Aqueous Extracts of the Edible Gracilaria tenuistipitata are Protective Against H2O2-Induced DNA Damage, Growth Inhibition, and Cell Cycle Arrest
Abstract
:1. Introduction
2. Results
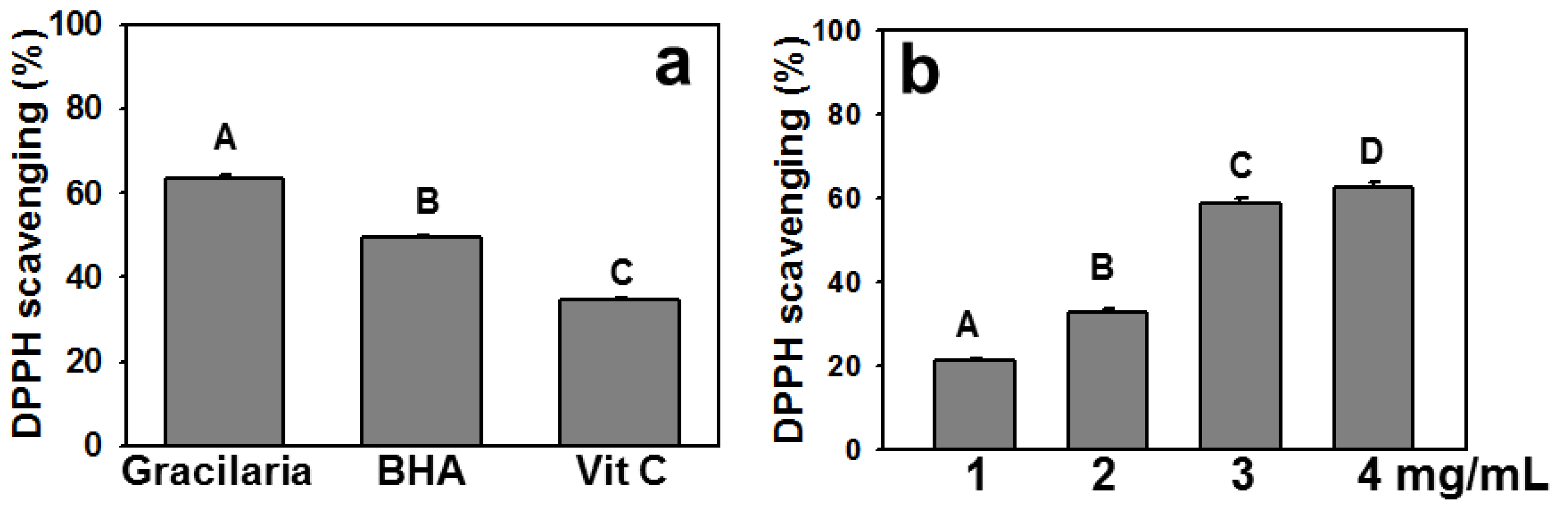
2.1. Polyphenols, Flavonoids, and Ascorbic Acid Contents of AEGT and Its DPPH Radical Scavenging Activity
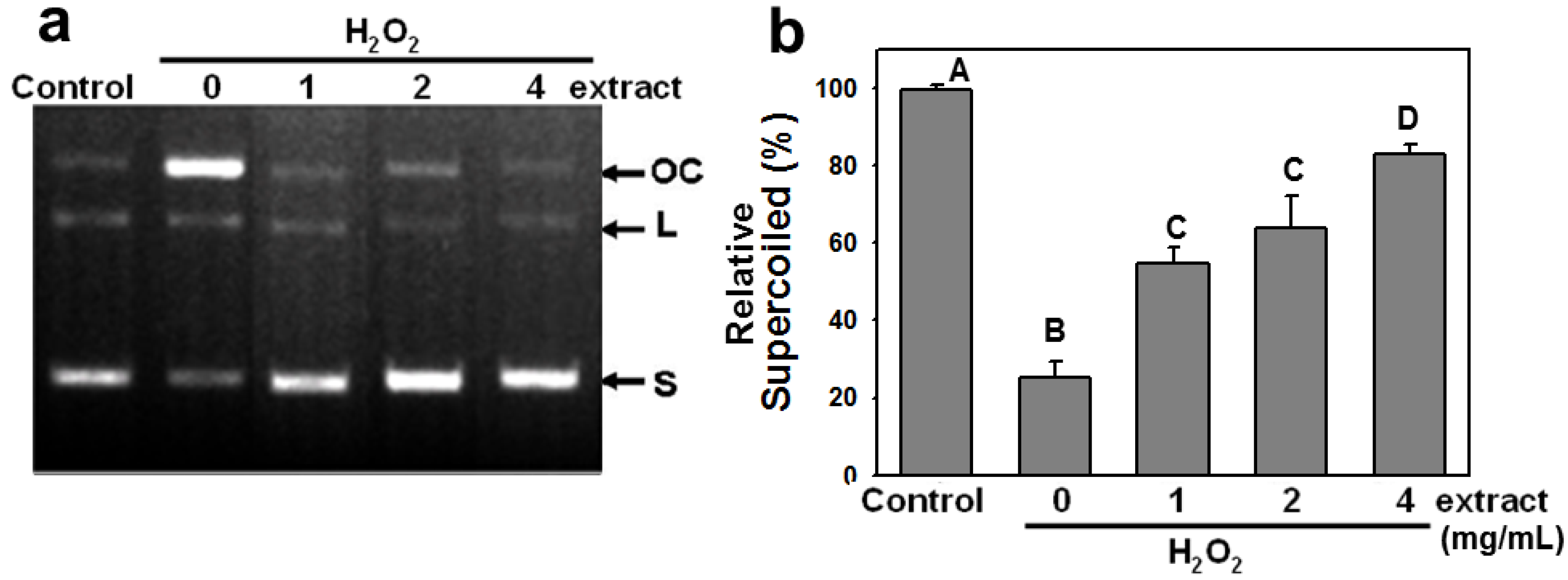
2.2. AEGT Modulates H2O2-Induced Plasmid DNA Strand Breaks
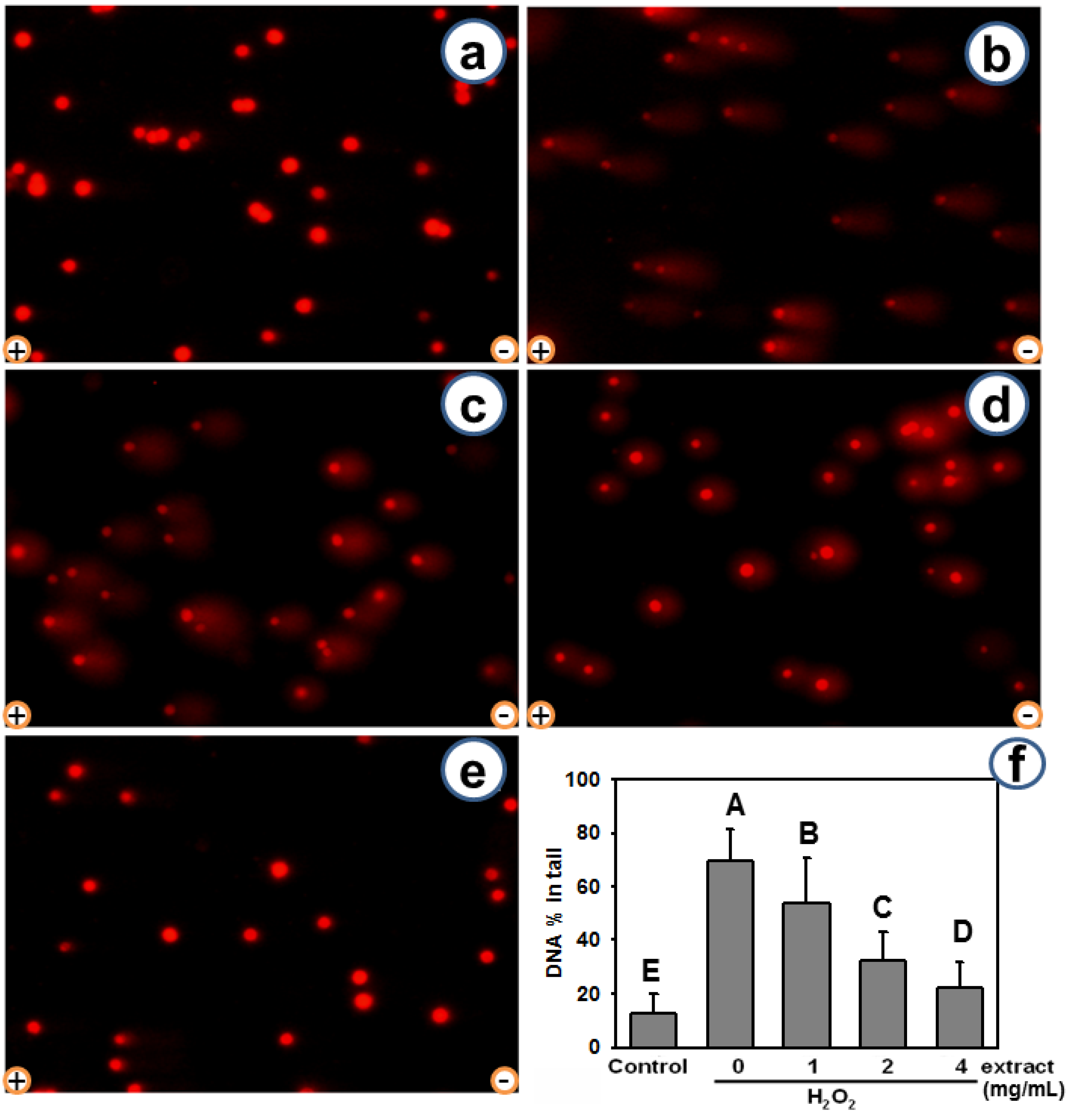
2.3. AEGT Modulates H2O2-Induced Cellular DNA Damage
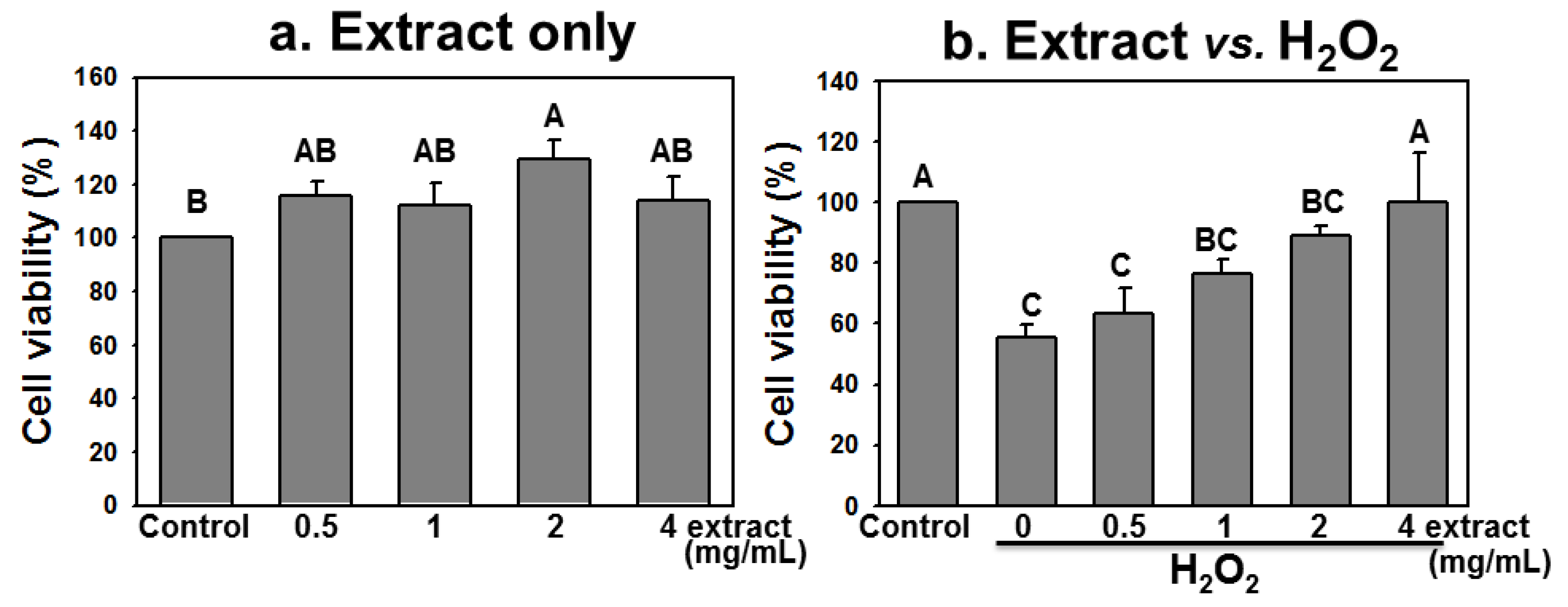
2.4. AEGT Promotes Cell Survival under H2O2 Treatment
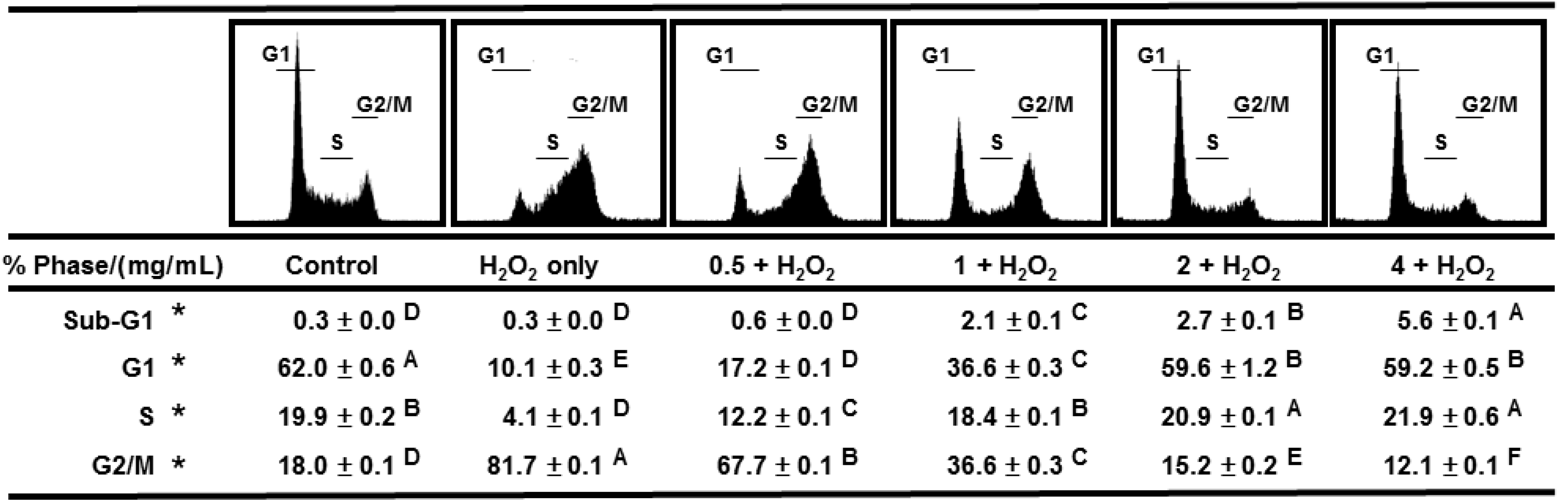
2.5. AEGT Prevents Cell Cycle Arrest by H2O2
2.6. Discussion
3. Experimental
3.1. Raw Materials
3.2. Extraction and Isolation of Seaweed G. tenuistipitata
3.3. Determination of Total Phenolics, Flavonoid, and Ascorbic Acid of AEGT
3.4. Free Radical Scavenging Activity
3.5. Cell Cultures
3.6. Plasmid DNA Cleavage Assay
3.7. Comet-NE Assay
3.8. Cell Viability Assay
3.9. Cell Cycle Histogram Obtained by Propidium Iodide Staining in Flow Cytometry
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chiang, Y.M.; Lin, J.L. Nitrate uptake by nitrogen-starved plants of the red alga Gracilaria tenuistipitata var. Liui. Jpn. J. Phycol. 1989, 37, 187–193. [Google Scholar]
- Armisen, R. World-wide use and importance of Gracilaria. J. Appl. Phycol. 1995, 7, 231–243. [Google Scholar] [CrossRef]
- Ajisaka, T.; Chiang, Y.M. Recent status of Gracilaria cultivation in Taiwan. Hydrobiologia 1993, 260/261, 335–338. [Google Scholar] [CrossRef]
- Jha, R.K.; Zi-rong, X. Biomedical compounds from marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2002, 19, 1–48. [Google Scholar]
- Hsu, B.Y.; Tsao, C.Y.; Chiou, T.K.; Hwang, P.A.; Hwang, D.F. HPLC determination for prostaglandins from seaweed Gracilaria gigas. Food Control 2007, 18, 639–645. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Bourret, E. Polysaccharides from the red seaweed Gracilaria dura (Gracilariales, Rhodophyta). Bioresour. Technol. 2005, 96, 379–382. [Google Scholar] [CrossRef]
- de Almeida, C.L.; Falcao Hde, S.; Lima, G.R.; Montenegro Cde, A.; Lira, N.S.; de Athayde-Filho, P.F.; Rodrigues, L.C.; de Souza Mde, F.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef]
- Lin, Y.H.; Tsai, J.S.; Hung, L.B.; Pan, B.S. Hypocholesterolemic effect of compounded freshwater clam protein hydrolysate and Gracilaria. Food Chem. 2010, 123, 395–399. [Google Scholar] [CrossRef]
- Yangthong, M.; Hutadilok-Towatana, N.; Phromkunthong, W. Antioxidant activities of four edible seaweeds from the southern coast of Thailand. Plant Foods Hum. Nutr. 2009, 64, 218–223. [Google Scholar] [CrossRef]
- Ganesan, P.; Kumar, C.S.; Bhaskar, N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour. Technol. 2008, 99, 2717–2723. [Google Scholar] [CrossRef]
- Vijayavel, K.; Martinez, J.A. In vitro antioxidant and antimicrobial activities of two Hawaiian marine Limu: Ulva fasciata (Chlorophyta) and Gracilaria salicornia (Rhodophyta). J. Med. Food 2010, 13, 1494–1499. [Google Scholar] [CrossRef]
- Souza, B.W.; Cerqueira, M.A.; Martins, J.T.; Quintas, M.A.; Ferreira, A.C.; Teixeira, J.A.; Vicente, A.A. Antioxidant potential of two red seaweeds from the Brazilian coasts. J. Agric. Food Chem. 2011, 59, 5589–5594. [Google Scholar]
- Dang, H.T.; Lee, H.J.; Yoo, E.S.; Shinde, P.B.; Lee, Y.M.; Hong, J.; Kim, D.K.; Jung, J.H. Anti-inflammatory constituents of the red alga Gracilaria verrucosa and their synthetic analogues. J. Nat. Prod. 2008, 71, 232–240. [Google Scholar] [CrossRef]
- Coura, C.O.; de Araujo, I.W.; Vanderlei, E.S.; Rodrigues, J.A.; Quindere, A.; Fontes, B.P.; de Queiroz, I.N.; de Menezes, D.B.; Bezerra, e Silver, A.A.; et al. Antinociceptive and anti-inflammatory activities of sulphated polysaccharides from the red seaweed gracilaria cornea. Basic Clin. Pharmacol. Toxicol. 2011, 110, 335–341. [Google Scholar]
- Yeh, S.T.; Lin, Y.C.; Huang, C.L.; Chen, J.C. White shrimp Litopenaeus vannamei that received the hot-water extract of Gracilaria tenuistipitata showed protective innate immunity and up-regulation of gene expressions after low-salinity stress. Fish Shellfish Immunol. 2010, 28, 887–894. [Google Scholar] [CrossRef]
- Martinet, W.; Knaapen, M.W.; De Meyer, G.R.; Herman, A.G.; Kockx, M.M. Oxidative DNA damage and repair in experimental atherosclerosis are reversed by dietary lipid lowering. Circ. Res. 2001, 88, 733–739. [Google Scholar] [CrossRef]
- Rigoulet, M.; Yoboue, E.D.; Devin, A. Mitochondrial ROS generation and its regulation: Mechanisms involved in H(2)O(2) signaling. Antioxid. Redox Signal. 2011, 14, 459–468. [Google Scholar] [CrossRef]
- Cao, W.; Chen, W.J.; Suo, Z.R.; Yao, Y.P. Protective effects of ethanolic extracts of buckwheat groats on DNA damage caused by hydroxyl radicals. Food Res. Int. 2008, 41, 924–929. [Google Scholar] [CrossRef]
- Wijeratne, S.S.; Cuppett, S.L.; Schlegel, V. Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colon cells. J. Agric. Food Chem. 2005, 53, 8768–8774. [Google Scholar] [CrossRef]
- Wood, J.M.; Decker, H.; Hartmann, H.; Chavan, B.; Rokos, H.; Spencer, J.D.; Hasse, S.; Thornton, M.J.; Shalbaf, M.; Paus, R.; et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009, 23, 2065–2075. [Google Scholar] [CrossRef]
- Spencer, J.D.; Gibbons, N.C.; Rokos, H.; Peters, E.M.; Wood, J.M.; Schallreuter, K.U. Oxidative stress via hydrogen peroxide affects proopiomelanocortin peptides directly in the epidermis of patients with vitiligo. J. Invest. Dermatol. 2007, 127, 411–420. [Google Scholar] [CrossRef]
- Seomun, Y.; Kim, J.T.; Kim, H.S.; Park, J.Y.; Joo, C.K. Induction of p21Cip1-mediated G2/M arrest in H2O2-treated lens epithelial cells. Mol. Vis. 2005, 11, 764–774. [Google Scholar]
- Thorn, T.; Gniadecki, R.; Petersen, A.B.; Vicanova, J.; Wulf, H.C. Differences in activation of G2/M checkpoint in keratinocytes after genotoxic stress induced by hydrogen peroxide and ultraviolet A radiation. Free Radic. Res. 2001, 35, 405–416. [Google Scholar] [CrossRef]
- Chien, M.; Rinker-Schaeffer, C.; Stadler, W.M. A G2/M growth arrest response to low-dose intermittent H2O2 in normal uroepithelial cells. Int. J. Oncol. 2000, 17, 425–432. [Google Scholar]
- Lu, S.; Guo, X.; Zhao, P. Effect of Ginkgo biloba extract 50 on immunity and antioxidant enzyme activities in ischemia reperfusion rats. Molecules 2011, 16, 9194–9206. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, C.; Zu, Y.; Song, Z.; Zhang, B.; Meng, X.; Qiu, W.; Zhang, L. SFE-CO2 extract from Typhonium giganteum Engl. tubers, induces apoptosis in human hepatoma SMMC-7721 cells involvement of a ROS-mediated mitochondrial pathway. Molecules 2011, 16, 8228–8242. [Google Scholar] [CrossRef]
- Simula, M.P.; De Re, V. Hepatitis C virus-induced oxidative stress and mitochondrial dysfunction: A focus on recent advances in proteomics. Proteom. Clin. Appl. 2010, 4, 782–793. [Google Scholar] [CrossRef]
- Dumont, M.; Beal, M.F. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic. Biol. Med. 2011, 51, 1014–1026. [Google Scholar] [CrossRef]
- de Oliveira, C.B.; Comunello, L.N.; Lunardelli, A.; Amaral, R.H.; Pires, M.G.; da Silva, G.L.; Manfredini, V.; Vargas, C.R.; Gnoatto, S.C.; de Oliveira, J.R.; et al. Phenolic enriched extract of Baccharis trimera presents anti-inflammatory and antioxidant activities. Molecules 2012, 17, 1113–1123. [Google Scholar] [CrossRef]
- Pieroni, L.G.; de Rezende, F.M.; Ximenes, V.F.; Dokkedal, A.L. Antioxidant activity and total phenols from the methanolic extract of Miconia albicans (Sw.) Triana leaves. Molecules 2011, 16, 9439–9450. [Google Scholar] [CrossRef]
- Heo, S.J.; Jeon, Y.J.; Lee, J.; Kim, H.T.; Lee, K.W. Antioxidant effect of enzymatic hydrolyzate from a Kelp, Ecklonia cava. Algae 2003, 18, 341–347. [Google Scholar] [CrossRef]
- Munda, I.M. Preliminary information on the ascorbic acid content in some Adriatic seaweeds. Hydrobiologia 1987, 151/152, 477–481. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Lee, K.W.; Kim, S.H.; Ha, J.W.; Park, G.T.; Jeon, Y.J. Lipid peroxidation inhibitory effects of Hizikia fusiformis methanolic extract on fish oil and linoleic acid. Food Sci. Technol. Int. 2004, 10, 65–72. [Google Scholar] [CrossRef]
- Nakamura, T.; Nagayama, K.; Uchida, K.; Tanaka, R. Antioxidant activity of phlorotannins from the brown alga Eisenia bicyclis. Fish. Sci. 1996, 62, 923–926. [Google Scholar]
- Scalzo, R.L. Organic acids influence on DPPH scavenging by ascorbic acid. Food Chem. 2008, 107, 40–43. [Google Scholar] [CrossRef]
- Yang, J.I.; Ho, H.Y.; Chu, Y.J.; Chow, C.J. Characteristic and antioxidant activity of retorted gelatin hydrolysate from cobia (Rachycentron canadum) skin. Food Chem. 2008, 110, 128–136. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Jimenez-Escrig, A.; Rincon, M.; Pulido, R.; Saura-Calixto, F. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. J. Agric. Food Chem. 2001, 49, 5489–5493. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative process in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and diseas. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Onuki, J.; Medeiros, M.H.; Bechara, E.J.; Di Mascio, P. 5-Aminolevulinic acid induces single-strand breaks in plasmid pBR322 DNA in the presence of Fe2+ ions. Biochim. Biophys. Acta 1994, 1225, 259–263. [Google Scholar] [CrossRef]
- Karawita, R.; Senevirathne, M.; Athukorala, Y.; Affan, A.; Lee, Y.J.; Kim, S.K.; Lee, J.B.; Jeon, Y.J. Protective effect of enzymatic extracts from microalgae against DNA damage induced by H2O2. Mar. Biotechnol. (NY) 2007, 9, 479–490. [Google Scholar] [CrossRef]
- Nizard, C.; Poggioli, S.; Heusele, C.; Bulteau, A.L.; Moreau, M.; Saunois, A.; Schnebert, S.; Mahe, C.; Friguet, B. Algae extract protection effect on oxidized protein level in human stratum corneum. Ann. NY Acad. Sci. 2004, 1019, 219–222. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Jones, E.; Hurghes, R.E. Foliar ascorbic acid in some angiosperms. Phytochemistry 1983, 22, 2493–2499. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Agric. Food Chem. 1998, 37, 99–105. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 26, 1199–1200. [Google Scholar] [CrossRef]
- Chen, B.H.; Chang, H.W.; Huang, H.M.; Chong, I.W.; Chen, J.S.; Chen, C.Y.; Wang, H.M. (−)-Anonaine induces DNA damage and inhibits growth and migration of human lung carcinoma H1299 cells. J. Agric. Food Chem. 2011, 59, 2284–2290. [Google Scholar] [CrossRef]
- Li, P.Y.; Chang, Y.C.; Tzang, B.S.; Chen, C.C.; Liu, Y.C. Antibiotic amoxicillin induces DNA lesions in mammalian cells possibly via the reactive oxygen species. Mutat. Res. 2007, 629, 133–139. [Google Scholar] [CrossRef]
- Wang, A.S.; Ramanathan, B.; Chien, Y.H.; Goparaju, C.M.; Jan, K.Y. Comet assay with nuclear extract incubation. Anal. Biochem. 2005, 337, 70–75. [Google Scholar]
- Chang, Y.C.; Liao, C.B.; Hsieh, P.Y.; Liou, M.L.; Liu, Y.C. Expression of tumor suppressor p53 facilitates DNA repair but not UV-induced G2/M arrest or apoptosis in Chinese hamster ovary CHO-K1 cells. J. Cell. Biochem. 2008, 103, 528–537. [Google Scholar] [CrossRef]
- Chang, Y.C.; Jan, K.Y.; Cheng, C.A.; Liao, C.B.; Liu, Y.C. Direct involvement of the tumor suppressor p53 in nucleotide excision repair. DNA Repair (Amst) 2008, 7, 751–761. [Google Scholar] [CrossRef]
- Cemeli, E.; Mirkova, E.; Chiuchiarelli, G.; Alexandrova, E.; Anderson, D. Investigation on the mechanisms of genotoxicity of butadiene, styrene and their combination in human lymphocytes using the Comet assay. Mutat. Res. 2009, 664, 69–76. [Google Scholar] [CrossRef]
- CometScore, version 1.5. Available online: http://www.tritekcorp.com (accessed on 11 June 2012).
- Wong, V.W.C.; Szeto, Y.T.; Collins, A.R.; Benzie, I.F.F. The comet assay: A biomonitoring tool for nutraceutical research. Curr. Top. Nutraceut. Res. 2005, 3, 1–14. [Google Scholar]
- Collins, A.R. The comet assay for DNA damage and repair: Principles, applications, and limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, J.Y.; Wu, J.E.; Pu, Y.S.; Liu, G.Y.; Pan, M.H.; Huang, Y.T.; Huang, A.M.; Hwang, C.C.; Chung, S.J.; et al. Ling-Zhi polysaccharides potentiate cytotoxic effects of anticancer drugs against drug-resistant urothelial carcinoma cells. J. Agric. Food Chem. 2010, 58, 8798–8805. [Google Scholar]
- Chiu, C.C.; Li, C.H.; Fuh, T.S.; Chen, W.L.; Huang, C.S.; Chen, L.J.; Ung, W.H.; Fang, K. The suppressed proliferation and premature senescence by ganciclovir in p53-mutated human non-small-lung cancer cells acquiring herpes simplex virus-thymidine kinase cDNA. Cancer Detect. Prev. 2005, 29, 286–293. [Google Scholar] [CrossRef]
- Nunez, R. DNA measurement and cell cycle analysis by flow cytometry. Curr. Issues Mol. Biol. 2001, 3, 67–70. [Google Scholar]
- JMP® 9 software. Available online: http://www.jmp.com/ (accessed on 11 June 2012).
- Sample Availability: Samples of aqueous extract of Gracilaria tenuistipitata (AEGT) are available upon request from Dr. Jing-Iong Yang.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, J.-I.; Yeh, C.-C.; Lee, J.-C.; Yi, S.-C.; Huang, H.-W.; Tseng, C.-N.; Chang, H.-W. Aqueous Extracts of the Edible Gracilaria tenuistipitata are Protective Against H2O2-Induced DNA Damage, Growth Inhibition, and Cell Cycle Arrest. Molecules 2012, 17, 7241-7254. https://doi.org/10.3390/molecules17067241
Yang J-I, Yeh C-C, Lee J-C, Yi S-C, Huang H-W, Tseng C-N, Chang H-W. Aqueous Extracts of the Edible Gracilaria tenuistipitata are Protective Against H2O2-Induced DNA Damage, Growth Inhibition, and Cell Cycle Arrest. Molecules. 2012; 17(6):7241-7254. https://doi.org/10.3390/molecules17067241
Chicago/Turabian StyleYang, Jing-Iong, Chi-Chen Yeh, Jin-Ching Lee, Szu-Cheng Yi, Hurng-Wern Huang, Chao-Neng Tseng, and Hsueh-Wei Chang. 2012. "Aqueous Extracts of the Edible Gracilaria tenuistipitata are Protective Against H2O2-Induced DNA Damage, Growth Inhibition, and Cell Cycle Arrest" Molecules 17, no. 6: 7241-7254. https://doi.org/10.3390/molecules17067241
APA StyleYang, J.-I., Yeh, C.-C., Lee, J.-C., Yi, S.-C., Huang, H.-W., Tseng, C.-N., & Chang, H.-W. (2012). Aqueous Extracts of the Edible Gracilaria tenuistipitata are Protective Against H2O2-Induced DNA Damage, Growth Inhibition, and Cell Cycle Arrest. Molecules, 17(6), 7241-7254. https://doi.org/10.3390/molecules17067241




