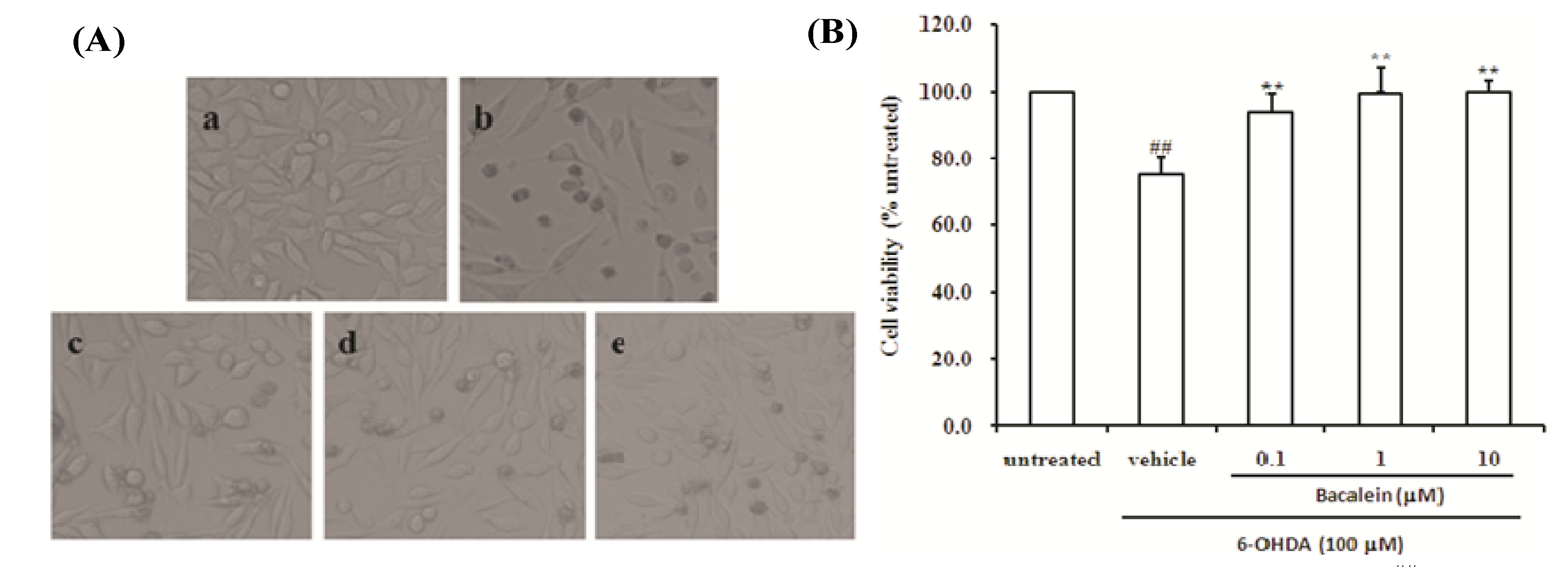
2.1. Effect of Baicalein on Morphology and Cell Viability in SH-SY5Y Cells Damaged by 6-OHDA
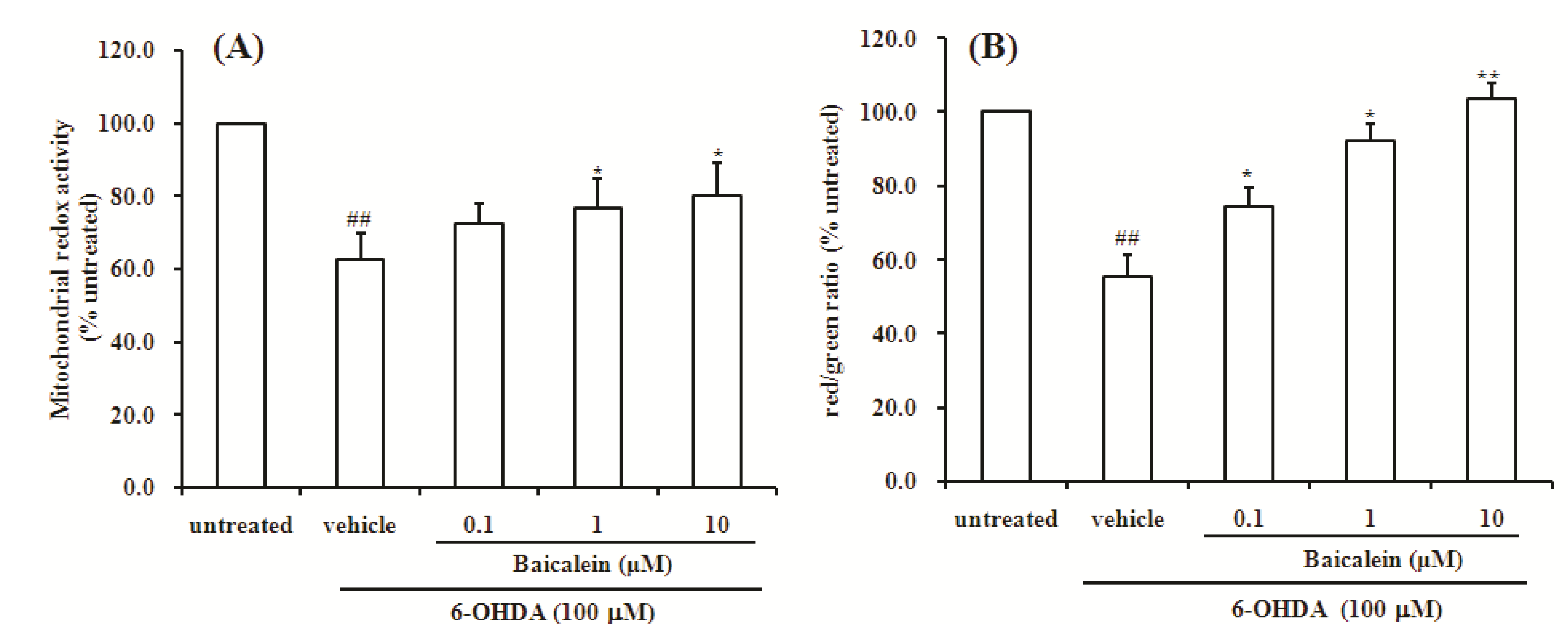
2.2. Baicalein Attenuates the Decrease of Mitochondria Redox Activity and the Collapse of Mitochondrial Membrane Potential Induced by 6-OHDA in SH-SY5Y Cells
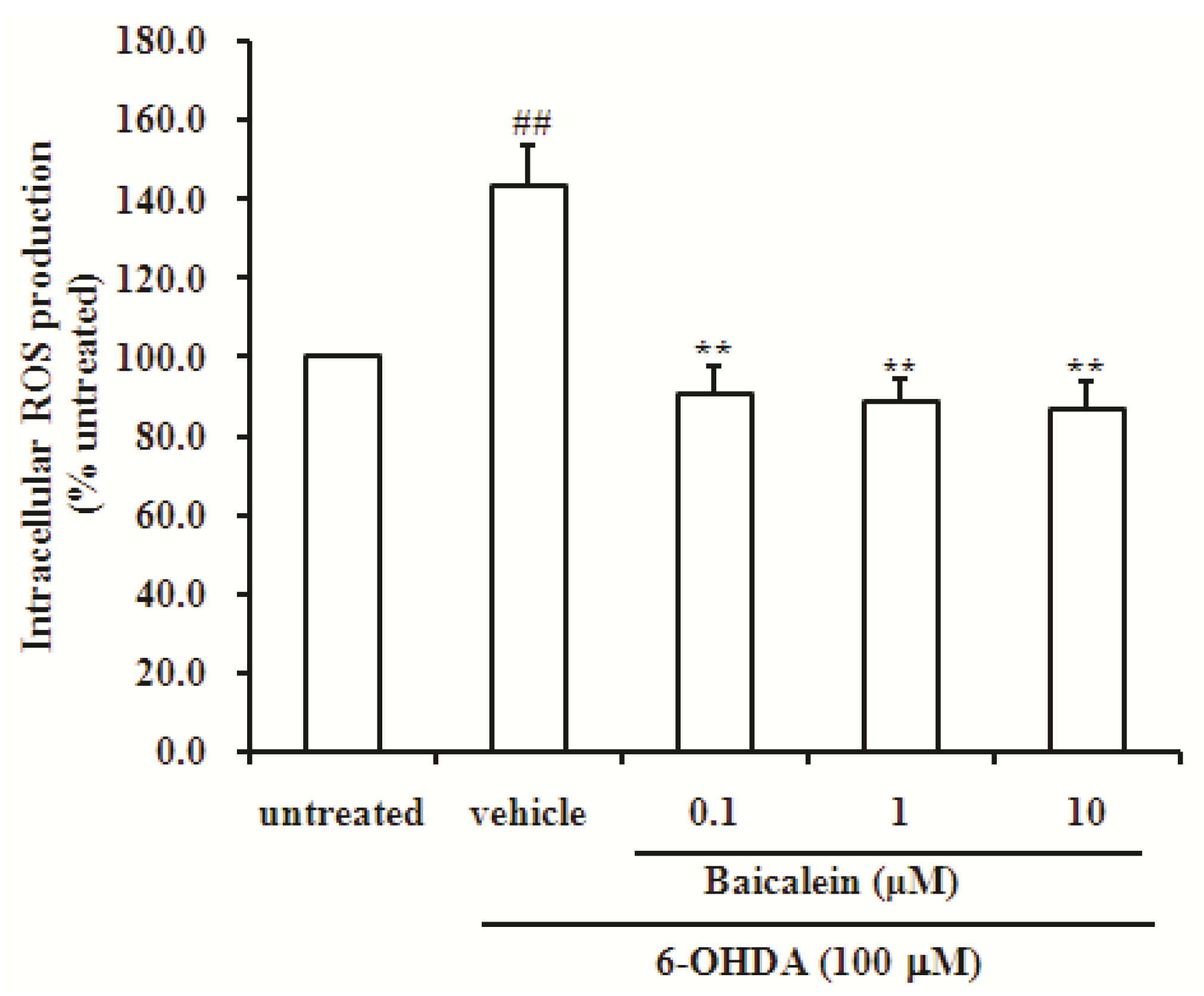
2.3. Baicalein Reduces the Production of ROS Induced by 6-OHDA in SH-SY5Y Cells
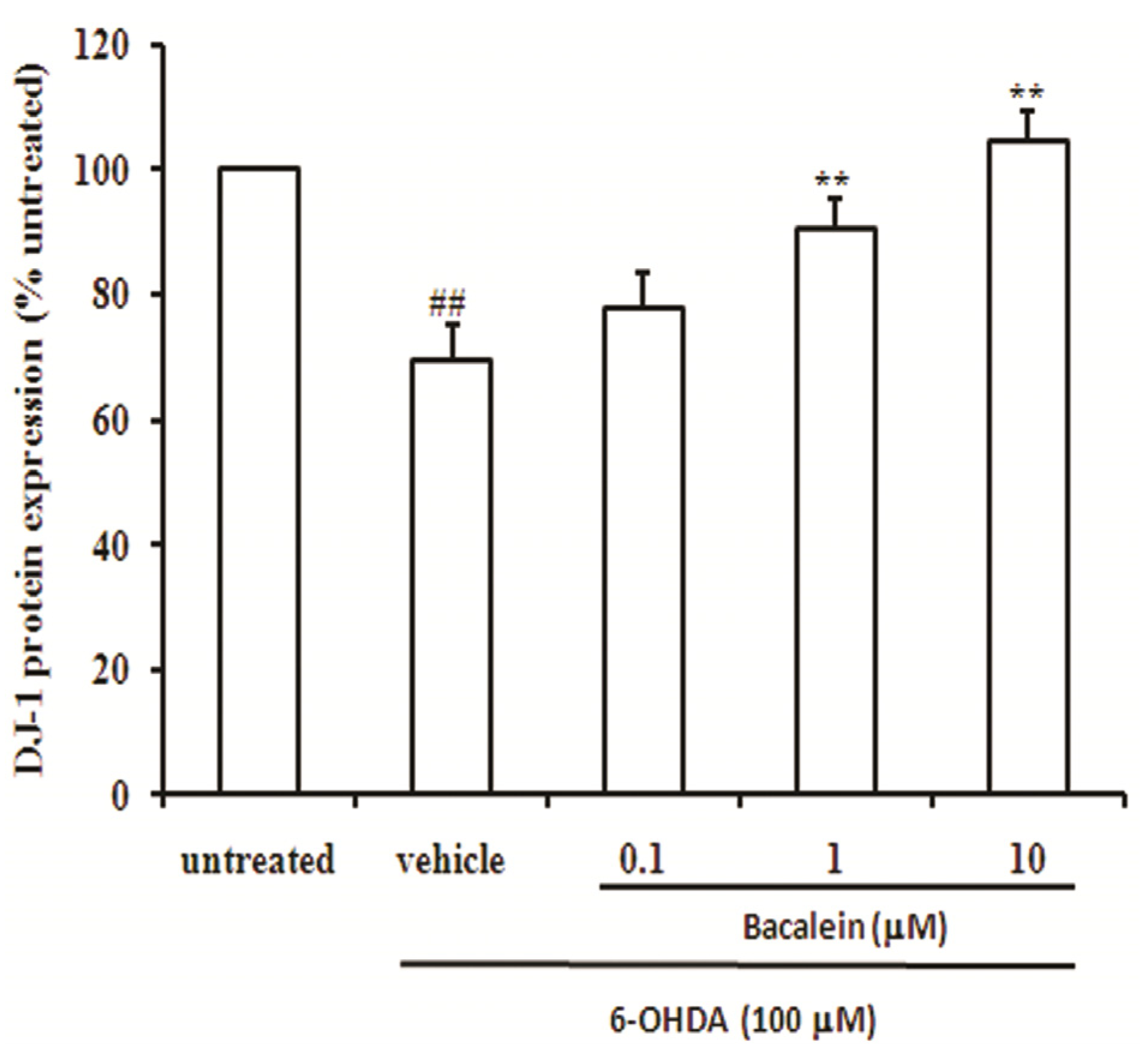
2.4. Baicalein Up-Regulates DJ-1 Protein Expression in SH-SY5Y Cells
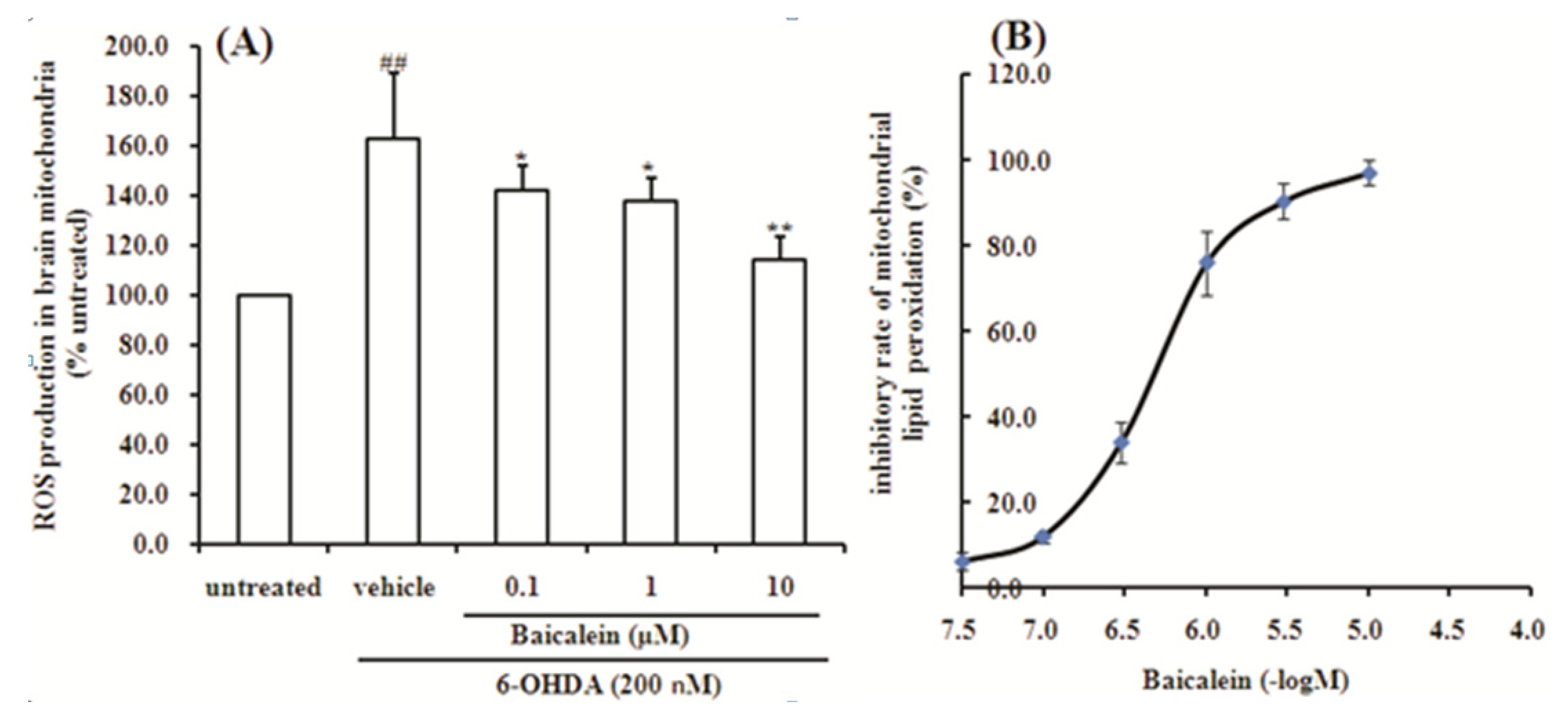
2.5. Baicalein Suppresses the ROS Production Induced by 6-OHDA and Lipid Peroxidation in Rat Brain Mitochondria
3. Experimental
3.1. Materials
3.2. Cell Culture and Treatment
3.3. Morphological Observations and Viability Assay
3.4. Measurement of Mitochondrial Redox Activity by Resazurin
3.5. Measurement of Mitochondrial Membrane Potential (Δψm) by JC-1
3.6. Determination of Intracellular ROS Level
3.7. DJ-1 Protein Expression Assay Using Cell-Based ELISA
3.8. Isolation of Rat Brain Mitochondria
3.9. Detection of ROS Production in Rat Brain Mitochondria
3.10. Lipid Peroxidation of Rat Brain Mitochondria Induced by FeSO4-Cystine
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kim, I.S.; Choi, D.K.; Jung, H.J. Neuroprotective effects of vanillyl alcohol in Gastrodia elata Blume through suppression of oxidative stress and anti-apoptotic activity in toxin-induced dopaminergic MN9D cells. Molecules 2011, 16, 5349–5361. [Google Scholar] [CrossRef]
- Swerdlow, R.H. The neurodegenerative mitochondriopathies. J. Alzheimers Dis. 2009, 17, 737–751. [Google Scholar]
- Mosley, R.L.; Benner, E.J.; Kadiu, I.; Thomas, M.; Boska, M.D.; Hasan, K.; Laurie, C.; Gendelman, H.E. Neuroinflammation, oxidative stress and the pathogenesis of Parkinson’s disease. Clin. Neurosci. Res. 2006, 6, 261–281. [Google Scholar] [CrossRef]
- Winklhofer, K.F.; Haass, C. Mitochondrial dysfunction in Parkinson’s disease. Biochim. Biophys. Acta 2010, 1802, 29–44. [Google Scholar] [CrossRef]
- Frazier, A.E.; Kiu, C.; Stojanovski, D.; Hoogenraad, N.J.; Ryan, M.T. Mitochondrial morphology and distribution in mammalian cells. Biol. Chem. 2006, 387, 1551–1558. [Google Scholar]
- Westermann, B. Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys. Acta 2012, 1817, 1833–1838. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondria: Dynamic organelles in disease, aging, and development. Cell 2006, 125, 1241–1252. [Google Scholar] [CrossRef]
- Dröge, W.; Schipper, H.M. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell 2007, 6, 361–370. [Google Scholar] [CrossRef]
- Koppula, S.; Kumar, H.; More, S.V.; Lim, H.W.; Hong, S.M.; Choi, D.K. Recent updates in redox regulation and free radical scavenging effects by herbal products in experimental models of Parkinson’s disease. Molecules 2012, 17, 11391–11420. [Google Scholar] [CrossRef]
- Nagakubo, D.; Taira, T.; Kitaura, H.; Ikeda, M.; Tamai, K.; Iguchi-Ariga, S.M.; Ariga, H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem. Biophys. Res. Commun. 1997, 231, 509–513. [Google Scholar] [CrossRef]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef]
- Clements, C.M.; McNally, R.S.; Conti, B.J.; Mak, T.W.; Ting, J.P. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Nat. Acad. Sci. USA 2006, 103, 15091–15096. [Google Scholar] [CrossRef]
- Taira, T.; Saito, Y.; Niki, T.; Iguchi-Ariga, S.M.; Takahashi, K.; Ariga, H. DJ-1has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004, 5, 213–218. [Google Scholar] [CrossRef]
- Yokota, T.; Sugawara, K.; Ito, K.; Takahashi, R.; Ariga, H.; Mizusawa, H. Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem. Biophys. Res. Commun. 2003, 312, 1342–1348. [Google Scholar] [CrossRef]
- Canet-Avilés, R.M.; Wilson, M.A.; Miller, D.W.; Ahmad, R.; McLendon, C.; Bandyopadhyay, S.; Baptista, M.J.; Ringe, D.; Petsko, G.A.; Cookson, M.R. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfonic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA 2004, 101, 9103–9108. [Google Scholar] [CrossRef]
- Martinat, C.; Shendelman, S.; Jonason, A.; Leete, T.; Beal, M.F.; Yang, L.; Floss, T.; Abeliovich, A. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: An ES-derived cell model of primary Parkinsonism. PLoS Biol. 2004, 2, e327. [Google Scholar] [CrossRef]
- Bonifati, V.; Rizzu, P.; Squitieri, F.; Krieger, E.; Vanacore, N.; van Swieten, J.C.; Brice, A.; van Duijn, C.M.; Oostra, B.; Meco, G.; et al. DJ-1( PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol. Sci. 2003, 24, 159–160. [Google Scholar] [CrossRef]
- Kahle, P.J.; Waak, J.; Gasser, T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic. Biol. Med. 2009, 47, 1354–1361. [Google Scholar] [CrossRef]
- Soto-Otero, R.; Méndez-Alvarez, E.; Sánchez-Iglesias, S.; Zubkov, F.I.; Voskressensky, L.G.; Varlamov, A.V.; de Candia, M.; Altomare, C. Inhibition of 6-hydroxydopamine-induced oxidative damage by 4,5-dihydro-3H-2-benzazepine N-oxides. Biochem. Pharmacol. 2008, 75, 1526–1537. [Google Scholar] [CrossRef]
- Soto-Otero, R.; Méndez-Alvarez, E.; Hermida-Ameijeiras, A.; López-Real, A.M.; Labandeira-García, J.L. Effects of (−)-nicotine and (−)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: Relevance for Parkinson’s disease. Biochem. Pharmacol. 2002, 64, 125–135. [Google Scholar] [CrossRef]
- Glinka, Y.Y.; Tipton, K.F.; Youdim, M.B. Nature of inhibition of mitochondrial respiratory complex I by 6-Hydroxydopamine. J. Neurochem. 1996, 66, 2004–2010. [Google Scholar] [CrossRef]
- Glinka, Y.Y.; Youdim, M.B. Inhibition of mitochondrial complexes I and IV by 6-hydroxydopamine. Eur. J. Pharmacol. 1995, 292, 329–332. [Google Scholar]
- Xie, H.R.; Hu, L.S.; Li, G.Y. SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar]
- Elkon, H.; Melamed, E.; Offen, D. 6-Hydroxydopamine increases ubiquitin-conjugates and protein degradation: Implications for the pathogenesis of Parkinson’s disease. Cell Mol. Neurobiol. 2001, 21, 771–781. [Google Scholar] [CrossRef]
- Jordán, J.; Galindo, M.F.; Tornero, D.; González-García, C.; Ceña, V. Bcl-x L blocks mitochondrial multiple conductance channel activation and inhibits 6-OHDA-induced death in SH-SY5Y cells. J. Neurochem. 2004, 89, 124–133. [Google Scholar] [CrossRef]
- Lin, Y.C.; Uang, H.W.; Lin, R.J.; Chen, I.J.; Lo, Y.C. Neuroprotective effects of glyceryl nonivamide against microglia-like cells and 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y human dopaminergic neuroblastoma cells. J. Pharmacol. Exp. Ther. 2007, 323, 877–887. [Google Scholar] [CrossRef]
- Gomez-Lazaro, M.; Bonekamp, N.A.; Galindo, M.F.; Jordán, J.; Schrader, M. 6-Hydroxydopamine (6-OHDA) induces Drp1-dependent mitochondrial fragmentation in SH-SY5Y cells. Free Radic. Biol. Med. 2008, 44, 1960–1969. [Google Scholar] [CrossRef]
- Xiang, B.; Fei, X.; Zhuang, W.; Fang, Y.; Qin, Z.; Liang, Z. Cathepsin L is involved in 6-hydroxydopamine induced apoptosis of SH-SY5Y neuroblastoma cells. Brain Res. 2011, 1387, 29–38. [Google Scholar] [CrossRef]
- Ciesielska, E.; Gwardys, A.; Metodiewa, D. Anticancer, antiradical and antioxidative actions of novel Antoksyd S and its major components, baicalin and baicalein. Anticancer Res. 2002, 22, 2885–2891. [Google Scholar]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Cheng, Y.; He, G.; Mu, X.; Zhang, T.; Li, X.; Hu, J.; Xu, B.; Du, G. Neuroprotective effect of baicalein against MPTP neurotoxicity: Behavioral, biochemical and immunohistochemical profile. Neurosci. Lett. 2008, 441, 16–20. [Google Scholar] [CrossRef]
- Mu, X.; He, G.R.; Yuan, X.; Li, X.X.; Du, G.H. Baicalein protects the brain against neuron impairments induced by MPTP in C57BL/6 mice. Pharmacol. Biochem. Behav. 2011, 98, 286–291. [Google Scholar] [CrossRef]
- Mu, X.; He, G.; Cheng, Y.; Li, X.; Xu, B.; Du, G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol. Biochem. Behav. 2009, 92, 642–648. [Google Scholar] [CrossRef]
- Xu, R.; Liu, J.; Chen, X.; Xu, F.; Xie, Q.; Yu, H.; Guo, Q.; Zhou, X.; Jin, Y. Ribozyme-mediated inhibition of caspase-3 activity reduces apoptosis induced by 6-hydroxydopamine in PC12 cells. Brain Res. 2001, 899, 10–19. [Google Scholar] [CrossRef]
- Jin, C.F.; Shen, S.R.; Zhao, B.L. Different effects of five catechins on 6-hydroxydopamine-induced apoptosis in PC12 cells. J. Agric. Food Chem. 2001, 49, 6033–6038. [Google Scholar] [CrossRef]
- Beal, M.F. Parkinson’s disease: A model dilemma. Nature 2010, 466, S8–S10. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Starkov, A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef]
- Lambert, A.J.; Brand, M.D. Reactive oxygen species production by mitochondria. Methods Mol. Biol. 2009, 554, 165–181. [Google Scholar] [CrossRef]
- Nakai, M.; Mori, A.; Watanabe, A.; Mitsumoto, Y. 1-methyl-4-phenylpyridinium (MPP+) decreases mitochondrial oxidation-reduction (REDOX) activity and membrane potential (Deltapsi(m)) in rat striatum. Exp. Neurol. 2003, 179, 103–110. [Google Scholar] [CrossRef]
- Levites, Y.; Youdim, M.B.; Maor, G.; Mandel, S. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-kappaB) activation and cell death by tea extracts in neuronal cultures. Biochem. Pharmacol. 2002, 63, 21–29. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species, and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef]
- Yanagida, T.; Takata, K.; Inden, M.; Kitamura, Y.; Taniguchi, T.; Yoshimoto, K.; Taira, T.; Ariga, H. Distribution of DJ-1, Parkinson’s diseaserelated protein PARK7, and its alteration in 6-hydroxydopamine-treated hemiparkinsonian rat brain. J. Pharmacol. Sci. 2006, 102, 243–247. [Google Scholar] [CrossRef]
- Yanagisawa, D.; Kitamura, Y.; Inden, M.; Takata, K.; Taniguchi, T.; Morikawa, S.; Morita, M.; Inubushi, T.; Tooyama, I.; Taira, T.; et al. DJ-1 protects against neurodegeneration caused by focal cerebral ischemia and reperfusion in rats. J. Cereb. Blood Flow Metab. 2008, 28, 563–578. [Google Scholar] [CrossRef]
- Lev, N.; Ickowicz, D.; Melamed, E.; Offen, D. Oxidative insults induce DJ-1 upregulation and redistribution: Implications for neuroprotection. Neurotoxicology 2008, 29, 397–405. [Google Scholar] [CrossRef]
- Yanagida, T.; Kitamura, Y.; Yamane, K.; Takahashi, K.; Takata, K.; Yanagisawa, D.; Yasui, H.; Taniguchi, T.; Taira, T.; Honda, T.; et al. Protection against oxidative stress-induced neurodegeneration by a modulator for DJ-1, the wild-type of familial Parkinson’s disease-linked PARK7. J. Pharmacol. Sci. 2009, 109, 463–468. [Google Scholar] [CrossRef]
- Yanagida, T.; Tsushima, J.; Kitamura, Y.; Yanagisawa, D.; Takata, K.; Shibaike, T.; Yamamoto, A.; Taniguchi, T.; Yasui, H.; Taira, T.; et al. Oxidative stress induction of DJ-1 protein in reactive astrocytes scavenges free radicals and reduces cell injury. Oxid. Med. Cell Longev. 2009, 2, 36–42. [Google Scholar] [CrossRef]
- Hayashi, T.; Ishimori, C.; Takahashi-Niki, K.; Taira, T.; Kim, Y.C.; Maita, H.; Maita, C.; Ariga, H.; Iguchi-Ariga, S.M. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem. Biophys. Res. Commun. 2009, 390, 667–672. [Google Scholar] [CrossRef]
- Schapira, A.H. Mitochondrial dysfunction in neurodegenerative disorders. Biochim. Biophys. Acta 1998, 1366, 225–233. [Google Scholar] [CrossRef]
- Wang, Y.H.; Du, G.H. Ginsenoside Rg1 inhibits beta-secretase activity in vitro and protects against Abeta-induced cytotoxicity in PC12 cells. J. Asian Nat. Prod. Res. 2009, 11, 604–612. [Google Scholar] [CrossRef]
- Magnani, E.; Bettini, E. Resazurin detection of energy metabolism changes in serum-starved PC12 cells and of neuroprotective agent effect. Brain Res. Protoc. 2000, 5, 266–272. [Google Scholar] [CrossRef]
- Mashimo, K.; Ohno, Y. Ethanol hyperpolarizes mitochondrial membrane potential and increases mitochondrial fraction in cultured mouse myocardial cells. Arch. Toxicol. 2006, 80, 421–428. [Google Scholar] [CrossRef]
- Hwang, D.S.; Kim, H.G.; Kwon, H.J.; Cho, J.H.; Lee, C.H.; Lee, J.M.; Jang, J.B.; Kim, Y.S.; Lee, K.S.; Oh, M.S. Dangguijakyak-san, a medicinal herbal formula, protects dopaminergic neurons from 6-hydroxydopamine-induced neurotoxicity. J. Ethnopharmacol. 2011, 133, 934–939. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xuan, Z.H.; Tian, S.; He, G.R.; Du, G.H. Myricitrin attenuates 6-hydroxydopamine-induced mitochondrial damage and apoptosis in PC12 cells via inhibition of mitochondrial oxidation. J. Func. Foods 2013, 5, 337–345. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Y.-H.; Yu, H.-T.; Pu, X.-P.; Du, G.-H. Baicalein Prevents 6-Hydroxydopamine-Induced Mitochondrial Dysfunction in SH-SY5Y Cells via Inhibition of Mitochondrial Oxidation and Up-Regulation of DJ-1 Protein Expression. Molecules 2013, 18, 14726-14738. https://doi.org/10.3390/molecules181214726
Wang Y-H, Yu H-T, Pu X-P, Du G-H. Baicalein Prevents 6-Hydroxydopamine-Induced Mitochondrial Dysfunction in SH-SY5Y Cells via Inhibition of Mitochondrial Oxidation and Up-Regulation of DJ-1 Protein Expression. Molecules. 2013; 18(12):14726-14738. https://doi.org/10.3390/molecules181214726
Chicago/Turabian StyleWang, Yue-Hua, Hai-Tao Yu, Xiao-Ping Pu, and Guan-Hua Du. 2013. "Baicalein Prevents 6-Hydroxydopamine-Induced Mitochondrial Dysfunction in SH-SY5Y Cells via Inhibition of Mitochondrial Oxidation and Up-Regulation of DJ-1 Protein Expression" Molecules 18, no. 12: 14726-14738. https://doi.org/10.3390/molecules181214726
APA StyleWang, Y.-H., Yu, H.-T., Pu, X.-P., & Du, G.-H. (2013). Baicalein Prevents 6-Hydroxydopamine-Induced Mitochondrial Dysfunction in SH-SY5Y Cells via Inhibition of Mitochondrial Oxidation and Up-Regulation of DJ-1 Protein Expression. Molecules, 18(12), 14726-14738. https://doi.org/10.3390/molecules181214726





