Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Xanthones Isolated from Artocarpus obtusus F.M. Jarrett
Abstract
:1. Introduction
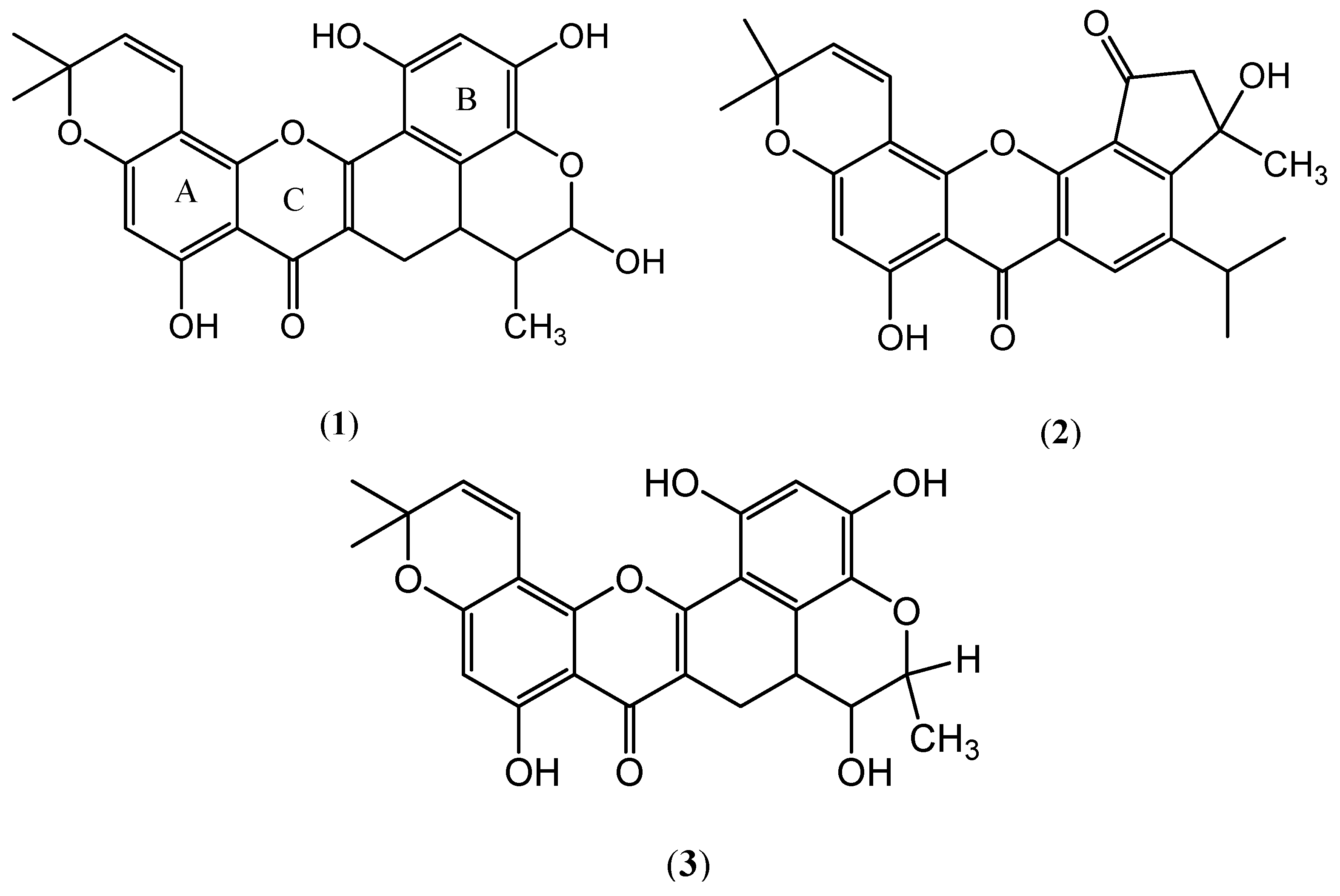
2. Results and Discussion
2.1. Antioxidant Activity
2.2. Antimicrobial Activity
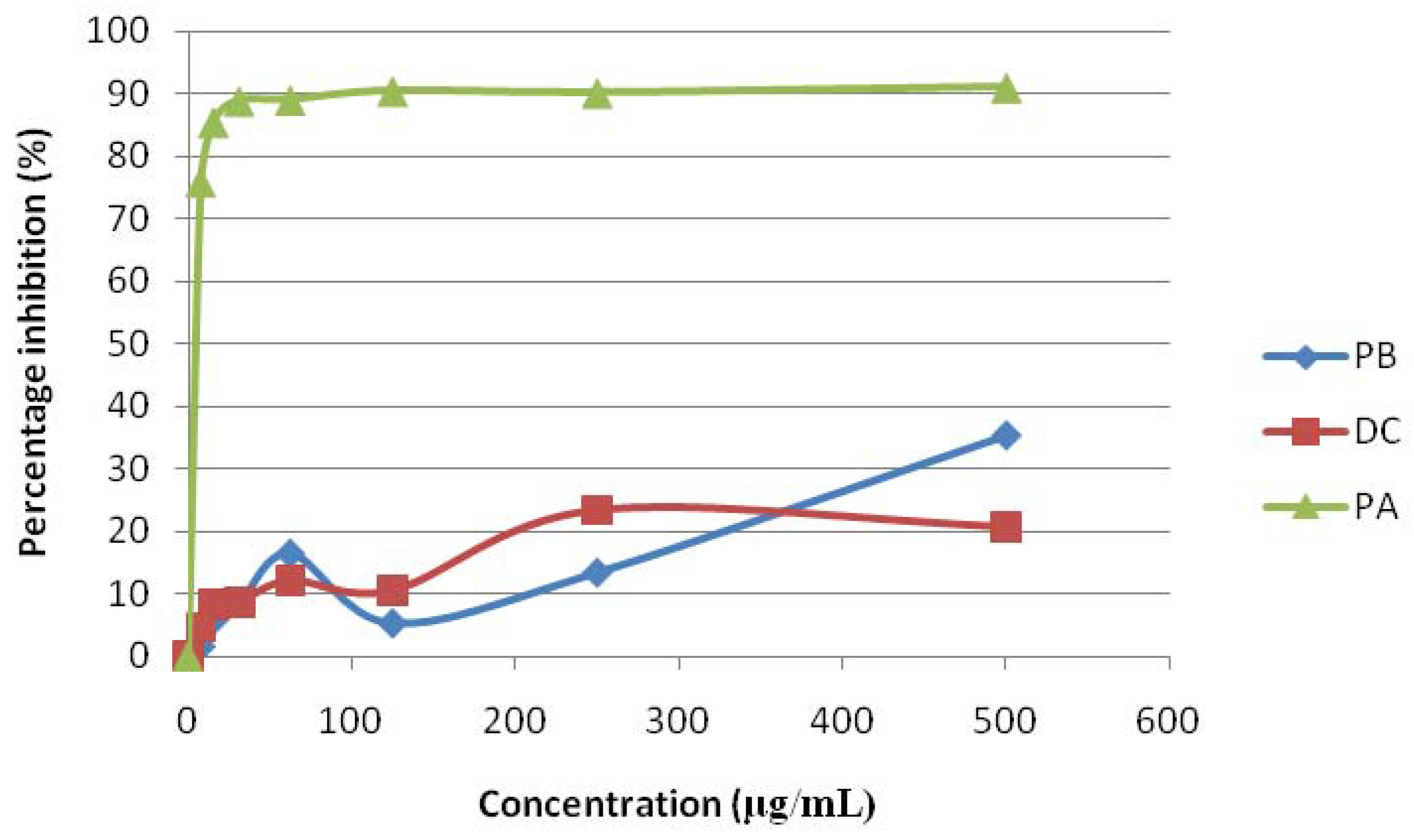
2.3. Tyrosinase Inhibitory Activity
3. Experimental
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Preparation of Plant Extracts and Isolation Procedure
3.4. DPPH Free Radical Scavenging Activity Assay
3.5. Bacterial and Fungal Cultures
3.6. Disc Diffusion Assay
3.7. Tyrosinase Inhibitory Activity Assay
- (A)
- 20 μL of mushroom tyrosinase in 20 mM phosphate buffer (480 units/mL) Sigma
- (B)
- 140 μL of 20 mM phosphate buffer (pH 6.8), and 20 μL of methanol
- (C)
- 20 μL of mushroom tyrosinase solution (480 units/mL), 140 μL of 20 mM phosphate buffer (pH 6.8), and 20 μL of sample solution
- (D)
- 160 μL of 20 mM phosphate buffer (ph 6.8) and 20 μL of sample solution.
4. Conclusions
Acknowledgements
References and Notes
- McChesney, J.D.; Venkataraman, S.K.; Henri, J.T. Plant natural products: Back to the future or into extinction. Phytochemistry 2007, 68, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Narváez-Mastache, J.M.; Novillo, F.; Delgado, G. Antioxidant aryl-prenylcoumarin, flavan-3-ols and flavonoids from Eysenhardtia subcoriacea. Phytochemistry 2008, 69, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Tadhani, M.B.; Patel, V.H.; Subash, R. In-vitro antioxidant activities of Stevia rebaudiana leaves and callus. J. Food Compos. Anal. 2007, 20, 323–329. [Google Scholar] [CrossRef]
- Kochummen, K.M.; Go, R. Moraceae. In Tree Flora Sabah and Sarawak; Ampang Press: Kuala Lumpur, Malaysia, 2000; pp. 181–212. [Google Scholar]
- Jamil, S.; Sirat, H.M.; Jantan, I.; Aimi, N.; Kitajima, M. A new prenylated dihydrochalcone from the leaves of Artocarpus Iowii. J. Nat. Med. 2008, 62, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Hakim, E.H.; Achmad, S.A.; Juliawaty, L.D.; Makmur, L.; Syah, Y.M.; Aimi, N.; Kitajima, M.; Takayama, H.; Ghisalberti, E.L. Prenylated flavonoids and related compounds of the Indonesian Artocarpus (Moraceae). J. Nat Med. 2006, 60, 161–184. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Omoloso, A.D.; Kinara, M. Antibacterial activity of Artocarpus heterophyllus. Fitoterapia 2003, 74, 501–505. [Google Scholar] [CrossRef]
- Su, B.N.; Cuendet, M.; Hawthorne, M.E.; Kardono, L.B.S.; Riswan, S.; Fong, H.H.S.; Mehta, R.G.; Pezzuto, J.M.; Kinghorn, A.D. Constituents of the bark and twigs of Artocarpus dadah with cyclooxgenase inhibitory activity. J. Nat. Prod. 2002, 65, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Killmer, L.; Offen, P.; Taylor, P.B.; Votta, B.J.; Johnson, R.K. A new dimeric dihydrochalcone and a new prenylated flavone from the bud covers of Artocarpus altilis: Potent inhibitors of cathepsin. J. Nat. Prod. 2002, 65, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Boonlaksiri, C.; Oonanant, W.; Kongsaeree, P.; Kittakoop, P.; Tanticharoen, M.; Thebtaranonth, Y. An antimalarial stilbene from Artocarpus integer. Phytochemistry 2000, 54, 415–417. [Google Scholar] [CrossRef]
- Lin, C.N.; Shieh, W.L.; Jong, T.T. A pyranodihydrobenzoxanthone epoxide from Artocarpus communis. Phytochemistry 1992, 31, 2563–2564. [Google Scholar]
- Sultanbawa, M.U.; Surendrakumar, S. Two pyranodihydrobenzoxanthones from Artocarpus nobilis. Phytochemistry 1989, 28, 599–605. [Google Scholar]
- Arung, E.T.; Muladi, S.; Sukaton, E.; Shimizu, K.; Kondo, R. Artocarpin, a promosing compound as whitening agent and anti-skin cancer. J. Trop. Wood Sci. Technol. 2008, 6, 33–36. [Google Scholar]
- Jayasinghe, L.; Balasooriya, B.A.I.S.; Padmini, W.C.; Hara, N.; Fujimoto, Y. Geranyl chalcone derivatives with antifungal and radical scavenging properties from the leaves of Artocarpus nobilis. Phytochemistry 2004, 65, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Hashim, N.M.; Sukari, M.A.; Ee, G.C.L.; Ali, A.M.; Alitheen, N.B.; Go, R. Antioxidant, cytotoxic and antibacterial activities of thirteen species of Artocarpus (Moraceae). J. Med. Arom. Plant Sci. 2009, 31, 142–146. [Google Scholar]
- Hashim, N.M.; Rahmani, M.; Sukari, M.A.; Ali, A.M.; Alitheen, N.B.; Ismail, H.B.M.; Go, R. Two new xanthones from Artocarpus obtusus. J. Asian Nat. Prod. Res. 2010, 12, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.M.; Rahmani, M.; Ee, G.C.L.; Sukari, M.A.; Yahayu, M.; Oktima, W.; Ali, A.M.; Go, R. Antiproliferative activity of xanthones isolated from Artocarpus obtusus. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.M.; Ali, A.M.; Rahmani, M.; Wiart, C.; Dhaliwal, J.S.; Yusoff, K. Apoptotic and necrotic cell death as manifestation in leukemic cell treated with methylgerambullin, a sulphone from Glycosmis calcicola. J. Biochem. Mol. Biol. Biophys. 2000, 4, 253–262. [Google Scholar]
- Ee, G.C.L.; Lim, C.M.; Rahmani, M.; Shaari, K.; Bong, C.F.J. Pellitorine, a potential anticancer lead compound against HL60 and MCF-7 cell lines and microbial transformation of piperine from Piper nigrum. Molecules 2000, 15, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Susidarti, R.A.; Rahmani, M.; Ismail, H.B.M.; Sukari, M.A.; Taufiq-Yap, Y.H.; Ee, G.C.L.; Ali, A.M. Cytotoxic activity of coumarins from Micromelum minutum. Pharm. Biol. 2009, 47, 182–185. [Google Scholar] [CrossRef]
- Jagtap, U.B.; Bapat, V.A. Artocarpus: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2010, 129, 142–166. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Noguchi, N.; Niki, E. Galvinoxyl method for standardizing electron and proton donation activity. In Methods in Enzymology: Flavonoids and other Polyphenols; Packer, L., Ed.; Academic Press: London, UK, 2001; pp. 157–165. [Google Scholar]
- Bors, W.; Michel, C.; Stettmaier, K. Structure-activity relationships governing antioxidant capacities of plant polyphenols. In Methods in Enzymology: Flavonoids and other Polyphenols; Packer, L., Ed.; Academic Press: London, UK, 2001; pp. 166–180. [Google Scholar]
- Dangles, O.; Dufour, C.; Manach, C.; Morand, C.; Remesy, C. Binding of flavonoids to plasma protein. In Methods in Enzymology: Flavonoids and other Polyphenols; Packer, L., Ed.; Academic Press: London, UK, 2001; pp. 319–333. [Google Scholar]
- Haraguchi, H. Antioxidative plants constituents. In Bioactive Compounds from Natural Sources; Tringali, C., Ed.; Taylor & Francis: London, UK, 2001; pp. 337–378. [Google Scholar]
- Gould, K.S.; Lister, C. Flavonoids functions in plants. In Flavonoids; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press: London, UK, 2006; pp. 397–441. [Google Scholar]
- Pannala, A.S.; Rice-Evans, C. Rapid screening method for relative antioxidant activities of flavonoids and phenolics. In Methods in Enzymology: Flavonoids and other Polyphenols; Packer, L., Ed.; Academic Press: London, UK, 2001; pp. 266–272. [Google Scholar]
- Burton, G.W.; Hughes, L.; Foster, D.O.; Pietrzak, E.; Samson, M.A.G.; Muller, D.P.R. Antioxidants mechanisms of vitamin E and β-carotene. In Free Radicals from Basic Science to Medicine; Poli, G., Albano, E., Dianzani, M.U., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1993; pp. 388–399. [Google Scholar]
- Suhartati, T.; Achmad, S.A.; Aimi, N.; Hakim, E.H.; Kitajima, M.; Takayama, H.; Takeya, K. Artoindonesianin L, a new prenylated flavones with cytotoxic activity from Artocarpus rotunda. Fitoterapia 2001, 72, 912–918. [Google Scholar] [CrossRef]
- Sato, M.; Fujiwara, S.; Tsuchiya, H.; Fujii, T.; Linuma, M.; Tosa, H.; Ohkawa, Y. Flavones with antibacterial activity against cariogenic bacteria. J. Ethnopharmacol. 1996, 54, 171–176. [Google Scholar] [CrossRef]
- Shimizu, K.; Kondo, R.; Sakai, K. Inhibition of tyrosinase by flavonoids, stilbenes and related 4-substituted resorcinols: structure-activity investigations. Planta Med. 2000, 66, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K.; Sritularak, B. A new dimeric stilbene with tyrosinase inhibitory activity from Artocarpus gomezianus. J. Nat. Prod. 2001, 64, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K.; Sritularak, B.; De-Eknamkul, W. Tyrosinase inhibitors from Artocarpus gomezianus. Planta Med. 2000, 66, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.K.; Lee, D.; Shin, Y.G.; Navarro, H.A.; Kardono, L.B.S.; Rahman, I.; Cordell, G.A.; Farnsworth, N.R.; Pezzuto, J.M.; Kinghorn, A.D.; Wani, M.C.; Wall, M.E. Bioactive prenylated flavonoids from the stem bark of Artocarpus kemando. Phytochemistry 2003, 31, 364–367. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Tutck, M. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Pathol. 1966, 45, 493–496. [Google Scholar]
Sample Availability: Samples of the three compounds are available from the authors. |
| Compounds | IC50 (μg/mL) | % Inhibition |
|---|---|---|
| Pyranocycloartobiloxanthone A (1) | 2 ± 1.2 | 80 |
| Dihydroartoindonesianin C (2) | >500 | not tested |
| Pyranocycloartobiloxanthone B (3) | >500 | not tested |
| Standard | ||
| Ascorbic acid | 20 ± 1.2 | |
| α-tocopherol | 60 ± 2.9 | |
| Quercetin | 40 ± 5.8 | |
| Kojic Acid | 96 |
| Microorganisms | Inhibition zone diameter (in mm) | |||||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | Gentamicin sulfate | Streptomycin sulfate | Nystatin | |
| Bacteria | ||||||
| Reference strains | ||||||
| Bacillus subtilis ATCC6633 | 8 | - | - | 15 | nt | |
| Escherichia coli ATCC25922 | 7 | - | - | 25 | nt | |
| Micrococcus luteus ATCC10240 | - | - | - | nt | nt | |
| Methicillin resistant Staphylococcus aureus ATCC33591 | 20 | - | - | nt | 20 | |
| Pseudomonas aeruginosa JCM2412 | - | nt | nt | 15 | 21 | |
| Staphylococcus aureus ATCC6538 | 9 | - | - | 11 | nt | |
| Salmonella typhimurium S865B (IMR culture) | 9 | - | - | nt | nt | |
| Clinically isolated strain Bacillus subtilis | 12 | nt | nt | nt | - | |
| Fungi | ||||||
| Reference strains | ||||||
| Aspergillus niger ATCC16404 | - | - | - | nt | ||
| Candida albican ATCC1023 | 7 | - | - | 15 | ||
| Saccharomyces cerevisiae S617 (IMR culture) | 8 | - | - | 23 | ||
© 2012 by the authors; This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hashim, N.M.; Rahmani, M.; Ee, G.C.L.; Sukari, M.A.; Yahayu, M.; Amin, M.A.M.; Ali, A.M.; Go, R. Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Xanthones Isolated from Artocarpus obtusus F.M. Jarrett. Molecules 2012, 17, 6071-6082. https://doi.org/10.3390/molecules17056071
Hashim NM, Rahmani M, Ee GCL, Sukari MA, Yahayu M, Amin MAM, Ali AM, Go R. Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Xanthones Isolated from Artocarpus obtusus F.M. Jarrett. Molecules. 2012; 17(5):6071-6082. https://doi.org/10.3390/molecules17056071
Chicago/Turabian StyleHashim, Najihah Mohd., Mawardi Rahmani, Gwendoline Cheng Lian Ee, Mohd Aspollah Sukari, Maizatulakmal Yahayu, Muhamad Aizat Mohd Amin, Abd Manaf Ali, and Rusea Go. 2012. "Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Xanthones Isolated from Artocarpus obtusus F.M. Jarrett" Molecules 17, no. 5: 6071-6082. https://doi.org/10.3390/molecules17056071
APA StyleHashim, N. M., Rahmani, M., Ee, G. C. L., Sukari, M. A., Yahayu, M., Amin, M. A. M., Ali, A. M., & Go, R. (2012). Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Xanthones Isolated from Artocarpus obtusus F.M. Jarrett. Molecules, 17(5), 6071-6082. https://doi.org/10.3390/molecules17056071




